Deep Removal of Fluoride Ions from Spent Ternary Lithium-Ion Batteries Leachate Using Porous La@Zr Adsorbent
Abstract
1. Introduction
2. Experiment
2.1. Reagents and Materials
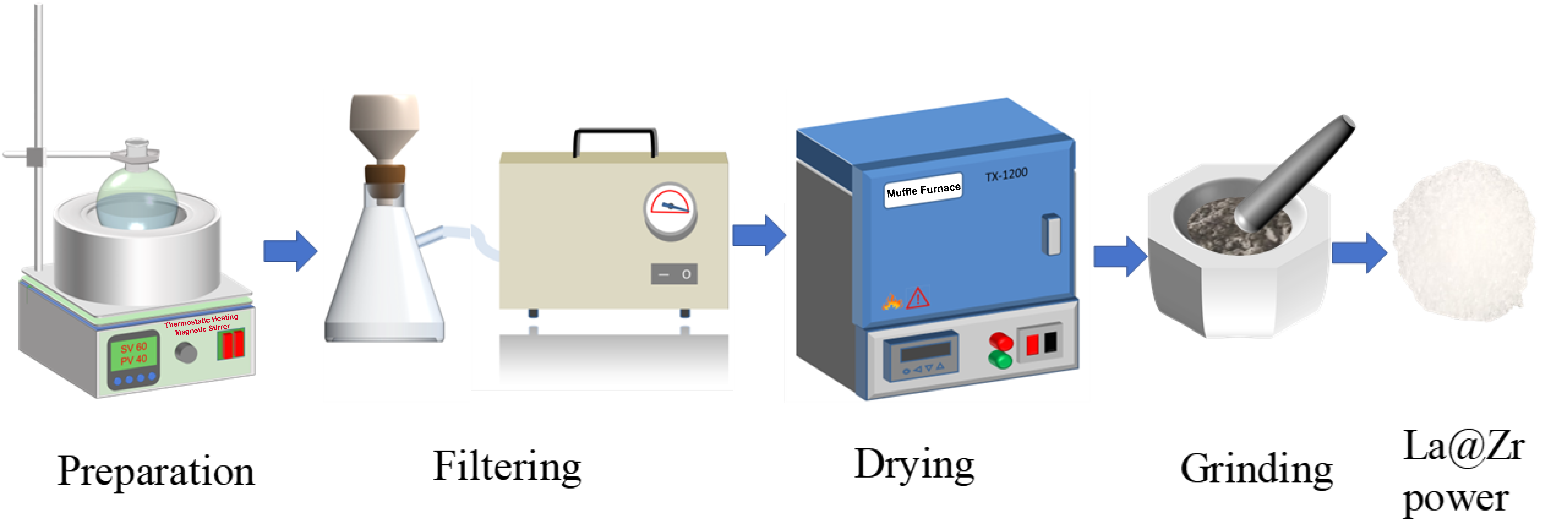
2.2. Preparation of La@Zr Adsorbent
2.3. Adsorption Experiments
2.3.1. Adsorption of Fluoride Ions
2.3.2. Adsorption Isothermal and Adsorption Kinetics Processes
2.4. Desorption and Regeneration Processes
2.5. Analytical Methods
3. Results and Discussion
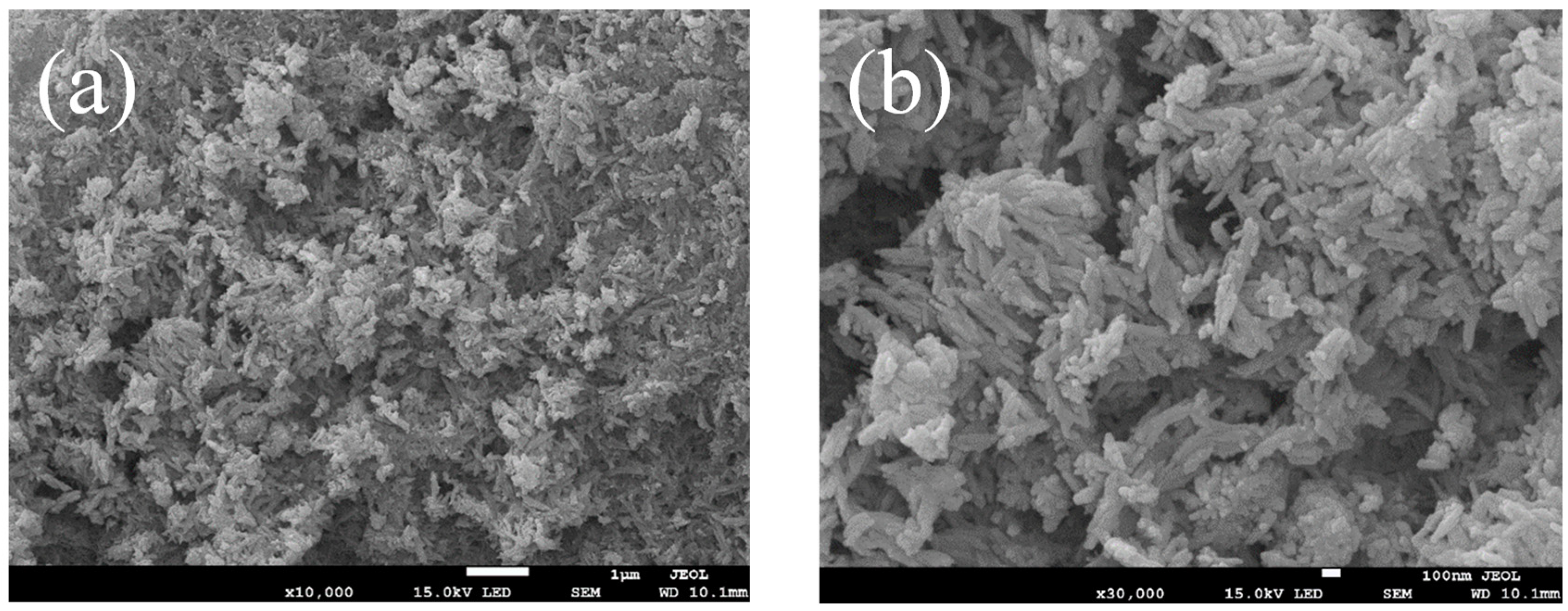
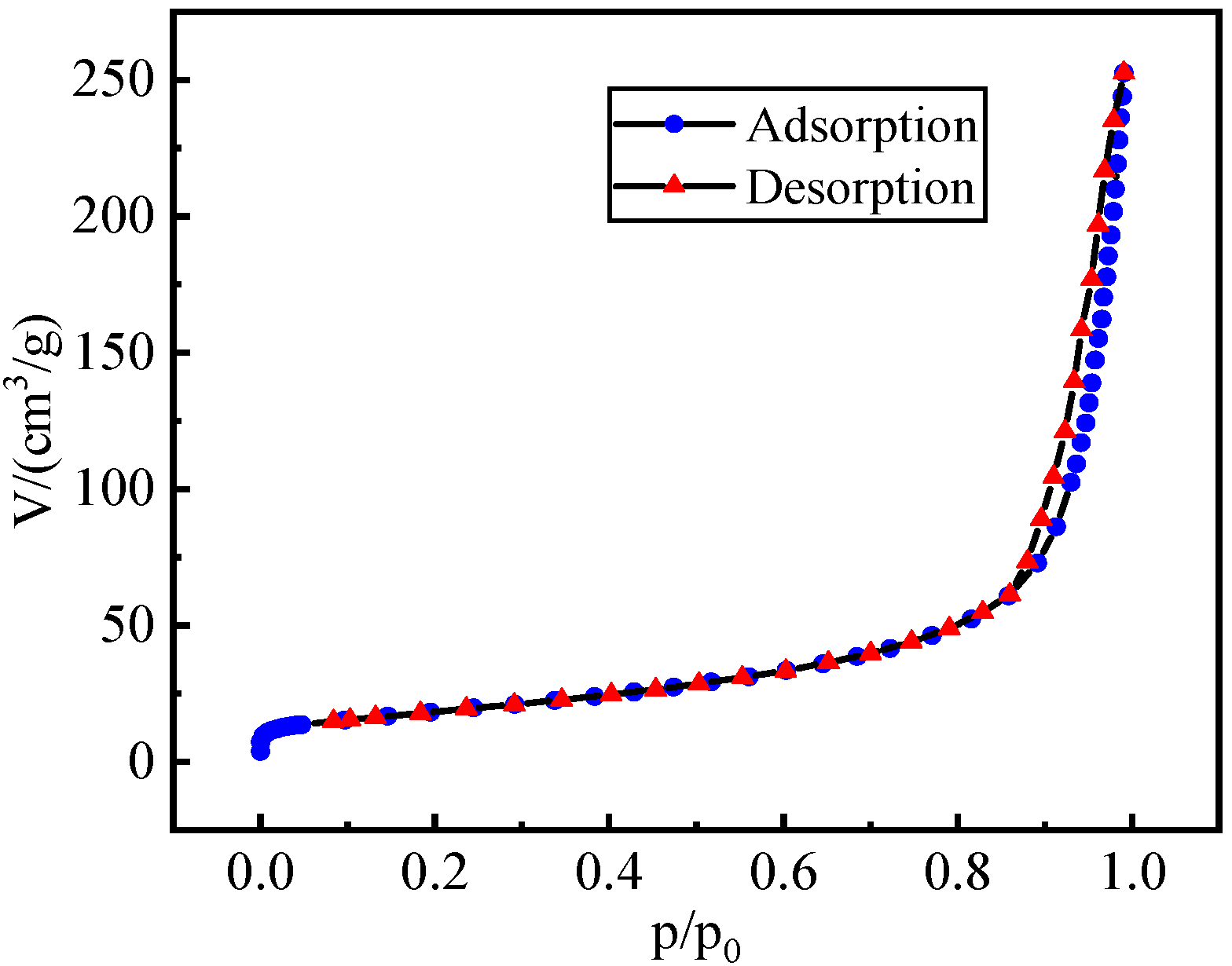
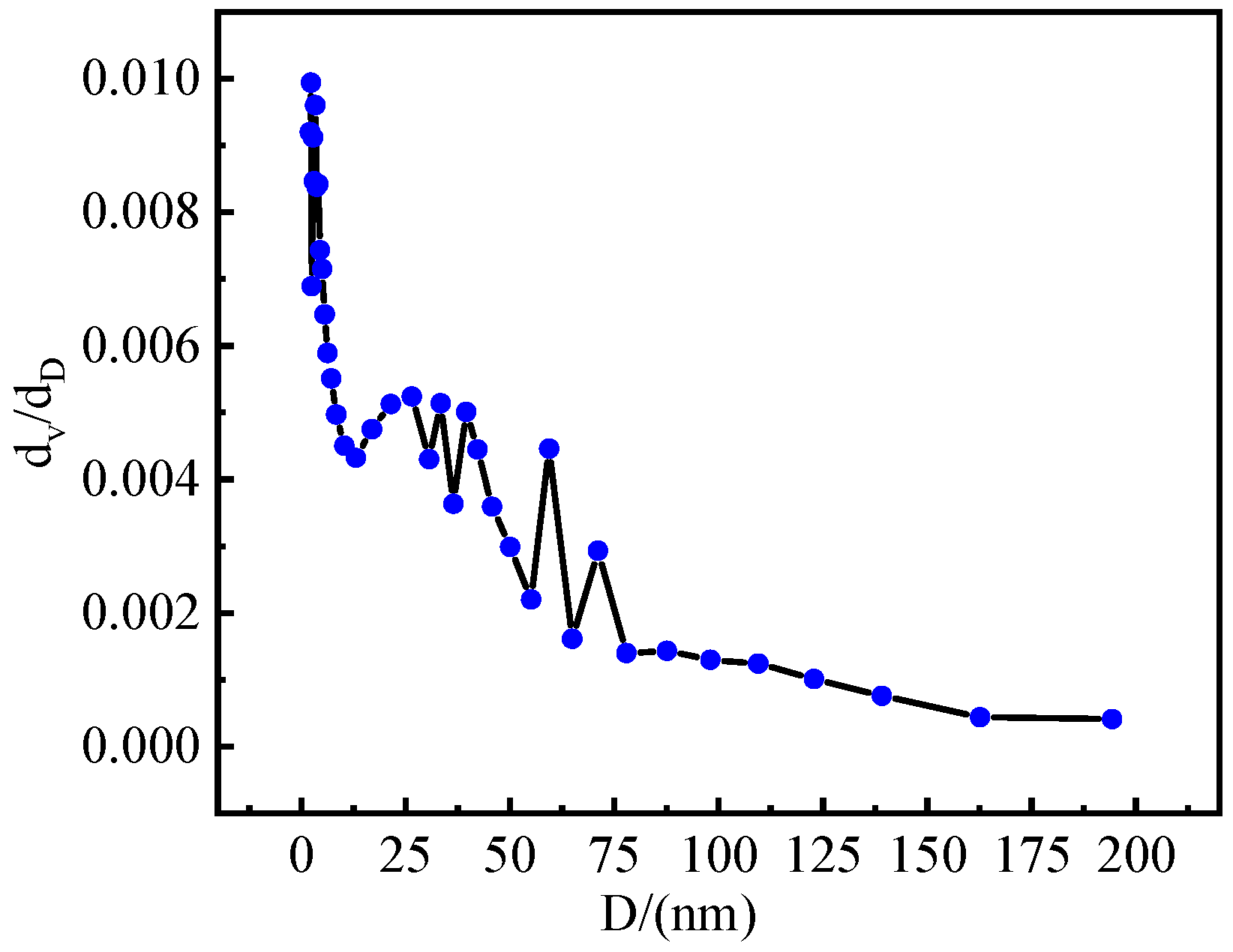
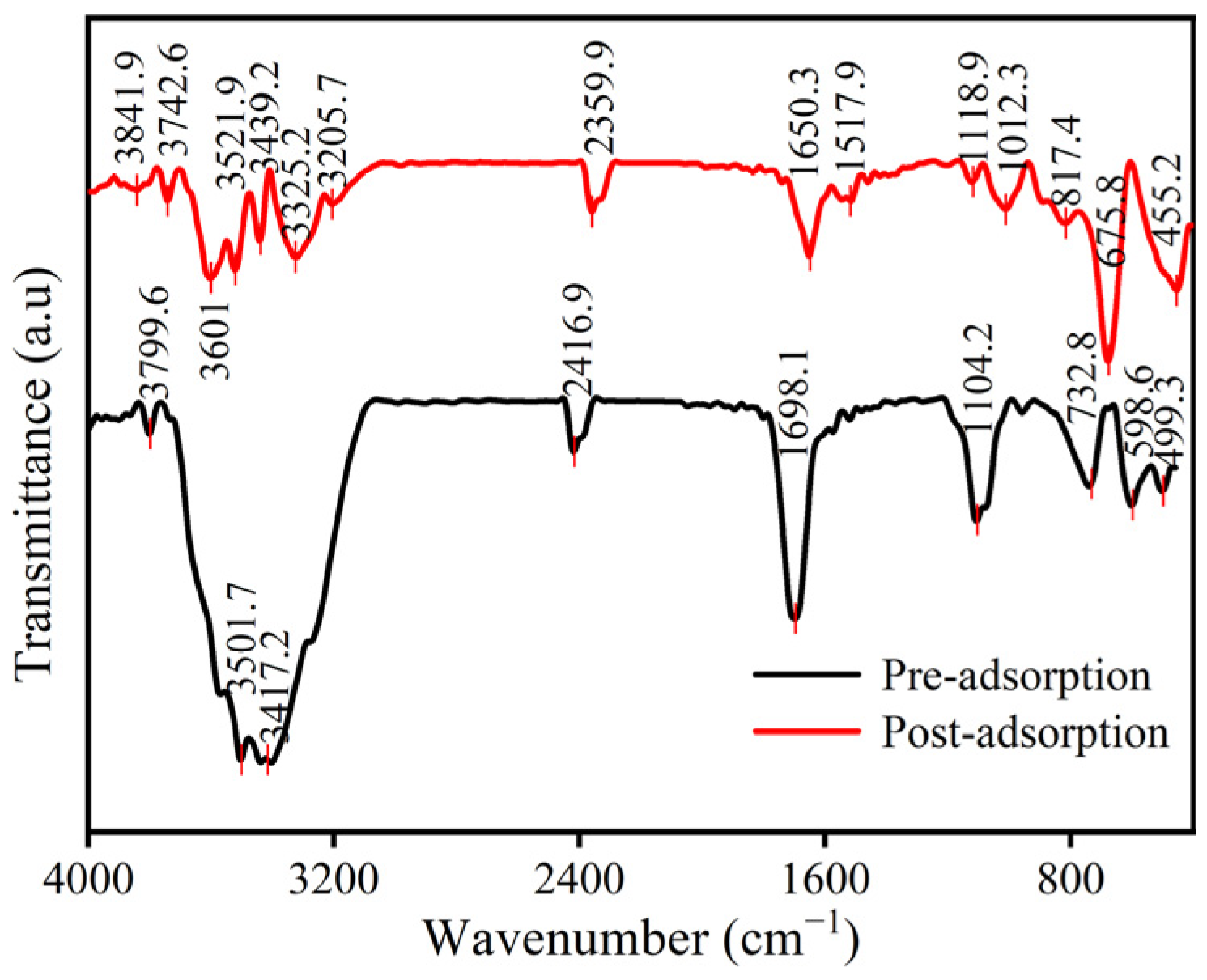
3.1. Characterization of La@Zr Adsorbent
3.2. Selective Removal of Fluoride Ions
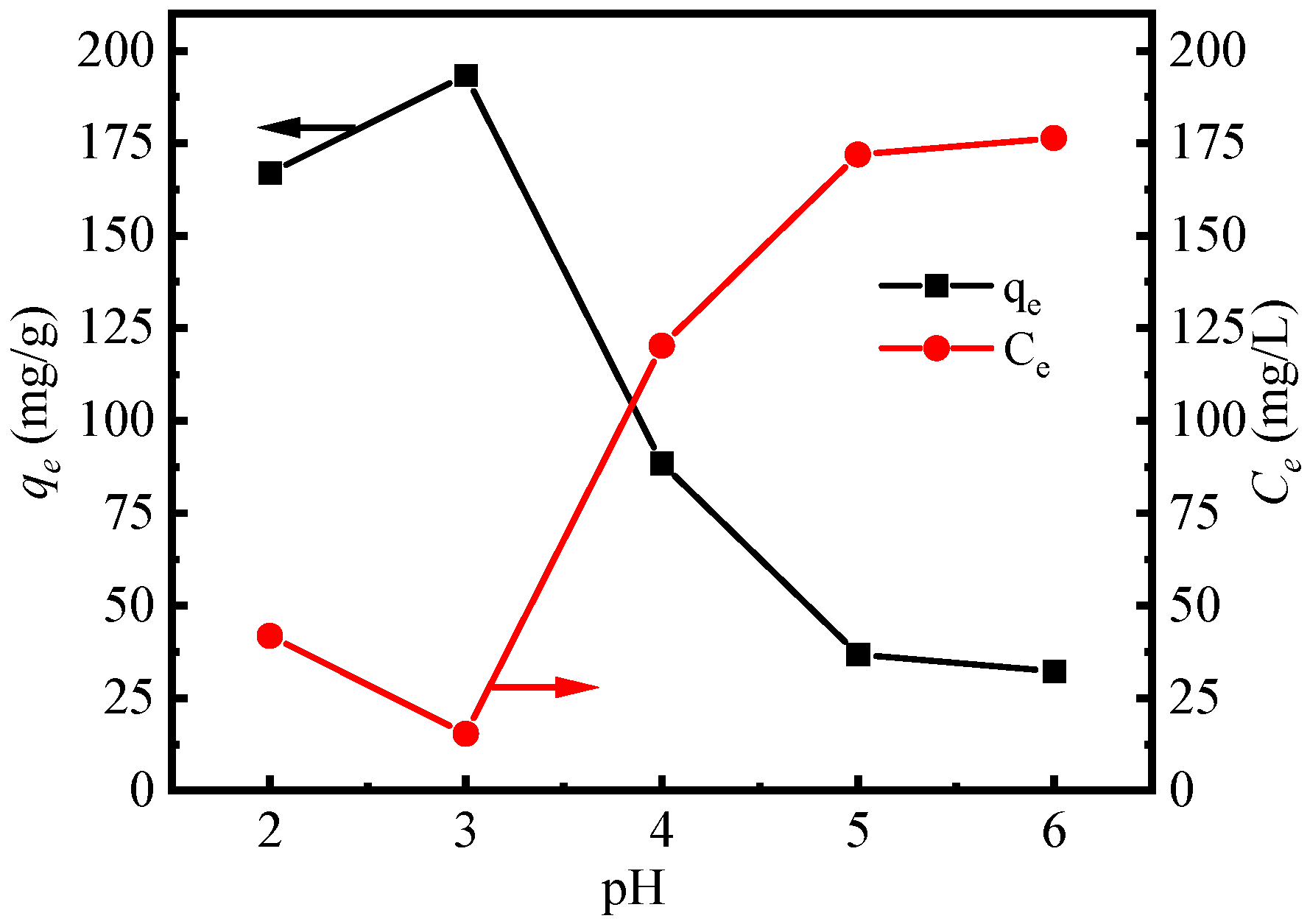
3.2.1. Effect of Initial pH
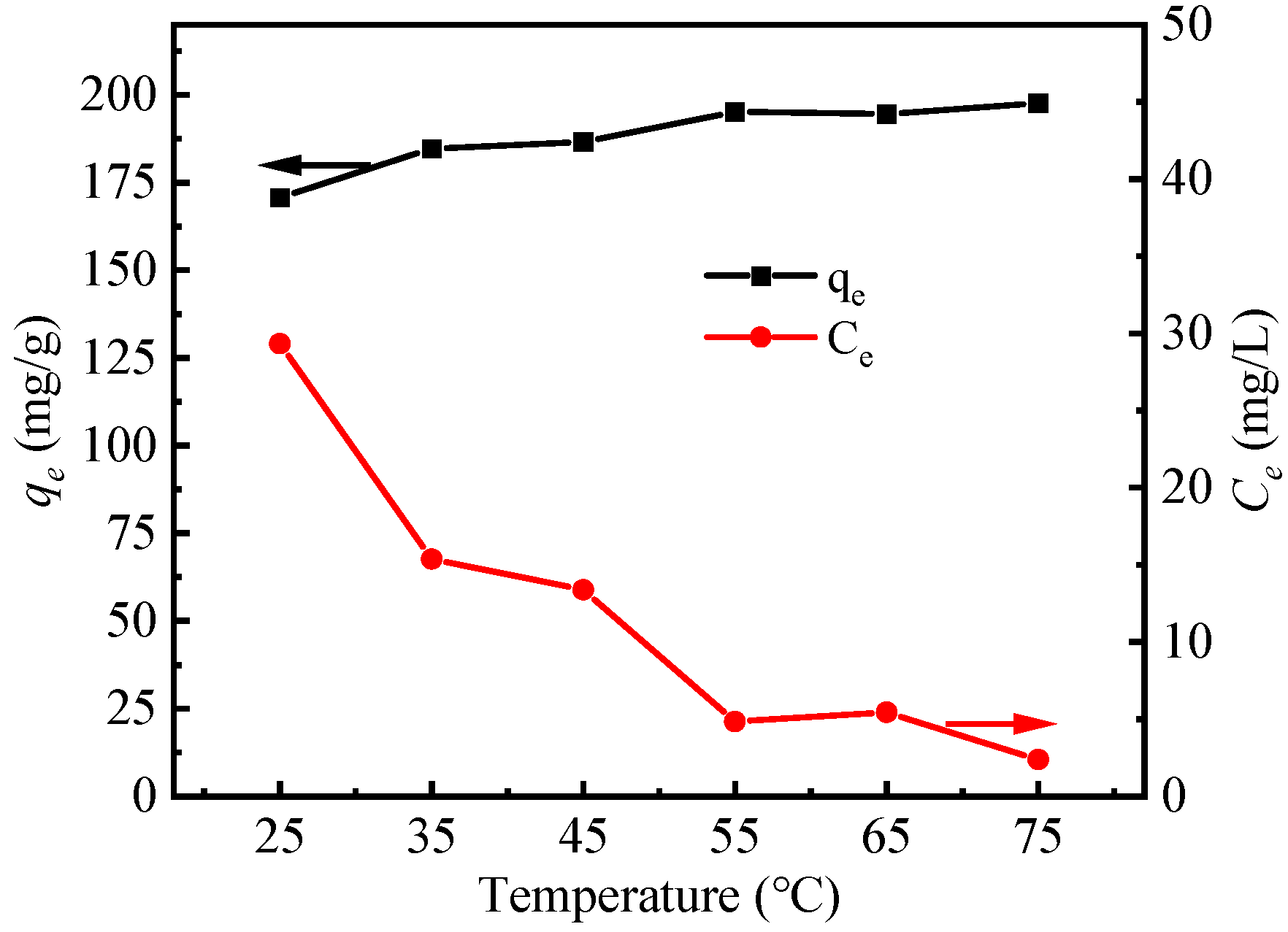
3.2.2. Effect of Reaction Temperature
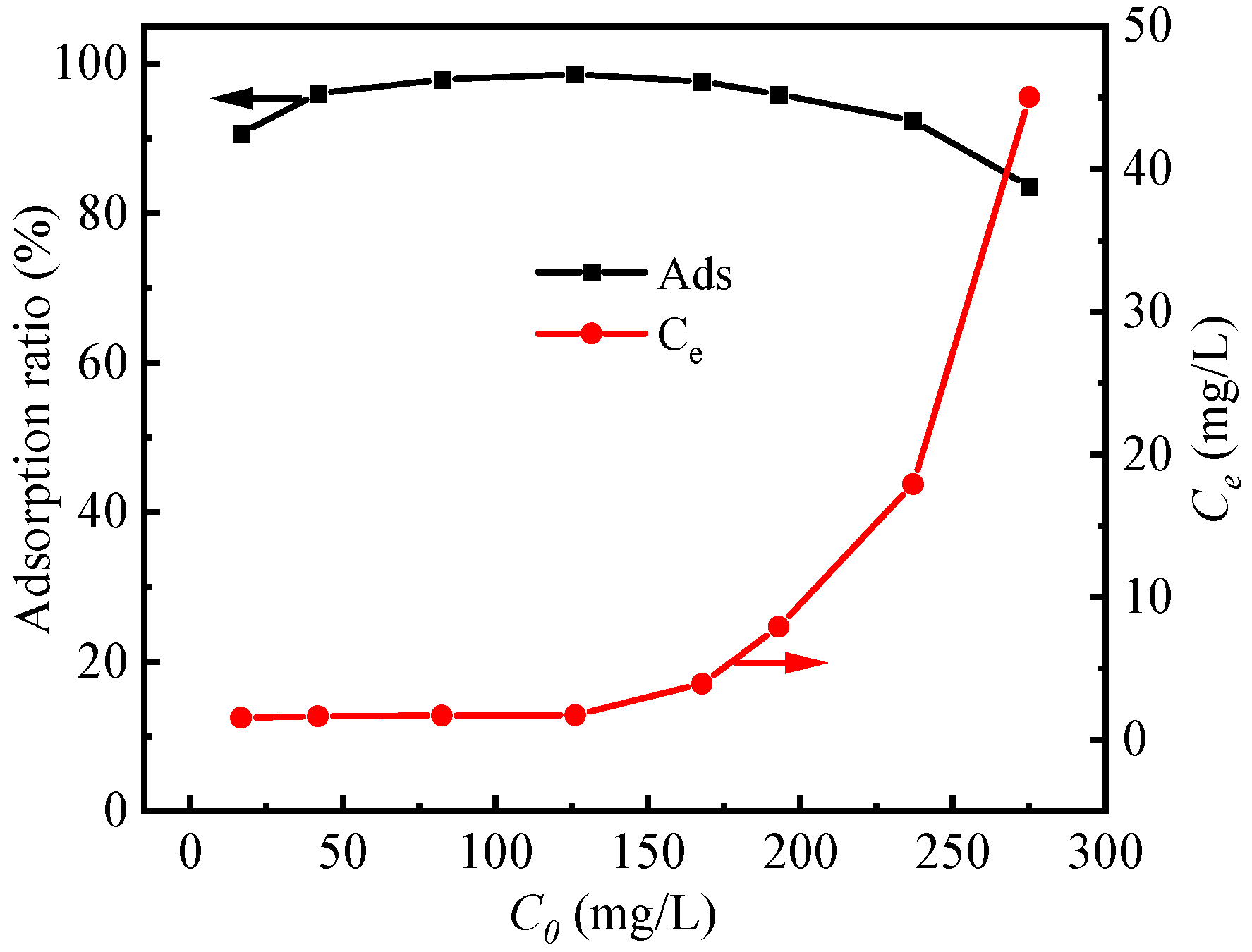
3.2.3. Effect of Initial Fluoride Concentration
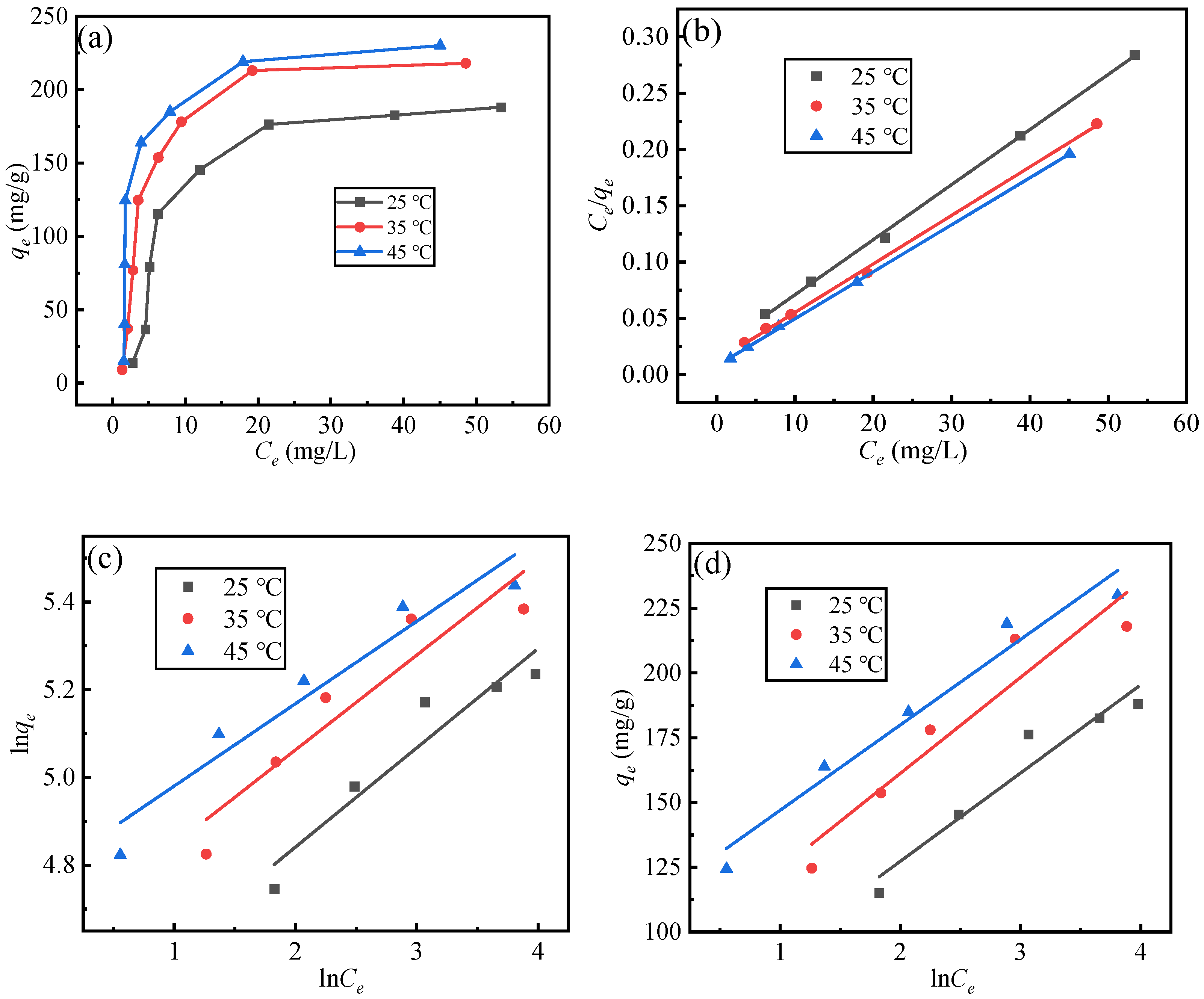
3.3. Adsorption Isotherms
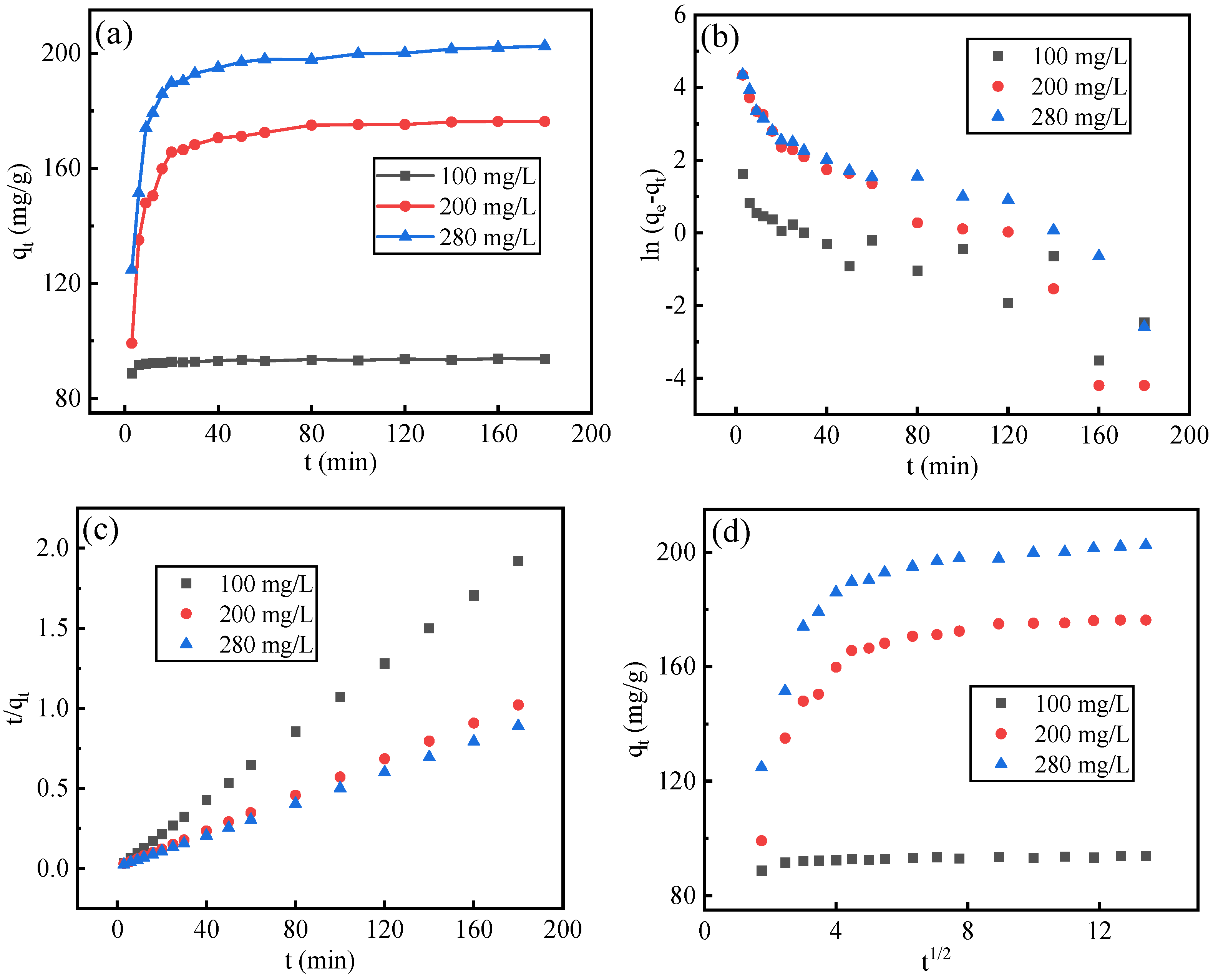
3.4. Adsorption Kinetics
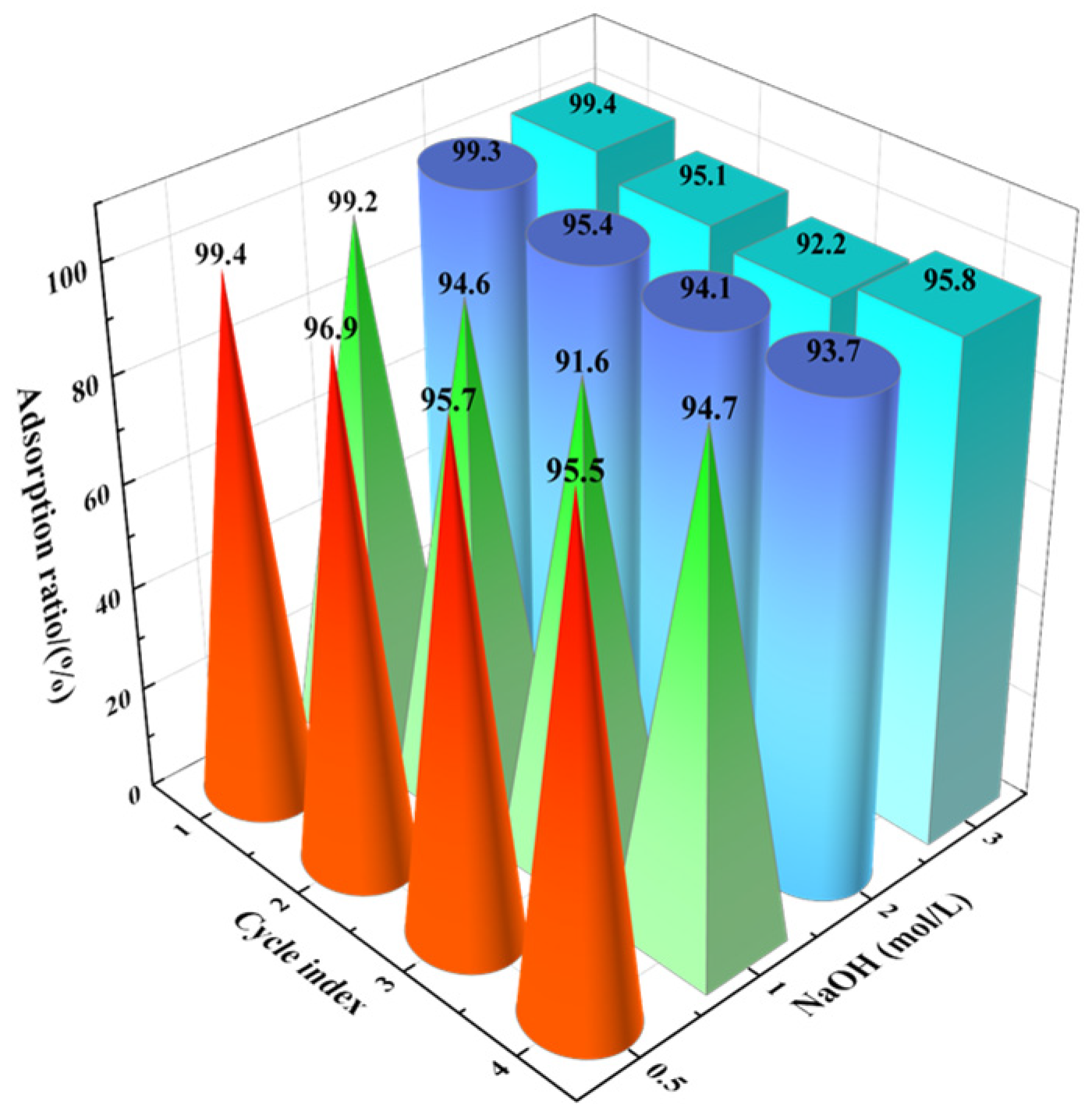
3.5. Desorption and Regeneration
4. Conclusions
- (1)
- The La@Zr composite adsorbent prepared using the hydrothermal synthesis method has microporous structure with a specific surface area of 67.41 m2/g and a pore size of 2–50 nm, and its pore size distribution is mesoporous.
- (2)
- In acidic solutions, the adsorbent has the maximum adsorption capacity of 193.4 mg/g. Moreover, it can selectively adsorb fluoride in mixed solution containing Li, Ni, Co, Mn, and other metal ions.
- (3)
- The adsorption of fluoride ions by adsorbents is more consistent with the Langmuir model, the secondary adsorption process is mainly chemical adsorption, and the adsorption reaction is a multi-stage control process. The adsorption by adsorbents is a monolayer adsorption, and the adsorption process is an endothermic reaction, and increasing temperature is conducive to the adsorption reaction.
- (4)
- The recycling and regeneration of the adsorbent is a vital index when measuring its practical performance. The fluoride removal performance of the adsorbent can be restored after alkali washing and regeneration, and the adsorption rate of fluoride ions can still reach 95% after four cycles of use.
- (5)
- Through the development of new adsorbents and optimization of adsorption processes, clean production of waste lithium batteries has been achieved.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prabhu, S.M.; Yusuf, M.; Ahn, Y.; Park, H.B.; Choi, J.; Amin, M.A.; Yadav, K.K.; Jeon, B.-H. Fluoride occurrence in environment, regulations, and remediation methods for soil: A comprehensive review. Chemosphere 2023, 324, 138334. [Google Scholar] [CrossRef]
- Srivastava, A.; Kumari, M.; Prasad, K.S. Fluoride occurrence, health issues, and removal using adsorption process. Proc. Indian Natl. Sci. Acad. 2022, 88, 129–141. [Google Scholar] [CrossRef]
- Hou, C.; Xiu, W.; Guo, H.; Li, S.; Jiang, C. Application of Al-Fe Co-modified Rice-Straw Biochar to Fluoride Removal: Synthesis, Optimization, and Performance. Water Air Soil Pollut. 2023, 234, 169. [Google Scholar] [CrossRef]
- Wu, L.; Fan, C.; Zhang, Z.; Zhang, X.; Lou, Q.; Guo, N.; Huang, W.; Zhang, M.; Yin, F.; Guan, Z.; et al. Association between fluoride exposure and kidney function in adults: A cross-sectional study based on endemic fluorosis area in China. Ecotoxicol. Environ. Saf. 2021, 225, 112735. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, S.J.; Sankannavar, R.; Madhu, G. Fluoride sources, toxicity and fluorosis management techniques—A brief review. J. Hazard. Mater. Lett. 2021, 2, 100033. [Google Scholar] [CrossRef]
- Zhang, Q.X.; Zhang, C.; Xu, Y.; Wan, L. Treatment status of high fluorine wate. J. Green Sci. Technol. 2021, 23, 46–49. [Google Scholar] [CrossRef]
- Kumar, P.S.; Suganya, S.; Srinivas, S.; Priyadharshini, S.; Karthika, M.; Sri, R.K.; Swetha, V.; Naushad, M.; Lichtfouse, E. Treatment of fluoride-contaminated water. A review. Environ. Chem. Lett. 2019, 17, 1707–1726. [Google Scholar] [CrossRef]
- Dai, D.Q.; Zhang, X.; Li, H. Study on sources and control of fluorine and chlorine in zinc hydrometallurgy. Jiangsu Sci. Technol. Inf. 2018, 35, 39–41. [Google Scholar] [CrossRef]
- Li, F.Y.; Jiang, T.Y.; Yu, T.; Yang, Z.F.; Hou, Q.Y.; Wang, L.X. Review on sources of fluorine in the environment and health risk as-sessment. Rock Miner. Anal. 2021, 40, 793–807. [Google Scholar] [CrossRef]
- Xu, X.; Liao, Y.; Sun, J.; Wang, X.; Chen, S.; Lv, Z.; Song, J. Removal of Fluorides from Aqueous Solutions Using Fresh and Regenerated Activated Alumina. Acta Phys. Chim. Sin. 2019, 35, 317–326. [Google Scholar] [CrossRef]
- Jumari, A.; Yudha, C.S.; Dyartanti, E.R.; Nizam, M.; Suranto; Purwanto, A. A facile approach for the selective recovery of lithium from spent lithium-ion batteries. J. Mater. Res. Technol. 2022, 18, 3640–3651. [Google Scholar] [CrossRef]
- Jian, Y.; Zongliang, Z.; Gang, Z.; Liangxing, J.; Fangyang, L.; Ming, J.; Yanqing, L. Process study of chloride roasting and water leaching for the extraction of valuable metals from spent lithium-ion batteries. Hydrometallurgy 2021, 203, 105638. [Google Scholar] [CrossRef]
- Ma, S.; Liu, F.; Li, K.; Chen, Z.; Chen, F.; Wang, J.; Zhong, S.; Wilson, B.P.; Lundström, M. Separation of Li and Al from spent ternary Li-ion batteries by in-situ aluminum-carbon reduction roasting followed by selective leaching. Hydrometallurgy 2022, 213, 105941. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, N.; Li, W.; Zhao, R.; Dong, Y.; Liu, J.; Su, C.; Wang, J.; Zhang, C. Effect of trace hydrofluoric acid in a LiPF6 electrolyte on the performance of a Li–organic battery with an N-heterocycle based conjugated microporous polymer as the cathode. J. Mater. Chem. A 2019, 7, 16347–16355. [Google Scholar] [CrossRef]
- Jie, Y.; Yang, S.; Shi, P.; Chang, D.; Fang, G.; Mo, C.; Ding, J.; Liu, Z.; Lai, Y.; Chen, Y. Thermodynamic Analysis and Experimental Investigation of Al and F Removal from Sulfuric Acid Leachate of Spent LiFePO4 Battery Powder. Metals 2021, 11, 1641. [Google Scholar] [CrossRef]
- Hernández, G.; Naylor, A.J.; Chien, Y.-C.; Brandell, D.; Mindemark, J.; Edström, K. Elimination of Fluorination: The Influence of Fluorine-Free Electrolytes on the Performance of LiNi1/3Mn1/3Co1/3O2/Silicon–Graphite Li-Ion Battery Cells. ACS Sustain. Chem. Eng. 2020, 8, 10041–10052. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.B.; Ren, G.X.; Xiao, S.W. Effect of fluorine and phosphorus impurity on recovery of lithium from spent NCM cathode powder by reduction roasting-water leaching process. Nonferrous Met. Extr. Metall. 2020, 12, 42–47. [Google Scholar] [CrossRef]
- Yu, B.; Ren, T.; Du, X.H.; Chu, Z.Q.; Guo, M.Y. Study on the treatment process of fluorine-containing wastewater. China Resour. Compr. Util. 2020, 38, 192–195. [Google Scholar] [CrossRef]
- Chen, D.; Zhao, M.; Tao, X.; Ma, J.; Liu, A.; Wang, M. Exploration and Optimisation of High-Salt Wastewater Defluorination Process. Water 2022, 14, 3974. [Google Scholar] [CrossRef]
- Dou, R.A.; Chen, B.B.; Luo, S.Q.; Luo, K. Study on the treatment of high concentration fluoride-containing wastewater by chemical precipitation process. Organo-Fluor. Ind. 2016, 2, 9–11. [Google Scholar]
- Wang, X.; Zhang, F.; Cui, J.G. Research on treatment of low concentration fluoride-containing organic wastewater by potassium ferrate-PAC coagulation. Mod. Chem. Ind. 2020, 40, 170–175. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, K.; Zhang, X.; Wang, M. Removal of Fluorine from RECl3 in Solution by Adsorption, Ion Exchange and Precipitation. Minerals 2021, 12, 31. [Google Scholar] [CrossRef]
- Fan, J.; Yu, L.; Zhou, X.; Liu, J. Synthesis and characterization of cross linked N-methylene phosphonic chitosan resin chelated with Al(III) for use as adsorbent for fluoride removal from aqueous solutions. Korean J. Chem. Eng. 2022, 39, 377–388. [Google Scholar] [CrossRef]
- Ni, C.; Liu, C.; Xie, Y.; Xie, W.; He, Z.; Zhong, H. A critical review on adsorption and recovery of fluoride from wastewater by metal-based adsorbents. Environ. Sci. Pollut. Res. 2022, 29, 82740–82761. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, B.; Chen, Q.; Peng, X.; Yang, D.; Qiu, F. Layered double hydroxide functionalized biomass carbon fiber for highly efficient and recyclable fluoride adsorption. Appl. Biol. Chem. 2019, 62, 12. [Google Scholar] [CrossRef]
- Hafshejani, L.D.; Tangsir, S.; Daneshvar, E.; Maljanen, M.; Lähde, A.; Jokiniemi, J.; Naushad, M.; Bhatnagar, A. Optimization of fluoride removal from aqueous solution by Al2O3 nanoparticles. J. Mol. Liq. 2017, 238, 254–262. [Google Scholar] [CrossRef]
- Díaz, I.; Gómez-Hortigüela, L.; Gálvez, P.; Pérez-Pariente, J.; Ólavsdóttir, J. Composite materials based on zeolite stilbite from Faroe Islands for the removal of fluoride from drinking water. Am. Miner. 2019, 104, 1556–1564. [Google Scholar] [CrossRef]
- Yapo, N.S.; Aw, S.; Briton, B.G.H.; Drogui, P.; Yao, K.B.; Adouby, K. Removal of fluoride in groundwater by adsorption using hydroxyapatite modified Corbula trigona shell powder. Chem. Eng. J. Adv. 2022, 12, 100386. [Google Scholar] [CrossRef]
- Rahman, N.; Nasir, M. Development of Zr(IV)—Doped polypyrrole/zirconium (IV) iodate composite for efficient removal of fluoride from water environment. J. Water Process. Eng. 2017, 19, 172–184. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Y. Characterization and adsorption properties of a lanthanum-loaded magnetic cationic hydrogel composite for fluoride removal. Water Res. 2016, 88, 852–860. [Google Scholar] [CrossRef]
- Wang, M.; Yu, X.; Yang, C.; Yang, X.; Lin, M.; Guan, L.; Ge, M. Removal of fluoride from aqueous solution by Mg-Al-Zr triple-metal composite. Chem. Eng. J. 2017, 322, 246–253. [Google Scholar] [CrossRef]
- Xiao, J.; Wu, M.R.; Dong, Z.Y.; Li, Y.T.; Sun, W.; Yang, Y. Synthesis of γ-Al2O3 for fluoride removal from spent Lithium-Ion battery leaching Solution: Performance and mechanism. Sep. Purif. Technol. 2025, 378, 134822. [Google Scholar] [CrossRef]
- Hu, H.P.; Dong, H.D.; Peng, Q.F. Study on removal of fluoride by hydrous zirconium oxide adsorbent in battery grade manganese sulfate solution. J. Cent. South Univ. (Sci. Technol.) 2022, 53, 4234–4241. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Liang, L.S.; Mi, H.; Ma, H.Y.; Li, L.B. Defluorination performance of lanthanum and zirconium modified aluminum hydroxide in zinc sulfate electrolyte. J. Cent. South Univ. (Sci. Technol.) 2024, 55, 895–906. [Google Scholar] [CrossRef]
- Blackwell, J.; Carr, P. Study of the fluoride adsorption characteristics of porous microparticulate zirconium oxide. J. Chromatogr. A 1991, 549, 43–57. [Google Scholar] [CrossRef]
- Adkins, B.D.; Davis, B.H. Comparison of Nitrogen Adsorption and Mercury Penetration Results: II. Pore size Distributions Calculated from Type IV Isotherm Data. Adsorpt. Sci. Technol. 1988, 5, 168–190. [Google Scholar] [CrossRef]
- El Mahbouby, A.; Ezaierb, Y.; Mechnoua, I.; Rajia, Y.; Zyade, S. Elaboration and Characterization of Organo-Ghassoul (Moroccan Clay) as an Adsorbent Using Cationic Surfactant for Anionic Dye Adsorption. Phys. Chem. Res. 2023, 11, 913–928. [Google Scholar]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for The Use and Interpretation of Adsorption Isotherm Models: A Review; Department of Environmental Engineering, School of Environment, Tsinghua University: Beijing, China, 2020. [Google Scholar]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum; Affiliations Tsinghua-Berkeley Shenzhen Institute and Tsinghua Shenzhen International Graduate School; Tsinghua University: Shenzhen, China, 1918. [Google Scholar]
- Ho, Y.S.; McKay, G. The Kinetics of Sorption of Divalent Metal Ions onto Sphagnum Moss Peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Rethinking of the intraparticle diffusion adsorption kinetics model: Interpretation, solving methods and applications. Chemosphere 2022, 309, 136732. [Google Scholar] [CrossRef]
- Mechnou, I.; Meskini, S.; Elqars, E.; El Had, M.A.; Hlaibi, M. Efficient CO2 capture using a novel Zn-doped activated carbon developed from agricultural liquid biomass: Adsorption study, mechanism and transition state. Surf. Interfaces 2024, 52, 104846. [Google Scholar] [CrossRef]

| Element | Li | Ni | Co | Mn | F |
|---|---|---|---|---|---|
| Concentration(mg/L) | 6065 | 24,780 | 9565 | 14,195 | 200 |
| Langmuir Isotherm | Freundlich Isotherm | Temkin Isotherm | |||||||
|---|---|---|---|---|---|---|---|---|---|
| T (°C) | KL | qm (mg/g) | R12 | KF | n | R22 | KT | B | R32 |
| 25 | 0.22 | 204.50 | 0.9991 | 80.57 | 4.42 | 0.9134 | 5.68 | 34.06 | 0.9347 |
| 35 | 0.36 | 232.02 | 0.9989 | 102.59 | 4.63 | 0.8802 | 10.47 | 37.07 | 0.9066 |
| 45 | 0.54 | 239.23 | 0.9998 | 120.64 | 5.33 | 0.9301 | 31.68 | 32.98 | 0.9617 |
| Adsorbents | Maximum qe(mg/g) | pH | References |
|---|---|---|---|
| Al-CPCM resin | 5.68 | 7 | [23] |
| LDH-BCF | 15.21 | 5.71 | [25] |
| Al2O3 nanoparticles | 13.70 | 4 | [26] |
| Zeolite hydroxyapatite composite | 0.3 | 6 | [27] |
| HAP3 | 4.52 | 7.5 | [28] |
| Zr-PZI | 183.5 | 7.0 | [29] |
| MCH-La | 136.78 | 7.0 | [30] |
| Mg-Al-Zr composite | 22.9 | 7.0 | [31] |
| La@Zr composite | 239.23 | 3.0 | Present study |
| Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | Intra-Particle Diffusion Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C0 (mg/L) | Qe,exp (mg/g) | K1 | qe,calcd (mg/g) | R12 | K2 | qe,calcd (mg/g) | R22 | Kp | R32 | C |
| 100 | 93.93 | 1.02 | 93.04 | 0.7483 | 0.0681 | 93.61 | 0.9585 | 0.24 | 0.5587 | 91.11 |
| 200 | 176.36 | 0.25 | 171.03 | 0.9229 | 0.0027 | 179.61 | 0.9887 | 3.96 | 0.5454 | 134.79 |
| 280 | 202.55 | 0.28 | 195.63 | 0.9035 | 0.0025 | 204.50 | 0.9896 | 4.08 | 0.5498 | 158.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Liu, F.; Liao, B.; Zhang, T.; Chen, F.; Wang, J.; Liao, C.; Xu, S. Deep Removal of Fluoride Ions from Spent Ternary Lithium-Ion Batteries Leachate Using Porous La@Zr Adsorbent. Inorganics 2025, 13, 369. https://doi.org/10.3390/inorganics13110369
Chen Z, Liu F, Liao B, Zhang T, Chen F, Wang J, Liao C, Xu S. Deep Removal of Fluoride Ions from Spent Ternary Lithium-Ion Batteries Leachate Using Porous La@Zr Adsorbent. Inorganics. 2025; 13(11):369. https://doi.org/10.3390/inorganics13110369
Chicago/Turabian StyleChen, Zaoming, Fupeng Liu, Bin Liao, Tao Zhang, Feixiong Chen, Jie Wang, Chunfa Liao, and Shengming Xu. 2025. "Deep Removal of Fluoride Ions from Spent Ternary Lithium-Ion Batteries Leachate Using Porous La@Zr Adsorbent" Inorganics 13, no. 11: 369. https://doi.org/10.3390/inorganics13110369
APA StyleChen, Z., Liu, F., Liao, B., Zhang, T., Chen, F., Wang, J., Liao, C., & Xu, S. (2025). Deep Removal of Fluoride Ions from Spent Ternary Lithium-Ion Batteries Leachate Using Porous La@Zr Adsorbent. Inorganics, 13(11), 369. https://doi.org/10.3390/inorganics13110369





