Blue and Green Phosphorescent Organic Light-Emitting Diodes Based on Bis(cyclometalated) Tetrahydrocurcuminate Iridium(III) Complexes
Abstract
1. Introduction
2. Results and Discussion
- (i)
- Preparation of the known chloro-bridged dimer [Ir(N,C-F2ppy)2(µ-Cl)]2 from F2ppyH and IrCl3.3H2O [20];
- (ii)
- Bridge-splitting reaction with the tetrahydrocurcumin anion THC obtained upon treatment of THC–H with sodium methoxide in methanol.
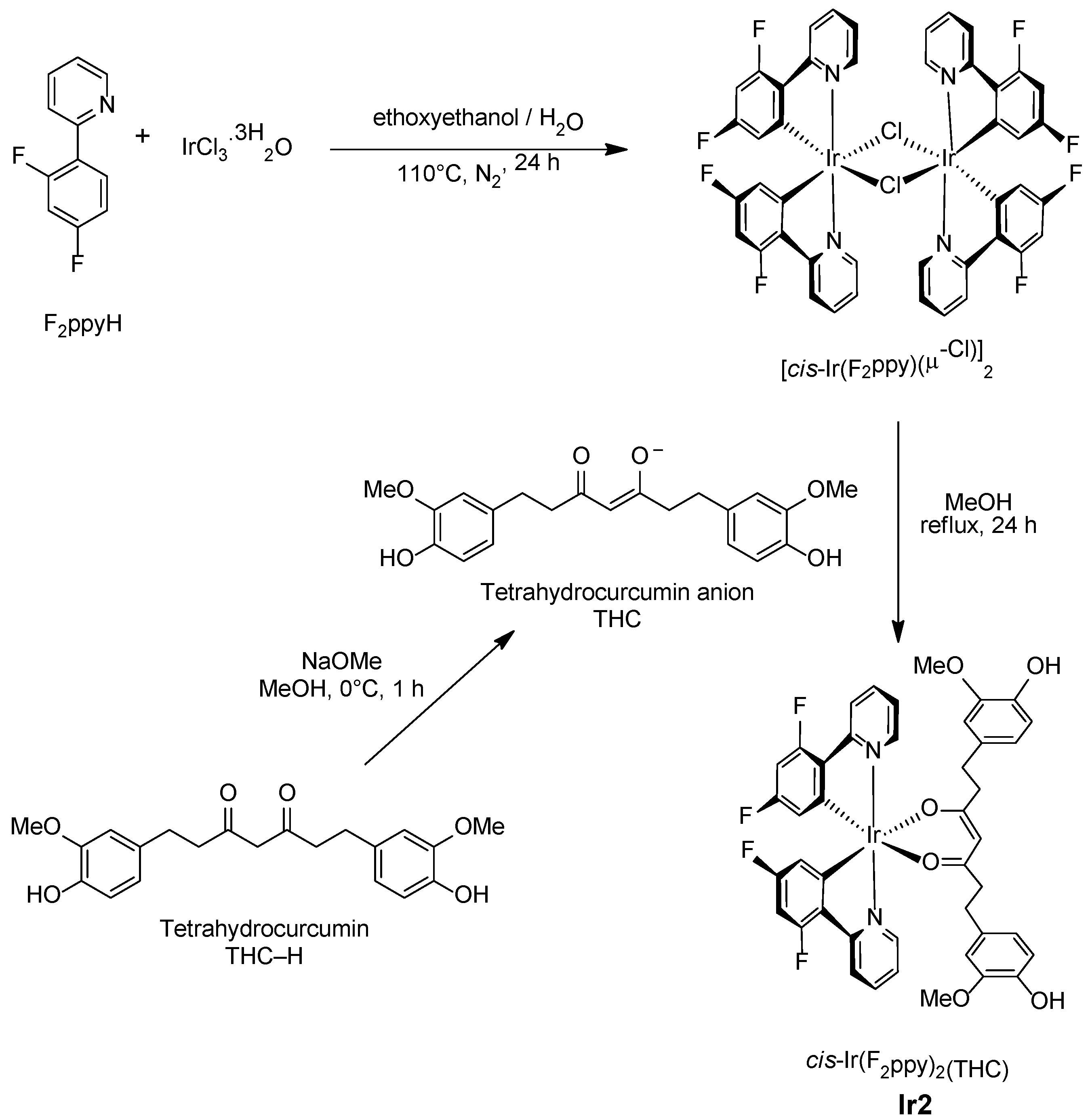
3. Materials and Methods
3.1. General Comments
3.2. Synthesis of Complex Ir2
3.3. Photophysical Characterization
3.4. OLED Fabrication
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIE | Commission Internationale de L’Éclairage |
| EL | Electroluminescence |
| EPA | Diethyl ether/isopentane/ethanol, 2:2:1 v/v |
| EQE | External quantum efficiency |
| ITO | Indium tin oxide |
| OLED | Organic light-emitting diode |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| SOC | Spin–orbit coupling |
| TCSPC | Time-correlated single-photon counting |
| TCTA | 4,4′,4′′-Tris (N-carbazolyl-triphenylamine) |
| TPBi | 2,2′,2′′-(1,3,5-benzinetriyl)-tris(1-phenyl-1H-benzimidazole) |
References
- Gildea, L.F.; Williams, J.A.G. Iridium and platinum complexes for OLEDs. In Organic Light-Emitting Diodes: Materials, Devices and Applications; Buckley, A., Ed.; Woodhead: Cambridge, UK, 2013. [Google Scholar]
- Baldo, M.A.; O’Brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Cebrian, C.; Mauro, M. Recent advances in phosphorescent platinum complexes for organic light-emitting diodes. Beilstein J. Org. Chem. 2018, 14, 1459–1481. [Google Scholar] [CrossRef]
- Pal, A.K.; Krotkus, S.; Fontani, M.; Mackenzie, C.F.R.; Cordes, D.B.; Slawin, A.M.Z.; Samuel, I.D.W.; Zysman-Colman, E. High-Efficiency Deep-Blue-Emitting Organic Light-Emitting Diodes Based on Iridium(III) Carbene Complexes. Adv. Mater. 2018, 30, 1804231. [Google Scholar] [CrossRef]
- Tao, P.; Jin, J.; Zheng, X.; Pu, Y.-J.; Wong, W.-Y. Engineering emissive excited states in organic electroluminescent materials. Matter 2025, 8, 102142. [Google Scholar] [CrossRef]
- Tao, P.; Lü, X.; Zhou, G.; Wong, W.-Y. Asymmetric Tris-Heteroleptic Cyclometalated Phosphorescent Iridium(III) Complexes: An Emerging Class of Metallophosphors. Acc. Mater. Res. 2022, 3, 830–842. [Google Scholar] [CrossRef]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razaq, F.; Kwong, R.; Tsyba, I.; Bortz, M.; Mui, B.; Bau, R.; Thompson, M.E. Synthesis and characterization of phosphorescent cyclometalated iridium complexes. Inorg. Chem. 2001, 40, 1704–1711. [Google Scholar] [CrossRef]
- Adachi, C.; Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Nearly 100% internal phosphorescence efficiency in an organic light emitting device. J. Appl. Phys. 2001, 90, 5048–5051. [Google Scholar] [CrossRef]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razzaq, F.; Lee, H.E.; Adachi, C.; Burrows, P.E.; Forrest, S.R.; Thompson, M.E. Highly Phosphorescent Bis-Cyclometalated Iridium Complexes: Synthesis, Photophysical Characterization, and Use in Organic Light Emitting Diodes. J. Am. Chem. Soc. 2001, 123, 4304–4312. [Google Scholar] [CrossRef]
- Chen, H.-F.; Yang, S.-J.; Tsai, Z.-H.; Hung, W.-Y.; Wang, T.-C.; Wong, K.-T. 1,3,5-Triazine derivatives as new electron transport–type host materials for highly efficient green phosphorescent OLEDs. J. Mater. Chem. 2009, 19, 8112–8118. [Google Scholar] [CrossRef]
- Wang, Z.B.; Helander, M.G.; Qiu, J.; Puzzo, D.P.; Greiner, M.T.; Liu, Z.W.; Lu, Z.H. Highly simplified phosphorescent organic light emitting diode with >20% external quantum efficiency at >10,000 cd/m2. Appl. Phys. Lett. 2011, 98, 073310. [Google Scholar] [CrossRef]
- Helander, M.G.; Wang, Z.B.; Qiu, J.; Greiner, M.T.; Puzzo, D.P.; Liu, Z.W.; Lu, Z.H. Chlorinated Indium Tin Oxide Electrodes with High Work Function for Organic Device Compatibility. Science 2011, 332, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Liehm, P.; Murawski, C.; Furno, M.; Leussem, B.; Leo, K.; Gather, M.C. Comparing the emissive dipole orientation of two similar phosphorescent green emitter molecules in highly efficient organic light-emitting diodes. Appl. Phys. Lett. 2012, 101, 253304. [Google Scholar] [CrossRef]
- Park, Y.S.; Lee, S.; Kim, K.-H.; Kim, S.-Y.; Lee, J.-H.; Kim, J.-J. Exciplex-Forming Co-host for Organic Light-Emitting Diodes with Ultimate Efficiency. Adv. Funct. Mater. 2013, 23, 4914–4920. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Wang, D.-B.; Zeng, W.-J.; Yan, M.-N. High-Efficiency Green Phosphorescent Organic Light-Emitting Diode Based on Simplified Device Structures. Chin. Phys. Lett. 2015, 32, 097803. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Jiao, M.; Lee, W.-K.; Chen, C.-Y.; Tsai, W.L.; Lin, C.-Y.; Wu, C.C. Achieving Above 60% External Quantum Effi ciency in Organic Light-Emitting Devices Using ITO-Free Low-Index Transparent Electrode and Emitters with Preferential Horizontal Emitting Dipoles. Adv. Funct. Mater. 2016, 26, 3250–3258. [Google Scholar] [CrossRef]
- Lee, S.; Kim, K.-H.; Limbach, D.; Park, Y.-S.; Kim, J.-J. Low Roll-Off and High Efficiency Orange Organic Light Emitting Diodes with Controlled Co-Doping of Green and Red Phosphorescent Dopants in an Exciplex Forming Co-Host. Adv. Funct. Mater. 2013, 23, 4105–4110. [Google Scholar] [CrossRef]
- Sun, N.; Zhao, Y.; Zhao, F.; Chen, Y.; Yang, D.; Chen, J.; Ma, D. A white organic light-emitting diode with ultra-high color rendering index, high efficiency, and extremely low efficiency roll-off. Appl. Phys. Lett. 2014, 105, 013303. [Google Scholar] [CrossRef]
- Zhang, T.; He, S.-J.; Wang, D.-K.; Jiang, N.; Lu, Z.-H. A multi-zoned white organic light-emitting diode with high CRI and low color temperature. Sci. Rep. 2016, 6, 20517. [Google Scholar] [CrossRef]
- Dumur, F.; Lepeltier, M.; Siboni, H.Z.; Gigmes, D.; Aziz, H. Phosphorescent organic light-emitting devices (PhOLEDs) based on heteroleptic bis-cyclometalated complexes using acetylacetonate as the ancillary ligand. Synth. Met. 2014, 198, 131–136. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, J.-H.; Park, H.J.; Jung, B.J.; Song, W.; Lee, J.Y.; Hwang, D.-H. Highly efficient and spectrally stable white organic light-emitting diodes. Dye. Pigment. 2018, 149, 363–372. [Google Scholar] [CrossRef]
- Colombo, A.; Fontani, M.; Dragonetti, C.; Roberto, D.; Williams, J.A.G.; Scotto di Perrotolo, R.; Casagrande, F.; Barozzi, S.; Polo, S. A Highly Luminescent Tetrahydrocurcumin Ir III Complex with Remarkable Photoactivated Anticancer Activity. Chem. Eur. J. 2019, 25, 7948–7952. [Google Scholar] [CrossRef]
- Fontani, M.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D.; Marinotto, D. Cyclometalated Ir(III) Complexes with Curcuminoid Ligands as Active Second-Order NLO Chromophores and Building Blocks for SHG Polymeric Films. Inorganics 2020, 8, 36. [Google Scholar] [CrossRef]
- Margapoti, E.; Shukla, V.; Valore, A.; Sharma, A.; Dragonetti, C.; Kitts, C.C.; Roberto, D.; Murgia, M.; Ugo, R.; Muccini, M. Excimer Emission in Single Layer Electroluminescent Devices Based on [Ir(4,5-diphenyl-2-methylthiazolo)2(5-methyl-1,10-phenanthroline)]+[PF6]−. J. Phys. Chem. C 2009, 113, 12517–12522. [Google Scholar] [CrossRef]
- Beyer, B.; Ulbricht, C.; Escudero, D.; Friebe, C.; Winter, A.; Gonzalez, L.; Schubert, U.S. Phenyl-1H-[1,2,3]triazoles as New Cyclometalating Ligands for Iridium(III) Complexes. Organometallics 2009, 28, 5478–5488. [Google Scholar] [CrossRef]
- Rausch, A.F.; Thompson, M.E.; Yersin, H. Blue light emitting Ir(III) compounds for OLEDs—New insights into ancillary ligand effects on the emitting triplet state. J. Phys. Chem. A 2009, 113, 5927–5932. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Humphry-Baker, R.; Berner, D.; Rivier, S.; Zuppiroli, L.; Graetzel, M. Highly phosphorescence iridium complexes and their application in organic light-emitting devices. J. Am. Chem. Soc. 2003, 125, 8790–8797. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Djurovich, P.I.; Alleyne, B.D.; Yousufuddin, M.; Ho, N.N.; Thomas, J.C.; Peters, J.C.; Bau, R.; Thompson, M.E. Synthetic control of excited-state properties in cyclometalated Ir(III) complexes using ancillary ligands. Inorg. Chem. 2005, 44, 1713–1727. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Bian, Z.; Huang, C. Luminescent Iridium Complexes and Their Applications. Top. Organomet. Chem. 2010, 28, 113–142. [Google Scholar]
- Tselekidou, D.; Papadopoulos, K.; Zachariadis, A.; Kyriazopoulos, V.; Kassavetis, S.; Laskarakis, A.; Gioti, M. Solution-processable red phosphorescent OLEDs based on Ir(dmpq)2(acac) doped in small molecules as emitting layer. Mater. Sci. Semicond. Process. 2023, 163, 107546. [Google Scholar] [CrossRef]
- Kalinowski, J.; Stampor, W.; Szmytkowski, J.; Virgili, D.; Cocchi, M.; Fattori, V.; Sabatini, C. Coexistence of dissociation and annihilation of excitons on charge carriers in organic phosphorescent emitters. Phys. Rev. B 2006, 74, 85316. [Google Scholar] [CrossRef]
- Suzuki, K.; Kobayashi, A.; Kaneko, S.; Takehira, K.; Yoshihara, T.; Ishida, H.; Shiina, Y.; Oishi, S.; Tobita, S. Re-evaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector. Phys. Chem. Chem. Phys. 2009, 11, 9850–9860. [Google Scholar] [CrossRef]
- Colombo, A.; De Soricellis, G.; Dragonetti, C.; Fagnani, F.; Roberto, D.; Carboni, B.; Guerchais, V.; Roisnel, T.; Cocchi, M.; Fantacci, S.; et al. Introduction of a mesityl substituent on pyridyl rings as a facile strategy for improving the performance of luminescent 1,3-bis-(2-pyridyl)benzene platinum(ii) complexes: A springboard for blue OLEDs. J. Mater. Chem. C 2024, 12, 9702–9715. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01, Quantum Chemistry Software; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Miertus, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of Ab initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Wachters, A.J.H. Gaussian basis set for molecular wavefunctions containing third-row atoms. J. Chem. Phys. 1970, 52, 1033. [Google Scholar] [CrossRef]
- Petersson, A.; Al-Laham, M.A. A Complete Basis Set Model Chemistry. II. Open-Shell Systems and the Total Energies of the First-Row Atoms. J. Chem. Phys. 1991, 94, 6081–6090. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Valore, A.; Colombo, A.; Dragonetti, C.; Righetto, S.; Roberto, D.; Ugo, R.; De Angelis, F.; Fantacci, S. Luminescent cyclometallated Ir(iii) and Pt(ii) complexes with β-diketonate ligands as highly active second-order NLO chromophores. Chem. Commun. 2010, 46, 2414–2416. [Google Scholar] [CrossRef]
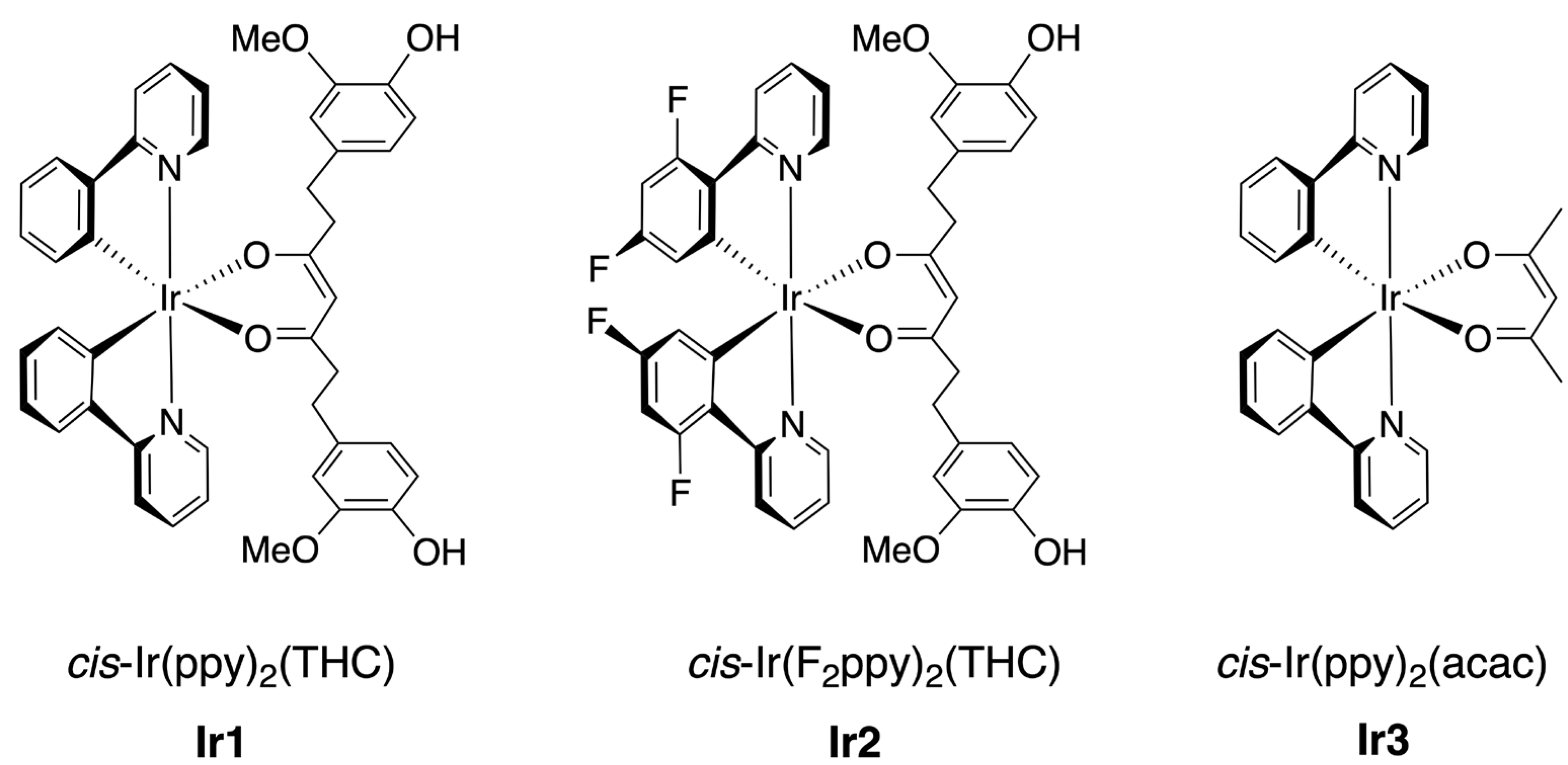
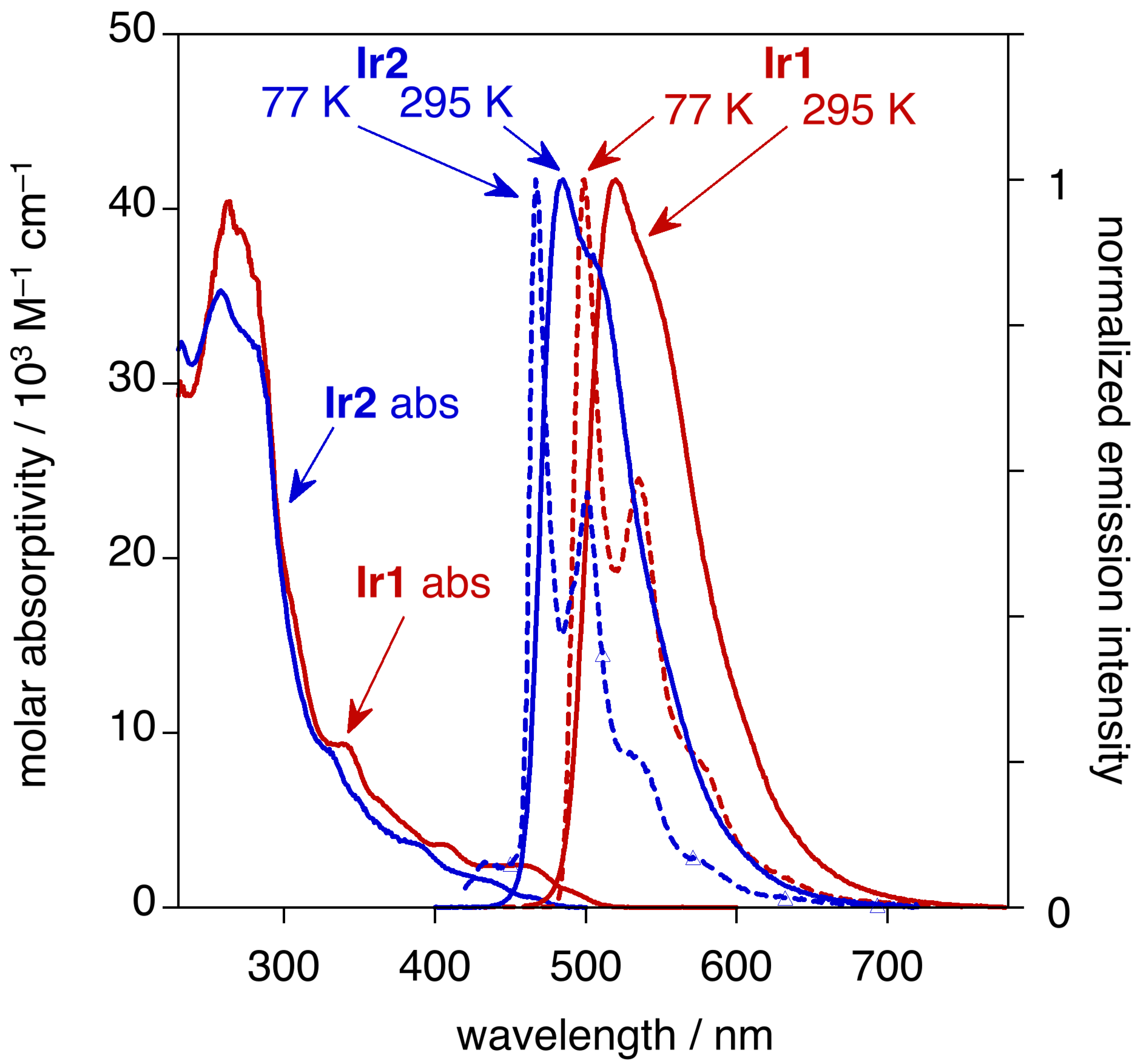
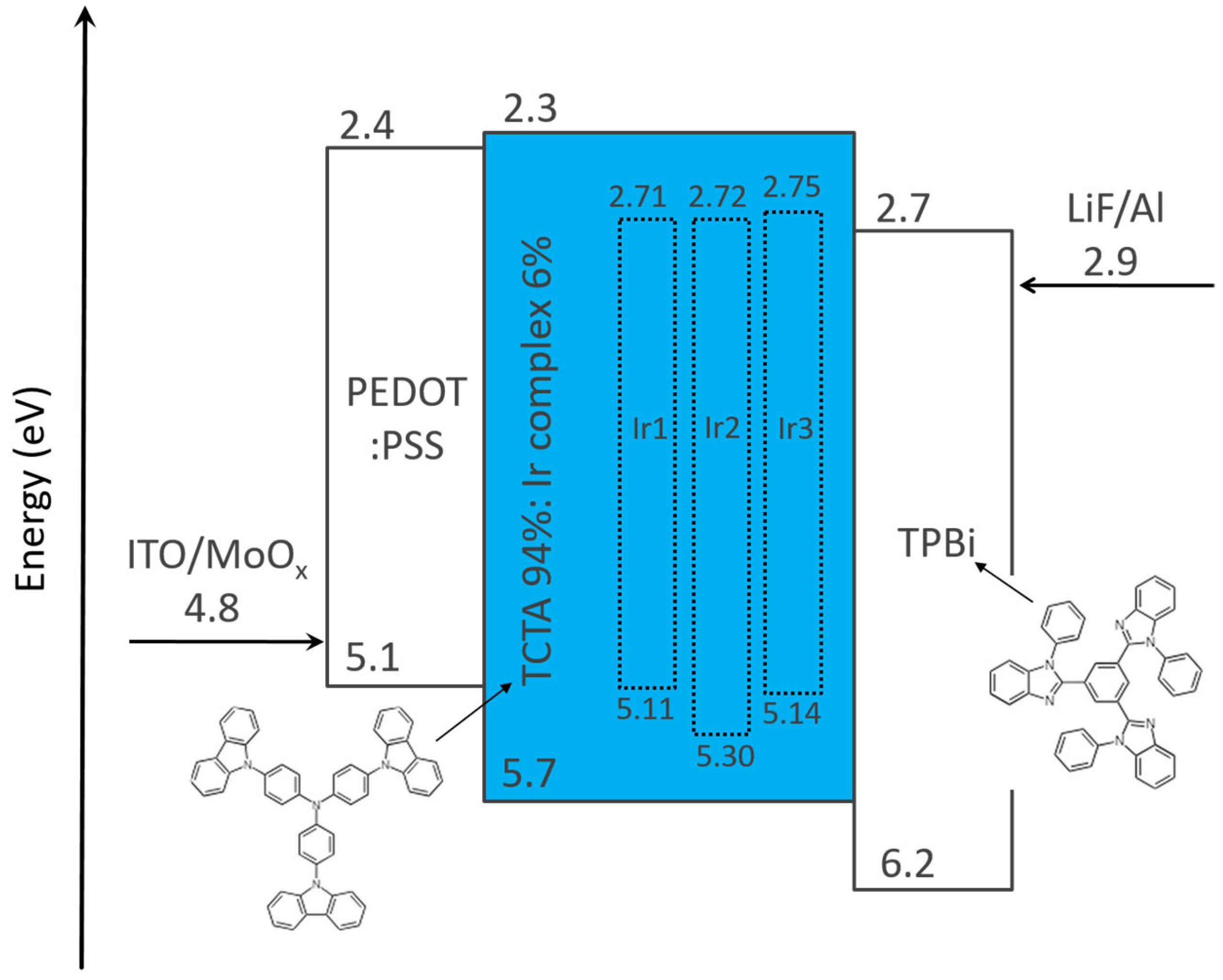
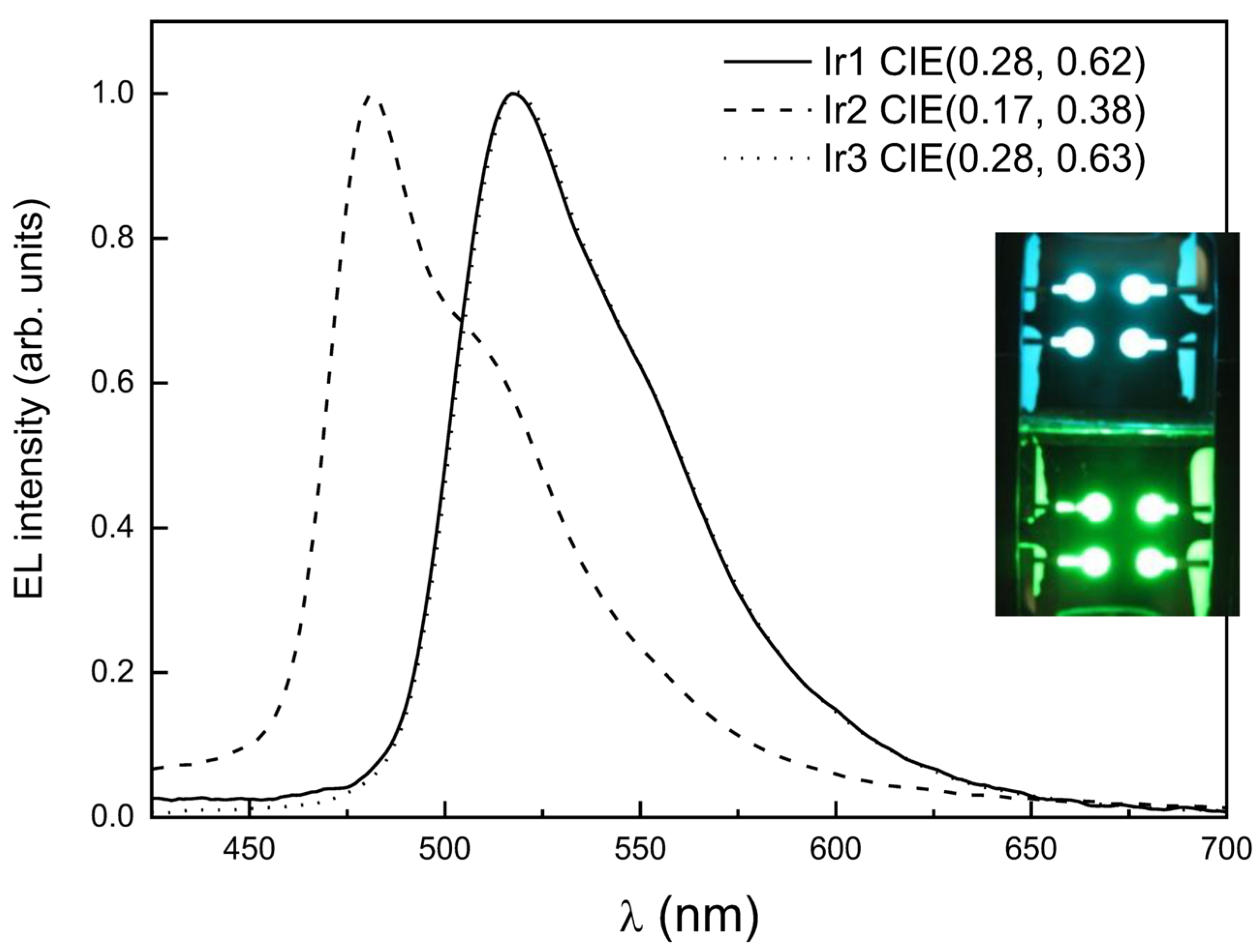
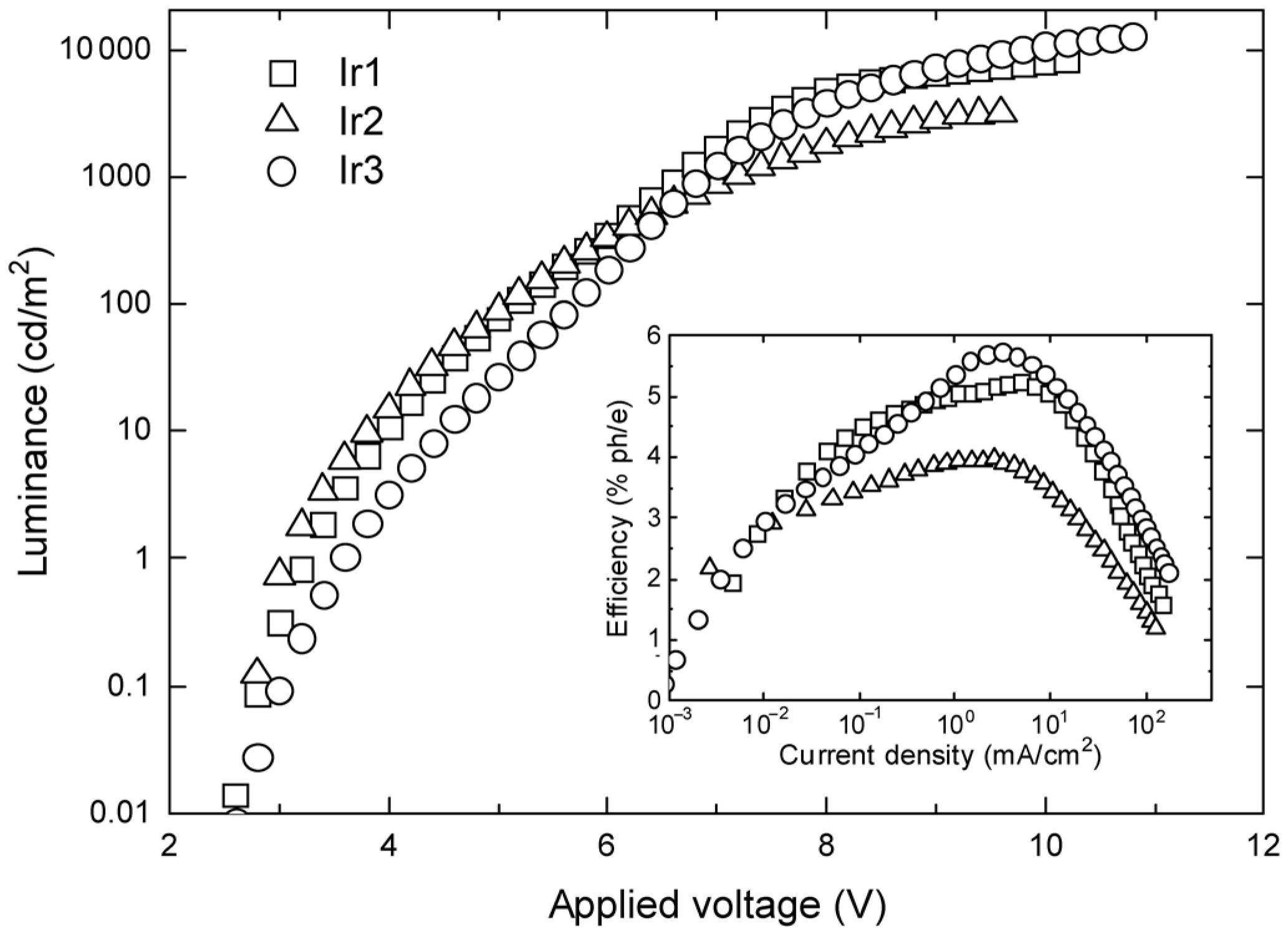
| Complex | λmax,em/nm | Φlum | τ/µs | T1/eV | HOMO/eV | LUMO/eV | OLED CIE (x, y) |
|---|---|---|---|---|---|---|---|
| Ir1 | 520 a | 0.90 a | 1.8 a | 2.40 b | −5.11 b,e | −2.71 b,f | 0.28, 0.62 b |
| Ir2 | 484 b | 0.32 b | 1.1 b | 2.58 b | −5.30 b,e | −2.72 b,f | 0.17, 0.38 b |
| Ir3 | 516 c | 0.53 d | 1.6 c | 2.39 b | −5.14 b,e | −2.75 b,f | 0.28, 0.63 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagnani, F.; Colombo, A.; Dragonetti, C.; Fontani, M.; Roberto, D.; Cocchi, M.; Fantacci, S.; Williams, J.A.G. Blue and Green Phosphorescent Organic Light-Emitting Diodes Based on Bis(cyclometalated) Tetrahydrocurcuminate Iridium(III) Complexes. Inorganics 2025, 13, 390. https://doi.org/10.3390/inorganics13120390
Fagnani F, Colombo A, Dragonetti C, Fontani M, Roberto D, Cocchi M, Fantacci S, Williams JAG. Blue and Green Phosphorescent Organic Light-Emitting Diodes Based on Bis(cyclometalated) Tetrahydrocurcuminate Iridium(III) Complexes. Inorganics. 2025; 13(12):390. https://doi.org/10.3390/inorganics13120390
Chicago/Turabian StyleFagnani, Francesco, Alessia Colombo, Claudia Dragonetti, Mattia Fontani, Dominique Roberto, Massimo Cocchi, Simona Fantacci, and J. A. Gareth Williams. 2025. "Blue and Green Phosphorescent Organic Light-Emitting Diodes Based on Bis(cyclometalated) Tetrahydrocurcuminate Iridium(III) Complexes" Inorganics 13, no. 12: 390. https://doi.org/10.3390/inorganics13120390
APA StyleFagnani, F., Colombo, A., Dragonetti, C., Fontani, M., Roberto, D., Cocchi, M., Fantacci, S., & Williams, J. A. G. (2025). Blue and Green Phosphorescent Organic Light-Emitting Diodes Based on Bis(cyclometalated) Tetrahydrocurcuminate Iridium(III) Complexes. Inorganics, 13(12), 390. https://doi.org/10.3390/inorganics13120390











