A Comprehensive Physicochemical Characterization of Silver Nanoparticles as a Prerequisite for Their Successful Biomedical Applications
Abstract
1. Introduction to Silver Nanoparticles (AgNPs)
2. Physicochemical Properties of Silver Nanoparticles Studied by Complementary Characterization Techniques
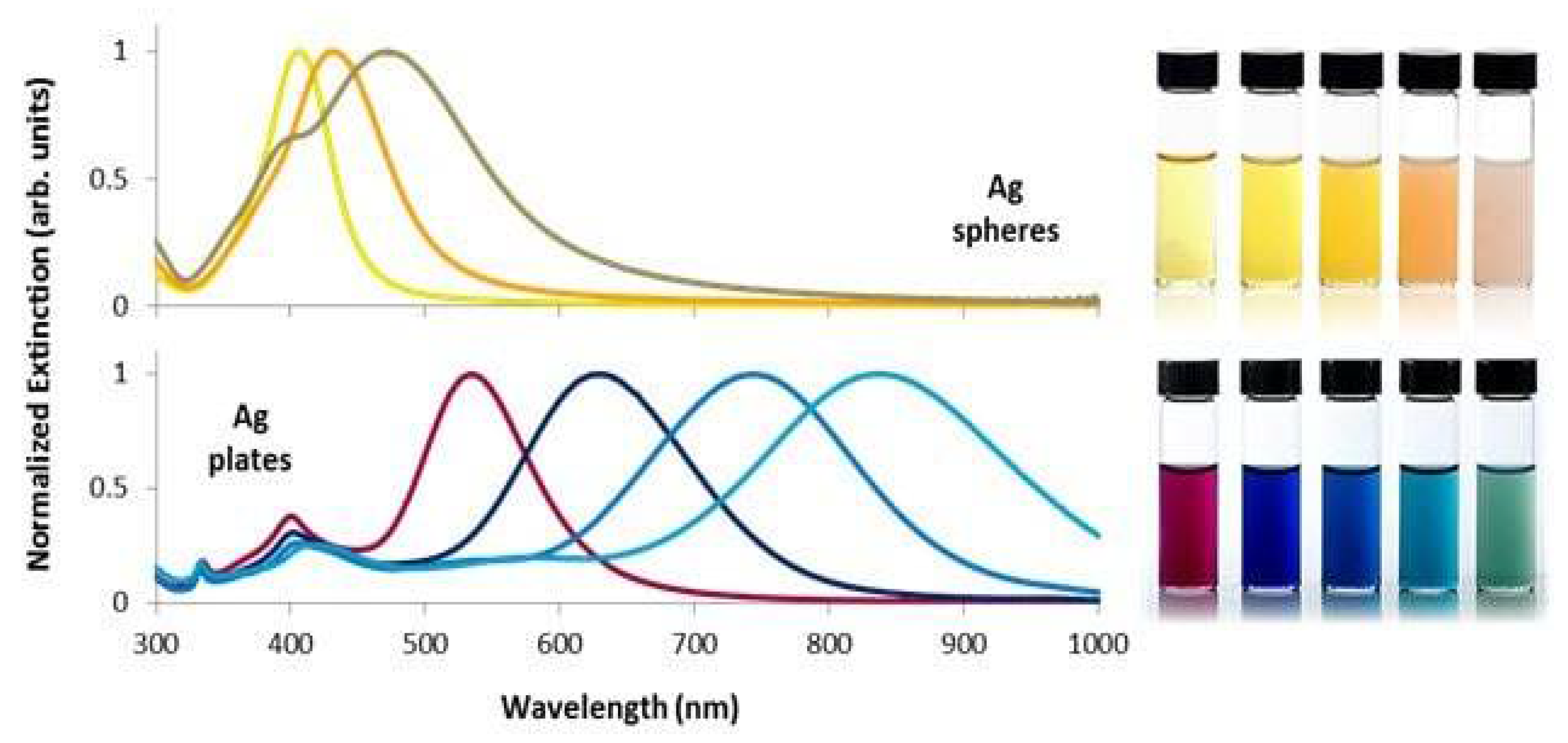
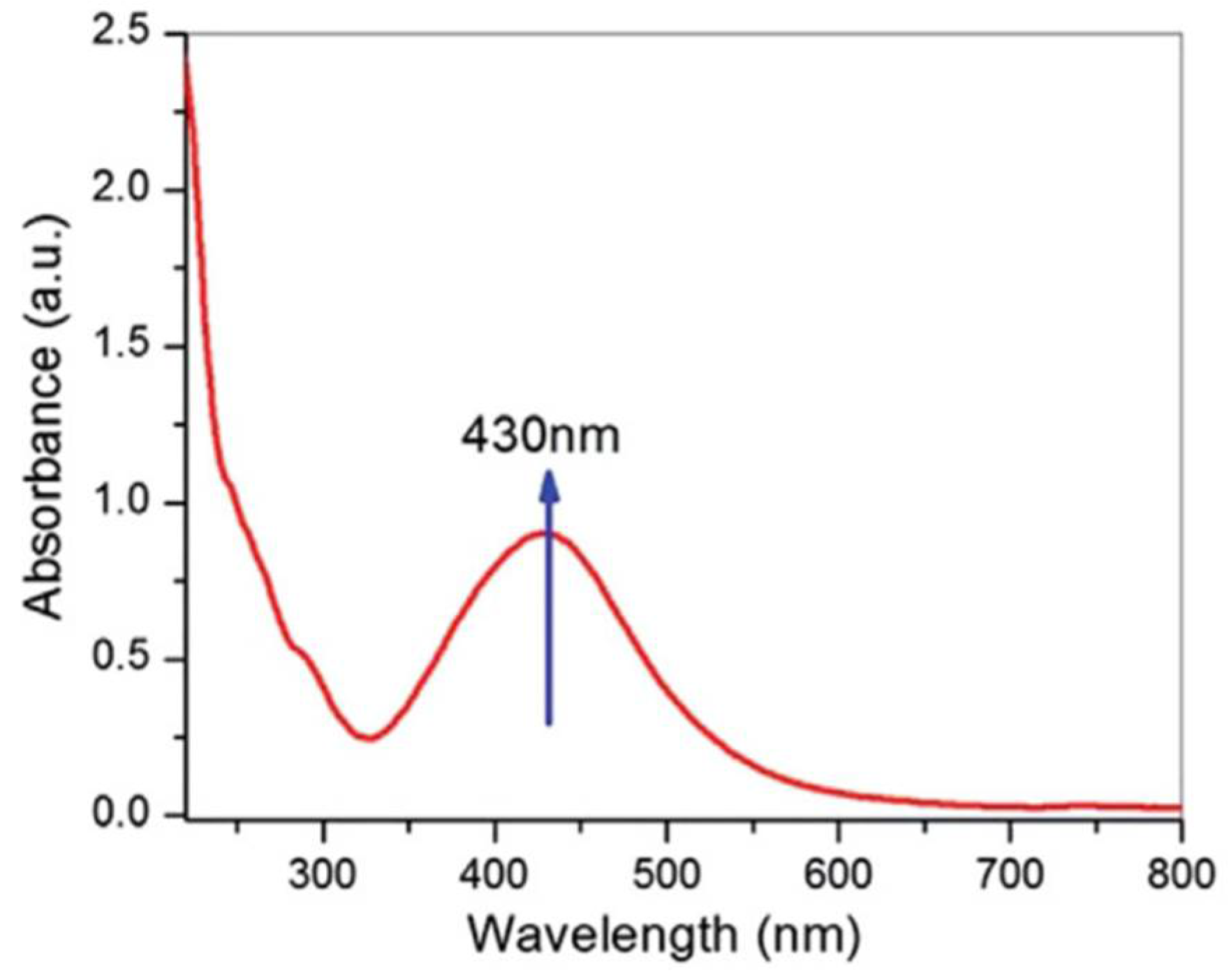
2.1. Ultraviolet-Visible Spectroscopy (UV–Vis)
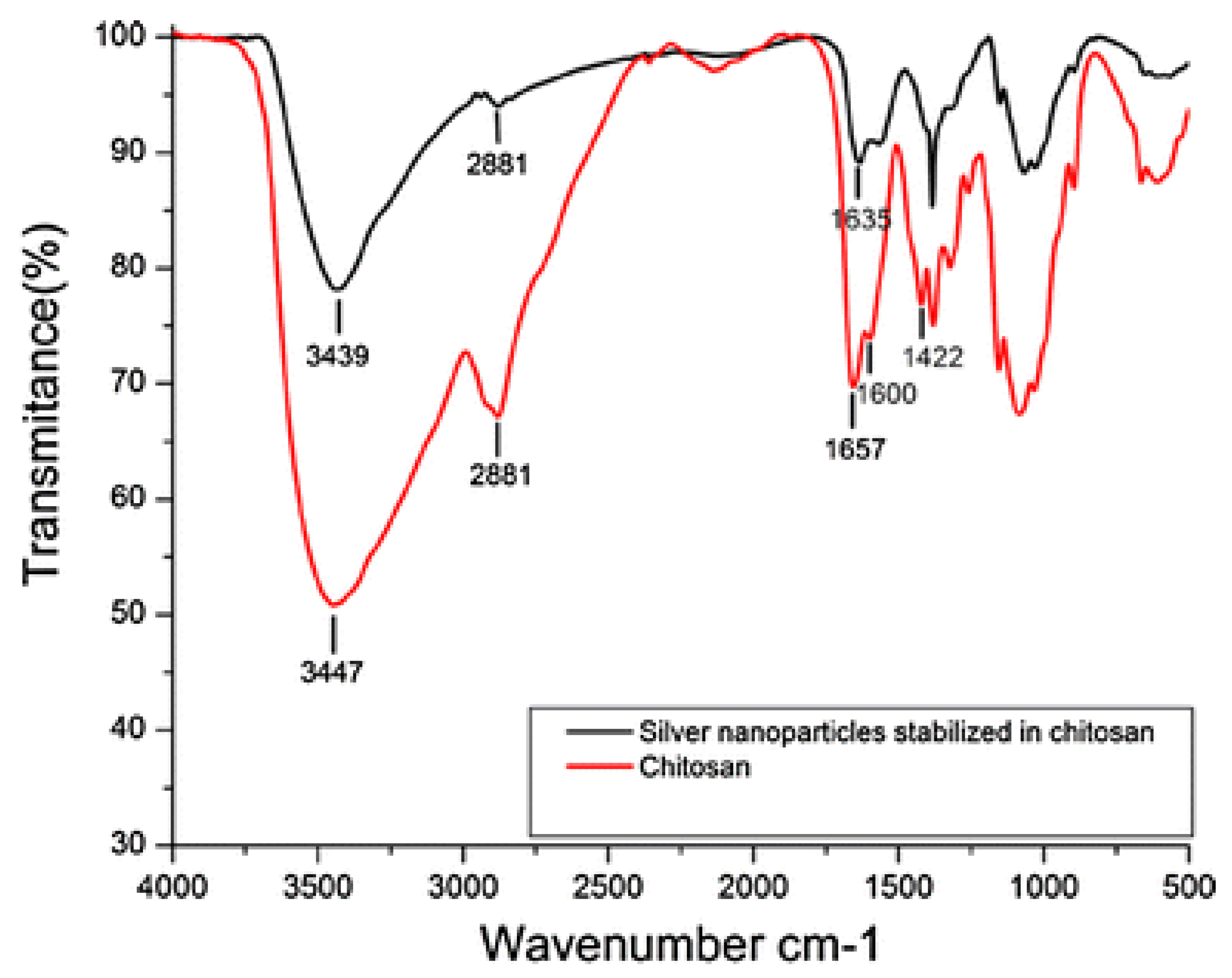
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
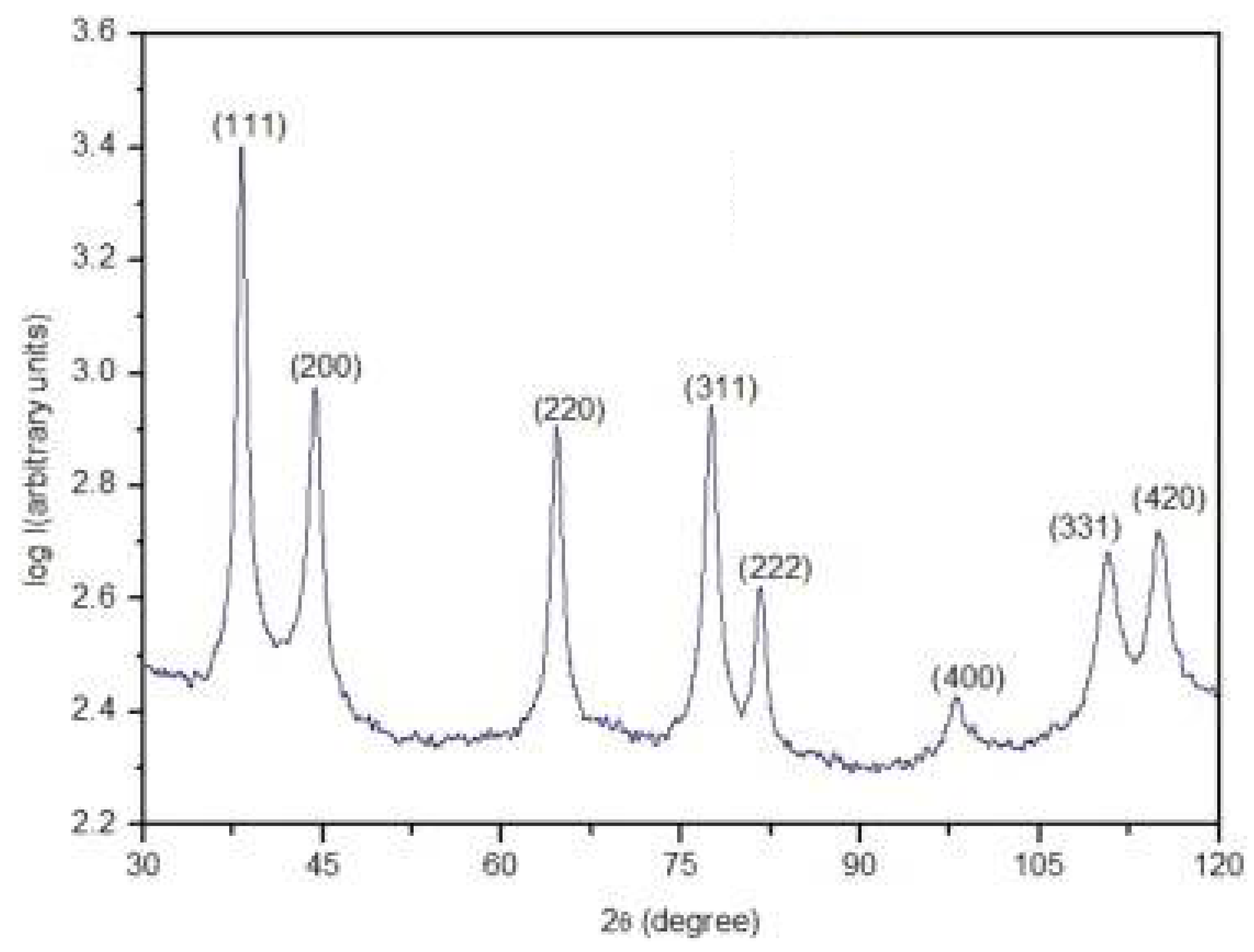
2.3. X-Ray Diffraction (XRD)
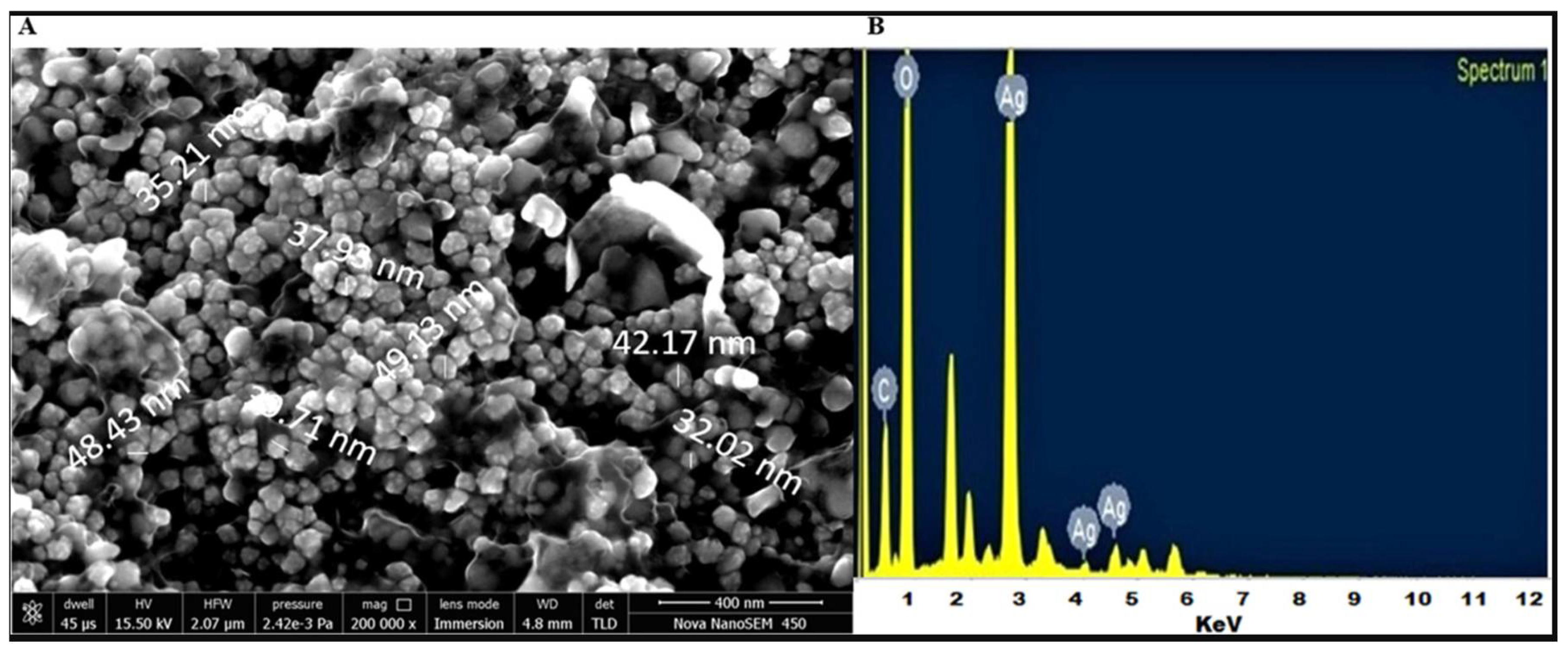
2.4. Scanning Electron Microscopy (SEM)
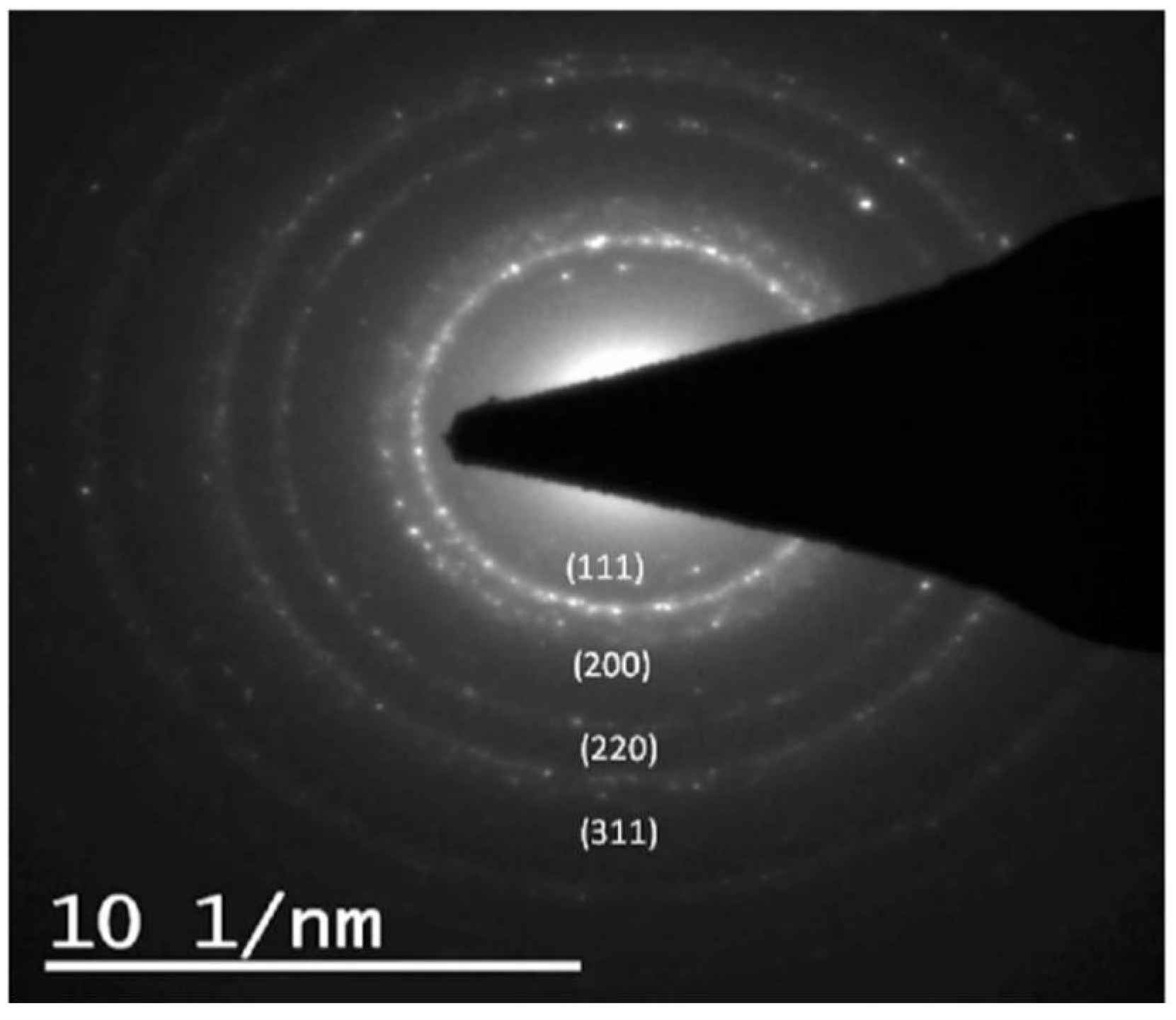
2.5. Tranmission Electron Microscopy (TEM)
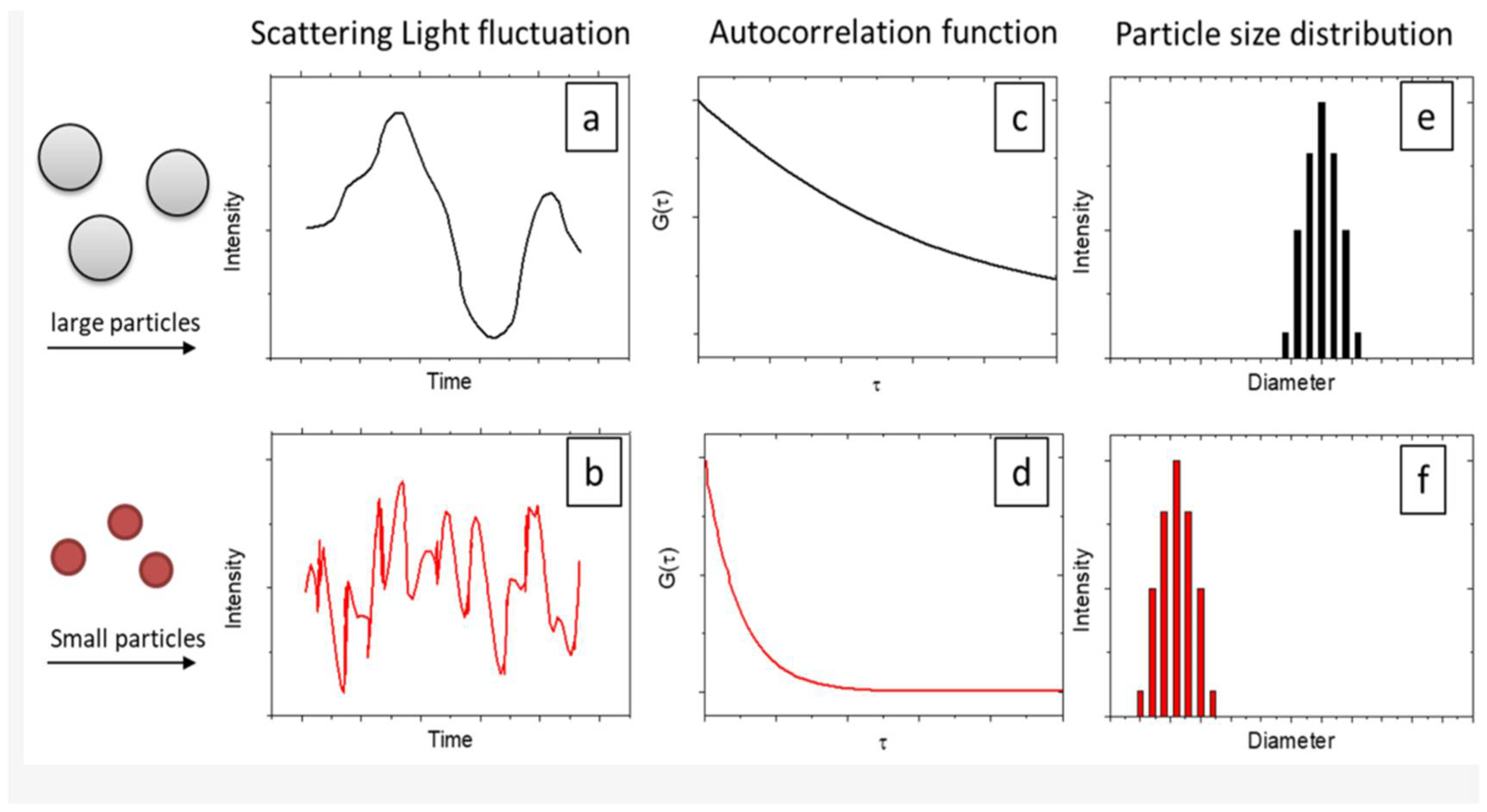
2.6. Dynamic Light Scattering (DLS)
2.7. Zeta-Potential
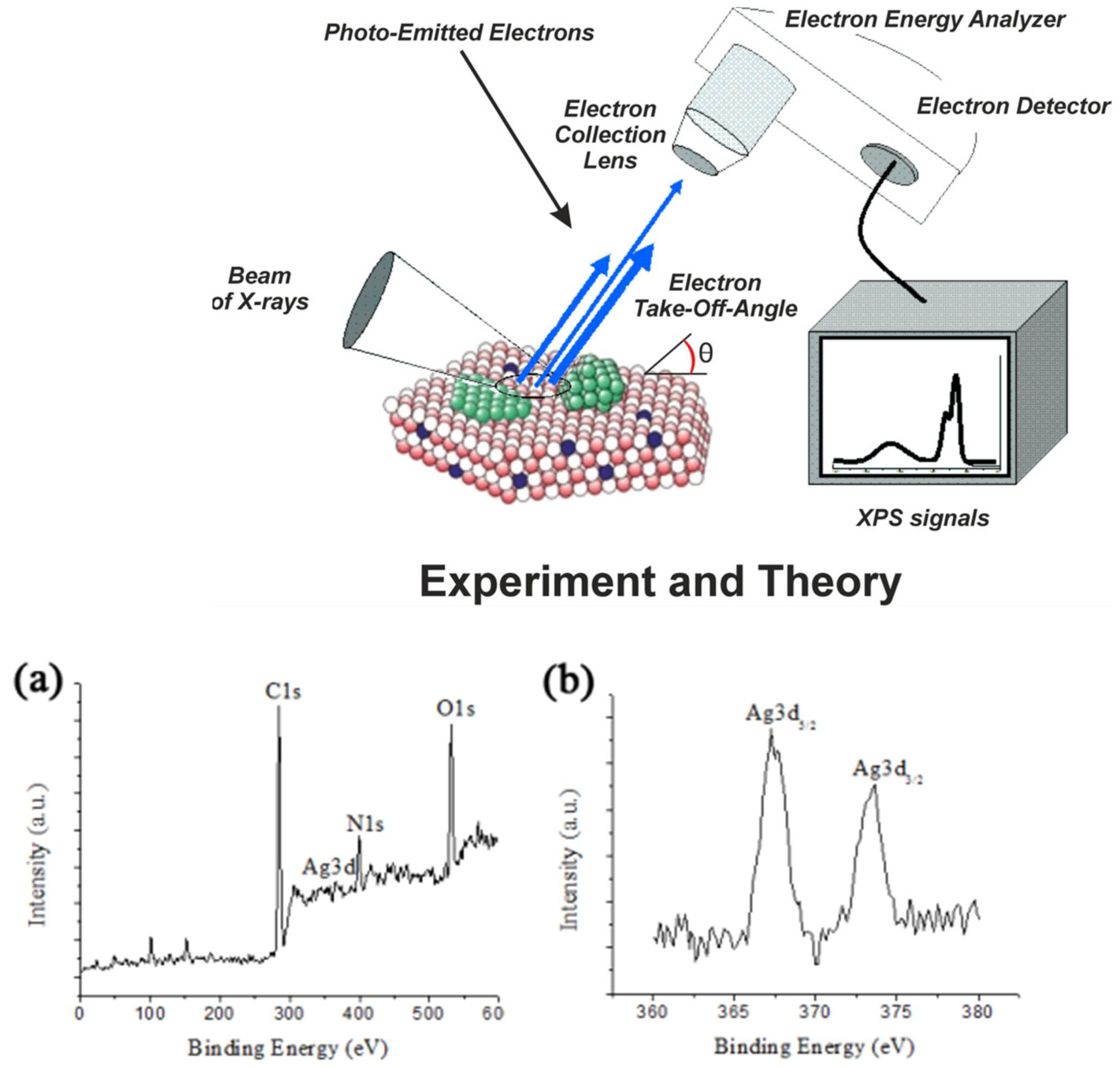
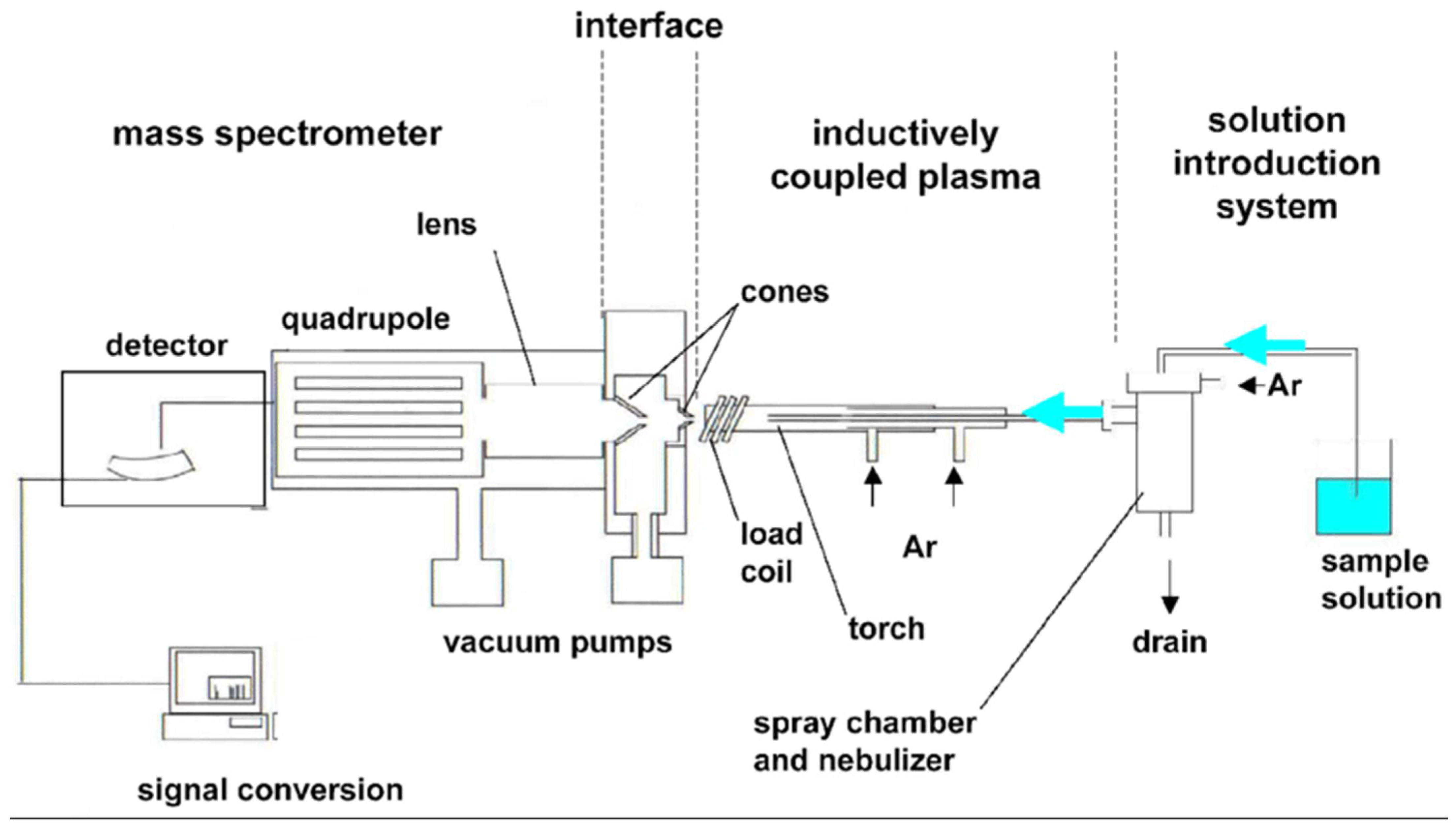
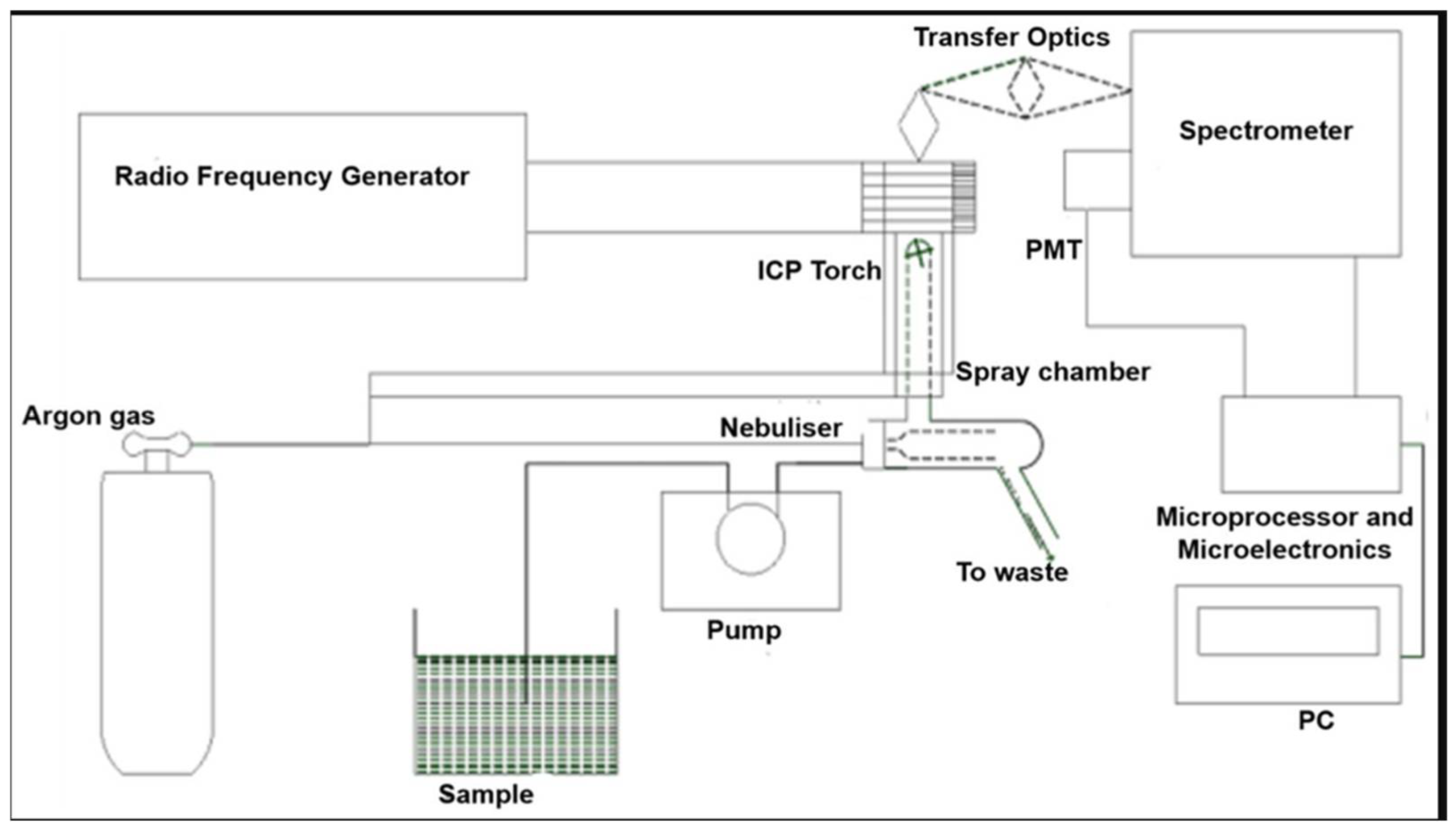
2.8. Advanced Techniques
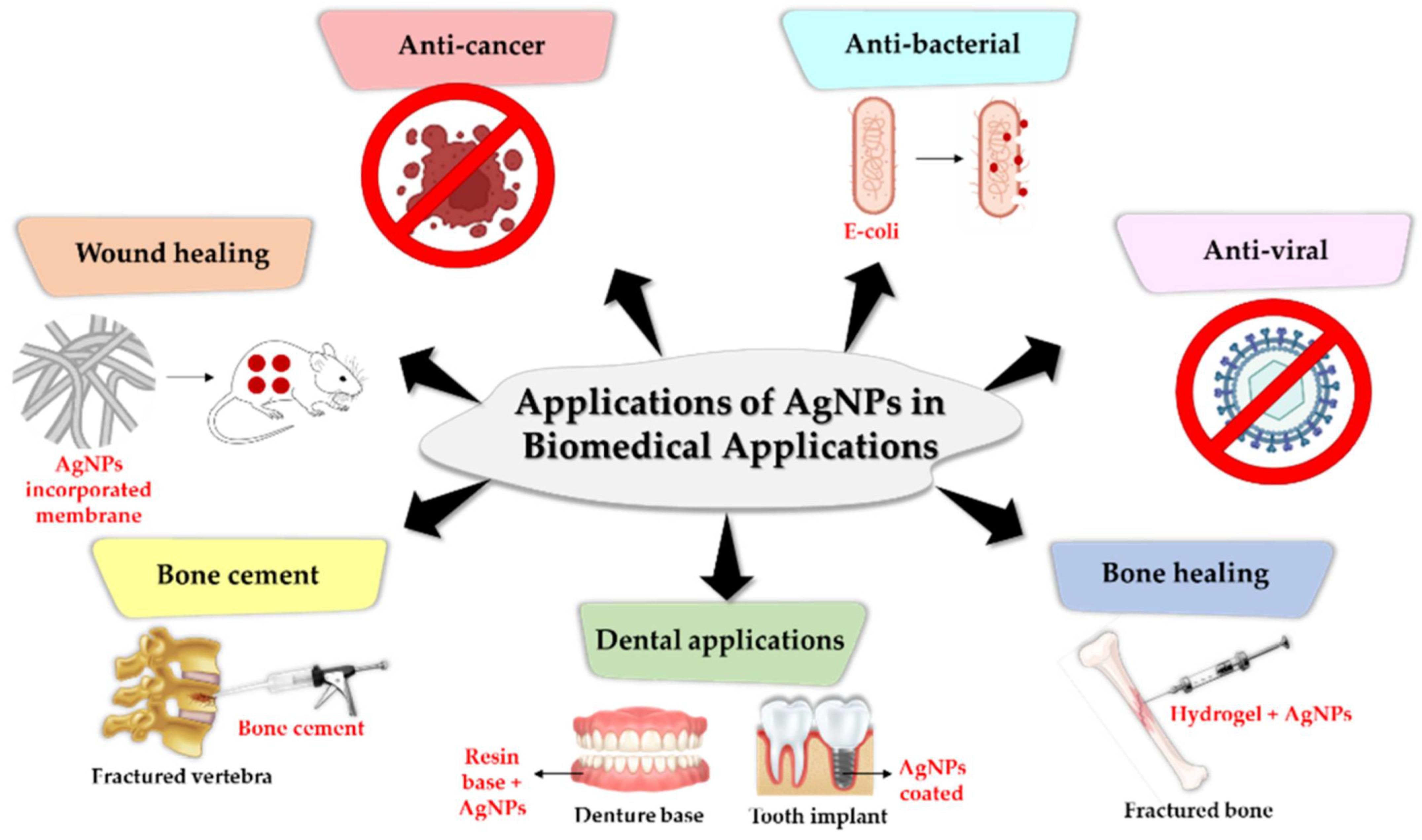
3. Biomedical Applications of Silver Nanoparticles
3.1. Antimicrobial Agents
3.2. Antiviral Agents
3.3. Wound Healing
3.4. Dental Products
3.5. Medical Device Coatings (Catheters with Embedded Silver Nanoparticles)
3.6. Tissue Engineering and Regeneration
3.7. Anticancer Agents
3.8. Biosensing and Diagnostics
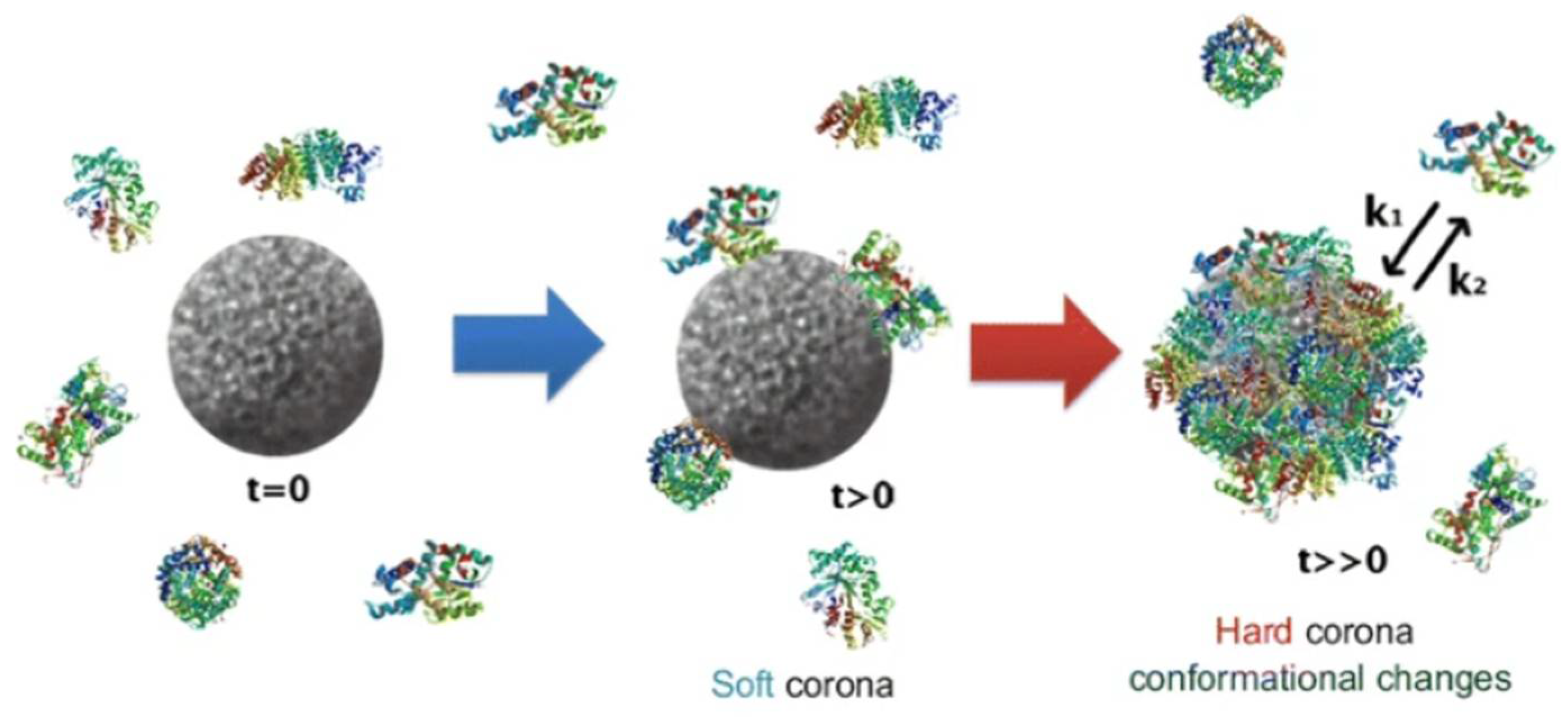
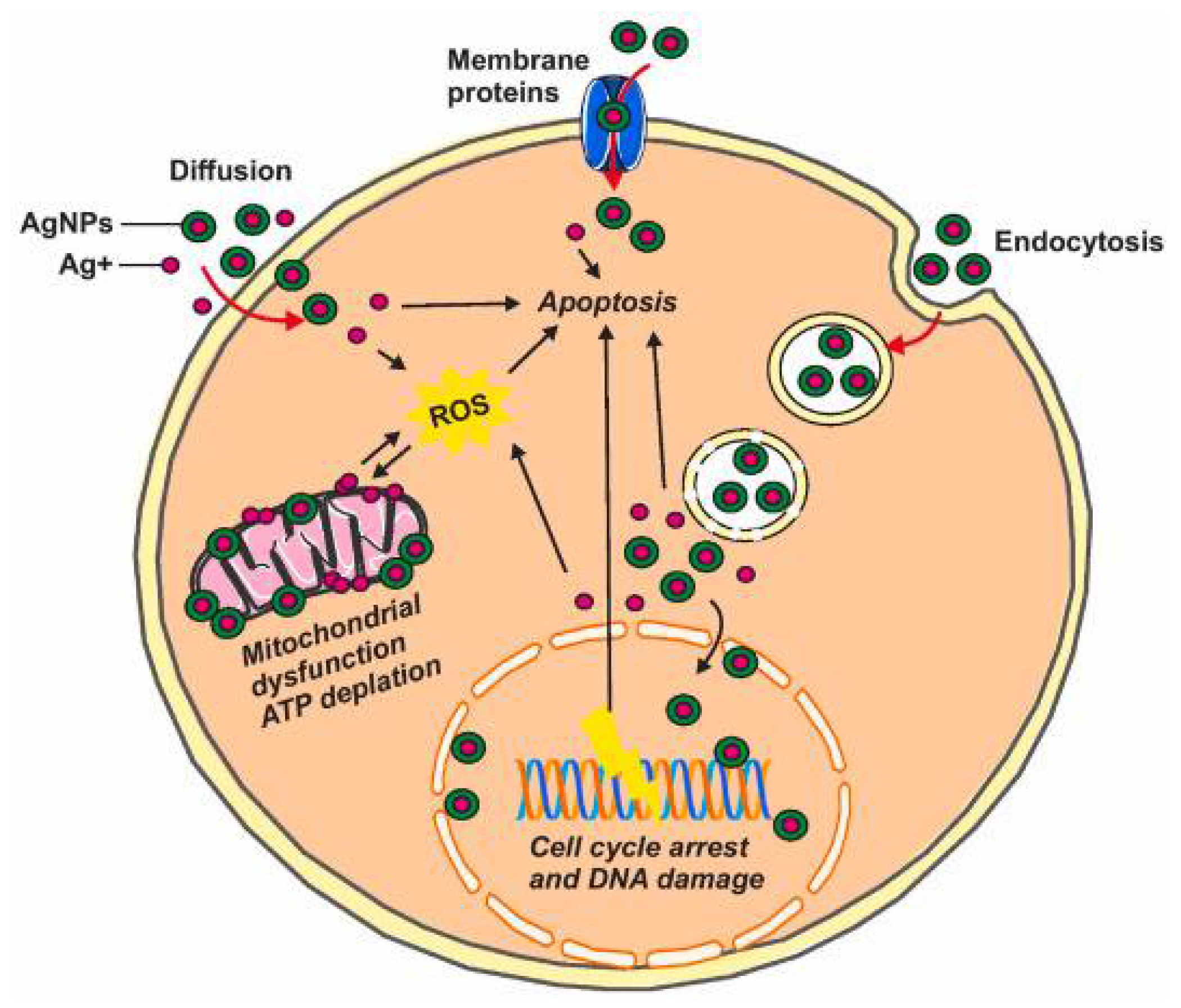
3.9. Toxicity of Silver Nanoparticles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silvestry-Rodriguez, N.; Sicairos-Ruelas, E.E.; Gerba, C.P.; Bright, K.R. Silver as a Disinfectant. Rev. Environ. Contam. Toxicol. 2007, 191, 23–45. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef]
- Abbas, R.; Luo, J.; Qi, X.; Naz, A.; Khan, I.A.; Liu, H.; Yu, S.; Wei, J. Silver nanoparticles: Synthesis, structure, properties and applications. Nanomaterials 2024, 14, 1425. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Wang, L.; Kafshgari, M.H.; Meunier, M. Optical Properties and Applications of Plasmonic-Metal Nanoparticles. Adv. Funct. Mater. 2020, 30, 2005400. [Google Scholar] [CrossRef]
- Abbasi, E.; Milani, M.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A.; Tayefi Nasrabadi, H.; Nikasa, P.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K. Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit. Rev. Microbiol. 2016, 42, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.B.; Chougale, A.D. Analytical Methods for the Identification and Characterization of Silver Nanoparticles: A Brief Review. Mater. Today Proc. 2021, 47, 5520–5532. [Google Scholar] [CrossRef]
- Ambreen, T.; Kim, M.-H. Influence of Particle Size on the Effective Thermal Conductivity of Nanofluids: A Critical Review. Appl. Energy 2020, 264, 114684. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Kandasamy, G.; Paramasivam, S.; Varudharajan, G.; Dhairiyasamy, R. Optimization of Silver Nanoparticle-Enhanced Nanofluids for Improved Thermal Management in Solar Thermal Collectors. Matéria 2024, 29, e20240363. [Google Scholar] [CrossRef]
- Lin, L.; Peng, X.; Voirin, E.; Donnio, B.; Rastei, M.V.; Vileno, B.; Gallani, J.L. Influence of the crystallinity of silver nanoparticles on their magnetic properties. Helv. Chim. Acta 2023, 106, e202200165. [Google Scholar]
- Koopmans, R.J.; Aggeli, A. Nanobiotechnology—Quo Vadis? Curr. Opin. Microbiol. 2010, 13, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Stojkovska, J.; Zvicer, J.; Obradovic, B. Preclinical functional characterization methods of nanocomposite hydrogels containing silver nanoparticles for biomedical applications. Appl. Microbiol. Biotechnol. 2020, 104, 4643–4658. [Google Scholar] [CrossRef]
- Tassieri, M.; Waigh, T.A.; Trinick, J.; Aggeli, A.; Evans, R.M.L. Analysis of the Linear Viscoelasticity of Polyelectrolytes by Magnetic Microrheometry—Pulsed Creep Experiments and the One Particle Response. J. Rheol. 2010, 54, 117–131. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Quevedo, A.C.; Guggenheim, Ε.B.; Sophie, M.; Adams, J.; Lofts, S.; Kwak, M.; Lee, T.G.; Johnston, C.; Wagner, S.; Holbrook, T.R.; et al. UV-Vis Spectroscopic Characterization of Nanomaterials in Aqueous Media. J. Vis. Exp. 2021, 176, e61764. [Google Scholar] [CrossRef] [PubMed]
- Grand, J.; Auguié, B.; Le Ru, E.C. Combined Extinction and Absorption UV–Visible Spectroscopy as a Method for Revealing Shape Imperfections of Metallic Nanoparticles. Anal. Chem. 2019, 91, 14639–14648. [Google Scholar] [CrossRef] [PubMed]
- Duman, H.; Bölükbaşı, K.; Yıldırım, M. Silver nanoparticles: A comprehensive review of synthesis methods and chemical and physical properties. Nanomaterials 2024, 14, 1527. [Google Scholar] [CrossRef]
- Pryshchepa, O.; Pomastowski, P.; Buszewski, B. Silver Nanoparticles: Synthesis, Investigation Techniques, and Properties. Adv. Colloid Interface Sci. 2020, 284, 102246. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Venkatesham, M.; Ayodhya, D.; Madhusudhan, A.; Veera Babu, N.; Veerabhadram, G. A Novel Green One-Step Synthesis of Silver Nanoparticles Using Chitosan: Catalytic Activity and Antimicrobial Studies. Appl. Nanosci. 2012, 4, 113–119. [Google Scholar] [CrossRef]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021, 2021, e6687290. [Google Scholar] [CrossRef]
- Braidy, N.; Béchu, A.; de Souza Terra, J.C.; Patience, G.S. Experimental methods in chemical engineering: Transmission electron microscopy—TEM. Can. J. Chem. Eng. 2020, 98, 628–641. [Google Scholar] [CrossRef]
- Lara, H.H.; Romero-Urbina, D.G.; Pierce, C.; Lopez-Ribot, J.L.; Arellano-Jiménez, M.J.; Jose-Yacaman, M. Effect of silver nanoparticles on Candida albicans biofilms: An ultrastructural study. J. Nanobiotechnology 2015, 13, 91. [Google Scholar] [CrossRef] [PubMed]
- Bendersky, L.A.; Gayle, F.W. Electron Diffraction Using Transmission Electron Microscopy. J. Res. Natl. Inst. Stand. Technol. 2001, 106, 997. [Google Scholar] [CrossRef]
- Malatesta, M. Transmission Electron Microscopy as a Powerful Tool to Investigate the Interaction of Nanoparticles with Subcellular Structures. Int. J. Mol. Sci. 2021, 22, 12789. [Google Scholar] [CrossRef]
- Das, D.; Ghosh, R.; Mandal, P. Biogenic Synthesis of Silver Nanoparticles Using S1 Genotype of Morus Alba Leaf Extract: Characterization, Antimicrobial and Antioxidant Potential Assessment. SN Appl. Sci. 2019, 1, 498. [Google Scholar] [CrossRef]
- Jia, Z.; Li, J.; Gao, L.; Yang, D.; Kanaev, A. Dynamic light scattering: A powerful tool for in situ nanoparticle sizing. Colloids Interfaces 2023, 7, 15. [Google Scholar] [CrossRef]
- Sharma, S.; Shukla, P.; Misra, A.; Mishr, P.R.; Ohshima, H.; Makino, K. (Eds.) Colloid and Interface Science in Pharmaceutical Research and Development; Elsevier: Amsterdam, The Netherlands, 2014; pp. 149–172. [Google Scholar]
- Khan, S.S.; Mukherjee, A.; Chandrasekaran, N. Studies on interaction of colloidal silver nanoparticles (SNPs) with five different bacterial species. Colloids Surf. B Biointerfaces 2011, 87, 129–138. [Google Scholar] [CrossRef]
- Bagus, P.S.; Freund, H.-J. X-ray photoelectron spectroscopy as a useful tool to study surfaces and model systems for heterogeneous catalysts: A review and perspective. Surf. Sci. 2024, 745, 122471. [Google Scholar] [CrossRef]
- Huang, Z.; Jiang, H.; Liu, P.; Sun, J.; Guo, D.; Shan, J.; Gu, N. Continuous synthesis of size-tunable silver nanoparticles by a green electrolysis method and multi-electrode design for high yield. J. Mater. Chem. A 2015, 3, 1925–1929. [Google Scholar] [CrossRef]
- Prieto, E.I.; Kiat, A.A. The antimicrobial action of silver nanoparticles on Escherichia coli as revealed by atomic force microscopy. Philipp. Sci. Lett. 2017, 10, 123–129. [Google Scholar]
- Caniglia, G.; Valavanis, D.; Tezcan, G.; Magiera, J.; Barth, H.; Bansmann, J.; Kranz, C.; Unwin, P.R. Antimicrobial effects of silver nanoparticle-microspots on the mechanical properties of single bacteria. Analyst 2024, 149, 2637–2646. [Google Scholar] [CrossRef]
- Daphedar, A.; Taranath, T.C. Characterization and cytotoxic effect of biogenic silver nanoparticles on mitotic chromosomes of Drimia polyantha (Blatt. & McCann) Stearn. Toxicol. Rep. 2018, 5, 910–918. [Google Scholar] [CrossRef]
- Gilstrap, R.A., Jr. A Colloidal Nanoparticle Form of Indium Tin Oxide: System Development and Characterization. PhD Dissertation, Georgia Institute of Technology, Atlanta, GA, USA, 2009. [Google Scholar]
- Rievaj, M.; Culková, E.; Šandorová, D.; Durdiak, J.; Bellová, R.; Tomčík, P. A review of analytical techniques for the determination and separation of silver ions and its nanoparticles. Nanomaterials 2023, 13, 1262. [Google Scholar] [CrossRef]
- Laborda, F.; Abad-Álvaro, I.; Jiménez, M.S.; Bolea, E. Catching particles by atomic spectrometry: Benefits and limitations of single particle-inductively coupled plasma mass spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2023, 199, 106570. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Mo, J.; Zhang, G.; Chen, Y.; Huang, C. Imaging Gold Nanoparticles in Mouse Liver by Laser Ablation Inductively Coupled Plasma Mass Spectrometry. Sci. Rep. 2017, 7, 2965. [Google Scholar] [CrossRef]
- Nyika, J.; Onyari, E.; Dinka, M.O.; Mishra, S.B. A comparison of reproducibility of inductively coupled spectrometric techniques in soil metal analyses. Air Soil Water Res. 2019, 12, 1178622119869002. [Google Scholar]
- Almatroudi, A. Silver Nanoparticles: Synthesis, Characterisation and Biomedical Applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef] [PubMed]
- Almatroudi, A. Potential Toxic Effects and Clinical Implications of Silver Nanoparticles in Tissue Engineering: A Comprehensive Review. Toxicol. Rep. 2020, 7, 1039–1053. [Google Scholar]
- Balout, H.; Tarrat, N.; Puibasset, J.; Ispas, S.; Bonafos, C.; Benoit, M. Density functional theory study of the spontaneous formation of covalent bonds at the silver/silica interface in silver nanoparticles embedded in SiO2: Implications for Ag+ release. ACS Appl. Nano Mater. 2019, 2, 5179–5189. [Google Scholar] [CrossRef]
- Khan, J.; Naseem, I.; Bibi, S.; Ahmad, S.; Altaf, F.; Hafeez, Μ.; Almoneef, M.M.; Ahmad, K. Green Synthesis of Silver Nanoparticles (Ag-NPs) Using Debregeasia Salicifolia for Biological Applications. Materials 2022, 16, 129. [Google Scholar] [CrossRef]
- Zozulya, A.; Zyubin, A.; Samusev, I. A review for DFT in chemical mechanism of SERS studies. R. Soc. Open Sci. 2025, 12, 242000. [Google Scholar] [CrossRef]
- Bachler, G.; von Goetz, N.; Hungerbühler, K. A physiologically based pharmacokinetic model for ionic silver and silver nanoparticles. Int. J. Nanomed. 2013, 3365–3382. [Google Scholar] [CrossRef]
- Lasmi, F.; Hamitouche, H.; Laribi-Habchi, H.; Benguerba, Y.; Chafai, N. Silver Nanoparticles (AgNPs), Methods of Synthesis, Characterization, and Their Application: A Review. Plasmonics 2025, 1–34. [Google Scholar] [CrossRef]
- Malik, M.; Iqbal, M.A.; Iqbal, Y.; Malik, M.; Bakhsh, S.; Irfan, S.; Ahmad, R.; Pham, P.V. Biosynthesis of silver nanoparticles for biomedical applications: A mini review. Inorg. Chem. Commun. 2022, 145, 109980. [Google Scholar] [CrossRef]
- Kolahalam, L.A.; Viswanath, I.K.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y.L.N. Review on nanomaterials: Synthesis and applications. Mater. Today Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Bronzino, J.D.; Enderle, J.D.; Blanchard, S.M. Introduction to Biomedical Engineering; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Barua, N.; Buragohain, A.K. Therapeutic Potential of Silver Nanoparticles (AgNPs) as an Antimycobacterial Agent: A Comprehensive Review. Antibiotics 2024, 13, 1106. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.A.; Li, L.; Li, H.; Abdala, A. Silver nanoparticle-based nanocomposites for combating infectious pathogens: Recent advances and future prospects. Nanomaterials 2021, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- More, P.R.; Pandit, S.; Filippis, A.D.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver nanoparticles: Bactericidal and mechanistic approach against drug resistant pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Solomon, M.; Holban, A.M.; Bălăceanu-Gurău, B.; Dițu, L.M.; Alberts, A.; Grumezescu, A.M.; Manolescu, L.S.C.; Mihai, M.M. Silver Nanoparticles Functionalized with Polymeric Substances to Reduce the Growth of Planktonic and Biofilm Opportunistic Pathogens. Int. J. Mol. Sci. 2025, 26, 3930. [Google Scholar] [CrossRef]
- Campora, S.; Ghersi, G. Recent Developments and Applications of Smart Nanoparticles in Biomedicine. Nanotechnol. Rev. 2022, 11, 2595–2631. [Google Scholar] [CrossRef]
- Agrawal, S.; Bhatt, M.; Rai, S.K.; Bhatt, A.; Dangwal, P.; Agrawal, P.K. Silver nanoparticles and its potential applications: A review. J. Pharmacogn. Phytochem. 2018, 7, 930–937. [Google Scholar]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, F.; Yalamarty, S.S.K.; Filipczak, N.; Jin, Y.; Li, X. Nano Silver-Induced Toxicity and Associated Mechanisms. Int. J. Nanomed. 2022, 17, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Che, A.; Che, A.; Ahmad, S. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef]
- Bamal, D.; Singh, A.; Chaudhary, G.; Kumar, M.; Singh, M.; Rani, N.; Mundlia, P.; Sehrawat, A.R. Silver Nanoparticles Biosynthesis, Characterization, Antimicrobial Activities, Applications, Cytotoxicity and Safety Issues: An Updated Review. Nanomaterials 2021, 11, 2086. [Google Scholar] [CrossRef] [PubMed]
- Crisan, C.M.; Mocan, T.; Manolea, M.; Lasca, L.I.; Tăbăran, F.A.; Mocan, L. Review on silver nanoparticles as a novel class of antibacterial solutions. Appl. Sci. 2021, 11, 1120. [Google Scholar] [CrossRef]
- Aguilar-Garay, R.; Lopez, M.; Sanchez, F.; Garcia, M. A comprehensive review of silver and gold nanoparticles as effective antibacterial agents. Pharmaceuticals 2024, 17, 1134. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Banach, M. Silver Nanoparticles—A Material of the Future…? Open Chem. 2016, 14, 76–91. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Tamboli, D.P.; Lee, D.S. Mechanistic Antimicrobial Approach of Extracellularly Synthesized Silver Nanoparticles against Gram Positive and Gram Negative Bacteria. J. Hazard. Mater. 2013, 260, 878–884. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics 2024, 14, 5. [Google Scholar] [CrossRef]
- Vishwanath, R.; Negi, B. Conventional and Green Methods of Synthesis of Silver Nanoparticles and Their Antimicrobial Properties. Curr. Res. Green Sustain. Chem. 2021, 4, 100205. [Google Scholar] [CrossRef]
- Nguyen, N.P.U.; Dang, N.T.; Doan, L.; Nguyen, T.T.H. Synthesis of Silver Nanoparticles: From Conventional to ‘Modern’ Methods—A Review. Processes 2023, 11, 2617. [Google Scholar] [CrossRef]
- Chicea, D.; Ciocan, I.; Watz, C.; Socol, M. Comparative synthesis of silver nanoparticles: Evaluation of chemical reduction procedures, AFM and DLS size analysis. Materials 2023, 16, 5244. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Jain, S.; Rani, V. Nanotechnology: Emerging Tool for Diagnostics and Therapeutics. Appl. Biochem. Biotechnol. 2011, 165, 1178–1187. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Sampath, G.; Chen, Y.Y.; Rameshkumar, N.; Krishnan, M.; Nagarajan, K.; Shyu, D.J. Biologically synthesized silver nanoparticles and their diverse applications. Nanomaterials 2022, 12, 3126. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of size, shape and surface functionalization on the antibacterial activity of silver nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.U.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnology 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Trivedi, R.; Soni, V. Biogenic Synthesis of Silver Nanoparticles, Characterization and Their Applications—A Review. Surfaces 2021, 5, 67–90. [Google Scholar] [CrossRef]
- Qamer, S.; Romli, M.H.; Che-Hamzah, F.; Misni, N.; Joseph, N.M.; Al-Haj, N.A.; Amin-Nordin, S. Systematic review on biosynthesis of silver nanoparticles and antibacterial activities: Application and theoretical perspectives. Molecules 2021, 26, 5057. [Google Scholar] [CrossRef] [PubMed]
- Pedroso-Santana, S.; Fleitas-Salazar, N. The Use of Capping Agents in the Stabilization and Functionalization of Metallic Nanoparticles for Biomedical Applications. Part. Part. Syst. Charact. 2022, 40, 2200146. [Google Scholar] [CrossRef]
- Gibała, A.; Żeliszewska, P.; Gosiewski, T.; Krawczyk, A.; Duraczyńska, D.; Szaleniec, J.; Szaleniec, M.; Oćwieja, M. Antibacterial and antifungal properties of silver nanoparticles—Effect of a surface-stabilizing agent. Biomolecules 2021, 11, 1481. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Hussain, F.S.; Abro, N.Q.; Ahmed, N.; Memon, S.Q.; Memon, N. Nano-antivirals: A comprehensive review. Front. Nanotechnol. 2022, 4, 1064615. [Google Scholar] [CrossRef]
- Goel, M.; Sharma, A.; Sharma, B. Recent Advances in Biogenic Silver Nanoparticles for Their Biomedical Applications. Sustain. Chem. 2023, 4, 61–94. [Google Scholar] [CrossRef]
- Aldakheel, F.M.; Sayed, M.M.E.; Mohsen, D.; Fagir, M.H.; El Dein, D.K. Green synthesis of silver nanoparticles loaded hydrogel for wound healing; systematic review. Gels 2023, 9, 530. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Zhang, G.; He, S.; Xu, B. Inorganic-nanomaterial-composited hydrogel dressings for wound healing. J. Compos. Sci. 2024, 8, 46. [Google Scholar] [CrossRef]
- Paladini, F.; Di Franco, C.; Panico, A.; Scamarcio, G.; Sannino, A.; Pollini, M. In vitro assessment of the antibacterial potential of silver nano-coatings on cotton gauzes for prevention of wound infections. Materials 2016, 9, 411. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Guo, G.; Wang, Q.; Tang, J.; Zhao, Y.; Qin, H.; Wahafu, T.; Shen, H.; Liu, X.; et al. Silver-nanoparticles-modified biomaterial surface resistant to staphylococcus: New insight into the antimicrobial action of silver. Sci. Rep. 2016, 6, 32699. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Nicolae-Maranciuc, A.; Chicea, D.; Chicea, L.M. Ag Nanoparticles for Biomedical Applications—Synthesis and Characterization—A Review. Int. J. Mol. Sci. 2022, 23, 5778. [Google Scholar] [CrossRef]
- Singh, M.; Thakur, V.; Kumar, V.; Raj, M.; Gupta, S.; Devi, N.; Upadhyay, S.K.; Macho, M.; Banerjee, A.; Ewe, D.; et al. Silver nanoparticles and its mechanistic insight for chronic wound healing: Review on recent progress. Molecules 2022, 27, 5587. [Google Scholar] [CrossRef]
- Vijapur, L.S.; Shalavadi, M.; Desai, A.R.; Hiremath, J.N.; Gudigennavar, A.S.; Shidramshettar, S.L.; Hiremath, S.R.; Peram, M.R.; Kittur, B.S. Wound healing potential of green synthesized silver nanoparticles of Glycyrrhiza glabra linn root extract: A preclinical study. J. Trace Elem. Miner. 2025, 11, 100214. [Google Scholar] [CrossRef]
- Kumar, S.S.D.; Rajendran, N.K.; Houreld, N.N.; Abrahamse, H. Recent Advances on Silver Nanoparticle and Biopolymer-Based Biomaterials for Wound Healing Applications. Int. J. Biol. Macromol. 2018, 115, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hou, X.; Fan, S.; Jin, C. Research Status of Silver Nanoparticles for Dental Applications. Inorganics 2025, 13, 168. [Google Scholar] [CrossRef]
- Lee, J.J.; Niu, M.; Shakir, Z.; Hwang, G.; Chung, C.H.; Wolff, M.S.; Zheng, Z.; Li, C. Usage of Silver Nanoparticles in Orthodontic Bonding Reagents. J. Funct. Biomater. 2025, 16, 244. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.C.; Sokolonski, A.R.; Fonseca, M.S.; Stanisic, D.; Araújo, D.B.; Azevedo, V.; Portela, R.D.; Tasic, L. Applications of silver nanoparticles in dentistry: Advances and technological innovation. Int. J. Mol. Sci. 2021, 22, 2485. [Google Scholar] [CrossRef]
- Rivera-Cortés, M.A.; Niño-Martínez, N.; Ruiz, F.; Félix-Sicairos, B.K.; Martínez-Castañón, G.A. Evaluation of Deformation and Antibacterial Properties of Dental Alginates Mixed with Silver Nanoparticles. Materials 2025, 18, 2069. [Google Scholar] [CrossRef]
- Corrêa, J.M.; Mori, M.; Sanches, H.L.; Cruz, A.D.D.; Poiate, E., Jr.; Poiate, I.A.V.P. Silver nanoparticles in dental biomaterials. Int. J. Biomater. 2015, 2015, 485275. [Google Scholar] [CrossRef]
- Butrón Téllez Girón, C.; Hernández Sierra, J.F.; DeAlba-Montero, I.; Urbano Peña, M.D.L.A.; Ruiz, F. Therapeutic use of silver nanoparticles in the prevention and arrest of dental caries. Bioinorg. Chem. Appl. 2020, 2020, 8882930. [Google Scholar] [CrossRef]
- Mazumder, M.M.U.; Sukul, A.; Saha, S.K.; Chowdhury, A.A.; Mamun, Y. A comprehensive in vitro biological investigation of metal complexes of tolfenamic acid. Alex. J. Med. 2018, 54, 23–26. [Google Scholar] [CrossRef]
- Lampé, I.; Beke, D.; Biri, S.; Csarnovics, I.; Csik, A.; Dombrádi, Z.; Hajdu, P.; Hegedűs, V.; Rácz, R.; Varga, I.; et al. Investigation of silver nanoparticles on titanium surface created by ion implantation technology. Int. J. Nanomed. 2019, 14, 4709–4721. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Li, X.; Zhou, Y.; Kuang, Y.; Sang, S.; Zhang, J.; Wang, Y. Silver nanoparticle coatings enhance bone density and formation without adverse tissue effects. Mater. Sci. Eng. C 2017, 75, 1089–1101. [Google Scholar]
- Kumar, A.; Vishakha, D.A.; Jeet, K.; Kumar, S. A review on silver nanoparticles focusing on applications in biomedical sector. Int. J. Pharmaceut. Sci. Drug Res. 2022, 8, 57–63. [Google Scholar]
- Saravanan, S.; Kumar, V.; Rajendran, A.; Thomas, S. A review on silver nanoparticles-based biomaterials for bone tissue engineering. Int. J. Chem. 2024, 16, 123–135. [Google Scholar]
- Aubert, T.; Buffière, J.; Montalent, P.; Bérenguer, F.; Boisse-Laporte, C.; Granier, C. Nanoparticles in tissue engineering: Applications, challenges, and prospects. J. Biomed. Nanotechnol. 2018, 14, 765–781. [Google Scholar]
- Kargozar, S.; Nazarnezhad, S.; Kermani, F.; Baino, F. Nanomaterials and scaffolds for tissue engineering and regenerative medicine. In Nanomaterials and Nanotechnology in Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 279–302. [Google Scholar]
- Massironi, A.; Rizzo, F.; Gallo, A.; Giacomini, M.; Biffi, S.; Del Giudice, A. Development and characterization of highly stable silver nanoparticles as novel potential antimicrobial agents for wound healing hydrogels. Int. J. Mol. Sci. 2022, 23, 2161. [Google Scholar] [CrossRef]
- Przekora, A. A Concise Review on Tissue Engineered Artificial Skin Grafts for Chronic Wound Treatment: Can We Reconstruct Functional Skin Tissue In Vitro? Cells 2020, 9, 1622. [Google Scholar] [CrossRef]
- Chen, M. Application of Nanotechnology in Biomedicine: From Diagnosis to Therapy. J. Bioanal. Biomed. 2023, 15, 381. [Google Scholar]
- Takáč, P., Jr.; Michalková, R.; Čižmáriková, M.; Bedlovičová, Z.; Balážová, Ľ.; Laca Megyesi, Š.; Mačeková, Z.; Takáčová, G.; Moreno-Borrallo, A.; Ruiz-Hernandez, E.; et al. Do We Know Enough About the Safety Profile of Silver Nanoparticles in Oncology? A Focus on Novel Methods and Approaches. Int. J. Mol. Sci. 2025, 26, 5344. [Google Scholar] [CrossRef]
- Gherasim, O.; Puiu, R.A.; Bîrcă, A.C.; Burdușel, A.C.; Grumezescu, A.M. An updated review on silver nanoparticles in biomedicine. Nanomaterials 2020, 10, 2318. [Google Scholar] [CrossRef]
- Saikia, N. Functional Inorganic Biomaterials for Molecular Sensing and Biomedical Applications. Inorganics 2025, 13, 260. [Google Scholar] [CrossRef]
- Beck, F.; Loessl, M.; Baeumner, A.J. Signaling strategies of silver nanoparticles in optical and electrochemical biosensors: Considering their potential for the point-of-care. Microchim. Acta 2023, 190, 91. [Google Scholar] [CrossRef]
- Bouafia, A.; Gheribi, R.; Haddad, I.; Al-Douri, Y. The recent progress on silver nanoparticles: Synthesis and electronic applications. Nanomaterials 2021, 11, 2318. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Tang, G.; Zhao, C.; Li, J.; Wang, Y. Silver nanoparticles: Comprehensive insights into bioanalytical and biomedical applications. ACS Omega 2025, 10, 1234–1245. [Google Scholar]
- Li, Y.; Zhang, L.; Chen, G.; Zhou, Q.; Wang, H. Constructing Fe-MOF-derived Z-scheme photocatalysts with enhanced charge transport: Nanointerface and carbon sheath synergistic effect. ACS Appl. Mater. Interfaces 2020, 12, 25494–25502. [Google Scholar] [CrossRef] [PubMed]
- Sarău, O.S.; Moacă, E.A.; Semenescu, A.D.; Dumitru, R.; Jijie, A.R.; Poenaru, M.; Dehelean, C.A.; Chevereşan, A. Physicochemical and toxicological screening of silver nanoparticle biosynthesis from Punica granatum peel extract. Inorganics 2024, 12, 160. [Google Scholar] [CrossRef]
- Laib, I.; Bououdina, M.; Bensaoula, A.; Benmansour, S. Tailoring innovative silver nanoparticles for modern medicine: The importance of size and shape control and functional modifications. Mater. Today Bio. 2025, 33, 102071. [Google Scholar]
- Prabhu, S.; Poulose, E.K. Silver Nanoparticles: Mechanism of Antimicrobial Action, Synthesis, Medical Applications, and Toxicity Effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Ferdous, Z.; Nemmar, A. Health Impact of Silver Nanoparticles: A Review of the Biodistribution and Toxicity Following Various Routes of Exposure. Int. J. Mol. Sci. 2020, 21, 2375. [Google Scholar] [CrossRef] [PubMed]
- Noga, M.; Milan, J.; Frydrych, A.; Jurowski, K. Toxicological aspects, safety assessment, and green toxicology of silver nanoparticles (AgNPs)—Critical review: State of the art. Int. J. Mol. Sci. 2023, 24, 5133. [Google Scholar] [CrossRef]
- Lee, H.A.; Kim, M.H.; Oh, J.H.; Park, E.H.; Lee, S.Y. Rhizome of Anemarrhena asphodeloides as mediators of the eco-friendly synthesis of silver and gold spherical, face-centred cubic nanocrystals and its anti-migratory and cytotoxic potential in normal and cancer cell lines. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S2), 285–294. [Google Scholar]
- Selvan, D.A.; Karuppusamy, I.; Veerappan, A.; Priya, C. Garlic, green tea and turmeric extracts-mediated green synthesis of silver nanoparticles: Phytochemical, antioxidant and in vitro cytotoxicity studies. J. Photochem. Photobiol. B Biol. 2018, 180, 243–252. [Google Scholar]
- Plotnikov, E.V.; Tretayakova, M.S.; Garibo-Ruíz, D.; Rodríguez-Hernández, A.G.; Pestryakov, A.N.; Toledano-Magaña, Y.; Bogdanchikova, N. A comparative study of cancer cells susceptibility to silver nanoparticles produced by electron beam. Pharmaceutics 2023, 15, 962. [Google Scholar] [CrossRef]
- Burdușel, A.C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical applications of silver nanoparticles: An up-to-date overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef]
- Liu, J.; Sonshine, D.A.; Shervani, S.; Hurt, R.H. Controlled release of biologically active silver from nanosilver surfaces. ACS Nano 2010, 4, 6903–6913. [Google Scholar] [CrossRef]
- Shannahan, J.H.; Layne, J.; Gardella, J.; Ke, P.C. Formation of a protein corona on silver nanoparticles mediates cellular toxicity via scavenger receptors. Toxicol. Sci. 2015, 143, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Breznica, P.; Rozafa, K.; Arlinda, D. A review of the current understanding of nanoparticles protein corona composition. Med. Pharm. Rep. 2020, 93, 342. [Google Scholar] [CrossRef]
- Miclăuş, T.; O’Connell, D.; Melinte, G.; Ivan, V.; Cristian, L.; Dinescu, G. Dynamic protein coronas revealed as a modulator of silver nanoparticle sulphidation in vitro. Nat. Commun. 2016, 7, 11770. [Google Scholar] [CrossRef] [PubMed]
- Eigenheer, R.; Hutchison, J.E.; Hess, K.L. Silver nanoparticle protein corona composition compared across engineered particle properties and environmentally relevant reaction conditions. Environ. Sci. Nano 2014, 1, 238–247. [Google Scholar] [CrossRef]
- Barbalinardo, M.; Bertacchini, J.; Bergamini, L.; Magarò, M.S.; Ortolani, L.; Sanson, A.; Palumbo, C.; Cavallini, M.; Gentili, D. Surface properties modulate protein corona formation and determine cellular uptake and cytotoxicity of silver nanoparticles. Nanoscale 2021, 13, 14119–14129. [Google Scholar] [CrossRef]
- Nikzamir, M.; Akbarzadeh, A.; Panahi, Y. An Overview on Nanoparticles Used in Biomedicine and Their Cytotoxicity. J. Drug Deliv. Sci. Technol. 2021, 61, 102316. [Google Scholar] [CrossRef]
- Panyala, N.R.; Peña-Méndez, E.M.; Havel, J. Silver or Silver Nanoparticles: A Hazardous Threat to the Environment and Human Health? J. Appl. Biomed. 2008, 6, 117–129. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntolia, A.; Chatzigiannakou, T.; Michailidis, N.; Aggeli, A. A Comprehensive Physicochemical Characterization of Silver Nanoparticles as a Prerequisite for Their Successful Biomedical Applications. Inorganics 2025, 13, 341. https://doi.org/10.3390/inorganics13100341
Ntolia A, Chatzigiannakou T, Michailidis N, Aggeli A. A Comprehensive Physicochemical Characterization of Silver Nanoparticles as a Prerequisite for Their Successful Biomedical Applications. Inorganics. 2025; 13(10):341. https://doi.org/10.3390/inorganics13100341
Chicago/Turabian StyleNtolia, Anastasia, Theofania Chatzigiannakou, Nikolaos Michailidis, and Amalia Aggeli. 2025. "A Comprehensive Physicochemical Characterization of Silver Nanoparticles as a Prerequisite for Their Successful Biomedical Applications" Inorganics 13, no. 10: 341. https://doi.org/10.3390/inorganics13100341
APA StyleNtolia, A., Chatzigiannakou, T., Michailidis, N., & Aggeli, A. (2025). A Comprehensive Physicochemical Characterization of Silver Nanoparticles as a Prerequisite for Their Successful Biomedical Applications. Inorganics, 13(10), 341. https://doi.org/10.3390/inorganics13100341








