Review on Chemistry of Water-Containing Calcium Carbonates and Their Transformations into Amorphous and Crystalline Carbonate Modifications
Abstract
1. Introduction
2. Occurrence in Geological Environment and/or Biological Systems, and Preparation, Morphology, and Properties of Hydrated Calcium Carbonates
2.1. Ikaite
2.2. Monohydrocalcite, MHC
2.3. Calcium Carbonate Hemihydrate, CCHH
2.4. Amorphous Calcium Carbonate (ACC)
3. Spectroscopic and Structural Studies on Hydrated Calcium Carbonates
3.1. IR and Raman Spectroscopy
3.2. Structure of Ikaite
3.3. Structure of MHC
3.4. Structure of CCHH
3.5. Structure of ACC
4. Transformations of Hydrated Calcium Carbonates
4.1. Transformation of Ikaite
4.2. Transformation of MHC
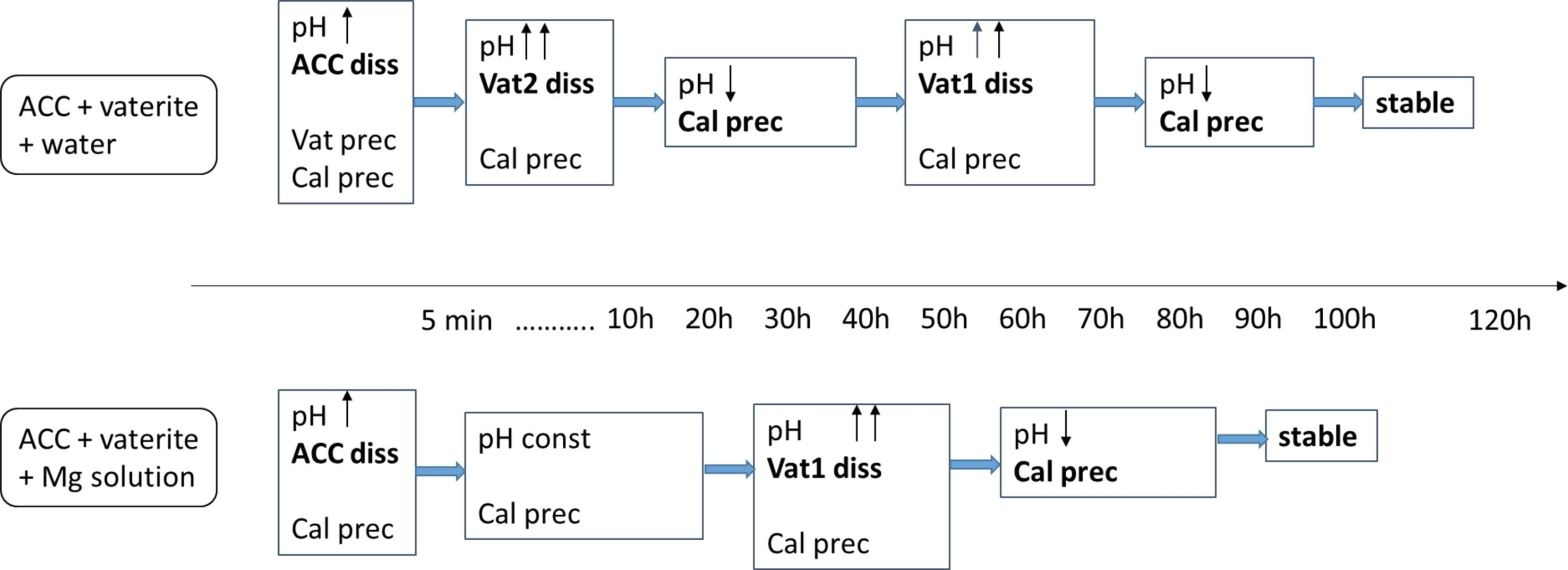
4.3. Transformation of Various ACCs
4.4. Transformation of CCHH
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leonard, J.E.; Cameron, B.; Pilkey, O.H.; Friedman, G.M. Evaluation of Cold-Water Carbonates as a Possible Paleoclimatic Indicator. Sediment. Geol. 1981, 28, 1–28. [Google Scholar] [CrossRef]
- Zervas, A.; Stougaard, P.; Thøgersen, M.S. Complete Genome Sequence of “Bacillaceae Sp. Strain IKA-2”: A Cold-Active, Amylase-Producing Bacterium from Ikaite Columns in SW Greenland. Microbiol. Resour. Announc. 2024, 13, e00887-23. [Google Scholar] [CrossRef]
- Schmidt, M.; Priemé, A.; Stougaard, P. Bacterial Diversity in Permanently Cold and Alkaline Ikaite Columns from Greenland. Extremophiles 2006, 10, 551–562. [Google Scholar] [CrossRef]
- Chekanov, G.S.; Shelgunov, V.V. Hexahydrate Deposits in Recirculating Systems for Hydraulic Ash Removal and Ways to Eliminate Them. Elektr. Stantsii 1991, 10, 10–13. [Google Scholar]
- Malkaj, P.; Chrissanthopoulos, A.; Dalas, E. The Overgrowth of Calcium Carbonate Hexahydrate on New Functionalized Polymers. J. Cryst. Growth 2002, 242, 233–238. [Google Scholar] [CrossRef]
- Thakur, S.K.; Tomar, N.K.; Pandeya, S.B. Influence of Phosphate on Cadmium Sorption by Calcium Carbonate. Geoderma 2006, 130, 240–249. [Google Scholar] [CrossRef]
- Trzcinski, A.P.; Stuckey, D.C. Inorganic Fouling of an Anaerobic Membrane Bioreactor Treating Leachate from the Organic Fraction of Municipal Solid Waste (OFMSW) and a Polishing Aerobic Membrane Bioreactor. Bioresour. Technol. 2016, 204, 17–25. [Google Scholar] [CrossRef]
- Kaplan, M.E. Calcite Pseudomorphs in Sedimentary Rocks and Their Paleogeographical Significance. Dokl. Akad. Nauk SSSR 1977, 237, 1467–1470. [Google Scholar]
- Suess, E.; Balzer, W.; Hesse, K.-F.; Müller, P.J.; Ungerer, C.A.; Wefer, G. Calcium Carbonate Hexahydrate from Organic-Rich Sediments of the Antarctic Shelf: Precursors of Glendonites. Science 1982, 216, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, K.; Vereshchagin, O.; Ershova, V.; Rogov, M.; Chernyshova, I.; Vishnevskaya, I.; Okuneva, T.; Pokrovsky, B.; Tuchkova, M.; Saphronova, N.; et al. Marine Diagenesis of Ikaite: Implications from the Isotopic and Geochemical Composition of Glendonites and Host Concretions (Palaeogene–Neogene Sediments, Sakhalin Island). Sedimentology 2021, 68, 2227–2251. [Google Scholar] [CrossRef]
- Politi, Y.; Arad, T.; Klein, E.; Weiner, S.; Addadi, L. Sea Urchin Spine Calcite Forms via a Transient Amorphous Calcium Carbonate Phase. Science 2004, 306, 1161–1164. [Google Scholar] [CrossRef]
- Radha, A.V.; Forbes, T.Z.; Killian, C.E.; Gilbert, P.U.P.A.; Navrotsky, A. Transformation and Crystallization Energetics of Synthetic and Biogenic Amorphous Calcium Carbonate. Proc. Natl. Acad. Sci. USA 2010, 107, 16438–16443. [Google Scholar] [CrossRef]
- Ghilardi, M.; Salter, M.A.; Parravicini, V.; Ferse, S.C.A.; Rixen, T.; Wild, C.; Birkicht, M.; Perry, C.T.; Berry, A.; Wilson, R.W.; et al. Temperature, Species Identity and Morphological Traits Predict Carbonate Excretion and Mineralogy in Tropical Reef Fishes. Nat. Commun. 2023, 14, 985. [Google Scholar] [CrossRef]
- Photong, C.; Pragot, W. Effect of Adding Monohydrocalcite on the Microstructural Change in Cement Hydration. ACS Omega 2022, 7, 36318–36329. [Google Scholar] [CrossRef]
- Lee, S.K.; Oh, T.; Kim, G.W.; Bae, S.; Yoo, D.-Y. Benefits of CaCO3 Nanoparticles for the Strain Hardening Behavior of High-Strength Alkali-Activated Composites Based on Blast Furnace Slag and Liquid Crystal Display Glass Powder. Constr. Build. Mater. 2024, 449, 138314. [Google Scholar] [CrossRef]
- Brečević, L.; Kralj, D. On Calcium Carbonates: From Fundamental Research to Application. Croat. Chem. Acta 2007, 80, 467–484. [Google Scholar]
- Nebel, H.; Neumann, M.; Mayer, C.; Epple, M. On the Structure of Amorphous Calcium Carbonate—A Detailed Study by Solid-State NMR Spectroscopy. Inorg. Chem. 2008, 47, 7874–7879. [Google Scholar] [CrossRef] [PubMed]
- Fischbeck, R.; Müller, G. Monohydrocalcite, Hydromagnesite, Nesquehonite, Dolomite, Aragonite, and Calcite in Speleothems of the Frankische Schweiz, Western Germany. Contrib. Mineral. Petrol. 1971, 33, 87–92. [Google Scholar] [CrossRef]
- Mackenzie, J.E. CCLXXIV.—Calcium Carbonate Hexahydrate. J. Chem. Soc. Trans. 1923, 123, 2409–2417. [Google Scholar] [CrossRef]
- Zou, Z.; Habraken, W.J.E.M.; Matveeva, G.; Jensen, A.C.S.; Bertinetti, L.; Hood, M.A.; Sun, C.; Gilbert, P.U.P.A.; Polishchuk, I.; Pokroy, B.; et al. A Hydrated Crystalline Calcium Carbonate Phase: Calcium Carbonate Hemihydrate. Science 2019, 363, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Lázár, A.; Molnár, Z.; Demény, A.; Kótai, L.; Trif, L.; Béres, K.A.; Bódis, E.; Bortel, G.; Aradi, L.E.; Karlik, M.; et al. Insights into the Amorphous Calcium Carbonate (ACC) → Ikaite → Calcite Transformations. CrystEngComm 2023, 25, 738–750. [Google Scholar] [CrossRef]
- Schmidt, C.A.; Tambutté, E.; Venn, A.A.; Zou, Z.; Castillo Alvarez, C.; Devriendt, L.S.; Bechtel, H.A.; Stifler, C.A.; Anglemyer, S.; Breit, C.P.; et al. Myriad Mapping of Nanoscale Minerals Reveals Calcium Carbonate Hemihydrate in Forming Nacre and Coral Biominerals. Nat. Commun. 2024, 15, 1812. [Google Scholar] [CrossRef]
- Suyama, M.; Kitajima, T.; Fukushi, K. Solubility of Calcium Carbonate Hemihydrate (CCHH): Where Does CCHH Occur? Geochem. Persp. Let. 2024, 31, 27–31. [Google Scholar] [CrossRef]
- Jiang, J.; Yan, P.; Liu, C.; Sun, T.; Xu, S.; Li, Q. Amorphous Calcium Carbonate (ACC): Structure, Preparation, Stability and Identification. Adv. Powder Technol. 2025, 36, 104850. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, W.; Wang, J.; Long, F.; Fu, Z.; Xie, J.; Zou, Z. Stabilization and Crystallization Mechanism of Amorphous Calcium Carbonate. J. Colloid Interface Sci. 2025, 680, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Marland, G. The Stability of CaCO3·6H2O (Ikaite). Geochim. Cosmochim. Acta 1975, 39, 83–91. [Google Scholar] [CrossRef]
- Elfil, H.; Roques, H. Prediction of the Limit of the Metastable Zone in the “CaCO3-CO2-H2O” System. AIChE J. 2004, 50, 1908–1916. [Google Scholar] [CrossRef]
- Sekkal, W.; Zaoui, A. Nanoscale Analysis of the Morphology and Surface Stability of Calcium Carbonate Polymorphs. Sci. Rep. 2013, 3, 1587. [Google Scholar] [CrossRef]
- Bushuev, Y.G.; Finney, A.R.; Rodger, P.M. Stability and Structure of Hydrated Amorphous Calcium Carbonate. Cryst. Growth Des. 2015, 15, 5269–5279. [Google Scholar] [CrossRef]
- Guo, D.; Ou, W.; Ning, F.; Fang, B.; Liang, Y.; Ud Din, S.; Zhang, L. Effects of Hydrophilic and Hydrophobic nano-CaCO3 on Kinetics of Hydrate Formation. Energy Sci. Eng. 2022, 10, 507–524. [Google Scholar] [CrossRef]
- Taylor, M.G.; Simmkiss, K.; Greaves, G.N.; Okazaki, M.; Mann, S. An X-Ray Absorption Spectroscopy Study of the Structure and Transformation of Amorphous Calcium Carbonate from Plant Cystoliths. Proc. R. Soc. Lond. B 1993, 252, 75–80. [Google Scholar] [CrossRef]
- Levi-Kalisman, Y.; Raz, S.; Weiner, S.; Addadi, L.; Sagi, I. Structural Differences Between Biogenic Amorphous Calcium Carbonate Phases Using X-Ray Absorption Spectroscopy. Adv. Funct. Mater. 2002, 12, 43–48. [Google Scholar] [CrossRef]
- DeVol, R.T.; Metzler, R.A.; Kabalah-Amitai, L.; Pokroy, B.; Politi, Y.; Gal, A.; Addadi, L.; Weiner, S.; Fernandez-Martinez, A.; Demichelis, R.; et al. Oxygen Spectroscopy and Polarization-Dependent Imaging Contrast (PIC)-Mapping of Calcium Carbonate Minerals and Biominerals. J. Phys. Chem. B 2014, 118, 8449–8457. [Google Scholar] [CrossRef]
- Endovitskii, A.P.; Minkin, M.B. Current Problems of the Carbonate System in Soil Solution Thermodynamics. Pochvovedenie 1986, 11, 76–88. [Google Scholar]
- Gal, J.; Bollinger, J.; Tolosa, H.; Gache, N. Calcium Carbonate Solubility: A Reappraisal of Scale Formation and Inhibition. Talanta 1996, 43, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Marion, G.M.; Millero, F.J.; Feistel, R. Precipitation of Solid Phase Calcium Carbonates and Their Effect on Application of Seawater SA–T–P Models. Ocean Sci. 2009, 5, 285–291. [Google Scholar] [CrossRef]
- Geilfus, N.-X.; Carnat, G.; Dieckmann, G.S.; Halden, N.; Nehrke, G.; Papakyriakou, T.; Tison, J.-L.; Delille, B. First Estimates of the Contribution of CaCO3 Precipitation to the Release of CO2 to the Atmosphere during Young Sea Ice Growth. JGR Ocean. 2013, 118, 244–255. [Google Scholar] [CrossRef]
- Papadimitriou, S.; Kennedy, H.; Kennedy, P.; Thomas, D.N. Kinetics of Ikaite Precipitation and Dissolution in Seawater-Derived Brines at Sub-Zero Temperatures to 265 K. Geochim. Cosmochim. Acta 2014, 140, 199–211. [Google Scholar] [CrossRef]
- Vasileva, K.; Zaretskaya, N.; Ershova, V.; Rogov, M.; Stockli, L.D.; Stockli, D.; Khaitov, V.; Maximov, F.; Chernyshova, I.; Soloshenko, N.; et al. New Model for Seasonal Ikaite Precipitation: Evidence from White Sea Glendonites. Mar. Geol. 2022, 449, 106820. [Google Scholar] [CrossRef]
- Zaoui, A.; Sekkal, W. Mechanisms behind the Ikaite-to-Calcite Phase Transformation from Molecular Dynamics Calculations. Geoderma 2014, 235–236, 329–333. [Google Scholar] [CrossRef]
- Galan, I.; Purgstaller, B.; Grengg, C.; Müller, B.; Dietzel, M. Amorphous and Crystalline CaCO3 Phase Transformation at High Solid/Liquid Ratio—Insight to a Novel Binder System. J. Cryst. Growth 2022, 580, 126465. [Google Scholar] [CrossRef]
- Sun, R.; Willhammar, T.; Svensson Grape, E.; Strømme, M.; Cheung, O. Mesoscale Transformation of Amorphous Calcium Carbonate to Porous Vaterite Microparticles with Morphology Control. Cryst. Growth Des. 2019, 19, 5075–5087. [Google Scholar] [CrossRef]
- Munemoto, T.; Fukushi, K. Transformation Kinetics of Monohydrocalcite to Aragonite in Aqueous Solutions. J. Mineral. Petrol. Sci. 2008, 103, 345–349. [Google Scholar] [CrossRef]
- Sawada, K. The Mechanisms of Crystallization and Transformation of Calcium Carbonates. Pure Appl. Chem. 1997, 69, 921–928. [Google Scholar] [CrossRef]
- Sánchez-Pastor, N.; Oehlerich, M.; Astilleros, J.M.; Kaliwoda, M.; Mayr, C.C.; Fernández-Díaz, L.; Schmahl, W.W. Crystallization of Ikaite and Its Pseudomorphic Transformation into Calcite: Raman Spectroscopy Evidence. Geochim. Cosmochim. Acta 2016, 175, 271–281. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, Y.-I.; Lee, K.; Bang, J.-H.; Jun, C.-W.; Jang, Y.-N. Effect of Serine and Arginine on the Phase Transition from Amorphous CaCO3 and CaCO3·6H2O to Calcite Film. Mater. Trans. 2012, 53, 1732–1738. [Google Scholar] [CrossRef]
- Larsen, D. Origin and Paleoenvironmental Significance of Calcite Pseudomorphs after Ikaite in the Oligocene Creede Formation, Colorado. J. Sediment. Res. 1994, 64, 593–603. [Google Scholar] [CrossRef]
- De Lurio, J.L.; Frakes, L.A. Glendonites as a Paleoenvironmental Tool: Implications for Early Cretaceous High Latitude Climates in Australia. Geochim. Cosmochim. Acta 1999, 63, 1039–1048. [Google Scholar] [CrossRef]
- Huggett, J.M.; Schultz, B.P.; Shearman, D.J.; Smith, A.J. The Petrology of Ikaite Pseudomorphs and Their Diagenesis. Proc. Geol. Assoc. 2005, 116, 207–220. [Google Scholar] [CrossRef]
- Rogov, M.; Ershova, V.; Vereshchagin, O.; Vasileva, K.; Mikhailova, K.; Krylov, A. Database of Global Glendonite and Ikaite Records throughout the Phanerozoic. Earth Syst. Sci. Data 2021, 13, 343–356. [Google Scholar] [CrossRef]
- Rachlin, A.L.; Henderson, G.S.; Goh, M.C. An Atomic Force Microscope (AFM) Study of the Calcite Cleavage Plane: Image Averaging in Fourier Space. Am. Min. 1992, 77, 904–910. [Google Scholar]
- Kwak, M.; Shindo, H. Atomic Force Microscopic Observation of Facet Formation on Various Faces of Aragonite in Aqueous Acetic Acid. J. Cryst. Growth 2005, 275, e1655–e1659. [Google Scholar] [CrossRef]
- Ranawat, Y.S.; Jaques, Y.M.; Foster, A.S. Predicting Hydration Layers on Surfaces Using Deep Learning. Nanoscale Adv. 2021, 3, 3447–3453. [Google Scholar] [CrossRef] [PubMed]
- Albright, J.N. Mineralogical Notes Vaterite Stability. Am. Mineral. 1971, 56, 620–624. [Google Scholar]
- Pelouze, M.J. Sur Une Combinaison Nouvelle d’eau et de Carbonate de Chaux. Chem. Rev. 1865, 60, 429–431. [Google Scholar]
- Pauly, H. “Ikaite”, a New Mineral from Greenland. ARCTIC 1963, 16, 263–264. [Google Scholar] [CrossRef]
- Copisarow, M. XCII.—Heteromorphism of Calcium Carbonate. Marble, Synthetic and Metamorphic. J. Chem. Soc., Trans. 1923, 123, 785–796. [Google Scholar] [CrossRef]
- Johnston, J.; Merwin, H.E.; Williamson, E.D. The Several Forms of Calcium Carbonate. Am. J. Sci. 1916, s4-41, 473–512. [Google Scholar] [CrossRef]
- Iwanoff, L.L. Ein Wasserhaltiges Calcium Carbonat Aussen Umgebungen von Nowo-Alexandria (Guv. Lublin). Ann. Der Geol. Und Mineral. Der Russl. 1906, 8, 23–25. [Google Scholar]
- Bischoff, J.L.; Stine, S.; Rosenbauer, R.J.; Fitzpatrick, J.A.; Stafford, T.W. Ikaite Precipitation by Mixing of Shoreline Springs and Lake Water, Mono Lake, California, USA. Geochim. Cosmochim. Acta 1993, 57, 3855–3865. [Google Scholar] [CrossRef]
- Feurdean, A.; Perşoiu, A.; Pazdur, A.; Onac, B.P. Evaluating the Palaeoecological Potential of Pollen Recovered from Ice in Caves: A Case Study from Scărişoara Ice Cave, Romania. Rev. Palaeobot. Palynol. 2011, 165, 1–10. [Google Scholar] [CrossRef]
- Boch, R.; Dietzel, M.; Reichl, P.; Leis, A.; Baldermann, A.; Mittermayr, F.; Pölt, P. Rapid Ikaite (CaCO3·6H2O) Crystallization in a Man-Made River Bed: Hydrogeochemical Monitoring of a Rarely Documented Mineral Formation. Appl. Geochem. 2015, 63, 366–379. [Google Scholar] [CrossRef]
- Mikkelsen, A.; Andersen, A.B.; Engelsen, S.B.; Hansen, H.C.B.; Larsen, O.; Skibsted, L.H. Presence and Dehydration of Ikaite, Calcium Carbonate Hexahydrate, in Frozen Shrimp Shell. J. Agric. Food Chem. 1999, 47, 911–917. [Google Scholar] [CrossRef]
- Swainson, I.P.; Hammond, R.P. Ikaite, CaCO3·6H2O: Cold Comfort for Glendonites as Paleothermometers. Am. Mineral. 2001, 86, 1530–1533. [Google Scholar] [CrossRef]
- Rickaby, R.E.M.; Shaw, S.; Bennitt, G.; Kennedy, H.; Zabel, M.; Lennie, A. Potential of Ikaite to Record the Evolution of Oceanic δ18O. Geol 2006, 34, 497. [Google Scholar] [CrossRef]
- Garvie, L.A.J. Seasonal Formation of Ikaite in Slime Flux Jelly on an Infected Tree (Populus fremontii) Wound from the Sonoran Desert. Sci. Nat. 2022, 109, 48. [Google Scholar] [CrossRef] [PubMed]
- Tansman, G.F.; Kindstedt, P.S.; Hughes, J.M. Crystallization and Demineralization Phenomena in Washed-Rind Cheese. J. Dairy Sci. 2017, 100, 8694–8704. [Google Scholar] [CrossRef]
- Bastianini, L.; Rogerson, M.; Brasier, A.; Prior, T.J.; Hardman, K.; Dempsey, E.; Bird, A.; Mayes, W.M. Ikaite Formation in Streams Affected by Steel Waste Leachate: First Report and Potential Impact on Contaminant Dynamics. Chem. Geol. 2024, 644, 121842. [Google Scholar] [CrossRef]
- Tollefsen, E.; Balic-Zunic, T.; Mörth, C.-M.; Brüchert, V.; Lee, C.C.; Skelton, A. Ikaite Nucleation at 35 °C Challenges the Use of Glendonite as a Paleotemperature Indicator. Sci. Rep. 2020, 10, 8141. [Google Scholar] [CrossRef] [PubMed]
- Németh, P.; Töchterle, P.; Dublyansky, Y.; Stalder, R.; Molnár, Z.; Klébert, S.; Spötl, C. Tracing Structural Relicts of the Ikaite-to-Calcite Transformation in Cryogenic Cave Glendonite. Am. Min. 2022, 107, 1960–1967. [Google Scholar] [CrossRef]
- Schultz, B.; Thibault, N.; Huggett, J. The Minerals Ikaite and Its Pseudomorph Glendonite: Historical Perspective and Legacies of Douglas Shearman and Alec K. Smith. Proc. Geol. Assoc. 2022, 133, 176–192. [Google Scholar] [CrossRef]
- Lázár, A.; Demény, A.; Hegyi, I.; Aradi, L.E.; Garvie, L.A.J.; Németh, P. Oxygen Isotopic Re-Equilibration during Transformation of Ikaite to Calcite via Amorphous Calcium Carbonate. Chem. Geol. 2025, 693, 122980. [Google Scholar] [CrossRef]
- Hu, Y.-B.; Wolf-Gladrow, D.A.; Dieckmann, G.S.; Völker, C.; Nehrke, G. A Laboratory Study of Ikaite (CaCO3·6H2O) Precipitation as a Function of pH, Salinity, Temperature and Phosphate Concentration. Mar. Chem. 2014, 162, 10–18. [Google Scholar] [CrossRef]
- Kimura, T.; Koga, N. Monohydrocalcite in Comparison with Hydrated Amorphous Calcium Carbonate: Precipitation Condition and Thermal Behavior. Cryst. Growth Des. 2011, 11, 3877–3884. [Google Scholar] [CrossRef]
- Brooks, R.; Clark, L.M.; Thurston, E.F. Calcium Carbonate and Its Hydrates. Phil. Trans. R. Soc. Lond. A 1950, 243, 145–167. [Google Scholar] [CrossRef]
- Van Tassel, R. Carbonatniederschläge Aus Gemischten Calcium-Magnesiumchloridlösungen. Z. Anorg. Allg. Chem. 1962, 319, 107–112. [Google Scholar] [CrossRef]
- Sapozhnikov, D.G.; Zvetkov, A.I. Precipitation of Hydrous Calcium Carbonate on the Bottom of Lake Issyk-Kul. Dokl. Akad. Nauk SSSR 1959, 124, 402–404. [Google Scholar]
- Carlström, D. A Crystallographic Study of Vertebrate Otoliths. Biol. Bull. 1963, 125, 441–463. [Google Scholar] [CrossRef]
- Dahl, K.; Buchardt, B. Monohydrocalcite in the Arctic Ikka Fjord, SW Greenland: First Reported Marine Occurrence. J. Sediment. Res. 2006, 76, 460–471. [Google Scholar] [CrossRef]
- Marschner, H. Hydrocalcite (CaCO3⋅H2O) and Nesquehonite (MgCO3⋅3H2O) in Carbonate Scales. Science 1969, 165, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Garvie, L.A.J. Decay-Induced Biomineralization of the Saguaro Cactus (Carnegiea gigantea). Am. Min. 2003, 88, 1879–1888. [Google Scholar] [CrossRef]
- Zaytseva, L.V.; Samylina, O.S.; Prokin, A.A. Formation of Monohydrocalcite in the Microbialites from Laguna de Los Cisnes (Isla Grande de Tierra Del Fuego, Chile). Environ. Sci. Proc. 2021, 6, 2. [Google Scholar]
- Krauss, F.; Schriever, W. Die Hydrate Des Calciumcarbonats. Z. Anorg. Allg. Chem. 1930, 188, 259–273. [Google Scholar] [CrossRef]
- Duedall, I.W.; Buckley, D.E. Calcium Carbonate Monohydrate in Seawater. Nat. Phys. Sci. 1971, 234, 39–40. [Google Scholar] [CrossRef]
- Nathan, C.C. Vaterite in Lake Water. Nat. Phys. Sci. 1971, 231, 158. [Google Scholar] [CrossRef]
- Vereshchagin, O.S.; Chernyshova, I.A.; Kuz’mina, M.A.; Frank-Kamenetskaya, O.V. Calcium Carbonate Precipitation Behavior in the System Ca-Me2+-CO3-H2O (Me2+ = Co, Ni, Cu, Fe): Ion Incorporation, Effect of Temperature and Aging. Minerals 2023, 13, 1497. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Bots, P.; Roncal-Herrero, T.; Benning, L.G. The Role of Mg in the Crystallization of Monohydrocalcite. Geochim. Cosmochim. Acta 2014, 127, 204–220. [Google Scholar] [CrossRef]
- Kitajima, T.; Fukushi, K.; Yoda, M.; Takeichi, Y.; Takahashi, Y. Simple, Reproducible Synthesis of Pure Monohydrocalcite with Low Mg Content. Minerals 2020, 10, 346. [Google Scholar] [CrossRef]
- Zhang, G.; Delgado-López, J.M.; Choquesillo-Lazarte, D.; García-Ruiz, J.M. Growth Behavior of Monohydrocalcite (CaCO3·H2O) in Silica-Rich Alkaline Solution. Cryst. Growth Des. 2015, 15, 564–572. [Google Scholar] [CrossRef]
- Kralj, D.; Brečević, L. Dissolution Kinetics and Solubility of Calcium Carbonate Monohydrate. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 287–293. [Google Scholar] [CrossRef]
- Taylor, G.F. The Occurrence of Monohydrocalcite in Two Small Lakes in the South-East of South Australia. Am. Mineral. 1975, 60, 690–697. [Google Scholar]
- Zhou, Y.; Liu, Q.; Hu, M.; Xu, G.; Xu, R.; Chong, X.; Feng, J. Investigation on the Stability, Electronic, Optical, and Mechanical Properties of Novel Calcium Carbonate Hydrates via First-principles Calculations. Int. J. Quantum Chem. 2020, 120, e26219. [Google Scholar] [CrossRef]
- Minchin, E.A. “Sponge-Spicules.” A Summary of Present Knowledge. Ergeb. Fortschr. Zool. 1909, 2, 171–274. [Google Scholar]
- Kendall, J. XCVI. The Solubility of Calcium Carbonate in Water. Lond. Edinb. Dubl. Phil. Mag. 1912, 23, 958–976. [Google Scholar] [CrossRef]
- Dorfmüller, G. Deut. Zuckerind 1938, 51, 1217. [Google Scholar]
- Schmidt, W.J. Die Bausteine Des Tierkörpers in Polarisiertem Lieht; Cohen: Bonn, Germany, 1924; pp. 528–529. [Google Scholar]
- Prenant, M. Les Formes Minéralogiques Du Calcaire Chez Les Êtres Vivants, et Le Problème de Leur Déterminisme. Biol. Rev. 1927, 2, 365–393. [Google Scholar] [CrossRef]
- Rinne, F. Quoted in a Footnote by Prenant, M. Bull. Biol. Fr. Belg. 1928, 62, 21. [Google Scholar]
- Odum, H.T. Nudibranch Spicules Made of Amorphous Calcium Carbonate. Science 1951, 114, 395. [Google Scholar] [CrossRef]
- Towe, K.M.; Malone, P.G. Precipitation of Metastable Carbonate Phases from Seawater. Nature 1970, 226, 348–349. [Google Scholar] [CrossRef]
- Enyedi, N.T.; Makk, J.; Kótai, L.; Berényi, B.; Klébert, S.; Sebestyén, Z.; Molnár, Z.; Borsodi, A.K.; Leél-Őssy, S.; Demény, A.; et al. Cave Bacteria-Induced Amorphous Calcium Carbonate Formation. Sci. Rep. 2020, 10, 8696. [Google Scholar] [CrossRef]
- Garvie, L.A.J.; Németh, P.; Trif, L. An Exceptionally Stable and Widespread Hydrated Amorphous Calcium Carbonate Precipitated by the Dog Vomit Slime Mold Fuligo septica (Myxogastria). Sci Rep 2022, 12, 3642. [Google Scholar] [CrossRef]
- Gago-Duport, L.; Briones, M.J.I.; Rodríguez, J.B.; Covelo, B. Amorphous Calcium Carbonate Biomineralization in the Earthworm’s Calciferous Gland: Pathways to the Formation of Crystalline Phases. J. Struct. Biol. 2008, 162, 422–435. [Google Scholar] [CrossRef]
- Marxen, J.C.; Becker, W.; Finke, D.; Hasse, B.; Epple, M. Early Mineralization in Biomphalaria Glabrata: Microscopic and Structural Results. J. Molluscan Stud. 2003, 69, 113–121. [Google Scholar] [CrossRef]
- Ito, T. Factors Controlling the Transformation of Natural Ikaite from Shiowakka, Japan. Geochem. J. 1998, 32, 267–273. [Google Scholar] [CrossRef]
- Weiner, S.; Levi-Kalisman, Y.; Raz, S.; Addadi, L. Biologically Formed Amorphous Calcium Carbonate. Connect. Tissue Res. 2003, 44, 214–218. [Google Scholar] [CrossRef]
- Meldrum, F.C. Calcium Carbonate in Biomineralisation and Biomimetic Chemistry. Int. Mater. Rev. 2003, 48, 187–224. [Google Scholar] [CrossRef]
- Faatz, M.; Gröhn, F.; Wegner, G. Amorphous Calcium Carbonate: Synthesis and Potential Intermediate in Biomineralization. Adv. Mater. 2004, 16, 996–1000. [Google Scholar] [CrossRef]
- Addadi, L.; Raz, S.; Weiner, S. Taking Advantage of Disorder: Amorphous Calcium Carbonate and Its Roles in Biomineralization. Adv. Mater. 2003, 15, 959–970. [Google Scholar] [CrossRef]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk Shell Formation: A Source of New Concepts for Understanding Biomineralization Processes. Chem.-Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef]
- Lam, R.S.K.; Charnock, J.M.; Lennie, A.; Meldrum, F.C. Synthesis-Dependant Structural Variations in Amorphous Calcium Carbonate. CrystEngComm 2007, 9, 1226–1236. [Google Scholar] [CrossRef]
- Xiao, J.; Yang, S. Bio-Inspired Synthesis: Understanding and Exploitation of the Crystallization Process from Amorphous Precursors. Nanoscale 2012, 4, 54–65. [Google Scholar] [CrossRef]
- Feng, Q. Müller, W.E.G., Ed.; Principles of Calcium-Based Biomineralization. In Molecular Biomineralization; Progress in Molecular and Subcellular Biology; Springer: Berlin/Heidelberg, Germany, 2011; Volume 52, pp. 141–197. ISBN 978-3-642-21229-1. [Google Scholar]
- Lippmann, F. Sedimentary Carbonate Minerals; Minerals, Rocks and Mountains Ser; Springer: Berlin/Heidelberg, Germany, 1973; ISBN 978-3-642-65474-9. [Google Scholar]
- Deng, S.; Dong, H.; Lv, G.; Jiang, H.; Yu, B.; Bishop, M.E. Microbial Dolomite Precipitation Using Sulfate Reducing and Halophilic Bacteria: Results from Qinghai Lake, Tibetan Plateau, NW China. Chem. Geol. 2010, 278, 151–159. [Google Scholar] [CrossRef]
- Pósfai, M.; Axisa, D.; Tompa, É.; Freney, E.; Bruintjes, R.; Buseck, P.R. Interactions of Mineral Dust with Pollution and Clouds: An Individual-Particle TEM Study of Atmospheric Aerosol from Saudi Arabia. Atmos. Res. 2013, 122, 347–361. [Google Scholar] [CrossRef]
- Ma, G.; He, X.; Jiang, X.; Liu, H.; Chu, J.; Xiao, Y. Strength and Permeability of Bentonite-Assisted Biocemented Coarse Sand. Can. Geotech. J. 2021, 58, 969–981. [Google Scholar] [CrossRef]
- Rostási, Á.; Rácz, K.; Fodor, M.A.; Topa, B.; Molnár, Z.; Weiszburg, T.G.; Pósfai, M. Pathways of Carbonate Sediment Accumulation in a Large, Shallow Lake. Front. Earth Sci. 2022, 10, 1067105. [Google Scholar] [CrossRef]
- Molnár, Z.; Dódony, I.; Pósfai, M. Transformation of Amorphous Calcium Carbonate in the Presence of Magnesium, Phosphate, and Mineral Surfaces. Geochim. Cosmochim. Acta 2023, 345, 90–101. [Google Scholar] [CrossRef]
- Larsen, D.; Crossey, L.J. Depositional Environments and Paleolimnology of an Ancient Caldera Lake: Oligocene Creede Formation, Colorado. Geol. Soc. America. Bull. 1996, 108, 526. [Google Scholar] [CrossRef]
- Dempster, T.; Jess, S.A. Ikaite Pseudomorphs in Neoproterozoic Dalradian Slates Record Earth’s Coldest Metamorphism. J. Geol. Soc. 2015, 172, 459–464. [Google Scholar] [CrossRef]
- Vickers, M.L.; Price, G.D.; Jerrett, R.M.; Sutton, P.; Watkinson, M.P.; FitzPatrick, M. The Duration and Magnitude of Cretaceous Cool Events: Evidence from the Northern High Latitudes. Geol. Soc. Am. Bull. 2019, 131, 1979–1994. [Google Scholar] [CrossRef]
- Vasileva, K.; Rogov, M.; Ershova, V.; Mikhailova, K.; Vereshchagin, O.; Pokrovsky, B. Ikaite versus Seep-Related Carbonate Precipitation in the Late Jurassic–Early Cretaceous of West Spitsbergen: Evidence for Cold versus Warm Climates? Int. J. Earth. Sci. (Geol. Rundsch.) 2024, 113, 417–439. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, J.; Zhang, J.; Xie, L.; Ji, Z.; Su, M.; Chen, J. A Different Approach for Seawater Decalcification Pretreatment Using Carbon Dioxide as Precipitator. Desalination 2013, 322, 151–158. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Petrova, N.L. Mineral Carbonation of Biomass Ashes in Relation to Their CO2 Capture and Storage Potential. ACS Omega 2021, 6, 14598–14611. [Google Scholar] [CrossRef]
- Gomez-Villalba, L.S.; Feijoo, J.; Rabanal, M.E.; Fort, R. In-Situ Electrochemical Synthesis of Inorganic Compounds for Materials Conservation: Assessment of Their Effects on the Porous Structure. Ceram. Int. 2021, 47, 30406–30424. [Google Scholar] [CrossRef]
- Jones, E.M.; Bakker, D.C.E.; Venables, H.J.; Whitehouse, M.J.; Korb, R.E.; Watson, A.J. Rapid Changes in Surface Water Carbonate Chemistry during Antarctic Sea Ice Melt. Tellus B: Chem. Phys. Meteorol. 2010, 62, 621–635. [Google Scholar] [CrossRef][Green Version]
- Mortenson, E.; Steiner, N.; Monahan, A.H.; Miller, L.A.; Geilfus, N.-X.; Brown, K. A Model-Based Analysis of Physical and Biogeochemical Controls on Carbon Exchange in the Upper Water Column, Sea Ice, and Atmosphere in a Seasonally Ice-Covered Arctic Strait. J. Geophys. Res. Ocean. 2018, 123, 7529–7549. [Google Scholar] [CrossRef]
- Skelton, A.; Tollefsen, E. Methode for Preparation of Ikaite Crystals and for Carbon Capture and Storage. 2021. Available online: https://worldwide.espacenet.com/patent/search/family/077745389/publication/SE543928C2?q=SE543928C2 (accessed on 21 September 2025).
- Liu, H.; Wen, Z.; Liu, Z.; Yang, Y.; Wang, H.; Xia, X.; Ye, J.; Liu, Y. Unlocking the Potential of Amorphous Calcium Carbonate: A Star Ascending in the Realm of Biomedical Application. Acta Pharm. Sin. B. 2024, 14, 602–622. [Google Scholar] [CrossRef]
- Konrad, F.; Gallien, F.; Gerard, D.E.; Dietzel, M. Transformation of Amorphous Calcium Carbonate in Air. Cryst. Growth Des. 2016, 16, 6310–6317. [Google Scholar] [CrossRef]
- Du, H.; Steinacher, M.; Borca, C.; Huthwelker, T.; Murello, A.; Stellacci, F.; Amstad, E. Amorphous CaCO3: Influence of the Formation Time on Its Degree of Hydration and Stability. J. Am. Chem. Soc. 2018, 140, 14289–14299. [Google Scholar] [CrossRef]
- Xto, J.M.; Borca, C.N.; Van Bokhoven, J.A.; Huthwelker, T. Aerosol-Based Synthesis of Pure and Stable Amorphous Calcium Carbonate. Chem. Commun. 2019, 55, 10725–10728. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-F.; Cölfen, H.; Antonietti, M.; Yu, S.-H. Ethanol Assisted Synthesis of Pure and Stable Amorphous Calcium Carbonate Nanoparticles. Chem. Commun. 2013, 49, 9564–9566. [Google Scholar] [CrossRef]
- Zou, Z.; Bertinetti, L.; Habraken, W.J.E.M.; Fratzl, P. Reentrant Phase Transformation from Crystalline Ikaite to Amorphous Calcium Carbonate. CrystEngComm 2018, 20, 2902–2906. [Google Scholar] [CrossRef]
- Coleyshaw, E.E.; Crump, G.; Griffith, W.P. Vibrational Spectra of the Hydrated Carbonate Minerals Ikaite, Monohydrocalcite, Lansfordite and Nesquehonite. Spectrochim. Acta-A Mol. Biomol. Spectrosc. 2003, 59, 2231–2239. [Google Scholar] [CrossRef]
- Aufort, J.; Demichelis, R. Magnesium Impurities Decide the Structure of Calcium Carbonate Hemihydrate. Cryst. Growth Des. 2020, 20, 8028–8038. [Google Scholar] [CrossRef]
- Avaro, J.T.; Ruiz-Agudo, C.; Landwehr, E.; Hauser, K.; Gebauer, D. Impurity-Free Amorphous Calcium Carbonate, a Preferential Material for Pharmaceutical and Medical Applications. Eur. J. Mineral. 2019, 31, 231–236. [Google Scholar] [CrossRef]
- Tlili, M.M.; Amor, M.B.; Gabrielli, C.; Joiret, S.; Maurin, G.; Rousseau, P. Characterization of CaCO3 Hydrates by micro-Raman Spectroscopy. J. Raman Spectrosc. 2002, 33, 10–16. [Google Scholar] [CrossRef]
- Effenberger, H. Kristallstruktur und Infrarot-Absorptionsspektrum von synthetischem Monohydrocalcit, CaCO3·H2O. Monatsh. Fur Chem. 1981, 112, 899–909. [Google Scholar] [CrossRef]
- Korneva, T.A.; Kovaleva, L.T.; Lyubushko, G.I. Thermal Analysis and IR-Spectroscopic Study of Synthetic Hydrocalcite. Term. An. I Faz. Ravnovesiya Perm 1983, 43, 94–97. [Google Scholar]
- Dickens, B.; Brown, W.E. Crystal Structure of Calcium Carbonate Hexahydrate at about −120°. Inorg. Chem. 1970, 9, 480–486. [Google Scholar] [CrossRef]
- Hesse, K.-F.; Küppers, H.; Suess, E. Refinement of the Structure of Ikaite, CaCO3·6H2O. Z. Für Krist.-Cryst. Mater. 1983, 163, 227–231. [Google Scholar] [CrossRef]
- Lennie, A.R.; Tang, C.C.; Thompson, S.P. The Structure and Thermal Expansion Behaviour of Ikaite, CaCO3·6H2O, from T = 114 to T = 293 K. Mineral. Mag. 2004, 68, 135–146. [Google Scholar] [CrossRef]
- Tateno, N.; Kyono, A. Structural Change Induced by Dehydration in Ikaite (CaCO3·6H2O). J. Min. Petr. Econ. Geol. 2014, 109, 157–168. [Google Scholar] [CrossRef]
- Chahi, G.; Bradai, D.; Belabbas, I. Structural and Elastic Properties of CaCO3 Hydrated Phases: A Dispersion-Corrected Density Functional Theory Study. J. Phys. Chem. Solids 2020, 138, 109295. [Google Scholar] [CrossRef]
- Singer, J.W.; Yazaydin, A.Ö.; Kirkpatrick, R.J.; Bowers, G.M. Structure and Transformation of Amorphous Calcium Carbonate: A Solid-State43 Ca NMR and Computational Molecular Dynamics Investigation. Chem. Mater. 2012, 24, 1828–1836. [Google Scholar] [CrossRef]
- Costa, S.N.; Freire, V.N.; Caetano, E.W.S.; Maia, F.F.; Barboza, C.A.; Fulco, U.L.; Albuquerque, E.L. DFT Calculations with van Der Waals Interactions of Hydrated Calcium Carbonate Crystals CaCO3·(H2O, 6H2O): Structural, Electronic, Optical, and Vibrational Properties. J. Phys. Chem. A 2016, 120, 5752–5765. [Google Scholar] [CrossRef]
- Swainson, I.P. The Structure of Monohydrocalcite and the Phase Composition of the Beachrock Deposits of Lake Butler and Lake Fellmongery, South Australia. Am. Mineral. 2008, 93, 1014–1018. [Google Scholar] [CrossRef]
- Mincheva-Stefanova, Y.; Neikov, K. Trigonal-Trapezohedral Monohydrocalcite from an Oxidation Zone. Dokl. Bulg. Acad. Nauk. 1990, 43, 57–60. [Google Scholar]
- Makovicky, E. The Order–Disorder Potential of the Crystal Structure of Monohydrocalcite, CaCO3·H2O. Miner. Petrol. 2018, 112, 105–109. [Google Scholar] [CrossRef]
- Demichelis, R.; Raiteri, P.; Gale, J.D.; Dovesi, R. Examining the Accuracy of Density Functional Theory for Predicting the Thermodynamics of Water Incorporation into Minerals: The Hydrates of Calcium Carbonate. J. Phys. Chem. C 2013, 117, 17814–17823. [Google Scholar] [CrossRef]
- Demichelis, R.; Raiteri, P.; Gale, J.D. Structure of Hydrated Calcium Carbonates: A First-Principles Study. J. Cryst. Growth 2014, 401, 33–37. [Google Scholar] [CrossRef]
- Michel, F.M.; MacDonald, J.; Feng, J.; Phillips, B.L.; Ehm, L.; Tarabrella, C.; Parise, J.B.; Reeder, R.J. Structural Characteristics of Synthetic Amorphous Calcium Carbonate. Chem. Mater. 2008, 20, 4720–4728. [Google Scholar] [CrossRef]
- Quigley, D.; Rodger, P.M. Free Energy and Structure of Calcium Carbonate Nanoparticles during Early Stages of Crystallization. J. Chem. Phys. 2008, 128, 221101. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, P.; Gale, J.D. Water Is the Key to Nonclassical Nucleation of Amorphous Calcium Carbonate. J. Am. Chem. Soc. 2010, 132, 17623–17634. [Google Scholar] [CrossRef]
- Goodwin, A.L.; Michel, F.M.; Phillips, B.L.; Keen, D.A.; Dove, M.T.; Reeder, R.J. Nanoporous Structure and Medium-Range Order in Synthetic Amorphous Calcium Carbonate. Chem. Mater. 2010, 22, 3197–3205. [Google Scholar] [CrossRef]
- Quigley, D.; Freeman, C.L.; Harding, J.H.; Rodger, P.M. Sampling the Structure of Calcium Carbonate Nanoparticles with Metadynamics. J. Chem. Phys. 2011, 134, 044703. [Google Scholar] [CrossRef] [PubMed]
- Saharay, M.; James Kirkpatrick, R. Onset of Orientational Order in Amorphous Calcium Carbonate (ACC) upon Dehydration. Chem. Phys. Lett. 2014, 591, 287–291. [Google Scholar] [CrossRef]
- Lopez-Berganza, J.A.; Diao, Y.; Pamidighantam, S.; Espinosa-Marzal, R.M. Ab Initio Studies of Calcium Carbonate Hydration. J. Phys. Chem. A 2015, 119, 11591–11600. [Google Scholar] [CrossRef]
- Innocenti Malini, R.; Bushuev, Y.G.; Hall, S.A.; Freeman, C.L.; Rodger, P.M.; Harding, J.H. Using Simulation to Understand the Structure and Properties of Hydrated Amorphous Calcium Carbonate. CrystEngComm 2016, 18, 92–101. [Google Scholar] [CrossRef]
- Saharuddin, T.S.T.; Samsuri, A.; Salleh, F.; Othaman, R.; Kassim, M.B.; Mohamed Hisham, M.W.; Yarmo, M.A. Studies on Reduction of Chromium Doped Iron Oxide Catalyst Using Hydrogen and Various Concentration of Carbon Monoxide. Int. J. Hydrogen Energy 2017, 42, 9077–9086. [Google Scholar] [CrossRef]
- Jensen, A.C.S.; Imberti, S.; Habraken, W.J.E.M.; Bertinetti, L. Small Ionic Radius Limits Magnesium Water Interaction in Amorphous Calcium/Magnesium Carbonates. J. Phys. Chem. C 2020, 124, 6141–6144. [Google Scholar] [CrossRef]
- Schmidt, M.P.; Ilott, A.J.; Phillips, B.L.; Reeder, R.J. Structural Changes upon Dehydration of Amorphous Calcium Carbonate. Cryst. Growth Des. 2014, 14, 938–951. [Google Scholar] [CrossRef]
- Jensen, A.C.S.; Imberti, S.; Parker, S.F.; Schneck, E.; Politi, Y.; Fratzl, P.; Bertinetti, L.; Habraken, W.J.E.M. Hydrogen Bonding in Amorphous Calcium Carbonate and Molecular Reorientation Induced by Dehydration. J. Phys. Chem. C 2018, 122, 3591–3598. [Google Scholar] [CrossRef]
- Sand, K.K.; Rodriguez-Blanco, J.D.; Makovicky, E.; Benning, L.G.; Stipp, S.L.S. Crystallization of CaCO3 in Water–Alcohol Mixtures: Spherulitic Growth, Polymorph Stabilization, and Morphology Change. Cryst. Growth Des. 2012, 12, 842–853. [Google Scholar] [CrossRef]
- Besselink, R.; Rodriguez-Blanco, J.D.; Stawski, T.M.; Benning, L.G.; Tobler, D.J. How Short-Lived Ikaite Affects Calcite Crystallization. Cryst. Growth Des. 2017, 17, 6224–6230. [Google Scholar] [CrossRef]
- Van Driessche, A.E.S.; Kellermeier, M.; Benning, L.G.; Gebauer, D. (Eds.) New Perspectives on Mineral Nucleation and Growth; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-45667-6. [Google Scholar]
- Huang, Y.; Rao, A.; Huang, S.; Chang, C.; Drechsler, M.; Knaus, J.; Chan, J.C.C.; Raiteri, P.; Gale, J.D.; Gebauer, D. Uncovering the Role of Bicarbonate in Calcium Carbonate Formation at Near-Neutral pH. Angew. Chem. Int. Ed. 2021, 60, 16707–16713. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, Z.; Wang, H.; Wang, W.; Fu, Z. Pressure-Induced Crystallization and Densification of Amorphized Calcium Carbonate Hexahydrate Controlled by Interfacial Water. J. Colloid Interface Sci. 2022, 611, 346–355. [Google Scholar] [CrossRef]
- Katsikopoulos, D.; Fernández-González, Á.; Prieto, A.C.; Prieto, M. Co-Crystallization of Co(II) with Calcite: Implications for the Mobility of Cobalt in Aqueous Environments. Chem. Geol. 2008, 254, 87–100. [Google Scholar] [CrossRef]
- Shuseki, Y.; Kohara, S.; Ohara, K.; Ohkubo, T.; Takei, K.; Tucker, M.G.; Kolesnikov, A.I.; Mcdonnell, M.T.; Sacci, R.L.; Neuefeind, J.C.; et al. Structural Analyses of Amorphous Calcium Carbonate before and after Removing Strontium Ions from an Aqueous Solution. J. Ceram. Soc. Jpn. 2022, 130, 225–231. [Google Scholar] [CrossRef]
- Molnár, Z.; Hegedűs, M.; Németh, P.; Pósfai, M. Competitive Incorporation of Ca, Sr, and Ba Ions into Amorphous Carbonates. Geochim. Cosmochim. Acta 2025, 393, 18–30. [Google Scholar] [CrossRef]
- Roberts, N.M.W.; Holdsworth, R.E. Timescales of Faulting through Calcite Geochronology: A Review. J. Struct. Geol. 2022, 158, 104578. [Google Scholar] [CrossRef]
- Fyfe, W.S.; Bischoff, J.L. The Calcite-Aragonite Problem1. In Dolomitization and Limestone Diagenesis; SEPM Society for Sedimentary Geology: Claremore, OK, USA, 1965; ISBN 978-1-56576-143-8. [Google Scholar]
- Zhao, S.; Schettino, E.; Merlini, M.; Poli, S. The Stability and Melting of Aragonite: An Experimental and Thermodynamic Model for Carbonated Eclogites in the Mantle. Lithos 2019, 324–325, 105–114. [Google Scholar] [CrossRef]
- Cooke, R.C.; Kepkay, P.E. The Solubility of Aragonite in Seawater—I. Effect of pH and Water Chemistry at One Atmosphere. Geochim. Cosmochim. Acta 1980, 44, 1071–1075. [Google Scholar] [CrossRef]
- Spanos, N.; Koutsoukos, P.G. The Transformation of Vaterite to Calcite: Effect of the Conditions of the Solutions in Contact with the Mineral Phase. J. Cryst. Growth 1998, 191, 783–790. [Google Scholar] [CrossRef]
- Vereshchagin, O.S.; Frank-Kamenetskaya, O.V.; Kuz’mina, M.A.; Chernyshova, I.A.; Shilovskikh, V.V. Effect of Magnesium on Monohydrocalcite Formation and Unit-Cell Parameters. Am. Mineral. 2021, 106, 1294–1305. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The Kinetics and Mechanisms of Amorphous Calcium Carbonate (ACC) Crystallization to Calcite, Viavaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- Lennie, A.R. Ikaite (CaCO3·6H2O) Compressibility at High Water Pressure: A Synchrotron X-Ray Diffraction Study. Mineral. mag. 2005, 69, 325–335. [Google Scholar] [CrossRef]
- Shahar, A. The Stability and Raman Spectra of Ikaite, CaCO3·6H2O, at High Pressure and Temperature. Am. Mineral. 2005, 90, 1835–1839. [Google Scholar] [CrossRef]
- Hu, Y.; Dieckmann, G.S.; Wolf-Gladrow, D.A.; Nehrke, G. Laboratory Study on Coprecipitation of Phosphate with Ikaite in Sea Ice. J. Geophys. Res. Ocean. 2014, 119, 7007–7015. [Google Scholar] [CrossRef]
- Purgstaller, B.; Dietzel, M.; Baldermann, A.; Mavromatis, V. Control of Temperature and Aqueous Mg2+/Ca2+ Ratio on the (Trans-)Formation of Ikaite. Geochim. Cosmochim. Acta 2017, 217, 128–143. [Google Scholar] [CrossRef]
- Strohm, S.B.; Saldi, G.D.; Mavromatis, V.; Schmahl, W.W.; Jordan, G. A Study on Ikaite Growth in the Presence of Phosphate. Aquat. Geochem. 2023, 29, 219–233. [Google Scholar] [CrossRef]
- Clarkson, J.R.; Price, T.J.; Adams, C.J. Role of Metastable Phases in the Spontaneous Precipitation of Calcium Carbonate. J. Chem. Soc., Faraday Trans. 1992, 88, 243–249. [Google Scholar] [CrossRef]
- Chaka, A.M. Quantifying the Impact of Magnesium on the Stability and Water Binding Energy of Hydrated Calcium Carbonates by Ab Initio Thermodynamics. J. Phys. Chem. A 2019, 123, 2908–2923. [Google Scholar] [CrossRef]
- Tang, C.C.; Thompson, S.P.; Parker, J.E.; Lennie, A.R.; Azough, F.; Kato, K. The Ikaite-to-Vaterite Transformation: New Evidence from Diffraction and Imaging. J. Appl. Crystallogr. 2009, 42, 225–233. [Google Scholar] [CrossRef]
- Shaikh, A.M. A New Crystal Growth Form of Vaterite, CaCO3. J. Appl. Crystallogr. 1990, 23, 263–265. [Google Scholar] [CrossRef]
- Hull, H.; Turnbull, A.G. A Thermochemical Study of Monohydrocalcite. Geochim. Cosmochim. Acta 1973, 37, 685–694. [Google Scholar] [CrossRef]
- Liu, R.; Liu, F.; Zhao, S.; Su, Y.; Wang, D.; Shen, Q. Crystallization and Oriented Attachment of Monohydrocalcite and Its Crystalline Phase Transformation. CrystEngComm 2013, 15, 509–515. [Google Scholar] [CrossRef]
- Nechiporenko, G.O.; Bondarenko, G.P.; Ermilov, V.V. Effect of Certain Factors on the Formation and Stability of Monohydrocalcite in Seawater. Zap. Vses. Mineral. O-Va. 1983, 112, 94–103. [Google Scholar]
- Nishiyama, R.; Munemoto, T.; Fukushi, K. Formation Condition of Monohydrocalcite from CaCl2–MgCl2–Na2CO3 Solutions. Geochim. Cosmochim. Acta 2013, 100, 217–231. [Google Scholar] [CrossRef]
- Dalas, E.; Kallitsis, J.; Koutsoukos, P.G. The Crystallization of Calcium Carbonate on Polymeric Substrates. J. Cryst. Growth 1988, 89, 287–294. [Google Scholar] [CrossRef]
- Giannimaras, E.K.; Koutsoukos, P.G. Precipitation of Calcium Carbonate in Aqueous Solutions in the Presence of Oxalate Anions. Langmuir 1988, 4, 855–861. [Google Scholar] [CrossRef]
- Zhang, G.; Delgado-López, J.M.; Choquesillo-Lazarte, D.; García-Ruiz, J.M. Crystallization of Monohydrocalcite in a Silica-Rich Alkaline Solution. CrystEngComm 2013, 15, 6526–6532. [Google Scholar] [CrossRef]
- Mucci, A.; Morse, J.W. The Incorporation of Mg2+ and Sr2+ into Calcite Overgrowths: Influences of Growth Rate and Solution Composition. Geochim. Cosmochim. Acta 1983, 47, 217–233. [Google Scholar] [CrossRef]
- Chen, T.; Neville, A.; Yuan, M. Assessing the Effect of on Scale Formation–Bulk Precipitation and Surface Deposition. J. Cryst. Growth 2005, 275, e1341–e1347. [Google Scholar] [CrossRef]
- Von Euw, S.; Azaïs, T.; Manichev, V.; Laurent, G.; Pehau-Arnaudet, G.; Rivers, M.; Murali, N.; Kelly, D.J.; Falkowski, P.G. Solid-State Phase Transformation and Self-Assembly of Amorphous Nanoparticles into Higher-Order Mineral Structures. J. Am. Chem. Soc. 2020, 142, 12811–12825. [Google Scholar] [CrossRef]
- Kimura, T.; Koga, N. Thermal Dehydration of Monohydrocalcite: Overall Kinetics and Physico-Geometrical Mechanisms. J. Phys. Chem. A 2011, 115, 10491–10501. [Google Scholar] [CrossRef]
- Zhang, G.; Verdugo-Escamilla, C.; Choquesillo-Lazarte, D.; García-Ruiz, J.M. Thermal Assisted Self-Organization of Calcium Carbonate. Nat. Commun. 2018, 9, 5221. [Google Scholar] [CrossRef]
- Termine, J.D.; Peckauskas, R.A.; Posner, A.S. Calcium Phosphate Formation in Vitro. Arch. Biochem. Biophys. 1970, 140, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Aizenberg, J.; Addadi, L.; Weiner, S.; Lambert, G. Stabilization of Amorphous Calcium Carbonate by Specialized Macromolecules in Biological and Synthetic Precipitates. Adv. Mater. 1996, 8, 222–226. [Google Scholar] [CrossRef]
- Kababya, S.; Gal, A.; Kahil, K.; Weiner, S.; Addadi, L.; Schmidt, A. Phosphate–Water Interplay Tunes Amorphous Calcium Carbonate Metastability: Spontaneous Phase Separation and Crystallization vs. Stabilization Viewed by Solid State NMR. J. Am. Chem. Soc. 2015, 137, 990–998. [Google Scholar] [CrossRef]
- Purgstaller, B.; Goetschl, K.E.; Mavromatis, V.; Dietzel, M. Solubility Investigations in the Amorphous Calcium Magnesium Carbonate System. CrystEngComm 2019, 21, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Maslyk, M.; Mondeshki, M.; Tremel, W. Amorphous Calcium Carbonate Monohydrate Containing a Defect Hydrate Network by Mechanochemical Processing of Mono-Hydrocalcite Using Ethanol as Auxiliary Solvent. CrystEngComm 2022, 24, 4687–4697. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawanobe, A.; Yasue, T.; Arai, Y. Controls of Polymorphism and Morphology of Calcium Carbonate Compounds Formed by Crystallizing Amorphous Calcium Carbonate Hydrate. J. Ceram. Soc. Jpn. 1994, 102, 1128–1136. [Google Scholar] [CrossRef]
- Kojima, Y.; Endo, N.; Yasue, T.; Arai, Y. Morphological Controls of Calcium Carbonate Hexahydrate and Its Dehydrating Substance. J. Ceram. Soc. Jpn. 1995, 103, 1282–1288. [Google Scholar] [CrossRef]
- Gower, L.A.; Tirrell, D.A. Calcium Carbonate Films and Helices Grown in Solutions of Poly(Aspartate). J. Cryst. Growth 1998, 191, 153–160. [Google Scholar] [CrossRef]
- Gower, L.B.; Odom, D.J. Deposition of Calcium Carbonate Films by a Polymer-Induced Liquid-Precursor (PILP) Process. J. Cryst. Growth 2000, 210, 719–734. [Google Scholar] [CrossRef]
- Sun, S.; Chevrier, D.M.; Zhang, P.; Gebauer, D.; Cölfen, H. Distinct Short-Range Order Is Inherent to Small Amorphous Calcium Carbonate Clusters (<2 Nm). Angew. Chem. Int. Ed. 2016, 55, 12206–12209. [Google Scholar] [CrossRef]
- Yasue, T.; Mamiya, A.; Fukushima, T.; Arai, Y. Synthesis and Characteristics of Amorphous Calcium Carbonate in Ethanol. Gypsum Lime 1985, 198, 245–252. [Google Scholar] [CrossRef]
- Ihli, J.; Wong, W.C.; Noel, E.H.; Kim, Y.-Y.; Kulak, A.N.; Christenson, H.K.; Duer, M.J.; Meldrum, F.C. Dehydration and Crystallization of Amorphous Calcium Carbonate in Solution and in Air. Nat. Commun. 2014, 5, 3169. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. How to Make ‘Stable’ ACC: Protocol and Preliminary Structural Characterization. Mineral. Mag. 2008, 72, 283–286. [Google Scholar] [CrossRef]
- Gebauer, D.; Gunawidjaja, P.N.; Ko, J.Y.P.; Bacsik, Z.; Aziz, B.; Liu, L.; Hu, Y.; Bergström, L.; Tai, C.; Sham, T.; et al. Proto-Calcite and Proto-Vaterite in Amorphous Calcium Carbonates. Angew. Chem. Int. Ed. 2010, 49, 8889–8891. [Google Scholar] [CrossRef]
- Gebauer, D.; Cölfen, H. Prenucleation Clusters and Non-Classical Nucleation. Nano Today 2011, 6, 564–584. [Google Scholar] [CrossRef]
- Bots, P.; Benning, L.G.; Rodriguez-Blanco, J.-D.; Roncal-Herrero, T.; Shaw, S. Mechanistic Insights into the Crystallization of Amorphous Calcium Carbonate (ACC). Cryst. Growth Des. 2012, 12, 3806–3814. [Google Scholar] [CrossRef]
- Cai, H.; Wang, Q.; Zou, Z. Crystallization Pathway of Monohydrocalcite via Amorphous Calcium Carbonate Regulated by Magnesium Ion. J. Inorg. Mater. 2024, 39, 1275–1282. [Google Scholar] [CrossRef]
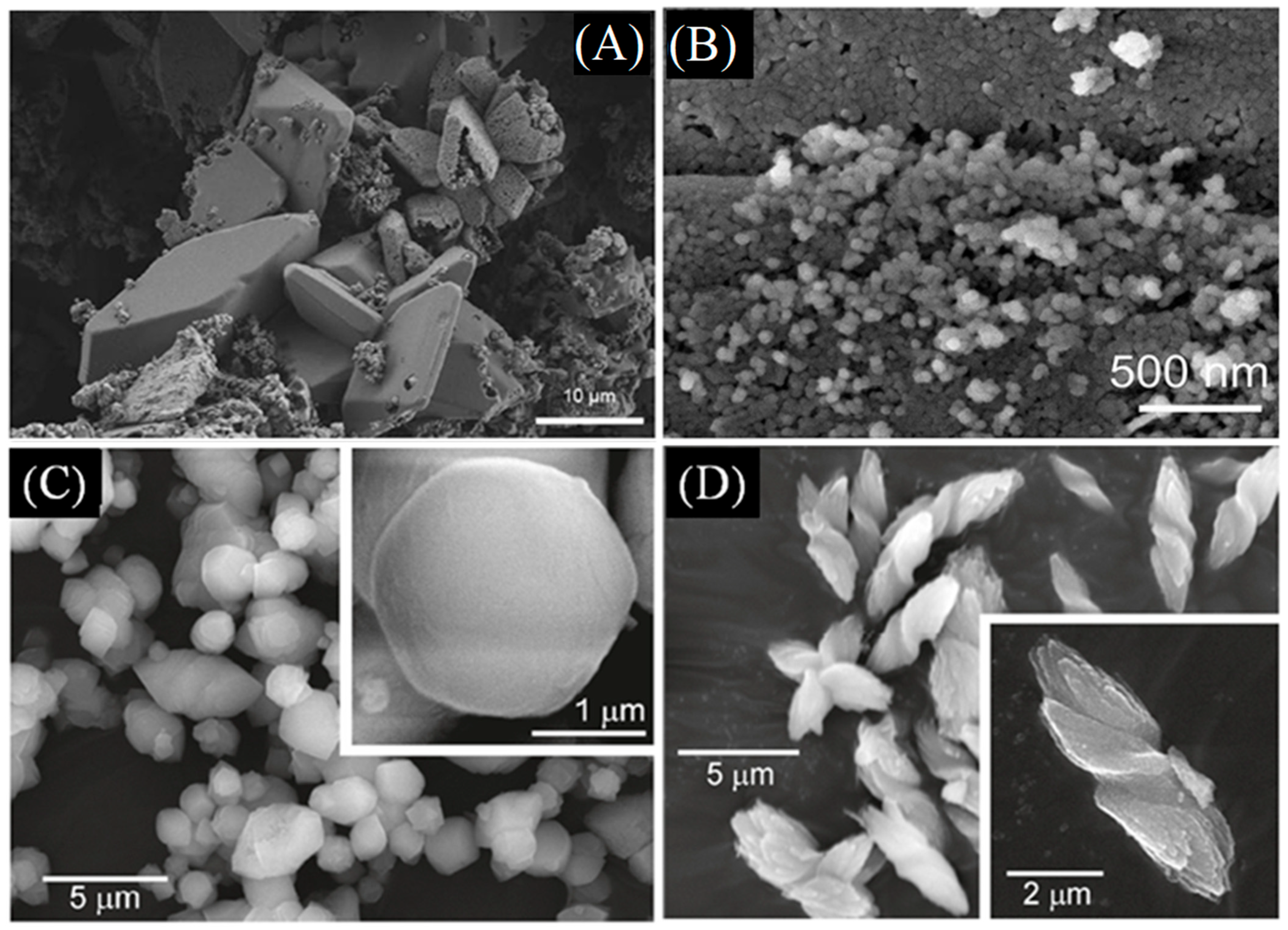
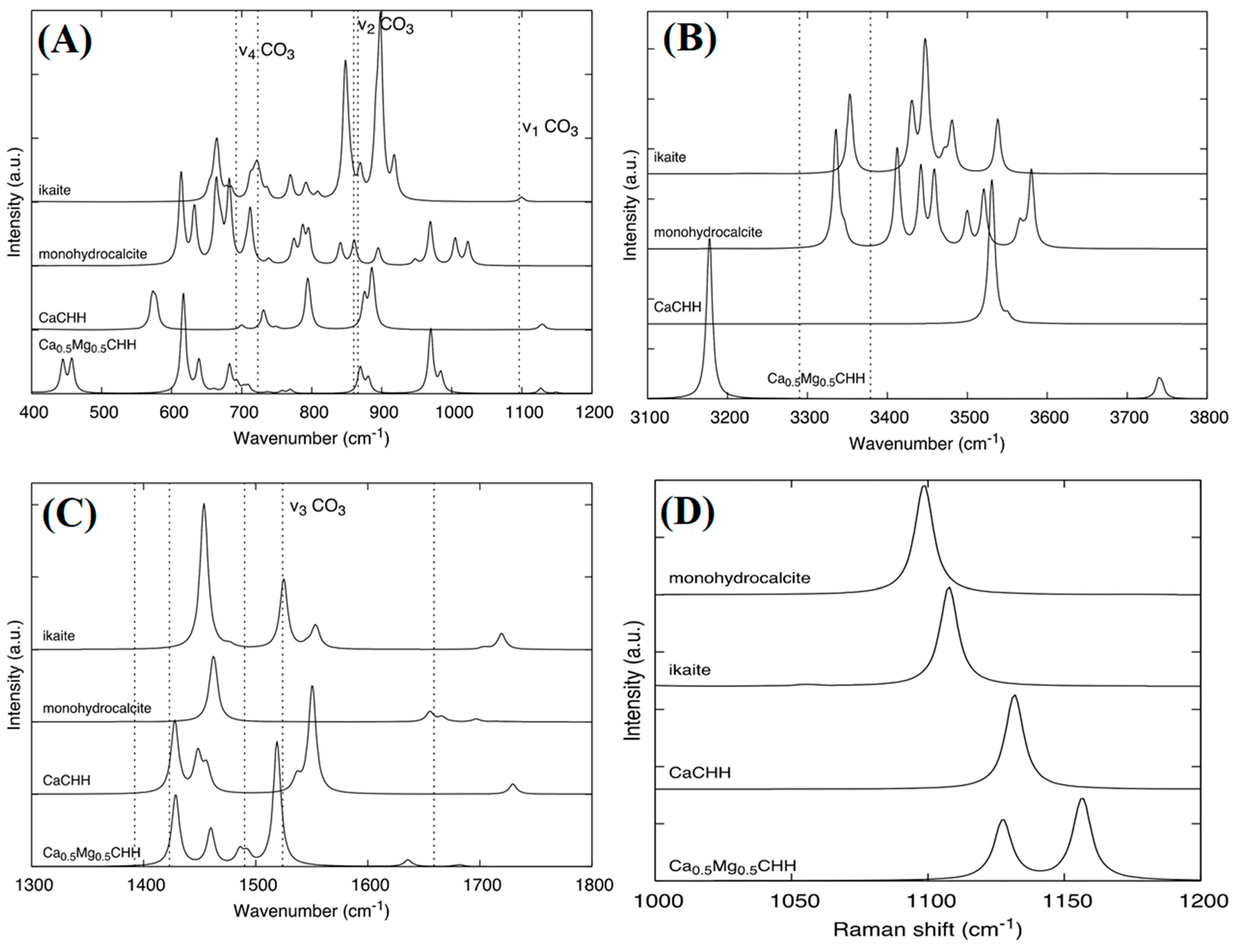
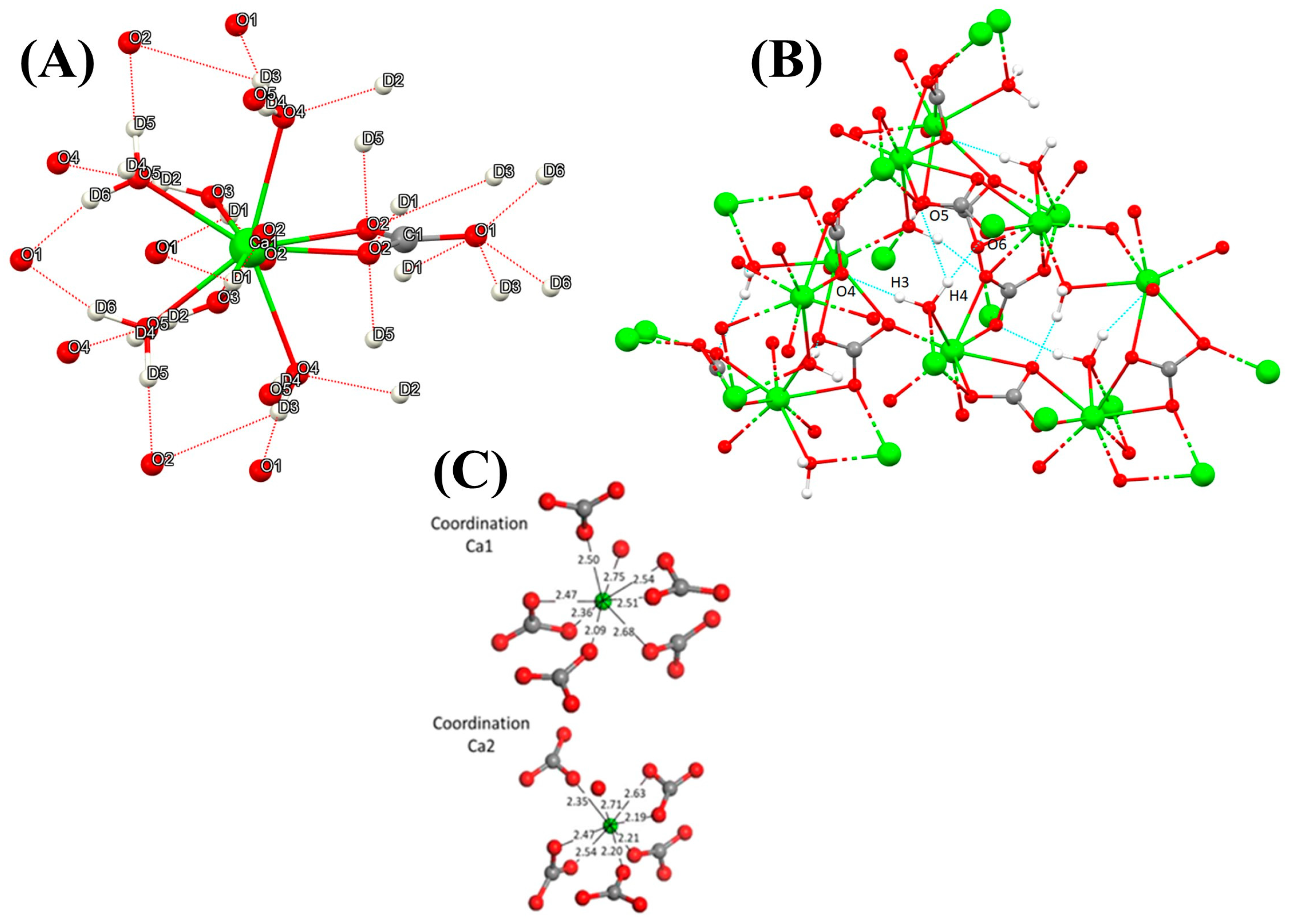

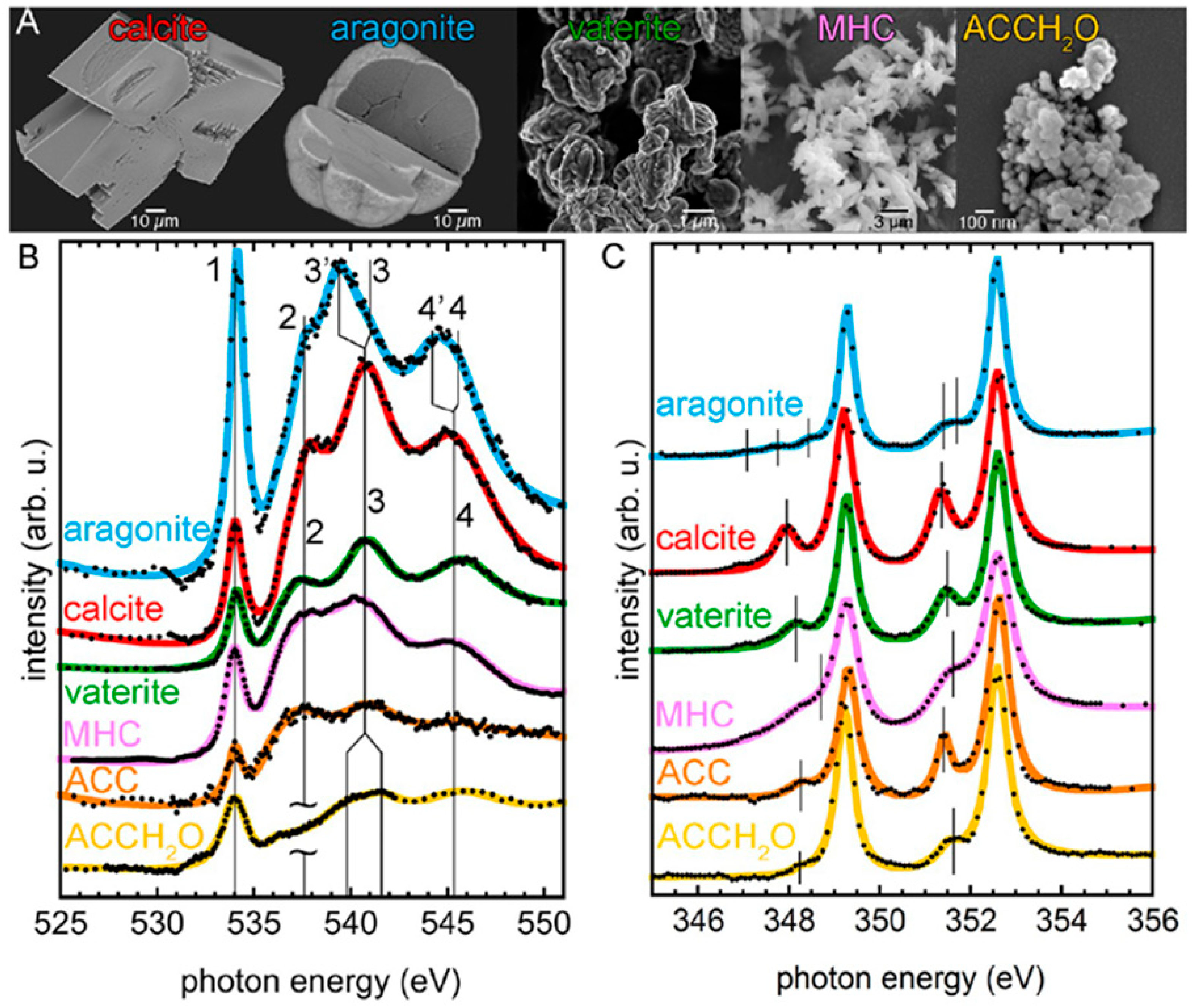
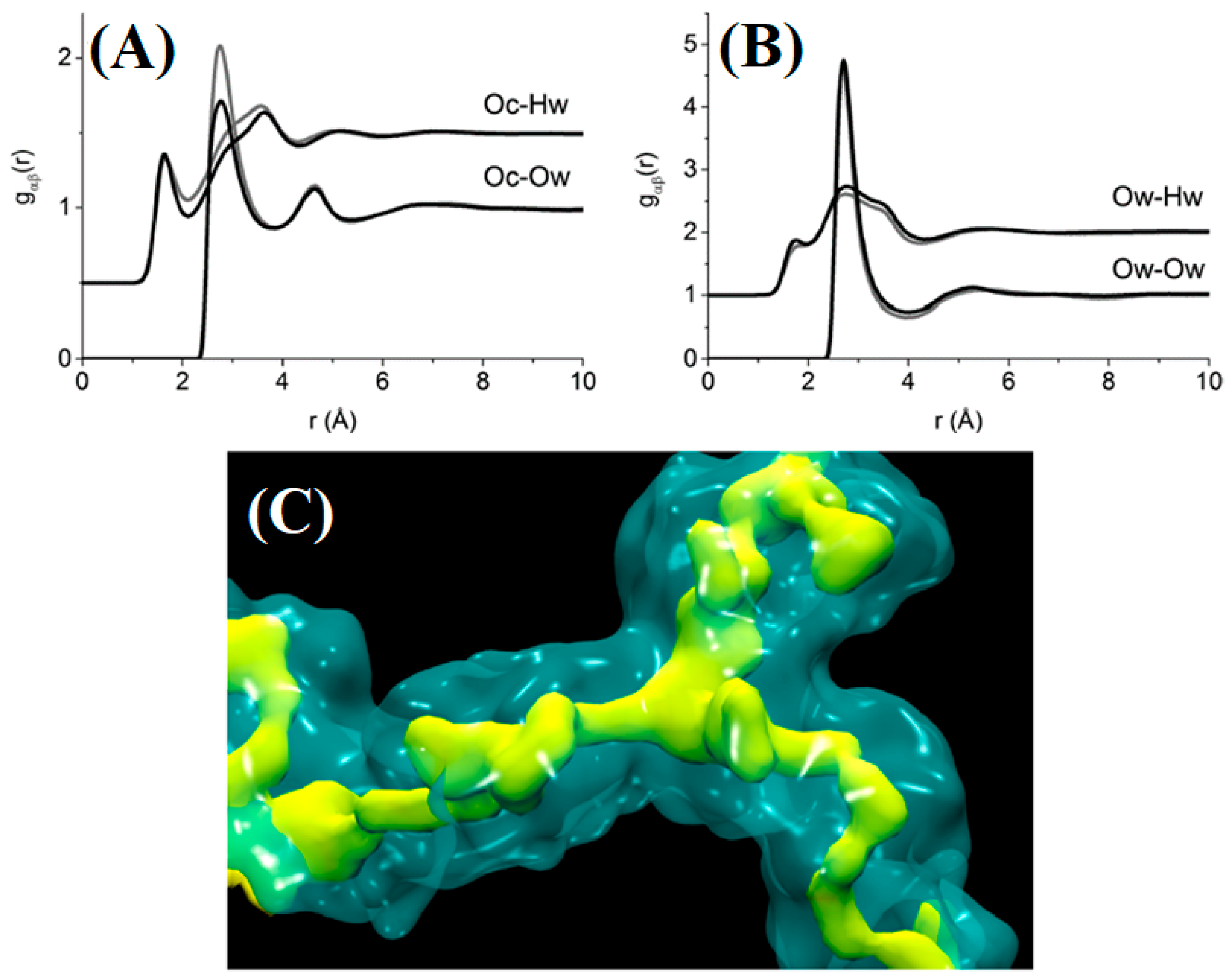
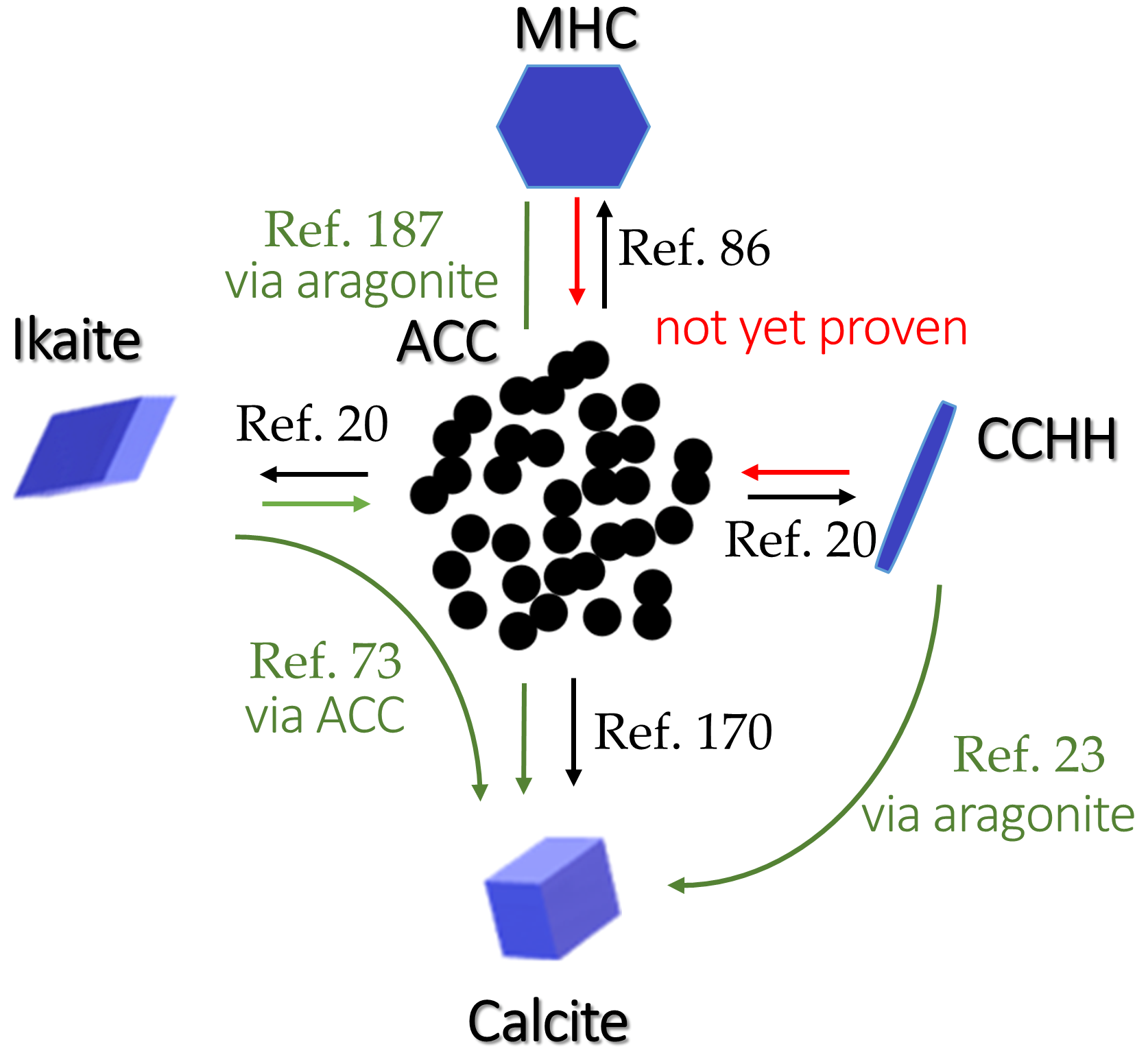
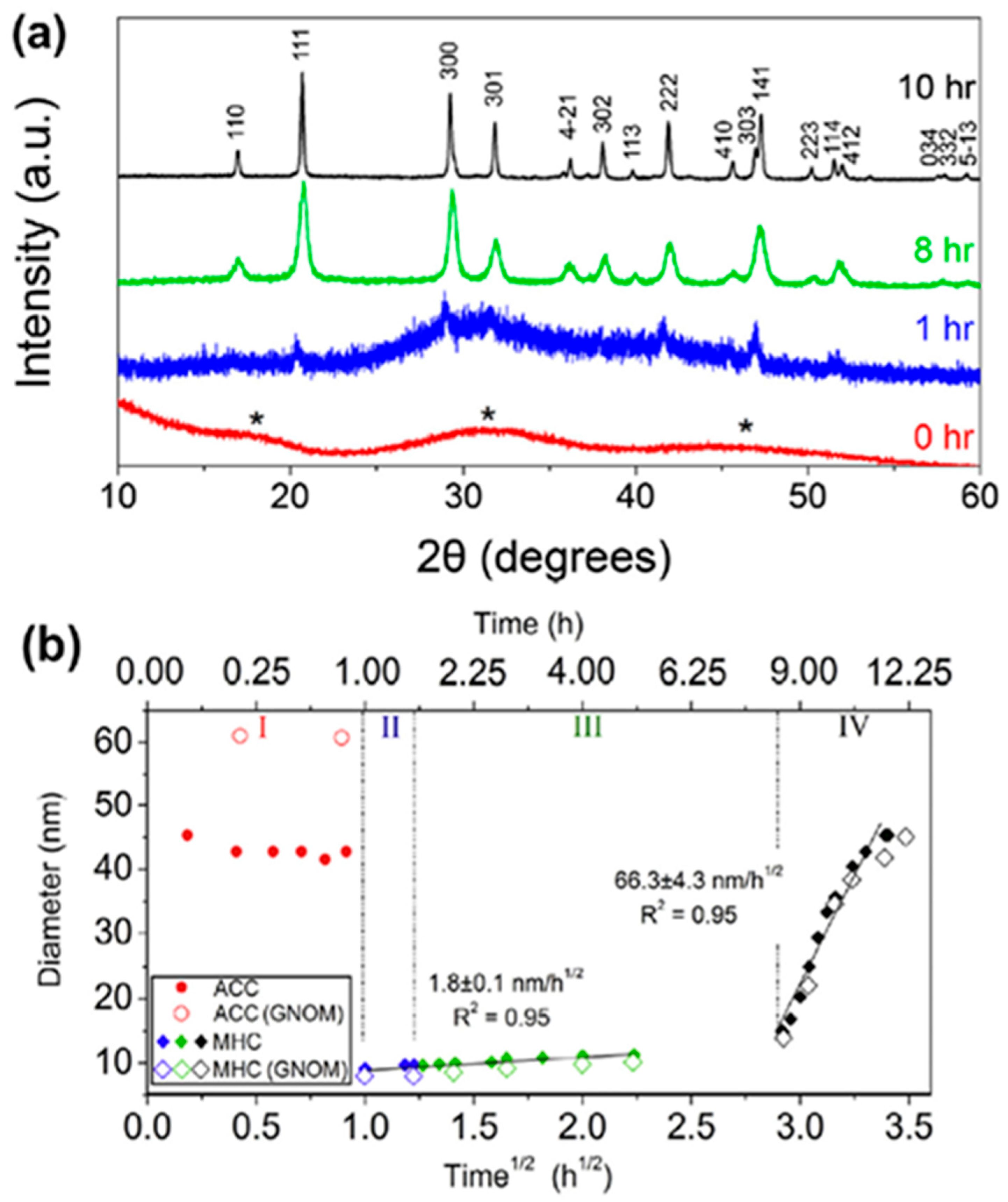
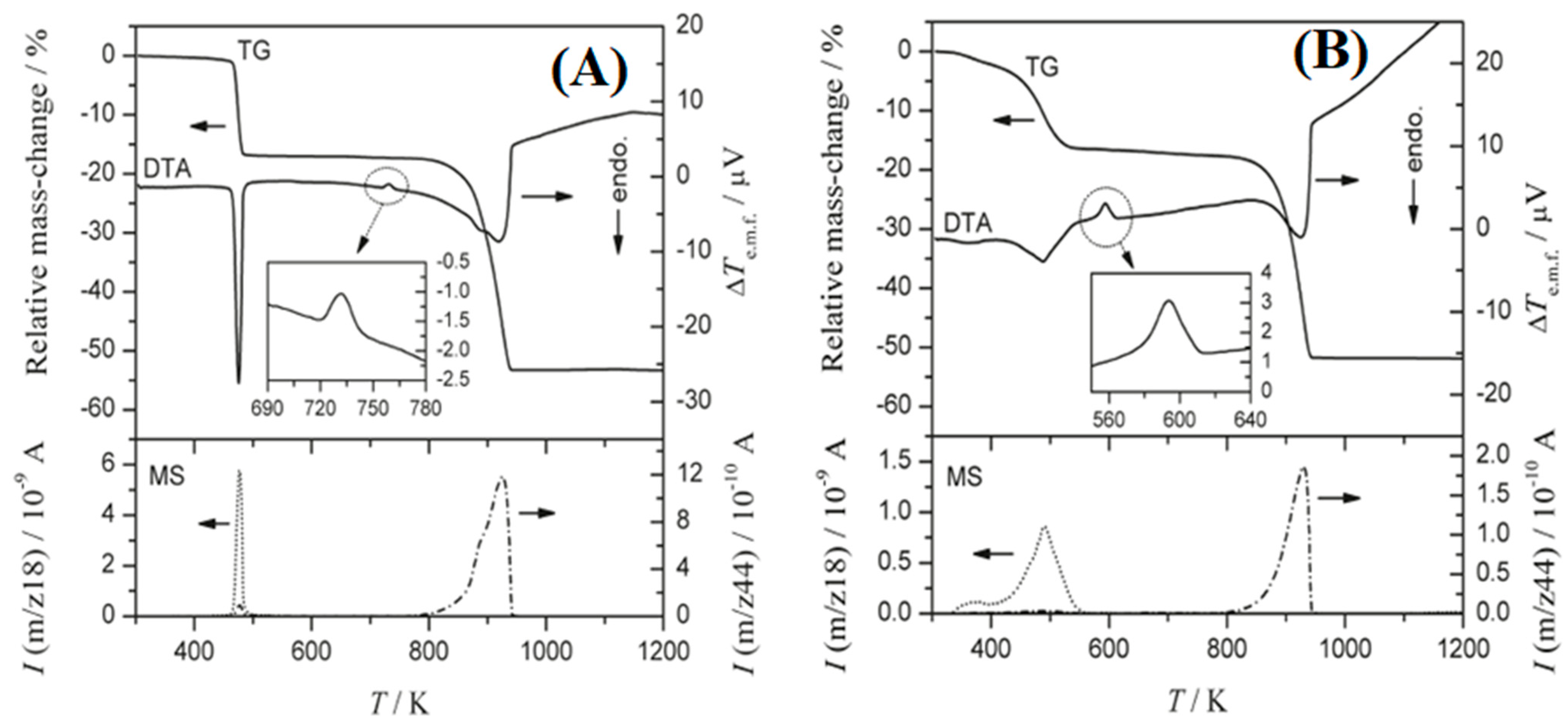
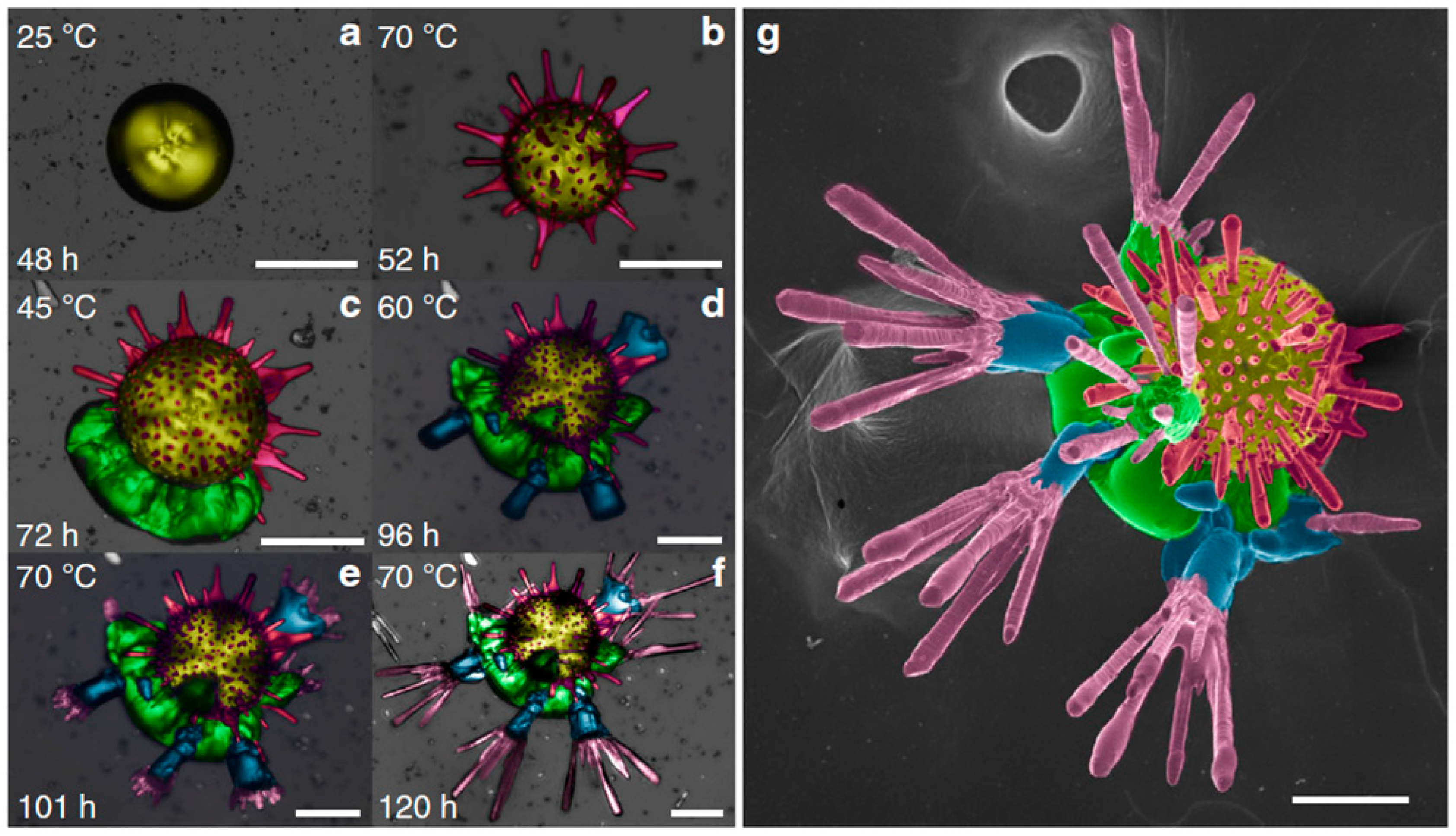
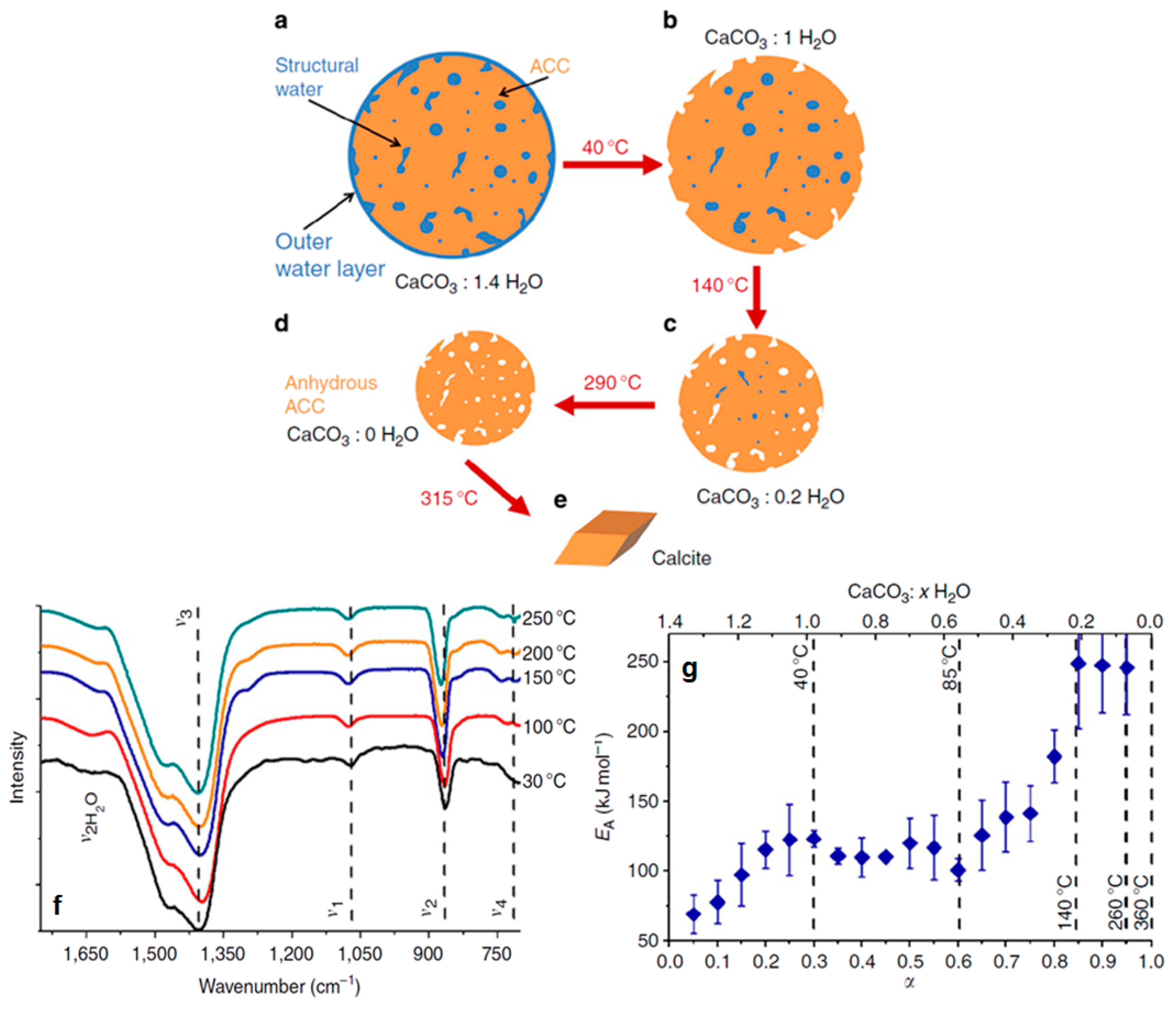
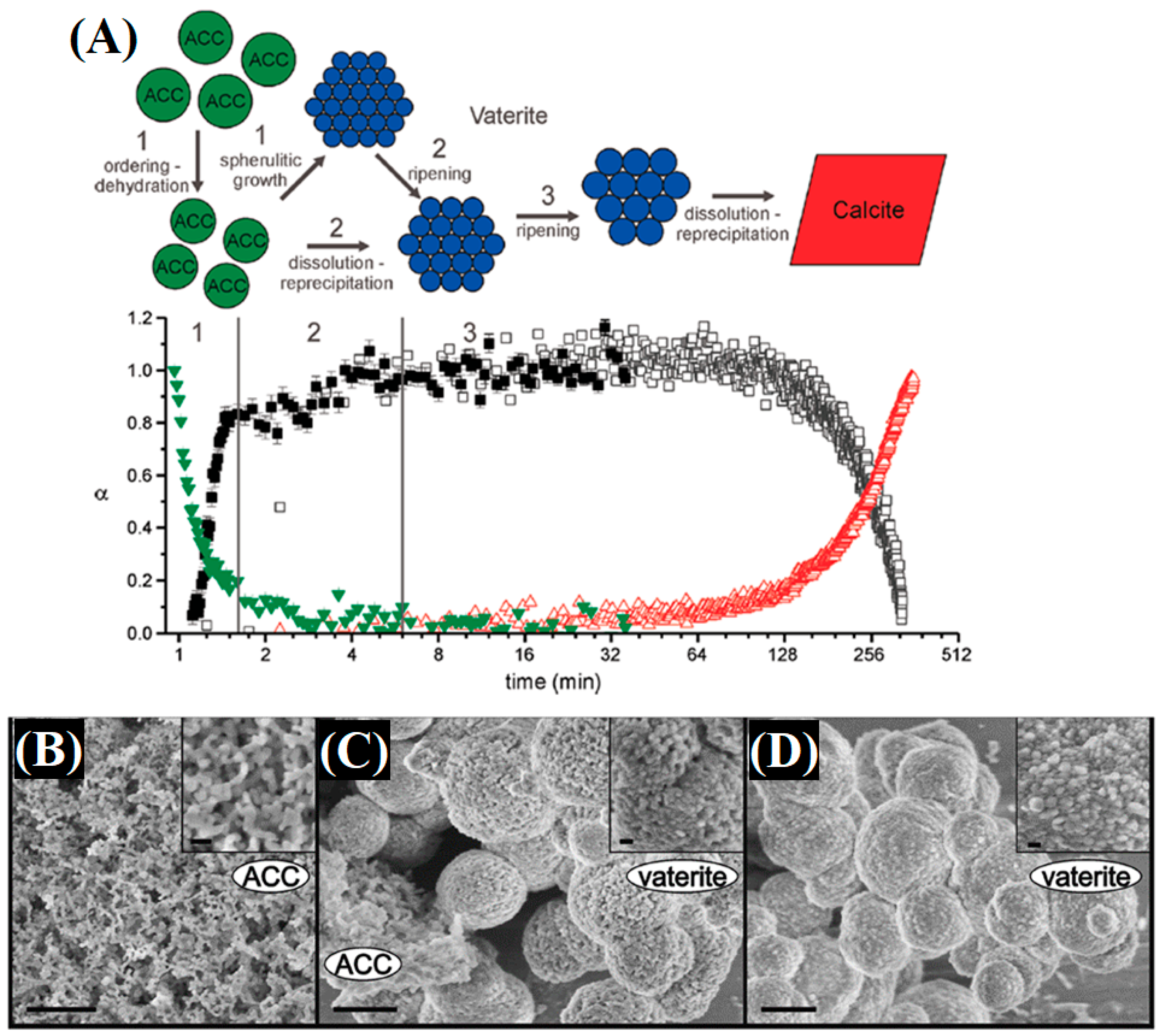
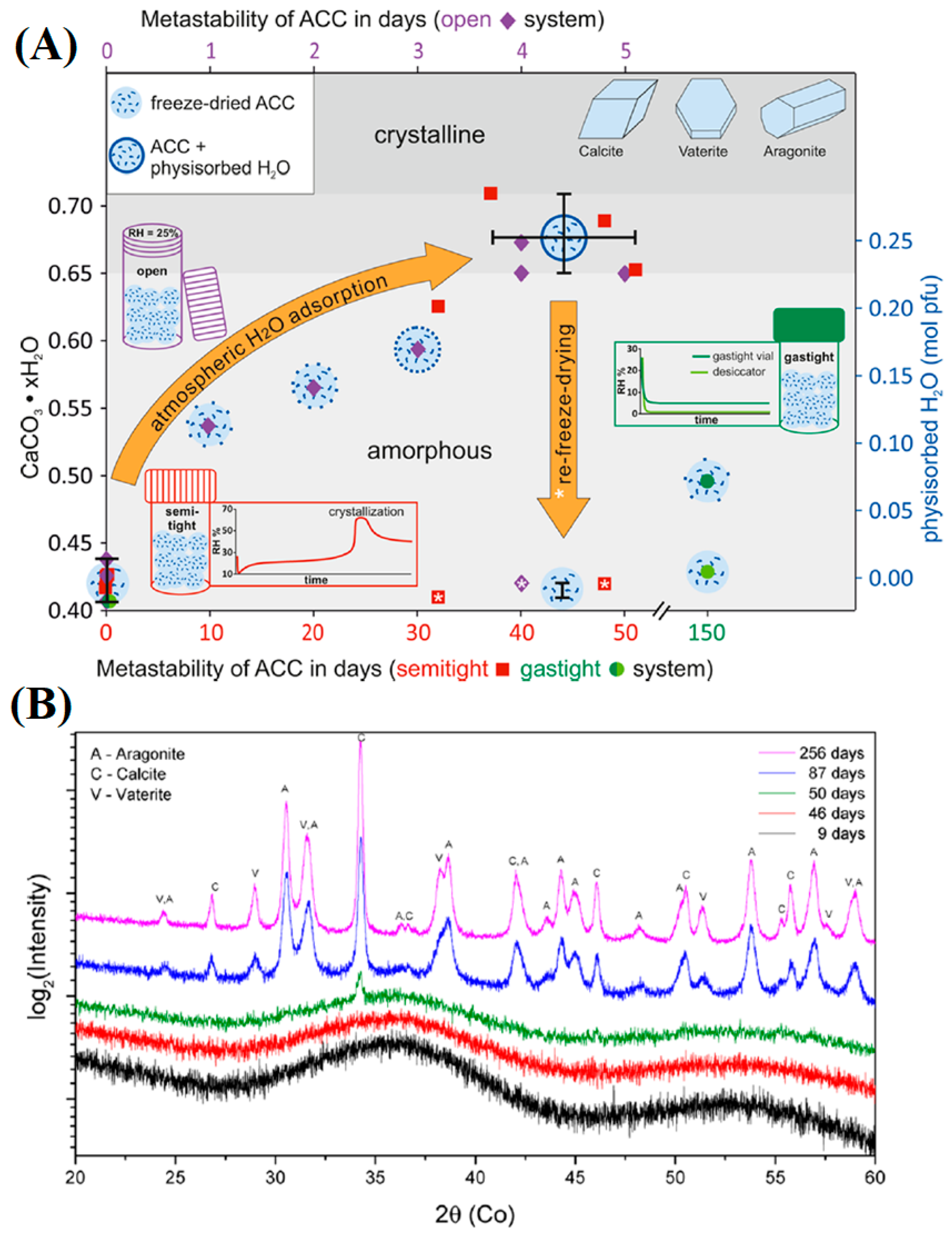

| Name | Method 1 | Method 2 | Method 3 |
|---|---|---|---|
| Ikaite | 0.1 M CaCl2 + 0.1 M K2CO3 + 0.04 M KOH; T = 0 °C [60] | 0.02 M Na2CO3 + 0.02 M CaCl2; pH = 12.8; T = 0–4 °C; t = 20–25 min [21] | 2.5 M CaCl2·2H2O + 0.5 M NaHCO3 + 0.5 M NaOH + seawater-similar mixture (presence of, e.g., PO43−), T = 0 and 4 °C [73] |
| Monohydrocalcite (MHC) | 700 mM Na2CO3 + 700 mM CaCl2 + MgCl2 (or CoCl2 2H2O [86]); T = 5 or 21 °C [87,88] | 0.05 M Na2CO3 + sodium silicate gel with 0.05 M CaCl2; pH = 10.5 [89] | 0.3 g K2CO3 + 250 mL seawater-similar mixture [90] |
| Calcium carbonate hemihydrate (CCHH) | 5–100 mM Na2CO3 ·10H2O + MgCl2·6H2O + CaCl2·2H2O (Mg2+ to Ca2+ ratio was 5 to 1); T = 25 °C; t = 4200 s [20] | ------ | ------ |
| Amorphous Calcium carbonate (ACC) | 0.25 M CaCl2·2H2O + Na2CO3, pH~10; T = 20 °C, filtration and freeze-drying [131] | 23 mM Ca(OH)2 solution, microfluidic spray-drying [132,133] | Gas diffusion of NH3/CO2 through 0.0136 mM CaCl2·2H2O in abs. EtOH, pH~8 [134] |
| Modes | Ikaite [136] | MHC [136] | ACC (Hydrated) | CCHH [20] | ||||
|---|---|---|---|---|---|---|---|---|
| IR | Raman | IR | Raman | IR [138] | Raman [139] | IR | Raman | |
| υas,OH | 3543, 3502, 3468, 3404, 3361, 3119 | 3423, 3182 | 3400, 3327 | 3425, 3326 | 3401, 3130 | 3430 | 3379 | 3382 |
| υs,OH | 3216 | 3240 | 3236 | 3224 | 3226 | --- | 3290 | 3285 |
| δs,HOH | 1673, 1644, 1616 | --- | 1700 | --- | 1650 | 1659 | 1661 | |
| υas,CO (υ3) | 1425, 1411 | 1483 | 1492, 1401 | --- | 1485 | 1540, 1460, 1390 | 1524, 1490, 1423, 1392, | 1579, 1483, 1423 |
| υs,CO (υ1) | 1085 | 1072 | 1063 | 1069 | 1064 | 1077 | 1096 | 1102 |
| π,CO (υ2) | 876 | 873 | 872 | 876 | 876, 856 | 868 | 866, 860 | 862 |
| δas,CO (υ4) | 743, 720 | 722 | 762, 698 | 723, 699 | 698 | 723, 698 | 723, 692 | 731, 700 |
| Name | Calcium Carbonate Hexahydrate [143] | Monohydrocalcite [140] | Calcium Carbonate Hemihydrate [20] |
|---|---|---|---|
| Acronym | Ikaite | MHC | CCHH |
| Formula | CaCO3·6H2O | CaCO3·H2O | CaCO3·0.5H2O |
| Shape of the crystalline | flat, platy, or tabular crystals with a prismatic habit | thin, sometimes rounded or plate-like crystals | needle-like morphology |
| Crystal System | Monoclinic | Trigonal | Monoclinic |
| Space group | C2/c | P31 | P21/c |
| Lattice parameters | a = 8.78 Å b = 8.28 Å c = 10.88 Å β = 109.59° | a = 10.554 Å b = 10.554 Å c = 7.454 Å | a = 9.33 Å b = 10.44 Å c = 6.16 Å β = 90.5° |
| Unit Cell Volume (Å3) | ~743.7 | ~751.6 | ~600.0 |
| Sample Composition | υ1 (Raman Shift) | Stability in Solution (Min) | logK |
|---|---|---|---|
| ACC | 1080 ± 1 | 15 | −6.20 ± 0.02 |
| Ca0.91Mg0.09 | 1082 ± 1 | 24 | −6.13 ± 0.02 |
| Ca0.85Mg0.15 | 1082 ± 1 | 24 | −6.13 ± 0.02 |
| Ca0.78Mg0.22 | 1083 ± 1 | 54 | −6.04 ± 0.03 |
| Ca0.69Mg0.31 | 1084 ± 1 | 85 | −6.00 ± 0.03 |
| Ca0.61Mg0.39 | 1085 ± 1 | 64 | −5.81 ± 0.07 |
| Ca0.47Mg0.53 | 1086 ± 1 | 51 | −5.71 ± 0.03 |
| Ca0.20Mg0.80 | 1088 ± 1 | 20 | −5.43 ± 0.02 |
| AMC | 1095 ± 1 | 9 | −4.96 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Béres, K.A.; Németh, P.; Kótai, L. Review on Chemistry of Water-Containing Calcium Carbonates and Their Transformations into Amorphous and Crystalline Carbonate Modifications. Inorganics 2025, 13, 321. https://doi.org/10.3390/inorganics13100321
Béres KA, Németh P, Kótai L. Review on Chemistry of Water-Containing Calcium Carbonates and Their Transformations into Amorphous and Crystalline Carbonate Modifications. Inorganics. 2025; 13(10):321. https://doi.org/10.3390/inorganics13100321
Chicago/Turabian StyleBéres, Kende Attila, Péter Németh, and László Kótai. 2025. "Review on Chemistry of Water-Containing Calcium Carbonates and Their Transformations into Amorphous and Crystalline Carbonate Modifications" Inorganics 13, no. 10: 321. https://doi.org/10.3390/inorganics13100321
APA StyleBéres, K. A., Németh, P., & Kótai, L. (2025). Review on Chemistry of Water-Containing Calcium Carbonates and Their Transformations into Amorphous and Crystalline Carbonate Modifications. Inorganics, 13(10), 321. https://doi.org/10.3390/inorganics13100321








