Ultra-Rapid Synthesis of Co3O4 Nanostructures with Tunable Morphology via Nickel-Assisted Anodization
Abstract
1. Introduction
2. Results
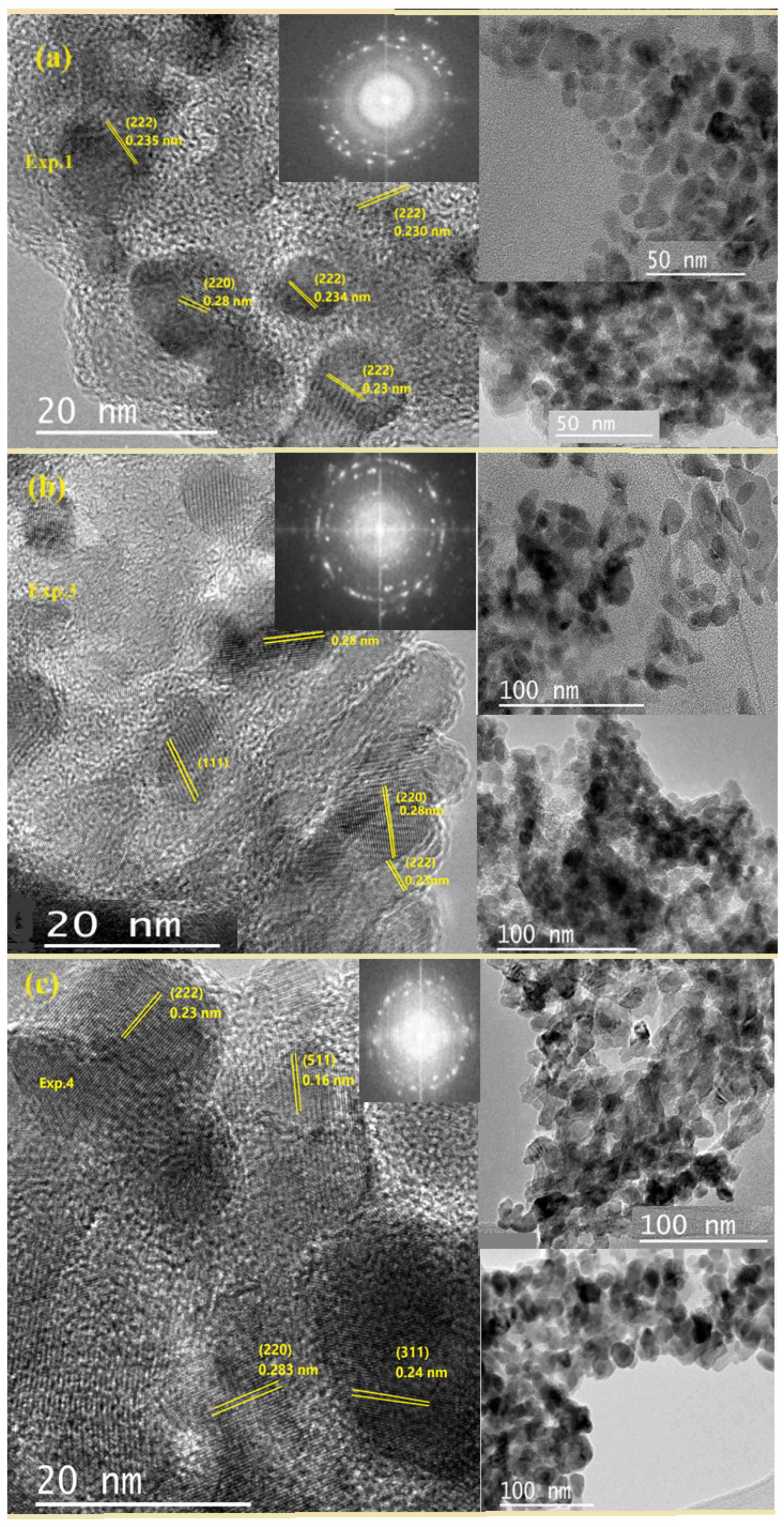
2.1. Structure Characterization
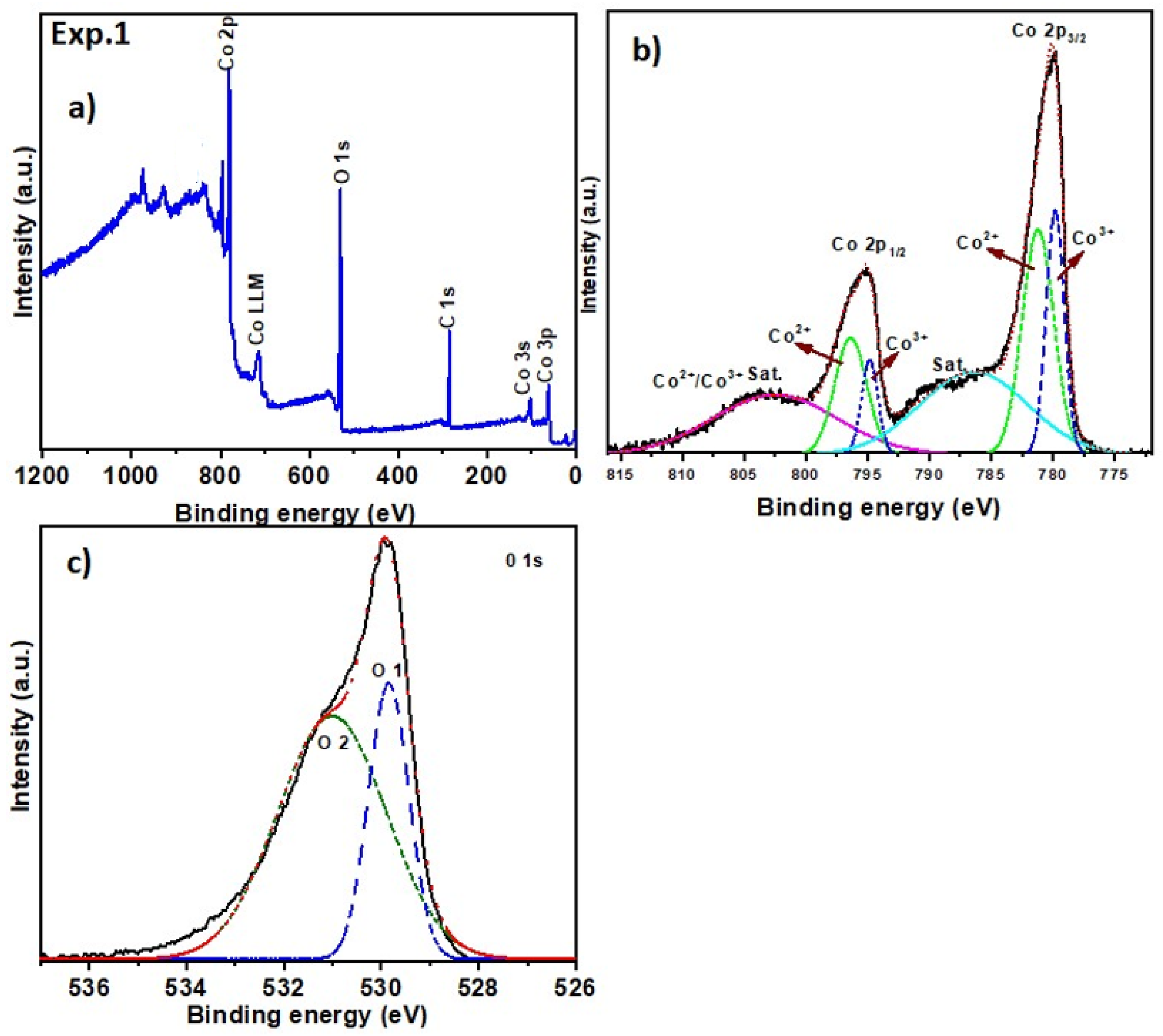
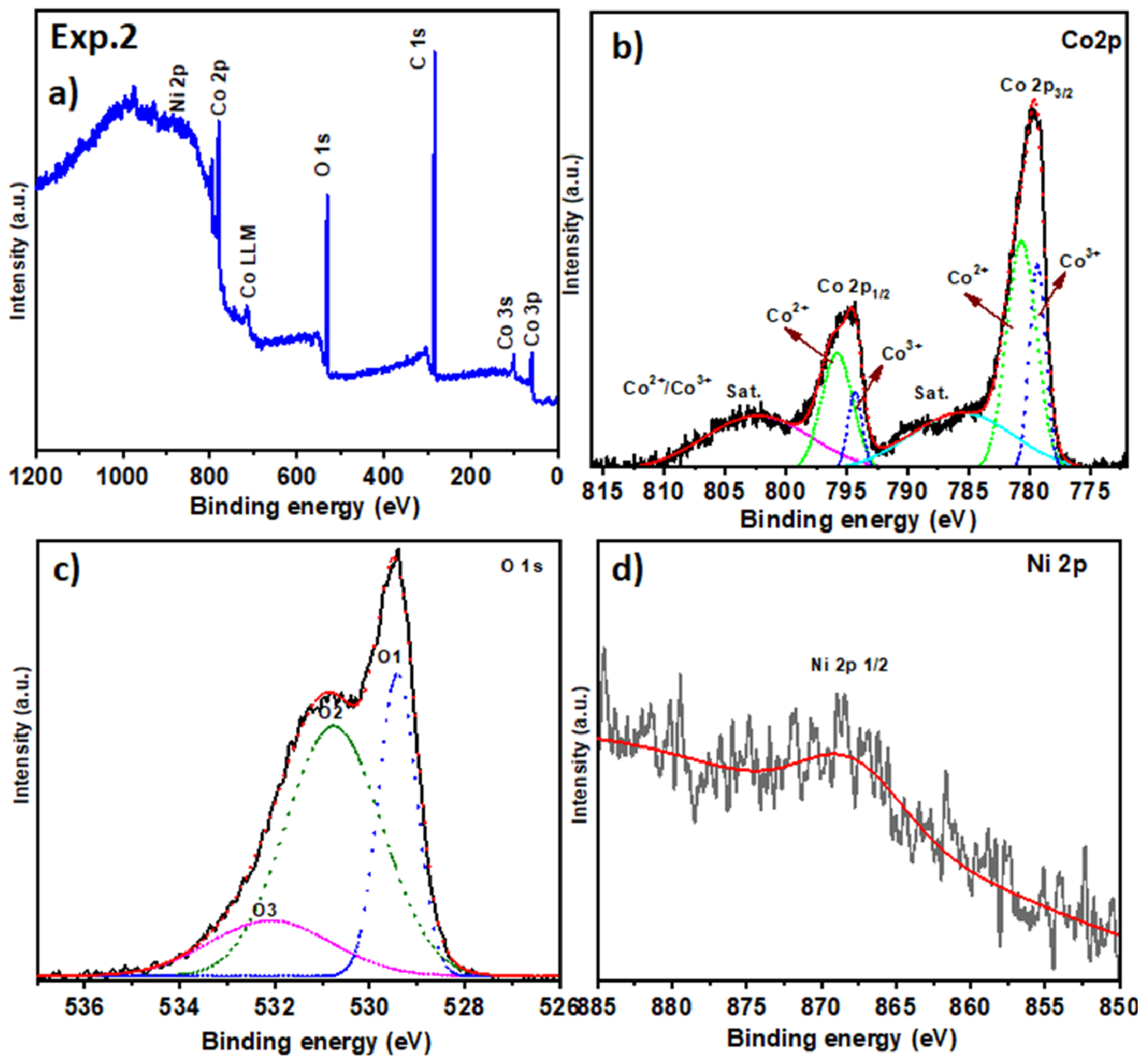
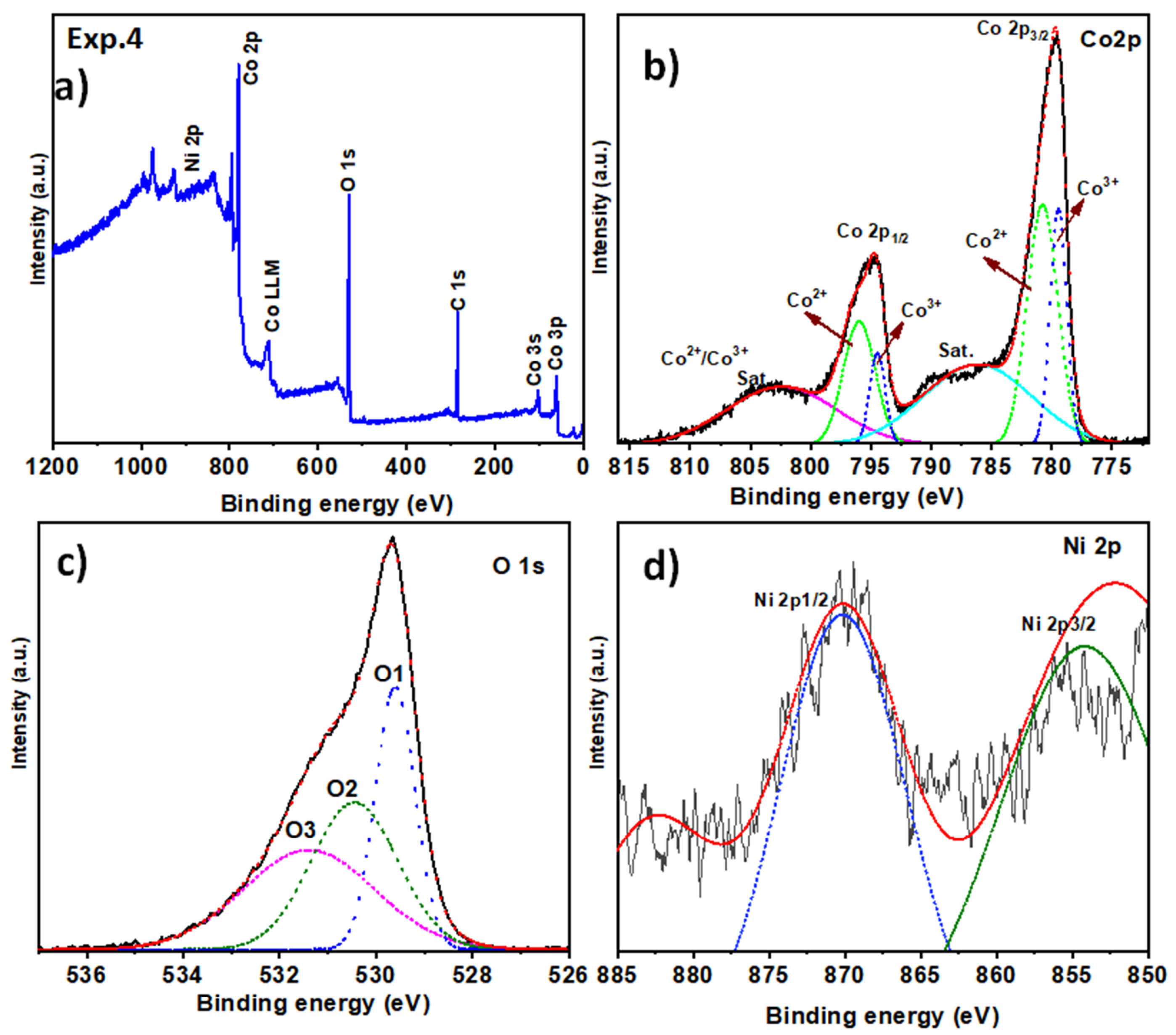
2.2. Surface Chemical Composition
3. Materials and Methods
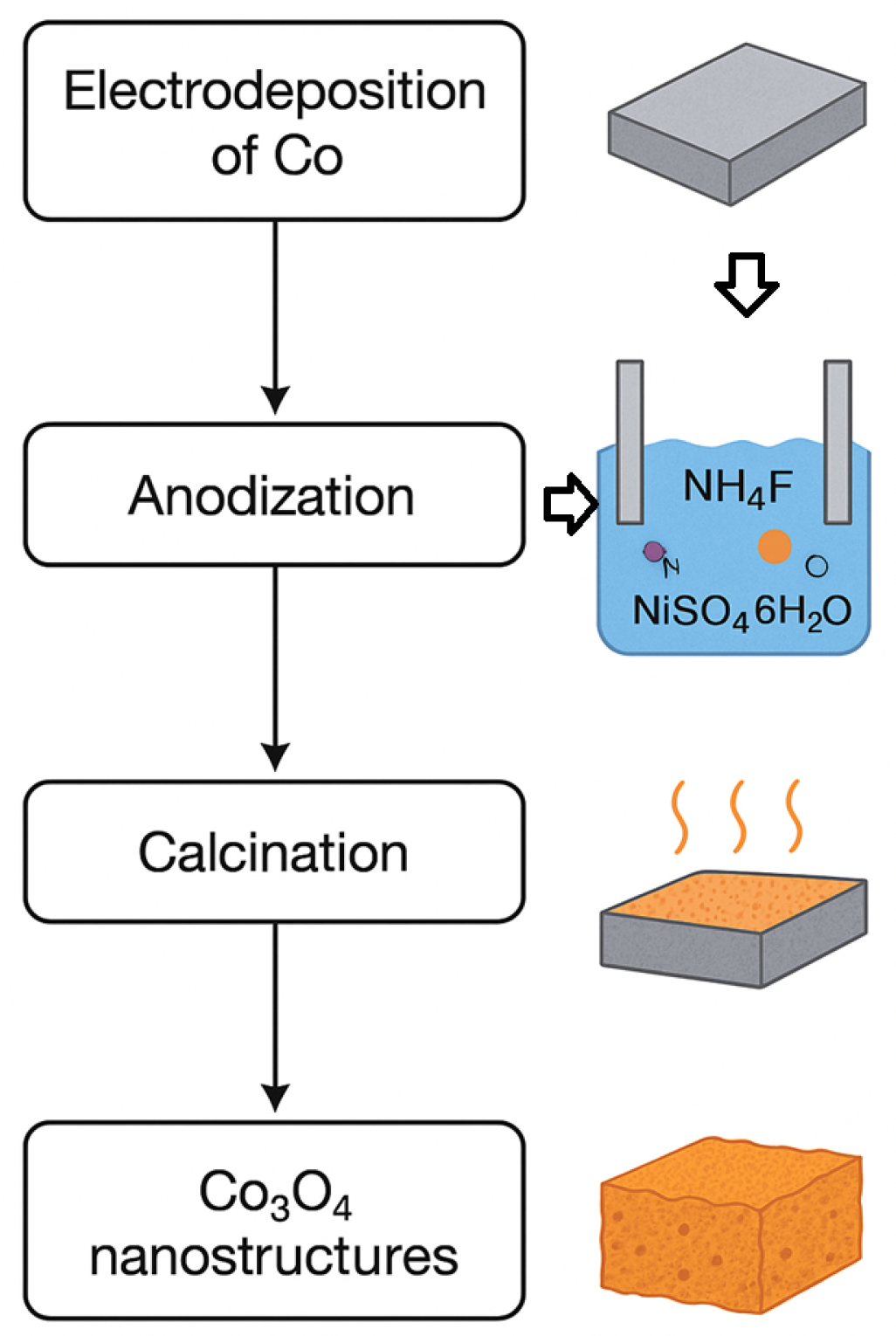
3.1. Anodization Process
3.2. Post-Anodization Thermal Treatment
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qorbani, M.; Naseri, N.; Moshfegh, A.Z. Hierarchical Co3O4/Co(OH)2 Nanoflakes as a Supercapacitor Electrode: Experimental and Semi-Empirical Model. ACS Appl. Mater. Interfaces 2015, 7, 11172–11179. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). World Energy Outlook 2021; International Energy Agency: Paris, France, 2021; Available online: https://www.iea.org/reports/world-energy-outlook-2021 (accessed on 23 October 2025).
- Statista Research Department. Percentage Distribution of Global Energy Consumption in 2019, by Energy Type; Statista: Hamburg, Germany, 2021; Available online: https://es.statista.com/estadisticas/633695/peso-de-los-diferentes-tipos-de-energias-en-el-consumo-energetico-mundial/statisticContainer (accessed on 23 October 2025).
- Ling, C.; Shi, L.; Ouyang, Y.; Chen, Q.; Wang, J. Transition Metal-Promoted V2CO2 (MXenes): A New and Highly Active Catalyst for Hydrogen Evolution Reaction. Adv. Sci. 2016, 3, 1600180. [Google Scholar] [CrossRef]
- Sheng, W.; Song, Y.; Dou, M.; Ji, J.; Wang, F. Constructing 1D Hierarchical Heterostructures of MoS2/In2S3 Nanosheets on CdS Nanorod Arrays for Enhanced Photoelectrocatalytic H2 Evolution. Appl. Surf. Sci. 2018, 436, 613–623. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development (A/RES/70/1); United Nations: New York, NY, USA, 2015; Available online: https://sdgs.un.org/2030agenda (accessed on 23 October 2025).
- Liu, Y.; Jia, Y.; Jia, H.; Gao, L. Recent Development on the Synthesis Strategies and Mechanisms of Co3O4-Based Electrocatalysts for Oxygen Evolution Reaction: A Review. Molecules 2025, 30, 3238. [Google Scholar] [CrossRef] [PubMed]
- Wagh, K.S.; Mane, S.M.; Teli, A.M.; Shin, J.C.; Lee, J. Recent Advancements in Co3O4-Based Composites for Enhanced Electrocatalytic Water Splitting. Micromachines 2024, 15, 1450. [Google Scholar] [CrossRef]
- Mondal, S.; Mondal, A.K.; Chintala, V.; Tauseef, S.M.; Kumar, S.; Pandey, J.K. Thermochemical Pyrolysis of Biomass Using Solar Energy for Efficient Biofuel Production: A Review. Biofuels 2021, 12, 125–134. [Google Scholar] [CrossRef]
- Sebestyén, V. Environmental Impact Networks of Renewable Energy Power Plants. Renew. Sustain. Energy Rev. 2021, 151, 111626. [Google Scholar] [CrossRef]
- El Haj Assad, M.; Zubayda, S.; Khuwaileh, B.; Hmida, A.; Al-Shabi, M. Geothermal Energy as Power Producer. Proc. SPIE 2021, 11722, 117220V. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nizetic, S.; Ong, H.C.; Chong, C.T.; Atabani, A.E.; Pham, V.V. Acid-Based Lignocellulosic Biomass Biorefinery for Bioenergy Production: Advantages, Application Constraints, and Perspectives. J. Environ. Manag. 2021, 296, 113194. [Google Scholar] [CrossRef]
- Aguilar Vargas, S.; Telles Esteves, G.R.; Medina Maçaira, P.; Quaresma Bastos, B.; Cyrino Oliveira, F.L.; Castro Souza, R. Wind Power Generation: A Review and a Research Agenda. J. Clean. Prod. 2019, 218, 850–870. [Google Scholar] [CrossRef]
- Worku, A.K.; Asfaw, A.A.; Ayele, D.W. Engineering of Co3O4 Electrodes via Ni and Cu-Doping for Supercapacitor Applications. Front. Chem. 2024, 12, 1357127. [Google Scholar] [CrossRef]
- Shinde, S.R.; Ruban, K. Advances in Cobalt Oxide-Based Supercapacitors: Recent Strategies and Performance Enhancement. ChemistrySelect 2025, 10, e01497. [Google Scholar] [CrossRef]
- Yang, J.; Shin, H.S. Recent Advances in Layered Transition Metal Dichalcogenides for Hydrogen Evolution Reaction. J. Mater. Chem. A 2014, 2, 5979–5985. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, Y.; Hao, Y.-C.; Ni, Y.-M.; Su, X.; Yin, A.-X.; Hu, C.-W. Ultrathin Cobalt Oxide Nanostructures with Morphology-Dependent Electrocatalytic Oxygen Evolution Activity. Nanoscale 2018, 10, 20313–20320. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble Metal-Free Hydrogen Evolution Catalysts for Water Splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- Wang, W.; Tian, Y.; Song, Y.; Ji, J.; Wang, F. Phase Controlled Synthesis and the Phase Dependent Photo- and Electrocatalysis of CdS@CoMo2S4/MoS2 Catalyst for HER. Int. J. Hydrogen Energy 2019, 44, 19890–19899. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, X.; Ma, H.; Sun, X.; Zhang, Y.; Yan, T.; Wei, Q.; Wu, D. Synthesis of Self-Supported Amorphous CoMoO4 Nanowire Array for Highly Efficient Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2017, 5, 10093–10098. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Zhan, C.; Yu, J.; Cao, Y.; Tu, J.; Shi, C. Phosphide–Oxide Honeycomb-Like Heterostructure CoP@CoMoOV/CC for Enhanced Hydrogen Evolution Reaction in Alkaline Solution. J. Mater. Sci. Technol. 2020, 46, 177–184. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, M.; Zhao, X.; Yang, J.; Fan, W. Synergistic Engineering of Architecture and Composition in NixCo1−xMoO4–CoMoO4 Nanobrush Arrays towards Efficient Overall Water Splitting Electrocatalysis. Nanoscale 2019, 11, 22820–22831. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, Z.; Lin, Y.; Wang, J.; Pan, H.; Xu, Z. Hierarchical Heterostructure NiCo2O4@CoMoO4/NF as an Efficient Bifunctional Electrocatalyst for Overall Water Splitting. J. Mater. Chem. A 2018, 6, 16950–16958. [Google Scholar] [CrossRef]
- Lu, W.; Song, Y.; Dou, M.; Ji, J.; Wang, F. Ni3S2@MoO3 Core/Shell Arrays on Ni Foam Modified with Ultrathin CdS Layer as a Superior Electrocatalyst for Hydrogen Evolution Reaction. Chem. Commun. 2018, 54, 646–649. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lee, K.; Schmuki, P. Anodic Formation of Self-Organized Cobalt Oxide Nanoporous Layers. Angew. Chem. Int. Ed. 2013, 52, 2077–2081. [Google Scholar] [CrossRef]
- Chondath, S.K.; Bansal, L.; Sahu, B.; Kumar, R. Dopant-Induced Defect Engineering in Transition Metal Oxide/Chalcogenide-Based Electrodes for High-Performance Supercapacitors: A Critical Review. ACS Appl. Energy Mater. 2025, 8, 8680–8709. [Google Scholar] [CrossRef]
- Jana, J.; Bhamu, K.C.; Ngo, Y.-L.T.; Kang, S.G.; Chung, J.S.; Hur, S.H. Designing a Bimetallic Transition Metal Oxide/Hydroxide Composite for Effective Electrocatalytic Oxygen Evolution Reaction. Appl. Surf. Sci. 2021, 562, 150253. [Google Scholar] [CrossRef]
- Pei, Z.; Xu, L.; Xu, W. Hierarchical Honeycomb-Like Co3O4 Pores Coating on CoMoO4 Nanosheets as Bifunctional Efficient Electrocatalysts for Overall Water Splitting. Appl. Surf. Sci. 2018, 433, 256–263. [Google Scholar] [CrossRef]
- Yan, Q.; Yang, X.; Wei, T.; Wu, W.; Yan, P.; Zeng, L.; Zhu, R.; Cheng, K.; Ye, K.; Zhu, K.; et al. Self-Supported Cobalt–Molybdenum Oxide Nanosheet Clusters as Efficient Electrocatalysts for Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2019, 44, 21220–21228. [Google Scholar] [CrossRef]
- Du, X.; Su, H.; Zhang, X. 3D Hierarchical Co3O4@Co3S4 Nanoarrays as Anode and Cathode Materials for Oxygen Evolution Reaction and Hydrogen Evolution Reaction. Dalton Trans. 2018, 47, 16305–16312. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Li, Y.; Jiang, H.; Jiang, H.; Li, C. Continuous Oxygen Vacancy Engineering of the Co3O4 Layer for an Enhanced Alkaline Electrocatalytic Hydrogen Evolution Reaction. J. Mater. Chem. A 2019, 7, 12240–12247. [Google Scholar] [CrossRef]
- Qu, C.; Cao, J.; Chen, Y.; Wei, M.; Fan, H.; Liu, X.; Li, X.; Wu, Q.; Feng, B.; Yang, L. In-Situ Growth of Hierarchical Trifunctional Co4S3/Ni3S2@MoS2 Core–Shell Nanosheet Array on Nickel Foam for Overall Water Splitting and Supercapacitor. Int. J. Hydrogen Energy 2023, 48, 648–661. [Google Scholar] [CrossRef]
- Xie, W.; Yu, T.; Ou, Z.; Zhang, J.; Li, R.; Song, S.; Wang, Y. Self-Supporting Clusters Constituted of Nitrogen-Doped CoMoO4 Nanosheets for Efficiently Catalyzing the Hydrogen Evolution Reaction in Alkaline Media. ACS Sustain. Chem. Eng. 2020, 8, 9070–9078. [Google Scholar] [CrossRef]
- Lu, W.; Song, Y.; Dou, M.; Ji, J.; Wang, F. Self-Supported Ni3S2@MoS2 Core/Shell Nanorod Arrays via Decoration with CoS as a Highly Active and Efficient Electrocatalyst for Hydrogen Evolution and Oxygen Evolution Reactions. Int. J. Hydrogen Energy 2018, 43, 8794–8804. [Google Scholar] [CrossRef]
- Xu, J.; Gao, L.; Cao, J.; Wang, W.; Chen, Z. Preparation and Electrochemical Capacitance of Cobalt Oxide (Co3O4) Nanotubes as Supercapacitor Material. Electrochim. Acta 2010, 56, 732–736. [Google Scholar] [CrossRef]
- Xiang, R.; Duan, Y.; Peng, L.; Wang, Y.; Tong, C.; Zhang, L.; Wei, Z. Three-Dimensional Core@Shell Co@CoMoO4 Nanowire Arrays as Efficient Alkaline Hydrogen Evolution Electrocatalysts. Appl. Catal. B Environ. 2019, 246, 41–49. [Google Scholar] [CrossRef]
- Lys, A.; Zabolotnii, V.; Čaplovičová, M.; Tepliakova, I.; Berzins, A.; Sahul, M.; Čaplovič, Ľ.; Pogrebnjak, A.; Iatsunskyi, I.; Viter, R. Core–Shell Nanofibers of ZnFe2O4/ZnO for Enhanced Visible-Light Photoelectrochemical Performance. J. Alloys Compd. 2024, 984, 173885. [Google Scholar] [CrossRef]
- Su, D.; Dou, S.; Wang, G. Single Crystalline Co3O4 Nanocrystals Exposed with Different Crystal Planes for Li–O2 Batteries. Sci. Rep. 2014, 4, 5767. [Google Scholar] [CrossRef]
- Gorimbo, J.; Chikati, R.; Khangale, P.; Beas, I.N.; Mguni, L.L.; Nkazi, D. Debunking the Impact of Crystallite/Particle Size in Cobalt-Based Fischer–Tropsch Synthesis. Chem. Eng. Commun. 2024, 211, 1262–1287. [Google Scholar] [CrossRef]
- Li, Y.; Keith, K.; Chopra, N. Structural and morphological evolution of free-standing Co3O4 nanowires via water vapor-assisted thermal oxidation of Co foil. J. Alloys Compd. 2017, 703, 414–423. [Google Scholar] [CrossRef]
- Abid, H.N.; Nayef, U.M.; Mutlak, F.A.H. Preparation and Characterization of Co3O4 Nanoparticles on Porous Silicon for Humidity Sensor by Photoluminescence. Optik 2019, 178, 379–383. [Google Scholar] [CrossRef]
- Mateus, H.M.; Bautista-Ruiz, J.; Barba-Ortega, J.; Rincón Joya, M. Formation of Titanium Oxide Nanotube Arrays by Controlling H2O and Time through Anodic Oxidation. Rasayan J. Chem. 2019, 12, 1304–1314. [Google Scholar] [CrossRef]
- Raja, K.S.; Misra, M.; Paramguru, K. Formation of Self-Ordered Nano-Tubular Structure of Anodic Oxide Layer on Titanium. Electrochim. Acta 2005, 51, 154–165. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Raja, A.; Devarajan, P.A. Structural Elucidation and Spectral Characterizations of Co3O4 Nanoflakes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 114, 586–591. [Google Scholar] [CrossRef]
- Tüysüz, H.; Liu, Y.; Weidenthaler, C.; Schüth, F. Pseudomorphic Transformation of Highly Ordered Mesoporous Co3O4 to CoO via Reduction with Glycerol. J. Am. Chem. Soc. 2008, 130, 14108–14110. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, S.; Lu, L.; Zeng, H.C. Preparation of Nanocomposites of Metals, Metal Oxides, and Carbon Nanotubes via Self-Assembly. J. Am. Chem. Soc. 2007, 129, 9401–9409. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Li, T.-T.; Zhu, H.-L.; Zheng, Y.-Q. Co3O4 Polyhedrons with Enhanced Electric Conductivity as Efficient Water Oxidation Electrocatalysts in Alkaline Medium. J. Mater. Sci. 2018, 53, 4323–4333. [Google Scholar] [CrossRef]
- Cárdenas, L.J.; Parra, C.A.; Cuervo, J.A.; Chiquito, A.J.; Moreno, L.C.; Rodríguez, J.E.; Rincón Joya, M. Electrical, Magnetic, and Sensing Properties of Acetone in Co3−xNixO4–rGO Samples. ACS Appl. Electron. Mater. 2024, 6, 4682–4697. [Google Scholar] [CrossRef]
- Babar, P.; Mahmood, J.; Maligal-Ganesh, R.V.; Kim, S.-J.; Xue, Z.; Yavuz, C.T. Electronic Structure Engineering for Electrochemical Water Oxidation. J. Mater. Chem. A 2022, 10, 20218–20241. [Google Scholar] [CrossRef]
- Zu, D.; Wang, H.; Lin, S.; Ou, G.; Wei, H.; Sun, S.; Wu, H. Oxygen-Deficient Metal Oxides: Synthesis Routes and Applications in Energy and Environment. Nano Res. 2019, 12, 2150–2163. [Google Scholar] [CrossRef]
- Vladimirova, S.; Krivetskiy, V.; Rumyantseva, M.; Gaskov, A.; Mordvinova, N.; Lebedev, O.; Martyshov, M.; Forsh, P. Co3O4 as p-Type Material for CO Sensing in Humid Air. Sensors 2017, 17, 2216. [Google Scholar] [CrossRef]
- Yin, H.; Zhu, J.; Chen, J.; Gong, J.; Nie, Q. PEG-Templated Assembling of Co3O4 Nanosheets with Nanoparticles for Enhanced Sensitive Non-Enzymatic Glucose Sensing Performance. J. Mater. Sci. Mater. Electron. 2018, 29, 17467–17475. [Google Scholar] [CrossRef]
- Rivas-Murias, B.; Salgueiriño, V. Thermodynamic CoO–Co3O4 Crossover Using Raman Spectroscopy in Magnetic Octahedron-Shaped Nanocrystals. J. Raman Spectrosc. 2017, 48, 837–841. [Google Scholar] [CrossRef]
- Luo, W.; Tian, H.; Li, Q.; Meng, G.; Chang, Z.; Chen, C.; Shen, R.; Yu, X.; Zhu, L.; Kong, F.; et al. Controllable Electron Distribution Reconstruction of Spinel NiCo2O4 Boosting Glycerol Oxidation at Elevated Current Density. Adv. Funct. Mater. 2024, 34, 2306995. [Google Scholar] [CrossRef]
- Cardenas-Flechas, L.J.; Freire, P.T.C.; Paris, E.C.; Moreno, L.C.; Joya, M.R. Temperature-Induced Structural Phase Transformation in Samples of Co3O4 and Co3−xNixO4 for CoO. Materialia 2021, 18, 101155. [Google Scholar] [CrossRef]
- Zhao, M.; Deng, J.; Liu, J.; Li, Y.; Liu, J.; Duan, Z.; Xiong, J.; Zhao, Z.; Wei, Y.; Song, W.; et al. Roles of Surface-Active Oxygen Species on 3DOM Cobalt-Based Spinel Catalysts MxCo3−xO4 (M = Zn and Ni) for NOx-Assisted Soot Oxidation. ACS Catal. 2019, 9, 7548–7567. [Google Scholar] [CrossRef]
- Mateus, H.M.; Barba-Ortega, J.; Rincón Joya, M. Comparison of the Growth of TiO2 Nanotubes in Different Solutions. J. Inorg. Organomet. Polym. 2018, 28, 612–623. [Google Scholar] [CrossRef]




| Element | Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 |
|---|---|---|---|---|
| Co (Cobalt) | 74.58 | 62.39 | 90.59 | 53.86 |
| O (Oxygen) | 21.32 | 32.32 | 6.92 | 40.04 |
| C (Carbon) | 4.10 | 4.44 | 2.50 | 5.20 |
| Ni (Nickel) | 0.00 | 0.75 | 0.00 | 0.90 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| Sample | Core Level | BE (eV) | Assignment | Lit. Range (eV) |
|---|---|---|---|---|
| Exp. 1 (Co3O4) | Co 2p3/2 | 780.4 | Co3+ | 779.6–780.2 [44] |
| Co 2p3/2 | 781.0 | Co2+ | 780.5–781.5 [44] | |
| Co 2p1/2 | 795.2 | Co3+ | 794.5–795.5 [44] | |
| Co 2p1/2 | 797.0 | Co2+ | 796.5–797.5 [44] | |
| O 1s | 530.0 | Lattice O (Co-O) | 529.6–530.2 [46] | |
| Exp. 2 (Co3O4 with Ni) | Co 2p3/2 | ∼780.0 | Co3+ | 779.6–780.2 [44] |
| Co 2p3/2 | ∼781.0 | Co2+ | 780.5–781.5 [44] | |
| Co 2p (Sat.) | 786 | Satellite peaks (Co2+) | – [47] | |
| Co 2p (shift) | ↓ | Partial reduction in Co2+ | 802.5 [47] | |
| Ni 2p3/2 | 867.0 | Ni0 or low-valence Ni | 866.5–867.5 [51] | |
| Exp. 4 (Co3O4 with Ni) | Co 2p3/2 | 780.0 | Co3+ | 779.6–780.2 [44] |
| Co 2p3/2 | 781.5 | Co2+ | 780.5–781.5 [44] | |
| Co 2p1/2 | 795.0 | Co3+ | 794.5–795.5 [44] | |
| Co 2p1/2 | 797.0 | Co2+ | 796.5–797.5 [44] | |
| O 1s | 529.5 | Lattice O (Co-O) | 529.6–530.2 [46] | |
| O 1s | 530.5 | Hydroxyl groups | 530.8–531.5 [46] | |
| O 1s | 531.8 | Oxygen vacancies or adsorbed H2O | 531.5–532.0 [47] | |
| Ni 2p3/2 | 855.0 | Ni2+ or Ni3+ | 854.5–855.5 [51] | |
| Ni 2p1/2 | 870.0 | Ni2+ or Ni3+ | 869.5–870.5 [51] |
| Sample | ν (cm−1) | Δν (cm−1) | FWHM (cm−1) |
|---|---|---|---|
| Exp. 0 | 702 | 0 | 15 |
| Exp. 3 | 700 | −2 | 14 |
| Exp. 2 | 698 | −4 | 16 |
| Exp. 1 | 696 | −6 | 17 |
| Exp. 4 | 694 | −8 | 18 |
| Samples | H2O (mL) | NH4F (g) | NiSO4·6H2O (g) | EG (mL) | Gly (mL) | V (V) | t (min.) |
|---|---|---|---|---|---|---|---|
| Exp. 0 | 0.5 | 0.3 | 0 | 145 | 0 | 30 | 30 |
| Exp. 1 | 2.0 | 0.0 | 0 | 145 | 0 | 30 | 30 |
| Exp. 2 | 0.5 | 0.3 | 1 | 145 | 48 | 30 | 30 |
| Exp. 3 | 2.0 | 0.3 | 0 | 145 | 48 | 30 | 30 |
| Exp. 4 | 0.5 | 0.3 | 1 | 145 | 48 | 40 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardenas Flechas, L.J.; Bautista-Ruiz, J.; Freire, P.T.C.; Paris, E.C.; Joya, M.R. Ultra-Rapid Synthesis of Co3O4 Nanostructures with Tunable Morphology via Nickel-Assisted Anodization. Inorganics 2025, 13, 350. https://doi.org/10.3390/inorganics13110350
Cardenas Flechas LJ, Bautista-Ruiz J, Freire PTC, Paris EC, Joya MR. Ultra-Rapid Synthesis of Co3O4 Nanostructures with Tunable Morphology via Nickel-Assisted Anodization. Inorganics. 2025; 13(11):350. https://doi.org/10.3390/inorganics13110350
Chicago/Turabian StyleCardenas Flechas, Leydi Julieta, Jorge Bautista-Ruiz, Paulo Tarso Cavalcante Freire, Elaine Cristina Paris, and Miryam Rincón Joya. 2025. "Ultra-Rapid Synthesis of Co3O4 Nanostructures with Tunable Morphology via Nickel-Assisted Anodization" Inorganics 13, no. 11: 350. https://doi.org/10.3390/inorganics13110350
APA StyleCardenas Flechas, L. J., Bautista-Ruiz, J., Freire, P. T. C., Paris, E. C., & Joya, M. R. (2025). Ultra-Rapid Synthesis of Co3O4 Nanostructures with Tunable Morphology via Nickel-Assisted Anodization. Inorganics, 13(11), 350. https://doi.org/10.3390/inorganics13110350








