Abstract
Significant efforts have been devoted to discovering novel metal-based complexes with better cytotoxicity and specificity to tumor cells. Within the range of complexes studied for cytotoxic activity, Ru complexes have gained significant attention as one of the most promising classes of compounds offering advantages such as good scaffolds for the construction of new bioactive molecules with a variety of ligands. Ruthenium-based compounds demonstrate efficient penetration into cancer cells and show affinity for DNA binding with antitumor mechanisms, other than those of cisplatin. They were identified as perfect chemotherapeutics for cancer treatment due to their good tolerance by normal cells, negligible toxic effects and stronger activity towards Pt-drug-resistant tumor cell lines. Ru-based complexes may interact with multiple targets and show selective accumulation in cancer cells, which enhances their therapeutic potential. In recent years, the design of polynuclear complexes has aroused considerable interest in drug discovery research. The strategy to incorporate two or more metal centers into one precise molecular structure may result in better cytotoxic activity compared to the mononuclear precursors. That is why ruthenium-based multinuclear anticancer organometallic and complex compounds have attracted lots of attention. The objective of the current review is to highlight the key results obtained in research on ruthenium complexes, presenting the up-to-date advances of multinuclear homometallic ruthenium complexes as promising anticancer candidates. The reported outcomes shed new light on the fundamental biological interactions and antineoplastic modes of action of ruthenium-based complexes and organometallic compounds as well as significant information for the prediction of novel anticancer drugs.
1. Introduction
Over the last years, metal complexes have been widely utilized in the field of medicinal chemistry [1,2]. The best-known platinum-based anticancer agents have shown many limitations, such as low selectivity, chemoresistance, and toxic side effects to normal cells, such as neurotoxicity, nephrotoxicity, ototoxicity, hepatotoxicity, skin toxicity, etc. Consequently, there is a necessity for new compounds containing metals other than platinum as anticancer candidates. In this regard, Ru-based complexes have gained consideration in many aspects and emerged as the most prominent candidates [3,4].
The anticancer profiles of ruthenium compounds include improved selectivity, prominent bioactivity and bioavailability, as well as lower toxicity against healthy cells. Various less toxic and more selective Ru-based compounds have been investigated [5,6]. Research into the medical applications of these compounds has been facilitated by the wide variety of ruthenium coordination and its organometallic chemical properties, as well as in connection with the usage of Ru-based complexes in catalysis, photodynamic therapy (PDT), and photoactivated chemotherapy (PACT) [7]. The promising activity of Ru complexes is supposed to be connected with the capability of ruthenium to mimic Fe in interactions with biomolecules, specifically serum proteins, such as albumin and transferrin, which are overexpressed in the tumor cells [8,9]. Thereby, Ru complexes can target cancer cells selectively.
It has been proven that Ru complexes showed synergistic action when combined with conventional chemotherapeutic drugs [10]. Moreover, these complexes have been extensively used as phototherapeutic and bioimaging agents [11]. Additionally, Ru complexes can be used as tumor theranostic candidates for diagnosis and therapy of different cancers with reduced toxic effects [12,13,14].
In a physiological environment, the ruthenium cation is stable in Ru(II) and Ru(III) oxidation states, the former being more reactive [15]. Ruthenium(III) complexes have stable thermodynamical and kinetical behaviors. They are effective as prodrugs in hypoxic and acidic environments [16]. The Ru(III) ion is more passive than Ru(II) because of its higher nuclear charge. That is why Ru(II) compounds have higher chemical reactivity than Ru(III) ones [17]. The reduction of Ru(III) complexes yields the more active Ru(II) form by the so-called “activation by reduction mechanism”, which depends on reducing compounds, such as glutathione, ascorbate, and hypoxic cancer environments [18,19]. Nevertheless, the “activation by reduction” theory is still controversial [20]. The complexes in both the oxidation states have octahedral geometry with six-coordinated configuration, which can easily take part in various redox reactions [21]. Being in the central location in the second period of d-block metals, ruthenium shows early and late properties of these elements equally. Its partially filled 4d electronic subshell allows different valencies which enable the metal to form a variety of complexes. Additionally, the ligands’ variation modulates their steric and electronic properties, and can allow large platforms of prominent structural Ru-based moieties and different ligand-exchange reactions with biotargets. Thus, Ru-based compounds (octahedral coordination geometry) with lower nonspecific toxicity and higher bioactivity against platinum-resistant tumor cells are considered to be a good alternative that may broaden the spectrum of activity and offer different modes of action and chemoreactivity of Pt-based drugs with typical square-planar geometry [22,23,24]. Furthermore, in comparison with typical platinum drugs, a number of ruthenium compounds possess better water solubility in physiological environments, leading to improved efficacy against Pt-resistant cells [25] and balancing the hydrophilicity and hydrophobicity of Ru-based complexes and their absorption in tumor cells [26].
Many mononuclear Ru coordination compounds have received consideration due to their binding capability to DNA and induction of cytotoxicity and their unique photochemical, photophysical, and electrochemical properties. Successful clinical trial entries of the Ru-based complexes NAMI-A, NKP1339, KP1019, and TLD1443 along with the information on in vitro and/or in vivo activity of other ruthenium-based chemotherapeutic candidates provide great, promising prospects in anticancer drug development. The cytotoxic activity of cisplatin is principally associated with binding to DNA, while the biotargets of clinically approved Ru complexes are not completely clarified [27,28,29,30].
Most Ru-based complexes presently being studied are mononuclear [15,16,23,24,25,29]. The design of polynuclear analogous compounds could lead to the development of new complexes with improved bioactivity [4,9,29,30,31]. An attractive approach to overcome the drawbacks, such as toxicity, selectivity, uptake, and biocompatibility problems, associated with Ru-based complexes could be the design of polynuclear complexes. Recent studies on multinuclear complexes cover structurally novel scaffolds, and many of these compounds have shown encouraging results.
2. Homodinuclear Ru Complexes
Ruthenium medicinal chemistry has generated many promising results. Considerable research attention has been devoted to the interaction of Ru complexes with different biologically active ligands with DNA, and numerous new structures were synthesized and investigated, where di- or polynuclear ruthenium complexes have attracted serious attention. Because Ru has a higher coordination number than Pt, it offers additional coordination sites that can be utilized to modulate the properties of the obtained complexes and the interactions with DNA [31]. The varied redox behavior of Ru is also important for both the transport of drug candidates in the body and their reactions with biorelevant proteins [32]. Other significant features are the variances in the kinetics of ligand substitution and their water solubilities. Many dinuclear Ru compounds have displayed a cytotoxicity better or analogous to that of platinum drugs [33]. A wide variation of potent Ru-based drugs has been obtained, incorporating different ligands (DMSO, arenes, amines, imines, and polypyridyl compounds) [34]. The structural diversity of these complexes suggests that different types of ruthenium compounds may operate through distinct mechanisms of action [35].
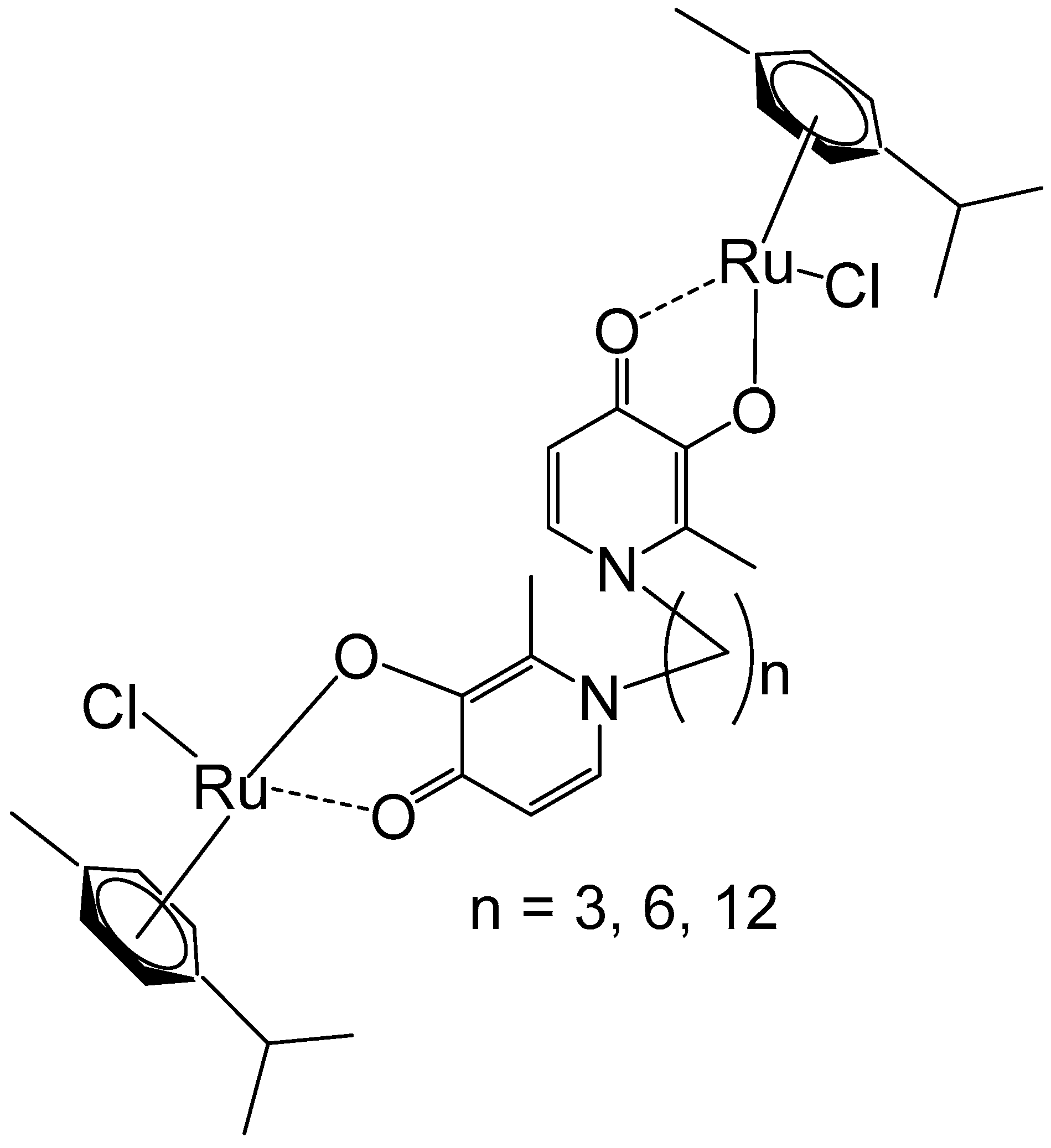
Mendoza-Ferri et al. have reported newly synthesized polynuclear ruthenium(II)–arene complexes holding bis(pyridinone)alkane linking ligands that contained 3, 6, and 12 methylene groups (Figure 1) [36]. The Ru–arene complexes displayed potent activity in various human ovarian cancer and colon cancer cells. A noticeable effect of their spacer length on the in vitro antitumor action was found, which increased with the dimension of the alkane linking ligand, correlating to the complexes’ lipophilicity. The studied dinuclear compounds were more active than the similar mononuclear analogues. IC50 in the same range as that recognized for Pt-based drugs was observed for the tested tumor cells. Based on water–octanol partition coefficients and hydrostability, the detailed SAR analysis revealed that the most lipophilic complex, containing a long alkyl chain (n = 12), exhibited high activity. The most active complex exhibited no cross-resistance to the cisplatin prodrug oxoplatin across the three resistant cell lines. Bioanalytical characterization of the compounds has shown that the studied ruthenium complexes hydrolyzed quickly, forming mainly diaqua species that exhibited affinity to transferrin and DNA, demonstrating that nucleobases and proteins could be possible targets for these ruthenium–arene polynuclear complex compounds.
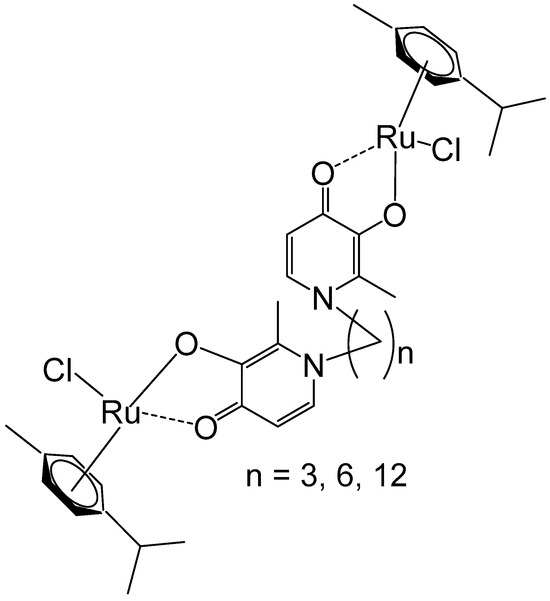
Figure 1.
Dinuclear ruthenium(II)–arene complexes of bis(pyridinone)alkane linker with 3, 6, or 12 methylenes in the alkane chain.
The dinuclear Ru(II) complexes of great length with ammine, amine, and imine ligands, which are able to participate in long-range DNA interactions, have attracted considerable interest. The observed excellent cytotoxic activity of these compounds is thought to result from the formation of intra- and inter-strand long-range DNA adducts. Dinuclear Ru(II) complexes are believed to be capable of overcoming both acquired and intrinsic resistance to the classical anticancer drug cisplatin. The lengths and flexibility of the chains, the hydrogen bonds and charges of the linking ligands, as well as the labile ligand geometry are the main aspects for the synthesis of dinuclear Ru(II) antineoplastic drug candidates. Additionally, the chlorido ions, as leaving ligands, can be replaced with an easily coordinating solvent, for instance, DMSO. The solvolysis reactions and the in vitro cytotoxic activity evaluation with murine L1210 leukemia cells of Ru(II) dinuclear complexes with several bridged ligands in different conditions (H2O, DMSO, H2O-DMSO) have been investigated and reported [37]. It was found that ruthenium(II) complexes with hydrophobic DMSO were more efficiently permeable into the tumor cells. Their IC50 values revealed that these complexes exhibited strong cytotoxic activity, signifying that the hydrophobic DMSO plays a significant role in their biological activity.
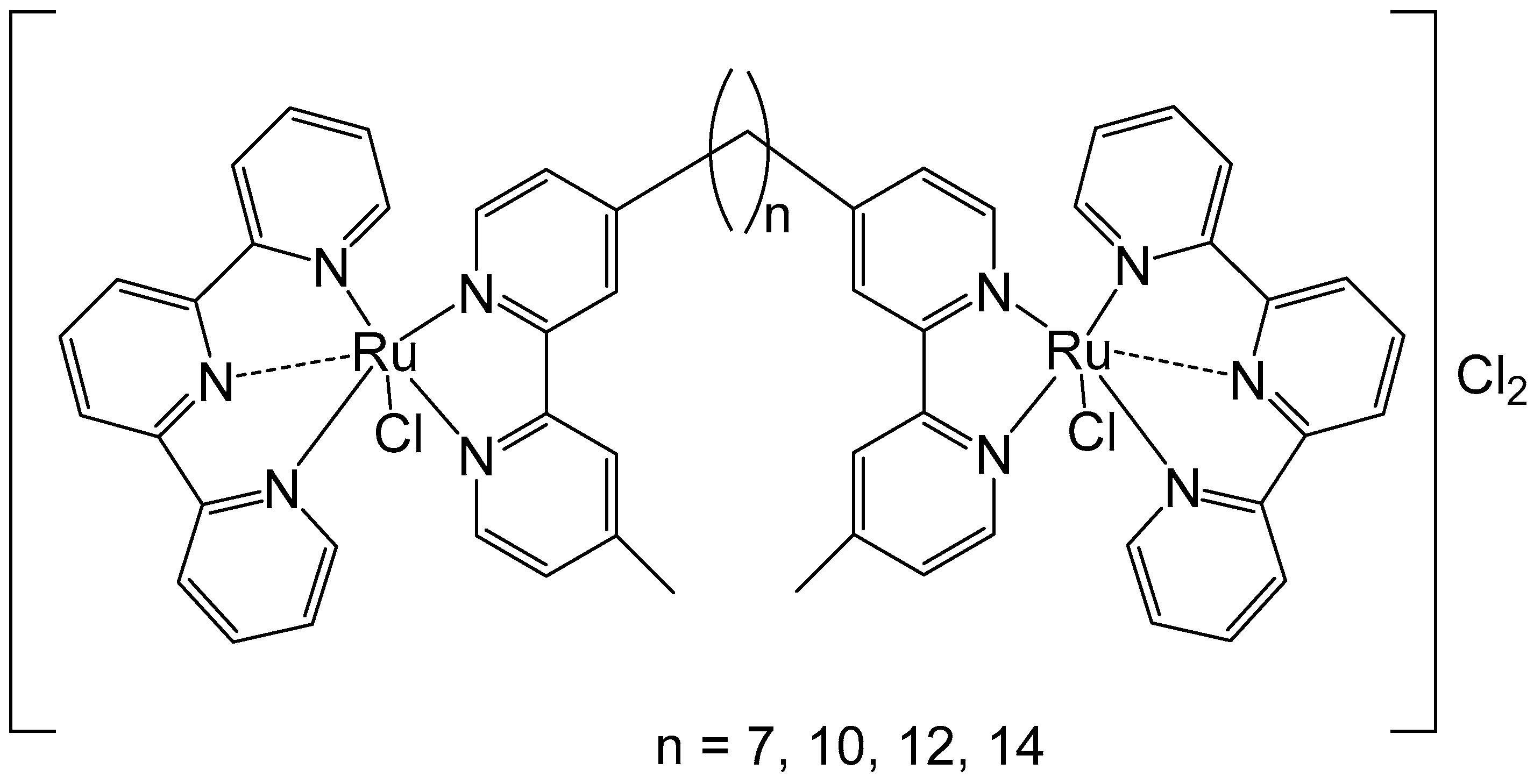
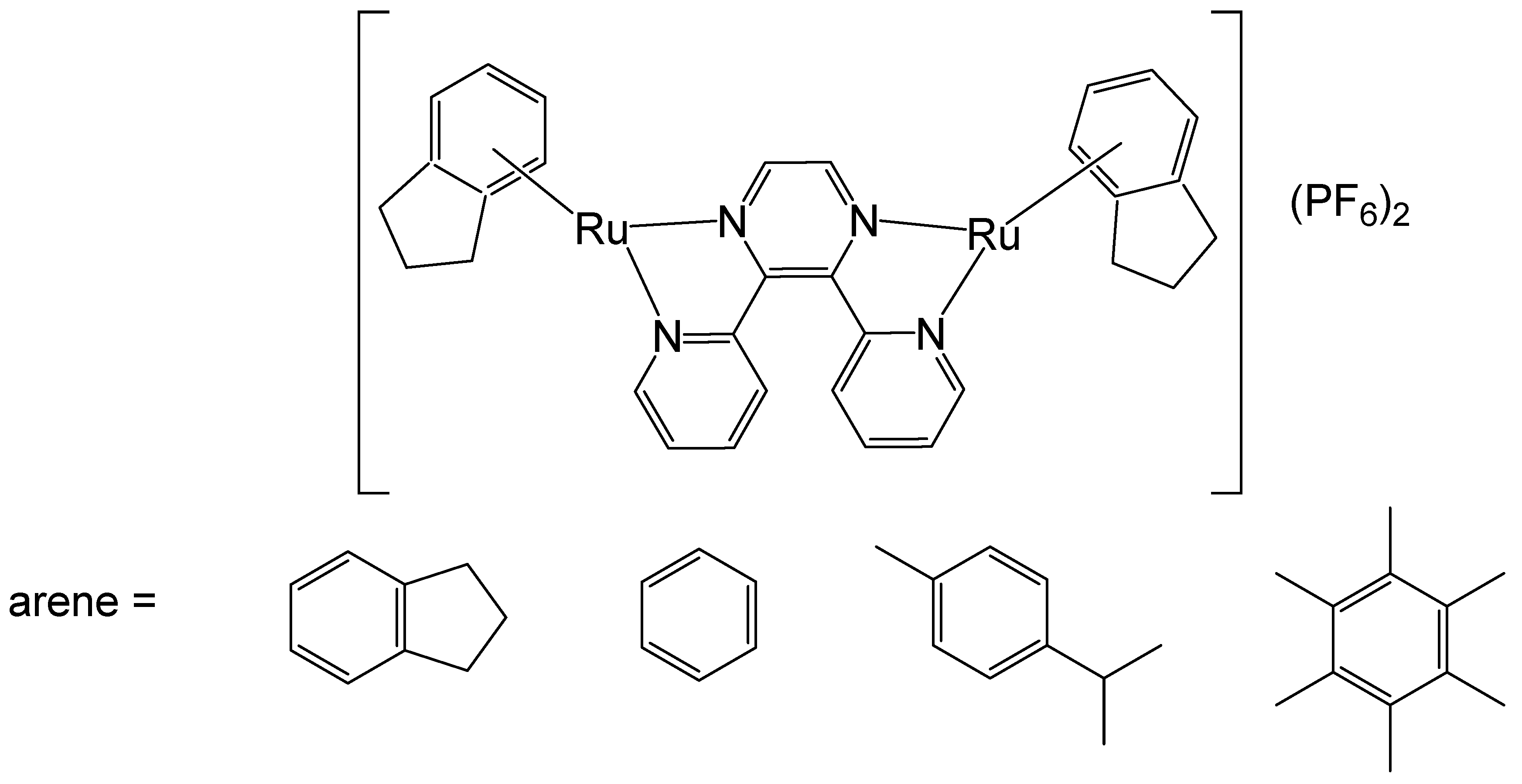
To improve the activity of ruthenium mononuclear 2,2′:6′,2″-terpyridine complexes [Ru(tpy)(L)(Cl)]Cl, where L = a non-labile bidentate ligand, Mulyana et al. obtained the dinuclear complexes with a general formula [{Ru(tpy)Cl}2{μ-bbn}]Cl2 (bbn = bis [4(4′-methyl-2,2′-bipyridyl)]-1, n-alkane, n = 7, 10, 12, 14), and investigated their nucleic acid binding and cytotoxicity [38]. The dinuclear complexes exhibited potent activity against the very sensitive murine leukemia L1210 cells with IC50 = 5–10 μM, and showed tenfold higher activity than the respective mononuclear compound [Ru(tpy)(Me2bpy)Cl]Cl (Me2bpy = 4,4′-dimethyl-2,2′-bipyridine). To expand the family of binuclear complexes, the authors synthesized new complexes with substituted bbn ligands (Figure 2) and studied their antineoplastic activity, hydrolysis rates, and binding capacity to guanosine-5′-monophosphate (5′-GMP) [39].
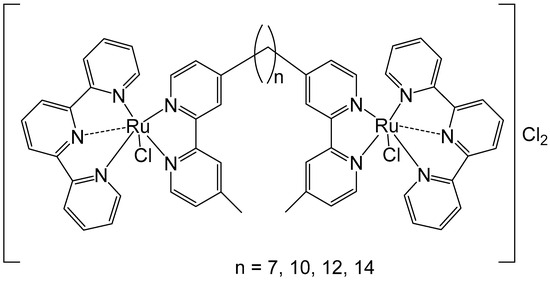
Figure 2.
Dinuclear ruthenium complexes [{Ru(tpy)Cl}2{μ-bbn}]Cl2, where tpy = 2,2′:6′,2″-terpyridine; bbn = bis [4(4′-methyl-2,2′-bipyridyl)]-1, n-alkane, n = 7, 10, 12, and 14.
The multinuclear Ru(II)-based complex [{Ru(tpy)Cl}2{μ-bbn}]Cl2, where the tpy is 2,2′:6′,2″-terpyridine, and the bbn is bis [4(4′-methyl-2,2′-bipyridyl)]-1,n-alkane (n = 12)}, showed higher anti-proliferative potential against two breast adenocarcinoma cell lines when compared both to cisplatin and carboplatin. The observed antitumor activity likely arises from both covalent and reversible interactions with DNA. Notably, the dinuclear ruthenium complexes with n = 10, 12, and 14 demonstrated higher potency than cisplatin across the tested cell lines. The complex with n = 12 exhibited the highest activity, while those with the shortest (n = 7) and longest (n = 16) linking chains showed the lowest activity. This superior activity was attributed to an optimal balance between lipophilicity, which facilitates cellular uptake, and the cytotoxicity resulting from the formation of covalent adducts with DNA. Nitro substituents in the tpy rings of these complexes decreased their activity, mainly in the most active compound (n = 12). The substitution of two methylene groups by two amine groups in the bridge ligand for the complexes (n = 7 and 16) decreased the activity. The highly potent complex (n = 12) was approximately four times more effective than cisplatin and then the dinuclear complexes [{Ru(bpy)2Cl}2{μBL}]Cl2, for instance, with bidentate ligands, such as, 2-phenylazopyridine, 2,2′-azobispyridine, and 2-phenylpyridinylmethylene amine with mechanisms of action quite diverse from that of the classical Pt anticancer agents [40].
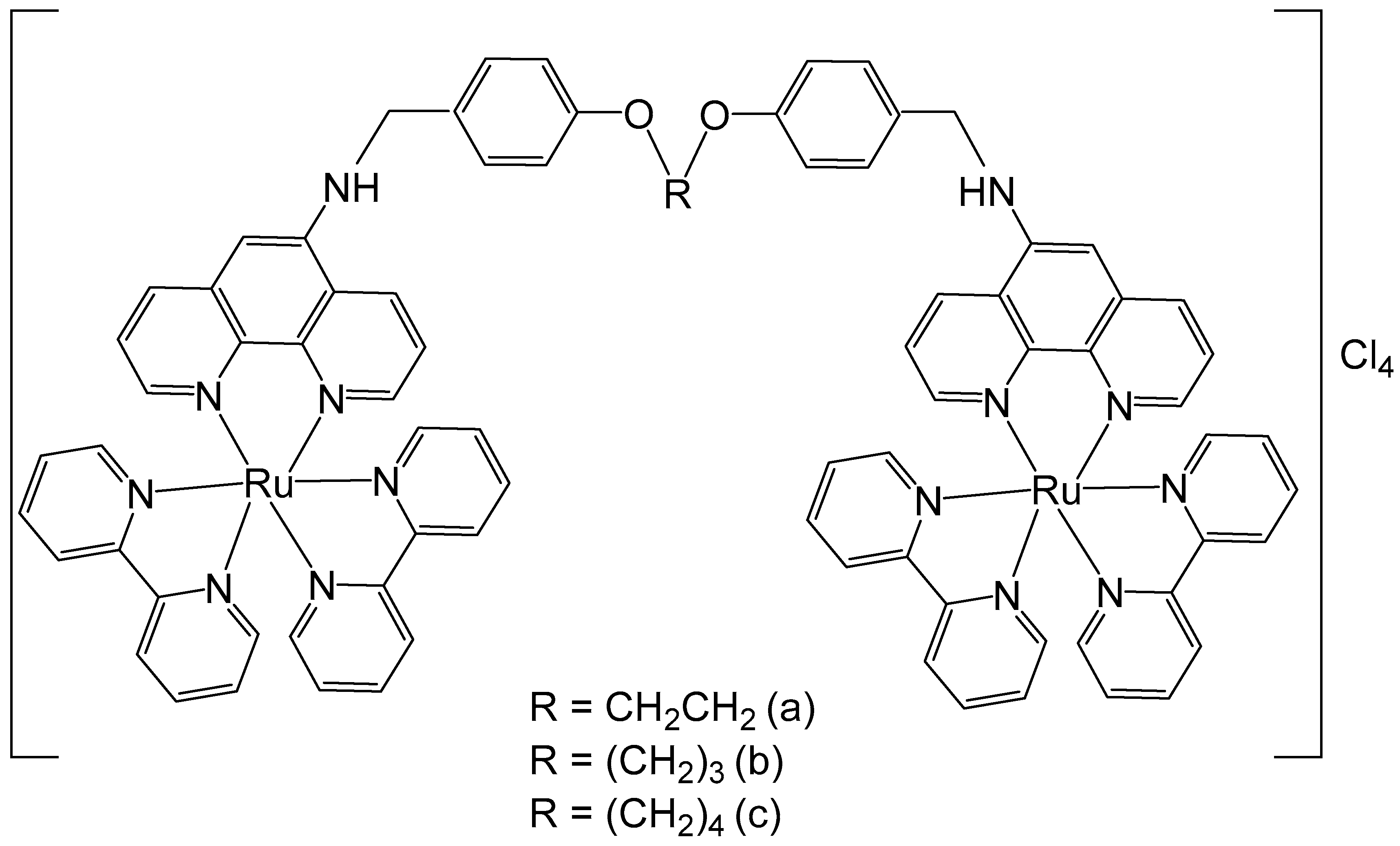
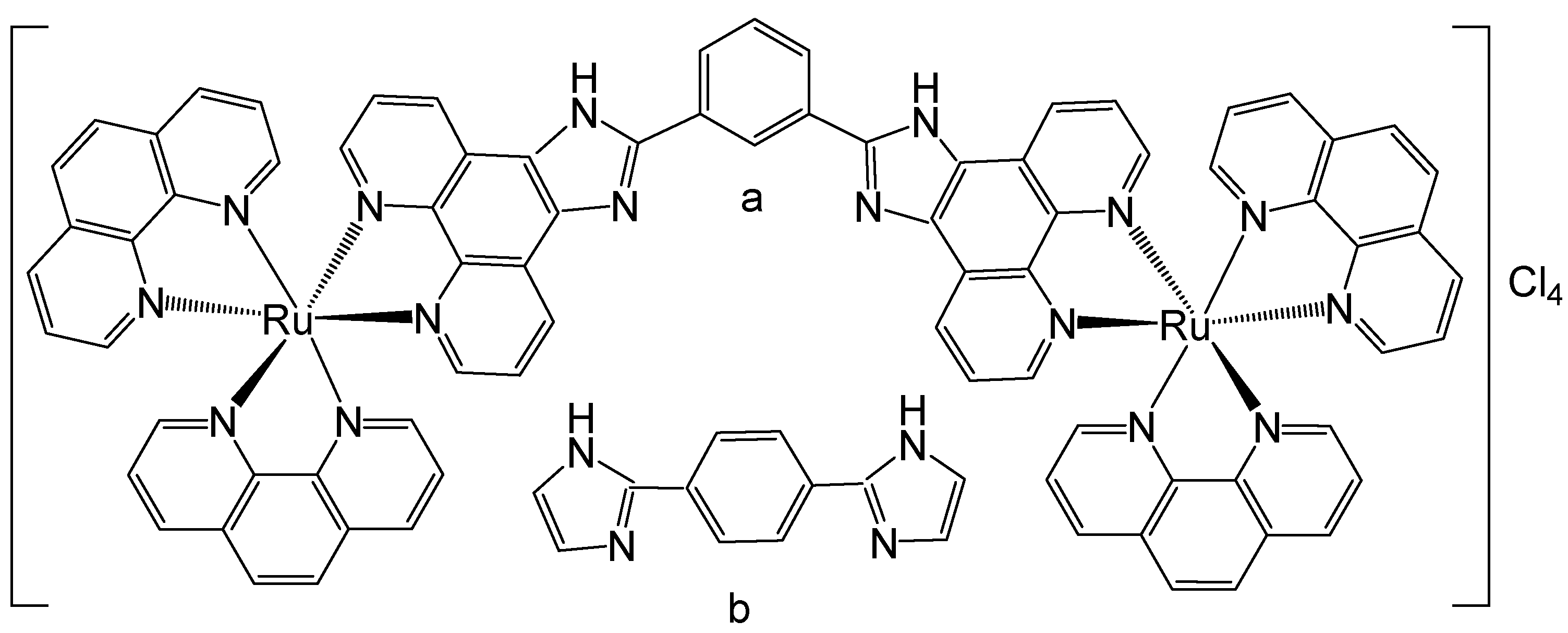
Some 1,10-phenanthroline bridging ligands BL and their ruthenium(II) complexes [(bpy)2Ru(BL)Ru(bpy)2][PF6]4, where bpy = 2,2′-bipyridine; PF6 = hexafluorophosphate; BL = polyaromatic ligands with two equivalent chelating sites on the phenanthroline rings, bridged by a flexible alcoxyphenyl spacer moiety (Figure 3a–c), have been obtained and investigated for their antineoplastic activity [41]. The symmetrical structure of the bridging ligands allowed the production of dinuclear ruthenium(II) complexes with equivalent metal centers. The photophysical and electrochemical behaviors of the compounds have also been studied. Their in vitro cytotoxic activity has been assessed against cervical cancer HeLa, gastric cancer SGC-7901, and gastric cancer BGC-823 cells by the MTT test. The data showed that the dinuclear Ru(II) complexes displayed substantial dose-dependent cytotoxic activity to the tested tumor cell lines. The complex (Figure 3c) was the most potent and exhibited low IC50 values (9.21 μM for BGC-823 and 10.38 μM for SGC7901 cells). The cytotoxicity of the complex, shown in Figure 3c, against HeLa cells was higher than that of [(bpy)2Ru(L)Ru(bpy)2][Cl4], where L = 1,6-bis(3-(1H-imidazo-[4,5-f][1,10]phenanthrolin-2-yl)-9H-carbazol-9-yl)hexane, with IC50 > 100 μM, which showed strong intercalative interaction and possible cell imaging application owing to its low antiproliferative activity, reported by Liu et al. [42]. The differences in cytotoxic activity between these series of complexes against HeLa, SGC-823, and SGC-7901 cells might be due to the different amount of methylene groups in their flexible alkane chains. The cytotoxicity of the complexes, depicted in Figure 3a–c, increased with the rise of methylene groups in the bridging ligands. The DNA quenching constants were found to be 1.34 × 105, 1.46 × 105, and 2.98 × 105 mol/L for the complexes shown in Figure 3a–c, demonstrating that there were different kinds of interactions between the complexes and DNA.
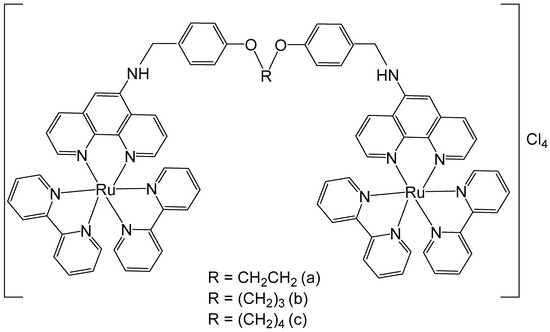
Figure 3.
Ru(II) dinuclear complexes of [(bpy)2Ru(BL)Ru(bpy)2]Cl4, where bpy = 2,2′-bipyridine; BL = polyaromatic 1,10-phenanthroline bridging ligands with 2 (a), 3 (b), or 4 (c) methylene groups in the alcoxyphenyl moiety.
Similar findings were reported by Beckford et al. for a series of trinuclear ruthenium complexes incorporating 1,3,5-triazine ligands and diverse η6–arene moieties. A set of trinuclear complexes comprising organometallic Ru fragments (-(arene)RuCl-) coordinated to 2,4,6-tris(di-2-pyridylamino)-1,3,5-triazine cores, with arene = benzene, p-cymene and hexamethylbenzene, have been synthesized. Their DNA interactions were broadly studied by various biophysical probes and by docking analysis. These compounds showed strong binding with DNA, found to be non-specific covalent or groove types of interactions. The complexes showed very low cytotoxic activity against tumor cell lines only at high (>100 μM) concentrations [43,44].
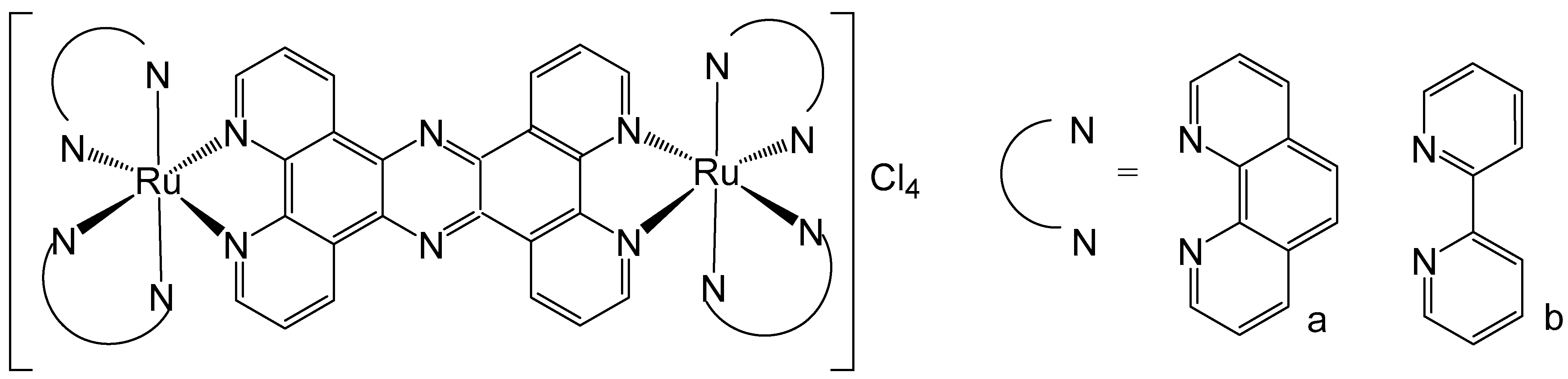
Tetrapyrido [3,2-a:2′,3′-c:3″,2″-h:2‴,3‴-j]phenazine (tpphz) ligands are a class of derivatives identified to interact with DNA due to their highly extended planar structure, similar to dipyridophenazine (dppz). They have been used by Gill et al. to obtain DNA-binding ruthenium(II) dinuclear complexes, shown in Figure 4. The complex in Figure 4a could specifically accumulate in the cellular nucleus coordinating to phenanthroline ligands alongside tpphz [45]. The complex (Figure 4a) was well tolerated by eukaryotic and prokaryotic cells, demonstrating suitability for applications in luminescence and transmission electron microscopy. Interestingly, despite its hydrophilic nature, the complex is internalized by live cells via a non-endocytotic mechanism. It enables visualization of structural changes in nuclear DNA through the cell cycle and shows promising potential for quadruplex DNA imaging [46].
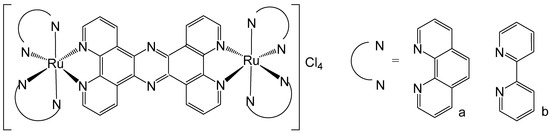
Figure 4.
Structures of dinuclear ruthenium(II)(tpphz) complexes, where tpphz = tetrapyrido [3,2-a:2′,3′-c:3″,2″-h:2‴,3‴-j]phenazine; N^N = 1,10-phenanthroline (a) and bipyridine (b).
Ruthenium binuclear polypyridyl complexes have been widely investigated over the past few decades due to their remarkable ability to selectively bind DNA. Svensson et al. have reported binuclear Ru(II) polypyridyl complex compounds, investigated their cell localization, uptake, and biomolecular interactions, and compared the results with those of well-known dipyridophenazine (dppz) complexes [47]. The structural isomers of the dinuclear ruthenium polypyridyl complex [μ-bipb(phen)4Ru2]Cl4, shown in Figure 5, are [μ-meta-bipb(phen)4Ru2]Cl4, Figure 5a, where meta-bipb = 1,3-bis(imidazo [4,5-f]-1,10-phenanthrolin-2-yl)benzene; phen = 1,10-phenanthroline; and [μ-para-bipb(phen)4Ru2]Cl4, Figure 5b, where para-bipb = 1,4-bis(imidazo [4,5-f]-1,10-phenanthrolin-2-yl)benzene. The interactions of the enantiomerically pure forms of these structural isomers of the dinuclear Ru(II) polypyridyl complex with the mammalian CHO-K1 cell line have been examined to elucidate the potential of Ru(II) polypyridyl complexes as possible cellular imaging probes. Interestingly, while the two structural isomers of [μ-bipb(phen)4Ru2]Cl4, Figure 5, showed similar staining patterns, the respective enantiomers (Δ and Λ) demonstrated significant localization differences. All complexes demonstrated the ability to penetrate the nucleus; however, the ΔΔ enantiomers of both isomers exhibited preferential accumulation within the nucleoplasm, whereas the ΛΛ enantiomers predominantly localized in the cytoplasm, displaying pronounced nucleolar staining. Notably, this differential subcellular distribution did not appear to arise from enantiomeric differences in the strength of DNA binding interactions. Although a definitive cause could not be established, it is plausible that variations in the nature rather than the strength of the interactions account for the observed localization patterns. The nucleoli are structures primarily composed of ribosomal RNA (rRNA), which exhibits substantial geometrical differences compared to the B-form of DNA. Consequently, the distinct intracellular distributions may reflect enantiomer-specific selectivity toward B-DNA versus rRNA [48]. Live-cell uptake of the complexes at 5 μM was both efficient and non-toxic, and proceeded through an energy-dependent pathway.
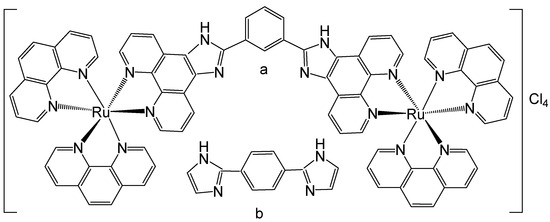
Figure 5.
Ru(II) complexes [μ-meta-bipb(phen)4Ru2]Cl4 (a) and [μ-para-bipb(phen)4Ru2]Cl4 (b), where meta-bipb is 1,3-bis(imidazo [4,5-f]-1,10-phenanthrolin-2-yl)benzene and para-bipb is 1,4-bis(imidazo [4,5-f]-1,10-phenanthrolin-2-yl)benzene.
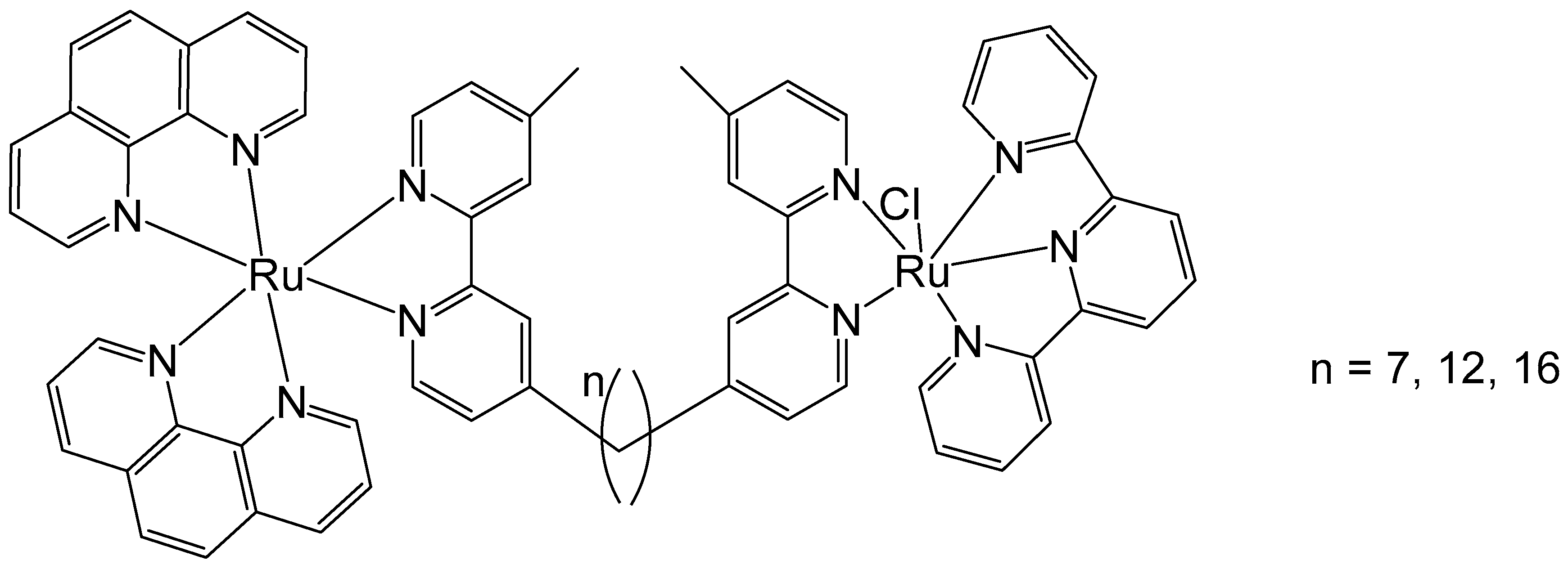
Li et al. have described the antineoplastic activity of some non-symmetric binuclear polypyridylruthenium(II) complexes (Rubbn-Cl), Figure 6. These complexes consisted of one inert metal center and one coordinatively labile metal center, bridged by a bis [4(4′-methyl-2,2′-bipyridyl)]-1,n-alkane ligand with varying alkyl chain lengths (n = 7, 12, 16). The cytotoxic activity of the complexes was assessed by MTT against three eukaryotic cell lines—two kidney lines (BHK and HEK-293) and a liver cell line (HepG2). The results showed that the complexes were approximately 30 to 80 times more cytotoxic toward cancer cells than toward bacteria. Furthermore, an increase in alkyl chain length correlated with improved cytotoxicity in the HEK-293 and BHK cells. With an increase in lipophilicity, the cellular uptake increased, and the complexes showed improved cytotoxicity. However, in the HepG2 cell line, the cytotoxicity of the least lipophilic complex (n = 7) was abnormally high with an IC50 value of 3.7 μM, which was lower than the values shown by the lipophilic complexes (n = 12, 16). Therefore, even though lipophilicity is a key factor in determining cellular uptake and cytotoxic activity, other parameters must also be taken into account. Moreover, the anticancer activity of these complexes has been attributed to their strong interactions with DNA [49]. To elucidate the unexpectedly large differences in the cytotoxic activities of Rubbn-Cl complexes against eukaryotic cells, their intracellular localization was investigated using confocal microscopy. The outcomes suggested that the complexes (n = 12, 16) had better selectivity for rRNA, compared to DNA, than does the complex (n = 7). The superior level of DNA localization in HepG2 by the complex (n = 7), compared to the complexes (n = 12, 16), could be associated with its higher cytotoxic activity against HepG2 cells.
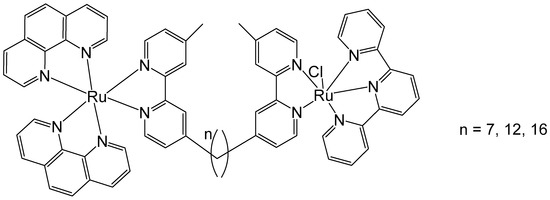
Figure 6.
Structure of binuclear polypyridyl-ruthenium(II) complexes (Rubbn-Cl) of bis [4(4′-methyl-2,2′-bipyridyl)]-1,n-alkane ligand with different alkyl chain lengths, n = 7, 12, 16.
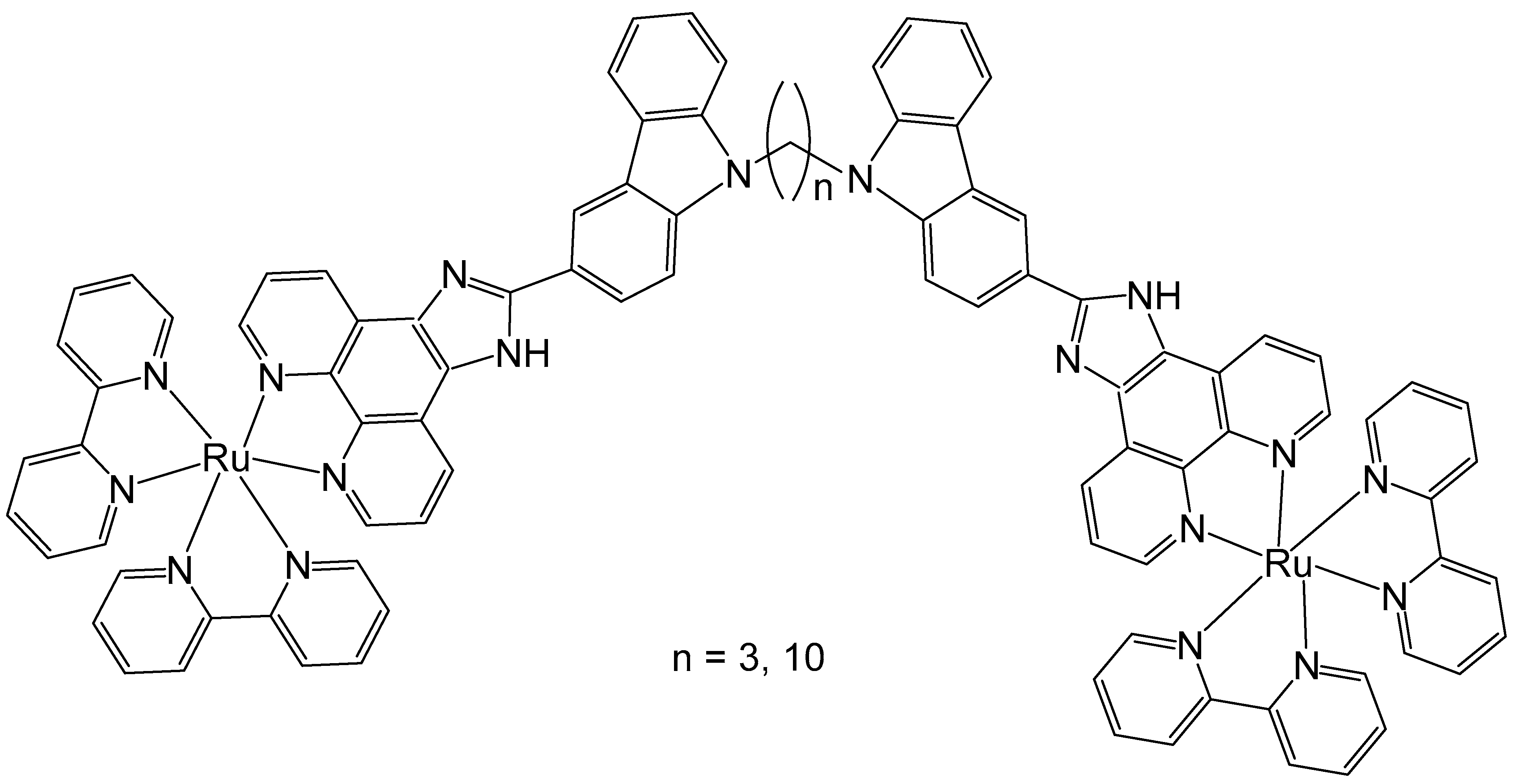
Liu et al. have also investigated the antitumor activity of two binuclear polypyridyl-ruthenium complexes (Figure 7) with variable bridging alkyl chains holding 3 and 10 carbon atoms. Their calf-thymus DNA-binding and plasmid-DNA photocleavage behaviors have been studied and the complexes showed partial DNA intercalation and selective localization in lysosomes. Theoretical calculations based on the density functional theory (DFT) method were performed to further understand the experimentally observed DNA-binding properties of the complexes. The results indicated that both complexes partially intercalate between the DNA base pairs. Cellular uptake and colocalization studies revealed that the complexes efficiently entered HeLa cells and localized within lysosomes. The in vitro antineoplastic activities of the complexes against HeLa and MCF-7 cancer cells were evaluated using an MTT cytotoxicity assay. In addition, high-content analysis (HCA) was employed to assess cytotoxic activity, apoptosis, and cell cycle arrest induced by the complexes. The complex (n = 10) exhibited moderate cytotoxic activity showing higher potency than the complex (n = 3), and the cytotoxic effects were not in line with DNA binding but depended on their lipophilicity. These results have shown that the length of the alkyl linkers could efficiently modulate their activity and that HCA was appropriate to quickly identify antiproliferation and could replace MTT assays to test the cytotoxic activity of chemotherapeutics. The complexes induced apoptosis through cell cycle arrest at the G0/G1 and S phases. Comprehensive elucidation of their mechanisms of action and structure–activity relationships is warranted to optimize and advance the therapeutic potential of these compounds [50]. Therefore, it can be settled that in most cases, the alkyl chain length is a critical factor influencing cytotoxic activity of the studied dinuclear ruthenium complexes, as it governs the lipophilicity and cellular uptake of the complexes.
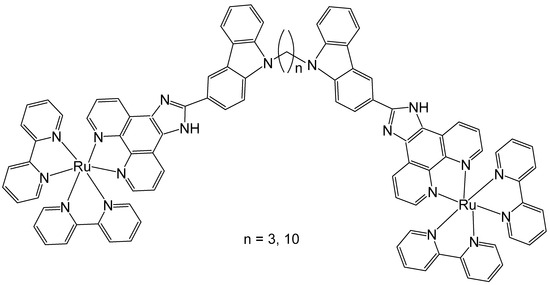
Figure 7.
Binuclear polypyridylruthenium(II) complexes with variable alkyl bridging chains holding 3 and 10 carbon atoms.
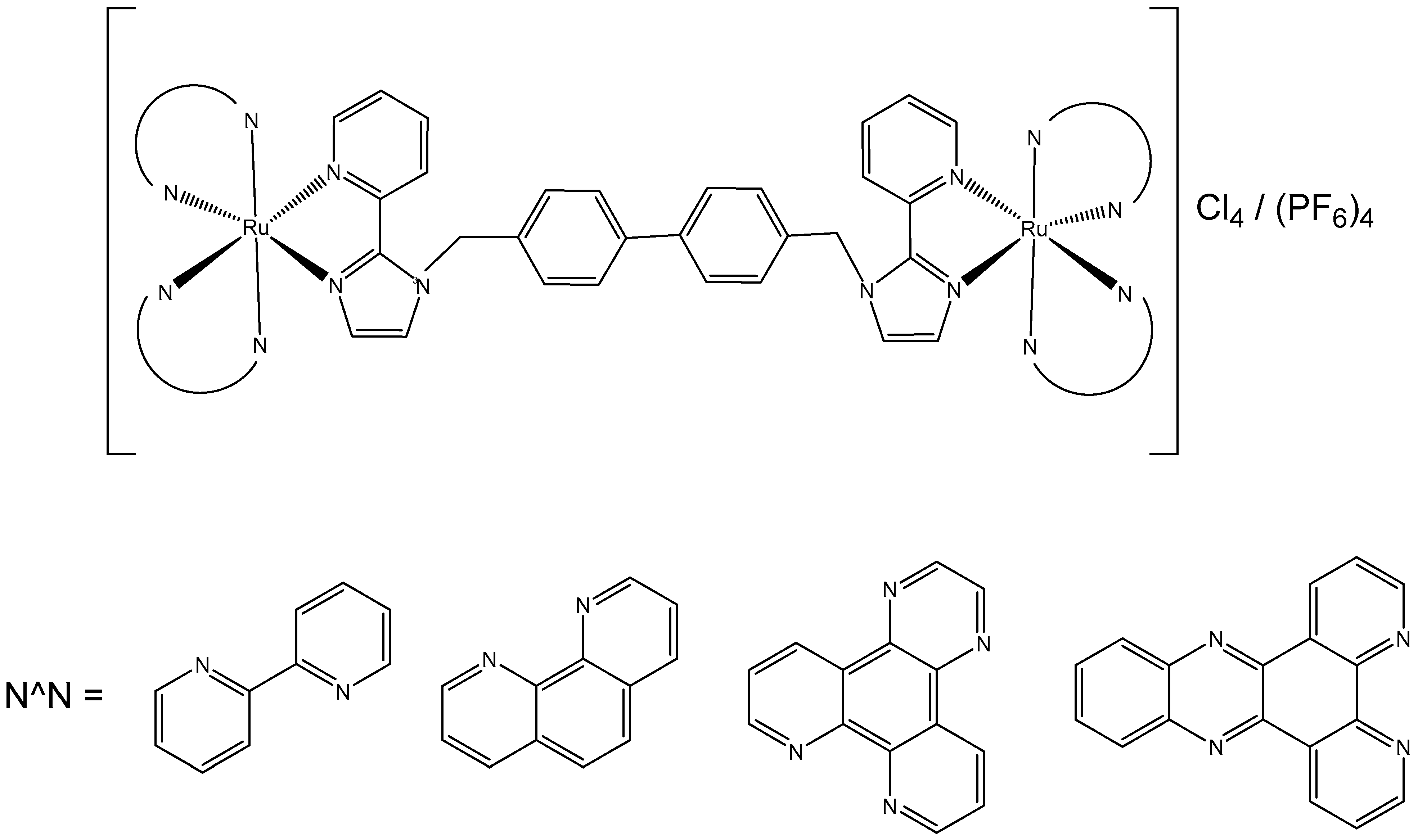
Singh et al. have studied the antineoplastic activity of new dinuclear Ru(II)–polypyridyl complexes [Ru2(N^N)4(BPIMBp)]Cl4, where N^N = 2,2′-bipyridine (bpy), 1,10-phenanthroline (phen), dipyrido [3,2-d:2′,3-f] quinoxaline (dpq), and [Ru2(N^N)4(BPIMBp)](PF6)4, where N^N = dipyrido [3,2-a:2′,3′-c] phenanzine (dppz), and 1,4′-bis[(2-pyridin-2-yl)-1H-imidazol-1-yl)methyl]-1,1′-biphenyl (BPIMBp) was used as a bridging ligand, Figure 8. These complexes were positively charged (IV+) cations and exhibited flexibility due to −CH2 groups of the bridging ligand. They also contained terminal ligands with variable intercalative capabilities. The interaction of these complexes with calf-thymus DNA (CT-DNA) was studied by different methods. The complexes exhibited DNA interaction through the spacer, consistent with a groove-binding mode. The complexes of dipyrido [3,2-d:2′,3-f] quinoxaline and dipyrido [3,2-a:2′,3′-c] phenanzine have also shown an intercalative interaction. These complexes demonstrated moderate cytotoxic effects on HeLa, MCF-7, and HL-60 cells with IC50 values in the range of 5–25 μM. The observed antitumor activity is mainly attributed to their ability to interact with DNA [51]. Liu et al. synthesized a series of twelve dinuclear Ru-arene metallacycles with 24-, 26-membered structures having 1-(3-((1H-imidazol-1-yl)methyl)benzyl)-1H-imidazole and 1-(4-((1H-imidazol-1-yl)methyl)benzyl)-1H-imidazole ligands and investigated their antineoplastic activity. The complexes demonstrated low to moderate cytotoxic activity against HeLa, MCF-7, and A549 tumor cells as well as the normal LO2 cells, suggesting a lack of selectivity toward cancer cells. The antineoplastic activity of these complexes is likely attributable to their capacity to induce DNA condensation [52].
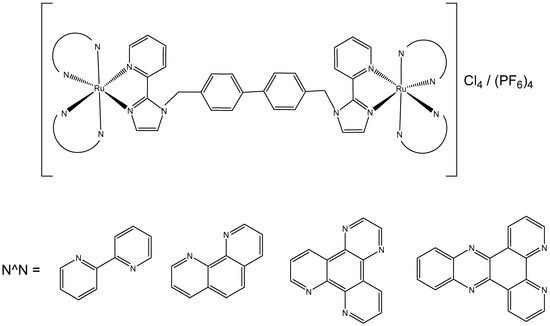
Figure 8.
Structure of dinuclear Ru(II)–polypyridyl complexes [Ru2(N^N)4(BPIMBp)]Cl4, where N^N = 2,2′-bipyridine (bpy), 1,10-phenanthroline (phen), dipyrido [3,2-d:2′,3-f] quinoxaline (dpq), and [Ru2(N^N)4(BPIMBp)](PF6)4, where N^N = dipyrido [3,2-a:2′,3′-c] phenanzine (dppz); BPIMBp = 1,4′-bis[(2-pyridin-2-yl)-1H-imidazol-1-yl)methyl]-1,1′-biphenyl.
The binuclear Ru(II)–arene complex compounds [{RuCl(η6-arene)}2(μ-2,3-dpp)](PF6)2, arene = indane, benzene, p-cymene, hexamethylbenzene, and 2,3-dpp = 2,3-bis(2-pyridyl)pyrazine have been reported [53,54]. The photoactivation of the binuclear Ru–arene complex [{RuCl(η6-indane)}2(μ-2,3-dpp)](PF6)2 (2,3-dpp = 2,3-bis(2-pyridyl)pyrazine) (Figure 9) has been studied in detail. Irradiation of the complexes had a substantial impact on their DNA interactions. The DNA-binding affinity of the complexes was markedly enhanced following irradiation. In the non-irradiated state, the complexes predominantly formed DNA adducts that only weakly impeded RNA polymerase progression, while irradiation of [{RuCl(η6-indane)}2(μ-2,3-dpp)](PF6)2, shown in Figure 9, promoted the formation of adducts with significantly greater transcription-blocking efficiency and transformed the adducts into stronger blocks for RNA polymerase. The thermal reactivity of the compounds was restricted to a stepwise double aquation process exhibiting biexponential kinetics. In contrast, irradiation of the samples induced photoactivation, leading to the loss of the coordinated arene and generating ruthenium species that were more reactive than their ruthenium–arene precursors. The results have shown that photoactivation of binuclear ruthenium(II)–arene complexes simultaneously generates a highly reactive ruthenium species capable of binding to DNA and a fluorescent marker (the released arene). Notably, this photoreactivity occurs independently of oxygen. Therefore, these complexes show great potential for combining photoinduced cell death with fluorescence imaging to monitor the location and efficiency of the photoactivation process. Upon photoactivation, the binuclear complex [{RuCl(η6-indane)}2(μ-2,3-dpp)](PF6)2, shown in Figure 9, can generate highly reactive and potentially cytotoxic ruthenium species along with the arene indane, which may serve as fluorescent probes. The photochemical studies of [{RuCl(η6-indane)}2(μ-2,3-dpp)](PF6)2 indicated that UV and visible light led to the separation of indane ligands, visualized by their fluorescence, and the formation of strong diruthenium DNA adducts. These complexes therefore have the potential for simultaneously inducing photoinduced cell death and facilitating fluorescence imaging to monitor the spatial distribution and effectiveness of photoactivation.
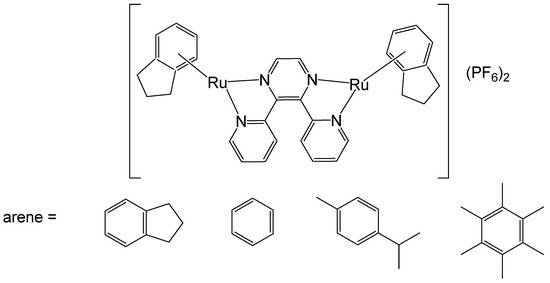
Figure 9.
Structure of the binuclear Ru(II)–arene complexes [{RuCl(η6-arene)}2(μ-2,3-dpp)](PF6)2, where arene = indane, benzene, p-cymene, hexamethylbenzene.
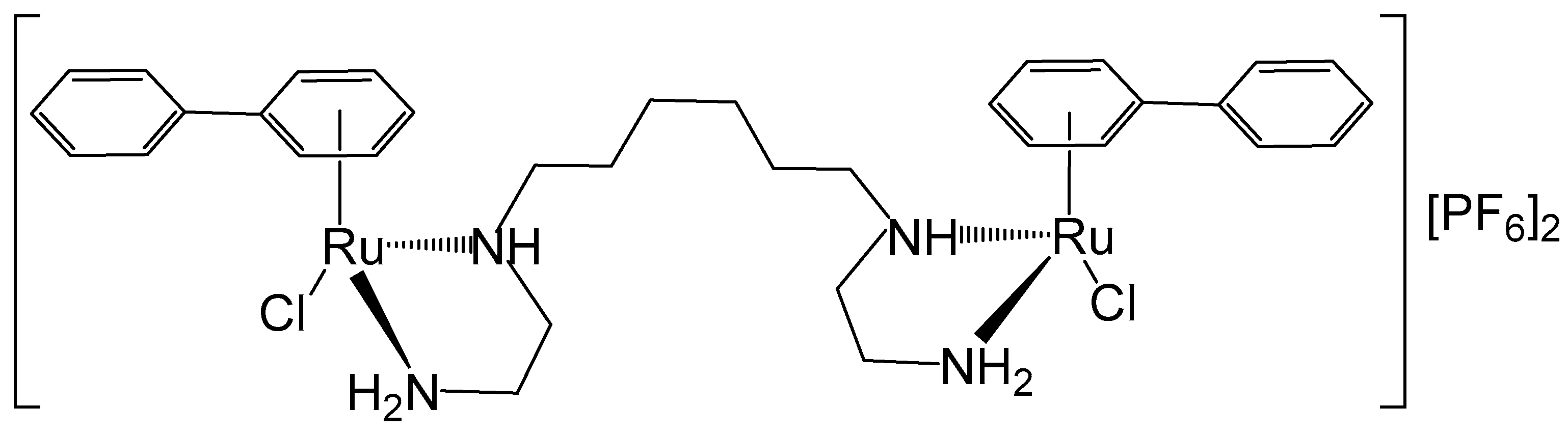
The stereochemistry of the binuclear complex [{RuCl(η6-biphenyl)}2(en)2-(CH2)6][PF6]2 (en = ethylenediamine), Figure 10, has been elucidated [55]. The anticancer potential of the mononuclear complex [(η6-arene)RuCl(en)], which exhibited in vitro and in vivo potency in a human ovarian cancer A2780 xenograft model (cisplatin-resistant), has also been described. The studied binuclear Ru–arene complex [{RuCl(η6-biphenyl)}2(en)2-(CH2)6][PF6]2 could bind quickly to CT-DNA, favorably to G bases. Complexes of this type exhibited remarkably higher selectivity for G compared to the other nucleobases in the potential target DNA. The cytotoxic activity tends to increase with larger η6-arenes. The inhibition of DNA-directed RNA synthesis was more effective than that observed with the corresponding mononuclear complex [56]. Further enhancement of DNA recognition could be achieved by modifying the chelating ethylenediamine moiety, the linkers, and the arene ring systems.
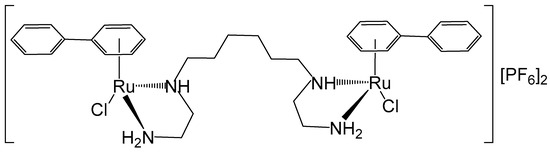
Figure 10.
Structure of the dinuclear complex [{RuCl(η6-biphenyl)}2(en)2-(CH2)6][PF6]2 (en = ethylenediamine).
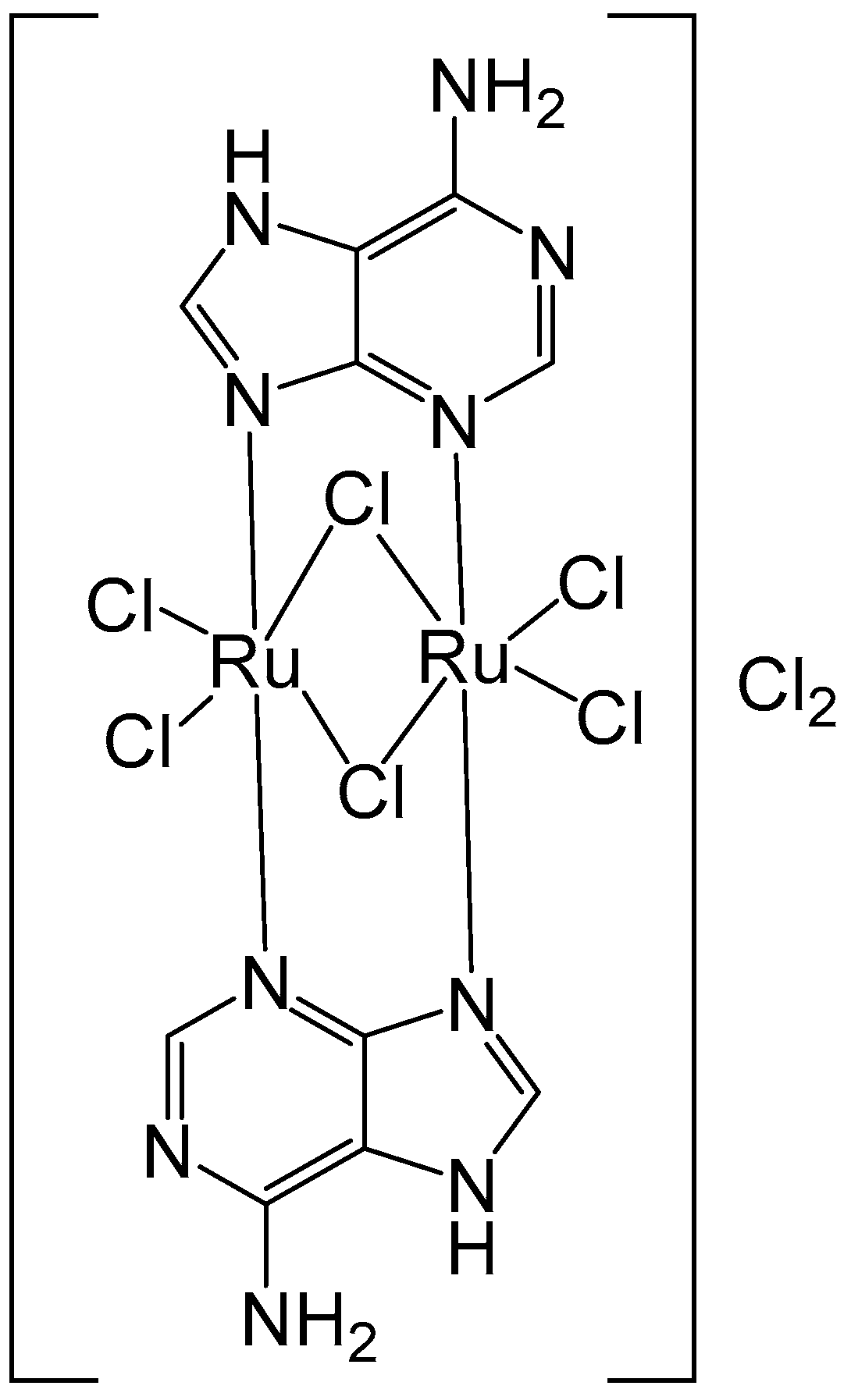
Metal complexes derived from purine nucleobases are valuable tools for cancer diagnosis and therapy, as well as for other biomedical uses. Orts-Arroyo and collaborators have recently reported the synthesis of a binuclear Ru(III-adenine protonated complex [57]. The new binuclear Ru(III) complex of the nucleobase adenine [{Ru(μ-Cl)(μ-Hade)}2Cl4]Cl2⋅2H2O (Figure 11), where Hade = protonated adenine, has been characterized using electrochemical and spectral methods. It has been tested against colon cancer HCT-116 and gastric cancer AGS cells by MTT and cell cytometry at 48 and 72 h treatments. The Ru(III) complex exhibited a specific cytotoxic activity against AGS tumor cells in a dose- and time-dependent manner with IC50 = 23.25 µM demonstrating an apoptotic pathway in the maximum concentration. McKenzie et al. have described the synthesis, characterization, and in vitro assessment of Ru(II) complexes bearing different oligonucleotides [58,59].
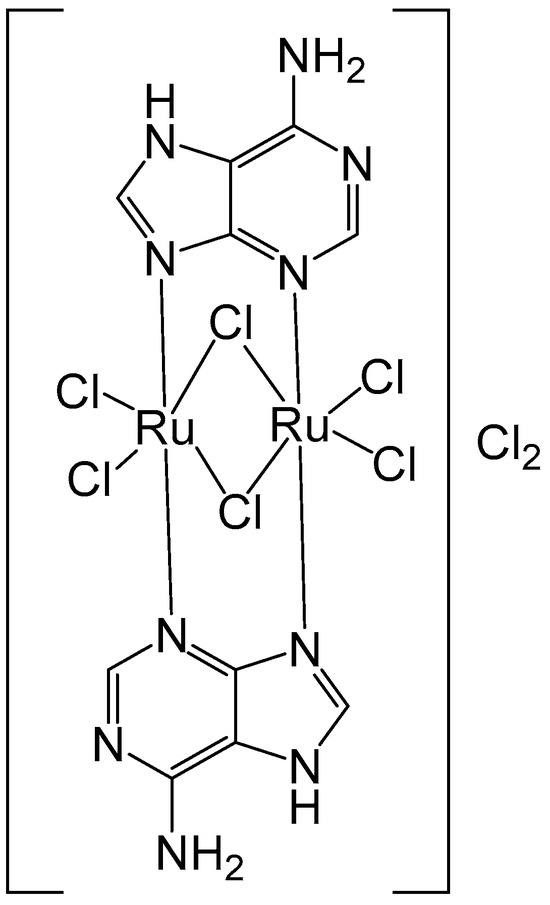
Figure 11.
Structure of the binuclear ruthenium(III) complex [{Ru(μ-Cl)(μ-Hade)}2Cl4]Cl2·2H2O, where Hade = protonated adenine.
3. Complexes with Arylphosphines as Ancillary Ligands
The ligands of the metal complexes play a substantial role in moderating bioactivity. Arylphosphines, as ancillary ligands for the construction of ruthenium half-sandwich complexes, were found effective in studies of anticancer activity. Partially oxidized phosphorus(III) ligands exhibit strong hydrogen-bond-accepting abilities. Among these, phosphine oxides are recognized as some of the most potent hydrogen-bond acceptors known to date. Nevertheless, their potential influence on the structural integrity of DNA remains largely unexplored.
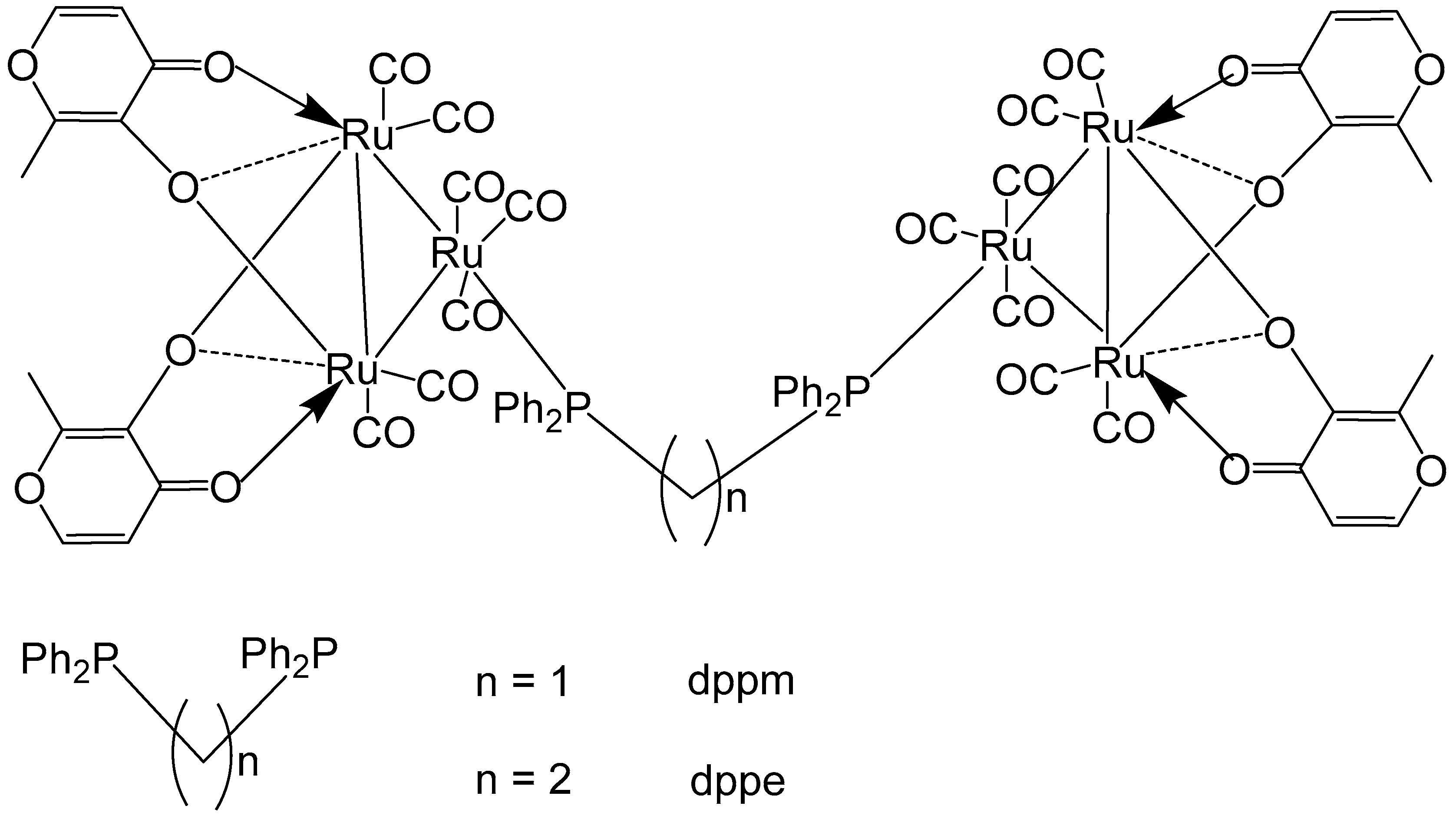
Reddy et al. have reported Ru–maltol complexes of the types [Ru3(CO)7(2L-2H)]2(dppm) and [Ru3(CO)7(2L-2H)]2(dppe), Figure 12, where L = 3-hydroxy-2-methyl-4H-pyran-4-one [60]. The compounds were synthesized by an interaction of [Ru3(CO)8(2L–2H)] with the bridging ligands 1,1-bis(diphenylphosphinomethane) (dppm) or 1,2-bis(diphenylphosphinoethane) (dppe). Cytotoxic evaluation of the complexes, along with the positive control cisplatin, has been performed on different tumor cells: K562 (chronic myelogenous leukemia, CML), HT-29 (colon), HL-60 (acute myloid leukemia, AML), DU-145 (prostate), MCF-7 (breast), GRANTA-519 (mantle cell leukemia, MCL), H460 (non-small cell lung carcinoma). The diphenylphosphinomethane- and diphenylphosphinoethane-bridged dimers of ruthenium–maltol compounds [Ru3(CO)7(2L-2H)]2(dppm), and [Ru3(CO)7(2L-2H)]2(dppe) exhibited significantly lower activity compared to their precursor complexes. A comparison between the two dimers revealed that increasing the chain length of the diphosphine bridge generally enhanced cytotoxic activity across most cell lines. However, the opposite trend was observed for the K562 cells, while no clear conclusions could be drawn for the HL-60 and GRANTA-519 cells. It is also noteworthy that [Ru3(CO)7(2L–2H)]2(dppe) could not achieve the reported IC50 values, as the complex showed poor solubility at concentrations above 25 μM. The diphenylphosphinomethane- and diphenylphosphinoethane-bridged dimers of Ru–maltol complexes, depicted in Figure 12, showed lower activity compared to the monomeric Ru–maltol complex comprising triphenylphosphine [Ru3(CO)7(2L-2H)(PPh3)]. The reduced activity may be attributed to limited cellular uptake caused by the intrinsic bulkiness of the hexanuclear complexes.
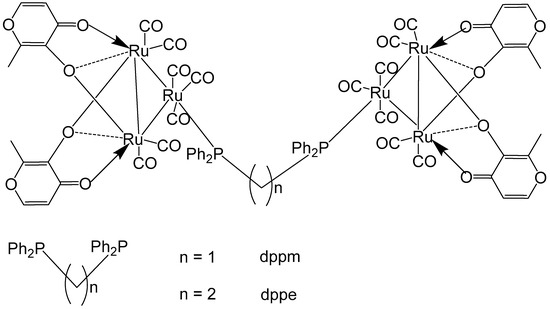
Figure 12.
Ru–maltol complexes [Ru3(CO)7(2L-2H)]2(dppm) and [Ru3(CO)7(2L-2H)]2(dppe), where L = 3-hydroxy-2-methyl-4H-pyran-4-one, dppm = 1,1-bis(diphenylphosphinomethane), dppe = 1,2-bis(diphenylphosphinoethane).
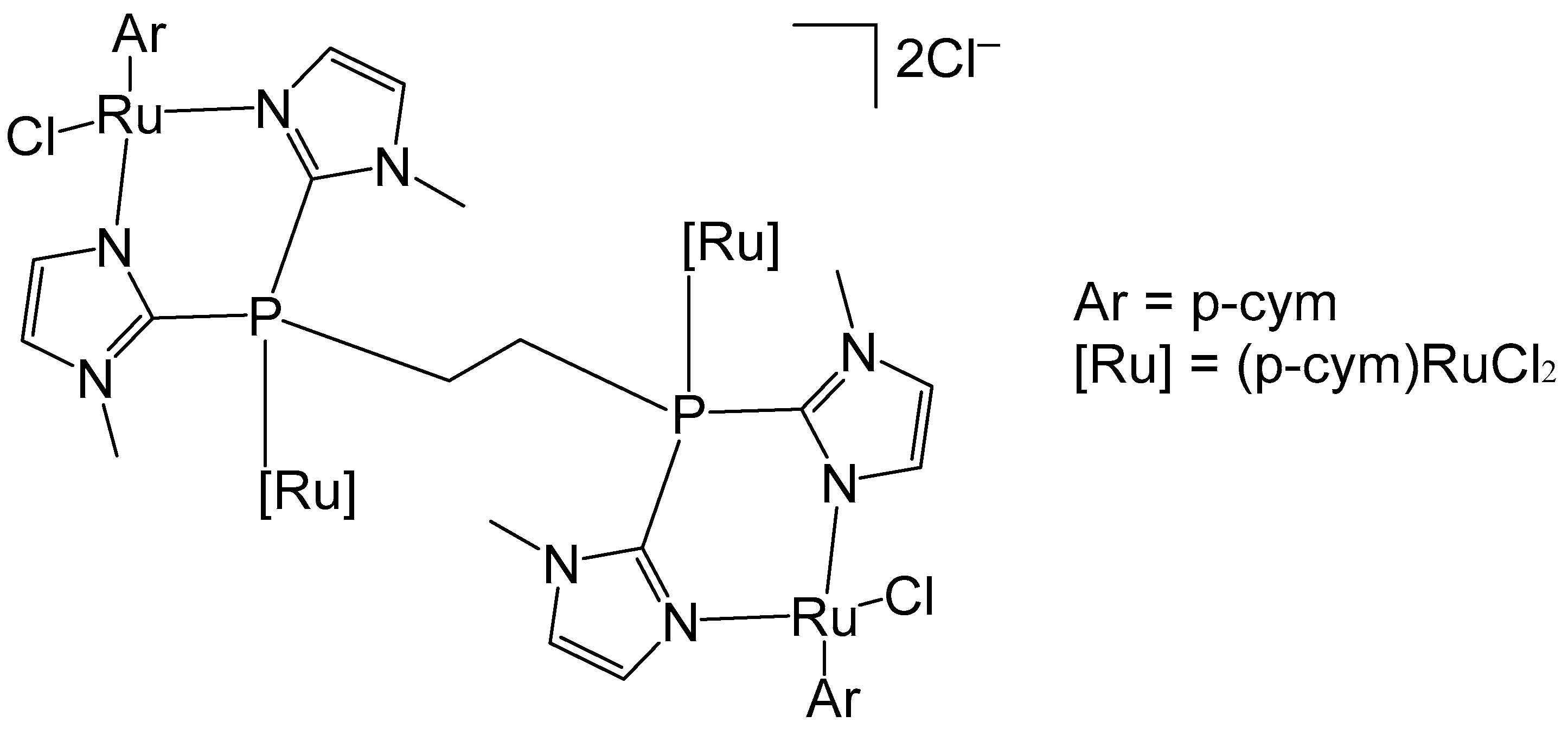
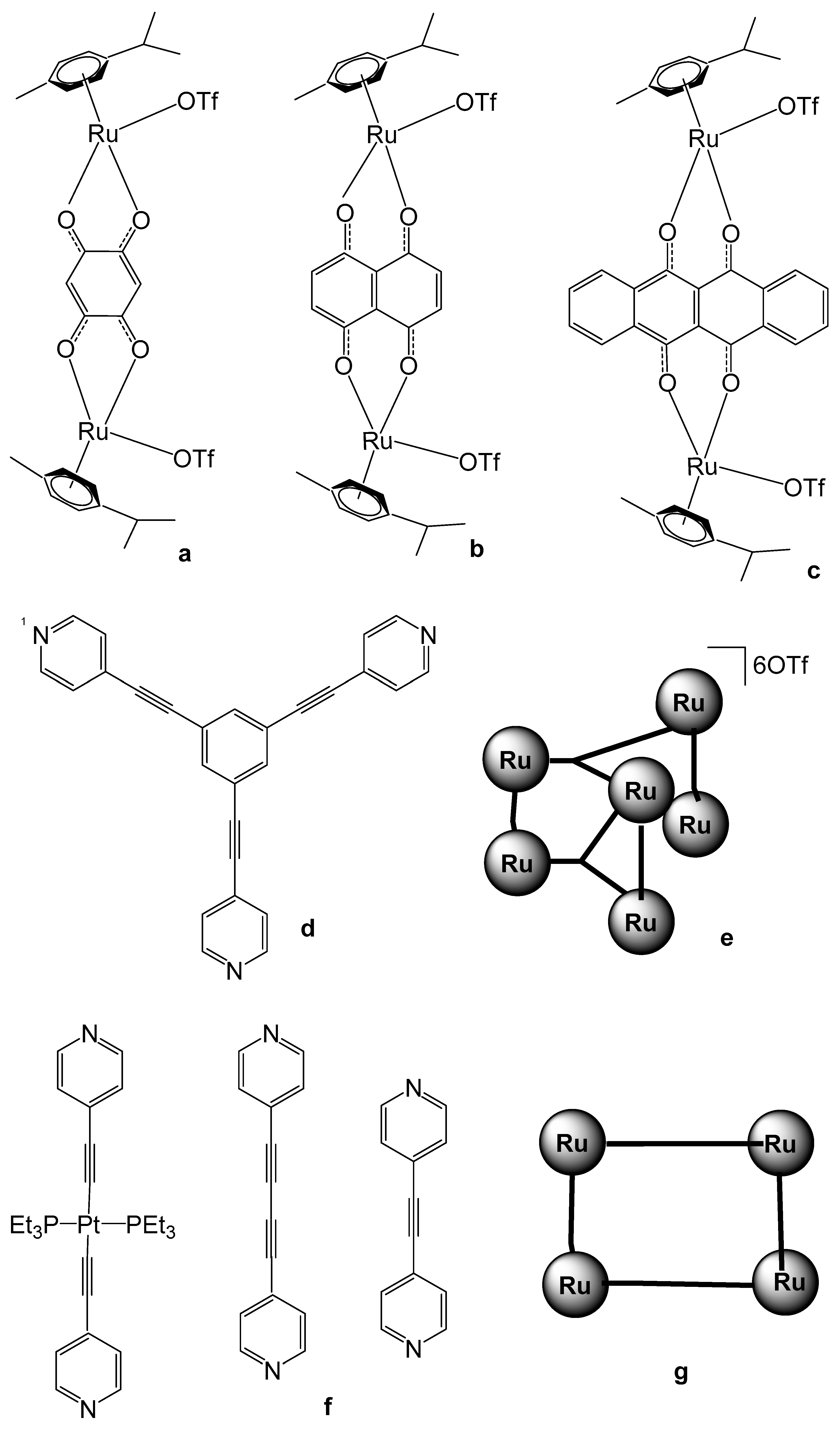
Noffke et al. have described the tetranuclear ruthenium–arene complex [(η6-p-cym)4Ru4(2-dimpeNMe)Cl6]Cl2, Figure 13, where 2-dimpeNMe = 1,2-bis(di-N-methylimidadol-2-ylphosphino)ethane [61]. Notably, this ligand exhibits higher water solubility compared to that of 1,2-bis(diphenylphosphinoethane), dppe [62]. The complex was synthesized by reacting 2-dimpeNMe with [(η6-p-cym)RuCl2]2. The four p-cymene ruthenium moieties exhibited a characteristic piano-stool geometry. The cytotoxic activity against different cell lines was evaluated using an MTT assay. With an approximate IC50 value greater than 100 μM, the ruthenium–arene complex can be considered non-toxic. The tested tumor cells were human colon carcinoma Hct116, human hepatoma Huh 7, rat hepatoma H4IIE, and human ovarian carcinoma A2780 (cisplatin sensitive).
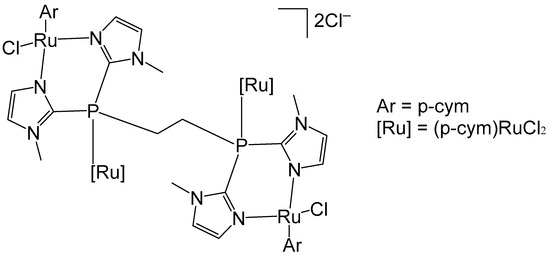
Figure 13.
Structure of tetranuclear ruthenium–arene complex [(η6-p-cym)4Ru4(2-dimpeNMe)Cl6]Cl2.
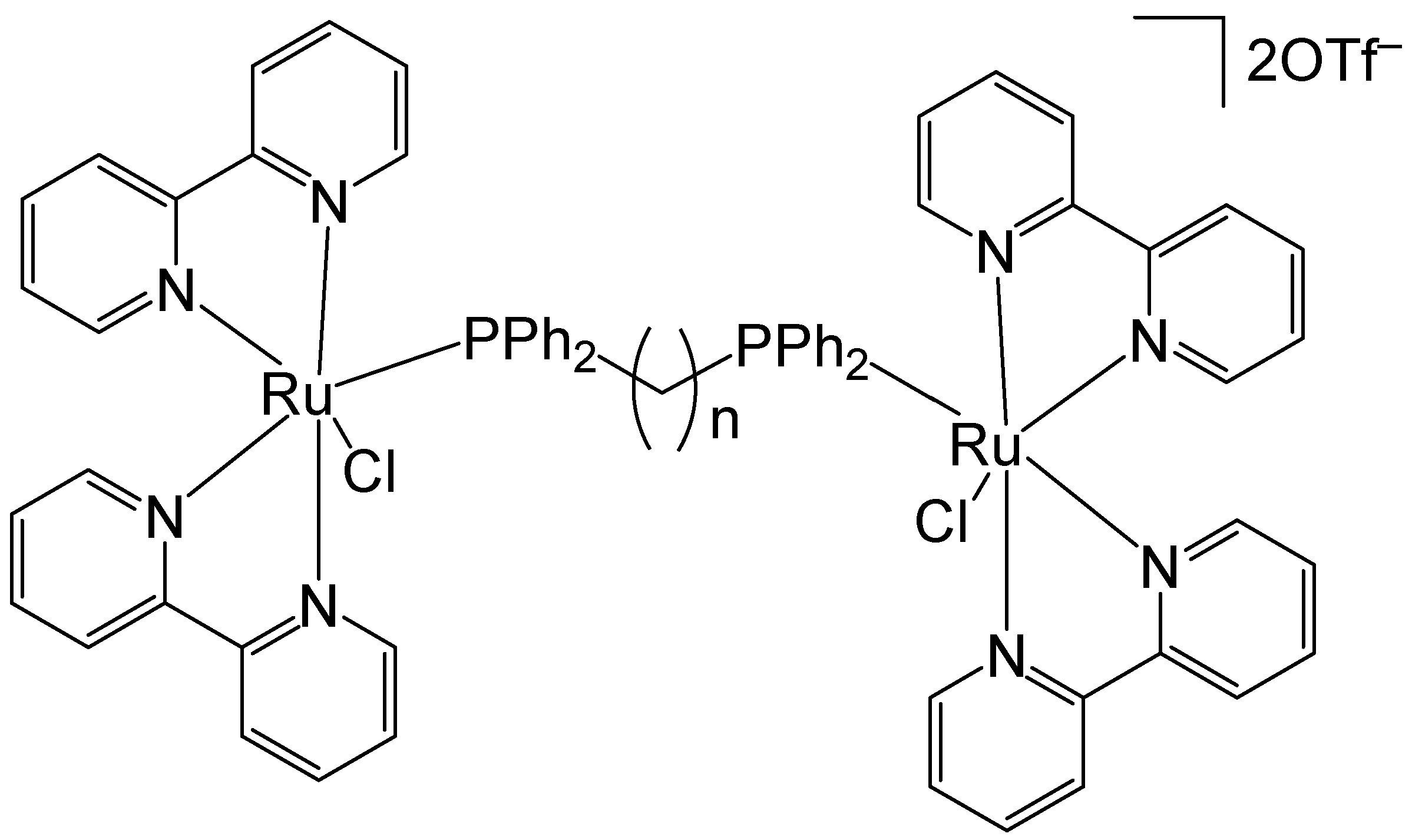
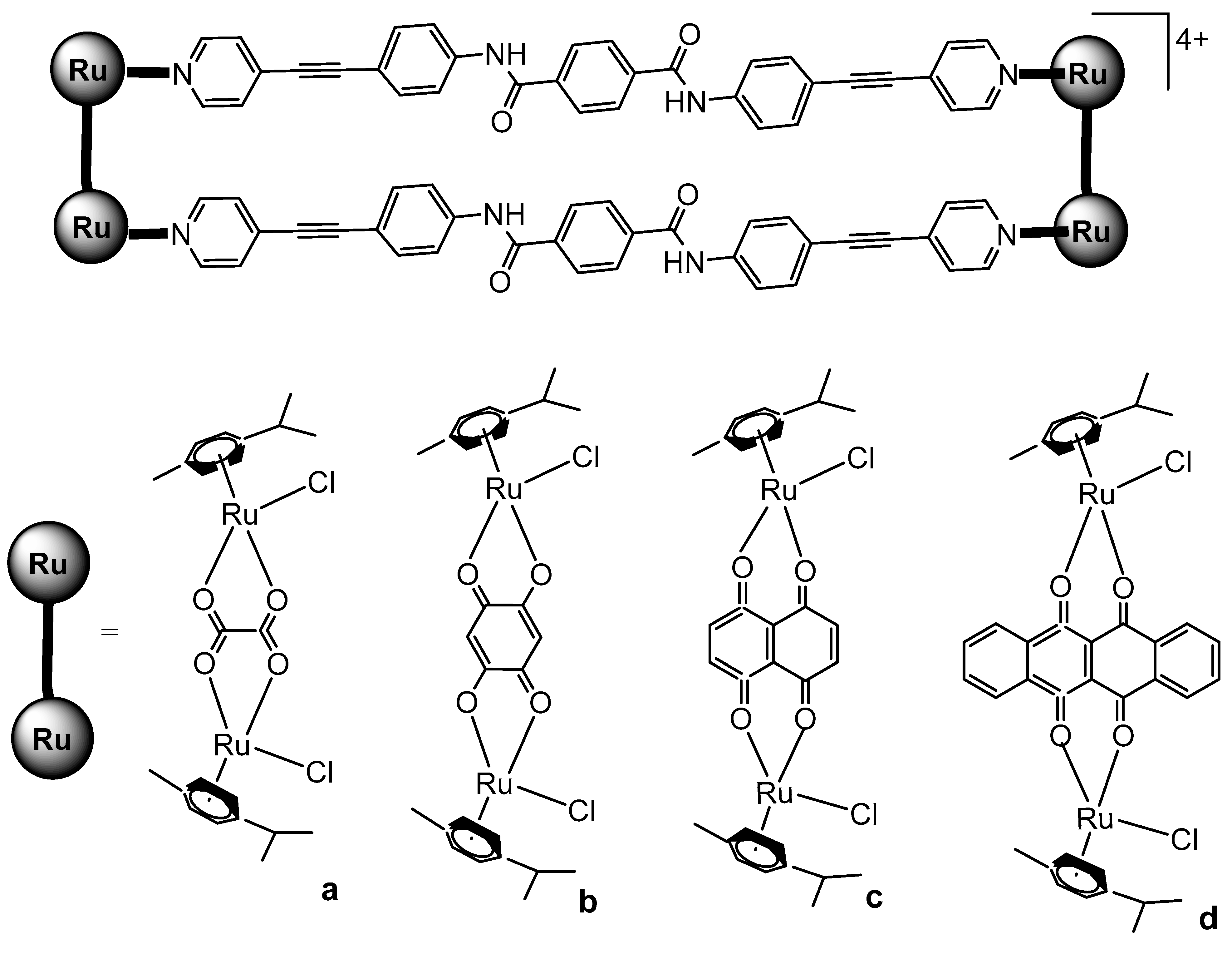
Lenis-Rojas et al. have reported the synthesis and structural characterization of the salt-type complex [(Ru(bipy)2Cl)2(μ-dppb)](OTf)2, Figure 14, where dppb = 1,4-bis(diphenylphosphino)butane and −OTf = trifluoromethanesulfonate anion CF3SO3− (triflate). Additional derivative complexes incorporating various bridging motifs have likewise been synthesized [63]. The complex [(Ru(bipy)2Cl)2(μ-dppb)](OTf)2 was synthesized by reacting the Ru(II) precursor complex [Ru(bipy)2Cl2] with AgOTf. The newly obtained complex and its derivatives have been tested in vitro for cytotoxicity on A2780, MCF7, and MDAMB231 human tumor cell lines. The complex [(Ru(bipy)2Cl)2(μ-dppb)](OTf)2 exhibited a higher percentage of A2780 cell apoptosis compared to the other derivatives. Considering the non-linear correlation between the IC50 values and the percentage of cell apoptosis, the authors proposed that these compounds might also trigger an alternative form of programmed cell death. This hypothesis was further supported by the observed increase in autophagy levels following treatment with these complexes. The newly obtained complexes interacted with CT-DNA via intercalation, which appeared to induce some DNA degradation extent. This effect appeared to be associated with the generation of ROS. In the in vivo toxicity evaluation, the complex [(Ru(bipy)2Cl)2(μ-dppb)](OTf)2 exhibited the highest toxicity toward zebrafish embryos, with an LC50 value of 5.397 mg/L, indicating it to be the least biocompatible among the tested compounds.
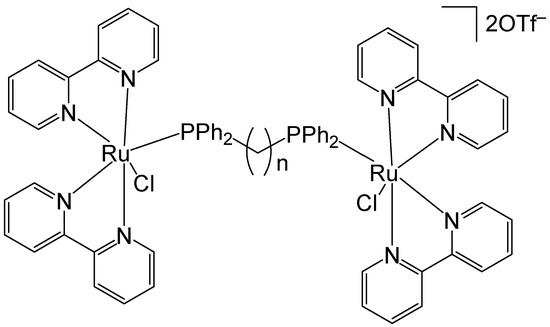
Figure 14.
Structure of the complex [(Ru(bipy)2Cl)2(μ-dppb)](OTf)2, where dppb = 1,4-bis(diphenylphosphino)butane and −OTf = trifluoromethanesulfonate anion CF3SO3− (triflate).
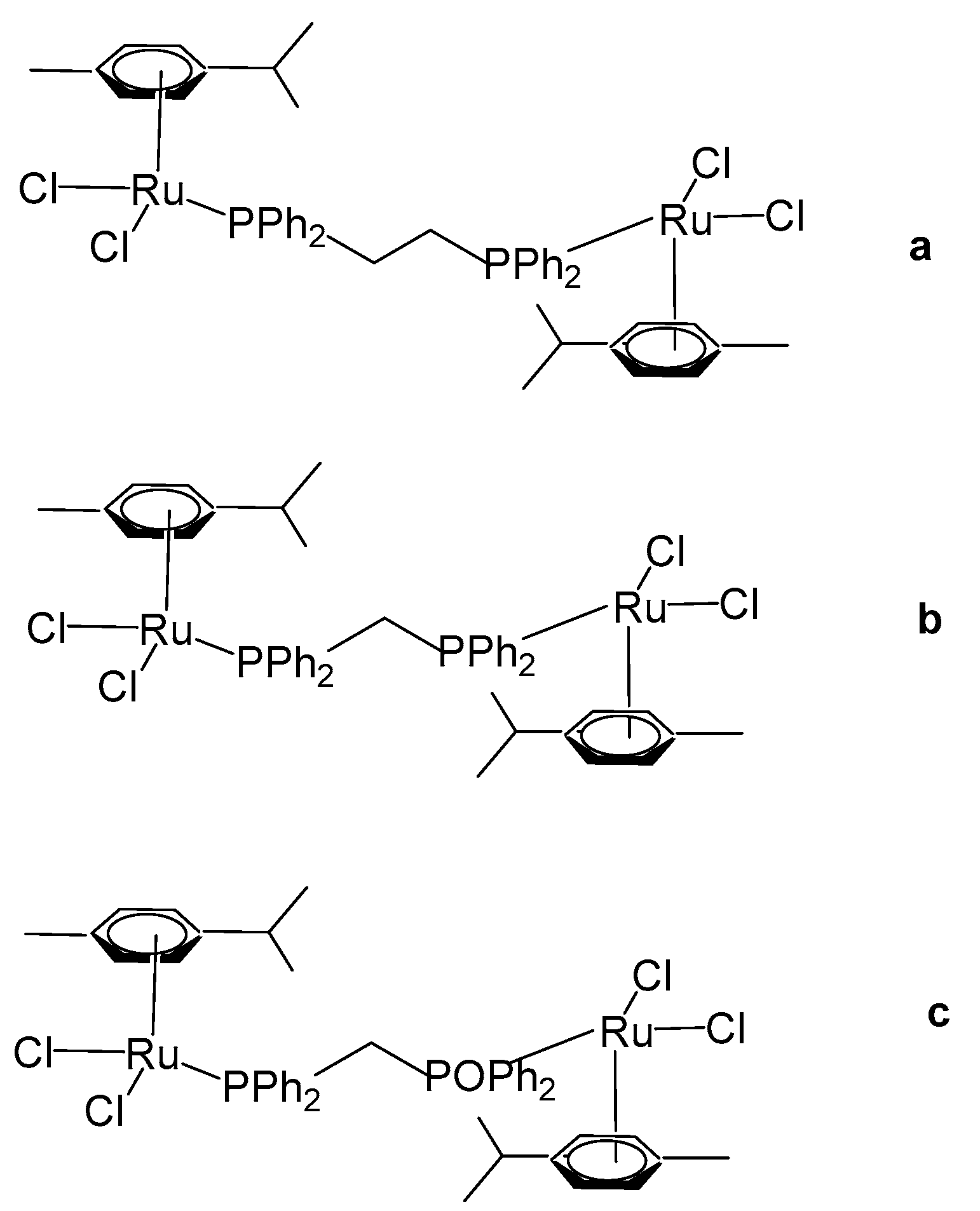
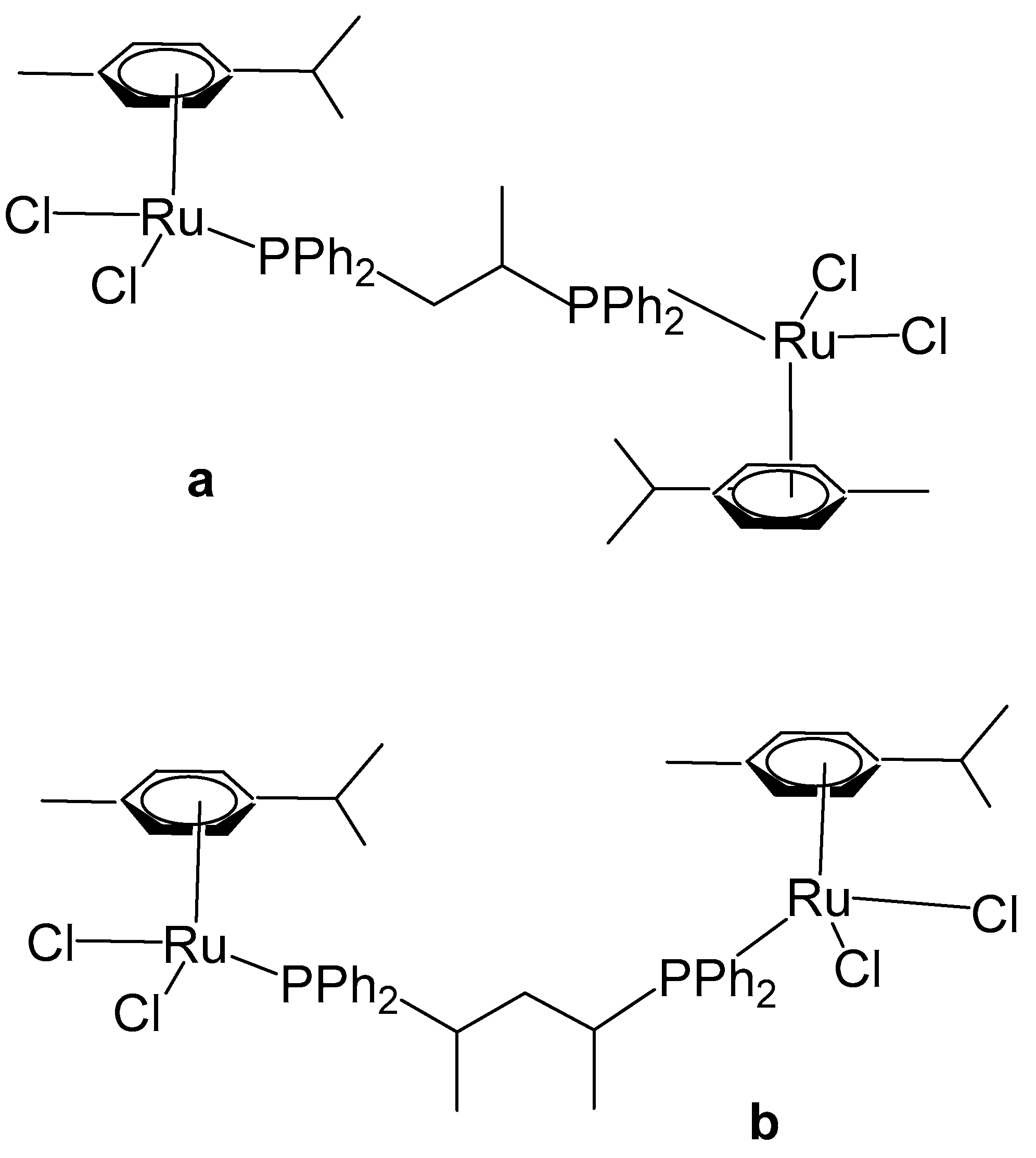
Das et al. have reported organoruthenium(II) complexes [(η6-p-cym)RuCl(X)(Y)], Figure 15 [64]. The compound [(η6-p-cym)RuCl2]2(μ-dppe), Figure 15a, where dppe = 1,2-bis(diphenylphosphino)ethane, was obtained using the dimer complex [(η6-p-cym)RuCl2]2 as a precursor, which interacted with dppe in benzene medium. The cytotoxicity was evaluated by MTT assays. The human lung non-small cell carcinoma H460 line treated with the complex [(η6-p-cym)RuCl2]2(μ-dppe) demonstrated limited cytotoxicity, exhibiting an IC50 value of 61.3 μM. This value is markedly higher than those observed for dppe alone (0.08 μM), cisplatin (1.7 μM), and several mononuclear ruthenium complexes reported within the same study. Owing to its comparatively poor cytotoxic performance, the complex was not subjected to further biological investigation. Furthermore, the complex was observed to show less cytotoxicity to non-cancerous HEL 299 cell lines. Additional neutral and cationic Ru(II) complexes [(η6-p-cym)RuCl(X)(Y)] with piano-stool geometries have been obtained and characterized by the same authors [64]. In the cationic complexes, the X and Y ligands were identified as either η2-coordinated phosphorus donors, such as 1,1-bis(diphenylphosphino)methane (dppm) and 1,2-bis(diphenylphosphino)ethane (dppe), or as their partially oxidized analogues, 1,2-bis(diphenylphosphino)methane monooxide (dppmo) and 1,2-bis(diphenylphosphino)ethane monooxide (dppeo), which exhibit strong hydrogen-bond-accepting capabilities. It has been found that the coordination of ruthenium to dppm and dppmo improved the cytotoxic activity of the complexes. The structurally similar compounds with the ligands dppm and dppmo [(η6-p-cym)Ru(η1-dppm)Cl2] (Figure 15b) and [(η6-p-cym)Ru(η1-dppmo)Cl2] (Figure 15c) showed good cytotoxic activity. The growth-inhibitory effects of these complexes on various human tumor cell lines have been investigated. The cytotoxic efficiency was tested against cervical cancer SIHA, breast cancer MCF7, prostate cancer PC3, ovarian cancer A2780, and hepatocellular carcinoma HEPG2 cell lines. Mechanistic studies on these complexes have revealed that their inhibitory effect on cancer cell growth involves the cell cycle arrest and the induction of apoptosis. Moreover, these complexes have demonstrated potent antimetastatic activity by preventing cancer cell invasion. They have been shown to interact with DNA through covalent binding without promoting its cleavage and to induce unwinding of plasmid DNA in a cell-free system. It can be concluded that the selection of ligands plays a pivotal role in maximizing cytotoxicity, and within the series examined, dppm and dppmo were identified as the most effective ligands.
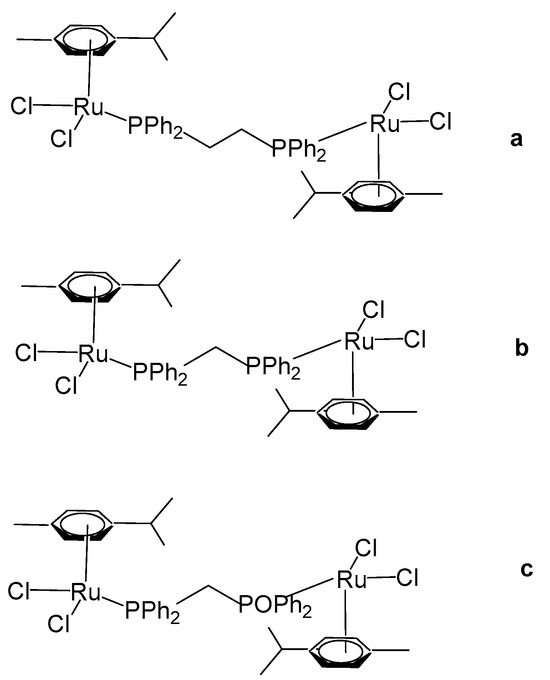
Figure 15.
Structures of ruthenium complexes [(η6-p-cym)RuCl2]2(μ-dppe) (a), [(η6-p-cym)Ru(η1-dppm)Cl2] (b), and [(η6-p-cym)Ru(η1-dppmo)Cl2] (c).
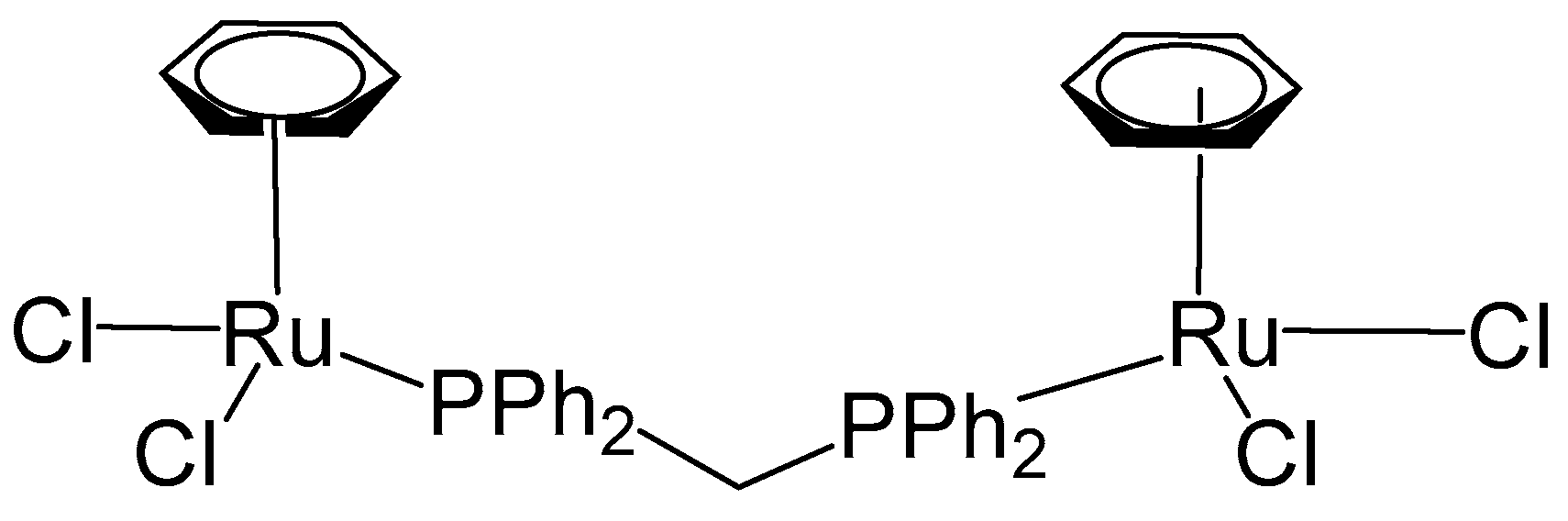
Herry et al. have recently described a related ruthenium complex featuring a distinct arene substituent and a short diphosphine bridging ligand, 1,1-bis(diphenylphosphino)methane (dppm) [65]. The mononuclear complex, [(η6-C6H6)RuCl2(κ1-dppm)], was initially prepared via the reaction of the dimeric precursor complex [(η6-C6H6)RuCl2]2 with dppm. Subsequent treatment of [(η6-C6H6)RuCl2(κ1-dppm)] with [(η6-C6H6)RuCl2]2 afforded the homodinuclear Ru,Ru complex [(η6-C6H6)RuCl2(μ-dppm)RuCl2(η6-C6H6)] (Figure 16), in a straightforward manner. Both monosubstituted and bimetallic complexes have been evaluated for cytotoxic activity against human ovarian cancer A2780 and the cisplatin resistant A2780cisR cancer cell lines along with the clinically approved cisplatin and RAPTA-C as positive and negative controls, respectively. The non-cancerogenic human embryonic kidney (HEK293) cell line was included to evaluate the interaction of the compounds with normal cells and to assess their selectivity. Cisplatin served as the positive control. The binuclear complex (Figure 16) exhibited substantially lower cytotoxic activity relative to both the mononuclear complex and cisplatin, with an IC50 value of 30 μM in A2780 cells, compared to 16 μM and 2.3 μM for the mononuclear complex and cisplatin, correspondingly. In the cisplatin-resistant A2780cisR cell line, the dinuclear complex demonstrated moderately enhanced activity (IC50 = 16 μM); however, this improvement was not significant when compared with cisplatin (IC50 = 17.6 μM), and the compound remained less potent than the mononuclear complex (IC50 = 13.8 μM). The observed reduction in cytotoxic activity of the dinuclear complex is likely attributable to diminished cellular uptake, possibly caused by the complex’s relatively large size, which may impede its ability to traverse the cellular membrane. The complex compounds showed somewhat lower activity against healthy cell line HEK293 than the malignant cells; however, they quite clearly were less toxic than cisplatin, which is attributed to the absence of Pt.
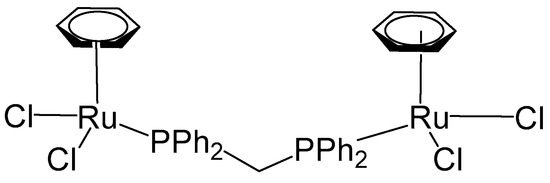
Figure 16.
The structure of the homobinuclear Ru,Ru complex [(η6-C6H6)RuCl2(μ-dppm)RuCl2(η6-C6H6)].
Arene-based organometallic ruthenium(II) complexes have garnered considerable pharmacological attention for their potent antitumor activities. Klaimanee et al. have recently reported similar complexes (Figure 17), with methyl-substituted ligands, 1,2-bis(diphenylphosphino)propane, and 2,4- bis(diphenylphosphino)pentane, producing the complex compounds [(η6-p-cym)RuCl2]2(μ-1,2-dppp), Figure 17a, and [(η6-p-cym)RuCl2]2(μ-2,4-dpppent), Figure 17b, correspondingly [66]. The complexes were synthesized by the interaction of the dimeric precursor [(η6-p-cym)RuCl2]2 with the corresponding ligand in THF medium under stirring. The obtained homobinuclear metallic compounds (Figure 17a,b) contained two distorted tetrahedral ruthenium(II) centers with η6-p-cymene, two chloride ligands, and the corresponding P-donor ligand. MTT assays against breast cancer cells, e.g., MCF-7, HCC1937, and MDA-MB-231, revealed that both complexes exhibited markedly higher anticancer activity than cisplatin. Complex [(η6-p-cym)RuCl2]2(μ-1,2-dppp), Figure 17a, was particularly potent against the tested tumor cell lines with IC50 values of 0.35, 0.54, and 0.89 μM, respectively. The authors observed that increasing the concentration of the Ru complex resulted in a decrease in cell viability. Among the tested cell lines, MCF-7 cells were notably more sensitive to the complexes than HCC1937 and MDA-MB-7 cells. Remarkably, these complexes exhibited greater effectiveness compared to previously studied analogues. This enhanced activity may be attributed to the increased lipophilicity conferred by the methyl groups on the diphosphine backbone or to a higher propensity for M–P bond dissociation due to their electron-donating properties.
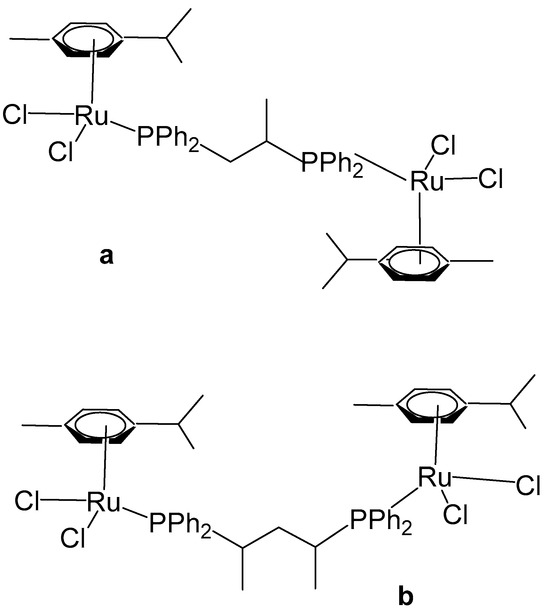
Figure 17.
Structures of [(η6-p-cym)RuCl2]2(μ-1,2-dppp) (a) and [(η6-p-cym)RuCl2]2(μ-2,4-dpppent) (b).
4. Ruthenium-Based Metalloassemblies
Supramolecular coordination complexes (SCCs), constructed via the self-assemblies of bioorganic ligands and metal cations, have been widely utilized across diverse applications. Their ability to interact with biomolecules through numerous non-covalent interactions is important for intense applicability in the biological fields, particularly in cancer therapy and cellular imaging. On the other hand, SCCs are attractive because of their solution capability due to their inherently charged assemblies and counter-ion effect. With their large, well-defined internal cavities, SCCs can encapsulate and deliver drugs at the anticipated locations, such as platinum complexes [67], porphin [68,69], pyrenyl-functionalized units [70,71,72], etc., to form the complex-in-a-complex systems with better cytotoxicity. Supramolecular coordination complexes can be principally separated into two classes: metallacycles with rhomboidal, triangular, rectangular, and hexagonal structures and metallacages with tetrahedral, hexahedral, and dodecahedral geometries.
The remarkable efficiency of bioactive Ru-based organometallic and coordination complexes has played a significant role in advancing the research on ruthenium supramolecular coordination complexes as prosperous antitumor systems. The ruthenium-based metalloassemblies can be obtained by integrating conventional polypyridine-coordinated Ru vertices or by introducing organoruthenium fragments containing Ru–C bonds. Working with Ru complexes in the +2 oxidation state offers an advantage. Furthermore, studies have revealed that kinetically inert ruthenium(II) ions are often favored in Ru-based species exhibiting bioactivity in vivo [73]. Rationalized synthetic processes for the formation of antineoplastic Ru-based metalloassemblies can also include reorganization of polypyridine–ruthenium fragments accompanied by coordination with organic linker ligands or additional metal cations as alternative strategies [74].
Arene–ruthenium organometallic complexes are undoubtedly an additional alternative for the construction of ruthenium metalloassemblies [75,76]. Arene–ruthenium complexes belong to the family of organometallic complexes with a half-sandwich piano-stool geometry, such as p-cymene (arene)–ruthenium compounds. Their octahedral geometry can be considered as pseudo-tetrahedral because of the presence of η6-arene or η5-Cp* ligands, described as monodentate. The organometallic Ru(II) half-sandwiched complexes are isostructural with their Os(II) [77], Rh(III) [78], and Ir(III) [79,80] analogues with anticancer activity [75]. The simplest arene–Ru complex is [(η6-benzene)RuCl2]2 with a dimeric structure consisting of two bridging and two terminal chloride ligands on the Ru(II) ions, together with two η6-coordinating benzene ligands [81]. The arenes coordinate in a facial arrangement, occupying three of the six coordination sites, while the remaining sites are occupied by labile ligands (H2O or Cl−). The arenes occupy three of the six coordination sites in a facial arrangement, while the other three are taken by labile ligands such as water or chloride. Thus, the octahedral arene–ruthenium complexes have three accessible coordination sites. Consequently, this coordination arrangement enables the design of biologically relevant ruthenium supramolecular coordination complexes [82].
In fact, a number of ruthenium organometallic and coordination supramolecular assemblies have been effectively designed in the last years [83,84,85,86,87]. Many of them have displayed various structure-inherent anticancer properties and substantial noncovalent targeting abilities to DNA, leading to superior antitumor activity [88,89,90,91,92]. Additionally, their capability to photosensitize oxygen and generate ROS makes them highly efficient PDT agents against numerous types of malignances [93]. Supramolecular chemistry offers better design capability for creating complex assemblies compared to traditional synthetic coordination chemistry. This is typically a controlled formation of structures that closely resemble the dimensions of DNA or protein recognition motifs [94]. For instance, metallohelices, such as helicates and their achiral counterparts, mesocates, are commonly assembled from rigid bioorganic ligands that coordinate around two or more metallic centers, resulting in the formation of two- or three-stranded helices [95,96]. Due to their defined 3D stereochemical structures, tunable configurations, charges, and diameters, they have been accepted as potent mimics of protein helices and efficient agents to cause DNA-–photoactive cleavage [97,98,99,100,101,102,103]. Recently, new binuclear ruthenium(II) double-stranded metallohelices for anticancer therapy that were controllably synthesized by changing the steric interactions between ligand strands have been reported [104]. Therefore, studying the metallohelicate systems might be an optimistic avenue to discover renewed antitumor therapeutics in the future. Therrien et al. have described water-soluble chloride or tetrafluoroborate salt-type compounds of tri-and tetranuclear Ru–arene clusters of the type [(η6-C6H6)(η6-C6Me6)2Ru3(μ-H)3(μ3-O)][BF4], [(η6-C6H6)(η6-1,4-iPrC6H4Me)(η6-C6Me6)Ru3(μ-H)3(μ3-O)][BF4], [(η6-C6H6)4Ru4(μ-H)4][BF4]2, [(η6-C6H5Me)4Ru4(μ-H)4][BF4]2, and [(η6-C6H6)4Ru4(μ-H)3(μ-OH)][Cl]2. The series of ruthenium clusters has been tested against ovarian cancer A2780 and A2780cisR cells. Both triruthenium clusters were very potent (IC50 < 10 µM) compared to cisplatin and Ru-based compounds on the whole, while the tetraruthenium clusters did not demonstrate substantial cytotoxicity. The pronounced difference observed between the Ru(III) and Ru(IV) cluster cations can be rationalized by their distinct supramolecular interactions with biomolecular environments. The triruthenium clusters exhibit an open hydrophobic cavity delineated by the three arene ligands, in conjunction with a hydrophilic oxo-cap that is predisposed to participate in hydrogen bonding. These complementary structural motifs facilitate specific supramolecular associations with aromatic systems and other functional groups within biomolecules. The same is not possible in the case of tetranuclear clusters. It is likely that these interactions are important in terms of their mode of action [105].
An alternative strategy has been confirmed to be relatively effective in formulating Ru–arene assemblies. This strategy includes stable binuclear clips, for instance, (p-cymene)2Ru2(oxalato)Cl2, and bidentate linear ligands, such as 4,4′-bipyridine, 4,4′-bipyridylethylene, etc. The binuclear Ru(II) clips may be obtained by utilizing another tetradentate bridging ligand, for instance, N/O or O/O derivatives, allowing the development of ruthenium supramolecular assemblies, such as metallocycles or metallocages. In the last decades, this approach has been widely applied to produce numerous 2D and 3D biologically active metal-based assemblies.
Among the varied range of metallomacrocycles that incorporate diverse metallic centers, geometries, sizes, and functionalities for biomedical applications, ruthenium(II) metallacycles have attracted considerable attention due to their unique structural versatility and promising therapeutic potential. Owing to the favorable photophysical and redox properties of the Ru(II) center, as well as its ability to form stable coordination assemblies, these metallacycles have been extensively explored for both chemotherapeutic and phototherapeutic interventions in malignant diseases. A particularly effective synthetic approach involves the use of binuclear Ru–arene clip modules, which can be linked through appropriate pyridine-containing bidentate linear ligands to afford discrete, well-defined tetranuclear Ru(II) metallacycles with square geometries, as reported by Yan et al. [106]. The oxalato-based complexes [Ru2(µ-η4-C2O4)Cl2(η6-p-PriC6H4Me)2] and [Ru(η2-C2O4)(NH3)(η6-p-PriC6H4Me)] reacted with triphenylphosphine to give [Ru2(µ-η4-C2O4)(PPh3)2(η6-p-PriC6H4Me)2][O3SCF3]2 and [Ru(η2-C2O4)(PPh3)(η6-p-PriC6H4Me)]. The dichloro complex Ru2(µ-η4-C2O4)Cl2(η6-p-PriC6H4Me)2] was transformed to the cationic di-methanol compound [Ru2(µ-η4-C2O4)(PPh3)2(η6-p-PriC6H4Me)2][O3SCF3]2 which interacted with 4,4′-bipyridine to form the new tetranuclear metallomacrocycle [Ru4(µ-η4-C2O4)2(µ-η1∶η1-bipy)2(η6-p-PriC6H4Me)4][O3SCF3]4 with alternating oxalato- and 4,4′-bipyridine bridge ligands [96].
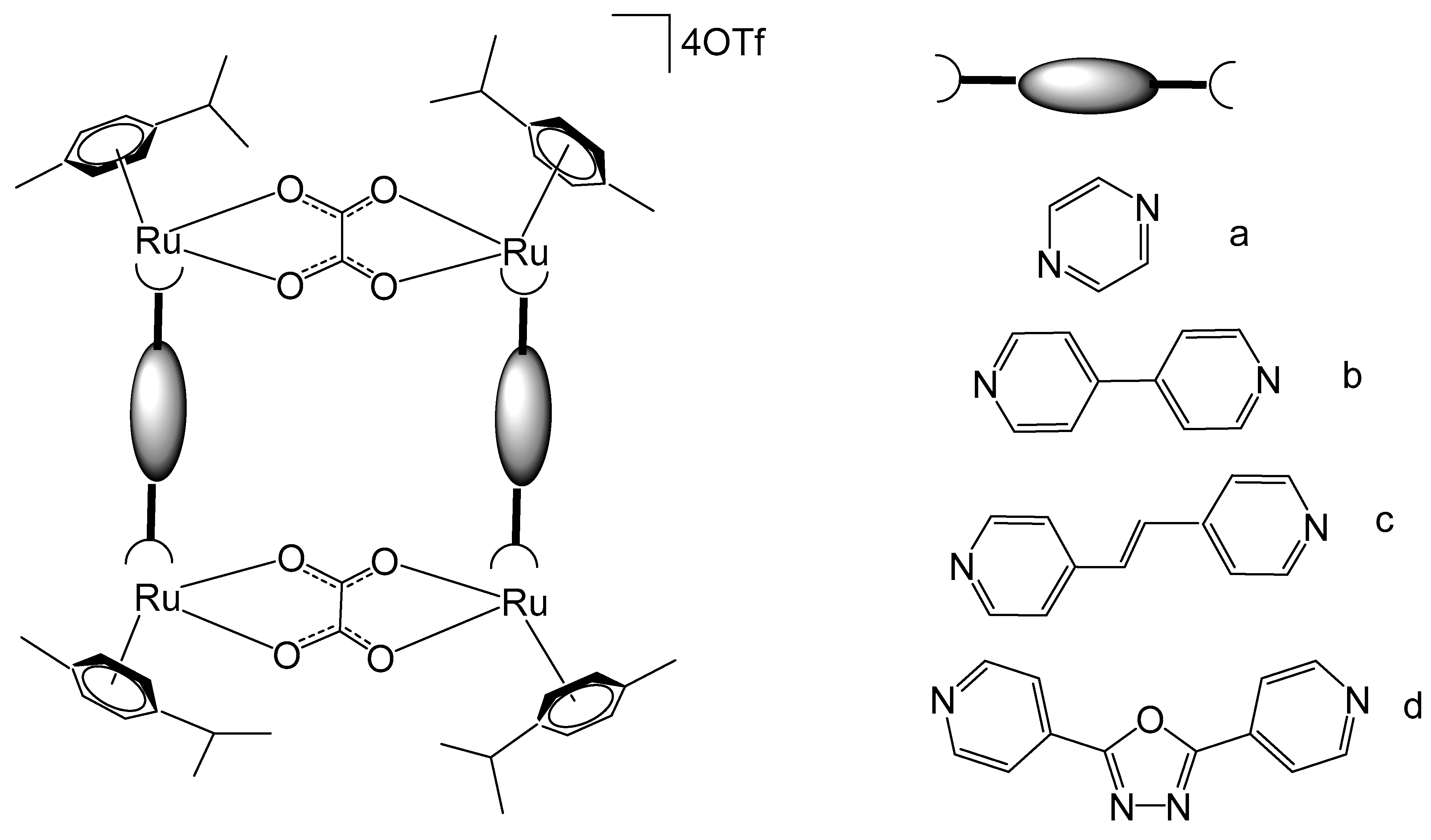
Han et al. have synthesized a series of metallarectangles, Figure 18a–d, through the coordination-driven self-assembly of an unsaturated binuclear Ru–arene clip with a range of linear bidentate pyridyl linkers. Binuclear complexes [(p-cymene)2Ru2(μ-CA)Cl2] (CA = chloranilate) have been synthesized by the reaction of [(p-cymene)RuCl(μ-Cl)]2 with H2CA in a basic medium. Interaction of [(p-cymene)2Ru2(μ-CA)Cl2] with bidentate ligands L such as pyrazine (prz), 4,4′-dipyridine (bpy), E-1,2-bis(4-pyridyl)ethene (bpe), and 2,5-bis(4-pyridyl)-1,3,5-oxadiazole (bpo), in the presence of AgOTf (OTf = CF3SO3) and CH3OH, afforded the respective tetranuclear complexes [(p-cymene)4Ru4(μ-CA)2(μ-L)2](OTf)4 (Figure 18a–d), correspondingly [107,108,109]. The single-crystal X-ray analysis of [(p-cymene)4Ru4(μ-CA)2(μ-L)2](OTf)4 revealed that the ruthenium centers were connected by pyridyl ligands and bis–bidentate chloranilate (CA) ligands to construct the rectangular cavity with different dimensions and strong π-interactions between independent molecules to produce rectangle channels in solid state. The metallocycles exhibited excellent aqueous solubility and possessed positive charges, facilitating their noncovalent binding with DNA and contributing to their antineoplastic activity. The tetracationic bowl-shaped rectangle, illustrated in Figure 18c, has been described to display strong non-covalent interactions with calf-thymus DNA, attributed to the dimensional complementarity between the metallarectangle and the major grooves of DNA [110]. The binding between the positively charged metallarectangle and the negatively charged DNA backbone is predominantly governed by electrostatic interactions.
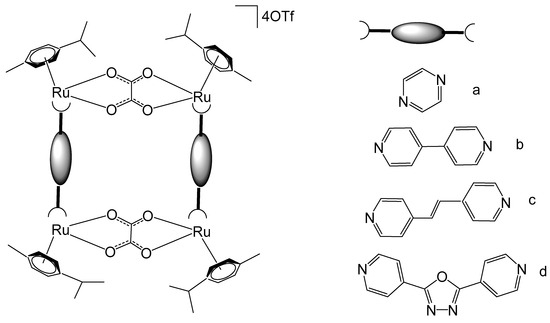
Figure 18.
Metallarectangles [(p-cymene)4Ru4(μ-CA)2(μ-L)2](OTf)4 with bidentate ligands (L): pyrazine (prz, (a)), 4,4′-dipyridine (bpy, (b)), E-1,2-bis(4-pyridyl)ethene (bpe, (c)), and 2,5-bis(4-pyridyl)-1,3,5-oxadiazole (bpo, (d)).
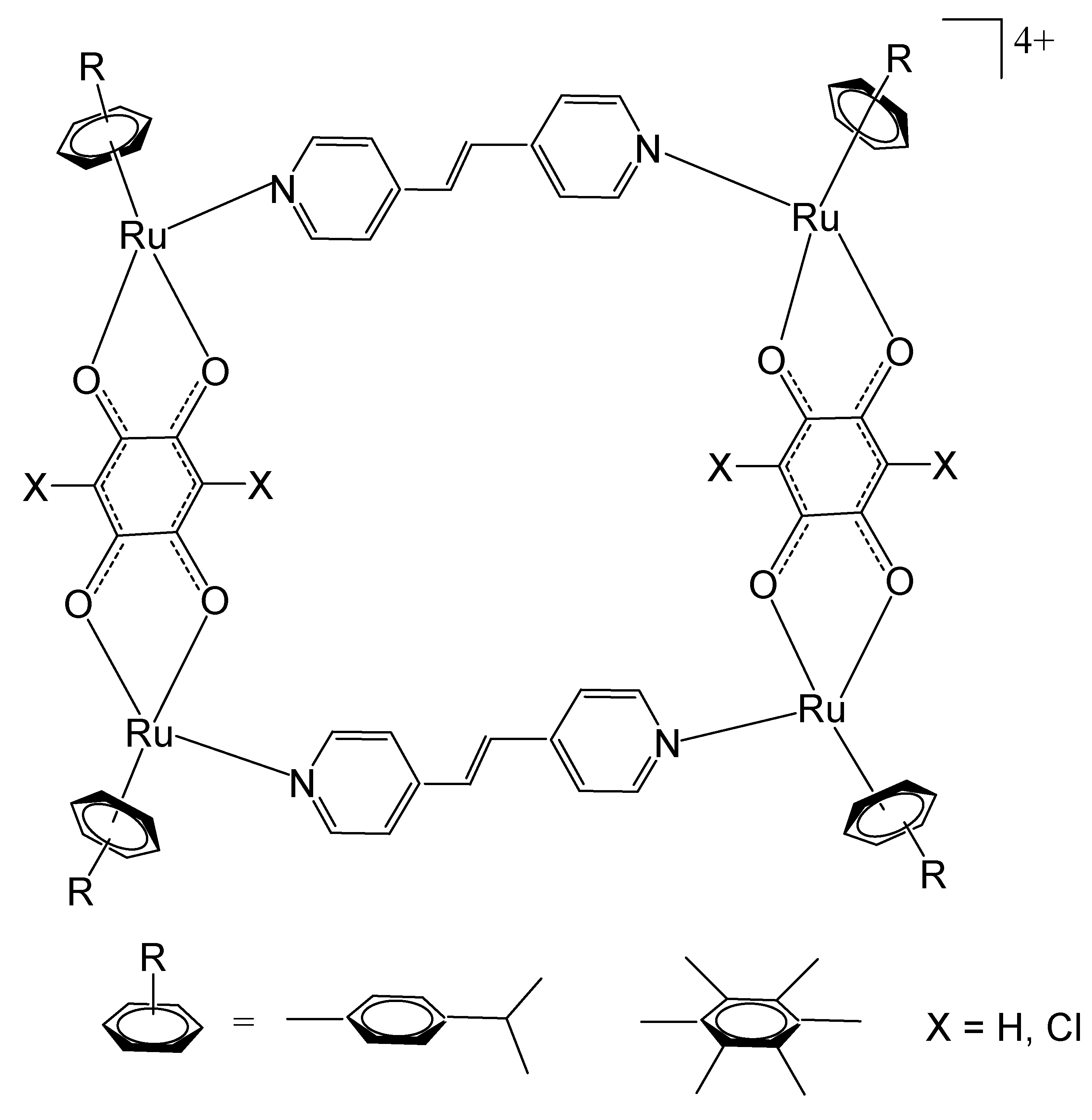
Building upon these preliminary studies, subsequent investigations into tetranuclear Ru-arene metallocycles have proliferated, focusing on their potential bioactive and antineoplastic properties. A series of such metallocyclic complexes were synthesized and systematically evaluated for their cytotoxic efficacy, as well as their interactions with DNA strands and protein targets. A set of ruthenium SCCs via [3 + 2] and [2 + 2] assembly have been reported (Figure 19) [111]. The set of [3 + 2] assembly with different biruthenium molecular clips, such as 2,5-dihydroxy-1,4-benzoquinone (dhbq, Figure 19a), 5,8-dioxydo-1,4-naphtaquinonato (donq, Figure 19b), and 6,11-dioxydo-5,12-naphtacenedionato (dotq, Figure 19c) and the ligand 1,3,5-tris(pyridine-4-ylethynyl) benzene, depicted in Figure 19d, have been synthesized [111]. The scheme for Ru-based SCCs via [3 + 2] assembly is presented in Figure 19e. The in vivo cytotoxic activity of the self-assemblies was assessed against liver SK-hep-1, cervix HeLa, colon HCT-15, lung A-549, and breast MDA-MB-231 cancer cell lines. The IC50 values of the obtained SCCs were comparatively low for some complexes, particularly those containing 2,5-dihydroxy-1,4-benzoquinone and 6,11-dioxydo-5,12-naphtacenedionato ligands. The same authors have reported a set of [2 + 2] ruthenium SCCs based on the 4-pyridyl ligands (Figure 19f). The produced [2 + 2] Ru-based SCCs had rectangular geometry. The schematic presentation for Ru-based SCCs via [2 + 2] assembly is shown in Figure 19g. The cytotoxicity of these compounds has been evaluated against SK-hep-1 (liver), HeLa (cervical), HCT-15 (colon), and AGS (gastric) cancer cells. The mixed ruthenium assemblies exhibited high antineoplastic activity. It has been found that the presence of longer dipyridyl ligands promotes the formation of complexes with enhanced anticancer activity. Furthermore, both the geometries and sizes of these complexes significantly affect their biological performance [111].
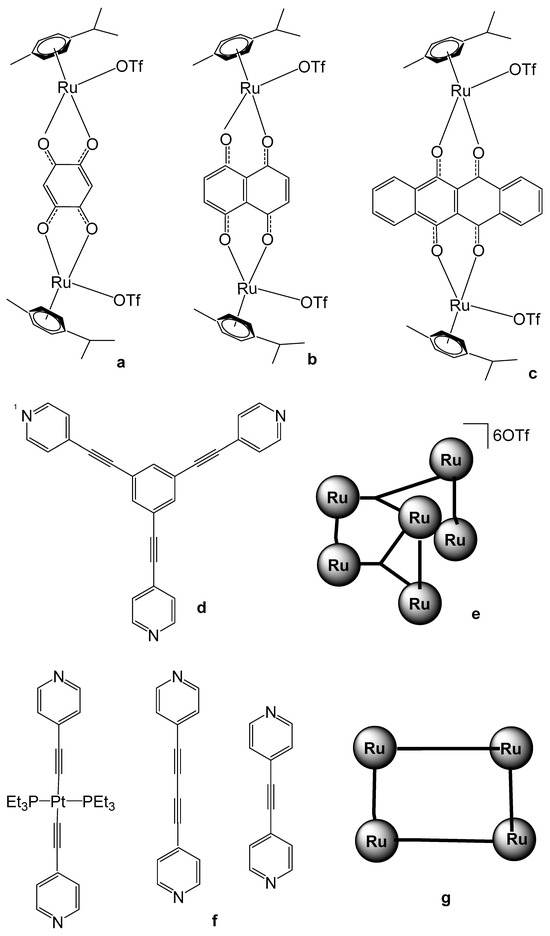
Figure 19.
Chemical structures of diruthenium molecular clips (a–c), the ligand 1,3,5-tris(pyridine-4-ylethynyl) benzene (d), schematic presentation for Ru-based SCCs via [3 + 2] assembly (e), 4-pyridyl ligands (f), and schematic presentation for ruthenium SCCs via [2 + 2] assembly (g).
Mishra et al. have synthesized ruthenium-based metallarectangles (Figure 20) by the interaction of various Ru–arene acceptors such as [Ru2(p-cymene)2(µ-η4-C2O4)Cl2] (Figure 20a) and [Ru2(p-cymene)2(L)(Cl)2] (Figure 20b–d), where L= 2,5-dihydroxy-1,4-benzoquinone (dhbq, Figure 20b), 5,8-dioxydo-1,4-naphtaquinonato (donq, Figure 20c), and 6,11-dioxydo-5,12-naphtacenedionato (dotq, Figure 20d) with a symmetrical donor ligand N,N′-bis(4-(pyridin-4-ylethynyl)phenyl)- terephthalamide [112]. The cytotoxicity of these metallarectangles was evaluated in human liver cancer SK-hep-1, gastric cancer AGS, and colorectal cancer HCT-15 cell lines. The studied metallarectangles exhibited greater cytotoxicity against all tested tumor cells compared to the recognized antitumor drugs doxorubicin and cisplatin. Moreover, exposure to the most active metallarectangle significantly upregulated the expression of the colorectal cancer suppressor genes APC and p53.
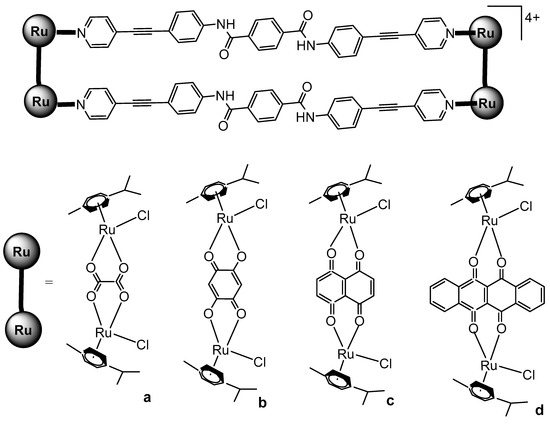
Figure 20.
Ruthenium-based metallarectangles of N,N′-bis(4-(pyridin-4-ylethynyl)phenyl)-terephthalamide ligand with the diruthenium molecular clips oxalate (a), dhbq (b), donq (c), and dotq (d).
The same authors have described large metallarectangles via self-assembly of an unsymmetrical amide donor (N-(4-(pyridin-4-ylethynyl)phenyl)isonicotinamide) ligand and two different lengths of Ru–arene acceptors [Ru2(μ-η4-C2O4)(MeOH)2(η6-p-PriC6H4Me)2][O3SCF3]2 and [Ru2(p-cymene)2(donq)(OH2)2 O3SCF3]2, where donq is 5,8-dioxydo-1,4-naphthaquinonato. They tested the new complexes in vitro for anticancer potency against human colorectal Colo320, lung A549, breast MCF-7, and lung H1299 cancer cell lines. The anticancer efficacy of the studied metallarectangles was observed to be significantly better against all the tested cell lines than that of the reference cisplatin [113].
Moreover, by choosing linear or V-shaped dipyridines to bind the different binuclear Ru(II)–arene clips, a series of tetranuclear metallocycles have been fabricated and their corresponding antineoplastic activity has been explored [114,115]. Metallacycles of dicarboxylate-bridged Ru–arene precursors [Ru2(μ-η4-OO∩OO)(η6-p-iPrC6H4Me)2][CF3SO3]2 (OO∩OO = oxalate, 2,5-dihydroxy-1,4-benzoquinonato (dobq), and 5,8-dihydroxy-1,4-naphthoquinonato (donq), reported in [114], effectively inhibited the growth in human colon HCT-15 and human gastric AGS cancer cell lines. The tetranuclear cationic metalla-bowls of bispyridine amide donor ligands, reported in [115], have the general formula [Ru4(p-cymene)4(N∩N)2(OO∩OO)2][CF3SO3]4 (N∩N = 2,6-bis(N-(4-pyridyl-carbamoyl)pyridine, where OO∩OO = 2,5-dihydroxy-1,4-benzoquinonato, 5,8-dioxydo-1,4-naphthaquinonato, or hoxonato ligands. Consistent with prior investigations of Ru–arene complexes, the obtained metalla-bowls exhibited pronounced antiproliferative activity against human liver (SK-hep-1), gastric (AGS), and colorectal (HCT-15) cancer cells. The extent of growth inhibition observed surpassed that of the reference chemotherapeutic agents doxorubicin and cisplatin. In colorectal tumor cells, exposure to the most active metalla-bowl led to the upregulation of the tumor suppressor genes APC and p53, indicating a potential mechanistic basis for the observed cytotoxic effects. Furthermore, Co–Ru bimetallic rectangles have been demonstrated to act as new possible agents for the induction of apoptosis and autophagy in human cancer cell lines [116]. Collectively, these investigations have deepened the understanding of the bioactivity of tetranuclear Ru–arene metallocycles, highlighting their promising antineoplastic potential.
Recently, the use of ruthenium-based complexes as PDT agents has further enriched their therapeutic effectiveness. Tetranuclear arene–ruthenium(II) complex compounds comprising the porphyrin scaffold have also been considered for PDT as promising photosensitizing chemotherapeutics. The bioactivity of these tetranuclear arene–ruthenium(II) complexes was evaluated on human melanoma tumor cells, and their intracellular localization and cellular uptake were studied [117]. With 5,10,15,20-tetra(4-pyridyl)porphyrin (4-tpp) as a central unit, the derivatives of para-cymene and toluene [(η6-p-MeC6H4Pri)RuCl2]4(4-tpp) and [(η6-C6H5Me)RuCl2]4(4-tpp) have been found to be very active against melanoma cell lines [117]. The tetranuclear ruthenium complexes exhibited excellent phototoxicity. Cellular localization and uptake studies of porphyrin arene–ruthenium(II) complexes [(η6-p-MeC6H4Pri)RuCl2]4(4-tpp) and [(η6-C6H5Me)RuCl2]4(4-tpp) showed that the studied compounds accumulated in the melanoma cellular cytoplasm differently than lysosomes, representing a new class of promising photosensitizers capable of associating chemotherapeutic potency with phototherapeutic anticancer therapy. The same authors have demonstrated that the isomeric 5,10,15,20- tetra(3-pyridyl)porphyrin (3-tpp) derivatives of para-cymene and toluene [(η6-p-MeC6H4Pri)RuCl2]4(3-tpp) and [(η6-C6H5Me)RuCl2]4(3-tpp) were very efficient at very low doses (5 µM) to induce antiproliferation of human Me300 melanoma cells [118]. The complexes displayed dual synergistic effects, integrating the favorable features of arene–ruthenium chemotherapeutic agents with those of porphyrin photosensitizers.
Mattsson et al. have described tetranuclear rectangular arene–ruthenium cationic complexes, Figure 21, combining 2,5-dihydroxy-1,4-benzoquinato (dbq) derivatives and a dipyridyl linker [119]. Cationic arene–ruthenium-based tetranuclear rectangulars have been obtained from the dinuclear arene–ruthenium complexes [Ru2(arene)2(OO∩OO)2Cl2] (arene = p-cymene, hexamethylbenzene; OO∩OO = 2,5-dihydroxy-1,4-benzoquinonato, 2,5-dichloro-1,4-benzoquinonato) by reaction with a bipyridine linker N∩N = E-1,2-bis(4-pyridyl)ethene in the presence of AgO3SCF3 in methanol, producing tetranuclear cations [Ru4(arene)4(N∩N)2(OO∩OO)2][CF3SO3]4. The cytotoxicity of these water-soluble complexes has been evaluated on the human ovarian cancer A2780 cell line. The studied compounds were found to be potent against the tested cancer cells [119].
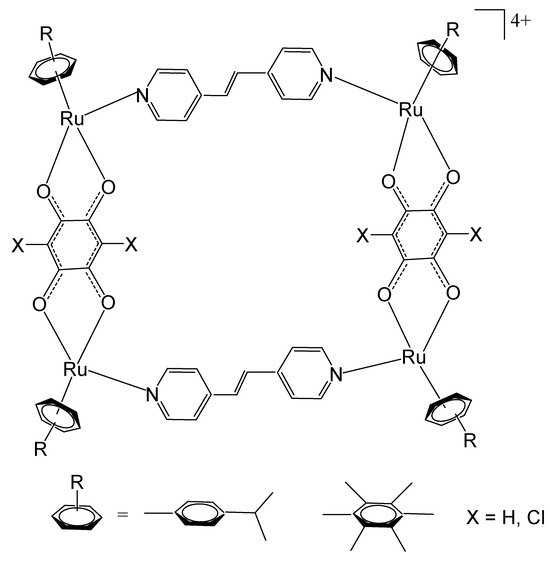
Figure 21.
Structures of rectangular tetranuclear arene–ruthenium cationic complexes [Ru4(arene)4(N∩N)2(OO∩OO)2][CF3SO3]4, where arene = p-cymene, hexamethylbenzene; OO∩OO = 2,5-dihydroxy-1,4-benzoquinonato, 2,5-dichloro-1,4-benzoquinonato derivatives.
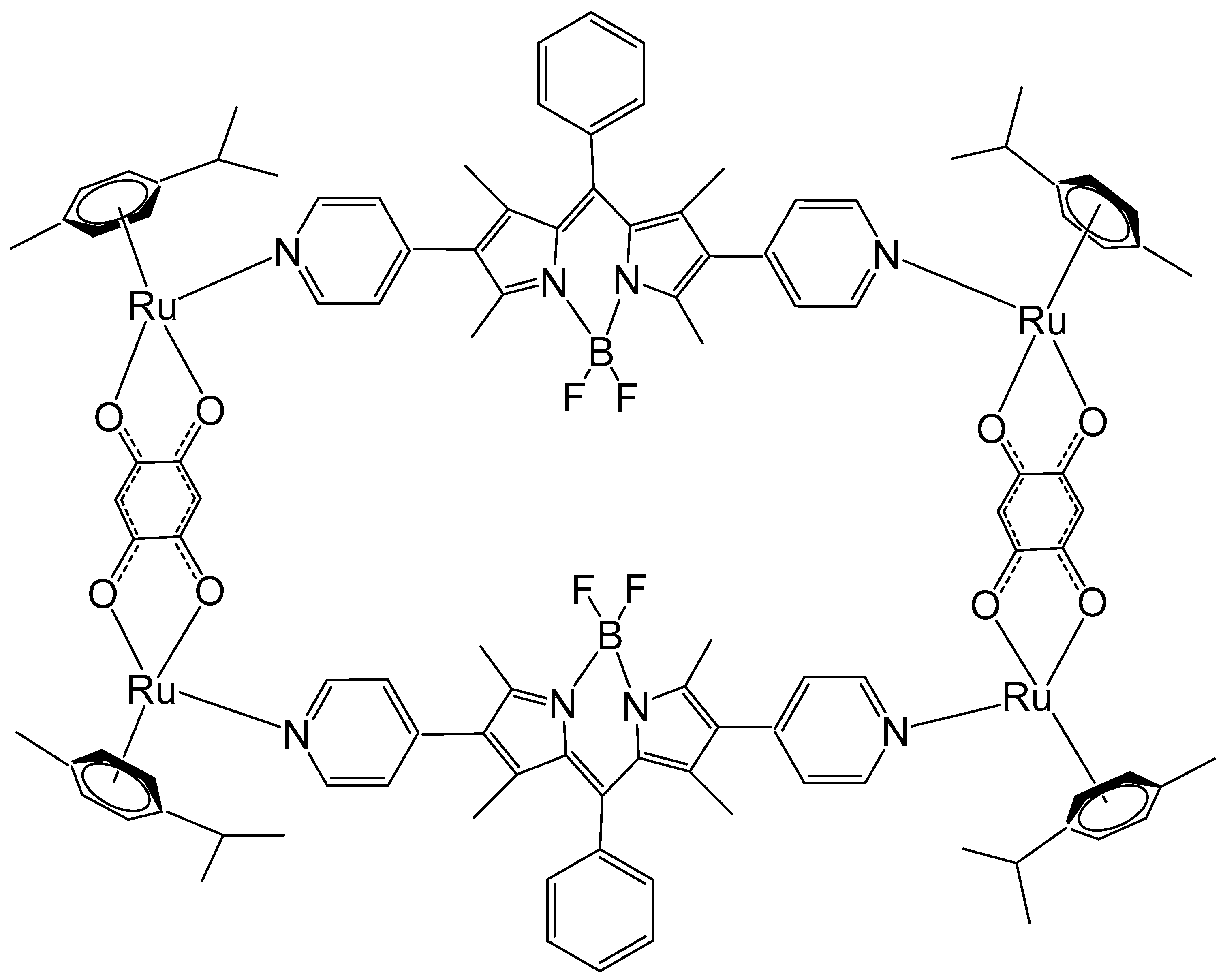
Motivated by the remarkable photophysical and photochemical characteristics of BODIPY, Gupta et al. have synthesized Ru metallorectangles containing a BODIPY-based dipyridine linker and evaluated their antineoplastic potential [120]. Some of the compounds displayed very selective antineoplastic potency and interacted strongly with DNA and proteins. Among them, the ruthenium metallorectangle, depicted in Figure 22, showed improved cytotoxicity against several tumor cell lines compared to that of cisplatin, e.g., lung A549 (IC50 = 3.09 ± 0.17 μM), breast MCF-7 (IC50 = 2.72 ± 0.21 μM), cervical HeLa (IC50 = 4.31 ± 0.22 μM), and brain U87 (IC50 = 0.24 ± 0.06 μM) cancer cell lines. The characteristic green fluorescence of the BODIPY ligand and associated aggregation-induced emission (AIE) allowed imagining of the compounds inside the cells using confocal microscopy.
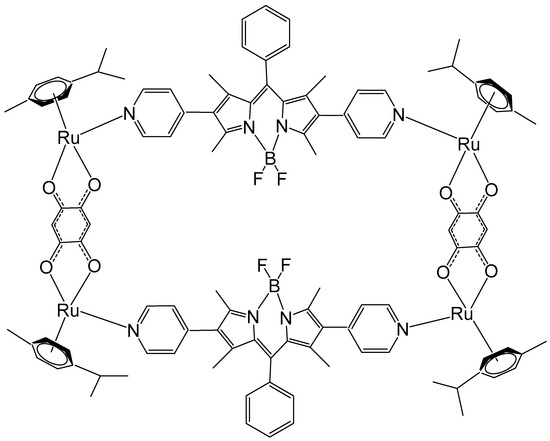
Figure 22.
The ruthenium metallorectangle, containing a BODIPY-based dipyridine linker.
To further enhance the biological performance of Ru-based supramolecular rectangles, the same research group recently reported a new series of Ru (2 + 2) assemblies constructed from thiophene-functionalized dipyridyl BODIPY ligands [121]. Confocal laser scanning microscopy analyses indicated that the obtained rectangles mainly localized in the cellular cytoplasm. Interestingly, the incorporation of the thiophene moiety into the BODIPY framework resulted in a pronounced enhancement of both cytotoxic activity and selectivity toward cancer cells, underscoring the critical role of ligand functionalization in modulating biological behavior. The complexes displayed dose-dependent cytotoxic activity against various tumor cell lines. The cytotoxicity of the studied rectangles showed selective activity against MCF-7 breast cancer cells while showing reduced toxic effects toward human non-malignant fibroblast WI38 cell lines. Moreover, the investigation revealed that cervical tumor cells also responded favorably to these compounds. The net fluorescence attributable to BODIPY permitted the visualization of their position inside tumor cells. Furthermore, the metallarectangles exhibited a considerable tendency to bind with biological macromolecules. Although previous binding studies have revealed substantial interactions between these metallorectangles and biomacromolecules such as DNA and proteins, additional investigations were warranted to elucidate their potential as photosensitizing agents in photodynamic therapy (PDT). Recently, Xu et al. have obtained a new Ru(II) metallacycle with a similar structure as that shown in Figure 22, by the inclusion of aza-BODIPY as a NIR-II fluorescence emitter. The constructed Ru(II) metalacyclic compound was able to emit over 1000 nm and to achieve precise NIR-II fluorescence for chemo- and phototherapy [122]. Its antineoplastic capability, assessed against lung (A549), cervix (Hela), and liver (HepG2) tumor cells, was notable in cisplatin-resistant cells with low toxic effects on healthy cells. In addition to its inhibitory effect on cell proliferation, the antimetastatic properties of the Ru(II) metallacycle were also investigated, and significant inhibition of the migration and invasion of tumor cells has been observed [122].
Ruthenium hexanuclear prismatic and octanuclear cubic metallocages are also of great interest. Water-soluble Ru–arene prismatic metallocages have demonstrated exceptional potential as drug delivery systems for encapsulating lipophilic antineoplastic agents in anticancer treatment. Therrien et al. have obtained a hexanuclear cation prismatic metallocage [Ru6(p-iPrC6H4Me)6(tpt)2-(dhbq)3](O3SCF3)6, which incorporated ruthenium p-cymene building blocks, bridged by 2,5-dihydroxy-1,4-benzoquinonato (dhbq) ligands with triangle-type pyridyl linkers, such as 2,4,6-tris (pyridin-4-yl)-1,3,5-triazine (tpt) [123]. The obtained metallocage [Ru6(p-iPrC6H4Me)6(tpt)2-(dhbq)3](O3SCF3)6 acted as a carrier, and its high charge may have promoted enhanced uptake in tumor cells. Many isostructural metalloprisms with slight modifications in the bridge ligands have been obtained in a similar approach, for instance, by using 5,8-dioxido-1,4-naphthoquinonato (donq) as a bridge ligand of ruthenium clips, which led to more spacious metalloprisms [124]. Cationic Ru–arene metalloprisms [Ru6(p-cymene)6(tpt)2(OO∩OO)3](O3SCF3)6, where OO∩OO represents 9,10-dioxo-9,10-dihydroanthracene-1,4-diolato and 6,11-dioxo-6,11-dihydronaphthacene-5,12-diolato, have been synthesized from the corresponding binuclear Ru–arene complexes [Ru2(p-cymene)2(OO∩OO)Cl2] via reaction with 2,4,6-tris (pyridin-4-yl)-1,3,5-triazine (tpt) [124].
On the other hand, with the substitution of the tridentate tpt derivatives with larger tridentate ligands, e.g., 1,3,5-tris{2-(pyridin-4-yl)vinyl}benzene (pvb), larger hexa- and octanuclear cages can be successfully produced [125]. Cationic Ru–arene assemblies [Ru6(p-cymene)6(tris-pvb)2(μ2-Cl)6][CF3SO3]6, [Ru6(p-cymene)6(tris-pvb)2(OO∩OO)3][CF3SO3]6 (tris-pvb = 1,3,5-tris{2-(pyridin-4-yl)vinyl}benzene), and [Ru8(p-cymene)8(NN∩NN)2(OO∩OO)4][CF3SO3]8 were successfully synthesized through coordination-driven self-assembly. The tritopic ligand 1,3,5-tris{2-(pyridin-4-yl)vinyl}benzene (tris-pvb) and the tetratopic ligands 1,2,4,5-tetrakis{2-(pyridin-4-yl)vinyl}benzene and 1,2,4,5-tetrakis{2-(pyridin-4-yl)ethynyl}benzene (NN∩NN) acted as bridging linkers to promote the formation of discrete, multinuclear architectures. These assemblies were obtained via the reaction of the corresponding binuclear Ru–arene precursors, [Ru2(p-cymene)2(μ-Cl)2Cl2] and [Ru2(p-cymene)2(OO∩OO)Cl2], with the appropriate multidentate ligands under mild conditions [125]. The OO∩OO bridging ligands employed include oxalato, 2,5-dioxido-1,4-benzoquinonato, 2,5-dichloro-1,4-benzoquinonato, 5,8-dioxido-1,4-naphthoquinonato, 5,8-dioxido-1,4-anthraquinonato, and 6,11-dioxido-5,12-naphthacenedionato, which impart distinct electronic and steric characteristics to the resulting assemblies. Variation of these bridging units allows fine-tuning of the redox properties and overall topology of the supramolecular frameworks. The resulting cationic species, stabilized by the η6-bound p-cymene ligands, display well-defined geometries characteristic of arene–ruthenium coordination-driven assemblies [125]. The large cationic metalla-assemblies allow encapsulation of various guest molecules [67,68,69,70,71,72]. The exceptional structural tunability of structures and molecular encapsulation characteristics toward aromatic planar guests, combined with the promising bioactivity of Ru–arene octanuclear and hexanuclear prismatic metallocages, highlight them as potential drug delivery vectors with remarkable selectivity for tumor cells [126,127,128].
Octanuclear cubic metallocages represent a class of attractive organometallic chemotherapeutics. Ru–arene octanuclear cubic metallocages can be conveniently assembled through the combination of appropriate Ru–arene clips and tetrapodal panel donors, e.g., the photoactive ligand tetrapyridyl porphyrin (tpp). Therrien et al. have synthesized a series of porphyrin-based organometallic cubes [129,130]. Some octanuclear ruthenium p-cymene and rhodium and iridium pentamethylcyclopentadienyl metalla-assemblies have been synthesized via coordination-driven self-assembly of tetrapyridyl porphyrins (tpp) with binuclear clips, (η6-MeC6H4Pri)2Ru2(μ4-C6HRO4)Cl2 (R = C11H23). All complexes displayed potent antiproliferative activity against MCF-7, B16, and A549 cancer cell lines, with IC50 values of about 0.1 μM, while showing similar effects in the non-tumorigenic NIH 3T3 cell line [129]. Ruthenium-based assemblies containing tetrapyridylporphyrins have been evaluated as photosensitizers (in photodynamic therapy) [130]. To further expand the development of new discrete ruthenium(II) octanuclear cubic cages with significant antineoplastic activity, Adeyemo et al. have recently obtained Ru(II) cubic cages constructed from tetradentate pyridyl ligands linked to various binuclear Ru(II) acceptor clips [131,132]. These novel octanuclear Ru(II) cages were prepared through coordination-driven self-assembly between a tetradentate pyridyl ligand, N,N,N′,N′-tetra(pyridin-4-yl)benzene-1,4-diamine, and different binuclear p-cymene Ru(II) acceptors: [Ru2(μ–η4-C2O4)(CH3OH)2(η6-p-cymene)2](O3SCF3)2, [Ru2(μ–η4-C6H2O4)(CH3OH)2(η6-p-cymene)2](O3SCF3)2, [Ru2(dhnq)(H2O)2(η6-p-cymene)2](O3SCF3)2, and [Ru2(dhtq)(H2O)2(η6-p-cymene)2](O3SCF3)2. The octanuclear self-assembled cages have demonstrated potent in vitro antineoplastic activity against human lung adenocarcinoma A549 and human cervical cancer HeLa cells, compared to cisplatin. Subsequent studies exploring the mechanism of action indicated that the observed cell death resulted from the generation of reactive oxygen species [132].
5. Conclusions
Ruthenium complex compounds have demonstrated substantial promise as antineoplastic agents with potentially reduced toxic side effects owing to their high selectivity against tumor cells. Ru-based complexes have demonstrated the capacity to associate with a broad spectrum of biological macromolecules, notably proteins and DNA.
While the predominant studies have focused on mononuclear ruthenium complexes, the potential of polynuclear Ru-based compounds remains rather underexplored. Despite the achieved promising prospects of these multifunctional drug candidates, some key issues of the currently known Ru polynuclear complexes still restrict their prevalent application. Some important aspects are still understudied: (1) detailed mechanistic studies on the possible mechanisms of action of homometallic ruthenium complexes are still scarce; (2) the SAR of these compounds remains basically unclear and necessitates further exploration; (3) their interactions with different targeting biomolecules are at their preliminary stage; (4) the in vivo experiments should be enhanced and systematically studied. Solving these issues is important and would lead to better design strategies for the development of new metallodrugs.
Significant approaches to increase the anticancer profile of structurally different polynuclear ruthenium complexes and organometallics are the incorporation of appropriate biologically active ligands with therapeutic values and the improvement of specific host–guest characteristics of Ru-based metalla-assemblies to enable better activity and selectivity, novel mechanisms of action, low toxicity, and modulated pharmacokinetic profiles. Through the functionalization of the obtained polynuclear ruthenium candidates, new targeting organelles could be revealed, contributing to the development of agents with a wide range of biological and medicinal applications. The reviewed findings and results clearly indicate that this field of drug discovery definitely needs further active exploration in the future in order to obtain alternative classes of effective anticancer chemotherapeutic and theranostic agents.
Funding
The financial support received by the European Union-Next Generation EU, through the National Recovery and Resilience Plan of the Republic of Bulgaria, project No. BG-RRP-2.004-0004-C01, is greatly acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal complexes in cancer therapy—An update from drug design perspective. Drug Des. Dev. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S. Recent advances in anticancer ruthenium Schiff base complexes. Appl. Organomet. Chem. 2020, 34, e5687. [Google Scholar] [CrossRef]
- Alessio, E. Bioinorganic Medicinal Chemistry; Wiley-VCH Verlag & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Kanaoujiya, R.; Srivastava, S.; Singh, R.; Mustafa, G. Recent advances and application of ruthenium complexes in tumor malignancy. Mater. Today Proc. 2023, 72, 2822–2827. [Google Scholar] [CrossRef]
- Mahmud, K.M.; Niloy, M.S.; Shakil, M.S.; Islam, M.A. Ruthenium Complexes: An Alternative to Platinum Drugs in Colorectal Cancer Treatment. Pharmaceutics 2021, 13, 1295. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Advances in the design of organometallic anticancer complexes. J. Organomet. Chem. 2017, 839, 5–14. [Google Scholar] [CrossRef]
- Vos, J.G.; Kelly, J.M. Ruthenium polypyridyl chemistry; from basic research to applications and back again. Dalton Trans. 2006, 41, 4869–4883. [Google Scholar] [CrossRef]
- Dyson, P.J.; Sava, G. Metal-based antitumour drugs in the post genomic era. Dalton Trans. 2006, 16, 1929–1933. [Google Scholar] [CrossRef]
- Sadique, S.; Baqer, A.A.; Salman, A.W.; Iqbal, M.A.; Kadim, M.M.; Jamil, F.; Altaf, A. Ruthenium complexes for breast cancer therapy. Rev. Inorg. Chem. 2024, 44, 191–208. [Google Scholar] [CrossRef]
- Silva, V.R.; Corrêa, R.S.; Santos, L.D.S.; Soares, M.B.P.; Batista, A.A.; Bezerra, D.P. A ruthenium-based 5-fluorouracil complex with enhanced cytotoxicity and apoptosis induction action in HCT116 cells. Sci. Rep. 2018, 8, 288. [Google Scholar] [CrossRef]
- Shum, J.; Leung, P.K.-K.; Lo, K.K.-W. Luminescent Ruthenium(II) Polypyridine Complexes for a Wide Variety of Biomolecular and Cellular Applications. Inorg. Chem. 2019, 58, 2231–2247. [Google Scholar] [CrossRef]
- Tan, C.-P.; Zhong, Y.-M.; Ji, L.-N.; Mao, Z.-W. Phosphorescent metal complexes as theranostic anticancer agents: Combining imaging and therapy in a single molecule. Chem. Sci. 2021, 12, 2357–2367. [Google Scholar] [CrossRef]
- Xu, M.; Wen, Y.; Liu, Y.; Tan, X.; Chen, X.; Zhu, X.; Wei, C.; Chen, L.; Wang, Z.; Liu, J. Hollow mesoporous ruthenium nanoparticles conjugated bispecific antibody for targeted anti-colorectal cancer response of combination therapy. Nanoscale 2019, 11, 9661–9678. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Gong, Y.; Liu, Y.; Yang, C.; Wu, S.; Yuan, G.; Guo, X.; Liu, J.; Qin, X. RuCeO2 yolk shell nanozymes: Oxygen supply in situ enhanced dual chemotherapy combined with photothermal therapy for orthotopic/subcutaneous colorectal cancer. Biomaterials 2020, 242, 119923. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska-Wawrzycka, A.; Rogala, P.; Michałkiewicz, S. Ruthenium complexes in different oxidation states: Synthesis, crystal structure, spectra and redox properties. Dalton Trans. 2013, 42, 6092–6101. [Google Scholar] [CrossRef]
- Lin, K.; Zhao, Z.-Z.; Bo, H.-B.; Hao, X.-J.; Wang, J.-Q. Applications of Ruthenium Complex in Tumor Diagnosis and Therapy. Front. Pharmacol. 2018, 9, 1323. [Google Scholar] [CrossRef]
- Riccardi, C.; Musumeci, D.; Trifuoggi, M.; Irace, C.; Paduano, L.; Montesarchio, D. Anticancer Ruthenium(III) Complexes and Ru(III)-Containing Nanoformulations: An Update on the Mechanism of Action and Biological Activity. Pharmaceuticals 2019, 12, 146. [Google Scholar] [CrossRef]
- Schluga, P.; Hartinger, C.G.; Egger, A.; Reisner, E.; Galanski, M.; Jakupec, M.A.; Keppler, B.K. Redox behavior of tumor-inhibiting ruthenium(III) complexes and effects of physiological reductants on their binding to GMP. Dalton Trans. 2006, 14, 1796–1802. [Google Scholar] [CrossRef]
- Wiśniewska, J.; Fandzloch, M.; Łakomska, I. The reduction of ruthenium(III) complexes with triazolopyrimidine ligands by ascorbic acid and mechanistic insight into their action in anticancer therapy. Inorg. Chim. Acta 2019, 484, 305–310. [Google Scholar] [CrossRef]
- Blazevic, A.; Hummer, A.A.; Heffeter, P.; Berger, W.; Filipits, M.; Cibin, G.; Keppler, B.K.; Rompel, A. Electronic State of Sodium trans-[Tetrachloridobis (1 H-indazole) ruthenate (III)](NKP-1339) in Tumor, Liver and Kidney Tissue of a SW480-bearing Mouse. Sci. Rep. 2017, 7, 40966. [Google Scholar] [CrossRef]
- Strasser, S.; Pump, E.; Fischer, R. On the chloride lability in electron-rich second-generation ruthenium benzylidene complexes. Monatsh Chem. 2015, 146, 1143–1151. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt (II) agents, nanoparticle delivery, and Pt (IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Kostova, I. Ruthenium complexes as anticancer agents. Curr. Med. Chem. 2006, 13, 1085–1107. [Google Scholar] [CrossRef] [PubMed]
- Motswainyana, W.M.; Ajibade, P.A. Anticancer Activities of Mononuclear Ruthenium(II) Coordination Complexes. Adv. Chem. 2015, 2015, 859730. [Google Scholar] [CrossRef]
- Savic, M.; Arsenijevic, A.; Milovanovic, J.; Stojanovic, B.; Stankovic, V.; Rilak Simovic, A.; Lazic, D.; Arsenijevic, N.; Milovanovic, M. Antitumor Activity of Ruthenium(II) Terpyridine Complexes towards Colon Cancer Cells In Vitro and In Vivo. Molecules 2020, 25, 4699. [Google Scholar] [CrossRef] [PubMed]
- Fukushi, S.; Yoshino, H.; Yoshizawa, A.; Kashiwakura, I. p53-independent structure-activity relationships of 3-ring mesogenic compounds’ activity as cytotoxic effects against human non-small cell lung cancer lines. BMC Cancer 2016, 16, 521. [Google Scholar] [CrossRef]
- Groessl, M.; Tsybin, Y.; Hartinger, C. Ruthenium versus platinum: Interactions of anticancer metallodrugs with duplex oligonucleotides characterised by electrospray ionisation mass spectrometry. J. Biol. Inorg. Chem. 2010, 15, 677–688. [Google Scholar] [CrossRef]
- Groessl, M.; Zava, O.; Dyson, P. Cellular uptake and subcellular distribution of ruthenium-based metallodrugs under clinical investigation versus cisplatin. Metallomics 2011, 3, 591–599. [Google Scholar] [CrossRef]
- Herić, A.; Dibranin, N.; Martić, L.; Hodžić, E.; Zahirović, A.; Kozarić, A.K. Ruthenium-based complexes as anti-tumor agents. J. Health Sci. 2024, 14, 70–83. [Google Scholar] [CrossRef]
- Kahrović, E.; Zahirović, A.; Pavelić, S.K.; Turkušić, E.; Harej, A. In vitro anticancer activity of binuclear Ru(II) complexes with Schiff bases derived from 5-substituted salicylaldehyde and 2-aminopyridine with notably low IC50 values. J. Coord. Chem. 2017, 70, 1683–1697. [Google Scholar] [CrossRef]
- Clarke, M.J. Ruthenium metallopharmaceuticals. Coord. Chem. Rev. 2002, 232, 69–93. [Google Scholar] [CrossRef]
- Davia, K.; King, D.; Hong, Y.; Swavey, S. A porphyrin–ruthenium photosensitizer as a potential photodynamic therapy agent. Inorg. Chem. Commun. 2008, 11, 584–586. [Google Scholar] [CrossRef]
- Vilaplana, R.A.; Delmani, F.; Manteca, C.; Torreblanca, J.; Moreno, J.; García-Herdugo, G.; González-Vílchez, F. Synthesis, interaction with double-helical DNA and biological activity of the water soluble complex cis-dichloro-1, 2-propylenediamine-N, N, N′, N′-tetraacetato ruthenium (III)(RAP). J. Inorg. Biochem. 2006, 100, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Han Ang, W.; Dyson, P.J. Classical and non-classical ruthenium-based anticancer drugs: Towards targeted chemotherapy. Eur. J. Inorg. Chem. 2006, 2006, 4003–4018. [Google Scholar] [CrossRef]
- Brabec, V.; Nováková, O. DNA binding mode of ruthenium complexes and relationship to tumor cell toxicity. Drug Resist. Upd. 2006, 9, 111–122. [Google Scholar] [CrossRef]
- Mendoza-Ferri, M.G.; Hartinger, C.G.; Mendoza, M.A.; Groessl, M.; Egger, A.E.; Eichinger, R.E.; Mangrum, J.B.; Farrell, N.P.; Maruszak, M.; Bednarski, P.J.; et al. Transferring the concept of multinuclearity to ruthenium complexes for improvement of anticancer activity. J. Med. Chem. 2009, 52, 916–925. [Google Scholar] [CrossRef]
- Yamada, T.; Noguchi, Y.; Nakai, M.; Yamauchi, O.; Nakabayashi, Y.; Sato, T.; Mino, Y. Solvolysis and cytotoxicity of dinuclear ruthenium (II)-2, 2′-bipyridine complexes with various bridged-ligands. Inorg. Chim. Acta 2013, 394, 190–195. [Google Scholar] [CrossRef]
- Mulyana, Y.; Collins, J.G.; Keene, F.R. Synthesis, nucleic acid binding and cytotoxicity of oligonuclear ruthenium complexes containing labile ligands. J. Incl. Phenom. Macrocycl. Chem. 2011, 71, 371–379. [Google Scholar] [CrossRef]
- Gorle, A.K.; Ammit, A.J.; Wallace, L.; Keene, F.R.; Collins, J.G. Multinuclear ruthenium(II) complexes as anticancer agents. New J. Chem. 2014, 38, 4049–4059. [Google Scholar] [CrossRef]
- Corral, E.; Hotze, A.C.; Den Dulk, H.; Leczkowska, A.; Rodger, A.; Hannon, M.J.; Reedijk, J. Ruthenium polypyridyl complexes and their modes of interaction with DNA: Is there a correlation between these interactions and the antitumor activity of the compounds? J. Biol. Inorg. Chem. 2009, 14, 439–448. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, L.; Cai, P.; Cheng, G.Z.; Xu, X.M.; Liu, Y. Synthesis, characterization and anticancer activity of dinuclear ruthenium(II) complexes linked by an alkyl chain. New J. Chem. 2015, 39, 5805–5812. [Google Scholar] [CrossRef]
- Liu, P.; Liu, J.; Zhang, Y.Q.; Wu, B.Y.; Wang, K.Z. Synthesis, DNA binding and photocleavage, and cellular uptake of an alkyl chain-linked dinuclear ruthenium(II) complex. J. Photochem. Photobiol. B 2015, 143, 89–99. [Google Scholar] [CrossRef]
- Beckford, F.A.; Niece, M.B.; Lassiter, B.P.; Beebe, S.J.; Holder, A.A. Polynuclear ruthenium organometallic complexes containing a 1, 3, 5-triazine ligand: Synthesis, DNA interaction, and biological activity. J. Biol. Inorg. Chem. 2018, 23, 1205–1217. [Google Scholar] [CrossRef]
- Lazarević, T.; Rilak, A.; Bugarčić, Ž.D. Platinum, palladium, gold and ruthenium complexes as anticancer agents: Current clinical uses, cytotoxicity studies and future perspectives. Eur. J. Med. Chem. 2017, 142, 8–31. [Google Scholar] [CrossRef]
- Gill, M.R.; Garcia-Lara, J.; Foster, S.J.; Smythe, C.; Battaglia, G.; Thomas, J.A. A ruthenium (II) polypyridyl complex for direct imaging of DNA structure in living cells. Nature Chem. 2009, 1, 662–667. [Google Scholar] [CrossRef]
- Baggaley, E.; Gill, M.R.; Green, N.H.; Turton, D.; Sazanovich, I.V.; Botchway, S.W.; Thomas, J.A. Dinuclear Ruthenium (II) Complexes as Two-Photon, Time-Resolved Emission Microscopy Probes for Cellular DNA. Angew. Chem. Intern. Ed. 2014, 53, 3367–3371. [Google Scholar] [CrossRef] [PubMed]
- Svensson, F.R.; Andersson, J.; Åmand, H.L.; Lincoln, P. Effects of chirality on the intracellular localization of binuclear ruthenium (II) polypyridyl complexes. J. Biol. Inorg. Chem. 2012, 17, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Huynh, M.; Vinck, R.; Gibert, B.; Gasser, G. Strategies for the nuclear delivery of metal complexes to cancer cells. Adv. Mater. 2024, 36, 2311437. [Google Scholar] [CrossRef]
- Li, X.; Heimann, K.; Li, F.; Warner, J.M.; Keene, F.R.; Collins, J.G. Dinuclear ruthenium (II) complexes containing one inert metal centre and one coordinatively-labile metal centre: Syntheses and biological activities. Dalt. Trans. 2016, 45, 4017–4029. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, B.Y.; Liu, J.; Dai, Y.C.; Wang, Y.J.; Wang, K.Z. DNA binding and photocleavage properties, cellular uptake and localization, and in-vitro cytotoxicity of dinuclear ruthenium (II) complexes with varying lengths in bridging alkyl linkers. Inorg. Chem. 2016, 55, 1412–1422. [Google Scholar] [CrossRef]
- Singh, S.B.; Kumbhar, A.S.; Khan, A. Honeycomb-like Ordered Assembly of DNA Induced by Flexible Binuclear Ruthenium (II)–Polypyridyl Complexes. Chem. A Eur. J. 2016, 22, 15760–15771. [Google Scholar] [CrossRef]
- Wang, H.Y.; Qian, Y.; Wang, F.X.; Habtemariam, A.; Mao, Z.W.; Sadler, P.J.; Liu, H.K. Ruthenium (II)–arene metallacycles: Crystal structures, interaction with DNA, and cytotoxicity. Eur. J. Inorg. Chem. 2017, 2017, 1792–1799. [Google Scholar] [CrossRef]
- Magennis, S.W.; Habtemariam, A.; Novakova, O.; Henry, J.B.; Meier, S.; Parsons, S.; Sadler, P.J. Dual triggering of DNA binding and fluorescence via photoactivation of a dinuclear ruthenium (II) arene complex. Inorg. Chem. 2007, 46, 5059–5068. [Google Scholar] [CrossRef] [PubMed]
- Peacock, A.F.; Sadler, P.J. Medicinal organometallic chemistry: Designing metal arene complexes as anticancer agents. Chem. Asian J. 2008, 3, 1890–1899. [Google Scholar] [CrossRef]
- Chen, H.; Parkinson, J.A.; Nováková, O.; Bella, J.; Wang, F.; Dawson, A.; Sadler, P.J. Induced-fit recognition of DNA by organometallic complexes with dynamic stereogenic centers. Proc. Nat. Acad. Sci. USA 2003, 100, 14623–14628. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.; Sadler, P.J. Controlling platinum, ruthenium, and osmium reactivity for anticancer drug design. Adv. Inorg. Chem. 2009, 61, 1–62. [Google Scholar] [PubMed]
- Orts-Arroyo, M.; Gutiérrez, F.; Gil-Tebar, A.; Ibarrola-Villava, M.; Jiménez-Martí, E.; Silvestre-Llora, A.; Castro, I.; Ribas, G.; Martínez-Lillo, J. A Novel Adenine-Based Diruthenium(III) Complex: Synthesis, Crystal Structure, Electrochemical Properties and Evaluation of the Anticancer Activity. J. Inorg. Biochem. 2022, 232, 111812. [Google Scholar] [CrossRef]
- McKenzie, L.K.; Flamme, M.; Felder, P.S.; Karges, J.; Bonhomme, F.; Gandioso, A.; Malosse, C.; Gasser, G.; Hollenstein, M. A ruthenium–oligonucleotide bioconjugated photosensitizing aptamer for cancer cell specific photodynamic therapy. RSC Chem. Biol. 2022, 3, 85–95. [Google Scholar] [CrossRef]
- Bortolamiol, E.; Visentin, F.; Scattolin, T. Recent advances in bioconjugated transition metal complexes for cancer therapy. Appl. Sci. 2023, 13, 5561. [Google Scholar] [CrossRef]
- Reddy, V.D.; Dayal, D.; Szalda, D.J.; Cosenza, S.C.; Reddy, M.R. Syntheses, structures, and anticancer activity of novel organometallic ruthenium–maltol complexes. J. Organomet. Chem. 2012, 700, 180–187. [Google Scholar] [CrossRef]
- Noffke, A.L.; Bongartz, M.; Wätjen, W.; Böhler, P.; Spingler, B.; Kunz, P.C. Synthesis, X-ray crystal structure and cytotoxicity of a new tetranuclear ruthenium arene complex. J. Organomet. Chem. 2011, 696, 1096–1101. [Google Scholar] [CrossRef]
- Kunz, P.C.; Wetzel, C.; Bongartz, M.; Noffke, A.L.; Spingler, B. Novel multitopic diphos-type ligands. J. Organomet. Chem. 2010, 695, 1891–1897. [Google Scholar] [CrossRef]
- Lenis-Rojas, O.A.; Roma-Rodrigues, C.; Fernandes, A.R.; Marques, F.; Perez-Fernandez, D.; Guerra-Varela, J.; Fernandez, J.J. Dinuclear RuII (bipy) 2 derivatives: Structural, biological, and in vivo zebrafish toxicity evaluation. Inorg. Chem. 2017, 56, 7127–7144. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sinha, S.; Britto, R.; Somasundaram, K.; Samuelson, A.G. Cytotoxicity of half sandwich ruthenium (II) complexes with strong hydrogen bond acceptor ligands and their mechanism of action. J. Inorg. Biochem. 2010, 104, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Herry, B.; Batchelor, L.K.; Roufosse, B.; Romano, D.; Baumgartner, J.; Borzova, M.; Reifenstahl, T.; Collins, T.; Benamrane, A.; Weggelaar, J.; et al. Heterobimetallic Ru (μ-dppm) Fe and homobimetallic Ru (μ-dppm) Ru complexes as potential anti-cancer agents. J. Organomet. Chem. 2019, 901, 120934. [Google Scholar] [CrossRef]
- Klaimanee, E.; Nhukeaw, T.; Saithong, S.; Ratanaphan, A.; Phongpaichit, S.; Tantirungrotechai, Y.; Leesakul, N. Half-sandwich ruthenium (II) p-cymene complexes based on organophosphorus ligands: Structure determination, computational investigation, in vitro antiproliferative effect in breast cancer cells and antimicrobial activity. Polyhedron 2021, 204, 115244. [Google Scholar] [CrossRef]
- Suntharalingam, K.; Łęczkowska, A.; Furrer, M.A.; Wu, Y.; Kuimova, M.K.; Therrien, B.; Vilar, R. A cyclometallated platinum complex as a selective optical switch for quadruplex DNA. Chem. Eur. J. 2012, 18, 16277. [Google Scholar] [CrossRef]
- Schmitt, F.; Freudenreich, J.; Barry, N.P.; Juillerat-Jeanneret, L.; Süss-Fink, G.; Therrien, B. Organometallic cages as vehicles for intracellular release of photosensitizers. J. Am. Chem. Soc. 2012, 134, 754. [Google Scholar] [CrossRef]
- Samaroo, D.; Perez, E.; Aggarwal, A.; Wills, A.; O’Connor, N. Strategies for delivering porphyrinoid-based photosensitizers in therapeutic applications. Therap. Deliv. 2014, 5, 859. [Google Scholar] [CrossRef]
- Yi, J.W.; Barry, N.P.; Furrer, M.A.; Zava, O.; Dyson, P.J.; Therrien, B.; Kim, B.H. Delivery of floxuridine derivatives to cancer cells by water-soluble organometallic cages. Bioconjugate Chem. 2012, 23, 461–471. [Google Scholar] [CrossRef]
- Furrer, M.A.; Schmitt, F.; Wiederkehr, M.; Juillerat-Jeanneret, L.; Therrien, B. Cellular delivery of pyrenyl-arene ruthenium complexes by a water-soluble arene ruthenium metalla-cage. Dalton Trans. 2012, 41, 7201–7211. [Google Scholar] [CrossRef]
- Pitto-Barry, A.; Barry, N.P.; Zava, O.; Deschenaux, R.; Dyson, P.J.; Therrien, B. Double Targeting of Tumours with Pyrenyl-Modified Dendrimers Encapsulated in an Arene–Ruthenium Metallaprism. Chem. Eur. J. 2011, 17, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Pascu, G.I.; Hotze, A.C.; Sanchez-Cano, C.; Kariuki, B.M.; Hannon, M.J. Dinuclear ruthenium (II) triple-stranded helicates: Luminescent supramolecular cylinders that bind and coil DNA and exhibit activity against cancer cell lines. Ang. Chem. Int. Ed. 2007, 46, 4374–4378. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Kimura, H.; Okuhara, T.; Yamamura, M.; Nabeshima, T. A hierarchical self-assembly system built up from preorganized tripodal helical metal complexes. J. Am. Chem. Soc. 2016, 138, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, X.; Wang, W.; Shi, Z.; Zhang, Y.; Tian, Q.; Duan, C. Biomedical applications of multinuclear Pt (II)/Ru (II)/Ir (III) metallo-supramolecular assemblies for intensive cancer therapy. Coord. Chem. Rev. 2023, 495, 215366. [Google Scholar] [CrossRef]
- Therrien, B. Biologically relevant arene ruthenium metalla-assemblies. Cryst. Eng. Comm. 2015, 17, 484–491. [Google Scholar] [CrossRef]
- Kostova, I. Recent Advances in Anticancer Research of Osmium and Rhodium Complexes. Med. Chem. 2024, 21, 619–645. [Google Scholar] [CrossRef]
- Kostova, I. Recent Developments in Antitumor Activity of Organometallic Rhodium Complex Compounds with Macrocyclic Ligands. In Anticancer Potential of Macrocyclic Metal Complexes; ACS Symposium Series; ACS Publications: Washington, DC, USA, 2025; Chapter 9; Volume 1492, pp. 197–233. [Google Scholar]
- Kostova, I. Homo-and Hetero-Multinuclear Iridium (III) Complexes with Cytotoxic Activity. Inorganics 2025, 13, 156. [Google Scholar] [CrossRef]
- Kostova, I. Cytotoxic Organometallic Iridium(III) Complexes. Molecules 2025, 30, 801. [Google Scholar] [CrossRef]
- Morozumi, T.; Matsuoka, R.; Nakamura, T.; Nabeshima, T. Solvent-dependent fac/mer-isomerization and self-assembly of triply helical complexes bearing a pivot part. Chem. Sci. 2021, 12, 7720–7726. [Google Scholar] [CrossRef]
- Therrien, B. Unmasking arene ruthenium building blocks. Chem. Record 2021, 21, 460–468. [Google Scholar] [CrossRef]
- Gong, Z.J.; Narayana, Y.S.; Lin, Y.C.; Huang, W.H.; Su, W.N.; Li, Y.P.; Yu, W.Y. Rational synthesis of ruthenium-based metallo-supramolecular polymers as heterogeneous catalysts for catalytic transfer hydrogenation of carbonyl compounds. Appl. Catal. B Environ. 2022, 312, 121383. [Google Scholar] [CrossRef]
- Mai, X.; Li, J.; Xie, M.; Wang, X.; Sun, M.; Wu, Y.; Yu, L. Self-assembled supramolecular materials based on ruthenium (ii) complexes with exceptional optical and photothermal properties. Mater. Chem. Front. 2025, 9, 1716–1725. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Li, P.; Bai, Y.; Pang, J.; Fan, L.; Tian, W. Ruthenium (II)-coordinated supramolecular metallodrug complex realizing oxygen self-supply in situ for overcoming hypoxic tumors. Adv. Funct. Mater. 2021, 31, 2105837. [Google Scholar] [CrossRef]
- Ma, H.; Jin, Y.; Li, J.; Xiao, X.; Liu, C.; Tian, W. Ruthenium (II)-Coordinated Supramolecular Drug Self-assembly for Efficient Combination Chemotherapy. Supramol. Mater. 2025, 4, 100113. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Huang, S.; Ma, X.; Sun, Y.; Zhao, J.; Gou, S. Boosting phototherapy efficacy of NIR-absorbing ruthenium (II) complex via supramolecular engineering. Mater. Today Nano 2022, 18, 100220. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Adon, T.; Dsouza, K.; Kumar, H.Y. Exploring the Future of Metal-Based Anticancer Agents: A Comprehensive Review of Ruthenium-Based Complexes. ChemistrySelect 2025, 10, e202404147. [Google Scholar] [CrossRef]
- Tu, L.; Li, C.; Ding, Q.; Sharma, A.; Li, M.; Li, J.; Sun, Y. Augmenting cancer therapy with a supramolecular immunogenic cell death inducer: A lysosome-targeted NIR-light-activated ruthenium (II) metallacycle. J. Am. Chem. Soc. 2024, 146, 8991–9003. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, D.; Le, Q.; Wang, Y.; Wang, W. Ruthenium-based antitumor drugs and delivery systems from monotherapy to combination therapy. Nanoscale 2022, 14, 16339–16375. [Google Scholar] [CrossRef]
- Kanaoujiya, R.; Singh, D.; Minocha, T.; Yadav, S.K.; Srivastava, S. Synthesis, characterization of ruthenium (III) macrocyclic complexes of 1, 4, 8, 11-tetraazacyclotetradecane (cyclam) and in vitro assessment of anti-cancer activity. Mater. Today Proc. 2022, 65, 3143–3149. [Google Scholar] [CrossRef]
- Xu, G.; Li, C.; Chi, C.; Wu, L.; Sun, Y.; Zhao, J.; Gou, S. A supramolecular photosensitizer derived from an Arene-Ru (II) complex self-assembly for NIR activated photodynamic and photothermal therapy. Nature Commun. 2022, 13, 3064. [Google Scholar] [CrossRef]
- Therrien, B. Transporting and shielding photosensitisers by using water-soluble organometallic cages: A new strategy in drug delivery and photodynamic therapy. Chem. Eur. J. 2013, 19, 8378–8386. [Google Scholar] [CrossRef]
- Lehn, J.M. Toward complex matter: Supramolecular chemistry and self-organization. Proc. Natl. Acad. Sci. USA 2002, 99, 4763–4768. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhao, Y.; Guo, D.; Lin, Z.; Duan, C. Chirality transfer through helical motifs in coordination compounds. Eur. J. Inorg. Chem. 2007, 2007, 3451–3463. [Google Scholar] [CrossRef]
- Howson, S.E.; Scott, P. Approaches to the synthesis of optically pure helicates. Dalton Trans. 2011, 40, 10268–10277. [Google Scholar] [CrossRef] [PubMed]
- Kaner, R.A.; Scott, P. Metallohelices: Potential mimetics of α-helical peptides in cancer treatment? Fut. Med. Chem. 2015, 7, 1–4. [Google Scholar] [CrossRef]
- Champness, N.R. The future of metal–organic frameworks. Dalton Trans. 2011, 40, 10311–10315. [Google Scholar] [CrossRef]
- Howson, S.E.; Bolhuis, A.; Brabec, V.; Clarkson, G.J.; Malina, J.; Rodger, A.; Scott, P. Optically pure, water-stable metallo-helical ‘flexicate’assemblies with antibiotic activity. Nature Chem. 2012, 4, 31–36. [Google Scholar] [CrossRef]
- Aldrich-Wright, J.R. Resolutely pure helices. Nature Chem. 2012, 4, 10–11. [Google Scholar] [CrossRef]
- Song, H.; Postings, M.; Scott, P.; Rogers, N.J. Metallohelices emulate the properties of short cationic α-helical peptides. Chem. Sci. 2021, 12, 1620–1631. [Google Scholar] [CrossRef]
- Lehn, J.M.; Rigault, A.; Siegel, J.; Harrowfield, J.; Chevrier, B.; Moras, D. Spontaneous assembly of double-stranded helicates from oligobipyridine ligands and copper (I) cations: Structure of an inorganic double helix. Proc. Nat. Acad. Sci. USA 1987, 84, 2565–2569. [Google Scholar] [CrossRef]
- Schoentjes, B.; Lehn, J.M. Interaction of Double-Helical Polynuclear Copper (I) complexes with double-stranded DNA. Helv. Chim. Acta 1995, 78, 1–12. [Google Scholar] [CrossRef]
- Allison, S.J.; Cooke, D.; Davidson, F.S.; Elliott, P.I.; Faulkner, R.A.; Griffiths, H.B.; Harper, O.J.; Hussain, O.; Owen-Lynch, P.J.; Phillips, R.M.; et al. Ruthenium-containing linear helicates and mesocates with tuneable p53-selective cytotoxicity in colorectal cancer cells. Angew. Chem. Int. Ed. 2018, 57, 9799–9804. [Google Scholar] [CrossRef]
- Therrien, B.; Ang, W.H.; Chérioux, F.; Vieille-Petit, L.; JuilleratJeanneret, L.; Süss-Fink, G.; Dyson, P.J. Remarkable anticancer activity of triruthenium-arene clusters compared to tetrarutheniumarene clusters. J. Clust. Sci. 2007, 18, 741–752. [Google Scholar] [CrossRef]
- Yan, H.; Süss-Fink, G.; Neels, A.; Stoeckli-Evans, H. Mono-, di-and tetra-nuclear p-cymeneruthenium complexes containing oxalato ligands. Dalton Trans. 1997, 22, 4345–4350. [Google Scholar] [CrossRef]
- Han, Y.F.; Jia, W.G.; Lin, Y.J.; Jin, G.X. Stepwise formation of molecular rectangles of half-sandwich rhodium and ruthenium complexes containing bridging chloranilate ligands. Organometallics 2008, 27, 5002–5008. [Google Scholar] [CrossRef]
- Han, Y.F.; Jia, W.G.; Lin, Y.J.; Jin, G.X. Extending Rectangular Metal–Organic Frameworks to the Third Dimension: Discrete Organometallic Boxes for Reversible Trapping of Halocarbons Occurring with Conservation of the Lattice. Angew. Chem. 2009, 121, 6352–6356. [Google Scholar] [CrossRef]
- Linares, F.; Galindo, M.A.; Galli, S.; Romero, M.A.; Navarro, J.A.; Barea, E. Tetranuclear coordination assemblies based on half-sandwich ruthenium (II) complexes: Noncovalent binding to DNA and cytotoxicity. Inorg. Chem. 2009, 48, 7413–7420. [Google Scholar] [CrossRef]
- Therrien, B. The role of the second coordination sphere in the biological activity of arene ruthenium metalla-assemblies. Front. Chem. 2018, 6, 602. [Google Scholar] [CrossRef]
- Cook, T.R.; Vajpayee, V.; Lee, M.H.; Stang, P.J.; Chi, K.W. Biomedical and biochemical applications of self-assembled metallacycles and metallacages. Acc. Chem. Res. 2013, 46, 2464–2474. [Google Scholar] [CrossRef]
- Mishra, A.; Jeong, Y.J.; Jo, J.H.; Kang, S.C.; Kim, H.; Chi, K.W. Coordination-Driven Self-Assembly and Anticancer Potency Studies of Arene-Ruthenium-Based Molecular Metalla-Rectangles. Organometallics 2014, 33, 1144–1151. [Google Scholar] [CrossRef]
- Mishra, A.; Jung, H.; Park, J.W.; Kim, H.K.; Kim, H.; Stang, P.J.; Chi, K.W. Anticancer Activity of Self-Assembled Molecular Rectangles via Arene-Ruthenium Acceptors and a New Unsymmetrical Amide Ligand. Organometallics 2012, 31, 3519–3526. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Jeong, Y.J.; Jo, J.H.; Woo, S.; Kim, D.H.; Kim, H.; Chi, K.W. Anticancer activity and autophagy involvement of self-assembled arene–ruthenium metallacycles. Organometallics 2015, 34, 4507–4514. [Google Scholar] [CrossRef]
- Mishra, A.; Jeong, Y.J.; Jo, J.H.; Kang, S.C.; Lah, M.S.; Chi, K.W. Anticancer Potency Studies of Coordination Driven Self-Assembled Arene–Ru-Based Metalla-Bowls. ChemBioChem 2014, 15, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jang, S.; Jo, J.H.; Kim, D.H.; Park, D.W.; Kim, I.; Chi, K.W. Coordination-driven self-assembly and anticancer potency studies of Ruthenium–Cobalt-Based heterometallic rectangles. Chem. Eur. J. 2016, 22, 16157–16164. [Google Scholar] [CrossRef]
- Schmitt, F.; Govindaswamy, P.; Süss-Fink, G.; Ang, W.H.; Dyson, P.J.; Juillerat-Jeanneret, L.; Therrien, B. Ruthenium porphyrin compounds for photodynamic therapy of cancer. J. Med. Chem. 2008, 51, 1811–1816. [Google Scholar] [CrossRef]
- Schmitt, F.; Govindaswamy, P.; Zava, O.; Süss-Fink, G.; Juillerat, J.L.; Therrien, B. Combined arene ruthenium porphyrins as chemotherapeutics and photosensitizers for cancer therapy. J. Biol. Inorg. Chem. 2009, 14, 101–109. [Google Scholar] [CrossRef]
- Mattsson, J.; Govindaswamy, P.; Renfrew, A.K.; Dyson, P.J.; Štěpnička, P.; Süss-Fink, G.; Therrien, B. Synthesis, molecular structure, and anticancer activity of cationic arene ruthenium metallarectangles. Organometallics 2009, 28, 4350–4357. [Google Scholar] [CrossRef]
- Gupta, G.; Das, A.; Ghate, N.B.; Kim, T.; Ryu, J.Y.; Lee, J.; Lee, C.Y. Novel BODIPY-based Ru (II) and Ir (III) metalla-rectangles: Cellular localization of compounds and their antiproliferative activities. Chem. Comm. 2016, 52, 4274–4277. [Google Scholar] [CrossRef]
- Gupta, G.; Das, A.; Panja, S.; Ryu, J.Y.; Lee, J.; Mandal, N.; Lee, C.Y. Self-assembly of novel thiophene-based BODIPY RuII rectangles: Potential antiproliferative agents selective against cancer cells. Chem. Eur. J. 2017, 23, 17199–17203. [Google Scholar] [CrossRef]
- Xu, Y.; Li, C.; Lu, S.; Wang, Z.; Liu, S.; Yu, X.; Sun, Y. Construction of emissive ruthenium (II) metallacycle over 1000 nm wavelength for in vivo biomedical applications. Nature Commun. 2022, 13, 2009. [Google Scholar] [CrossRef]
- Therrien, B.; Süss-Fink, G.; Govindaswamy, P.; Renfrew, A.K.; Dyson, P.J. The “Complex-in-a-Complex” Cations [(acac)2M⊂Ru6 (p!iPrC6H4Me)6(tpt)2(dhbq)3]6+: A Trojan Horse for Cancer Cells. Angew. Chem. Int. Ed. Engl. 2008, 47, 3773–3776. [Google Scholar] [CrossRef] [PubMed]
- Freudenreich, J.; Barry, N.P.; Süss-Fink, G.; Therrien, B. Permanent encapsulation or host–guest behavior of aromatic molecules in hexanuclear arene ruthenium prisms. Eur. J. Inorg. Chem. 2010, 2010, 2400–2405. [Google Scholar] [CrossRef]
- Freudenreich, J.; Dalvit, C.; Süss-Fink, G.; Therrien, B. Encapsulation of photosensitizers in hexa-and octanuclear organometallic cages: Synthesis and characterization of carceplex and host–guest systems in solution. Organometallics 2013, 32, 3018–3033. [Google Scholar] [CrossRef]
- Mattsson, J.; Govindaswamy, P.; Furrer, J.; Sei, Y.; Yamaguchi, K.; Süss-Fink, G.; Therrien, B. Encapsulation of aromatic molecules in hexanuclear arene ruthenium cages: A strategy to build up organometallic carceplex prisms with a dangling arm standing out. Organometallics 2008, 27, 4346–4356. [Google Scholar] [CrossRef]
- Barry, N.P.E.; Therrien, B. Host–Guest Chemistry in the Hexanuclear (Arene) ruthenium Metalla-Prismatic cage [Ru6(p-cymene)6(tpt)2(dhnq)3]6+. Eur. J. Inorg. Chem. 2009, 2009, 4695–4700. [Google Scholar] [CrossRef]
- Garci, A.; Dobrov, A.A.; Riedel, T.; Orhan, E.; Dyson, P.J.; Arion, V.B.; Therrien, B. Strategy to Optimize the Biological Activity of Arene Ruthenium Metalla-Assemblies. Organometallics 2014, 33, 3813–3822. [Google Scholar] [CrossRef]
- Gupta, G.; Oggu, G.S.; Nagesh, N.; Bokara, K.K.; Therrien, B. Anticancer activity of large metalla-assemblies built from half-sandwich complexes. Cryst. Eng. Comm. 2016, 18, 4952–4957. [Google Scholar] [CrossRef]
- Gallardo-Villagrán, M.; Paulus, L.; Charissoux, J.L.; Leger, D.Y.; Vergne-Salle, P.; Therrien, B.; Liagre, B. Ruthenium-based assemblies incorporating tetrapyridylporphyrin panels: A photosensitizer delivery strategy for the treatment of rheumatoid arthritis by photodynamic therapy. Dalton Trans. 2022, 51, 9673–9680. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Shettar, A.; Bhat, I.A.; Kondaiah, P.; Mukherjee, P.S. Self-assembly of discrete RuII8 molecular cages and their in vitro anticancer activity. Inorg. Chem. 2017, 56, 608–617. [Google Scholar] [CrossRef]
- Adeyemo, A.A.; Shettar, A.; Bhat, I.A.; Kondaiah, P.; Mukherjee, P.S. Coordination-driven self-assembly of ruthenium (II) architectures: Synthesis, characterization and cytotoxicity studies. Dalton Trans. 2018, 47, 8466–8475. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).