Synthesis and Biological Evaluation of Novel Mixed-Ligand 99mTc-Labeled Anthraquinone Complexes as Potential DNA-Targeted Imaging Agents
Abstract
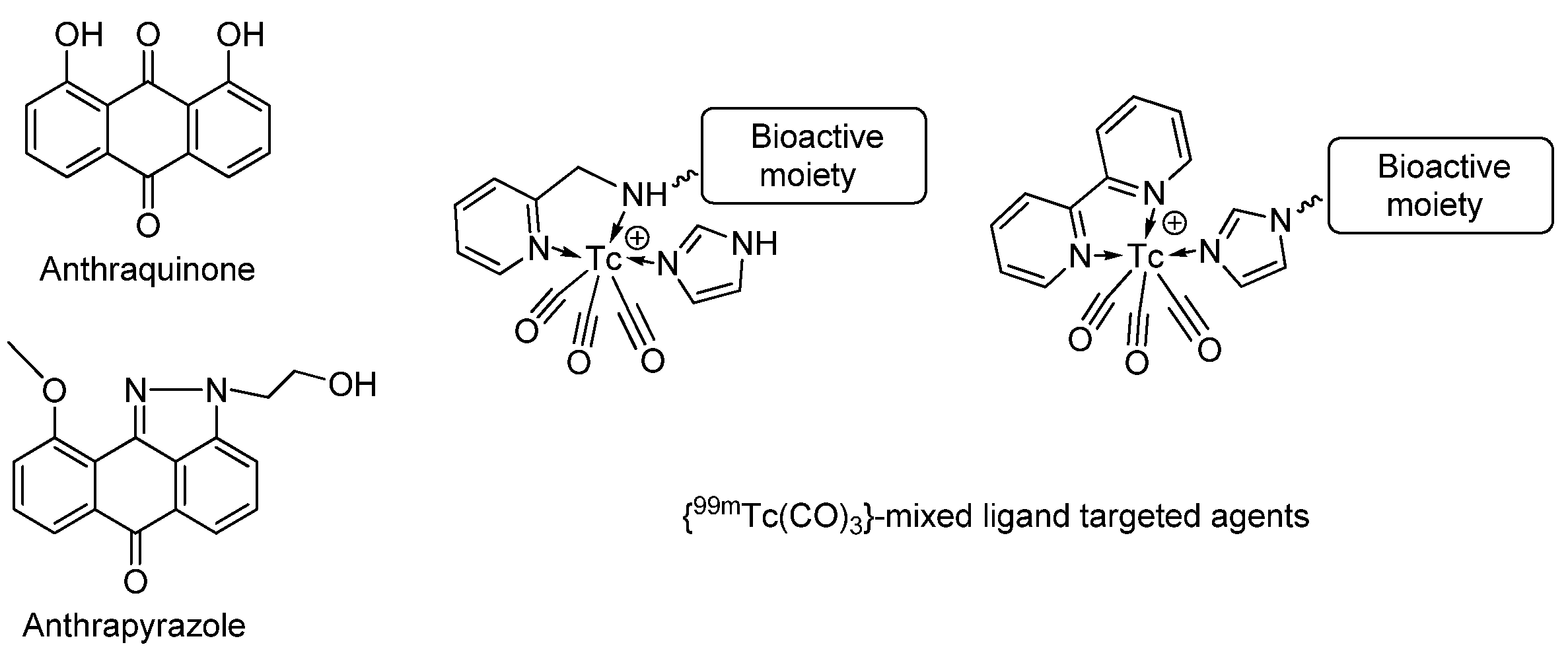
1. Introduction
2. Results and Discussion
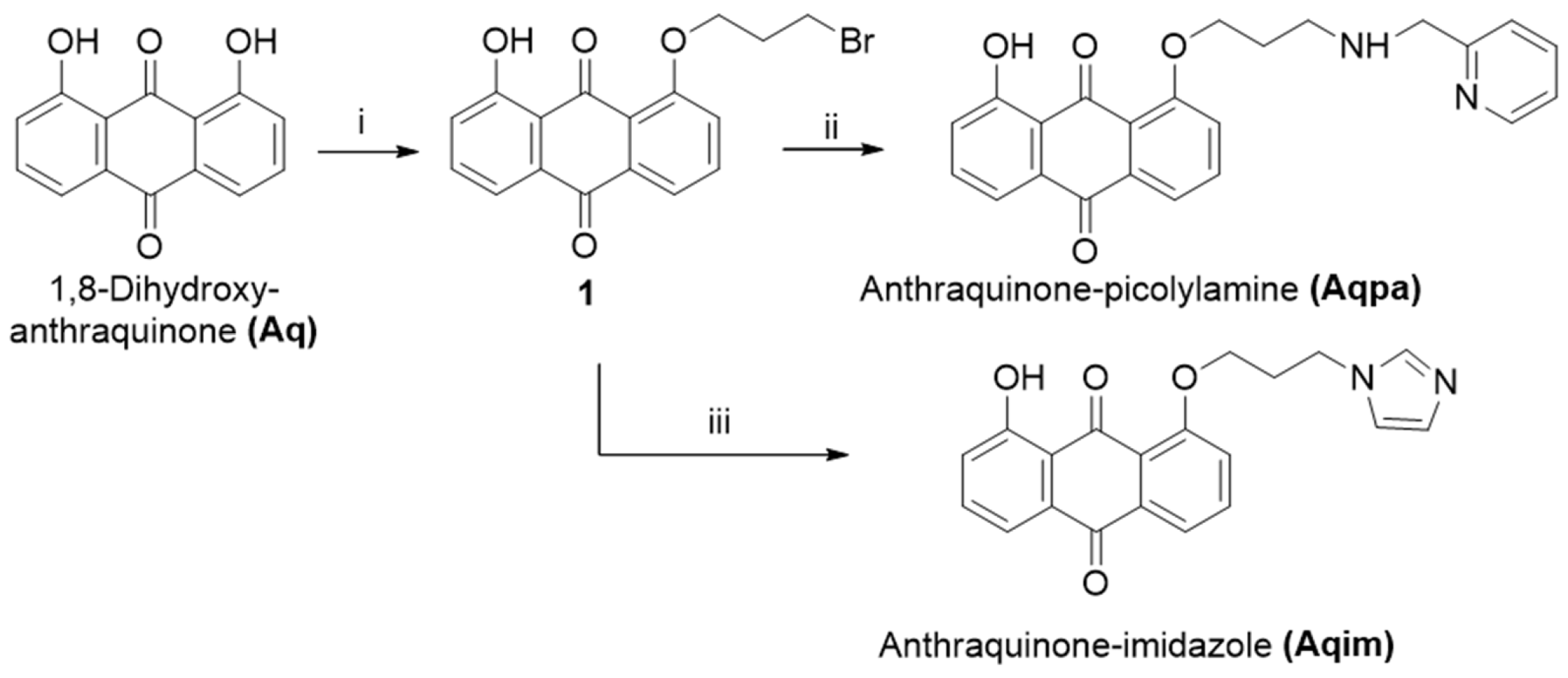
2.1. Synthesis and Spectroscopic Characterization of Rhenium Complexes
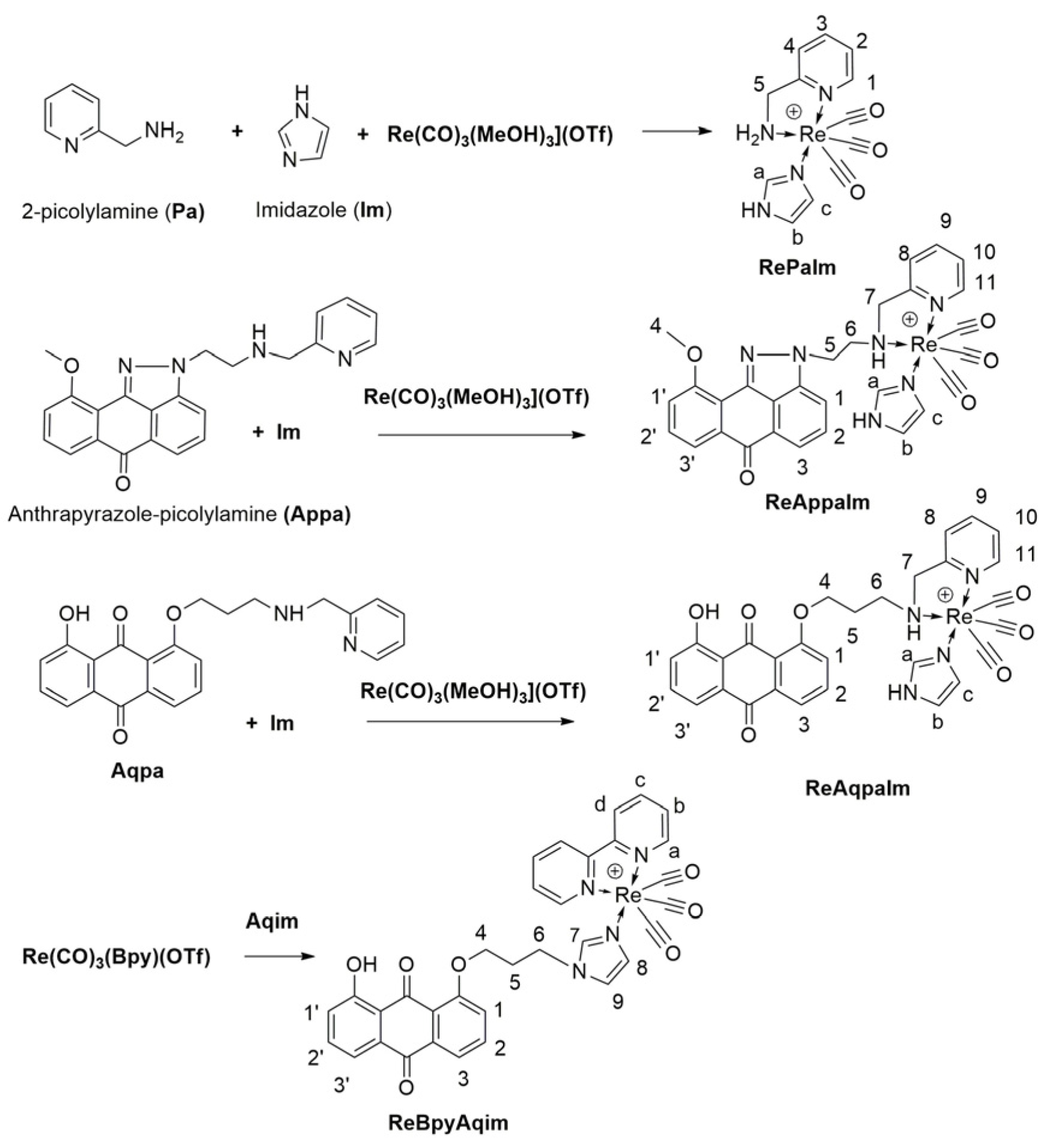
| 1H NMR/ppm | RePaIm | ReAqpaIm (Major) | ReAqpaIm (Minor) | ReAppaIm (Major) | ReAppaIm (Minor) |
|---|---|---|---|---|---|
| Py-CH2-NH | 4.38, 4.09 (dt) | 5.52, 4.14 (dd) | 4.75, 3.6 (dd) | 5.22, 4.50 (dd) | 4.8, 3.7 |
| Py-CH2-NH | 5.89, 4.81 | 5.46 | 7.08 | 5.74 | |
| Imidazole | 7.79, 7.18, 6.80 | 7.81, 6.64, 6.46 | 7.38, 7.17, 6.66 | 7.82, 6.77, 6.79 | 7.94, 7.64, 7.3 |
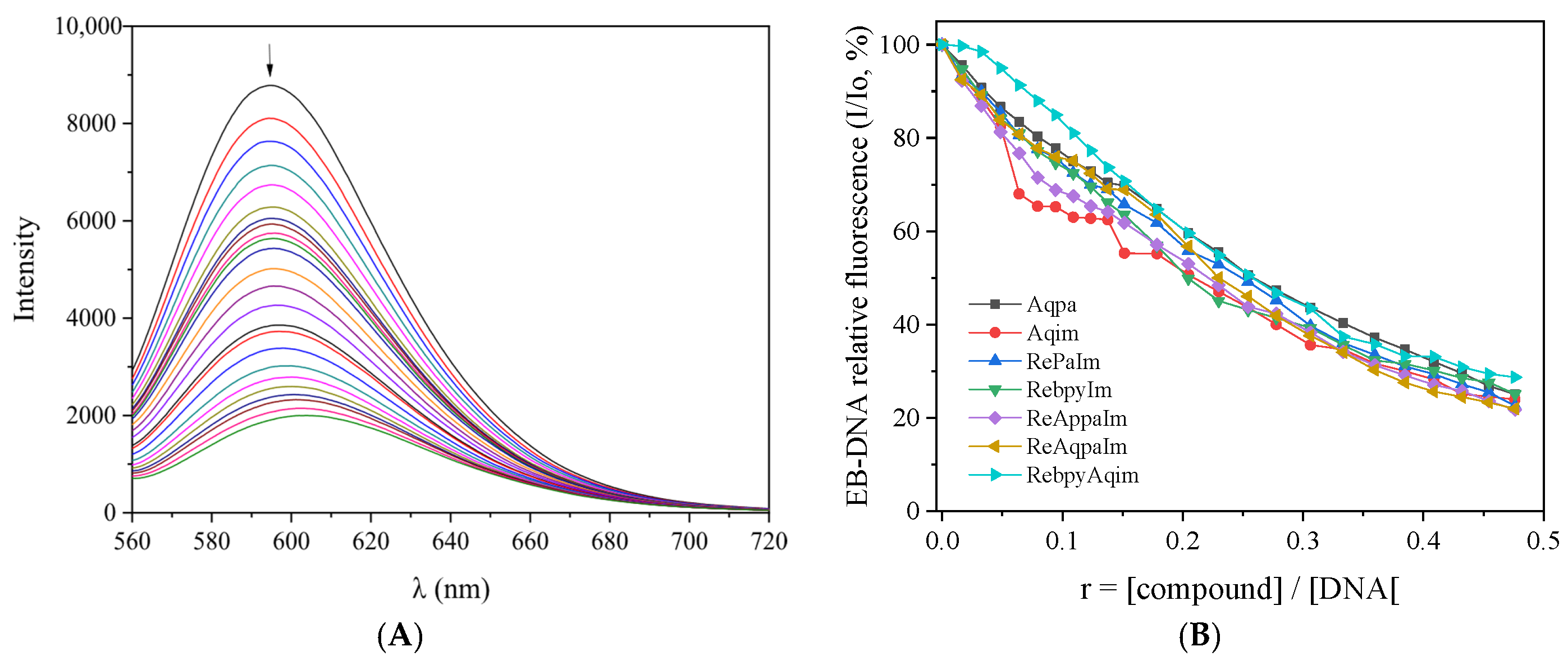
2.2. DNA-Binding Studies of the Compounds
2.3. Radiochemistry
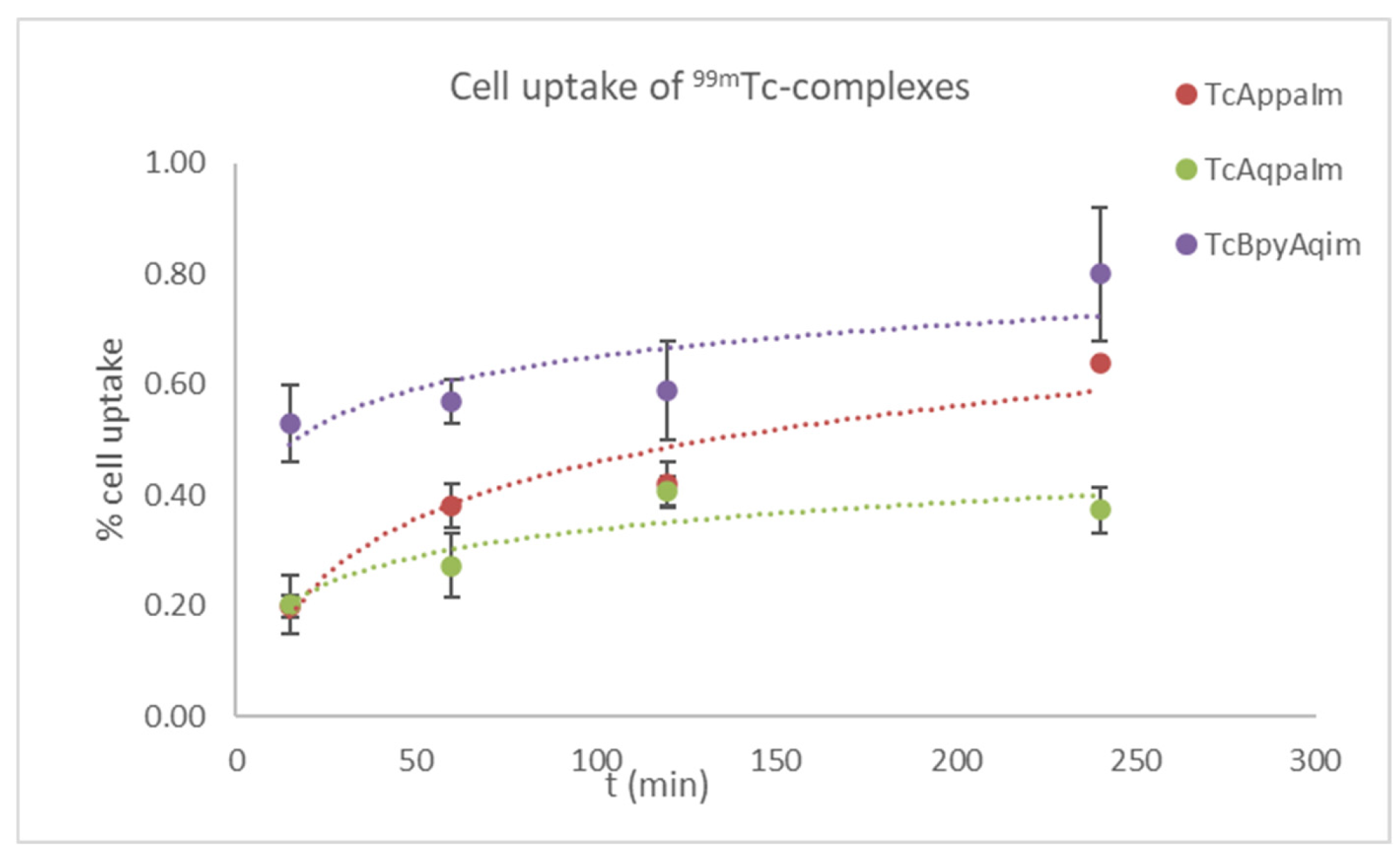
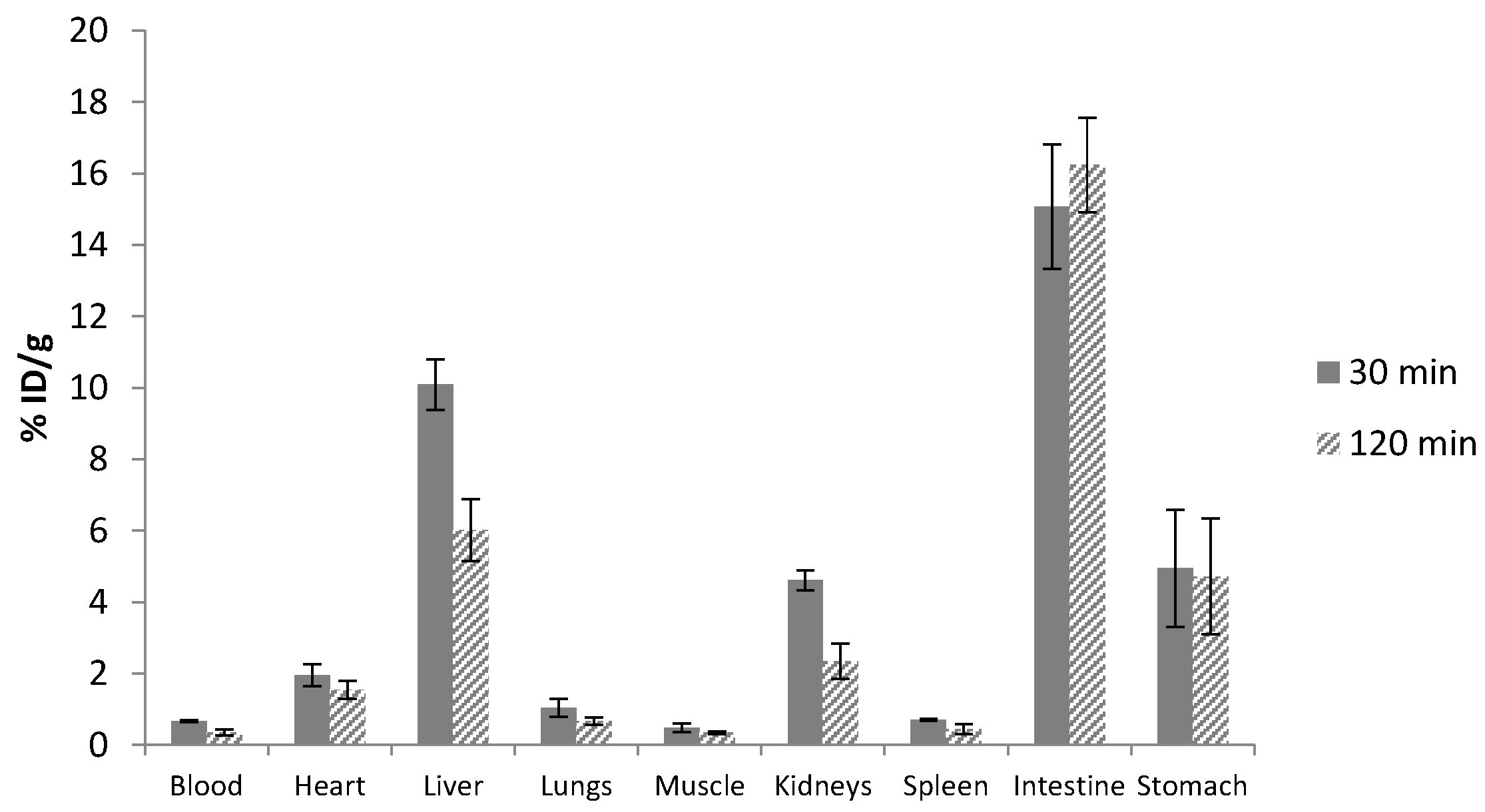
2.4. Biological Evaluation of 99mTc Tracers
3. Materials and Methods
3.1. General
3.2. Chemistry
3.3. In Vitro DNA-Binding Studies
3.4. Radiochemistry
3.5. Biological Evaluation of the 99mTc Complexes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duatti, A. Review on 99mTc Radiopharmaceuticals with Emphasis on New Advancements. Nucl. Med. Biol. 2021, 92, 202–216. [Google Scholar] [CrossRef]
- Papagiannopoulou, D. Technetium-99m Radiochemistry for Pharmaceutical Applications. J. Label. Compd. Radiopharm. 2017, 60, 502–520. [Google Scholar] [CrossRef]
- Rathmann, S.M.; Ahmad, Z.; Slikboer, S.; Bilton, H.A.; Snider, D.P.; Valliant, J.F. The Radiopharmaceutical Chemistry of Technetium-99m. In Radiopharmaceutical Chemistry; Springer International Publishing: Cham, Switzerland, 2019; pp. 311–333. [Google Scholar] [CrossRef]
- Alberto, R. [Tc(CO)3]+ Chemistry: A Promising New Concept for SPET? Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1299–1302. [Google Scholar] [CrossRef]
- Alberto, R.; Braband, H.; Qaisar, N. Bioorganometallic Technetium and Rhenium Chemistry: Fundamentals for Applications. Chim. Int. J. Chem. 2020, 74, 953–959. [Google Scholar] [CrossRef]
- Makris, G.; Kuchuk, M.; Gallazzi, F.; Jurisson, S.S.; Smith, C.J.; Hennkens, H.M. Somatostatin Receptor Targeting with Hydrophilic [99mTc/186Re]Tc/Re-Tricarbonyl NODAGA and NOTA Complexes. Nucl. Med. Biol. 2019, 71, 39–46. [Google Scholar] [CrossRef]
- Riondato, M.; Rigamonti, D.; Martini, P.; Cittanti, C.; Boschi, A.; Urso, L.; Uccelli, L. Oldie but Goodie: Is Technetium-99m Still a Treasure Trove of Innovation for Medicine? A Patents Analysis (2000–2022). J. Med. Chem. 2023, 66, 4532–4547. [Google Scholar] [CrossRef]
- Goffin, K.E.; Joniau, S.; Tenke, P.; Slawin, K.; Klein, E.A.; Stambler, N.; Strack, T.; Babich, J.; Armor, T.; Wong, V. Phase 2 Study of 99mTc-Trofolastat SPECT/CT to Identify and Localize Prostate Cancer in Intermediate- and High-Risk Patients Undergoing Radical Prostatectomy and Extended Pelvic LN Dissection. J. Nucl. Med. 2017, 58, 1408–1413. [Google Scholar] [CrossRef]
- Cook, G.J.R.; Wong, W.-L.; Sanghera, B.; Mangar, S.; Challapalli, A.; Bahl, A.; Bassett, P.; Leaning, D.; Schmidkonz, C. Eligibility for 177Lu-PSMA Therapy Depends on the Choice of Companion Diagnostic Tracer: A Comparison of 68Ga-PSMA-11 and 99mTc-MIP-1404 in Metastatic Castration-Resistant Prostate Cance. J. Nucl. Med. 2023, 64, 227–231. [Google Scholar] [CrossRef]
- Koehler, D.; Sauer, M.; Klutmann, S.; Apostolova, I.; Lehnert, W.; Budäus, L.; Knipper, S.; Maurer, T. Feasibility of 99mTc-MIP-1404 for SPECT/CT Imaging and Subsequent PSMA-Radioguided Surgery in Early Biochemically Recurrent Prostate Cancer: A Case Series of 9 Patients. J. Nucl. Med. 2023, 64, 59–62. [Google Scholar] [CrossRef]
- Tu, H.Y.; Huang, A.M.; Teng, C.H.; Hour, T.C.; Yang, S.C.; Pu, Y.S.; Lin, C.N. Anthraquinone Derivatives Induce G2/M Cell Cycle Arrest and Apoptosis in NTUB1 Cells. Bioorg Med. Chem. 2011, 19, 5670–5678. [Google Scholar] [CrossRef]
- Rescifina, A.; Zagni, C.; Varrica, M.G.; Pistarà, V.; Corsaro, A. Recent Advances in Small Organic Molecules as DNA Intercalating Agents: Synthesis, Activity, and Modeling. Eur. J. Med. Chem. 2014, 74, 95–115. [Google Scholar] [CrossRef]
- Kapuscinski, J.; Darzynkiewicz, Z. Interactions of antitumor agents Ametantrone and Mitoxantrone (Novatrone) with double-stranded DNA. Biochem. Pharmacol. 1985, 34, 4203–4213. [Google Scholar] [CrossRef] [PubMed]
- Frederick, C.A.; Williams, L.D.; Ughetto, G.; Van der Marel, G.A.; Van Boom, J.H.; Rich, A.; Wang, A.H.J. Structural Comparison of Anticancer Drug-DNA Complexes: Adriamycin and Daunomycin. Biochemistry 1990, 29, 2538–2549. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.S.; Alsantali, R.I.; Jassas, R.S.; Alsimaree, A.A.; Syed, R.; Alsharif, M.A.; Kalpana, K.; Morad, M.; Althagafi, I.I.; Ahmed, S.A. Journey of Anthraquinones as Anticancer Agents—A Systematic Review of Recent Literature. RSC Adv. 2021, 11, 35806–35827. [Google Scholar] [CrossRef]
- Nurhidayah, W.; Setyawati, L.U.; Daruwati, I.; Gazzali, A.M.; Subroto, T.; Muchtaridi, M. Future Prospective of Radiopharmaceuticals from Natural Compounds Using Iodine Radioisotopes as Theranostic Agents. Molecules 2022, 27, 8009. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, S.; Jiang, C.; Li, J.; Wang, C.; Chen, L.; Jin, Q.; Song, S.; Feng, Y.; Ni, Y.; et al. Discovery of Radioiodinated Monomeric Anthraquinones as a Novel Class of Necrosis Avid Agents for Early Imaging of Necrotic Myocardium. Sci. Rep. 2016, 6, 21341. [Google Scholar] [CrossRef]
- Vitor, R.F.; Esteves, T.; Marques, F.; Raposinho, P.; Paulo, A.; Rodrigues, S.; Rueff, J.; Casimiro, S.; Costa, L.; Santos, I. 99m Tc-Tricarbonyl Complexes Functionalized with Anthracenyl Fragments: Synthesis, Characterization, and Evaluation of Their Radiotoxic Effects in Murine Melanoma Cells. Cancer Biother. Radiopharm. 2009, 24, 551–563. [Google Scholar] [CrossRef]
- Imstepf, S.; Pierroz, V.; Raposinho, P.; Bauwens, M.; Felber, M.; Fox, T.; Shapiro, A.B.; Freudenberg, R.; Fernandes, C.; Gama, S.; et al. Nuclear Targeting with an Auger Electron Emitter Potentiates the Action of a Widely Used Antineoplastic Drug. Bioconjug Chem. 2015, 26, 2397–2407. [Google Scholar] [CrossRef]
- Pereira, E.; do Quental, L.; Palma, E.; Oliveira, M.C.; Mendes, F.; Raposinho, P.; Correia, I.; Lavrado, J.; Di Maria, S.; Belchior, A.; et al. Evaluation of Acridine Orange Derivatives as DNA-Targeted Radiopharmaceuticals for Auger Therapy: Influence of the Radionuclide and Distance to DNA. Sci. Rep. 2017, 7, 42544. [Google Scholar] [CrossRef]
- Ji, A.-Y.; Jin, Q.-M.; Zhang, D.-J.; Zhu, H.; Su, C.; Duan, X.-H.; Bian, L.; Sun, Z.-P.; Ni, Y.-C.; Zhang, J.; et al. Novel 18F-Labeled 1-Hydroxyanthraquinone Derivatives for Necrotic Myocardium Imaging. ACS Med. Chem. Lett. 2017, 8, 191–195. [Google Scholar] [CrossRef]
- Liang, J.; Luo, Q.; Zhang, D.; Jin, Q.; Liu, L.; Liu, W.; Gao, M.; Zhang, J.; Yin, Z. SPECT Imaging of Treatment-Related Tumor Necrosis Using Technetium-99m-Labeled Rhein. Mol. Imaging Biol. 2019, 21, 660–668. [Google Scholar] [CrossRef]
- Imstepf, S.; Pierroz, V.; Rubbiani, R.; Felber, M.; Fox, T.; Gasser, G.; Alberto, R. Organometallic Rhenium Complexes Divert Doxorubicin to the Mitochondria. Angew. Chem.-Int. Ed. 2016, 55, 2792–2795. [Google Scholar] [CrossRef] [PubMed]
- Paparidis, G.; Akrivou, M.; Psomas, G.; Vizirianakis, I.S.; Hatzidimitriou, A.; Gabriel, C.; Sarigiannis, D.; Papagiannopoulou, D. Novel Tricarbonylrhenium-Anthrapyrazole Complexes with DNA-Binding and Antitumor Properties: In Vitro and In Vivo Pharmacokinetic Studies with 99mTc-Analogue. Inorganics 2024, 12, 254. [Google Scholar] [CrossRef]
- Schibli, R.; La Bella, R.; Alberto, R.; Garcia-Garayoa, E.; Ortner, K.; Abram, U.; Schubiger, P.A. Influence of the Denticity of Ligand Systems on the in Vitro and in Vivo Behavior of 99mTc(I)-Tricarbonyl Complexes: A Hint for the Future Functionalization of Biomolecules. Bioconjug Chem. 2000, 11, 345–351. [Google Scholar] [CrossRef]
- Yazdani, A.; Janzen, N.; Banevicius, L.; Czorny, S.; Valliant, J.F. Imidazole-Based [2 + 1] Re(I)/99mTc(I) Complexes as Isostructural Nuclear and Optical Probes. Inorg. Chem. 2015, 54, 1728–1736. [Google Scholar] [CrossRef]
- Wolfe, A.; Shimer, G.; Meehan, T. Polycyclic Aromatic Hydrocarbons Physically Intercalate into Duplex Regions of Denatured DNA. Biochemistry 1987, 26, 6392–6396. [Google Scholar] [CrossRef]
- Tan, J.-H.; Zhang, Q.-X.; Huang, Z.-S.; Chen, Y.; Wang, X.-D.; Gu, L.-Q.; Wu, J.Y. Synthesis, DNA Binding and Cytotoxicity of New Pyrazole Emodin Derivatives. Eur. J. Med. Chem. 2006, 41, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakopoulou, A.; Dendrinou-Samara, C.; Pantazaki, A.A.; Alexiou, M.; Nordlander, E.; Kessissoglou, D.P. Synthesis, Structure and Interactions with DNA of Novel Tetranuclear, [Mn4(II/II/II/IV)] Mixed Valence Complexes. J. Inorg. Biochem. 2008, 102, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Luis García-Giménez, J.; González-Álvarez, M.; Liu-González, M.; Macías, B.; Borrás, J.; Alzuet, G. Toward the Development of Metal-Based Synthetic Nucleases: DNA Binding and Oxidative DNA Cleavage of a Mixed Copper(II) Complex with N-(9H-Purin-6-Yl)Benzenesulfonamide and 1,10-Phenantroline. Antitumor Activity in Human Caco-2 Cells and Jurkat T Lymphocy. J. Inorg. Biochem. 2009, 103, 923–934. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Plenum Press: New York, NY, USA, 2006. [Google Scholar]
- Wilson, W.D.; Ratmeyer, L.; Zhao, M.; Strekowski, L.; Boykin, D. The Search for Structure-Specific Nucleic Acid-Interactive Drugs: Effects of Compound Structure on RNA versus DNA Interaction Strength. Biochemistry 1993, 32, 4098–4104. [Google Scholar] [CrossRef]
- Zhao, G.; Lin, H.; Zhu, S.; Sun, H.; Chen, Y. Dinuclear Palladium(II) Complexes Containing Two Monofunctional [Pd(En)(Pyridine)Cl]+ Units Bridged by Se or S. Synthesis, Characterization, Cytotoxicity and Kinetic Studies of DNA-Binding. J. Inorg. Biochem. 1998, 70, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.P.; Greenstock, C.L. Fluorescence Lifetime Analysis of DNA Intercalated Ethidium Bromide and Quenching by Free Dye. Biophys. Chem. 1994, 50, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, A.; Janzen, N.; Czorny, S.; Valliant, J.F. Technetium(I) Complexes of Bathophenanthrolinedisulfonic Acid. Inorg. Chem. 2017, 56, 2958–2965. [Google Scholar] [CrossRef] [PubMed]
- Tsantili-Kakoulidou, A.; Demopoulos, V.J. Drug-like Properties and Fraction Lipophilicity Index as a Combined Metric. ADMET DMPK 2021, 9, 177–190. [Google Scholar] [CrossRef]
- Schmidt, S.; Trogler, W.; Basolo, F. Pentacarbonyl Rhenium Halides. Inorg. Synth. 1985, 23, 41. [Google Scholar]
- Nitschke, J.; Schmidt, S.P.; Trogler, W.C. Properties of (Trifluoromethanesulfonato)Pentacarbonylmanganese(I) and -Rhenium(I). Reactions in Superacid Solvents. Inorg. Chem. 1985, 24, 1972–1978. [Google Scholar] [CrossRef]
- Alberto, R.; Schibli, R.; Egli, A.; Schubiger, A.P.; Abram, U.; Kaden, T.A. A Novel Organometallic Aqua Complex of Technetium for the Labeling of Biomolecules: Synthesis of [99mTc(OH2)3(CO)3]+ from [99mTcO4]− in Aqueous Solution and Its Reaction with a Bifunctional Ligand. J. Am. Chem. Soc. 1998, 120, 7987–7988. [Google Scholar] [CrossRef]
- Marmur, J. A Procedure for the Isolation of Deoxyribonucleic Acid from Micro-Organisms. J. Mol. Biol. 1961, 3, 208–218. [Google Scholar] [CrossRef]
- Reichmann, M.E.; Rice, S.; Thomas, C.; Doty, P. A Further Examination of the Molecular Weight and Size of Desoxypentose Nucleic Acid. J. Am. Chem. Soc. 1954, 76, 3047–3053. [Google Scholar] [CrossRef]
- Mantso, T.; Vasileiadis, S.; Lampri, E.; Botaitis, S.; Perente, S.; Simopoulos, C.; Chlichlia, K.; Pappa, A.; Panayiotidis, M.I. Hyperthermia Suppresses Post—In Vitro Proliferation and Tumor Growth in Murine Malignant Melanoma and Colon Carcinoma. Anticancer Res. 2019, 39, 2307–2315. [Google Scholar] [CrossRef]
- Aindelis, G.; Tiptiri-Kourpeti, A.; Lampri, E.; Spyridopoulou, K.; Lamprianidou, E.; Kotsianidis, I.; Ypsilantis, P.; Pappa, A.; Chlichlia, K. Immune Responses Raised in an Experimental Colon Carcinoma Model Following Oral Administration of Lactobacillus Casei. Cancers 2020, 12, 368. [Google Scholar] [CrossRef]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lamprianidou, E.E.; Saxami, G.; Ypsilantis, P.; Lampri, E.S.; Simopoulos, C.; et al. Lactobacillus Casei Exerts Anti-Proliferative Effects Accompanied by Apoptotic Cell Death and up-Regulation of TRAIL in Colon Carcinoma Cells. PLoS ONE 2016, 11, e0147960. [Google Scholar] [CrossRef]
- Shao, J.; Zhang, F.; Bai, Z.; Wang, C.; Yuan, Y.; Wang, W. Synthesis and Antitumor Activity of Emodin Quaternary Ammonium Salt Derivatives. Eur. J. Med. Chem. 2012, 56, 308–319. [Google Scholar] [CrossRef]
- Liu, Q.-X.; Hu, Z.-L.; Zhao, Z.-X. A New Fluorescent–Colorimetric Chemosensor for Cobalt(Ii) Ions Based on Bis-Benzimidazolium Salt with Three Anthraquinone Groups. New J. Chem. 2018, 42, 20049–20055. [Google Scholar] [CrossRef]
- Kydonaki, T.E.; Tsoukas, E.; Mendes, F.; Hatzidimitriou, A.G.; Paulo, A.; Papadopoulou, L.C.; Papagiannopoulou, D.; Psomas, G. Synthesis, Characterization and Biological Evaluation of 99mTc/Re-Tricarbonyl Quinolone Complexes. J. Inorg. Biochem. 2016, 160, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Hevia, E.; Pérez, J.; Riera, L.; Riera, V.; del Río, I.; García-Granda, S.; Miguel, D. Insertion of Unsaturated Organic Electrophiles into Molybdenum—Alkoxide and Rhenium—Alkoxide Bonds of Neutral, Stable Carbonyl Complexes. Chem. Eur. J. 2002, 8, 4510–4521. [Google Scholar] [CrossRef] [PubMed]
- Pitchumony, T.S.; Banevicius, L.; Janzen, N.; Zubieta, J.; Valliant, J.F. Isostructural Nuclear and Luminescent Probes Derived from Stabilized [2 + 1] Rhenium(I)/Technetium(I) Organometallic Complexes. Inorg. Chem. 2013, 52, 13521–13528. [Google Scholar] [CrossRef]
- Tzovas, G.; Papadimitriou, N.; Angelakarou, E.; Bompola, G.; Miliotou, A.N.; Kakafika, M.G.; Papadopoulou, L.C.; Iakovou, Ι.; Gabriel, C.; Sarigiannis, D.; et al. Synthesis and Biological Evaluation of 99mTc-Tricarbonyl Complexes of C-3 Carboxy Derivatives of Fluoroquinolones in Infection and Tumor Animal Models. Inorganica Chim. Acta 2024, 562, 121894. [Google Scholar] [CrossRef]
- Paparidis, G.; Akrivou, M.; Malis, G.; Lazou, M.; Psomas, G.; Vizirianakis, I.; Papagiannopoulou, D. Novel Technetium-99m Anthrapyrazole Derivatives as DNA-Targeted Agents. In Proceedings of the European Molecular Imaging Meeting 2022, Thessaloniki, Greece, 15–8 March 2022; p. 507. [Google Scholar]



| Compound | Band, λ (nm) (ΔA/Aο a (%), Δλ b (nm)) | Kb (M−1) |
|---|---|---|
| Appa [24] | 278 (−10, 0); 326 (sh) (−3.5, 0); 441(−4, +2) | 7.74 (±0.03) × 104 |
| Aqpa | 266 (−12 a, +2 b); 414 (−13, 0); 435 sh (−13, 0) | 7.64 (±0.11) × 105 |
| Aqim | 270 (−17, 0); 417 (−2, 0) | 1.62 (±0.27) × 106 |
| RePaIm | 268 (−17, +3) | 9.09 (±0.42) × 104 |
| RebpyIm | 282 (+30 a, 0); 320 sh (−12, elim); 352 (−15, +4) | 6.54 (±0.55) × 105 |
| ReAppaIm | 274 (−4, 0); 299 sh (−5, +2); 323 sh (+5, 0); 437 (−2, 0) | 5.65 (±0.45) × 105 |
| ReAqpaIm | 277 (+10, 0); 416 (−8, 0); 437 sh (−7, 0) | 1.61 (±0.17) × 105 |
| RebpyAqim | 266 (−20, +2); 310 sh (−8, 0); 320 (−7,0); 411 (−6, 0) | 5.90 (±0.18) × 105 |
| Compound | ΔΙ/Ιο (%) | KSV (M−1) | Kq (M−1s−1) |
|---|---|---|---|
| Aqpa | 74.9 | 1.26 (±0.06) × 105 | 5.47 (±0.24) × 1012 |
| Aqim | 76.1 | 2.02 (±0.06) × 105 | 8.77 (±0.27) × 1012 |
| RePaIm | 77.4 | 6.83 (±0.25) × 104 | 2.97 (±0.11) × 1012 |
| RebpyIm | 74.9 | 1.00 (±0.25) × 105 | 4.35 (±0.11) × 1012 |
| ReAppaIm | 78.2 | 8.24 (±0.30) × 104 | 3.58 (±0.13) × 1012 |
| ReAqpaIm | 78.0 | 5.56 (±0.26) × 104 | 2.42 (±0.11) × 1012 |
| RebpyAqim | 71.3 | 1.96 (±0.07) × 105 | 8.50 (±0.30) × 1012 |
| Complex | HPLC Elution Time (min) | RCY * (%) |
|---|---|---|
| fac-[M(CO)3(Pa)(Im)]+ | 14.7 (M = Re), 15.8 (M = 99mTc) | 73 ± 4 |
| fac-[M(CO)3(Appa)(Im)]+ | 19.85/20.35, 7:3 (M = Re), 20.00/20.42, 7:3 (M = 99mTc) | 65 ± 3 |
| fac-[M(CO)3(Aqpa)(Solv)]+ | 19.89/20.55, 45:55 (Re), 20.01/20.55, 33:67(99mTc) | 75 ± 6 |
| fac-[M(CO)3(Aqpa)(Im)]+ | 19.25/20.75, 7:3 (Re), 19.48/20.87, 7:3 (99mTc) | 40 ± 5 |
| fac-[M(CO)3(bpy)(Aqim)]+ | 20.87 (Re)/20.85 (99mTc) | 75 ± 5 |
| Complex | Histidine Stability (%) | Rat Plasma Stability (%) * | Lipophilicity (logD7.4) | ||||
|---|---|---|---|---|---|---|---|
| 1 h | 4 h | 24 h | 1 h | 4 h | 24 h | ||
| 99m TcPaIm | 92 | 85 | 57 | 44 (20) | 37 (21) | 27 (47) | 0.39 ± 0.13 |
| 99m TcAppaIm | 97 | 97 | 96 | 95 (21) | 95 (27) | 91(34) | 1.62 ± 0.04 |
| 99m TcAqpaIm | 98 | 98 | 92 | 97 (14) | 90 (19) | 98 (22) | 1.98 ± 0.01 |
| 99m TcbpyAqim | 96 | 96 | 89 | 93 (28) | 97 (35) | 90 (38) | 0.89 ± 0.15 |
| Organ | 99mTcAqpa | 99mTcAqpaIm | 99mTcAppaIm | 99mTcbpyAqim | ||||
|---|---|---|---|---|---|---|---|---|
| 30 min | 120 min | 30 min | 120 min | 30 min | 120 min | 30 min | 120 min | |
| Blood | 5.70 ± 1.00 | 2.62 ± 1.14 | 0.89 ± 0.04 | 0.47 ± 0.31 | 6.79 ± 1.84 | 1.39 ± 0.10 | 1.58 ± 0.59 | 0.64 ± 0.33 |
| Tumor | 1.83 ±0.18 | 1.23 ± 0.23 | 0.90 ± 0.05 | 0.85 ± 0.61 | 4.24 ± 1.39 | 2.34 ± 1.29 | 2.51 ± 1.76 | 0.63 ± 0.42 |
| Heart | 6.44 ± 0.55 | 4.65 ± 1.17 | 1.29 ± 0.07 | 0.89 ± 0.27 | 1.79 ± 0.36 | 0.81 ± 0.07 | 2.87 ± 0.70 | 1.63 ± 0.64 |
| Liver | 34.01 ±5.75 | 29.20 ± 3.47 | 35.52 ± 6.50 | 17.15 ± 3.46 | 26.08 ± 8.50 | 21.16 ± 3.79 | 31.12 ± 11.02 | 19.20 ± 5.95 |
| Lungs | 9.38 ± 1.30 | 4.26 ± 0.35 | 0.98 ± 0.05 | 0.69 ± 0.09 | 2.34 ± 0.44 | 0.96 ± 0.15 | 2.17 ± 0.63 | 1.05 ± 0.29 |
| Muscle | 1.27 ± 0.26 | 0.94 ± 0.11 | 0.38 ± 0.03 | 0.32 ± 0.15 | 0.88 ± 0.14 | 0.50 ± 0.11 | 1.34 ± 0.53 | 0.83 ± 0.71 |
| Kidneys | 12.48 ± 0.28 | 9.90 ± 0.91 | 53.03 ± 3.09 | 36.50 ± 11.04 | 24.09 ± 3.69 | 24.06 ± 3.89 | 29.44 ± 16.81 | 24.56 ± 12.09 |
| Spleen | 3.74 ± 0.34 | 1.92 ± 0.57 | 0.80 ± 0.12 | 0.59 ± 0.22 | 1.44 ± 0.31 | 0.76 ± 0.11 | 2.40 ± 0.73 | 1.15 ± 0.25 |
| Intestine | 5.10 ± 1.05 | 11.62 ± 2.51 | 23.41 ± 3.59 | 31.40 ± 7.59 | 12.78 ± 2.28 | 34.13 ± 4.42 | 6.97 ± 2.60 | 20.68 ± 7.51 |
| Stomach | 13.57 ±7.33 | 12.21 ± 6.47 | 3.54 ± 2.17 | 5.09 ± 2.78 | 3.59 ± 2.42 | 12.84 ± 7.06 | 54.67 ± 28.86 | 26.25 ± 17.65 |
| Tu/Bl | 0.33 ± 0.05 | 0.49 ± 0.13 | 0.99 ± 0.09 | 2.30 ± 0.61 | 0.70 ± 0.45 | 1.63 ± 0.92 | 1.28 ± 0.85 | 1.16 ± 0.02 |
| Tu/Mu | 1.48 ± 0.31 | 1.34 ± 0.66 | 2.49 ± 0.21 | 2.42 ± 1.42 | 4.76 ± 0.98 | 5.10 ± 3.55 | 1.51 ± 0.60 | 1.36 ± 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migkos, T.M.; Glykofridi, P.; Paparidis, G.; Psomas, G.; Vizirianakis, I.S.; Gabriel, C.; Sarigiannis, D.; Iakovou, I.; Papagiannopoulou, D. Synthesis and Biological Evaluation of Novel Mixed-Ligand 99mTc-Labeled Anthraquinone Complexes as Potential DNA-Targeted Imaging Agents. Inorganics 2025, 13, 368. https://doi.org/10.3390/inorganics13110368
Migkos TM, Glykofridi P, Paparidis G, Psomas G, Vizirianakis IS, Gabriel C, Sarigiannis D, Iakovou I, Papagiannopoulou D. Synthesis and Biological Evaluation of Novel Mixed-Ligand 99mTc-Labeled Anthraquinone Complexes as Potential DNA-Targeted Imaging Agents. Inorganics. 2025; 13(11):368. https://doi.org/10.3390/inorganics13110368
Chicago/Turabian StyleMigkos, Theofanis Matthaios, Pigi Glykofridi, Georgios Paparidis, George Psomas, Ioannis S. Vizirianakis, Catherine Gabriel, Dimosthenis Sarigiannis, Ioannis Iakovou, and Dionysia Papagiannopoulou. 2025. "Synthesis and Biological Evaluation of Novel Mixed-Ligand 99mTc-Labeled Anthraquinone Complexes as Potential DNA-Targeted Imaging Agents" Inorganics 13, no. 11: 368. https://doi.org/10.3390/inorganics13110368
APA StyleMigkos, T. M., Glykofridi, P., Paparidis, G., Psomas, G., Vizirianakis, I. S., Gabriel, C., Sarigiannis, D., Iakovou, I., & Papagiannopoulou, D. (2025). Synthesis and Biological Evaluation of Novel Mixed-Ligand 99mTc-Labeled Anthraquinone Complexes as Potential DNA-Targeted Imaging Agents. Inorganics, 13(11), 368. https://doi.org/10.3390/inorganics13110368








