Structural and Magneto-Optical Study on the Tetrahedrally Configured [CoCl2(1-allylimidazole)2] and Molecular Docking to Hypoxia-Inducible Factor-1α
Abstract
1. Introduction
2. Results and Discussion
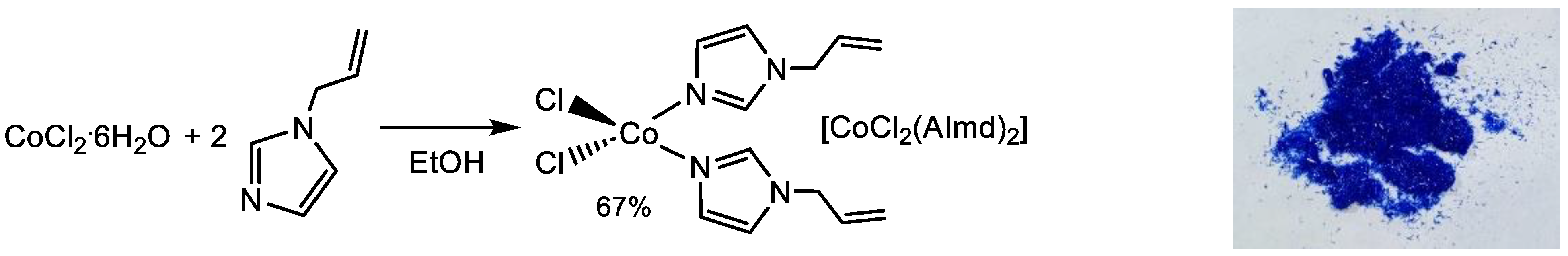
2.1. Synthesis and Characterization
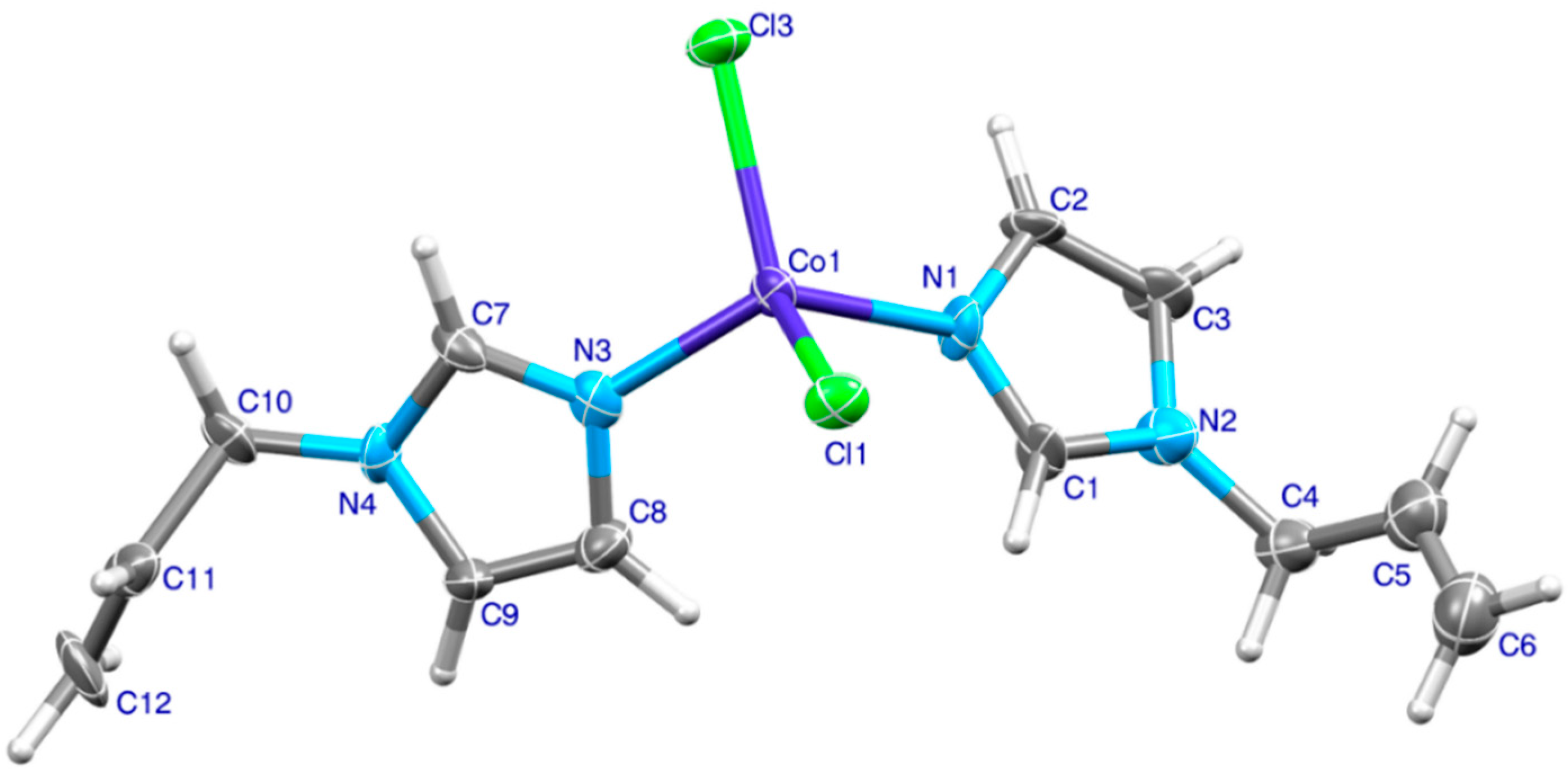
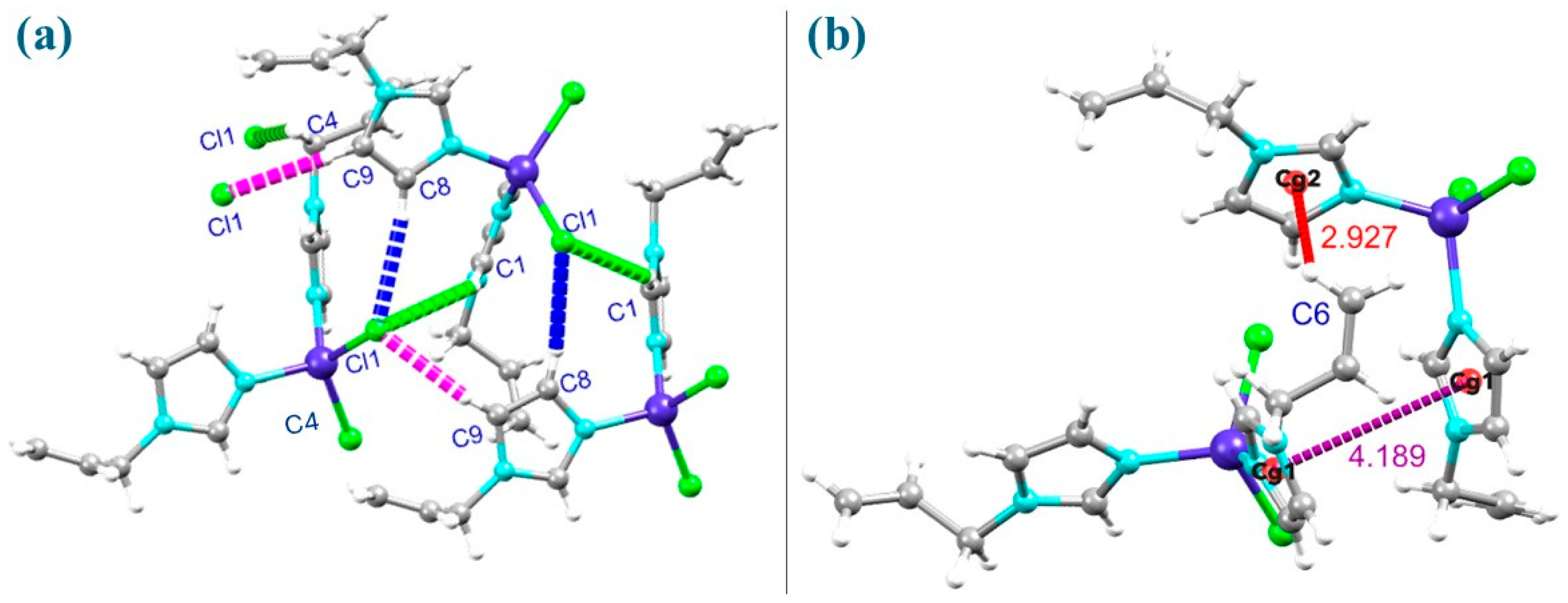
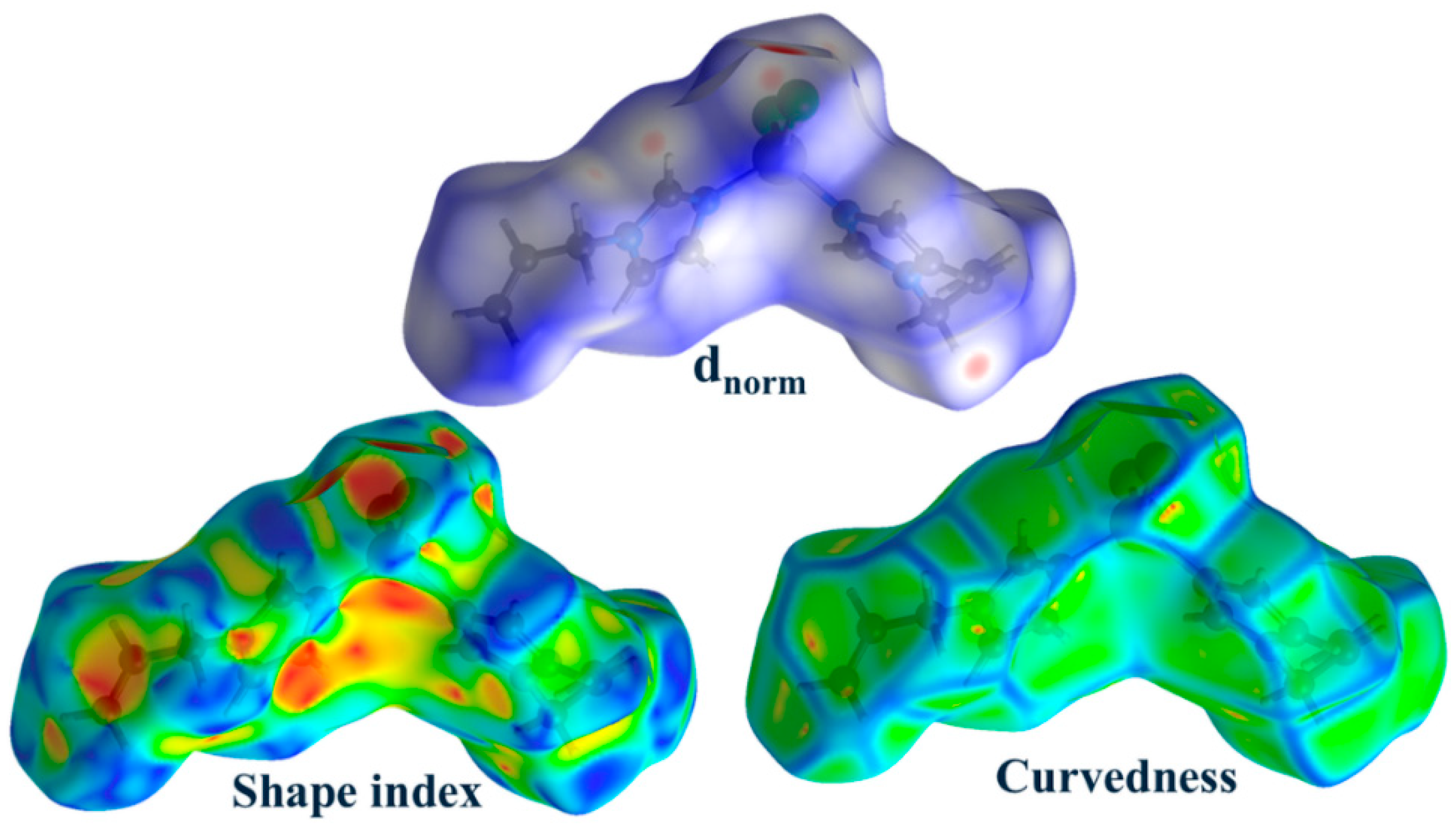
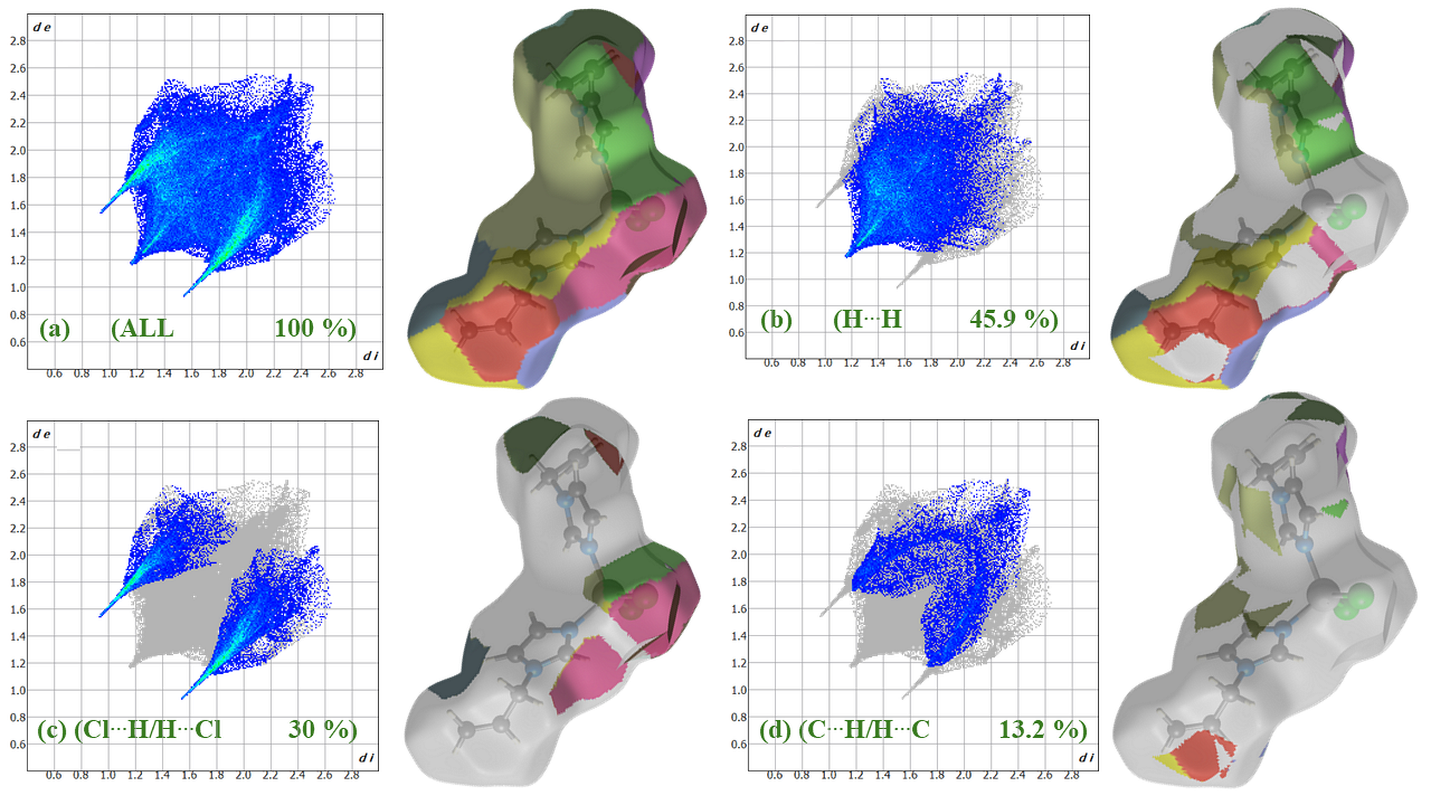
2.2. Structure Description, Hirshfeld Surface and Enrichment Ratio Analysis
2.3. QTAIM/NCI-RDG Analysis of a Dimer Model of [CoCl2(AImd)2]
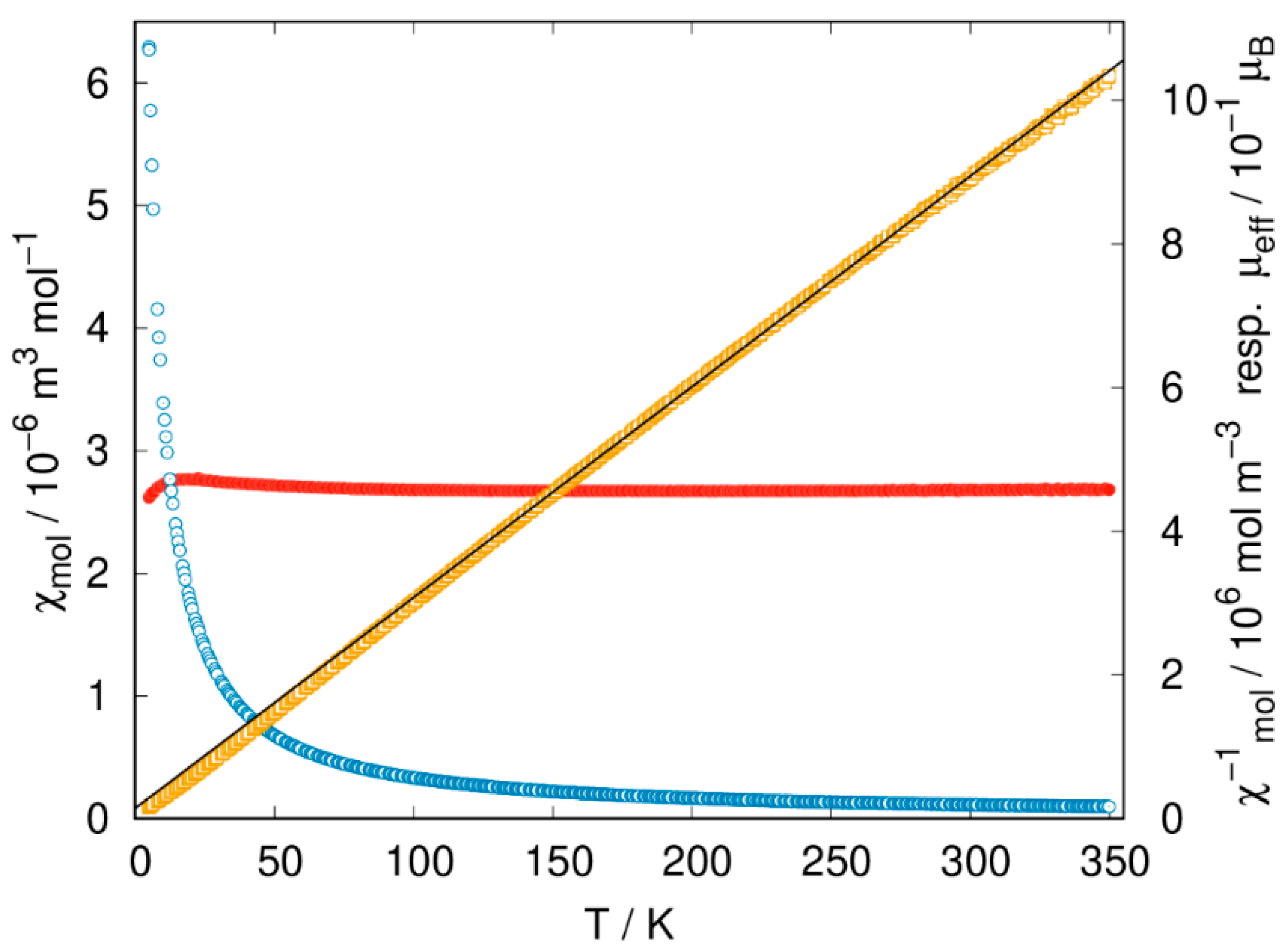
2.4. Magnetic Properties
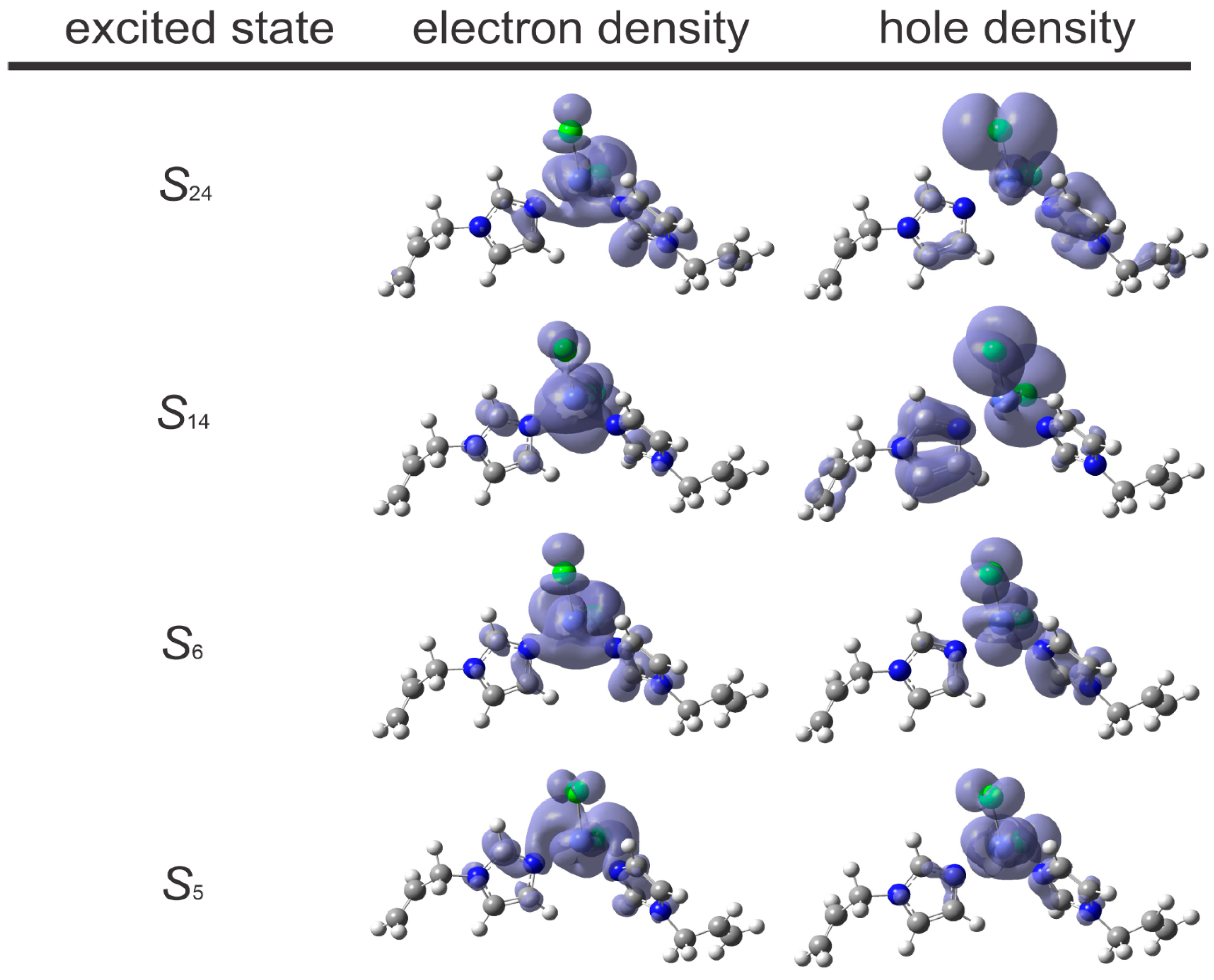
2.5. Experimental and TD-DFT-Calculated UV-Vis Absorptions in Solution
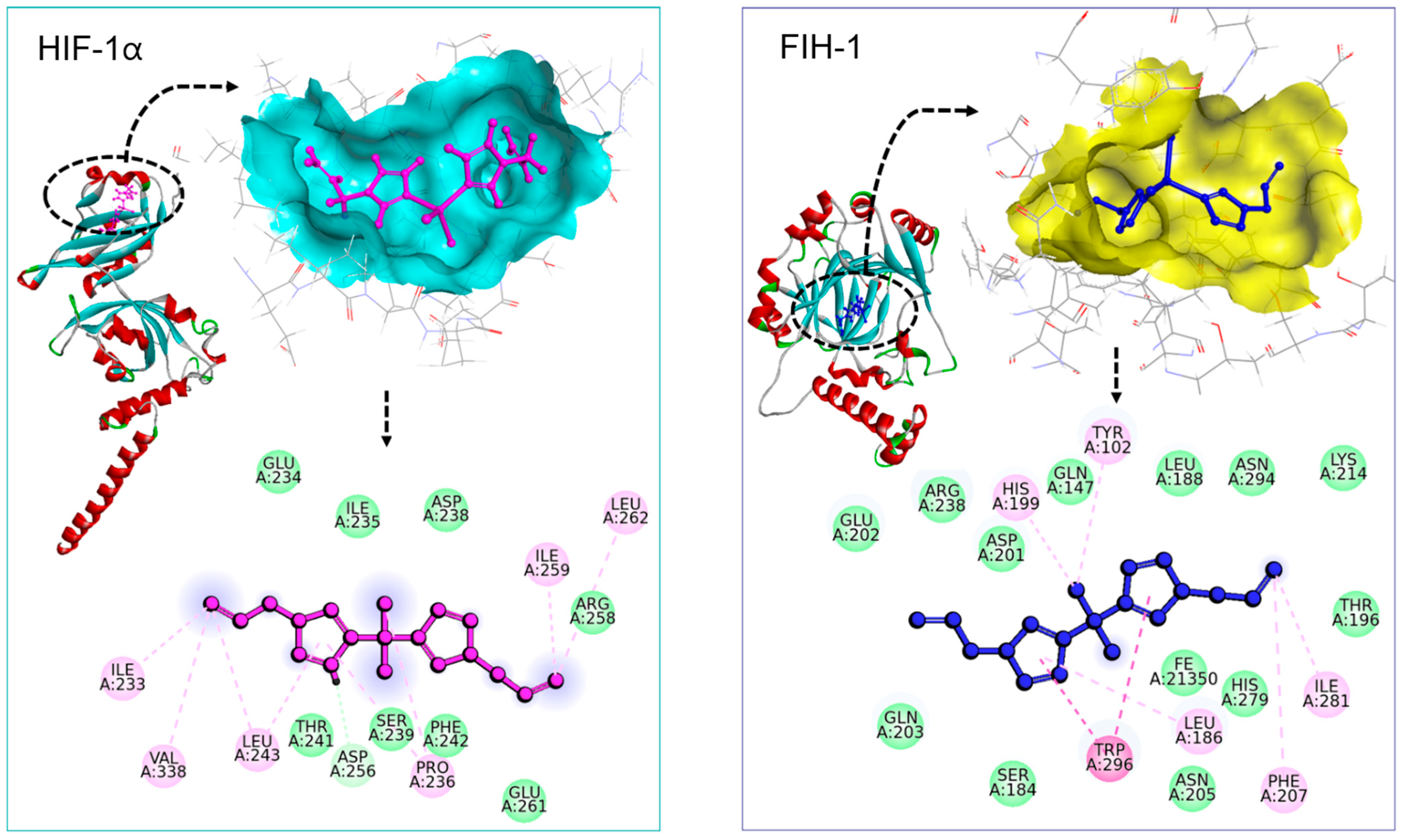
2.6. Molecular Docking
3. Materials and Methods
3.1. Materials
3.2. Synthesis of [CoCl2(AImd)2]
3.3. Instrumentation
3.4. Single Crystal X-Ray Diffraction
3.5. Hirshfeld Surface Analysis and Enrichment Ratio Calculations
3.6. DFT Calculations
3.7. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stroek, W.; Albrecht, M. Application of First-Row Transition Metal Complexes Bearing 1,2,3-Triazolylidene Ligands in Catalysis and Beyond. Chem. Soc. Rev. 2024, 53, 6322–6344. [Google Scholar] [CrossRef]
- Massoud, S.S.; Mautner, F.A.; Sakiyama, H.; Louka, F.R.; Salem, N.H.M.; Fischer, R.C.; Torvisco, A.; Guizouarn, T.; Velmurugan, G.; Comba, P.; et al. SMM Behavior in Distorted Trigonal Bipyramidal and Tetrahedral Cobalt(II) Complexes Based on Tripodal Tetradentate Phenolic Amines. Eur. J. Inorg. Chem. 2025, 28, e202400777. [Google Scholar] [CrossRef]
- Bielková, Z.; Titiš, J.; Marek, J.; Moncol, J.; Klokočíková, A.; Wesemann, C.; Renz, F.; Rajná, C. Zero-Field Splitting in Tetracoordinate Co(II) Complexes Containing Heterocyclic Aromatic Ligands. J. Mol. Struct. 2024, 1295, 136667. [Google Scholar] [CrossRef]
- Dutta, B.; Guizouarn, T.; Kotrle, K.; Pointillart, F.; Herchel, R.; Ray, D. Tuning Self-Aggregation and Nuclearity in Multinuclear Co(II) Complexes: Synthesis, Structural Diversity, and Changes in Magnetic Anisotropy and Relaxation Behavior. Cryst. Growth Des. 2025, 25, 3915–3927. [Google Scholar] [CrossRef]
- Oleksii, Y.; El-Ghayoury, A. Beyond Spin Crossover: Optical and Electronic Horizons of 2,6-Bis(pyrazol-1-yl)pyridine Ligands and Complexes. Molecules 2025, 30, 1314. [Google Scholar] [CrossRef]
- Sônego Milani, J.L.; Arruda da Mata, A.F.; Santos Oliveira, I.; Mendanha Valdo, A.K.S.; Terra Martins, F.; Rabelo, R.; Cangussu, D.; Cano, J.; Lloret, F.; Julve, M.; et al. Single-molecule magnet behaviour and catalytic properties of tetrahedral Co(II) complexes bearing chloride and 1,2-disubstituted benzimidazole as ligands. Dalton Trans. 2022, 51, 12258–12270. [Google Scholar] [CrossRef] [PubMed]
- Unavane, S.; Patil, R.; Syed, S.; Jain, H.K. Exploring the Therapeutic Potential of Copper and Cobalt Complexes as Anticancer Agents: A Comprehensive Review. Transit. Met. Chem. 2025, 50, 407–430. [Google Scholar] [CrossRef]
- Albobaledi, Z.; Tarahhomi, A.; Khaleghian, A.; van der Lee, A.; Excoffier, G. Novel Co2+ and Cd2+ Complexes Derived from a New N-Donor Pyridyl-Functionalized Thiophosphoric Triamide Ligand: Structural Investigation, DNA/COVID-19/Monkeypox Molecular Docking, and Biological Assays. Appl. Organomet. Chem. 2025, 39, e7750. [Google Scholar] [CrossRef]
- Chinnasamy, M.; Venkatesh, N.; Marimuthu, P.; Sathiyan, G.; Alharbi, S.A.; Venkatesan, G. Synthesis, Characterization, and Biological Evaluation of Novel Bioactive Schiff Base Metal Complexes and Their Molecular Docking Studies. J. Environ. Chem. Eng. 2025, 13, 115835. [Google Scholar] [CrossRef]
- Gispert, J.R. Coordination Chemistry; Wiley-VCH: Weinheim, Germany, 2008; ISBN 978-3-527-31802-5. [Google Scholar]
- Kaim, W.; Schwederski, B.; Klein, A. Bioinorganic Chemistry: Inorganic Elements in the Chemistry of Life—An Introduction and Guide; John Wiley & Sons, Ltd.: Chichester, UK, 2013; ISBN 978-0-470-97524-4. [Google Scholar]
- Borghesani, V.; Zastrow, M.L.; Tolbert, A.E.; Deb, A.; Penner-Hahn, J.E.; Pecoraro, V.L. Co(II) Substitution Enhances the Esterase Activity of a de Novo Designed Zn(II) Carbonic Anhydrase. Chem.–Eur. J. 2024, 30, e202304367. [Google Scholar] [CrossRef]
- Sterkhova, I.V.; Parshina, L.N.; Grishchenko, L.A.; Borodina, T.N.; Belovezhets, L.A.; Semenov, V.A. Metal Complexes of Cobalt Chloride with Vinyl-, Allyl- and Styrylbenzimidazole Ligands: Synthesis, Structure, and Properties. ChemistrySelect 2024, 9, e202403393. [Google Scholar] [CrossRef]
- Daisylet, B.S.; Raphael, S.J.; Kumar, P.; Rajan, P.P.; Dasan, A. Exploring the versatility of sulfur-containing heterocyclic metal complexes: Application in medical and prospects of visible-light-driven photocatalysis. J. Inorg. Biochem. 2024, 257, 112603. [Google Scholar] [CrossRef]
- Sterkhova, I.V.; Parshina, L.N.; Grishchenko, L.A.; Borodina, T.N.; Belovezhets, L.A.; Semenov, V.A. Six-coordinated complexes of Co(II), Ni(II) and Cu(II) chlorides with N-propargylimidazoles: Synthesis, structure, antimicrobial activity, AIM analysis and molecular docking. Polyhedron 2024, 260, 117093. [Google Scholar] [CrossRef]
- Yılmaz, Ü.; Apohan, E.; Küçükbay, H.; Yılmaz, Ö.; Tatlıcı, E.; Yesilada, Ö. Synthesis a group of 5(6)-substituted benzimidazole Zn(II) and Co(II) complexes and investigation their cytotoxic and antimicrobial activities. J. Heterocycl. Chem. 2022, 59, 1241–1246. [Google Scholar] [CrossRef]
- Parshina, L.N.; Grishchenko, L.A.; Smirnov, V.I.; Borodina, T.N.; Shakhmardanova, S.A.; Tarasov, V.V.; Apartsin, K.A.; Kireeva, V.V.; Trofimov, B.A. Synthesis, Characterization and Biological Evaluation of Zn(II) and Co(II) Complexes of N-Allylimidazole as Potential Hypoxia-Targeting Agents. Polyhedron 2019, 161, 126–131. [Google Scholar] [CrossRef]
- Yılmaz, Ü.; Tekin, S.; Buğday, N.; Yavuz, K.; Küçükbay, H.; Sandal, S. Synthesis and evaluation of anticancer properties of novel benzimidazole ligand and their cobalt(II) and zinc(II) complexes against cancer cell lines A-2780 and DU-145. Inorg. Chim. Acta 2019, 495, 118977. [Google Scholar] [CrossRef]
- Sahin, N.; Yıldırım, I.; Özdemir, N.; Gürbüz, N.; Özdemir, I. First used of Alkylbenzimidazole-Cobalt(II) complexes as a catalyst for the N-Alkylation of amines with alcohols under solvent-free medium. J. Organomet. Chem. 2020, 918, 121285. [Google Scholar] [CrossRef]
- Behera, N.; Manivannan, V. Molecular structures of some bivalent metal complexes of 1-(4-acetylphenyl)imidazole and co-ligands. Polyhedron 2018, 149, 84–94. [Google Scholar] [CrossRef]
- Bouchouit, M.; Bouacida, S.; Zouchoune, B.; Merazig, H.; Bua, S.; Bouaziz, Z.; Le Borgne, M.; Supuran, C.T.; Bouraiou, A. Synthesis, X-ray structure, in silico calculation, and carbonic anhydrase inhibitory properties of benzylimidazole metal complexes. J. Enzyme Inhib. Med. Chem. 2018, 33, 1150–1159. [Google Scholar] [CrossRef]
- Betanzos-Lara, S.; Chmel, N.P.; Zimmerman, M.T.; Barrón-Sosa, L.R.; Garino, C.; Salassa, L.; Rodger, A.; Brumaghim, J.L.; Gracia-Mora, I.; Barba-Behrens, N. Redox-active and DNA-binding coordination complexes of clotrimazole. Dalton Trans. 2015, 44, 3673–3685. [Google Scholar] [CrossRef]
- Mahdy, A.R.E.; Ali, O.A.A.; Serag, W.M.; Fayad, E.; Elshaarawy, R.F.M.; Gad, E.M. Synthesis, characterization, and biological activity of Co(II) and Zn(II) complexes of imidazoles-based azo-functionalized Schiff bases. J. Mol. Struct. 2022, 1259, 132726. [Google Scholar] [CrossRef]
- Sahin, N.; Üstün, E.; Özdemir, I.; Günal, S.; Özdemir, N.; Bülbül, H.; Gürbüz, N.; Özdemir, I.; Sémeril, D. Antimicrobial activities of bis-(N-alkylbenzimidazole)-cobalt(II) and zinc(II) complexes. Inorg. Chem. Commun. 2023, 157, 111396. [Google Scholar] [CrossRef]
- Sterkhova, I.V.; Parshina, L.N.; Grishchenko, L.A.; Borodina, T.N.; Belovezhets, L.A.; Semenov, V.A. Synthesis, Structure, and Antimicrobial Properties of New Cobalt(II) Complexes with 1-Propargylimidazoles. Curr. Org. Chem. 2024, 28, 708–715. [Google Scholar] [CrossRef]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef]
- Shi, Y.; Lin, X.; Wang, J.; Zhou, Z.; Chen, S.; Chen, G. Advances of HIF-1α/glycolysis axis in non-small cell lung cancer (Review). Oncol. Rep. 2024, 51, 55. [Google Scholar] [CrossRef]
- Tian, Y.-M.; Yeoh, K.K.; Lee, M.K.; Eriksson, T.; Kessler, B.M.; Kramer, H.B.; Edelmann, M.J.; Willam, C.; Pugh, C.W.; Schofield, C.J.; et al. Differential Sensitivity of Hypoxia Inducible Factor Hydroxylation Sites to Hypoxia and Hydroxylase Inhibitors. J. Biol. Chem. 2011, 286, 13041–13051. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Vandghanooni, S.; Dinparast, L.; Eskandani, M.; Ayatollahi, S.A.; Ata, A.; Nazemiyeh, H. Triterpenoid corosolic acid attenuates HIF-1 stabilization upon cobalt (II) chloride-induced hypoxia in A549 human lung epithelial cancer cells. Filoterapia 2019, 134, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Corner, T.P.; Teo, R.Z.R.; Wu, Y.; Salah, E.; Nakashima, Y.; Fiorini, G.; Tumber, A.; Brasnett, A.; Holt-Martyn, J.P.; Figg, W.D., Jr.; et al. Structure-guided optimisation of N-hydroxythiazole-derived inhibitors of factor inhibiting hypoxia-inducible factor-α. Chem. Sci. 2023, 14, 12098–12120. [Google Scholar] [CrossRef]
- Yadav, P.K.; Singh, S.; Singh, A.K. ‘3D-QSAR-based, pharmacophore modelling, virtual screening, and molecular docking studies for identification of hypoxia-inducible factor-1 inhibitor with potential bioactivity. Comp. Biol. Med. 2023, 166, 107557. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, K.K.; Chan, M.C.; Thalhammer, A.; Chowdhury, M.D.R.; Tian, Y.-M.; Stolze, I.; McNeill, L.A.; Lee, M.K.; Woon, E.C.Y.; Mackeen, M.M.; et al. Dual-action inhibitors of HIF prolyl hydroxylases that induce binding of a second iron ion. Org. Biomol. Chem. 2013, 11, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Jagathesan, K.; Roy, S. Recent Development of Transition Metal Complexes as Chemotherapeutic Hypoxia Activated Prodrug (HAP). ChemMedChem 2024, 19, e202400127. [Google Scholar] [CrossRef]
- Xue, J.-J.; Chen, Q.-Y.; Kong, M.-Y.; Zhu, C.-Y.; Gen, Z.-R.; Wang, Z.-L. Synthesis, cytotoxicity for mimics of catalase: Inhibitors of lactate dehydrogenase and hypoxia inducible factor. Eur. J. Med. Chem. 2014, 80, 1–7. [Google Scholar] [CrossRef]
- Wahengbam, S.; Sharma, H.; Chanu, P.R.; Masarkar, N.; Mukherjee, S.; Menon, M.B.; Malakar, C.C.; Roy, M. Bio-reductive Co(III)–doxorubicin complex for cancer cell-selective delivery of doxorubicin and potent anticancer activity. RSC Med. Chem. 2025, online. [Google Scholar] [CrossRef]
- Ke, Q.; Kluz, T.; Costa, M. Down-Regulation of the Expression of the FIH-1 and ARD-1 Genes at the Transcriptional Level by Nickel and Cobalt in the Human Lung Adenocarcinoma A549 Cell Line. Int. J. Environ. Res. Public Health 2005, 2, 10–13. [Google Scholar] [CrossRef]
- Sun, L.; Ding, X.; Kang, Y.J. ABCE1 selectively promotes HIF-1α transactivation of angiogenic gene expression. J. Trace Elem. Med. Biol. 2023, 80, 127307. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, W.; Li, G.-D.; Zhong, H.-J.; Dong, Z.-Z.; Wong, C.-Y.; Kwong, D.W.J.; Ma, D.-L.; Leung, C.-H. Anticancer osmium complex inhibitors of the HIF-1α and p300 protein-protein interaction. Sci. Rep. 2017, 7, 42860. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, D.; Wu, C.; Li, S.; Chen, F.; Peng Li, P.; Ko, C.-N.; Wang, W.; Lee, S.M.-Y.; Lin, L.; et al. Homocysteine-targeting compounds as a new treatment strategy for diabetic wounds via inhibition of the histone methyltransferase SET7/9. Nat. Exp. Mol. Med. 2022, 54, 988–998. [Google Scholar] [CrossRef]
- Li, G.; Ko, C.-N.; Li, D.; Yang, C.; Wang, W.; Yang, G.-J.; Di Primo, C.; Wong, V.K.W.; Xiang, Y.; Lin, L.; et al. A small molecule HIF-1α stabilizer that accelerates diabetic wound healing. Nat. Commun. 2021, 12, 3363. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Luo, J.; Wang, K.; Li, J.; Ma, Y.; Li, J.; Yang, H.; Chen, T.; Zhang, G.; Ji, X.; et al. Iridium metal complex targeting oxidation resistance 1 protein attenuates spinal cord injury by inhibiting oxidative stress-associated reactive oxygen species. Redox Biol. 2023, 67, 102913. [Google Scholar] [CrossRef]
- Che, X.; Liu, Q.; Zhang, L. An accurate and universal protein-small molecule batch docking solution using Autodock Vina. Res. Engin. 2023, 19, 101335. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Sherwood, A.M.; Kargbo, R.B.; Kaylo, K.W.; Cozzi, N.V.; Meisenheimer, P.; Kaduk, J.A. Psilocybin: Crystal Structure Solutions Enable Phase Analysis of Prior Art and Recently Patented Examples. Acta Crystallogr. C 2022, 78, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Bergese, P.; Bontempi, E.; Colombo, I.; Depero, L.E. Micro X-ray Diffraction on Capillary Powder Samples: A Novel and Effective Technique for Overcoming Preferred Orientation. J. Appl. Crystallogr. 2001, 34, 663–665. [Google Scholar] [CrossRef]
- Pınar, S.; Akkurt, M.; Kücükbay, H.; Orhan, E.; Büyükgüngör, O. Bis[1-(but-2-enyl)-5-nitro-1H-benzimidazole-κN3]-dichlorocobalt(II). Acta Crystallogr. E Struct. Rep. 2006, 62, m1663–m1665. [Google Scholar] [CrossRef]
- Vershinin, M.A.; Adonin, S.A. Crystal Structure of CoCl2.6H2O Reaction Products with 2-Methylpyridine and 2,6-Dimethylpyridine. J. Struct. Chem. 2021, 62, 90–94. [Google Scholar] [CrossRef]
- Adonin, S.A.; Bondarenko, M.A.; Novikov, A.S.; Sokolov, M.N. Halogen Bonding in Isostructural Co(II) Complexes with 2-Halopyridines. Crystals 2020, 10, 289. [Google Scholar] [CrossRef]
- Ahmadi, R.A.; Safari, N.; Khavasi, H.R.; Amani, S. Four new Co(II) complexes with 2-amino-4-methylpyridine, 2-amino-3-methylpyridine, or 2-amino-5-chloropyridine: Synthesis, spectroscopy, magnetic properties, and crystal structure. J. Coord. Chem. 2011, 64, 2056–2065. [Google Scholar] [CrossRef]
- Jelsch, C.; Ejsmont, K.; Huder, L. The enrichment ratio of atomic contacts in crystals, an indicator derived from the Hirshfeld surface analysis. IUCrJ 2014, 1, 119–128. [Google Scholar] [CrossRef]
- Ferjani, H. Structural, Hirshfeld Surface Analysis, Morphological Approach, and Spectroscopic Study of New Hybrid Iodobismuthate Containing Tetranuclear 0D Cluster Bi4I16·4(C6H9N2)·2(H2O). Crystals 2020, 10, 397. [Google Scholar] [CrossRef]
- Ferjani, H.; Lemine, O.M.; Ben Smida, Y.; Salah, N.; Kaouach, H.; Saadi, F.; Wahbi, H.I.; Almashnowi, M.Y.; Ramadan, R.; Onwudiwe, D.C. Visible Emission from a Zero-Dimensional Tin-Based Organic–Inorganic Metal Halide for Luminescent Devices: Experimental and Theoretical Investigation. J. Mol. Struct. 2025, 1327, 141210. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H⋯F–Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Rozas, I.; Elguero, J.; Molins, E. About the evaluation of the local kinetic, potential and total energy densities in closed-shell interactions. Chem. Phys. Lett. 2001, 336, 457–461. [Google Scholar] [CrossRef]
- Murrie, M. Cobalt(II) single-molecule magnets. Chem. Soc. Rev. 2010, 39, 1986–1995. [Google Scholar] [CrossRef]
- Ostrovsky, S.; Tomkowicz, Z.; Haase, W. High-spin Co(II) in monomeric and exchange coupled oligomeric structures: Magnetic and magnetic circular dichroism investigations. Coord. Chem. Rev. 2009, 253, 2363–2375. [Google Scholar] [CrossRef]
- Murray, K.S. Advances in Polynuclear Iron(II), Iron(III) and Cobalt(II) Spin-Crossover Compounds. Eur. J. Inorg. Chem. 2008, 2008, 3101–3121. [Google Scholar] [CrossRef]
- Titiš, J.; Miklovič, J.; Boča, R. Magnetostructural Study of Tetracoordinate Cobalt(II) Complexes. Inorg. Chem. Commun. 2013, 35, 72–75. [Google Scholar] [CrossRef]
- Criado, J.J.; Jimenez-Sanchez, A.; Cano, F.H.; Saez-Puche, R.; Rodríguez-Fernandez, E. Preparation and characterization of tetrachlorocobaltates(II) of α,ω-alkylenediammonium. Magnetic and thermal properties. Crystal structure of [NH3(CH2)5NH3]CoCl4. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1999, 5, 947–952. [Google Scholar] [CrossRef]
- Liu, J.-J.; Jiang, S.-D.; Neugebauer, P.; van Slageren, J.; Lan, Y.; Wernsdorfer, W.; Wang, B.-W.; Gao, S. Magnetic and HFEPR Studies of Exchange Coupling in a Series of μ-Cl Dicobalt Complexes. Inorg. Chem. 2017, 56, 2417–2425. [Google Scholar] [CrossRef]
- Protsenko, A.N.; Shakirova, O.G.; Protsenko, A.E.; Kuratieva, N.V.; Fowles, S.M.; Turnbull, M.M. Effect of isomeric cations of 3(2)-(chloromethyl)pyridine on the structure and properties of copper(II) and cobalt(II) complexes. J. Mol. Struct. 2021, 1240, 130561. [Google Scholar] [CrossRef]
- Garci, F.; Chebbi, H.; Rouzbeh, N.; Rochels, L.; Disch, S.; Klein, A.; Faouzi Zid, M. Structure, optical and magnetic properties of the pyridinium cobaltate (C6H9N2)2[CoCl4]. Inorg. Chim. Acta 2022, 539, 121003. [Google Scholar] [CrossRef]
- Issaoui, F.; Amamou, W.; Bekri, M.; Zouari, F.; Dhahri, E.; Valente, M.A. Structural, magnetic and vibrational characterization of the new organic-inorganic hybrid material, (C9H14N)2CoCl4. J. Mol. Struct. 2019, 1189, 175–180. [Google Scholar] [CrossRef]
- Pietrzyk, P.; Srebro, M.; Radoń, M.; Sojka, Z.; Michalak, A. Spin Ground State and Magnetic Properties of Cobalt(II): Relativistic DFT Calculations Guided by EPR Measurements of Bis(2,4-acetylacetonate)cobalt(II)-Based Complexes. J. Phys. Chem. A 2011, 115, 2316–2324. [Google Scholar] [CrossRef]
- Ferjani, H.; Bechaeib, R.; Gil, D.M.; Klein, A. The 1D Hybrid Material Allylimidazolium Iodoantimonate: A Combined Experimental and Theoretical Study. Inorganics 2025, 13, 243. [Google Scholar] [CrossRef]
- Figgis, B.N.; Hitchman, M.A. Ligand Field Theory and Its Applications; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Klein, A.; Elmas, S.; Butsch, K. Oxido Pincer Ligands—Exploring their Coordination Chemistry of Bis(hydroxymethyl)pyridine Ligands for the Late Transition Metals. Eur. J. Inorg. Chem. 2009, 2009, 2271–2281. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Mukund, V.; Saddala, M.S.; Farran, B.; Mannavarapu, M.; Alam, A.; Nagaraju, G.P. Molecular docking studies of angiogenesis target protein HIF-1α and genistein in breast cancer. Gene 2019, 701, 169–172. [Google Scholar] [CrossRef]
- Tang, X.; Lu, J.; Chen, H.; Zhai, L.; Zhang, Y.; Lou, H.; Wang, Y.; Sun, L.; Song, B. Underlying Mechanism and Active Ingredients of Tianma Gouteng Acting on Cerebral Infarction as Determined via Network Pharmacology Analysis Combined with Experimental Validation. Front. Pharmacol. 2021, 12, 760503. [Google Scholar] [CrossRef]
- He, Y.; Diao, S.; Hou, S.; Li, T.; Meng, W.; Zhang, J. Identification of novel potential hypoxia-inducible factor-1α inhibitors through machine learning and computational simulations. Front. Chem. 2025, 13, 1585882. [Google Scholar] [CrossRef]
- Riccardi, L.; Genna, V.; De Vivo, M. Metal–ligand interactions in drug design. Nat. Rev. Chem. 2018, 2, 100–112. [Google Scholar] [CrossRef]
- Lee, K.; Kang, J.E.; Park, S.-K.; Jin, Y.; Chung, K.-S.; Kim, H.-M.; Lee, K.; Kang, M.R.; Lee, M.K.; Song, K.B.; et al. LW6, a novel HIF-1 inhibitor, promotes proteasomal degradation of HIF-1α via upregulation of VHL in a colon cancer cell line. Biochem. Pharmacol. 2010, 80, 982–989. [Google Scholar] [CrossRef]
- Esakkimuthukumar, M.; Swaroop, A.K.; Patnaik, S.K.; Kumar, R.R.; Praveen, T.K.; Naik, M.R.; Selvaraj, J. A novel family of small molecule HIF-1 alpha stabilizers for the treatment of diabetic wounds; an integrated in silico, in vitro, and in vivo strategy. RSC Adv. 2022, 12, 31293–31302. [Google Scholar] [CrossRef]
- Lueken, H.; Schilder, H.; Eifert, T.; Handrick, K.; Hüning, F. Magnetochemistry: Compounds and Concepts. In Advances in Solid State Physics; Kramer, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; Volume 41. [Google Scholar] [CrossRef]
- APEX4—Software Suite for Crystallographic Programs; Bruker AXS, Inc.: Madison, WI, USA, 2021.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A Found. Adv. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Byrom, P.G. A Novel Definition of a Molecule in a Crystal. Chem. Phys. Lett. 1997, 267, 215–220. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel Tools for Visualizing and Exploring Intermolecular Interactions in Molecular Crystals. Acta Crystallogr. Sect. B Struct. Sci. Crystal Engin. Mater. 2004, 60, 627–668. [Google Scholar] [CrossRef]
- Wood, P.A.; McKinnon, J.J.; Parsons, S.; Pidcock, E.; Spackman, M.A. Analysis of the Packing of Organic Molecules Using Hirshfeld Surfaces. CrystEngComm 2008, 10, 368–376. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic Interaction of a Solute with a Continuum. A Direct Utilization of AB Initio Molecular Potentials for the Prevision of Solvent Effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Bacskay, G.B. A Quadratically Convergent Hartree—Fock (QC-SCF) Method. Application to Closed Shell Systems. Chem. Phys. 1981, 61, 385–404. [Google Scholar] [CrossRef]
- O’boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A Library for Package-Independent Computational Chemistry Algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef]
- Lu, T. A Comprehensive Electron Wavefunction Analysis Toolbox for Chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-Atom Fragments for Describing Molecular Charge Densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- BIOVIA, Dassault Systèmes, Discovery Studio Visualizer, Version 20.1.0.19295 SDDS 2020. Available online: https://discover.3ds.com/discovery-studio-visualizer-download (accessed on 2 October 2025).


| Atoms | H | Cl | C | N | Co |
|---|---|---|---|---|---|
| Surface % | 70.9 | 15 | 8 | 4.25 | 1.2 |
| EXY = CXY/RXY | |||||
| Co | 1.41 | - | - | - | - |
| Cl | 1.41 | - | - | - | - |
| N | 0.93 | - | 3.38 | 1.67 | - |
| C | 1.16 | - | 2.19 | 3.38 | - |
| H | 0.91 | 1.41 | 1.16 | 0.93 | 1.41 |
| State (Wavelength) | osc. Strength | Main Transition | Contribution (%) |
|---|---|---|---|
| S5 (685.34 nm) | 0.0370 | HOMO(β)→LUMO(β) | 52 |
| S6 (667.07 nm) | 0.0040 | HOMO–1(β)→LUMO+1(β) | 52 |
| S14 (248.14 nm) | 0.0095 | HOMO–4(β)→LUMO(β) | 68 |
| S24 (226.35 nm) | 0.0103 | HOMO–5(β)→LUMO+1(β)/ HOMO–7(β)→LUMO+1(β) | 27/ 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferjani, H.; Silva, B.P.e.; Azam, F.; Abou El-Reash, Y.G.; Yousef, T.; Rouzbeh, N.; Rochels, L.; Disch, S.; Schäfer, S.A.; Klein, A. Structural and Magneto-Optical Study on the Tetrahedrally Configured [CoCl2(1-allylimidazole)2] and Molecular Docking to Hypoxia-Inducible Factor-1α. Inorganics 2025, 13, 344. https://doi.org/10.3390/inorganics13110344
Ferjani H, Silva BPe, Azam F, Abou El-Reash YG, Yousef T, Rouzbeh N, Rochels L, Disch S, Schäfer SA, Klein A. Structural and Magneto-Optical Study on the Tetrahedrally Configured [CoCl2(1-allylimidazole)2] and Molecular Docking to Hypoxia-Inducible Factor-1α. Inorganics. 2025; 13(11):344. https://doi.org/10.3390/inorganics13110344
Chicago/Turabian StyleFerjani, Hela, Bruno Poti e Silva, Faizul Azam, Yasmeen G. Abou El-Reash, Tarek Yousef, Nahal Rouzbeh, Leonhard Rochels, Sabrina Disch, Sascha A. Schäfer, and Axel Klein. 2025. "Structural and Magneto-Optical Study on the Tetrahedrally Configured [CoCl2(1-allylimidazole)2] and Molecular Docking to Hypoxia-Inducible Factor-1α" Inorganics 13, no. 11: 344. https://doi.org/10.3390/inorganics13110344
APA StyleFerjani, H., Silva, B. P. e., Azam, F., Abou El-Reash, Y. G., Yousef, T., Rouzbeh, N., Rochels, L., Disch, S., Schäfer, S. A., & Klein, A. (2025). Structural and Magneto-Optical Study on the Tetrahedrally Configured [CoCl2(1-allylimidazole)2] and Molecular Docking to Hypoxia-Inducible Factor-1α. Inorganics, 13(11), 344. https://doi.org/10.3390/inorganics13110344











