Smart Inorganic Nanomaterials for Tumor Microenvironment Modulation
Abstract
1. Introduction
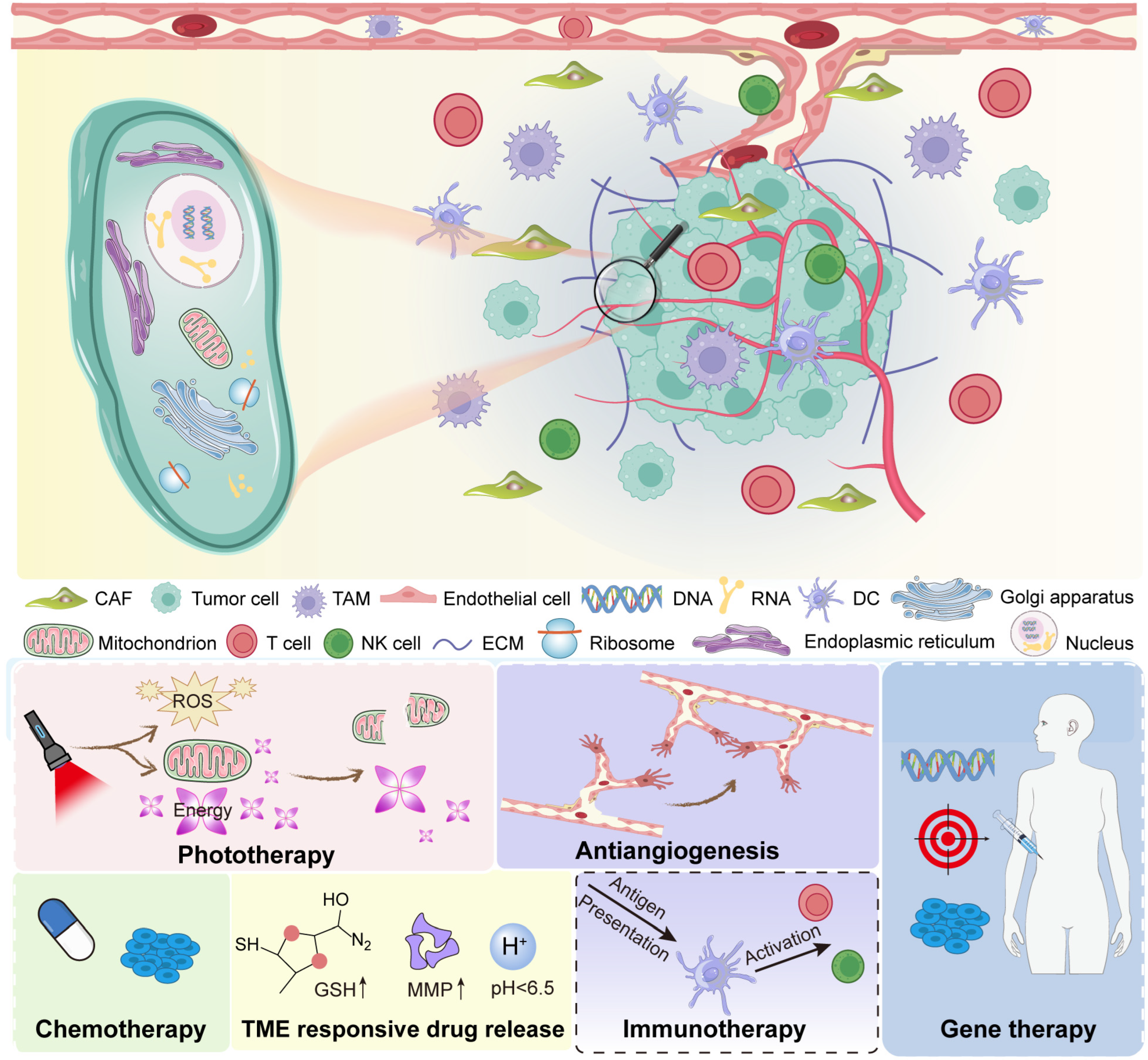
2. Overview of the Tumor Microenvironment (TME)
2.1. Key Components of the TME
2.2. Major Abnormalities in the Tumor Microenvironment
2.3. The Tumor Microenvironment as a Therapeutic Target
3. Classes of Inorganic Nanomaterials Used in TME Modulation
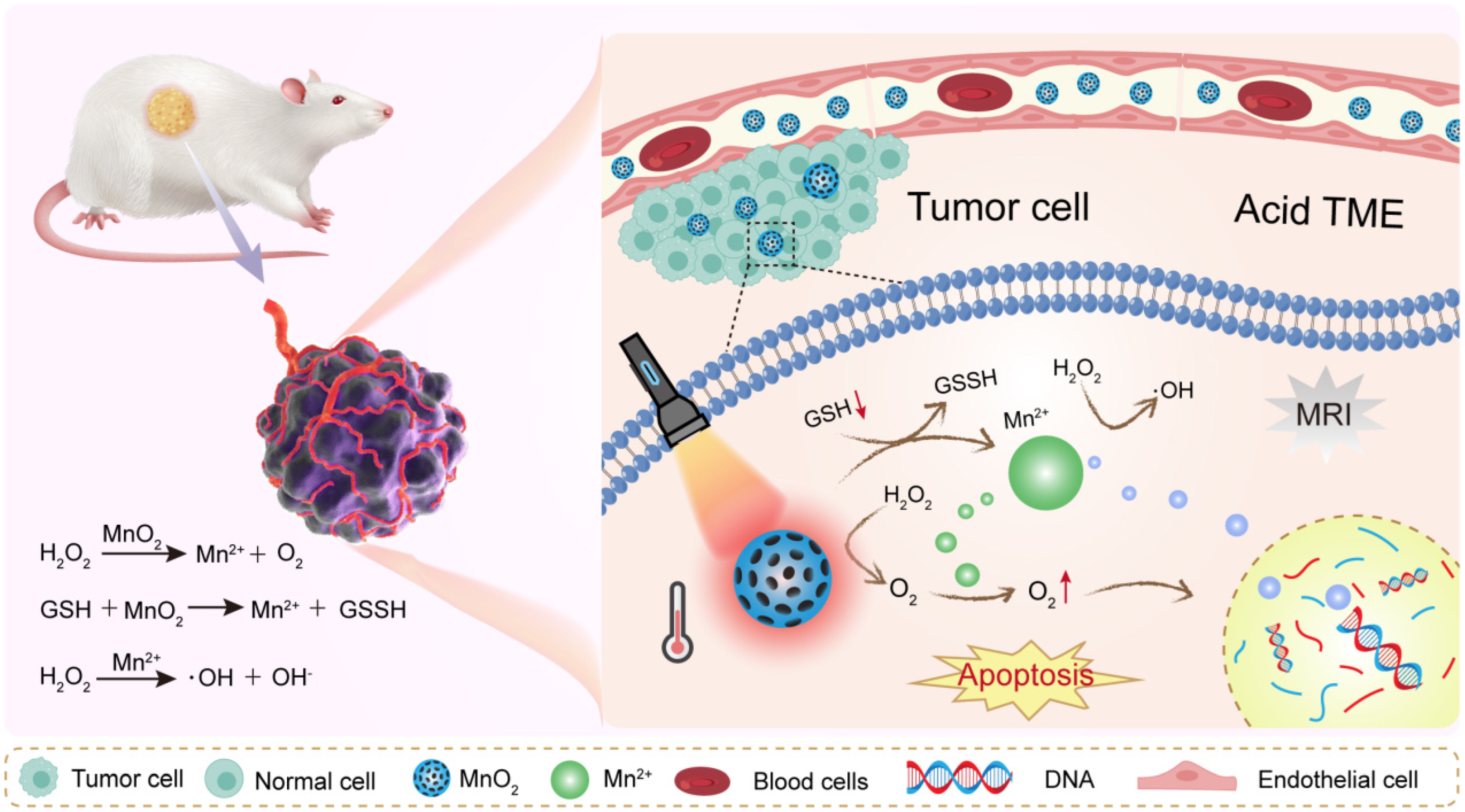
3.1. Metal Oxides
3.2. Noble Metals
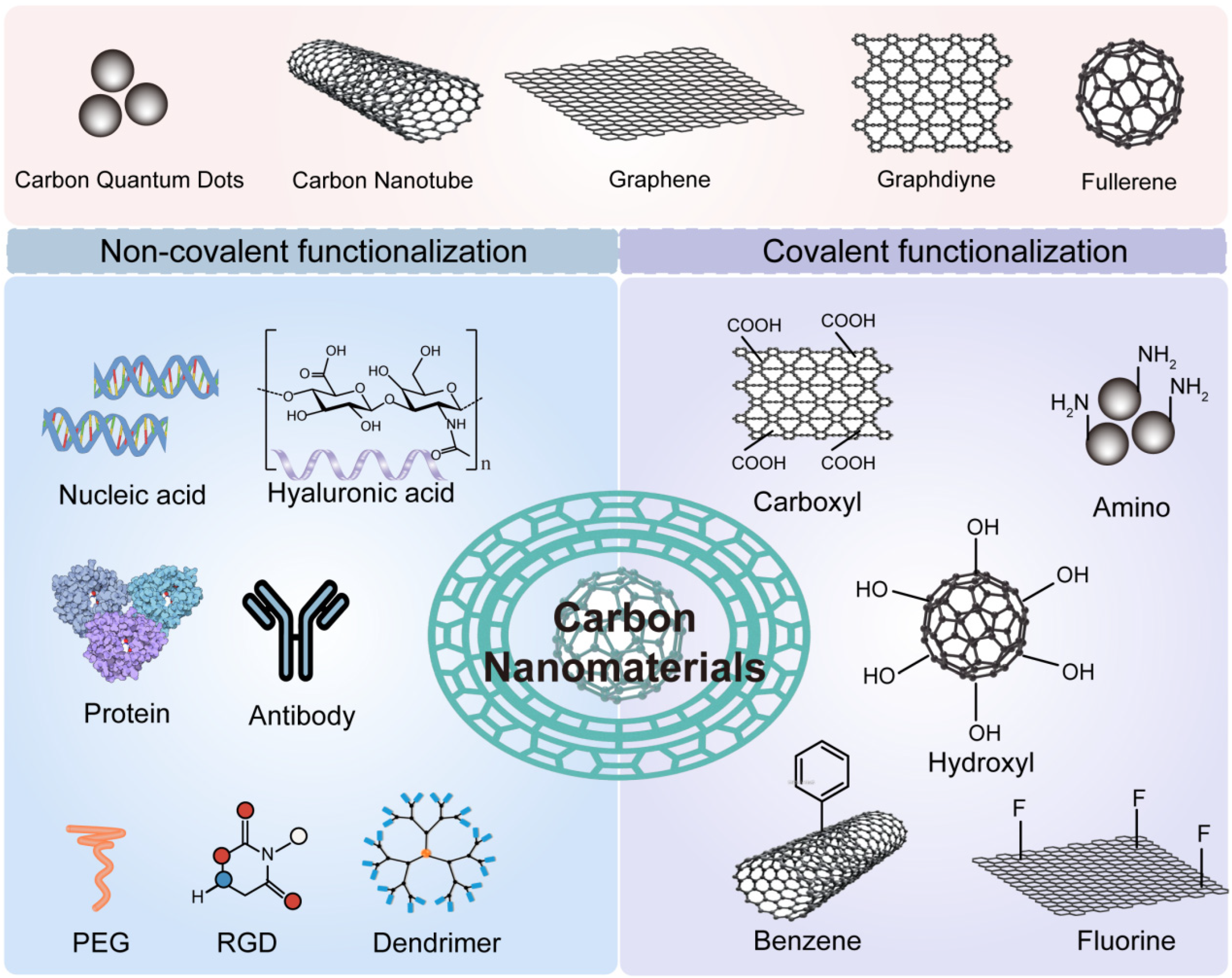
3.3. Carbon-Based Nanomaterials
3.4. Other Inorganic Materials
4. Strategies for Tumor Microenvironment Modulation Using Inorganic Nanomaterials
4.1. Alleviating Hypoxia
4.2. pH Modulation
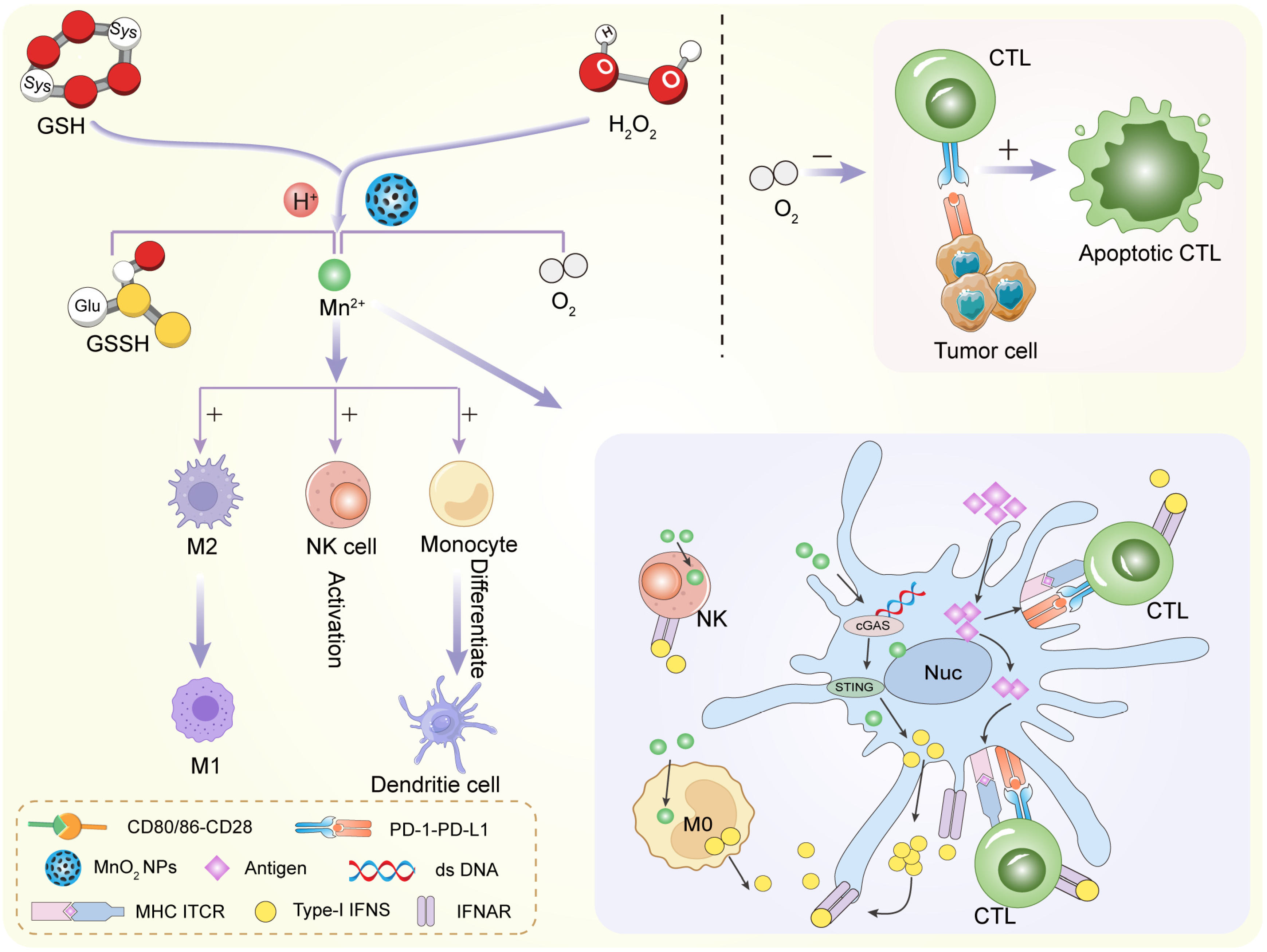
4.3. Redox Balance Regulation
4.4. TME-Triggered Drug Release
4.5. Immune Modulation
5. Multimodal Therapies Enabled by Tumor Microenvironment (TME) Modulation
5.1. Chemo/Chemodynamic Therapy (CDT)
5.2. Photothermal and Photodynamic Therapy
5.3. Radiotherapy Enhancement
5.4. Synergistic Immunotherapy
6. Design Considerations for Smart TME-Modulating Nanoplatforms
6.1. Surface Functionalization and Targeting
6.2. Biodegradability and Biosafety
6.3. Stimuli-Responsiveness (pH, Redox, Enzyme, Light)
6.4. Size, Charge, and Biodistribution
7. Challenges and Future Perspectives
7.1. Tumor Heterogeneity in TME Responsiveness
7.2. Immune Evasion and Nanoparticle–Immune System Interaction
7.3. Integration of AI for Nanoparticle Design
7.4. Potential Nanoparticle Toxicity
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2021, 221, 107753. [Google Scholar] [CrossRef]
- Elhanani, O.; Ben-Uri, R.; Keren, L. Spatial profiling technologies illuminate the tumor microenvironment. Cancer Cell 2023, 41, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.-Z.; Jin, W.-L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Yang, C.; Shao, F.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef]
- Chen, S.; Liao, C.; Hu, H.; Liao, J.; Chen, Z.; Li, S.; Zeng, X.; Peng, B.; Shen, S.; Li, D.; et al. Hypoxia-driven tumor stromal remodeling and immunosuppressive microenvironment in scirrhous HCC. Hepatology 2024, 79, 780–797. [Google Scholar] [CrossRef]
- Wu, Q.; You, L.; Nepovimova, E.; Heger, Z.; Wu, W.; Kuca, K.; Adam, V. Hypoxia-inducible factors: Master regulators of hypoxic tumor immune escape. J. Hematol. Oncol. 2022, 15, 77. [Google Scholar] [CrossRef]
- Wang, C.; Xu, S.; Yang, X. Hypoxia-Driven Changes in Tumor Microenvironment: Insights into Exosome-Mediated Cell Interactions. Int. J. Nanomed. 2024, 19, 8211–8236. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef]
- Malla, R.; Kumari, S.; Ganji, S.P.; Srilatha, M.; Nellipudi, H.R.; Nagaraju, G.P. Reactive oxygen species of tumor microenvironment: Harnessing for immunogenic cell death. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189154. [Google Scholar] [CrossRef]
- Malla, R.; Surepalli, N.; Farran, B.; Malhotra, S.V.; Nagaraju, G.P. Reactive oxygen species (ROS): Critical roles in breast tumor microenvironment. Crit. Rev. Oncol. 2021, 160, 103285. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Ding, Y.; Qin, Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1133308. [Google Scholar] [CrossRef] [PubMed]
- DePeaux, K.; Rivadeneira, D.B.; Lontos, K.; Dean, V.G.; Gunn, W.G.; Watson, M.J.; Yao, T.; Wilfahrt, D.; Hinck, C.; Wieteska, L.; et al. An oncolytic virus–delivered TGFβ inhibitor overcomes the immunosuppressive tumor microenvironment. J. Exp. Med. 2023, 220, e20230053. [Google Scholar] [CrossRef] [PubMed]
- O’COnnell, B.C.; Hubbard, C.; Zizlsperger, N.; Fitzgerald, D.; Kutok, J.L.; Varner, J.; Ilaria, R.; Cobleigh, M.A.; Juric, D.; Tkaczuk, K.H.R.; et al. Eganelisib combined with immune checkpoint inhibitor therapy and chemotherapy in frontline metastatic triple-negative breast cancer triggers macrophage reprogramming, immune activation and extracellular matrix reorganization in the tumor microenvironment. J. ImmunoTher. Cancer 2024, 12, e009160. [Google Scholar] [CrossRef] [PubMed]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichoń, T.; Kułach, N. Tumor Microenvironment as A Game Changer in Cancer Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef]
- Ozpiskin, O.M.; Zhang, L.; Li, J.J. Immune targets in the tumor microenvironment treated by radiotherapy. Theranostics 2019, 9, 1215–1231. [Google Scholar] [CrossRef]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.C.; Kroemer, G.; Zitvogel, L. Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immunotherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef]
- Khosravi, G.; Mostafavi, S.; Bastan, S.; Ebrahimi, N.; Gharibvand, R.S.; Eskandari, N. Immunologic tumor microenvironment modulators for turning cold tumors hot. Cancer Commun. 2024, 44, 521–553. [Google Scholar] [CrossRef]
- Song, X.; Hao, C.; Li, Y.; Li, Y.; Dong, H.; Wei, Q.; Wei, M.; Li, H.; Zhao, L. Chiral inorganic nanomaterials in the tumor microenvironment: A new chapter in cancer therapy. Pharmacol. Res. 2024, 208, 107386. [Google Scholar] [CrossRef]
- Ding, H.; Tan, P.; Fu, S.; Tian, X.; Zhang, H.; Ma, X.; Gu, Z.; Luo, K. Preparation and application of pH-responsive drug delivery systems. J. Control. Release 2022, 348, 206–238. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.; Luo, Y.; Ning, T.; Liu, P.; Chen, Q.; Chu, Y.; Guo, Q.; Zhang, Y.; Zhou, W.; et al. Macrophage-Disguised Manganese Dioxide Nanoparticles for Neuroprotection by Reducing Oxidative Stress and Modulating Inflammatory Microenvironment in Acute Ischemic Stroke. Adv. Sci. 2021, 8, 2101526. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Chao, Y.; Xu, J.; Sun, X.; Wu, Y.; Peng, R.; Liu, Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, D.; Zhao, Y.; Wang, X.; Yao, S.; Huang, W.; Yang, Y.; Dong, X.; Zhang, L.; Yang, J. Tumor microenvironment-responsive manganese-based nano-modulator activate the cGAS-STING pathway to enhance innate immune system response. J. Nanobiotechnol. 2024, 22, 535. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Barkley, D.; Moncada, R.; Pour, M.; Liberman, D.A.; Dryg, I.; Werba, G.; Wang, W.; Baron, M.; Rao, A.; Xia, B.; et al. Cancer cell states recur across tumor types and form specific interactions with the tumor microenvironment. Nat. Genet. 2022, 54, 1192–1201. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, M.; Wu, L.; Yang, H.; Yao, Y.; Yang, Q.; Du, J.; Liu, L.; Li, Y.; Bai, Y. Stromal cells in the tumor microenvironment: Accomplices of tumor progression? Cell Death Dis. 2023, 14, 587. [Google Scholar] [CrossRef]
- Gabai, Y.; Assouline, B.; Ben-Porath, I. Senescent stromal cells: Roles in the tumor microenvironment. Trends Cancer 2023, 9, 28–41. [Google Scholar] [CrossRef]
- Jin, H.R.; Wang, J.; Wang, Z.J.; Xi, M.J.; Xia, B.H.; Deng, K.; Yang, J.L. Lipid metabolic reprogramming in tumor microenvironment: From mechanisms to therapeutics. J. Hematol. Oncol. 2023, 16, 103. [Google Scholar] [CrossRef]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- Mellman, I.; Chen, D.S.; Powles, T.; Turley, S.J. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity 2023, 56, 2188–2205. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Sleeboom, J.J.F.; van Tienderen, G.S.; Schenke-Layland, K.; van der Laan, L.J.W.; Khalil, A.A.; Verstegen, M.M.A. The extracellular matrix as hallmark of cancer and metastasis: From biomechanics to therapeutic targets. Sci. Transl. Med. 2024, 16, eadg3840. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, H.; Wang, J.; Liu, Y.; Luo, T.; Hua, H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J. Hematol. Oncol. 2022, 15, 34. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.K. Imitating Hypoxia and Tumor Microenvironment with Immune Evasion by Employing Three Dimensional In vitro Cellular Models: Impressive Tool in Drug Discovery. Recent Pat. Anti-Cancer Drug Discov. 2022, 17, 80–91. [Google Scholar] [CrossRef]
- Bai, R.; Li, Y.; Jian, L.; Yang, Y.; Zhao, L.; Wei, M. The hypoxia-driven crosstalk between tumor and tumor-associated macrophages: Mechanisms and clinical treatment strategies. Mol. Cancer 2022, 21, 177. [Google Scholar] [CrossRef]
- Dekker, Y.; Le Dévédec, S.E.; Danen, E.H.J.; Liu, Q. Crosstalk between Hypoxia and Extracellular Matrix in the Tumor Microenvironment in Breast Cancer. Genes 2022, 13, 1585. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, D.; Taniguchi, C.M. Hypoxia inducible factor (HIF) in the tumor microenvironment: Friend or foe? Sci. China Life Sci. 2017, 60, 1114–1124. [Google Scholar] [CrossRef]
- Dharmaratne, N.U.; Kaplan, A.R.; Glazer, P.M. Targeting the Hypoxic and Acidic Tumor Microenvironment with pH-Sensitive Peptides. Cells 2021, 10, 541. [Google Scholar] [CrossRef]
- Corn, K.C.; Windham, M.A.; Rafat, M. Lipids in the tumor microenvironment: From cancer progression to treatment. Prog. Lipid Res. 2020, 80, 101055. [Google Scholar] [CrossRef]
- Chen, X.; Song, M.; Zhang, B.; Zhang, Y. Reactive Oxygen Species Regulate T Cell Immune Response in the Tumor Microenvironment. Oxidative Med. Cell Longev. 2016, 2016, 1580967. [Google Scholar] [CrossRef]
- Jafari, M.; Sriram, V.; Premnauth, G.; Merino, E.; Lee, J.-Y. Modified peroxamide-based reactive oxygen species (ROS)-responsive doxorubicin prodrugs. Bioorganic Chem. 2022, 127, 105990. [Google Scholar] [CrossRef]
- Saikolappan, S.; Kumar, B.; Shishodia, G.; Koul, S.; Koul, H.K. Reactive oxygen species and cancer: A complex interaction. Cancer Lett. 2019, 452, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Zong, L.; Chen, X.; Chen, K.; Jiang, Z.; Nan, L.; Li, X.; Li, W.; Shan, T.; et al. Reactive Oxygen Species and Targeted Therapy for Pancreatic Cancer. Oxidative Med. Cell Longev. 2016, 2016, 1616781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Wu, Q.; Chen, Y.; Deng, Y.; Yang, Z.; Zhang, L.; Liu, B. Tumoral NOX4 recruits M2 tumor-associated macrophages via ROS/PI3K signaling-dependent various cytokine production to promote NSCLC growth. Redox Biol. 2019, 22, 101116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, L.; Lin, Z.; Jiang, C.; Chen, X.; Wang, K.; Liu, L.; Shao, L.; Pan, J.; Li, J.; et al. Methylglyoxal from gut microbes boosts radiosensitivity and radioimmunotherapy in rectal cancer by triggering endoplasmic reticulum stress and cGAS-STING activation. J. ImmunoTher. Cancer 2023, 11, e007840. [Google Scholar] [CrossRef]
- Xu, Q.; Zhan, G.; Zhang, Z.; Yong, T.; Yang, X.; Gan, L. Manganese porphyrin-based metal-organic framework for synergistic sonodynamic therapy and ferroptosis in hypoxic tumors. Theranostics 2021, 11, 1937–1952. [Google Scholar] [CrossRef]
- Wen, X.; Wang, C.; Bi, S.; Xu, Y.; Wu, Z.; Huang, H.; Liu, Z.; Zeng, S. Tumor Microenvironment Cascade Activated Biodegradable Nano-Enzymes for Glutathione-Depletion and Ultrasound-Enhanced Chemodynamic Therapy. Small 2024, 20, e2405457. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, J.; Zhang, D. Manganese Dioxide-Based Nanomaterials for Medical Applications. ACS Biomater. Sci. Eng. 2024, 10, 2680–2702. [Google Scholar] [CrossRef]
- Huang, Y.; Ruan, Y.; Ma, Y.; Chen, D.; Zhang, T.; Fan, S.; Lin, W.; Huang, Y.; Lu, H.; Xu, J.-F.; et al. Immunomodulatory activity of manganese dioxide nanoparticles: Promising for novel vaccines and immunotherapeutics. Front. Immunol. 2023, 14, 1128840. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Guo, Y.; Wu, F. Chemodynamic Therapy via Fenton and Fenton-Like Nanomaterials: Strategies and Recent Advances. Small 2022, 18, 2103868. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.; Zhang, Z.; He, D.; Zhu, J.; Chen, Y.; Zhang, Y. Remodeling of tumor microenvironment for enhanced tumor chemodynamic/photothermal/chemo-therapy. J. Nanobiotechnol. 2022, 20, 388. [Google Scholar]
- Tu, C.; Lu, H.; Zhou, T.; Zhang, W.; Deng, L.; Cao, W.; Yang, Z.; Wang, Z.; Wu, X.; Ding, J.; et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials 2022, 286, 121597. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, W.; Xiao, X.; Liu, S.; Liu, L.; Zhang, L.; Li, L.; Li, Z.; Li, Z.; Xu, M.; et al. Reprogramming exosomes for immunity-remodeled photodynamic therapy against non-small cell lung cancer. Bioact. Mater. 2024, 39, 206–223. [Google Scholar] [CrossRef]
- Liang, L.; Jia, M.; Zhao, M.; Deng, Y.; Tang, J.; He, X.; Liu, Y.; Yan, K.; Yu, X.; Yang, H.; et al. Progress of Nanomaterials Based on Manganese Dioxide in the Field of Tumor Diagnosis and Therapy. Int. J. Nanomed. 2024, 19, 8883–8900. [Google Scholar] [CrossRef]
- Yue, Z.; Zhao, Q.; Wang, S.; Yao, S.; Wan, X.; Hu, Q.; Wen, K.; Zhao, Y.; Li, L. Manganese Dioxide Coated Piezoelectric Nanosonosensitizer for Cancer Therapy with Tumor Microenvironment Remodeling and Multienzyme-Like Catalysis. Small Methods 2024, 8, e2400018. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, Y.; Zheng, R.; Xu, K.; Yan, J.; Song, P.; Wang, Y.; Rauf, A.; Pan, Y.; Zhang, H. Immunomodulation of Tumor Microenvironment by Arginine-Loaded Iron Oxide Nanoparticles for Gaseous Immunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 19825–19835. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, D.; Ding, M.; Yu, N.; Liu, J.; Li, J.; Lin, L. Tumor-targeting biomimetic sonosensitizer-conjugated iron oxide nanocatalysts for combinational chemodynamic–sonodynamic therapy of colorectal cancer. J. Mater. Chem. B 2022, 10, 4595–4604. [Google Scholar] [CrossRef]
- Zhang, W.; Li, L.; Wu, Y.; Li, C.; Xu, Z.; Zhang, N.; Wang, X.; Zhao, Y.; Zu, T.; He, Q.; et al. Biomimetic Iron-Based Nanoparticles Remodel Immunosuppressive Tumor Microenvironment for Metabolic Immunotherapy. Int. J. Nanomed. 2024, 19, 9333–9349. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Xia, Q.; Shang, J.; He, Y.; Li, Z.; Chen, Y.; Gao, F.; Yu, X.; Yuan, Z.; et al. Photothermal Fe3O4 nanoparticles induced immunogenic ferroptosis for synergistic colorectal cancer therapy. J. Nanobiotechnol. 2024, 22, 630. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Q.; Yang, G.; Zhang, L.; Liu, Z.; Cheng, Z.; Zhu, X. Magnetic nanomaterials with near-infrared pH-activatable fluorescence via iron-catalyzed AGET ATRP for tumor acidic microenvironment imaging. J. Mater. Chem. B 2015, 3, 2786–2800. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wang, S.; Zheng, H.; Yang, S.; Zhou, L.; Liu, K.; Zhang, Q.; Zhang, H. Cu-doped cerium oxide-based nanomedicine for tumor microenvironment-stimulative chemo-chemodynamic therapy with minimal side effects. Colloids Surf. B Biointerfaces 2021, 205, 111878. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Cheng, H.; Dai, Y.; Su, Z.; Wang, C.; Lei, L.; Lin, D.; Li, X.; Chen, H.; Fan, K.; et al. In Vivo Regenerable Cerium Oxide Nanozyme-Loaded pH/H2O2-Responsive Nanovesicle for Tumor-Targeted Photothermal and Photodynamic Therapies. ACS Appl. Mater. Interfaces 2021, 13, 233–244. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, N.; Wu, Q.; Chen, Z.; Wang, Y.; Fu, C.; Huang, Z.; Meng, X.; Qiao, B. Cerium oxide nanozymes enhance microwave immunotherapy by reshaping the tumor microenvironment. Nanoscale 2025, 17, 14614–14623. [Google Scholar] [CrossRef]
- Yan, S.; Gao, Z.; Ding, J.; Chen, S.; Wang, Z.; Jin, W.; Qu, B.; Zhang, Y.; Yang, L.; Guo, D.; et al. Nanocomposites based on nanoceria regulate the immune microenvironment for the treatment of polycystic ovary syndrome. J. Nanobiotechnol. 2023, 21, 412. [Google Scholar] [CrossRef]
- Li, X.; Yu, L.; Zhang, C.; Niu, X.; Sun, M.; Yan, Z.; Wang, W.; Yuan, Z. Tumor acid microenvironment-activated self-targeting & splitting gold nanoassembly for tumor chemo-radiotherapy. Bioact. Mater. 2021, 7, 377–388. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, P.; Hu, B.; Zhong, S.; Yan, K.; Wu, Y.; Li, S.; Yang, Y.; Xu, Z.; Lu, Y.; et al. MDSC-targeting gold nanoparticles enhance PD-1 tumor immunotherapy by inhibiting NLRP3 inflammasomes. Biomaterials 2024, 307, 122533. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, Z.; Tong, Z.; Zhang, S.; Min, J.; Xu, Q.; Zhou, L.; Mao, Z.; Xia, H.; Wang, W. Tumor microenvironment-responsive multifunctional peptide coated ultrasmall gold nanoparticles and their application in cancer radiotherapy. Theranostics 2020, 10, 5195–5208. [Google Scholar] [CrossRef]
- Jiang, W.; Wei, L.; Chen, B.; Luo, X.; Xu, P.; Cai, J.; Hu, Y. Platinum prodrug nanoparticles inhibiting tumor recurrence and metastasis by concurrent chemoradiotherapy. J. Nanobiotechnol. 2022, 20, 129. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, L.; Lu, Y.; Chen, X.; Zhang, Y.; Zhou, W.; Guo, Q.; Li, C.; Zhang, Y.; Zhang, Y.; et al. Tumor Microenvironment-Triggered Aggregated Magnetic Nanoparticles for Reinforced Image-Guided Immunogenic Chemotherapy. Adv. Sci. 2019, 6, 1802134. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Ma, W.; Zhang, Y.; Wang, Y.; Sun, L.; Jiang, J.; Jiao, L.; Li, R.; Zhang, Y.; Zhang, M.; et al. A versatile nanoplatform carrying cascade Pt nanozymes remodeling tumor microenvironment for amplified sonodynamic/chemo therapy of thyroid cancer. Biomaterials 2025, 313, 122778. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Luo, W.; Zhu, M.; Zhao, L.; Gao, L.; Liang, H.; Zhang, Z.; Yang, F. Human Serum Albumin-Platinum(II) Agent Nanoparticles Inhibit Tumor Growth Through Multimodal Action Against the Tumor Microenvironment. Mol. Pharm. 2023, 21, 346–357. [Google Scholar] [CrossRef]
- Cai, C.; Zhang, Q.; Ye, J.; Yao, S.; Li, Q.; Fan, Z.; Ge, S.; Wang, Y.; Xu, D.; Zhou, J.; et al. Tumor microenvironment modulation innovates combinative cancer therapy via a versatile graphene oxide nanosystem. Biomater. Sci. 2025, 13, 3123–3148. [Google Scholar] [CrossRef]
- Lin, B.; Chen, H.; Liang, D.; Lin, W.; Qi, X.; Liu, H.; Deng, X. Acidic pH and High-H2O2 Dual Tumor Microenvironment-Responsive Nanocatalytic Graphene Oxide for Cancer Selective Therapy and Recognition. ACS Appl. Mater. Interfaces 2019, 11, 11157–11166. [Google Scholar] [CrossRef]
- Tao, Y.; Zhu, L.; Zhao, Y.; Yi, X.; Zhu, L.; Ge, F.; Mou, X.; Chen, L.; Sun, L.; Yang, K. Nano-graphene oxide-manganese dioxide nanocomposites for overcoming tumor hypoxia and enhancing cancer radioisotope therapy. Nanoscale 2018, 10, 5114–5123. [Google Scholar] [CrossRef]
- Du, P.; Yan, J.; Long, S.; Xiong, H.; Wen, N.; Cai, S.; Wang, Y.; Peng, D.; Liu, Z.; Liu, Y. Tumor microenvironment and NIR laser dual-responsive release of berberine 9-O-pyrazole alkyl derivative loaded in graphene oxide nanosheets for chemo-photothermal synergetic cancer therapy. J. Mater. Chem. B 2020, 8, 4046–4055. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, M.-M.; Luo, Q.-W.; Zhang, Y.-C.; Liu, T.-T.; Yang, Z.; Liao, M.; Tu, P.; Zeng, K.-W. Carbon Quantum Dots-Based Nanozyme from Coffee Induces Cancer Cell Ferroptosis to Activate Antitumor Immunity. ACS Nano 2022, 16, 9228–9239. [Google Scholar] [CrossRef]
- Bao, Y.; Li, G.; Li, S.; Zhang, H.; Wu, X.; Yan, R.; Wang, Z.; Guo, C.; Jin, Y. Multifunctional Tumor-Targeting Carbon Dots for Tumor Microenvironment Activated Ferroptosis and Immunotherapy in Cancer Treatment. ACS Appl. Mater. Interfaces 2023, 15, 56834–56845. [Google Scholar] [CrossRef]
- Guo, L.; Ding, J.; Zhou, W. Converting bacteria into autologous tumor vaccine via surface biomineralization of calcium carbonate for enhanced immunotherapy. Acta Pharm. Sin. B 2023, 13, 5074–5090. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Z.; Deng, L.; Yang, Y.; Zhu, Y.; Sun, X.; Hao, Y.; He, Y.; Fu, J. Hydrogen Therapy Reverses Cancer-Associated Fibroblasts Phenotypes and Remodels Stromal Microenvironment to Stimulate Systematic Anti-Tumor Immunity. Adv. Sci. 2024, 11, e2401269. [Google Scholar] [CrossRef]
- Gharehbaba, A.M.; Soltanmohammadi, F.; Eskandani, M.; Adibkia, K. From barriers to breakthroughs: Mesoporous silica nanoparticles in targeting the tumor microenvironment. Int. J. Pharm. 2025, 682, 125979. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Z.; Du, H.; Chen, X.; Hu, N.; Yu, T.; Hou, M.; Yu, X. An enzyme-responsive hydrogel functionalized with mesoporous silica nanoparticles for co-delivery of cisplatin and shRNA to overcome chemotherapy resistance in non-small cell lung cancer. RSC Adv. 2025, 15, 23966–23977. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Dong, H.; Gao, L.; Liu, M.; Wang, C. Mechanism of selenium-doped black phosphorus nanosheets wrapped with biomimetic tumor cell membrane for prostate cancer immunotherapy. Mater. Sci. Eng. C 2025, 176, 214339. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Mu, M.; Wei, Y.; Yan, B.; Liu, H.; Guo, K.; Zhang, M.; Dai, X.; Sun, X.; Leong, D.T. Novel ultrathin ferrous sulfide nanosheets: Towards replacing black phosphorus in anticancer nanotheranostics. Bioact. Mater. 2025, 43, 564–578. [Google Scholar] [CrossRef]
- Rahimkhoei, V.; Akbari, A.; Jassim, A.Y.; Hussein, U.A.-R.; Salavati-Niasari, M. Recent advances in targeting cancer stem cells by using nanomaterials. Int. J. Pharm. 2025, 673, 125381. [Google Scholar] [CrossRef]
- Saini, S.; Kumar, K.; Saini, P.; Sethi, M.; Meena, P.; Dandia, A.; Weigand, W.; Parewa, V. Elucidating the Synergistic Promotional Mechanism of Water and Oxygen in the Aerobic C–C Homocoupling Reaction Catalyzed by Visible-Light-Derived Core–Shell Pd@A-CQDs Nanostructures. ACS Appl. Mater. Interfaces 2024, 16, 46200–46215. [Google Scholar] [CrossRef]
- Mali, A.; Nayak, N.U.; van Doesburg, J.; Fokkink, R.; van Riessen, K.; de Kruijf, R.; Srinivas, M. Polymeric (Poly(lactic-co-glycolic acid)) Particles Entrapping Perfluorocarbons Are Stable for a Minimum of Six Years. ACS Omega 2025, 10, 6768–6779. [Google Scholar] [CrossRef]
- Krafft, M.; Riess, J. Corrigendum to Therapeutic oxygen delivery by perfluorocarbon-based colloids. Adv. Colloid Interface Sci. 2021, 295, 102505. [Google Scholar] [CrossRef]
- Srinivasan, M.K.; Aruchamy, M.; Namasivayam, N.; Muthupandian, S. Protective effect of pH-sensitive carvacrol zinc oxide quantum dots on Nrf-2, MAPK and NF-κB pathways and protein-bound carbohydrates in DMBA-induced mammary carcinogenesis. Pathol. Res. Pract. 2025, 272, 156117. [Google Scholar] [CrossRef]
- Aydın, B.; Bozoğlu, S.; Karatepe, N.; Güner, F.S. Folic acid-conjugated magnetic carbon nanotube nanocarriers for targeted delivery of mitoxantrone. Nanotechnology 2025, 36, 305701. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhou, Z.; Gu, Y.; Bing, L.; Xie, Y.; Shen, Z.; Chen, W. Powerful Tribocatalytic Degradation of Methyl Orange Solutions with Concentrations as High as 100 mg/L by BaTiO3 Nanoparticles. Nanomaterials 2025, 15, 1135. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Q.; Chen, X.; Yan, B.; Li, S.; Deng, H.; Lu, H. Efficacy, Kinetics, and Mechanism of Tetracycline Degradation in Water by O3/PMS/FeMoBC Process. Nanomaterials 2025, 15, 1108. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, A.; Kornaś, A.; Gil, J.; Sękara, A.; Húska, D.; Rudolphi-Szydło, E.; Sieprawska, A.; Gawrońska, K.; Kulak, M.; Fotopoulos, V.; et al. Exogenously applied putrescine and chitosan–putrescine nanocomposite alleviate the negative effects of chilling stress on iceberg lettuce seedlings. Sci. Rep. 2025, 15, 26963. [Google Scholar] [CrossRef]
- Madbouly, N.A.; Ali, D.M.; Farid, A.A. Nanoparticles from grape seed extract inhibit inflammatory cytokines and ameliorate CCl4-induced hepatotoxicity. BMC Complement. Med. Ther. 2025, 25, 276. [Google Scholar] [CrossRef]
- Chauhan, A.; Saini, A.; Sharma, D. The evolution of integrated magnetic hyperthermia and chemodynamic therapy for combating cancer: A comprehensive viewpoint. Nanoscale Adv. 2025, 7, 4820–4836. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Chen, H.; Chen, Y.; Ni, Y.; Ji, J.; Wang, K.; Jin, Q. Self-assembled copper-amino acid nanoleaves for targeted treatment of deep-seated bacterial infections via chemodynamic therapy and cuproptosis-like death. Biomaterials 2025, 325, 123566. [Google Scholar] [CrossRef]
- Kim, E.; Jang, D.; Kim, M.; Heo, J.; Shin, J.; Chung, J.-Y.; Choi, M.; Yang, W.-H.; Lee, H.; Cha, J.-H. High-throughput screening system for immunogenic cell death inducers using artificial intelligence-based real-time image analysis. Comput. Biol. Med. 2025, 196, 110727. [Google Scholar] [CrossRef]
- Isaeva, A.S.; Yeriomenko, A.D.T.; Idota, E.; Volodina, S.I.; Porozova, N.O.; Bezsonov, E.E.; Malogolovkin, A.S. Shaping viral immunotherapy towards cancer-targeted immunological cell death. Front. Oncol. 2025, 15, 1540397. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, J.; Chang, Y.; Tang, L.; Ma, G.; Liu, X.; Gao, F.; Ma, X.; Guo, Y. Tumor microenvironment responsive smart nanoplatform for synergistic tumor therapy through co-enhancement of GSH depletion and hypoxia relief. J. Inorg. Biochem. 2025, 272, 113005. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Tong, W.; Peng, B.; Gao, W.; Wang, Y.; Wang, H.; Liu, J.; Liu, Y.; Sun, B.; Ma, J.; et al. Mesoporous Nanoplatform That Efficiently Delivers DOX as an H2O2 Generator to Trigger Mutual Amplification of Cuproptosis and Chemodynamic Therapy. Adv. Healthc. Mater. 2025, 14, e2500933. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lu, H.; Lei, L.; Scherman, D.; Liu, Y. Tumor microenvironment-responsive MIL-53(Fe)@MnO2- induced glutathione depletion and sustained hydroxyl radical generation for enhanced chemodynamic cancer therapy. J. Colloid Interface Sci. 2025, 700, 138342. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, H.; Hou, Y.; Yunlong, W.; Feng, X. Immunomodulatory effects of photothermal therapy in breast cancer: Advances and challenges. Front. Immunol. 2025, 16, 1544693. [Google Scholar] [CrossRef]
- Zhang, W.; Yue, W.; Shi, X.; Lv, L.; Fang, Z.; Li, J. A TME-activated photothermal agent with photodegradability for accurate breast tumor photothermal therapy. RSC Adv. 2025, 15, 25062–25066. [Google Scholar] [CrossRef]
- Peng, W.; Zhou, T.; Hu, L.; Vankann, V.; Bohn, T.; Bopp, T.; Kuan, S.L.; Weil, T. Autonomous Activation of a Gated Chemiluminescent Photosensitizer Enables Targeted Photodynamic Therapy in Tumor Cells. J. Am. Chem. Soc. 2025, 147, 27822–27834. [Google Scholar] [CrossRef]
- Yang, Z.; Deng, L.; Pan, Z.; Wang, X.; Lin, L.; Fang, Y.; Huang, Y.; Feng, X.; Chen, X. Oxygen-boosted nanodrug for amplified ferroptosis-photodynamic immunotherapy together with PD-1 checkpoint blockade against triple-negative breast cancer. Colloids Surf. B Biointerfaces 2025, 255, 114963. [Google Scholar] [CrossRef]
- Wang, L.; Xu, D.; Hu, X.; Quan, R.; Lu, D.; Li, Z.; Yu, C.; Li, X.; Ma, S.; Li, X.; et al. Cascade reaction–driven biomimetic scintillant/metal–organic frameworks for X-ray triggered combinational therapy against glioma. Mater. Today Bio 2025, 33, 102069. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Liu, L.; Wu, Y.; Li, W.; Liang, J.; Shen, H.; Fang, S.; Huang, X.; Chu, Z.; et al. Gelatin Methacryloyl Xerogel Puncture Implants Loaded with Cu0.5Mn2.5O4 Nanoparticles Synergizes Cuproptosis and STING Activation for Enhanced Breast Cancer Immunotherapy. ACS Nano 2025, 19, 27902–27918. [Google Scholar] [CrossRef]
- Graván, P.; Peña-Martín, J.; de Andrés, J.L.; Pedrosa, M.; Villegas-Montoya, M.; Galisteo-González, F.; Marchal, J.A.; Sánchez-Moreno, P. Exploring the Impact of Nanoparticle Stealth Coatings in Cancer Models: From PEGylation to Cell Membrane-Coating Nanotechnology. ACS Appl. Mater. Interfaces 2024, 16, 2058–2074. [Google Scholar] [CrossRef]
- Luo, Q.; Shi, W.; Wang, P.; Zhang, Y.; Meng, J.; Zhang, L. Tumor Microenvironment-Responsive Shell/Core Composite Nanoparticles for Enhanced Stability and Antitumor Efficiency Based on a pH-Triggered Charge-Reversal Mechanism. Pharmaceutics 2021, 13, 895. [Google Scholar] [CrossRef]
- Liu, B.; Shen, J.; Wu, Y.; Wong, C.F.; Hei, T.Y.; Li, J.W.-T.; Hummel, S.N.; Jin, G.; Petrucci, N.R.; Wang, D.M.; et al. Novel strategy for tumor immunotherapy using FITC-folate bispecific adapter bridged CAR immune cell cocktails. Bioact. Mater. 2025, 52, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Dong, J.; Xie, F.; Feng, X.; Wang, J.; Xu, X.; Tang, B.; Sun, C.; Wang, Y.; Zhong, W.; et al. Polyvalent folate receptor-targeting chimeras for degradation of membrane proteins. Nat. Chem. Biol. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Wang, M.; Mubeen, F.; Sundas, A.; Naz, R.; Hu, X.; Chen, X. CDKN2A and matrix metalloproteinases: Key regulators of cellular senescence in squamous cell carcinoma. Am. J. Transl. Res. 2025, 17, 4573–4589. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, Y.; Wei, W. Research advances of matrix metalloproteinases family in uveal melanoma. Eur. J. Med. Res. 2025, 30, 609. [Google Scholar] [CrossRef]
- Sun, M.; Ni, C.; Li, A.; Liu, J.; Guo, H.; Xu, F.; Li, K.; Cao, X.; Shi, X.; Guo, R. A biomimetic nanoplatform mediates hypoxia-adenosine axis disruption and PD-L1 knockout for enhanced MRI-guided chemodynamic-immunotherapy. Acta Biomater. 2025, 201, 618–632. [Google Scholar] [CrossRef]
- Tang, Z.; Luo, W.; Xu, M.; Liu, Y.; Yu, Q.; Wang, L. A TME-responsive oxygen-self-supplying hybridized polymersome for synergistic triple-modal therapy and precision theranostics in hypoxic tumors. J. Mater. Chem. B 2025, 13, 8136–8148. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Y.; Cui, H.; Geng, H.; Ruan, L.; Zhao, Y.; Zhou, C.; Dai, W.; Chen, J.; Yu, J.; et al. EGCG and DOX dual-drug-loaded enzyme-responsive nanovesicles boost mitochondrial-mediated ICD for improved immunotherapy. Front. Pharmacol. 2025, 16, 1624109. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Xiang, H.; Tang, X. Smart Inorganic Nanomaterials for Tumor Microenvironment Modulation. Inorganics 2025, 13, 337. https://doi.org/10.3390/inorganics13100337
Jiang Z, Xiang H, Tang X. Smart Inorganic Nanomaterials for Tumor Microenvironment Modulation. Inorganics. 2025; 13(10):337. https://doi.org/10.3390/inorganics13100337
Chicago/Turabian StyleJiang, Zhenqi, Hui Xiang, and Xiaoying Tang. 2025. "Smart Inorganic Nanomaterials for Tumor Microenvironment Modulation" Inorganics 13, no. 10: 337. https://doi.org/10.3390/inorganics13100337
APA StyleJiang, Z., Xiang, H., & Tang, X. (2025). Smart Inorganic Nanomaterials for Tumor Microenvironment Modulation. Inorganics, 13(10), 337. https://doi.org/10.3390/inorganics13100337





