High-Pressure Intrusion of Saline Solutions in Hydrophobic STT-Type Zeosil
Abstract
1. Introduction
2. Results and Discussion
2.1. Intrusion–Extrusion Experiments
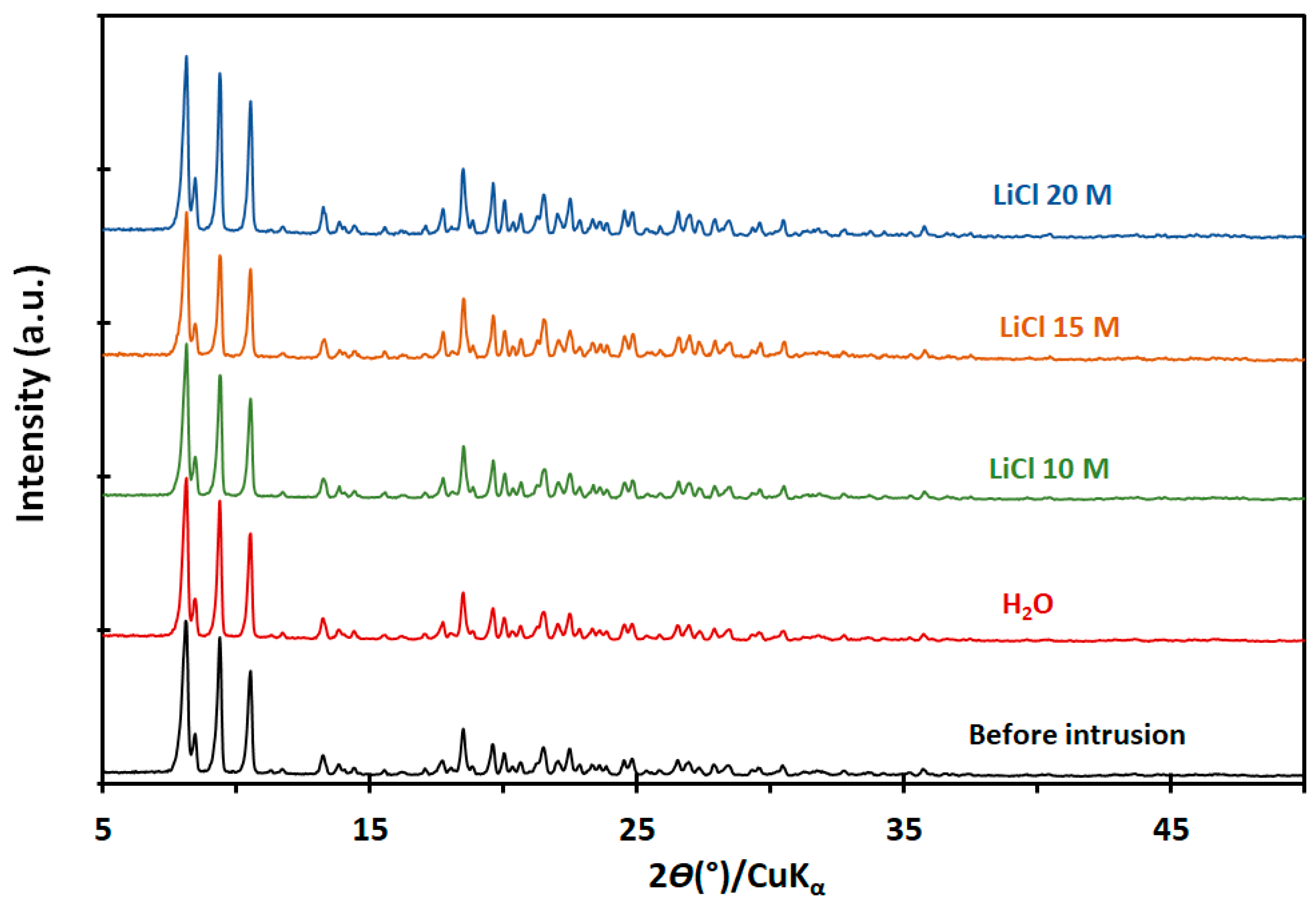
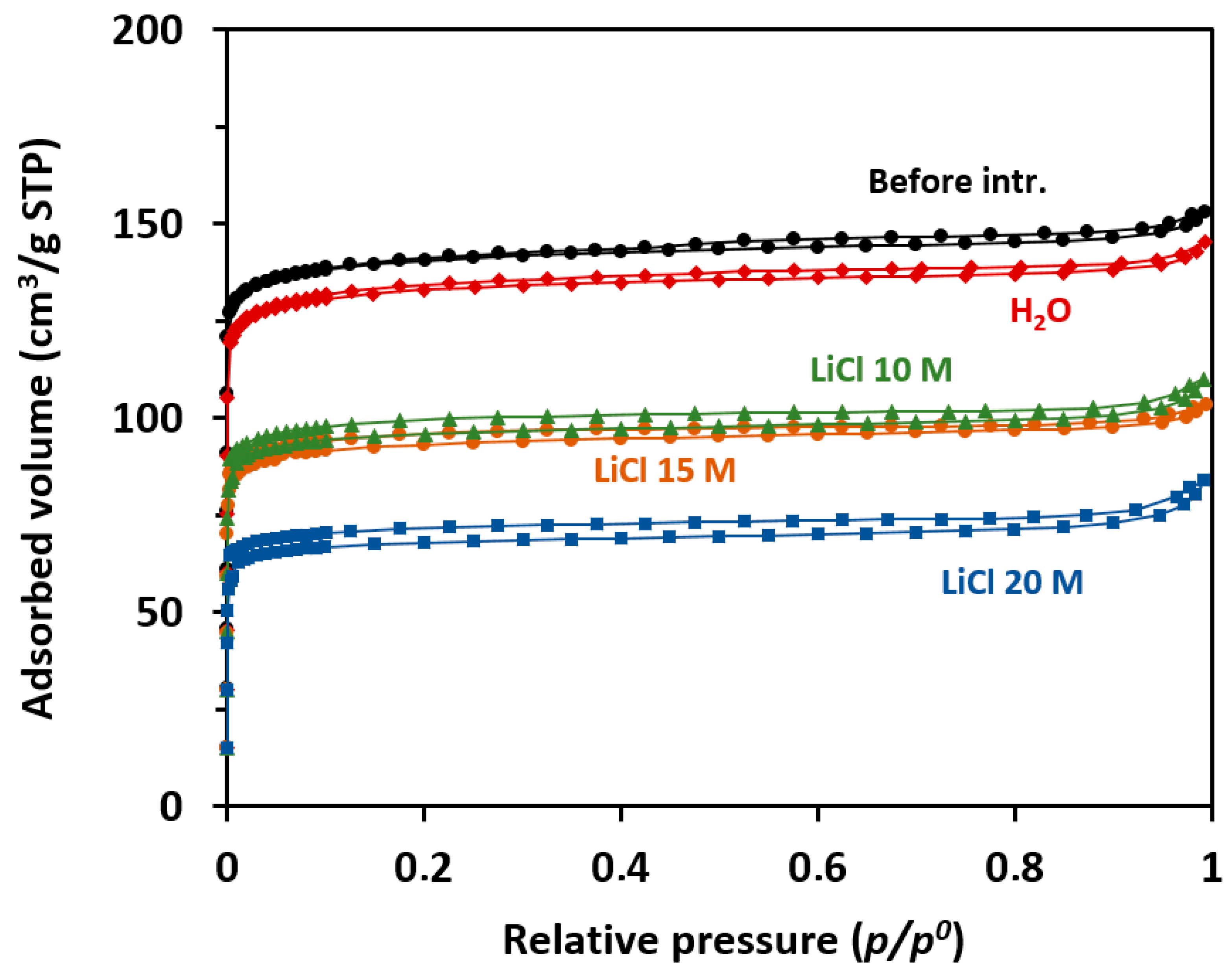
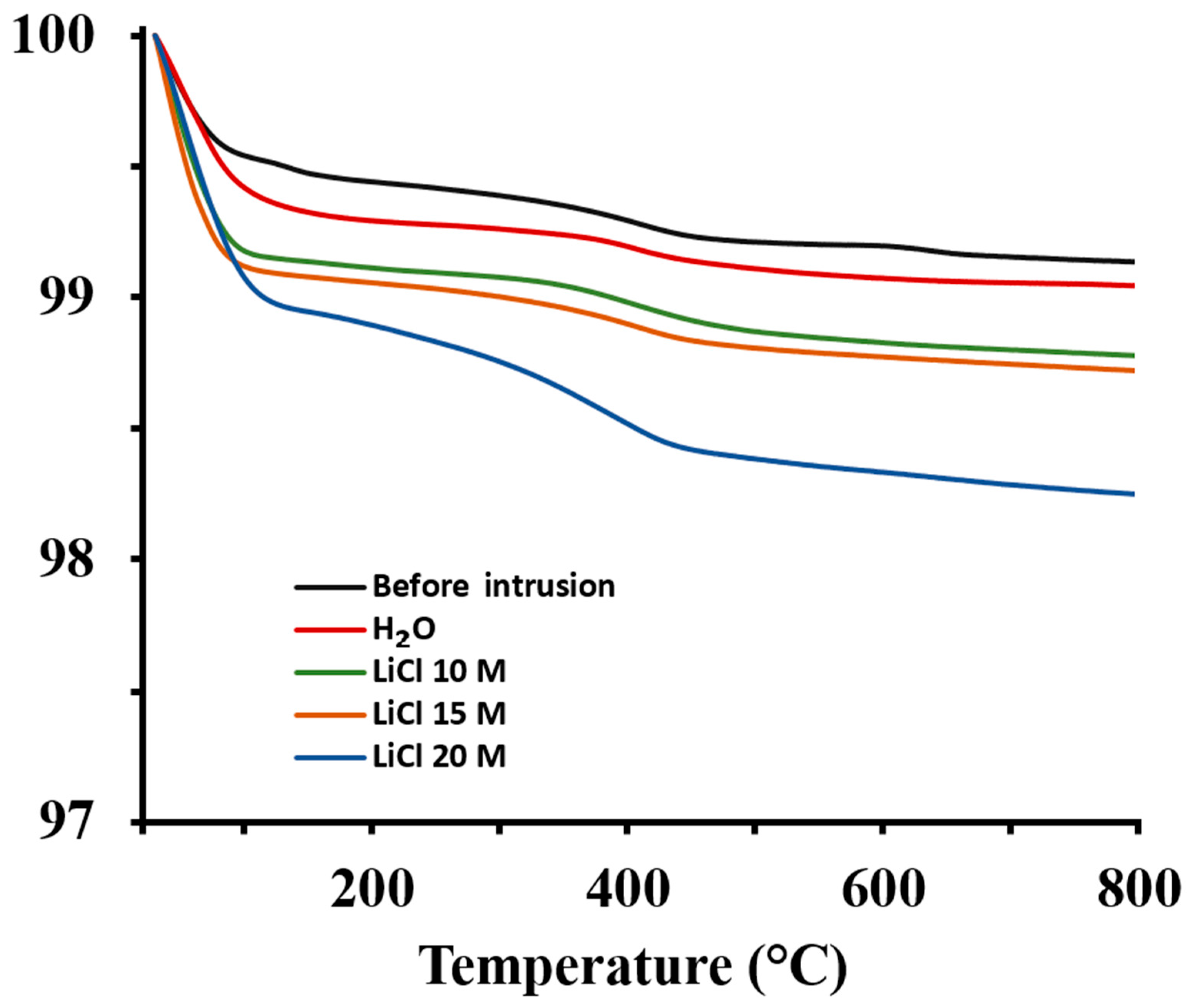
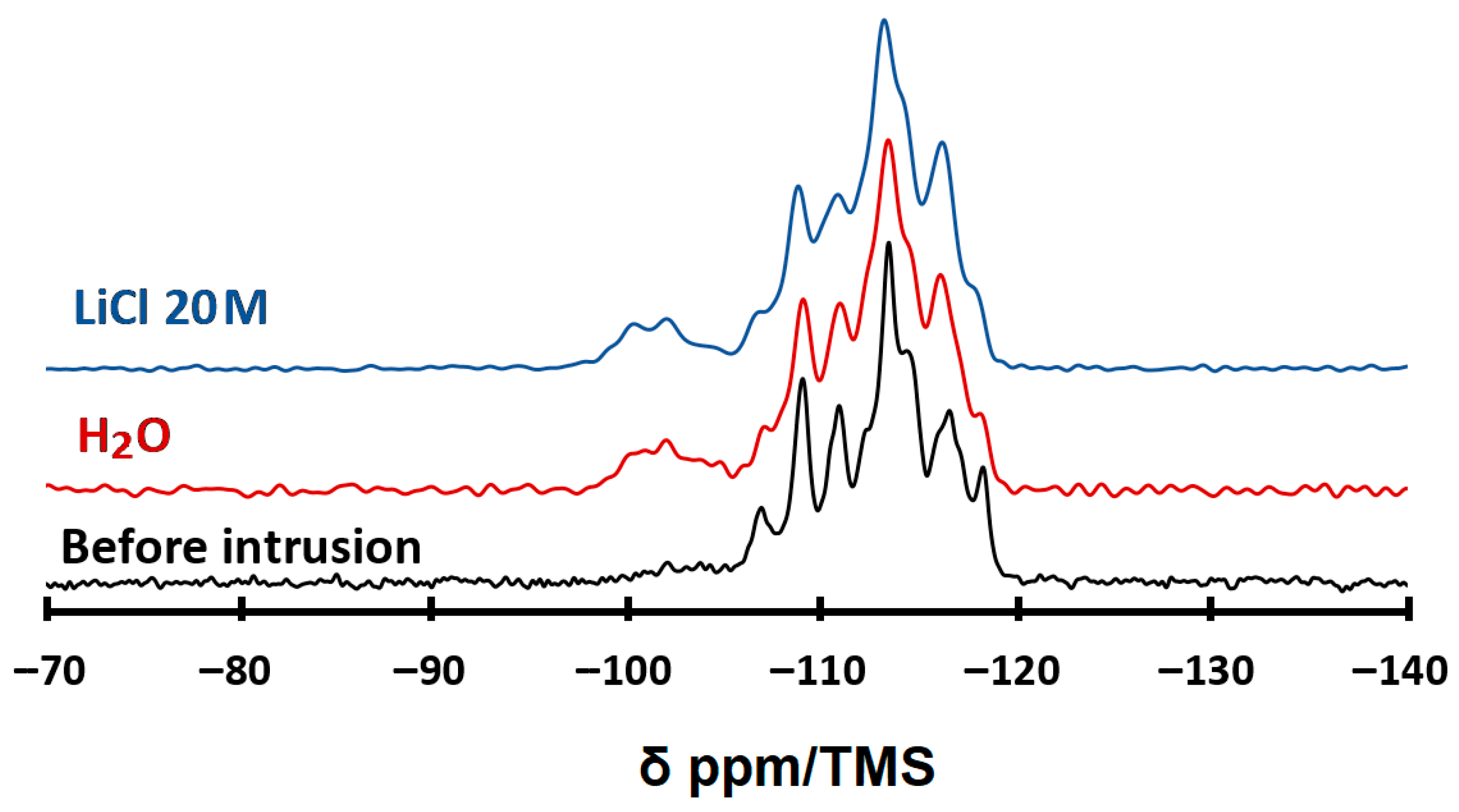
2.2. Characterization
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eroshenko , V.A. Hydrocapillary Accumulator. URSS Patent 1333870, 24 May 1985. [Google Scholar]
- Eroshenko, V.A. Heterogeneous Structure for Accumulation or Dissipation of Energy, Process to Use it and Associated Devices. International Patent WO 96/18040, 13 June 1996. [Google Scholar]
- Fraux, G.; Coudert, F.-X.; Boutin, A.; Fuchs, A.H. Forced intrusion of water and aqueous solutions in microporous materials: From fundamental thermodynamics to energy storage devices. Chem. Soc. Rev. 2017, 46, 7421–7437. [Google Scholar] [CrossRef] [PubMed]
- Le Donne, A.; Tinti, A.; Amayuelas, E.; Kashyap, H.K.; Camisasca, G.; Remsing, R.C.; Roth, R.; Grosu, Y.; Meloni, S. Intrusion and extrusion of liquids in highly confining media: Bridging fundamental research to applications. Adv. Phys. X 2022, 7, 2052353. [Google Scholar] [CrossRef]
- Fadeev, A.Y.; Eroshenko, V.A. Study of penetration of water into hydrophobized porous silicas. J. Coll. Inter. Sci. 1997, 187, 275–282. [Google Scholar] [CrossRef]
- Martin, T.; Lefevre, B.; Brunel, D.; Galarneau, A.; Di Renzo, F.; Fajula, F.; Gobin, P.F.; Quinson, J.; Vigier, G. Dissipative water intrusion in hydrophobic MCM-41 type materials. Chem. Commun. 2002, 1, 24–25. [Google Scholar] [CrossRef]
- Han, A.; Kong, X.; Qiao, Y. Temperature dependence of working pressure of a nanoporous liquid spring. Appl. Phys. Lett. 2006, 100, 014308. [Google Scholar]
- Amabili, M.; Grosu, Y.; Giacomello, A.; Meloni, S.; Zaki, A.; Bonilla, F.; Faik, A.; Casciola, C.M. Pore morphology determines spontaneous liquid extrusion from nanopores. ACS Nano 2019, 13, 1728–1738. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, C.; Lu, W.; Li, Y. Rate effect of liquid infiltration into mesoporous materials. RSC Adv. 2017, 7, 971–974. [Google Scholar] [CrossRef]
- Grosu, Y.; Giacomello, A.; Meloni, S.; González-Fernández, L.; Chorazewski, M.; Geppert-Rybczynska, M.; Faik, A.; Nedelec, J.-M.; Grolier, J.-P. Viscosity at the nanoscale: Confined liquid dynamics and thermal effects in self-recovering nanobumpers. J. Phys. Chem. C 2018, 122, 14248. [Google Scholar] [CrossRef]
- Suciu, C.V.; Iwatsubo, T.; Yaguchi, K.; Ikenaga, M. Novel and global approach of the complex and interconnected phenomena related to the contact line movement past a solid surface from hydrophobized silica gel. J. Coll. Inter. Sci. 2005, 283, 196–214. [Google Scholar] [CrossRef]
- Michel, L.; Ludescher, L.; Cristiglio, V.; Charlaix, E.; Paris, O.; Picard, C. The bowtie-shaped deformation isotherm of superhydrophobic cylindrical mesopores. Langmuir 2022, 38, 211–220. [Google Scholar] [CrossRef]
- Eroshenko, V.; Regis, R.C.; Soulard, M.; Patarin, J. Energetics: A new field of applications for hydrophobic zeolites. J. Am. Chem. Soc. 2001, 123, 8129–8130. [Google Scholar] [CrossRef] [PubMed]
- Tzanis, L.; Trzpit, M.; Soulard, M.; Patarin, J. High pressure water intrusion investigation of pure silica 1D channel AFI, MTW and TON-type zeolites. Micropor. Mesopor. Mater. 2011, 146, 119–126. [Google Scholar] [CrossRef]
- Tzanis, L.; Trzpit, M.; Soulard, M.; Patarin, J. Energetic performances of channel and cage-type zeosils. J. Phys. Chem. C 2012, 116, 20389–20395. [Google Scholar] [CrossRef]
- Khay, I.; Tsanis, L.; Daou, T.J.; Nouali, H.; Ryzhikov, A.; Patarin, J. Energetic behavior of the pure silica ITQ-12 (ITW) zeolite under high pressure water intrusion. Phys. Chem. Chem. Phys. 2013, 15, 20320–20325. [Google Scholar] [CrossRef] [PubMed]
- Ievtushenko, O.V.; Eroshenko, V.A.; Grosu, Y.G.; Nedelec, J.M.; Grolier, J.P.E. Evolution of the energetic characteristics of silicalite-1 + water repulsive clathrates in a wide temperature range. Phys. Chem. Chem. Phys. 2013, 15, 4451–4457. [Google Scholar] [CrossRef]
- Humplik, T.; Shalabh, R.; Maroo, C.; Laoui, T.; Wang, E.N. Framework water capacity and infiltration pressure of MFI zeolites. Micropor. Mesopor. Mater. 2014, 190, 84–91. [Google Scholar] [CrossRef]
- Fasano, M.; Humplik, T.; Bevilacqua, A.; Tsapatsis, M.; Chiavazzo, E.; Wang, E.N.; Asinari, P. Interplay between hydrophilicity and surface barriers on water transport in zeolite membranes. Nat. Comm. 2016, 7, 12762. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, L.; Chen, X. Pressurized liquid in nanopores: A modified Laplace-Young equation. Nano Lett. 2009, 9, 984–988. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Z.; Xu, J.; Xu, X.; Liu, C.; Li, Y. A candidate of mechanical energy mitigation system: Dynamic and quasi-static behaviors and mechanisms of zeolite β/water system. Mater. Des. 2015, 66B, 545–551. [Google Scholar] [CrossRef]
- Ortiz, G.; Nouali, H.; Marichal, C.; Chaplais, G.; Patarin, J. Energetic performances of the metal–organic framework ZIF-8 obtained using high pressure water intrusion–extrusion experiments. Phys. Chem. Chem. Phys. 2013, 15, 4888–4891. [Google Scholar] [CrossRef]
- Khay, I.; Chaplais, G.; Nouali, H.; Ortiz, G.; Marichal, C.; Patarin, J. Assessment of the energetic performances of various ZIFs with SOD or RHO topology using high pressure water intrusion–extrusion experiments. Dalton Trans. 2016, 45, 4392–4400. [Google Scholar] [CrossRef]
- Grosu, Y.; Li, M.; Peng, Y.-L.; Luo, D.; Li, D.; Faik, A.; Nedelec, J.-M.; Grolier, J.-P. A highly stable nonhysteretic {Cu2(tebpz) mof+water} molecular spring. ChemPhysChem 2016, 17, 3359–3364. [Google Scholar] [CrossRef]
- Grosu, Y.; Renaudin, G.; Eroshenko, V.; Nedelec, J.M.; Grolier, J.P.E. Synergetic effect of temperature and pressure on energetic and structural characteristics of {ZIF-8 + water} molecular spring. Nanoscale 2015, 7, 8803–8810. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Rogge, S.M.J.; Lamaire, A.; Vandenbrande, S.; Wieme, J.; Siviour, C.R.; Van Speybroeck, V.; Tan, J.-C. High-rate nanofluidic energy absorption in porous zeolitic frameworks. Nat. Mater. 2021, 20, 1015–1023. [Google Scholar] [CrossRef]
- Tzanis, L.; Nouali, H.; Daou, T.J.; Soulard, M.; Patarin, J. Influence of the aqueous medium on the energetic performances of silicalite-1. Mater. Lett. 2014, 115, 229–232. [Google Scholar] [CrossRef]
- Qiao, Y.; Han, K. Infiltration pressure of a nanoporous liquid spring modified by an electrolyte. J. Mater. Res. 2007, 22, 644–648. [Google Scholar] [CrossRef]
- Astafan, A.; Chaplais, G.; Nouali, H.; Daou, T.J.; Ryzhikov, A. Influence of cation nature on high pressure intrusion of aqueous salt solutions in pure silica MFI-type zeolite. Phys. Chem. Chem. Phys. 2025, 27, 8552–8558. [Google Scholar] [CrossRef] [PubMed]
- Ryzhikov, A.; Khay, I.; Nouali, H.; Daou, T.J.; Patarin, J. Drastic change of the intrusion-extrusion behavior of electrolyte solutions in pure silica *BEA-type zeolite. Phys. Chem. Chem. Phys. 2014, 16, 17893–17899. [Google Scholar] [CrossRef] [PubMed]
- Ryzhikov, A.; Ronchi, L.; Nouali, H.; Daou, T.J.; Paillaud, J.-L.; Patarin, J. High-pressure intrusion–extrusion of water and electrolyte solutions in pure-silica LTA zeolite. J. Phys. Chem. C 2015, 119, 28319–28325. [Google Scholar] [CrossRef]
- Khay, I.; Daou, T.J.; Nouali, H.; Ryzhikov, A.; Rigolet, S.; Patarin, J. High pressure intrusion-extrusion of LiCl aqueous solutions in silicalite-1 zeolite: Influence on energetic performances. J. Phys. Chem. C 2014, 118, 3935–3941. [Google Scholar] [CrossRef]
- Ronchi, L.; Ryzhikov, A.; Nouali, H.; Daou, T.J.; Patarin, J. Influence of LiCl aqueous solution concentration on the energetic performances of pure silica chabazite. New J. Chem. 2017, 47, 2586–2592. [Google Scholar] [CrossRef]
- Ronchi, L.; Ryzhikov, A.; Nouali, H.; Daou, T.J.; Patarin, J. Extra-large pore opening CFI and DON-type zeosils for mechanical energy storage. Micropor. Mesopor. Mater. 2018, 255, 211–219. [Google Scholar] [CrossRef]
- Isaac, C.; Confalonieri, G.; Nouali, H.; Paillaud, J.-L.; Arletti, R.; Daou, T.J.; Ryzhikov, A. Unusual high-pressure intrusion-extrusion behavior of electrolyte solutions in Mu-26, a pure silica zeolite of topology STF. Micropor. Mesopor. Mater. 2020, 298, 110047. [Google Scholar] [CrossRef]
- Ronchi, L.; Ryzhikov, A.; Nouali, H.; Daou, T.J.; Albrecht, S.; Patarin, J. Investigation of the energetic performance of pure silica BEC-type zeolite under high pressure water and 20 M LiCl intrusion-extrusion experiments. Micropor. Mesopor. Mater. 2017, 254, 153–159. [Google Scholar] [CrossRef]
- Ronchi, L.; Ryzhikov, A.; Nouali, H.; Daou, T.J.; Patarin, J. Heterogeneous lyophobic systems based on pure silica ITH-type zeolites: High pressure intrusion of water and electrolyte solutions. New J. Chem. 2017, 41, 15087–15093. [Google Scholar] [CrossRef]
- Confalonieri, G.; Daou, T.J.; Nouali, H.; Arletti, R.; Ryzhikov, A. Energetic performance of pure silica zeolites under high-pressure intrusion of LiCll aqueous solutions: An Overview. Molecules 2020, 25, 2145. [Google Scholar] [CrossRef]
- Tzanis, L.; Trzpit, M.; Soulard, M.; Patarin, J. Energetic performances of STT-type zeosil: Influence of the nature of the mineralizing agent used for the synthesis. J. Phys. Chem. C 2012, 116, 4802–4808. [Google Scholar] [CrossRef]
- D’Izarra, A.; Coudert, F.-X.; Fuchs, A.; Boutin, A. Predictive thermodynamic model for intrusion of electrolyte aqueous solutions in nanoporous materials. Chem. Mater. 2023, 35, 10606–10618. [Google Scholar] [CrossRef]
- Confalonieri, G.; Ryzhikov, A.; Arletti, R.; Quartieri, S.; Vezzalini, G.; Isaac, C.; Paillaud, J.-L.; Nouali, H.; Daou, T.J. Structural interpretation of the energetic performances of a pure silica LTA-type zeolite. Phys. Chem. Chem. Phys. 2020, 22, 5178–5187. [Google Scholar] [CrossRef]
- Desbiens, N.; Demachy, I.; Fuchs, A.H.; Kirsch-Rodeschini, H.; Soulard, M.; Patarin, J. Water condensation in hydrophobic nanopores. Angew. Chem. Int. Ed. 2005, 44, 5310–5313. [Google Scholar] [CrossRef]
- Bushuev, Y.G.; Sastre, G.; De Julian-Ortiz, J.V.; Galvez, J. Water−hydrophobic zeolite systems. J. Phys. Chem. C 2012, 116, 24916–24929. [Google Scholar] [CrossRef]
- Camblor, M.A.; Diaz-Cabanas, M.-J.; Perez-Pariente, J.; Teat, S.J.; Clegg, W.; Shannon, I.J.; Lightfoot, P.; Wright, P.A.; Morris, R.E. SSZ-23: An Odd Zeolite with Pore Openings of Seven and Nine Tetrahedral Atoms. Angew. Chem. Int. Ed. 1998, 37, 2122–2126. [Google Scholar] [CrossRef]
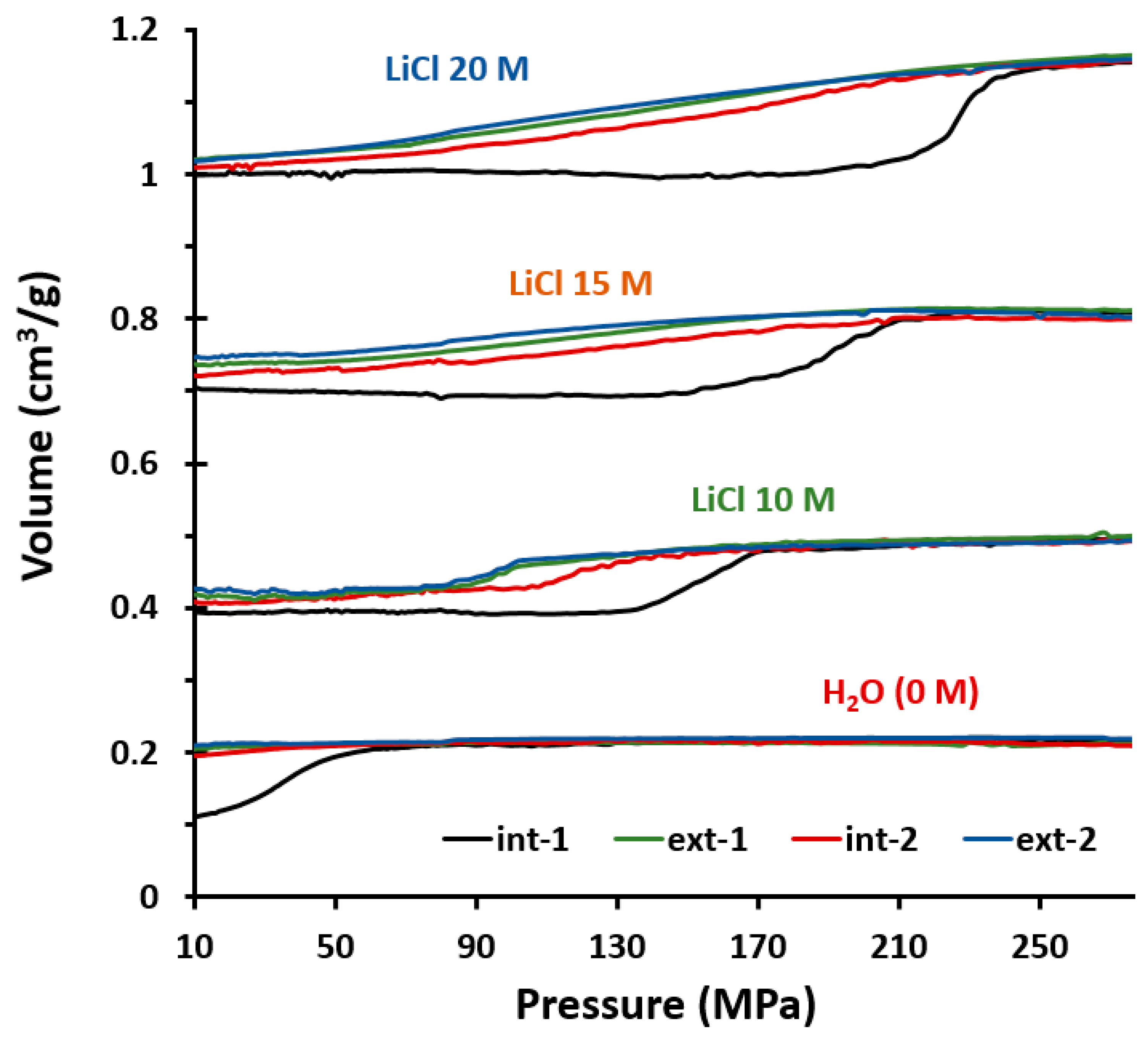
| System STT+ | Pint (MPa) | Pext (MPa) | Vint (mL/g) | Vext (mL/g) | Behavior |
|---|---|---|---|---|---|
| H2O | 35 | - | 0.10 | - | B |
| 10 M LiCl | 154 a/112 b | 95 a/95 b | 0.09 a/0.08 b | 0.08 | SA+B a/S b |
| 15 M LiCl | 191 a/131 b | 114 a/114 b | 0.10 a/0.08 b | 0.09 | SA+B a/S b |
| 20 M LiCl | 227 a/166 b | 136 a/133 b | 0.14 a/0.12 b | 0.12 | SA+B a/A b |
| Sample | Vmicro (cm3/g) | SBET (m2/g) |
|---|---|---|
| STT before intrusion | 0.21 | 536 |
| STT H2O (0 M) | 0.20 | 533 |
| STT—LiCl 10 M | 0.15 | 355 |
| STT—LiCl 15 M | 0.13 | 317 |
| STT—LiCl 20 M | 0.10 | 253 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaib-Draa, Y.-M.; Astafan, A.; Chaplais, G.; Nouali, H.; Rigolet, S.; Ryzhikov, A. High-Pressure Intrusion of Saline Solutions in Hydrophobic STT-Type Zeosil. Inorganics 2025, 13, 371. https://doi.org/10.3390/inorganics13110371
Chaib-Draa Y-M, Astafan A, Chaplais G, Nouali H, Rigolet S, Ryzhikov A. High-Pressure Intrusion of Saline Solutions in Hydrophobic STT-Type Zeosil. Inorganics. 2025; 13(11):371. https://doi.org/10.3390/inorganics13110371
Chicago/Turabian StyleChaib-Draa, Yacine-Malik, Amir Astafan, Gérald Chaplais, Habiba Nouali, Séverinne Rigolet, and Andrey Ryzhikov. 2025. "High-Pressure Intrusion of Saline Solutions in Hydrophobic STT-Type Zeosil" Inorganics 13, no. 11: 371. https://doi.org/10.3390/inorganics13110371
APA StyleChaib-Draa, Y.-M., Astafan, A., Chaplais, G., Nouali, H., Rigolet, S., & Ryzhikov, A. (2025). High-Pressure Intrusion of Saline Solutions in Hydrophobic STT-Type Zeosil. Inorganics, 13(11), 371. https://doi.org/10.3390/inorganics13110371







