A Review of Synthesis, Characterization, Properties, and Applications of Double Perovskite Oxides
Abstract
1. Introduction
2. Synthesis
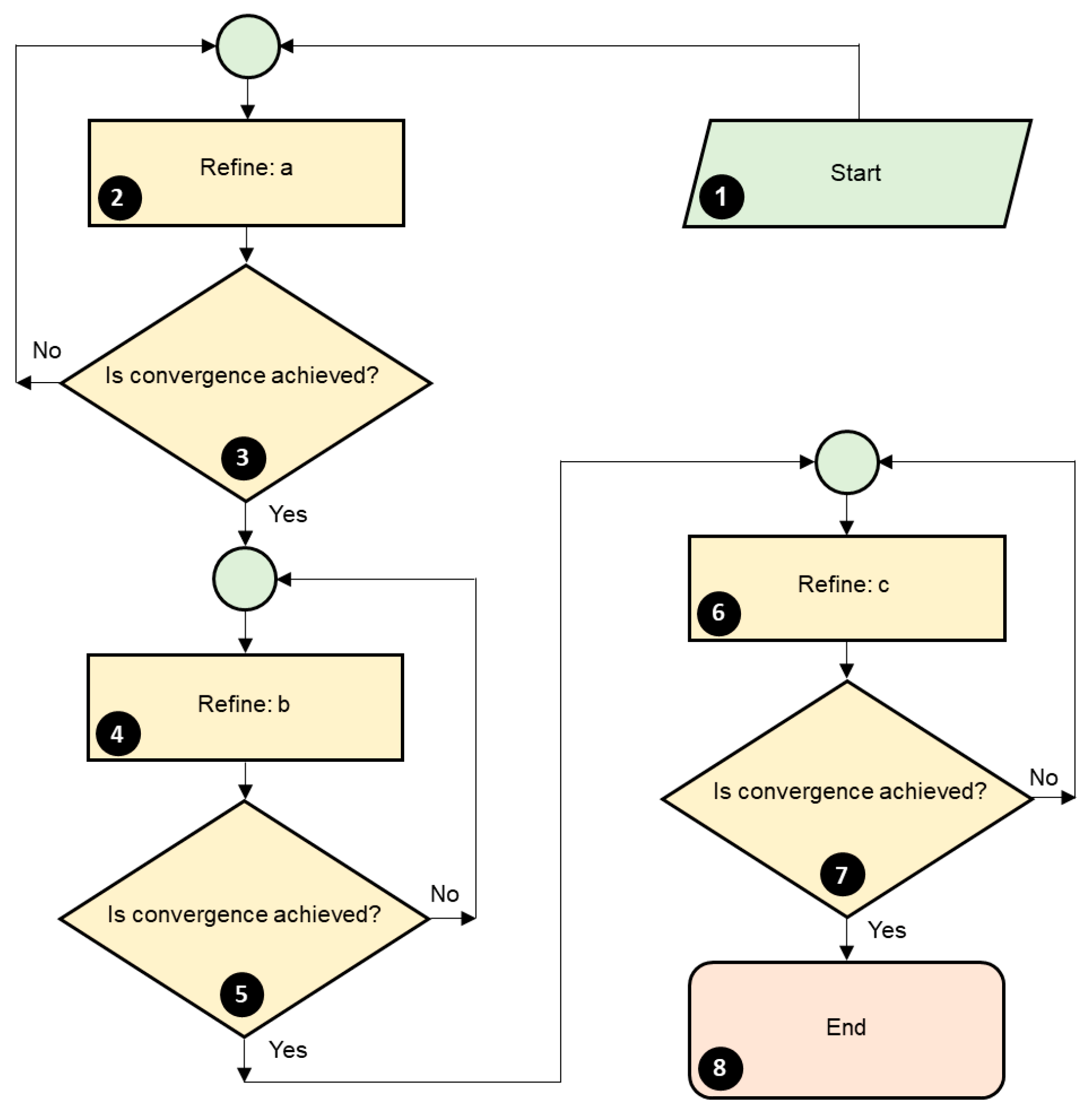
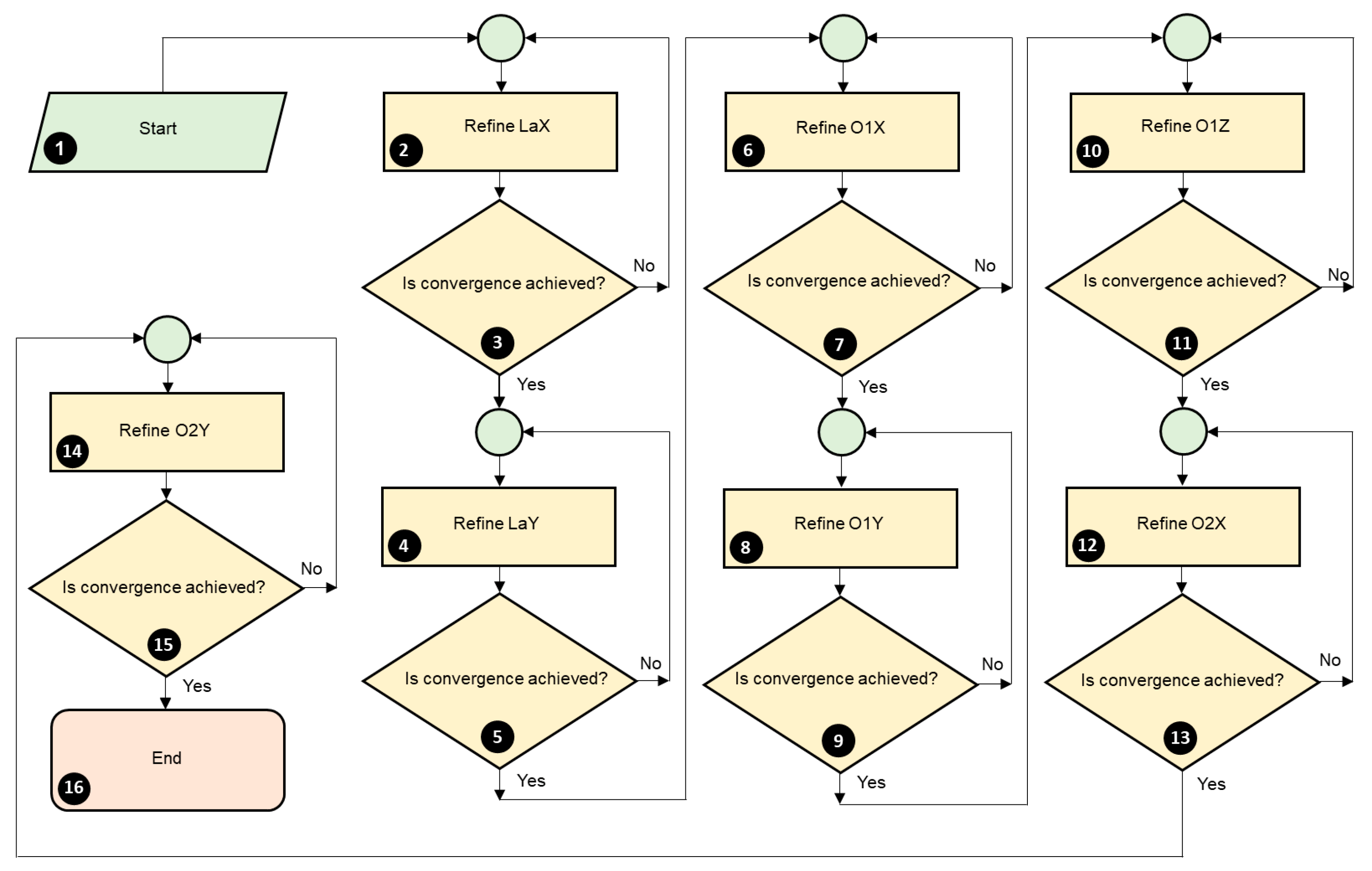
2.1. Solid-State Reaction Method
2.2. Sol–Gel Method
2.3. Pechini Method
2.4. Hydrothermal Method
3. Characterization
3.1. X-Ray Fluorescence
3.2. X-Ray Diffraction (XRD)
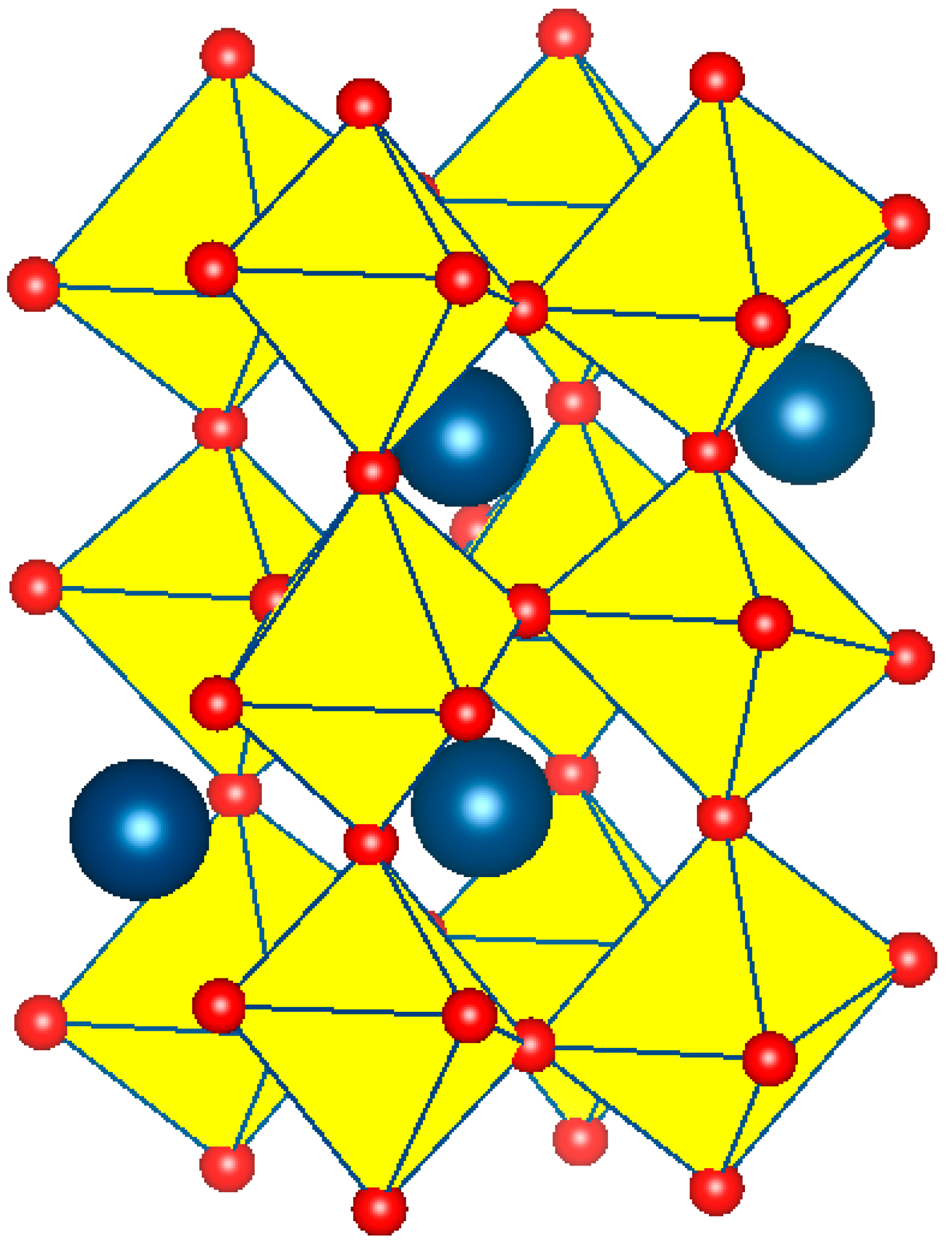
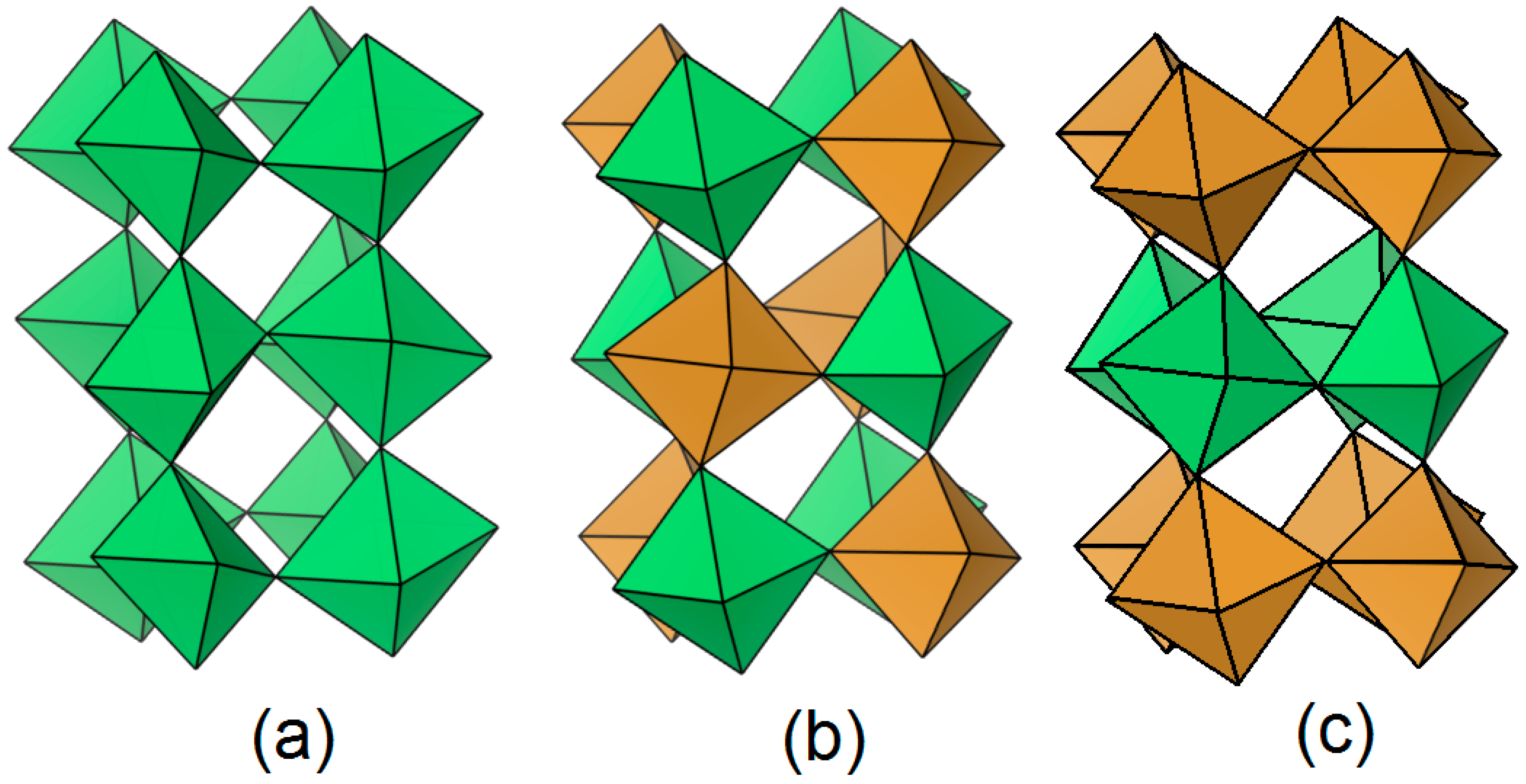
3.2.1. Crystal Structure Determination
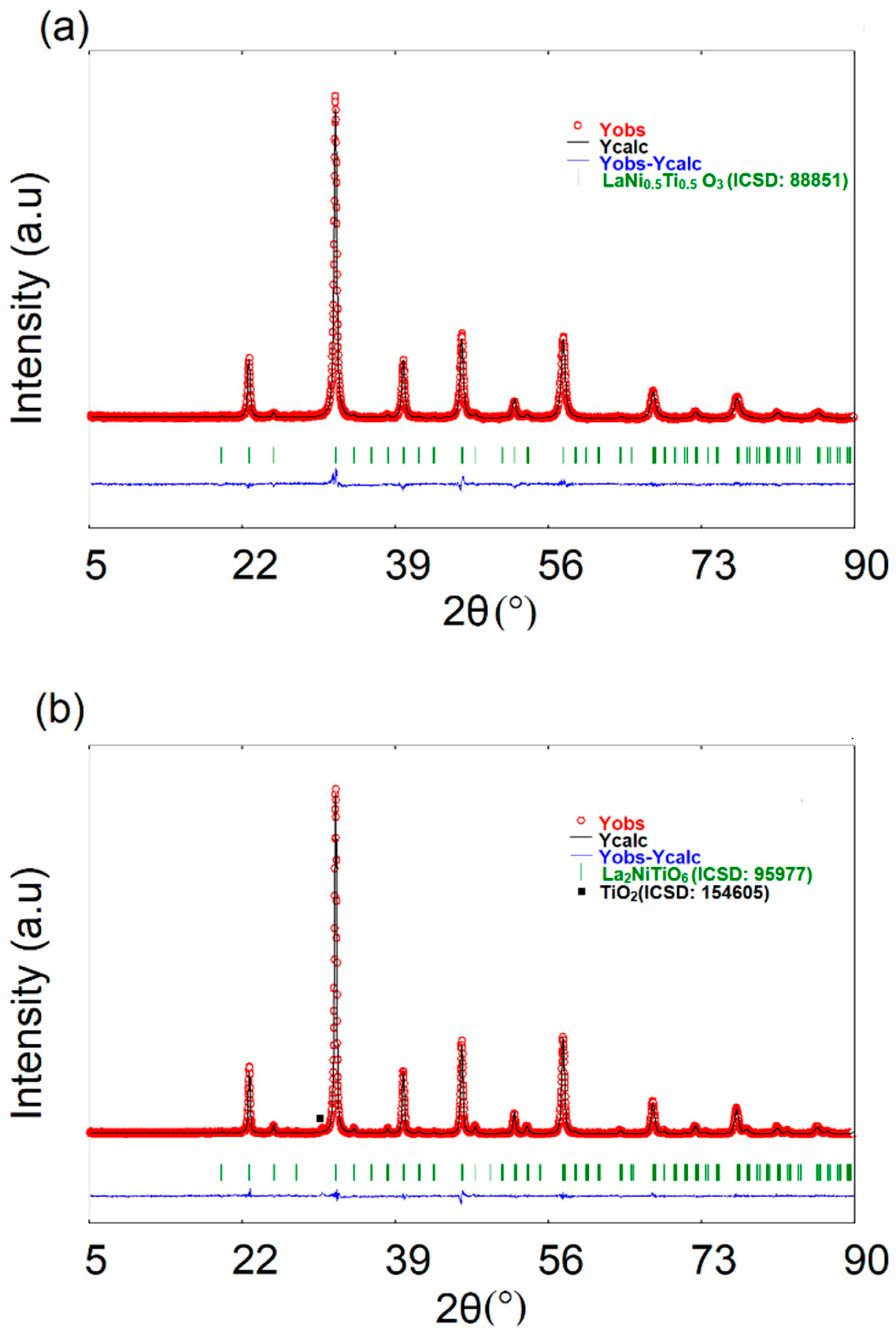
3.2.2. Graph of the Observed and Calculated XRD Pattern
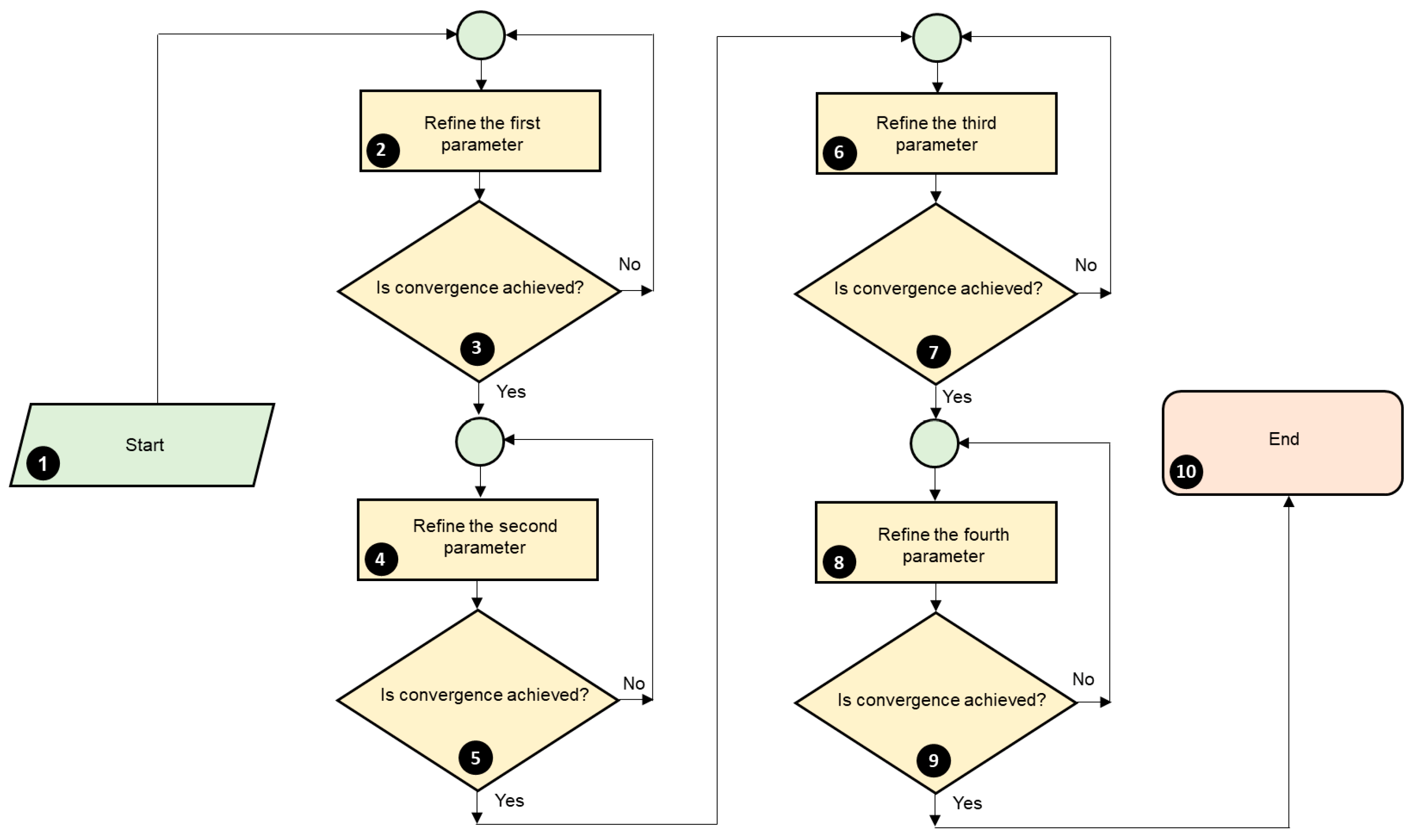
3.2.3. Criteria for Judging the Quality of the Refinement
3.2.4. CIF File Generation
3.2.5. Apparent Crystallite Size and Apparent Strain
3.3. Magnetic Measurements
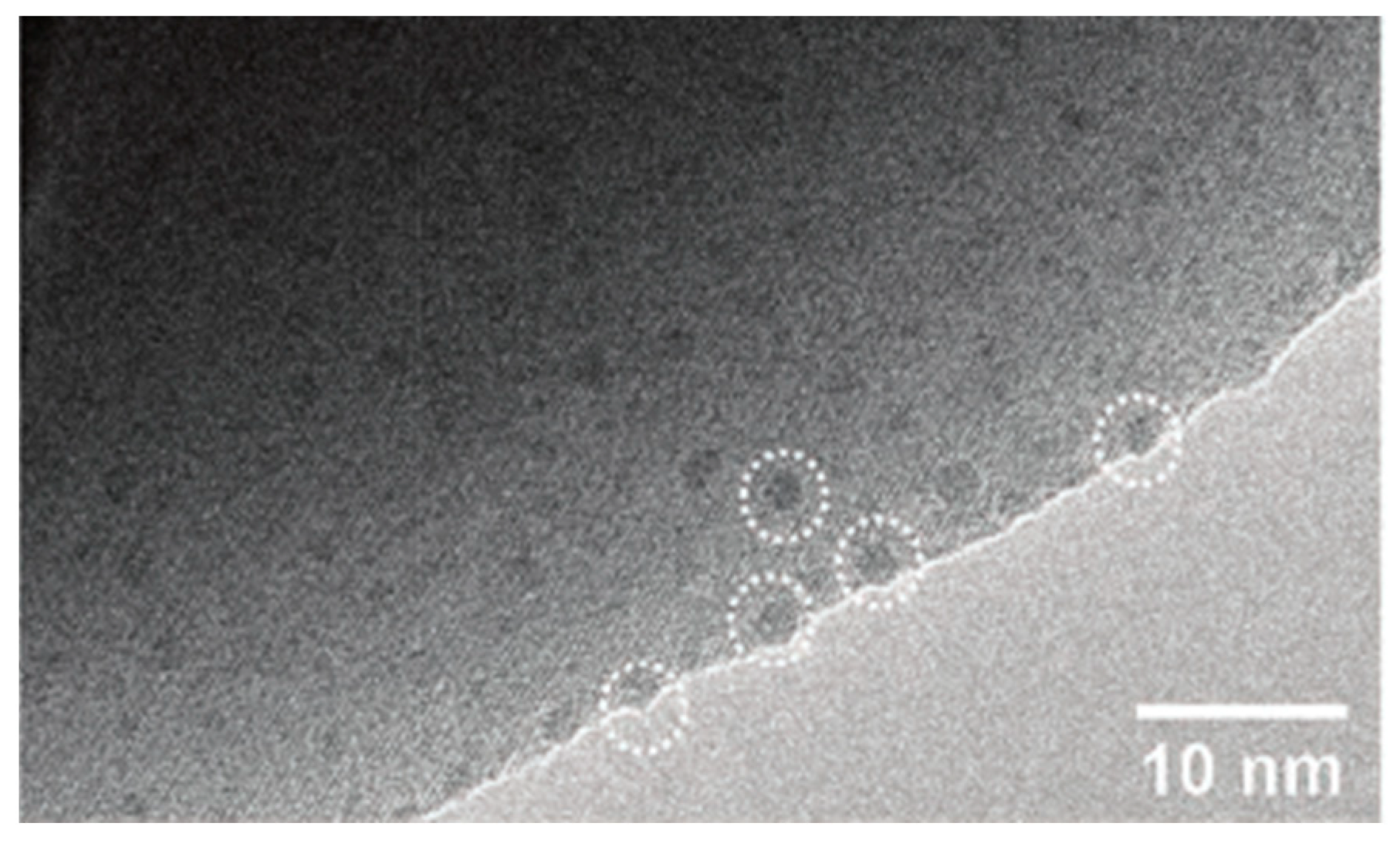
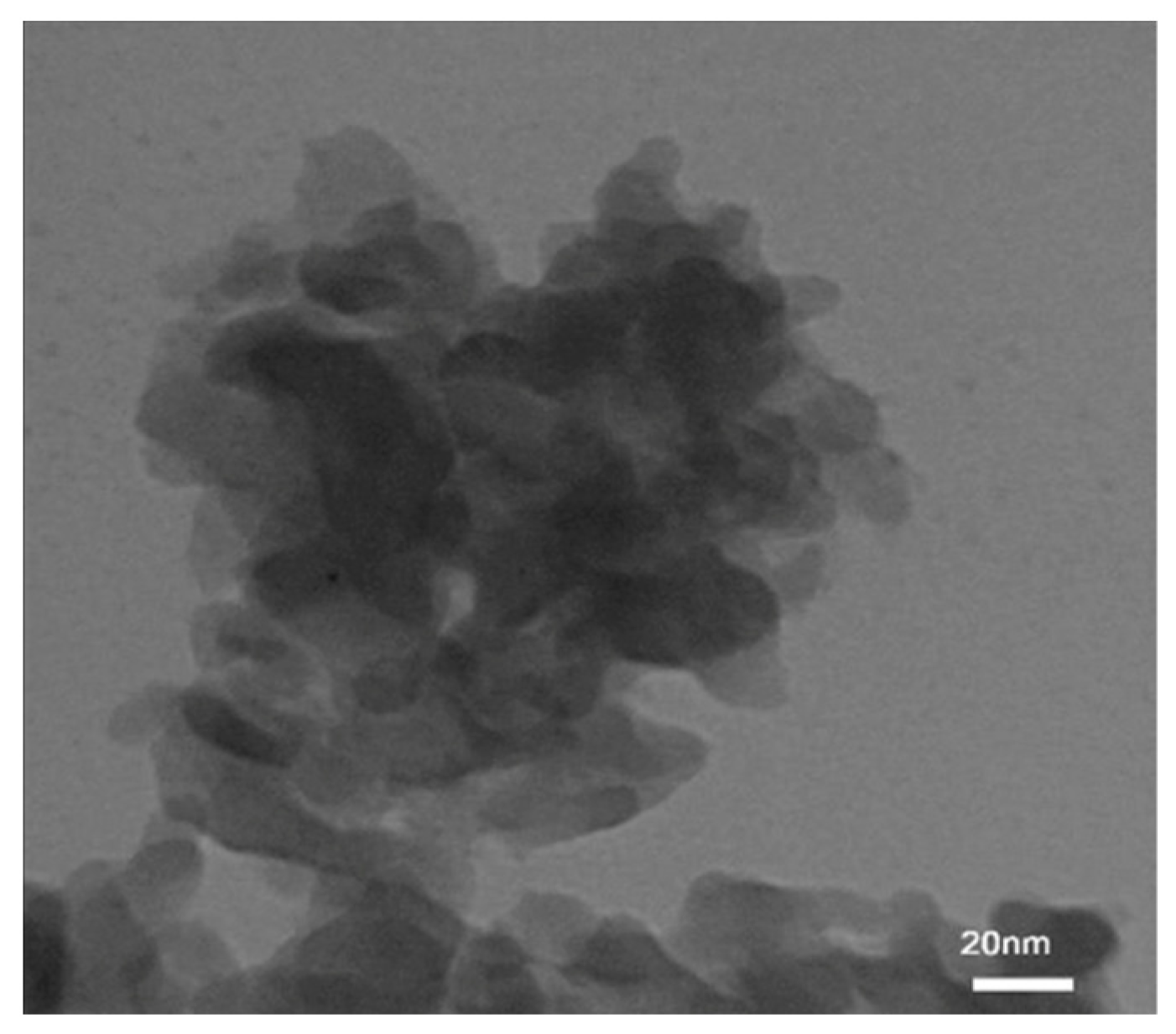
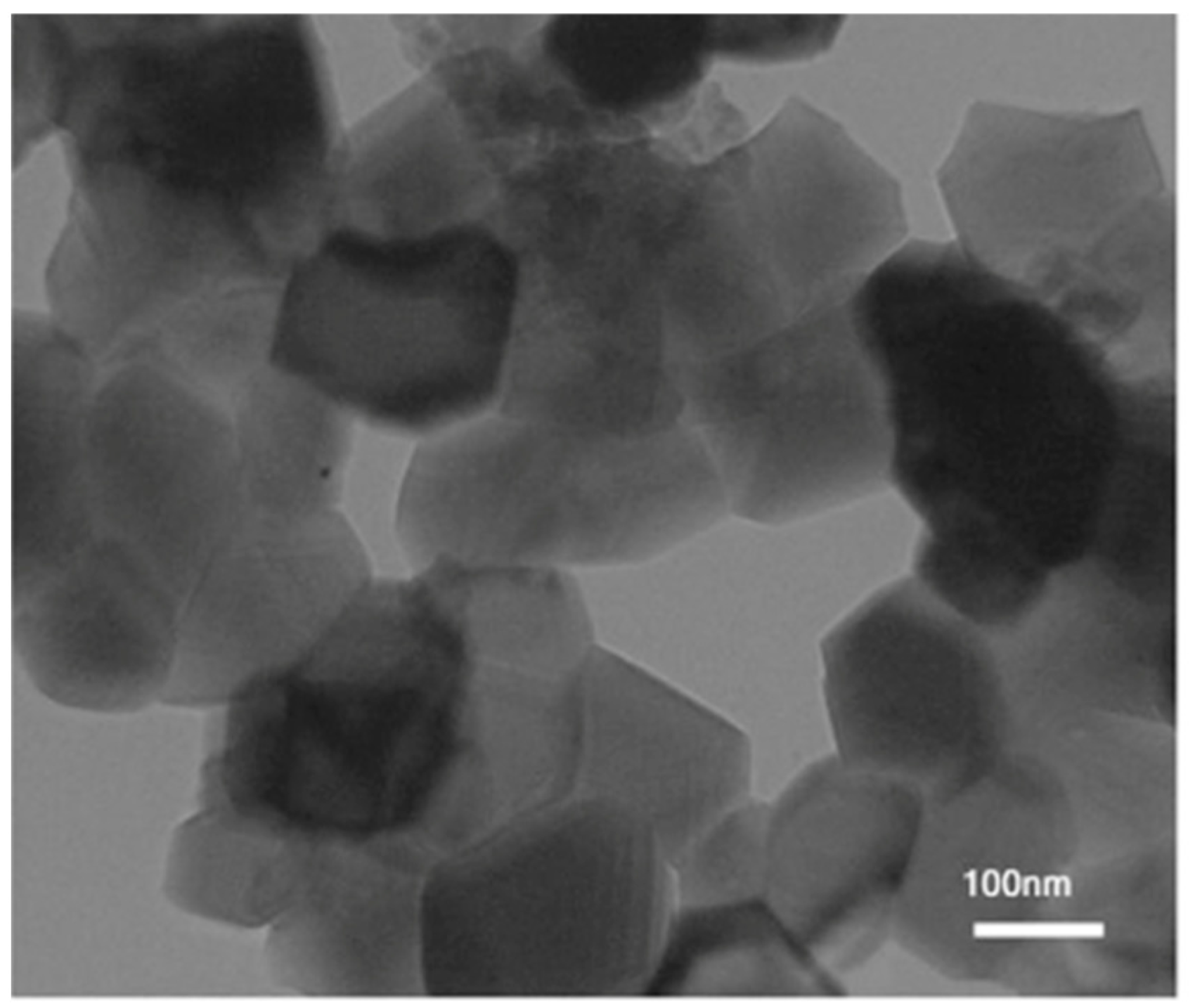
3.4. Transmission Electron Microscopy
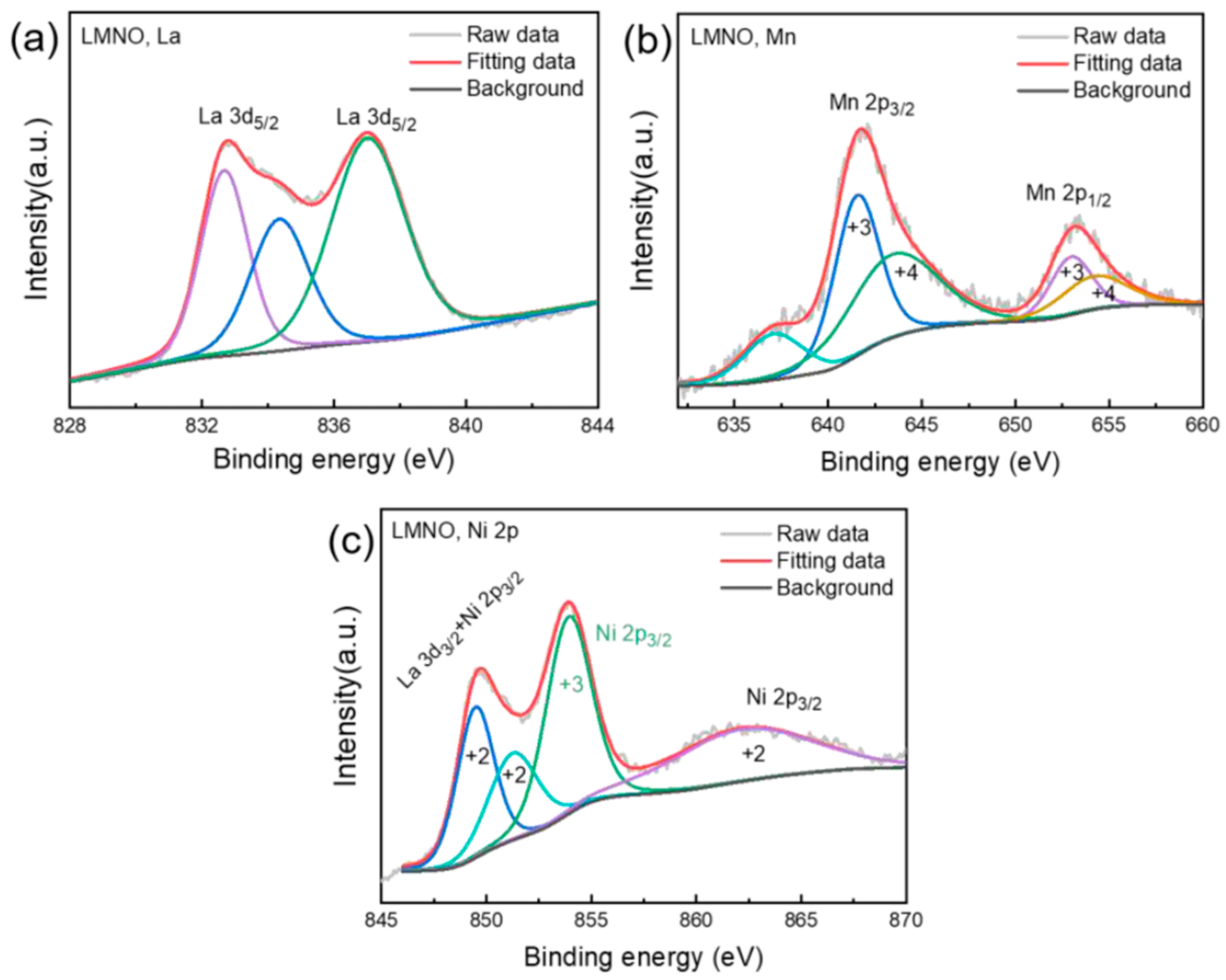
3.5. X-Ray Photoelectron Spectroscopy
3.6. Temperature-Programmed Reduction
3.7. Synchrotron X-Ray Diffraction (SXRD)
3.8. Neutron Powder Diffraction (NPD)
3.9. Extended X-Ray Absorption Fine Structure (EXAFS)
3.10. Raman Spectroscopy
4. Properties and Applications
5. Summary
6. Outlook and Challenges
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | Antiferromagnetic |
| DP | Double perovskite |
| BMP | File containing a figure of a crystal structure |
| CIF | Crystallographic information file |
| DAT | File containing observed X-ray diffraction data |
| EB | Exchange Bias |
| EG | Ethylene glycol |
| EXAFS | Extended X-ray absorption fine structure |
| FC | Field-cooled mode |
| FiM | Ferrimagnetic |
| FM | Ferromagnetic |
| HER | Hydrogen evolution reaction |
| IRF | File for an instrumental resolution function containing information of a standard like CeO2 |
| LED | Light-Emitting Diode |
| M | Magnetization |
| MIC | File containing the apparent size and apparent strain |
| OER | Oxygen evolution reaction |
| PCR | A tool of the Fullprof program that generates editing files used during the refinement of a crystal structure, called PCR files |
| PVA | Polyvinyl alcohol |
| NPD | Neutron Powder Diffraction |
| SXRD | Synchrotron X-ray diffraction |
| T | Temperature |
| TC | Curie Temperature |
| TEM | Transmission electron microscopy |
| Tg | Glass transition temperature |
| TN | Néel temperature |
| TPR | Temperature-programmed reduction |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray Diffraction |
| XRF | X-ray Fluorescence |
| ZFC | Zero-field cooled mode |
| χ | Magnetic susceptibility |
References
- Bibi, N.; Usman, M.; Noreen, S. Predictions on new Cu-based ABO3 (A=Cu and B=Lu, Y) oxide-perovskite for energy storage and optoelectronic applications: A DFT study. Mater. Sci. Semicond. Process. 2025, 185, 109001. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Malik, S.; Dutta, A. Examining the effects of sintering temperature on double perovskite La2NiTiO6: Analysis of structural, optical, electrical properties, and leakage current characteristics. J. Phys. Chem. Solids 2024, 190, 112027. [Google Scholar] [CrossRef]
- Banerjee, A.; Awasthi, M.K.; Maji, P.; Pal, M.; Aziz, S.T.; Lahiri, G.K.; Dutta, A. Double Perovskite Oxides Bringing a Revelation in Oxygen Evolution Reaction Electrocatalyst Design. ChemElectroChem 2023, 10, e202201098. [Google Scholar] [CrossRef]
- Tuza, P.V.; Souza, M.M.V.M. Steam Reforming of Methane over Catalyst Derived from Ordered Double Perovskite: Effect of Crystalline Phase Transformation. Catal. Lett. 2016, 146, 47–53. [Google Scholar] [CrossRef]
- Luo, H.; Li, Y.; Li, T.; Wang, Z.; Wang, L.; Liu, Y.-Q. Double perovskite type catalysts with improved anti-coking and sulfur-resisting performance for diesel reforming. Int. J. Hydrogen Energy 2023, 48, 9929–9944. [Google Scholar] [CrossRef]
- Kaczkowski, J.; Pugaczowa-Michalska, M.; Płowaś-Korus, I. Isovalent cation ordering in Bi-based double perovskites: A density functional analysis. J. Magn. Magn. Mater. 2022, 548, 168984. [Google Scholar] [CrossRef]
- Córdova-Calderón, J.; Tuza, P.V.; Souza, M.M.V.M. Synthesis and Characterization of LaNi0.5Ti0.5O3 and La2NiTiO6 Double Perovskite Nanoparticles. Materials 2022, 15, 2411. [Google Scholar] [CrossRef]
- Mohandas, M.; Ugendar, K.; Harsita, M.; Rao, T.D.; Kanakaraju, P.; Mondal, R.; Sattibabu, B. Structural, magnetic and dielectric properties in double perovskite La2-xScxCoMnO6 (x = 0.0, 0.1, and 0.2). Mater. Today Commun. 2024, 41, 110917. [Google Scholar] [CrossRef]
- Boudad, L.; Taibi, M.; Belayachi, A.; Abd-Lefdil, M. Structural, morphological, dielectric and optical properties of double perovskites RBaFeTiO6(R = La, Eu). RSC Adv. 2021, 11, 40205–40215. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Nie, Z.; Wu, C.; Gao, T.; Yang, N.; Yu, Y.; Cui, Y.; Gao, Y.; Liu, W. Double Perovskite La2MnNiO6 as a High-Performance Anode for Lithium-Ion Batteries. Adv. Sci. 2023, 10, 2300506. [Google Scholar] [CrossRef]
- Levitas, B.; Piligian, S.; Ireland, T.; Gopalan, S. Elucidating the influence of molten salt chemistries on the synthesis and stability of perovskites oxides. RSC Adv. 2021, 11, 29156–29163. [Google Scholar] [CrossRef]
- Wlodarczyk, D.; Amilusik, M.; Kosyl, K.M.; Chrunik, M.; Lawniczak-Jablonska, K.; Strankowski, M.; Zajac, M.; Tsiumra, V.; Grochot, A.; Reszka, A.; et al. Synthesis Attempt and Structural Studies of Novel A2CeWO6 Double Perovskites (A2+ = Ba, Ca) in and outside of Ambient Conditions. ACS Omega 2022, 7, 18382–18408. [Google Scholar] [CrossRef] [PubMed]
- Zouridi, L.; Vourros, A.; Garagounis, I.; Marnellos, G.E.; Binas, V. Solvent-free direct salt precursor mechanochemical synthesis of La0.5Sr0.5Ti0.5Mn0.5O3-δ oxide perovskite and its electrocatalytic behavior as oxygen electrode for solid oxide cells. J. Solid State Chem. 2023, 328, 124293. [Google Scholar] [CrossRef]
- Zouridi, L.; Totnios, D.; Papoutsakis, L.; Daskalos, E.; Karagiannakis, G.; Marnellos, G.E.; Binas, V. Direct Salt Precursor Mechanochemical Synthesis for La1– xSrxTi1– yMnyO3±δ Perovskite Nanomaterials as Solid Oxide Oxygen Electrodes. ACS Appl. Nano Mater. 2025, 8, 3389–3401. [Google Scholar] [CrossRef]
- Siemek, K.; Olejniczak, A.; Konieczny, P.; Perzanowski, M. Defect engineering in Ba-doped SrTiO3 ball-milled particles. Appl. Surf. Sci. 2025, 710, 163843. [Google Scholar] [CrossRef]
- Phuyal, D.; Mukherjee, S.; Panda, S.K.; Man, G.J.; Simonov, K.; Simonelli, L.; Butorin, S.M.; Rensmo, H.; Karis, O. Nonlocal Interactions in the Double Perovskite Sr2FeMoO6 from Core-Level X-ray Spectroscopy. J. Phys. Chem. C 2021, 125, 11249–11256. [Google Scholar] [CrossRef]
- Alvarado-Flores, J.J.; Mondragón-Sánchez, R.; Ávalos-Rodríguez, M.L.; Alcaraz-Vera, J.V.; Rutiaga-Quiñones, J.G.; Guevara-Martínez, S.J. Synthesis, characterization and kinetic study of the Sr2FeMoO6-δ double perovskite: New findings on the calcination of one of its precursors. Int. J. Hydrogen Energy 2021, 46, 26185–26196. [Google Scholar] [CrossRef]
- Nadig, P.R.; Murari, M.S.; Daivajna, M.D. Influence of heat sintering on the physical properties of bulk La0.67Ca0.33 MnO3 perovskite manganite: Role of oxygen in tuning the magnetocaloric response. Phys. Chem. Chem. Phys. 2024, 26, 5237–5252. [Google Scholar] [CrossRef]
- Hosen, M.J.; Basith, M.A.; Syed, I.M. Structural, magnetic and optical properties of disordered double perovskite Gd2CoCrO6 nanoparticles. RSC Adv. 2023, 13, 17545–17555. [Google Scholar] [CrossRef]
- Islam, M.A.; Sato, T.; Ara, F.; Basith, M.A. Sol-gel based synthesis to explore structure, magnetic and optical properties of double perovskite Y2FeCrO6 nanoparticles. J. Alloys Compd. 2023, 94, 169066. [Google Scholar] [CrossRef]
- Boudad, L.; Taibi, M.; Belayachi, A.; Abd-Lefdil, M. Sol-gel synthesis and characterization of novel double perovskites RBaFeTiO6 (R= Pr, Nd). Ceram. Int. 2022, 48, 6087–6096. [Google Scholar] [CrossRef]
- Zhao, X.; Ge, W.; Gu, J.; Tang, Q.; Wu, Z.; Yi, K.; Zhu, X. Effects of synthesis methods on the structural and magnetic properties of double perovskite Sr2CrReO6 oxide powders. J. Alloys Compd. 2023, 952, 170004. [Google Scholar] [CrossRef]
- Valdés, J.; Reséndiz, D.; Cuán, Á.; Nava, R.; Aguilar, B.; Cortés-Romero, C.M.; Navarro, O. Sol-Gel Synthesis of the Double Perovskite Sr2FeMoO6 by Microwave Technique. Materials 2021, 14, 3876. [Google Scholar] [CrossRef]
- Bartoletti, A.; Gondolini, A.; Sangiorgi, N.; Aramini, M.; Ardit, M.; Rancan, M.; Armelao, L.; Kondrat, S.A.; Sanson, A. Identification of structural changes in CaCu3Ti4O12 on high energy ball milling and their effect on photocatalytic performance. Catal. Sci. Technol. 2023, 13, 1041–1058. [Google Scholar] [CrossRef]
- Xu, Z.; Feng, Z.; Xu, Y. Preparation and characterization of R2CoMnO6 (R=La, Nd) via PVA sol-gel route. J. Asian Ceram. Soc. 2021, 9, 119–127. [Google Scholar] [CrossRef]
- Yousif, N.M.; Makram, N.; Wahab, L.A. Structural, dielectric, and magnetic properties of LaCo0.2Mn0.8O3 and La2CoMnO6 perovskite materials. J. Sol-Gel Sci. Technol. 2021, 98, 238–251. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, H.A.; Jurado, J.F.; Herrera-Pérez, G.; Espinoza-Magana, F.; Reyes-Rojas, A. Enhancing Pr1-xBaxMnO3-δ perovskite charge-transport by electronic structure modulation. J. Mater. Sci. 2021, 56, 16510–16523. [Google Scholar] [CrossRef]
- Dara, M.; Hassanpour, M.; Alshamsi, H.A.; Baladi, M.; Salavati-Niasari, M. Green sol-gel auto combustion synthesis and characterization of double perovskite Tb2ZnMnO6 nanoparticles and a brief study of photocatalytic activity. RSC Adv. 2021, 11, 8228–8238. [Google Scholar] [CrossRef]
- Dara, M.; Hassanpour, M.; Amiri, O.; Baladi, M.; Salavati-Niasari, M. Sol–gel auto combustion synthesis, characterization, and application of Tb2FeMnO6 nanostructures as an effective photocatalyst for the discoloration of organic dye contaminants in wastewater. RSC Adv. 2021, 11, 26844–26854. [Google Scholar] [CrossRef]
- Oroumi, G.; Monsef, R.; Dawi, E.A.; Aljeboree, A.M.; Alubiady, M.H.S.; Al-Ani, A.M.; Salavati-Niasari, M. Achieving new insights on rational design and application of double perovskite Y2CrMnO6 nanostructures as potential materials for electrochemical hydrogen storage performance. J. Energy Storage 2024, 85, 111161. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Abdelrahman, E.A. Simple Synthesis and Characterization of Cobalt Ferrite Nanoparticles for the Successful Adsorption of Indigo Carmine Dye from Aqueous Media. Inorganics 2023, 11, 453. [Google Scholar] [CrossRef]
- Guamán-Ayala, M.; Tuza, P.V.; Souza, M.M.V.M. Cation reducibility of LaNi0.5Ti0.5O3, LaNi0.5Ti0.45Co0.05O3, and LaNi0.45Co0.05Ti0.5O3 perovskites from X-ray powder diffraction data using the Rietveld method. Powder Diffr. 2022, 37, 84–90. [Google Scholar] [CrossRef]
- Swadchaipong, N.; Tongnan, V.; Maneesard, P.; Hartley, M.; Li, K.; Ampairojanawong, R.; Makdee, A.; Hartley, U.W.; Sereewatthanawut, I. Study of nitrous oxide utilization for syngas production via partial oxidation of methane using Ni-doped perovskite catalysts. RSC Adv. 2025, 15, 3080–3088. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Flores, J.C.; Castro-García, M.; Crespo-Muñoz, V.; Valera-Jiménez, J.F.; García-Alvarado, F.; Canales-Vázquez, J. Analysis of performance losses and degradation mechanism in porous La2−xNiTiO6−δ:YSZ electrodes. Materials 2021, 14, 2819. [Google Scholar] [CrossRef]
- Zhao, Z.; Zou, M.; Huang, H.; Zhai, X.; Wofford, H.; Tong, J. Insight of BaCe0.5Fe0.5O3−δ twin perovskite oxide composite for solid oxide electrochemical cells. J. Am. Ceram. Soc. 2023, 106, 186–200. [Google Scholar] [CrossRef]
- Zhai, X.; Ding, F.; Zhao, Z.; Santomauro, A.; Luo, F.; Tong, J. Predicting the formation of fractionally doped perovskite oxides by a function-confined machine learning method. Commun. Mater. 2022, 3, 42. [Google Scholar] [CrossRef]
- Cheraghi, A.; Davar, F.; Homayoonfal, M.; Hojjati-Najafabadi, A. Effect of lemon juice on microstructure, phase changes, and magnetic performance of CoFe2O4 nanoparticles and their use on release of anti-cancer drugs. Ceram. Int. 2021, 47, 20210–20219. [Google Scholar] [CrossRef]
- Al-Farraj, E.S.; Khairy, M.; Saad, F.A.; Shah, R.K.; Abdelrahman, E.A. Efficient Photocatalytic Decomposition of Acid Blue 25 Dye using Facilely Synthesized Magnesium Aluminate Nanoparticles. Water Conserv. Sci. Eng. 2024, 9, 3. [Google Scholar] [CrossRef]
- dos Santos, T.V.; Pryston, D.B.A.; Assis, G.C.; Meneghetti, M.R.; Meneghetti, S.M.P. Tin, Niobium and Tin-Niobium oxides obtained by the Pechini method using glycerol as a polyol: Synthesis, characterization and use as a catalyst in fructose conversion. Catal. Today 2021, 379, 62–69. [Google Scholar] [CrossRef]
- Bataliotti, M.D.; Costa, F.B.; Minussi, F.B.; Araújo, E.B.; de Lima, N.B.; Moraes, J.C.S. Characterization of tellurium dioxide thin films obtained through the Pechini method. J. Sol-Gel Sci. Technol. 2022, 103, 378–385. [Google Scholar] [CrossRef]
- Sharmili, T.; Preethi, A.J.; Ragam, M. Comprehensive study on double perovskite La2NiMnO6 structures synthesized via hydrothermal and sol-gel methods for energy storage applications. Mater. Today Commun. 2025, 48, 113315. [Google Scholar] [CrossRef]
- Wu, Z.; Lv, C.; Fang, J.; Wang, Y.; Jin, X.; Ge, J. Cobalt-doping-controlled synthesis of CoMnO3 as a novel electrode material for supercapacitors. Inorg. Chem. Commun. 2025, 180, 115005. [Google Scholar] [CrossRef]
- Priya, L.S.; Dhanemozhi, A.C.; Mayandi, J.; Pratha Govindaraj, B.S.; Somu, A.R. Structural, optical, morphological and electrochemical properties of dual-shaped SrTiO3 nanostructures with enhanced photocatalytic and supercapacitor performances. J. Indian Chem. Soc. 2025, 102, 101819. [Google Scholar] [CrossRef]
- Yi, K.; Wu, Z.; Tang, Q.; Gu, J.; Ding, J.; Chen, L.; Zhu, X. Microstructural Characterization and Magnetic, Dielectric, and Transport Properties of Hydrothermal La2FeCrO6 Double Perovskites. Nanomaterials 2023, 13, 3132. [Google Scholar] [CrossRef]
- Irfan, M.; Murtaza, G.; Muhammad, N.; Tahir, S.; Raza, H.H.; Sabir, B.; Iftikhar, M.; Sharif, S. Experimental and theoretical studies of structural, electronic and magnetic properties of RE2NiCrO6 (RE = Ce, Pr and Nd) double perovskites. Phys. E 2023, 148, 115635. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Yuan, L.; Ti, R.; Wu, H.; Yuan, H. Double perovskites R2NiMnO6 with small R3+ cations: Magnetic interactions tuned by R3+ ionic radius and the role of orbital ordering. J. Alloys Compd. 2025, 1039, 182997. [Google Scholar] [CrossRef]
- McGuire, S.C.; Wesley, W.; Sasaki, K.; Tong, X.; Wong, S.S. Yttrium-based Double Perovskite Nanorods for Electrocatalysis. ACS Appl. Mater. Interfaces 2022, 14, 30914–30926. [Google Scholar] [CrossRef]
- Sharma, A.; Bhardwaj, U.; Kushwaha, H.S. Efficacious visible-light photocatalytic degradation of toxics by using Sr2TiMnO6-rGO composite for the wastewater treatment. Clean. Eng. Technol. 2021, 2, 100087. [Google Scholar] [CrossRef]
- Khan, R.; Mehran, M.T.; Baig, M.M.; Sarfraz, B.; Naqvi, S.R.; K. Niazi, M.B.; Khan, M.Z.; Khoja, A.H. 3D hierarchical heterostructured LSTN@NiMn-layered double hydroxide as a bifunctional water splitting electrocatalyst for hydrogen production. Fuel 2021, 285, 119174. [Google Scholar] [CrossRef]
- Popov, D.V.; Batulin, R.G.; Cherosov, M.A.; Yatsyk, I.V.; Chupakhina, T.I.; Deeva, Y.A.; Makarchenko, A.S.; Fazlizhanova, D.I.; Shustov, V.A.; Eremina, R.M.; et al. Intermediate-spin state of Co ions in magnetic and thermoelectric properties of double perovskite Ba2CoNbO6. J. Alloys Compd. 2024, 1009, 176900. [Google Scholar] [CrossRef]
- Popov, D.V.; Batulin, R.G.; Cherosov, M.A.; Yatsyk, I.V.; Chupakhina, T.I.; Deeva, Y.A.; Eremina, R.M.; Maiti, T. Magnetic properties of the double perovskite Sr2CoNbO6-δ. Magn. Reson. Solids 2023, 25, 23301. [Google Scholar] [CrossRef]
- Jin, Z.; Xu, C.; Zhou, T.; Hu, J.; Hu, R.; Meng, H.; Shen, J.; Yang, M.; Motkuri, R.K. High surface area magnetic double perovskite La2AlFeO6 as an efficient and stable photo-Fenton catalyst under a wide pH range. Appl. Surf. Sci. 2022, 574, 151554. [Google Scholar] [CrossRef]
- Popov, D.V.; Batulin, R.G.; Cherosov, M.A.; Chupakhina, T.I.; Deeva, Y.A.; Shustov, V.A.; Agafonov, S.S.; Kolkov, M.I.; Kurbakov, A.I.; Eremina, R.M.; et al. Magnetic phase separation in double perovskite Sr2MnNbO6-δ. Mater. Chem. Phys. 2025, 345, 131139. [Google Scholar] [CrossRef]
- Chahla, S.; Gagou, Y.; El Marssi, M.; Chaker, H.; Ben Hassen, R. An analysis of the crystal structure, vibrational properties, and optoelectronic behavior of NdCaFeSnO6. RSC Adv. 2025, 15, 22843–22862. [Google Scholar] [CrossRef]
- Fabrelli, H.; Silva, A.G.; Boldrin, M.; Bufaiçal, L.; Bittar, E.M. Structural transitions and spontaneous exchange bias in La2−xBaxCoMnO6 series. J. Solid State Chem. 2023, 322, 123944. [Google Scholar] [CrossRef]
- Zaraq, A.; Orayech, B.; Igartua, J.M.; El Bouari, A.; Gregory, D.H.; Gesing, T.M. Crystallography at non-ambient conditions and physical properties of the synthesized double perovskites, Sr2(Co1−xFex)TeO6. Dalton Trans. 2023, 52, 4086–4102. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríquez-Carvajal, J. WinPLOTR: A Windows Tool for Powder Diffraction Pattern Analysis. In Proceedings of the Seventh European Powder Diffraction Conference (EPDIC 7), Barcelona, Spain, 20–23 May 2020. [Google Scholar]
- Graversen, L.G.; Juelsholt, M.; Aalling-Frederiksen, O.; Friis-Jensen, U.; Pittkowski, R.K.; Thomsen, M.S.; Kirsch, A.; Magnard, N.P.L.; Jensen, K.M.Ø. Mechanistic insights into solvent-guided growth and structure of MoO2 nanoparticles in solvothermal synthesis. Chem. Sci. 2025, 16, 14350–14365. [Google Scholar] [CrossRef]
- Souza, M.M.V.M.; Maza, A.; Tuza, P.V. X-ray powder diffraction data of LaNi0.5Ti0.45Co0.05O3, LaNi0.45Co0.05Ti0.5O3, and LaNi0.5Ti0.5O3 perovskites. Powder Diffr. 2021, 36, 29–34. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. An Introduction to the Program Fullprof 2000 (Version July 2001); Laboratoire Léon Brillouin (CEA-CNRS): Saclay, France, 2001. [Google Scholar]
- Silva, R.S.; Gainza, J.; dos Santos, C.; Rodrigues, J.E.F.S.; Dejoie, C.; Huttel, Y.; Biskup, N.; Nemes, N.M.; Martínez, J.L.; Ferreira, N.S.; et al. Magnetoelastic Coupling and Cryogenic Magnetocaloric Effect in Two-Site Disordered GdSrCoFeO6 Double Perovskite. Chem. Mater. 2023, 35, 2439–2455. [Google Scholar]
- Binns, J.; Darmanin, C.; Kewish, C.M.; Pathirannahalge, S.K.; Berntsen, P.; Adams, P.L.R.; Paporakis, S.; Wells, D.; Roque, F.G.; Abbey, B.; et al. Preferred orientation and its effects on intensity-correlation measurements. IUCrJ 2022, 9, 231–242. [Google Scholar] [CrossRef]
- Yotsumoto, Y.; Nakajima, Y.; Takamoto, R.; Takeichi, Y.; Ono, K. Autonomous robotic experimentation system for powder X-ray diffraction. Digit. Discov. 2024, 3, 2523–2532. [Google Scholar] [CrossRef]
- Bhuyan, M.D.I.; Das, S.; Basith, M.A. Sol-gel synthesized double perovskite Gd2FeCrO6 nanoparticles: Structural, magnetic and optical properties. J. Alloys Compd. 2021, 878, 160389. [Google Scholar] [CrossRef]
- Guo, J.; Cai, R.; Cali, E.; Wilson, G.E.; Kerherve, G.; Haigh, S.J.; Skinner, S.J. Low-Temperature Exsolution of Ni–Ru Bimetallic Nanoparticles from A-Site Deficient Double Perovskites. Small 2022, 18, 2107020. [Google Scholar] [CrossRef]
- El Hachmi, A.; Sadoune, Z. Synthesis and structural characterization of new perovskite phases, Ba2Bi0.572TeO6±δ and SrLa2NiFeNbO9. Powder Diffr. 2024, 39, 235–244. [Google Scholar] [CrossRef]
- Fullprof Suite. Fullprof News—2002. 2002. Available online: https://www.ill.eu/sites/fullprof/Old_FP/php/Fullprof_News_2002.htm (accessed on 8 September 2025).
- Rodríguez-Carvajal, J. Rietveld Refinement of Complex Inorganic Materials Using FullProf; Laboratoire Léon Brillouin (CEA-CNRS): Saclay, France, 2003; Available online: http://ccp14.cryst.bbk.ac.uk/projects/ecm21-durban2003/juan/ecm21_fullprof_juan.pdf (accessed on 9 September 2025).
- Almadhi, A.; Ji, K.; Injac, S.D.; Ritter, C.; Attfield, J.P. (Ca0.5Mn0.5)2MnTeO6—An Anomalously Stable High-Pressure Double Perovskite. Chem Asian J. 2024, 19, e202400280. [Google Scholar] [CrossRef]
- Shibuya, K.; Hirai, D.; Takenaka, K. Chemical pressure tuning of multipolar and magnetic orders in Ba2(Cd1-xCax)ReO6 double perovskites. Phys. Rev. Mater. 2025, 9, 034406. [Google Scholar] [CrossRef]
- Agarwal, C.; Verma, J.K.; Bano, T.; Kumawat, S.; Gora, M.K.; Kumar, A.; Chandra, S.; Gautam, Y.K.; Kumar, S. Effect of Cr Substitution for Co on the Structural, Optical, and Magnetic Properties of La2CoMnO6 Double Perovskite Nanomaterials: A Facile Auto-Combustion Sol-Gel Approach. ECS J. Solid State Sci. Technol. 2025, 14, 043012. [Google Scholar] [CrossRef]
- Macchiutti, C.; Jesus, J.R.; Carneiro, F.B.; Bufaiçal, L.; Klein, R.A.; Zhang, Q.; Kirkham, M.; Brown, C.M.; dos Reis, R.D.; Perez, G.; et al. Tuning the spontaneous exchange bias effect in La1.5Sr0.5CoMnO6 with sintering temperature. Phys. Rev. Mater. 2024, 8, 044408. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, W.; Xie, L.; Zhao, H.; Li, Q. Tunable exchange bias in La1.5Sr0.5CoMnO6 double perovskite doped with nonmagnetic Ga ions. Curr. Appl. Phys. 2022, 35, 58–66. [Google Scholar] [CrossRef]
- Fourmont, P.; Cho, E.; Cloutier, S.G.; Ross, C.A. Exchange Bias in La0.67Sr0.33MnO3/YFeO3 Ferromagnet/Antiferromagnet Multilayer Heterostructures. Small 2025, 21, 2501644. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, Z.; Chen, H.; Liang, Y.; Jiang, X.; Zhang, Y.; Ma, J.; Zhu, F.; Nan, T.; Chen, Z.; et al. Enhanced exchange bias in all-oxide heterostructures with cation-ordered ferrimagnetic double-perovskite. Npj Spintron. 2024, 2, 44. [Google Scholar] [CrossRef]
- Pal, K.; Mandal, S.; Dey, S.; Pradhan, K.; Das, I. Unraveling the origins of exchange bias and magnetic anomalies in Sr2FeRuO6: Experimental and theoretical insights. Mater. Today Commun. 2025, 46, 112814. [Google Scholar] [CrossRef]
- Deng, Z.; Wang, X.; Wang, M.; Shen, F.; Zhang, J.; Chen, Y.; Feng, H.L.; Xu, J.; Peng, Y.; Li, W.; et al. Giant Exchange-Bias-Like Effect at Low Cooling Fields Induced by Pinned Magnetic Domains in Y2NiIrO6 Double Perovskite. Adv. Mater. 2023, 35, 2370120. [Google Scholar] [CrossRef]
- Kush, L.; Srivastava, S. Observation of Giant Zero-Field Cooled Spontaneous Exchange Bias Effect in La2FeCoO6 Double Perovskite Ceramic. J. Supercond. Nov. Magn. 2025, 38, 82. [Google Scholar] [CrossRef]
- Datta, R.; Mondal, S.; Mondal, S.; Kalyan Pradhan, S.; Majumdar, S.; Kumar De, S. Evolution of structural, magnetic and transport properties of 3d-5d based double perovskites Nd2–xSrxCoIrO6. J. Phys. Condens. Matter 2025, 37, 065801. [Google Scholar] [CrossRef]
- Mahalle, P.; Kumar, A.; Cuello, G.J.; da Silva, I.; Krzystyniak, M.; Yusuf, S.M. Investigating the genesis of negative magnetization, exchange bias, and electrical properties in Gd2CuRuO6. Phys. Rev. Mater. 2025, 9, 104407. [Google Scholar] [CrossRef]
- Li, S.Z.; Rahman, A.; Ma, C.L.; Zhao, X.; Sun, Z.Y.; Liu, M.F.; Wang, X.Z.; Xu, X.F.; Liu, J.M. Exchange bias effect in polycrystalline Bi0.5Sr0.5Fe0.5Cr0.5O3 bulk. Sci. Rep. 2023, 13, 6333. [Google Scholar] [CrossRef]
- Patel, R.K.; Chikara, K.S.; Hossain, S.M.; Majumder, M.; Nath, C.; Yusuf, S.M.; Saravanan, M.P.; Bera, A.K.; Pramanik, A.K. Crystal structure, magnetic, and magnetostructural correlations in double perovskite antiferromagnets Sr2NiMo1−xWxO6 (0≤x≤1). Phys. Rev. Mater. 2025, 9, 044406. [Google Scholar] [CrossRef]
- Mohanty, S.; Satapathy, S.; Nayak, M.; Rai, S.; Singh, R.; Behera, S. Effect of low Ni- substitution on optical, dielectric and magnetic properties of double perovskite Mg2FeNbO6. Inorg. Chem. Commun. 2024, 165, 112513. [Google Scholar] [CrossRef]
- Sahoo, S.; Sahoo, L.; Nayak, N.C.; Parida, B.N.; Parida, R.K. Investigation of the structural, dielectric, magnetic properties and NTC-thermistor response of CaBiFeMnO6 double perovskites. Mater. Adv. 2024, 5, 5442–5457. [Google Scholar] [CrossRef]
- Wu, Z.; Yi, K.; Tang, Q.; Gu, J.; Ding, J.; Chen, L.; Zhu, X. Microstructures and physical properties of sol–gel derived Ba2FeMnO6 double perovskite. J. Am. Ceram. Soc. 2023, 106, 3663–3675. [Google Scholar] [CrossRef]
- Zaraq, A.; Gregory, D.H.; Orayech, B.; Igartua, J.M.; El Bouari, A.; Eales, J.D.; Bingham, P.A.; Gesing, T.M. Effects of iron substitution and anti-site disorder on crystal structures, vibrational, optical and magnetic properties of double perovskites Sr2(Fe1−xNix)TeO6. Dalton Trans. 2022, 51, 17368–17380. [Google Scholar] [CrossRef] [PubMed]
- García-Ruíz, D.L.; Aguilar, B.; Navarro, O.; de la Torre Medina, J.; Soto, T.E.; Cervantes-Solano, M. Effects of the electron-doping on the structural and magnetic properties of the Sr2−xCexFe1+x/2Mo1−x/2O6 double perovskite. Solid State Commun. 2025, 404, 116046. [Google Scholar] [CrossRef]
- Punitha, J.S.; Raji, R.K.; Ramachandran, T.; Kumar, K.S.; Dhilip, M.; Hamed, F.; Nataraj, A. Influence of Pr3+ substitution on the structural, optical, magnetic, and dielectric properties of Sr2FeTiO6−δ double perovskites. Solid State Sci. 2025, 160, 107825. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ: Image Processing and Analysis and Java. Available online: https://imagej.net/ij/ (accessed on 22 August 2025).
- Dara, M.; Hassanpour, M.; Amiri, O.; Baladi, M.; Salavati-Niasari, M. Tb2CoMnO6 double perovskites nanoparticles as photocatalyst for the degradation of organic dyes: Synthesis and characterization. Arab. J. Chem. 2021, 14, 103349. [Google Scholar] [CrossRef]
- Nartova, A.V.; Mashukov, M.Y.; Astakhov, R.R.; Kudinov, V.Y.; Matveev, A.V.; Okunev, A.G. Particle Recognition on Transmission Electron Microscopy Images Using Computer Vision and Deep Learning for Catalytic Applications. Catalysts 2022, 12, 135. [Google Scholar] [CrossRef]
- Rahimkhoei, V.; Salavati-Niasari, M.; Alsultany, F.H.; Aljeboree, A.M.; Hamadanian, M. Exploration of electrochemical energy storage potential of MWCNT scaffolds functionalized with Lu2FeMnO6 synthesized via a facile sol–gel Pechini chemical method. Appl. Water Sci. 2025, 15, 126. [Google Scholar] [CrossRef]
- Sha, Z.; Shen, Z.; Calì, E.; Kilner, J.A.; Skinner, S.J. Understanding surface chemical processes in perovskite oxide electrodes. J. Mater. Chem. A 2023, 11, 5645–5659. [Google Scholar] [CrossRef]
- Wlodarczyk, D.; Amilusik, M.; Kosyl, K.M.; Chrunik, M.; Lawniczak-Jablonska, K.; Przybylinska, H.; Kosmela, P.; Strankowski, M.; Bulyk, L.-I.; Tsiumra, V.; et al. Synthesis and Properties of the Ba2PrWO6 Double Perovskite. Inorg. Chem. 2024, 63, 10194–10206. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Gondal, M.A.; Khan, J.A.; Mustaqeem, M.; Almessiere, M.A.; Baykal, A. Optimizing La2MnXO6 Double Perovskite for Superior Electrochemical Efficiency in Supercapacitors. Energy Storage 2025, 7, e70123. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Parida, R.K.; Parida, B.N. Investigation of multifunction features of double perovskite oxide A2FeVO6 (where A = Ba, Ca). Phys. B 2023, 659, 414849. [Google Scholar] [CrossRef]
- Raji, R.K.; Ramachandran, T.; Dhilip, M.; Aravindan, V.; Punitha, J.S.; Hamed, F. Integrating Experimental and Computational Insights: A Dual Approach to Ba2CoWO6 Double Perovskites. Ceramics 2024, 7, 2006–2023. [Google Scholar] [CrossRef]
- Tang, Q.; Zhu, X. Structural Characterization and Physical Properties of Double Perovskite La2FeReO6+δ Powders. Nanomaterials 2022, 12, 244. [Google Scholar] [CrossRef]
- Islam, Z.U.; Want, B. Structural, optical, and dielectric properties of double perovskite NdBaFeTiO6. Ceram. Int. 2025, 51, 1585–1594. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Ehsani, M.H. Synthesis and magnetic properties of Ba2-xSmxFeMoO6 (0.0 ≤ x ≤ 0.1) Double perovskite. Ceram. Int. 2023, 49, 27362–27372. [Google Scholar] [CrossRef]
- Kumar, P.; Tripathi, A.; Verma, H.; Mittal, A.; Bhattacharya, B.; Upadhyay, S. Cation deficiency driven enhancement in electrochemical performance of Sr2-xFeCoO6-δ double perovskite for supercapacitor electrodes. Electrochim. Acta 2025, 538, 146992. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Ehsani, M.H. Double perovskite oxides Ba2-xAgxFeMoO6 (x = 0.0, 0.025, 0.05). Phys. B 2024, 682, 415867. [Google Scholar] [CrossRef]
- Yi, K.; Tang, Q.; Wu, Z.; Gu, J.; Zhu, X. Structural, magnetic, and electrical transport properties of half-metallic double perovskite La2CrNiO6 oxides. J. Alloys Compd. 2023, 933, 167742. [Google Scholar] [CrossRef]
- Solanki, N.; Choudhary, R.J.; Kaurav, N. Qualitative study of structural phase transition in nickel doped La2CoTi(1−x)NixO6 double perovskite. J. Alloys Compd. 2023, 943, 169126. [Google Scholar] [CrossRef]
- Roozbahania, H.; Maghsoodia, S.; Raeia, B.; Kootenaeia, A.S.; Azizi, Z. MgO support mediated enhancement of La2BMnO6 (B = Co, Ni) perovskite oxide in catalytic combustion of propane. Iran. J. Catal. 2023, 2, 211–222. [Google Scholar]
- Winterstein, T.F.; Malleier, C.; Mohammadi, A.; Krüger, H.; Kahlenberg, V.; Venter, A.M.; Bekheet, M.F.; Müller, J.T.; Gurlo, A.; Heggen, M.; et al. Lanthanum nickel titanate perovskites as model systems for Ni-perovskite interfacial engineering in methane dry reforming. Mater. Today Chem. 2025, 45, 102620. [Google Scholar] [CrossRef]
- Morimura, A.; Yamada, I. Pressure-Induced Order-Disorder Transition in the Double Perovskite Oxide La2CoRuO6. Mater. Trans. 2023, 64, 2093–2096. [Google Scholar] [CrossRef]
- Rodrigues, J.E.F.S.; Gainza, J.; Serrano-Sánchez, F.; Silva, R.S.; Dejoie, C.; Nemes, N.M.; Dura, O.J.; Martínez, J.L.; Alonso, J.A. Thermal Expansion and Rattling Behavior of Gd-Filled Co4Sb12 Skutterudite Determined by High-Resolution Synchrotron X-ray Diffraction. Materials 2022, 16, 370. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, X.; Shen, X.; Sahle, C.J.; Dong, C.; Hojo, H.; Sakai, Y.; Zhang, J.; Li, W.; Duan, L.; et al. Magnetic Ordering and Structural Transition in the Ordered Double-Perovskite Pb2NiMoO6. Chem. Mater. 2022, 34, 97–106. [Google Scholar] [CrossRef]
- Liang, X.; Yamaura, K.; Tsirlin, A.A.; Belik, A.A. Ca2CuWO6: A triclinically distorted double perovskite with low-dimensional magnetic behavior. Phys. Rev. B 2025, 111, 094432. [Google Scholar] [CrossRef]
- da Cruz Pinha Barbosa, V.; Maharaj, D.D.; Cronkright, Z.W.; Wang, Y.; Cong, R.; Garcia, E.; Reyes, A.P.; Yan, J.; Ritter, C.; Mitrović, V.F.; et al. Exploring the Links between Structural Distortions, Orbital Ordering, and Multipolar Magnetic Ordering in Double Perovskites Containing Re(VI) and Os(VII). Chem. Mater. 2024, 36, 11478–11489. [Google Scholar] [CrossRef]
- Silva, R.S.; Rodrigues, J.E.; Gainza, J.; Serrano-Sánchez, F.; Martínez, L.; Huttel, Y.; Martínez, J.L.; Alonso, J.A. Magnetoelastic Coupling Evidence by Anisotropic Crossed Thermal Expansion in Magnetocaloric RSrCoFeO6 (R = Sm, Eu) Double Perovskites. Inorg. Chem. 2024, 63, 7007–7018. [Google Scholar] [CrossRef]
- Artini, C.; Massardo, S.; Carnasciali, M.M.; Joseph, B.; Pani, M. Evaluation of the Defect Cluster Content in Singly and Doubly Doped Ceria through In Situ High-Pressure X-ray Diffraction. Inorg. Chem. 2021, 60, 7306–7314. [Google Scholar] [CrossRef]
- Sharma, S.; Ritter, C.; Adroja, D.T.; Stenning, G.B.; Sundaresan, A.; Langridge, S. Magnetic structure of the double perovskite La2NiIrO6 investigated using neutron diffraction. Phys. Rev. Mater. 2022, 6, 014407. [Google Scholar] [CrossRef]
- Naveen, K.; Rom, T.; Islam, S.S.; Reehuis, M.; Adler, P.; Felser, C.; Hoser, A.; Nath, R.C.; Yadav, A.K.; Jha, S.N.; et al. Evolution of transition metal charge states in correlation with the structural and magnetic properties in disordered double perovskites Ca2− xLaxFeRuO6 (0.5 ≤ x ≤ 2). Phys. Chem. Chem. Phys. 2021, 23, 21769–21783. [Google Scholar] [CrossRef]
- Ji, K.; Chen, R.; Manuel, P.; Attfield, J.P. Coexisting commensurate and incommensurate magnetic orders in the double double perovskite CaMnCoWO6. Z. Anorg. Allg. Chem. 2023, 649, e202200084. [Google Scholar] [CrossRef]
- Kearins, P.; Solana-Madruga, E.; Ji, K.; Ritter, C.; Attfield, J.P. Cluster Spin Glass Formation in the Double Double Perovskite CaMnFeTaO6. J. Phys. Chem. C 2021, 125, 9550–9555. [Google Scholar] [CrossRef]
- Dhawan, R.; Balasubramanian, P.; Nautiyal, T. Origins of multi-sublattice magnetism and superexchange interactions in double–double perovskite CaMnCrSbO6. J. Phys.:Condens. Matter 2024, 36, 305801. [Google Scholar] [CrossRef]
- Ji, K.; Alharbi, K.N.; Solana-Madruga, E.; Moyo, G.T.; Ritter, C.; Attfield, J.P. Double Double to Double Perovskite Transformations in Quaternary Manganese Oxides. Angew. Chem. Int. Ed. 2021, 60, 22248–22252. [Google Scholar] [CrossRef]
- Mustonen, O.H.J.; Fogh, E.; Paddison, J.A.M.; Mangin-Thro, L.; Hansen, T.; Playford, H.Y.; Diaz-Lopez, M.; Babkevich, P.; Vasala, S.; Karppinen, M.; et al. Structure, Spin Correlations, and Magnetism of the S = 1/2 Square-Lattice Antiferromagnet Sr2CuTe1– xWx O6 (0 ≤ x ≤ 1). Chem. Mater. 2024, 36, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Harsita, M.; Kodam, U.; Mishra, S.K.; Rao, T.D.; Borole, S.; Rayaprol, S.; Krishna, P.S.R.; Sattibabu, B. Combined structural refinement using x-ray and neutron diffraction and magnetic properties of double perovskites La2−xErxNiMnO6. Mater. Lett. 2025, 391, 138481. [Google Scholar] [CrossRef]
- Pal, A.; Anand, K.; Patel, N.; Das, A.; Ghosh, S.; Yen, P.T.-W.; Huang, S.-M.; Singh, R.K.; Yang, H.D.; Ghosh, A.K.; et al. Interplay of spin, phonon, and lattice degrees in a hole-doped double perovskite: Observation of spin–phonon coupling and magnetostriction effect. J. Appl. Phys. 2022, 132, 223906. [Google Scholar] [CrossRef]
- Solana-Madruga, E.; Kearins, P.S.; Ritter, C.; Arévalo-López, Á.M.; Attfield, J.P. 1:1 Ca2+:Cu2+ A-site Order in a Ferrimagnetic Double Double Perovskite. Angew. Chem. 2022, 134, e202209497. [Google Scholar] [CrossRef]
- Jin, L.; Ni, D.; Gui, X.; Straus, D.B.; Zhang, Q.; Cava, R.J. Ferromagnetic Double Perovskite Semiconductors with Tunable Properties. Adv. Sci. 2022, 9, 2104319. [Google Scholar] [CrossRef]
- López, C.A.; Singh, P.; Martínez-Coronado, R.; Alonso, J.A. Structural peculiarities of La2Ge1-xCrxMgO6-δ (0< x ≤0.5): A superior oxide-ion electrolyte for low-temperature solid-oxide fuel cells. Int. J. Hydrogen Energy 2023, 48, 12485–12492. [Google Scholar]
- Yatoo, M.A.; Seymour, I.D.; Skinner, S.J. Neutron diffraction and DFT studies of oxygen defect and transport in higher-order Ruddlesden–Popper phase materials. RSC Adv. 2023, 13, 13786–13797. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandham, K.; Annadata, H.; Vinod, K.; Sathyanarayana, A.T.; Pandian, R.; Chakraborty, S.; Goutam, U.K.; Ghosh, B. Investigations of structural, magnetic, and magnetocaloric properties of La2Ni1−xCuxMnO6 double perovskites. Phys. Rev. B 2025, 111, 214402. [Google Scholar] [CrossRef]
- Majumder, S.; Tripathi, M.; Raghunathan, R.; Rajput, P.; Jha, S.N.; de Souza, D.O.; Olivi, L.; Chowdhury, S.; Choudhary, R.J.; Phase, D.M. Mapping the magnetic state as a function of antisite disorder in Sm2NiMnO6 double perovskite thin films. Phys. Rev. B 2022, 105, 024408. [Google Scholar] [CrossRef]
- Kavaliuk, H.; Mielewczyk-Gryń, A.; Gazda, M.; Miruszewski, T.; Gajewska, M.; Wachowski, S.L. High entropy oxides: How many lanthanides can be introduced into Co-based double perovskite oxides? J. Eur. Ceram. Soc. 2025, 45, 117617. [Google Scholar] [CrossRef]
- Murthy, P.S.R.; Salkar, K.; Xec, S.; Zambaulikar, S. Unraveling the role of Mn doping in transforming SrLaLiTeO6 perovskites: Structural, optical, and dielectric insights. J. Mater. Sci. Mater. Electron. 2025, 36, 369. [Google Scholar] [CrossRef]
- Khan, A.; Banerjee, D.; Rawat, D.; Nath, T.K.; Soni, A.; Chatterjee, S.; Taraphder, A. Emergence of spin-phonon coupling in Gd-doped Y2CoMnO6 double perovskite oxide: A combined experimental and ab-initio study. Phys. Chem. Chem. Phys. 2025, 27, 18005–18014. [Google Scholar] [CrossRef]
- Meena, B.R.; Chatterjee, S.; Ghosh, A.K. Insight into charge conduction and relaxation in La2-xCaxFeMnO6 solid solution double perovskites. J. Alloys Compd. 2025, 1037, 182341. [Google Scholar] [CrossRef]
- Silva, A.V.S.; Silva, R.X.; Paschoal, C.W.A.; Paschoal, A.R.; Nonato, A. Dynamics of magnetic inhomogeneity in La2CoMnO6 films probed by Raman spectroscopy. Spectrochim. Acta Part A 2025, 325, 125112. [Google Scholar] [CrossRef]
- Apu, R.S.; Hasan, N.; Haque, R.I.; Kabir, A.; Rashid, M.H. Ferromagnetic double Sr2PrSnO6 perovskite: DFT analysis of physical, optoelectronic, and transport properties for advanced opto-spintronics. AIP Adv. 2025, 15, 035043. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Pan, Z.; Lu, D.; Pi, M.; Ye, X.; Dong, C.; Chen, J.; Chen, K.; Radu, F.; et al. X-ray Absorption Spectroscopic Study of the Transition-Metal-Only Double Perovskite Oxide Mn2CoReO6. J. Phys. Chem. C 2024, 128, 15668–15675. [Google Scholar] [CrossRef]
- Sahoo, L.; Parida, B.N.; Parida, R.K.; Padhee, R.; Mahapatra, A.K. Structural, optical dielectric and ferroelectric properties of double perovskite BaBiFeTiO6. Inorg. Chem. Commun. 2022, 146, 110102. [Google Scholar] [CrossRef]
- Ali, H.S.; Murtaza, G.; Ayyaz, A.; Ismail, K.; Touqir, M.; Rehman, H.U.; Alanazi, Y.M. First-principles predictions of structure, half-metallic antiferromagnetism, optoelectronic, and elastic properties of double perovskites A2TaNiO6 (A = Ca, Sr, and Ba) for energy harvesting. Mater. Sci. Semicond. Process. 2024, 181, 108638. [Google Scholar] [CrossRef]
- Tang, Q.; Zhu, X. Microstructure and physical properties of Sr2CrHfO6 ferrimagnetic double perovskite oxides. J. Am. Ceram. Soc. 2024, 107, 968–978. [Google Scholar] [CrossRef]
- Chatterjee, S.; Das, I. Structural, magnetic, magnetocaloric behavior and magneto-transport, electrical polarization study in 3d based bulk and nano-crystalline multiferroic double perovskite Dy2MnCoO6. J. Phys.: Condens. Matter 2024, 36, 385802. [Google Scholar] [CrossRef]
- Faiza-Rubab, S.; Nazir, S. Interplay between spin–orbital coupling and electron-correlation: Induction of phase transitions and giant magnetic anisotropy in strained LaSr1− xCaxNiReO6. Phys. Chem. Chem. Phys. 2022, 24, 17174–17184. [Google Scholar] [CrossRef]
- Hao, W.; Ji, R.; Zhu, L.; Huang, S.; Zhang, Y. Structural, magnetic and cryogenic magnetocaloric properties in Gd2CrFeO6 ceramic oxide. Solid State Commun. 2025, 399, 115876. [Google Scholar] [CrossRef]
- Verma, S.; Chaurasia, L.; Kumari, S.; Prasad, A.; Rao, A.S. Reddish-Orange emitting thermally stable Sm3+ doped Sr2LaSbO6 phosphor for applications in w-LEDs. J. Photochem. Photobiol. A 2025, 467, 116405. [Google Scholar] [CrossRef]
- Ali, M.L.; Hasan, Z.; Akter, M.S.; Khan, M. Pressure induced physical properties of lead-free double perovskite Ba2GdBiO6 for optoelectronic applications. Comput. Condens. Matter 2025, 44, e01078. [Google Scholar] [CrossRef]
- Rather, M.R.; Bilal, F.; Mushtaq, A.; Parvaiz, A.; Hassan, T.; Ghosh, S.; Sultan, K. Investigating the Multifunctional Role of Sr2FeMnO6 Double Perovskite in Spintronic and Thermoelectric Properties. Phys. Status Solidi B 2025, 262, 2400562. [Google Scholar] [CrossRef]
- Arif, S.; Ali, Y. The electronic, magnetic, and optical properties of double perovskite La2BB′O6 (B = Cr, V and B′ = Co, Ni and Sc). RSC Adv. 2022, 12, 35279–35289. [Google Scholar] [CrossRef]
- Amin, M.A.; Nazir, G.; Mahmood, Q.; Alzahrani, J.; Kattan, N.A.; Mera, A.; Mirza, H.; Mezni, A.; Refat, M.S.; Gobouri, A.A.; et al. Study of double perovskites X2InSbO6 (X = Sr, Ba) for renewable energy; alternative of organic-inorganic perovskites. J. Mater. Res. Technol. 2022, 18, 4403–4412. [Google Scholar] [CrossRef]
- Shereef, A.; Aleena, P.A.; Kunjumon, J.; Jose, A.K.; Thomas, S.A.; Tomy, M.; Xavier, T.S.; Hussain, S.; Sajan, D. Third order nonlinear optical properties and electrochemical performance of La2CoMnO6 double perovskite. Mater. Sci. Eng. B 2023, 289, 116262. [Google Scholar] [CrossRef]
- Alheibshy, F.; Belhachi, S.; Qureshi, M.T.; Hussein, W.; Alqarni, S.; Younes, K.M.; Abdel-Hameed, R.; Iqbal, M.W.; Sillanpää, M. Biomedical applications of double perovskites Sr2TmNbO6 and Sr2NdNbO6: A pathway to innovative healthcare solutions. Talanta 2026, 296, 128440. [Google Scholar] [CrossRef] [PubMed]
- Pattanaik, R.; Pradhan, D.; Dash, S.K. A Brief Review on Solar Light Assisted Photocatalytic Degradation of Dyes using Double/Layered Perovskites. Curr. Nanosci. 2024, 21, 201–217. [Google Scholar] [CrossRef]
- Roudgar-Amoli, M.; Abedini, E.; Alizadeh, A.; Shariatinia, Z. Understanding double perovskite oxides capabilities to improve photocatalytic contaminants degradation performances in water treatment processes: A review. J. Ind. Eng. Chem. 2024, 129, 579–619. [Google Scholar] [CrossRef]
- Mahdi, M.A.; Oroumi, G.; Samimi, F.; Dawi, E.A.; Abed, M.J.; Alzaidy, A.H.; Jasim, L.S.; Salavati-Niasari, M. Tailoring the innovative Lu2CrMnO6 double perovskite nanostructure as an efficient electrode materials for electrochemical hydrogen storage application. J. Energy Storage 2024, 88, 111660. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuza, P.V.; Souza, M.M.V.M. A Review of Synthesis, Characterization, Properties, and Applications of Double Perovskite Oxides. Inorganics 2025, 13, 372. https://doi.org/10.3390/inorganics13110372
Tuza PV, Souza MMVM. A Review of Synthesis, Characterization, Properties, and Applications of Double Perovskite Oxides. Inorganics. 2025; 13(11):372. https://doi.org/10.3390/inorganics13110372
Chicago/Turabian StyleTuza, Pablo V., and Mariana M. V. M. Souza. 2025. "A Review of Synthesis, Characterization, Properties, and Applications of Double Perovskite Oxides" Inorganics 13, no. 11: 372. https://doi.org/10.3390/inorganics13110372
APA StyleTuza, P. V., & Souza, M. M. V. M. (2025). A Review of Synthesis, Characterization, Properties, and Applications of Double Perovskite Oxides. Inorganics, 13(11), 372. https://doi.org/10.3390/inorganics13110372






