Synthesis and Use of Complex Titanium-Containing Coagulant in Water Purification Processes
Abstract
1. Introduction
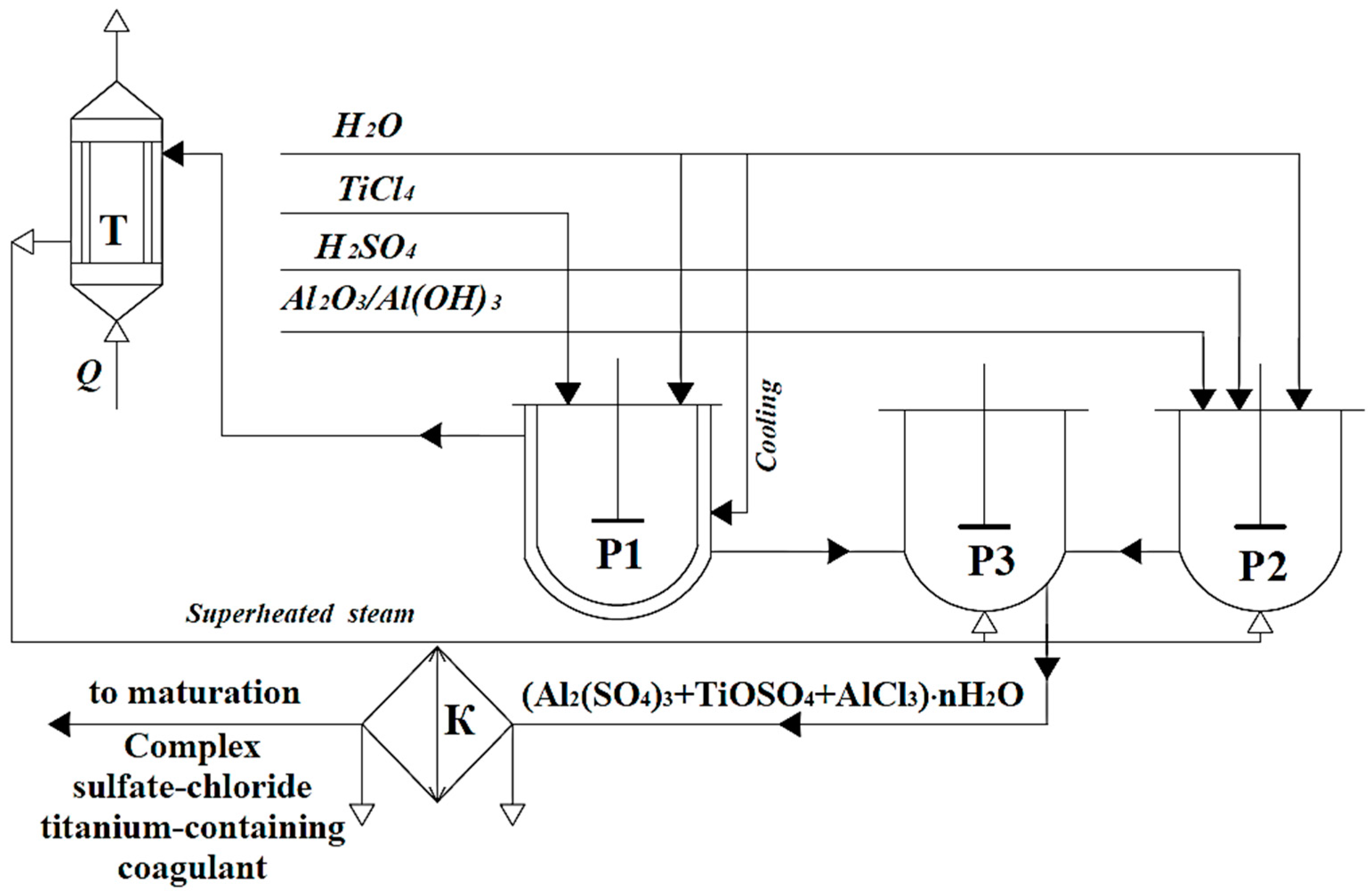
2. Results and Discussion
- The interaction of 50–80% sulfuric acid and 120% stoichiometric excess of aluminum hydroxide/oxide (reactions (1) and (2)) for 30 min at a temperature of 80–100 °C to form aluminum sulfate (reactor (3)):
- The hydrolytic decomposition of tetrachloride with the formation of hydrochloric acid (reaction (3), reactor (1)) and a wide range of titanium polyoxychlorides within 10–20 min:
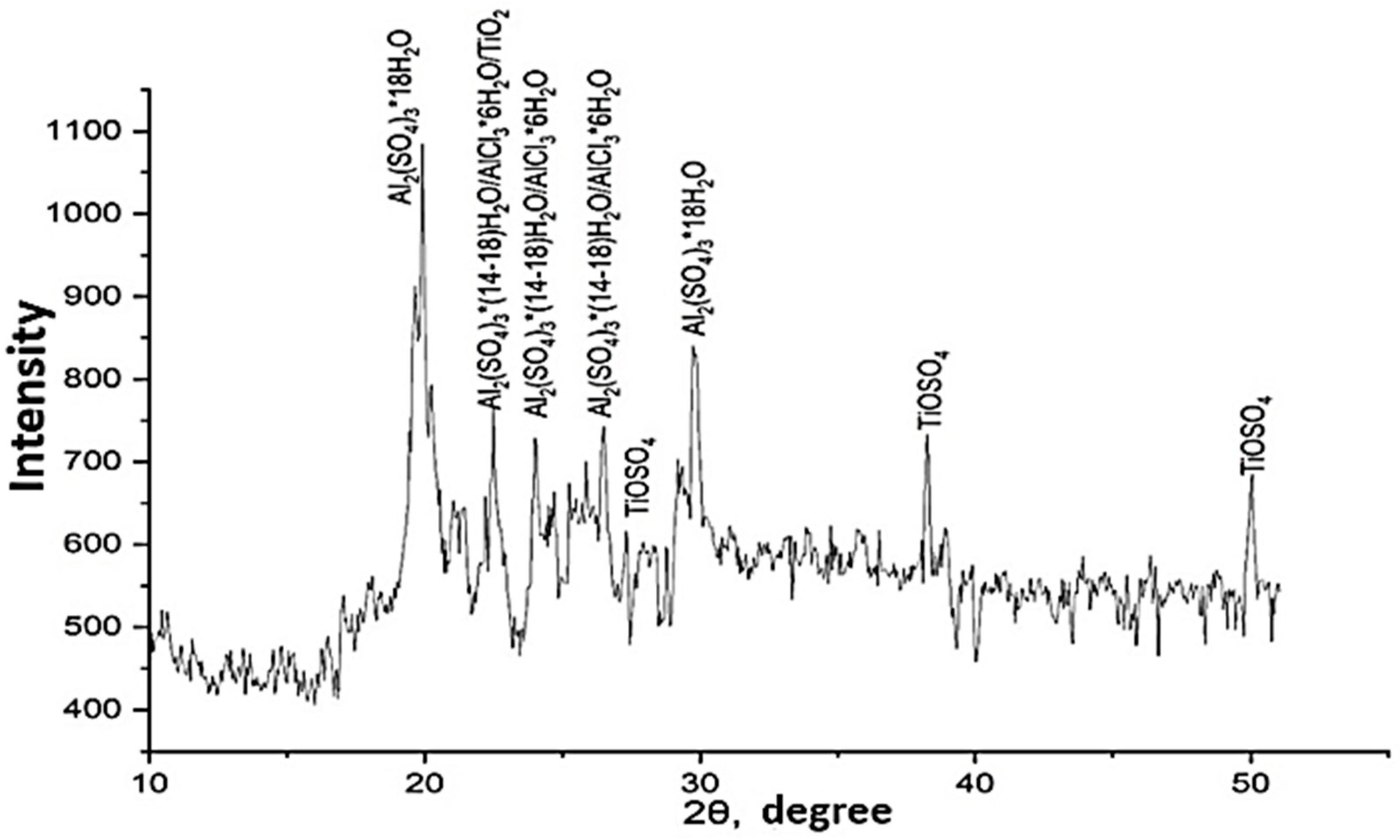
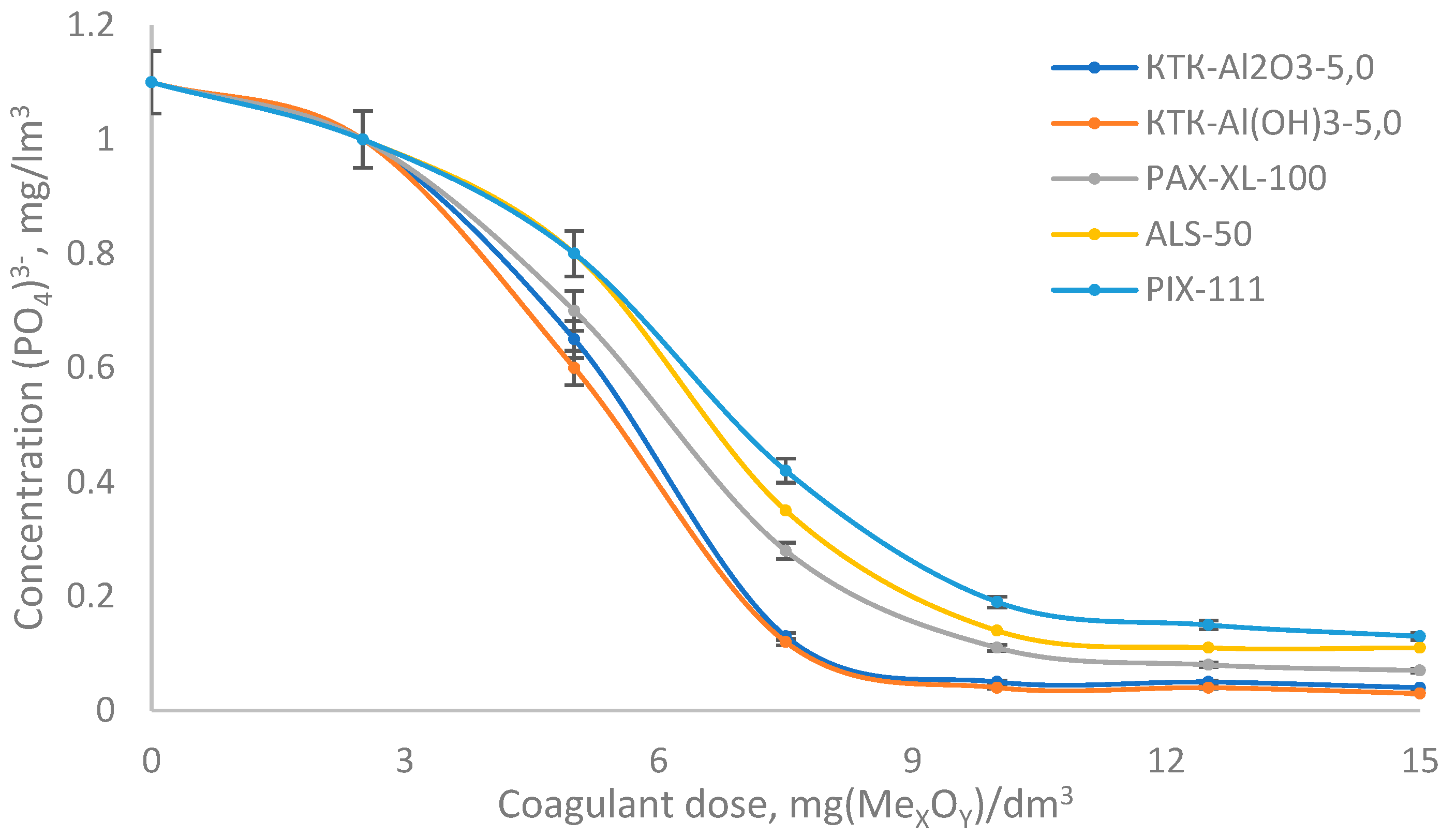
- Data on the surface characteristics of the hydrolysis products of different coagulants in the model (distilled water with pH correction with 1% NaOH to values of 7.1–7.2) confirming the advanced hypotheses are presented in Table 3.
3. Materials and Research Methods
- Titanium tetrachloride obtained by selective chlorination of quartz–leucoxene concentrate (waste from shale oil production) with an admixture of silicon tetrachloride to 0.4 wt.%; the total content of impurities of aluminum chlorides, iron, sodium, calcium, etc., does not exceed 0.1 wt.%;
- Aluminum oxide and hydroxide, special purity grade, Sigma-Aldrich (Taufkirchen, Germany);
- Sulfuric acid, chemically pure, Sigma-Aldrich (Germany).
- Aluminum sulfate (ALS-50), Kemira (Helsinki, Finland);
- Polyoxychloride (PAX-XL-100), Kemira (Helsinki, Finland);
- Iron chloride (PIX-111), Kemira (Helsinki, Finland).
- Water from the surface intake of the Danube River in Belgrade (Serbia);
- Wastewater that underwent the stage of deep biological treatment (nitrification/denitrification).
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moussas, P.; Tzoupanos, N.; Zouboulis, A. Advances in coagulation/flocculation field: Al- and Fe-based composite coagulation reagents. Desalination Water Treat. 2011, 33, 140–146. [Google Scholar] [CrossRef]
- Han, S.W.; Kang, L.S. Comparison of Al(III) and Fe(III) Coagulants for Improving Coagulation Effectiveness in Water Treatment. J. Korean Soc. Environ. Eng. 2015, 37, 325–331. [Google Scholar] [CrossRef]
- Hidayah, E.N.; Cahyonugroho, O.H.; Novembrianto, R.; Wahyusi, K.N.; Srihari, E. Comparison of Fe-based and Al-based coagulants in the removal of organic and disinfection by-product precursors. CLEAN—Soil. Air Water. 2024, 52, 2300177. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Y.; Gao, B.; Zhao, Q. Enhanced algae removal by Ti-based coagulant: Comparison with conventional Al- and Fe based coagulants. Environ. Sci. Pollut. Res. 2018, 25, 13147–13158. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.M.; Joo, J.C.; Kim, J.K.; Yeo, W.S. Evaluation of Titanium Tetrachloride (TiCl4;) as an Alternative Coagulant and Characterization of Recovered TiO2; J. Nanosci. Nanotechnol. 2021, 21, 4067–4072. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, Y.; Fan, L.; Zheng, X.; Wang, J.; Jiang, C.; Xu, S.; Xu, H.; Wang, D. Coagulation effect of polyaluminum-titanium chloride coagulant and the effect of floc aging in fluoride removal: A mechanism analysis. Sep. Purif. Technol. 2023, 325, 124674. [Google Scholar] [CrossRef]
- Gan, Y.; Li, J.; Zhang, L.; Wu, B.; Huang, W.; Li, H.; Zhang, S. Potential of titanium coagulants for water and wastewater treatment: Current status and future perspectives. Chem. Eng. J. 2020, 2020, 126837. [Google Scholar] [CrossRef]
- Thomas, M.; Bąk, J.; Królikowska, J. Efficiency of titanium salts as alternative coagulants in water and wastewater treatment: Short review. Desalination Water Treat. 2020, 208, 261–272. [Google Scholar] [CrossRef]
- Galloux, J.; Chekli, L.; Phuntsho, S.; Tijing, L.D.; Jeong, S.; Zhao, Y.X.; Gao, B.Y.; Park, S.H.; Shon, H.K. Coagulation performance and floc characteristics of polytitanium tetrachloride and titanium tetrachloride compared with ferric chloride for coal mining wastewater treatment. Sep. Purif. Technol. 2015, 152, 94–100. [Google Scholar] [CrossRef]
- Chekli, L.; Corjon, E.; Tabatabai, S.A.A.; Naidu, G.; Tamburic, B.; Park, S.H.; Shon, H.K. Performance of titanium salts compared to conventional FeCl3 for the removal of algal organic matter (AOM) in synthetic seawater: Coagulation performance, organic fraction removal and floc characteristics. J. Environ. Manag. 2017, 201, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, G.; Naidu, M.A.; Johir, J.; Kandasamy, S. Vigneswaran, Performance of flocculation titanium salts for seawater reverse osmosis pretreatment. Desalin. Water Treat. 2017, 98, 92–97. [Google Scholar] [CrossRef]
- Nawaz, A.; Ahmed, Z.; Shahbaz, A.; Khan, Z.; Javed, M. Coagulation-flocculation for lignin removal from wastewater—A review. Water Sci. Technol. 2014, 69, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.A.; Razak MH, A.; Rahim MZ, A.; Kamar WI, S.W.; Amr, S.S.A.; Hussain, S.; John, V.L. Evaluation and comparison the performance of titanium and zirconium(IV) tetrachloride in textile wastewater treatment. Data Brief. 2018, 18, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.-J.; Ahn, J.-H. Evaluation of titanium tetrachloride and polytitanium tetrachloride to remove phosphorus from wastewater. Sep. Purif. Technol. 2018, 197, 197–201. [Google Scholar] [CrossRef]
- Dubey, S.; Agrawal, M.; Gupta, A.B. Advances in coagulation technique for treatment of fluoride-contaminated water: A critical review. Rev. Chem. Eng. 2019, 35, 109–137. [Google Scholar] [CrossRef]
- Kuzin, E.N. Titanium Coagulants in Water Purification and Water Treatment Processes in Additive Manufacturing. Metall. Mater. Eng. 2024, 30, 25–33. [Google Scholar]
- Tsoutsa, E.K.; Tolkou, A.K.; Kyzas, G.Z.; Katsoyiannis, I.A. New Trends in Composite Coagulants for Water and Wastewater Treatment. Macromol 2024, 4, 509–532. [Google Scholar] [CrossRef]
- Xu, H.; Wei, S.; Li, G.; Guo, B. Advanced removal of phosphorus from urban sewage using chemical precipitation by Fe-Al composite coagulants. Sci. Rep. 2024, 14, 4918. [Google Scholar] [CrossRef]
- Liu, B.; Gao, B.; Guo, K.; Pan, J.; Yue, Q. The interactions between Al (III) and Ti (IV) in the composite coagulant polyaluminum-titanium chloride. Sep. Purif. Technol. 2022, 282, 120148. [Google Scholar] [CrossRef]
- Kuzin, E.; Averina, Y.; Kurbatov, A.; Kruchinina, N.; Boldyrev, V. Titanium-Containing Coagulants in Wastewater Treatment Processes in the Alcohol Industry. Processes 2022, 10, 440. [Google Scholar] [CrossRef]
- Kuzin, E.N.; Kruchinina, N.E.; Gromovykh, P.S.; TyaglovaYa, V. Coagulants in the Processes of Waste Water Treatment in Dairy Complex Industry. Chem. Sustain. Dev. 2020, 28, 388–393. [Google Scholar] [CrossRef]
- Shon, H.; Vigneswaran, S.; Kandasamy, J.; Zareie, M.; Kim, J.; Cho, D.; Kim, J.H. Preparation and characterization of titanium dioxide (TiO2) from sludge produced by TiCl4 flocculation with FeCl3, Al2(SO4)3 and Ca(OH)2 coagulantaids in wastewater. Sep. Sci. Technol. 2009, 44, 1525–1543. [Google Scholar] [CrossRef][Green Version]
- Wang, T.-H.; Navarrete-López, A.M.; Li, S.; Dixon, D.A.; Gole, J.L. Hydrolysis of TiCl4: Initial steps in the production of TiO2. J. Phys. Chem. A 2010, 114, 7561–7570. [Google Scholar] [CrossRef] [PubMed]
- Weisz, A.D.; Rodenas, L.G.; Morando, P.J.; Regazzoni, A.E.; Blesa, M.A. FTIR study of the adsorption of single pollutants and mixtures of pollutants onto titanium dioxide in water: Oxalic and salicylic acids. Catal. Today 2002, 76, 103–112. [Google Scholar] [CrossRef]
- Akhter, P.; Arshad, A.; Saleem, A.; Hussain, M. Recent Development in Non-Metal-Doped Titanium Dioxide Photocatalysts for Different Dyes Degradation and the Study of Their Strategic Factors: A Review. Catalysts 2022, 12, 1331. [Google Scholar] [CrossRef]

| Mass Ratio (Al2O3/Al(OH)3)/TiCl4 | Compositional Breakdown, % | |||
|---|---|---|---|---|
| Insoluble Part | AlCl3·6H2O | Al2(SO4)3·18H2O | Ti-Com. | |
| Aluminum oxide | ||||
| 2.1/1 | 0.5 | 9.7 | 87.3 | 2.5 |
| 1.1/1 | 0.9 | 19.1 | 75.1 | 4.9 |
| 0.9/1 | 1.6 | 29.6 | 61.2 | 7.6 |
| 0.7/1 | 2.4 | 38.0 | 49.7 | 9.9 |
| Aluminum hydroxide | ||||
| 4/1 | 0.3 | 10.1 | 87.1 | 2.5 |
| 2/1 | 0.5 | 19.8 | 74.8 | 4.9 |
| 1.4/1 | 0.8 | 30.3 | 61.3 | 7.6 |
| 1/1 | 1.3 | 39.2 | 49.6 | 9.9 |
| Coagulant Dose, mg(MeXOY)/dm3 | 0 | 2.5 | 5.0 | 7.5 | 10.0 | 12.5 | 15.0 | 17.5 | 20.0 |
|---|---|---|---|---|---|---|---|---|---|
| Turbidity, NTU | |||||||||
| KTK-Al2O3-2.5 | 29 | 25 | 18 | 5.6 | 1.3 | 0.8 | 0.8 | 0.8 | 0.8 |
| KTK-Al2O3-5.0 | 29 | 21 | 9 | 3.5 | 0.9 | 0.5 | 0.5 | 0.5 | 0.5 |
| KTK-Al2O3-7.5 | 29 | 22 | 8 | 2.9 | 0.9 | 0.5 | 0.5 | 0.4 | 0.4 |
| KTK-Al2O3-10.0 | 29 | 24 | 11 | 3.5 | 1.3 | 0.9 | 0.6 | 0.6 | 0.6 |
| KTK-Al(OH)3-2.5 | 29 | 24 | 16 | 4.8 | 1.2 | 0.7 | 0.7 | 0.7 | 0.7 |
| KTK-Al(OH)3-5.0 | 29 | 19 | 7 | 2.2 | 0.6 | 0.4 | 0.4 | 0.4 | 0.4 |
| KTK-Al(OH)3-7.5 | 29 | 20 | 8 | 1.9 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 |
| KTK-Al(OH)3-10.0 | 29 | 22 | 10 | 2.9 | 1.1 | 1 | 0.6 | 0.6 | 0.6 |
| PAX-XL-100 | 29 | 27 | 22 | 11 | 4.9 | 2.8 | 1.4 | 0.8 | 0.8 |
| ALS-50 | 29 | 28 | 28 | 20 | 8.6 | 5.2 | 2.2 | 1.4 | 1.4 |
| Oxidizability, mg(O)/dm3 | |||||||||
| KTK-Al2O3-2.5 | 15.6 | 14.9 | 11.3 | 6.1 | 3.8 | 3.8 | 3.6 | 3.5 | 3.5 |
| KTK-Al2O3-5.0 | 15.6 | 13.3 | 7.6 | 5.2 | 3.3 | 3.2 | 3.2 | 3.0 | 3.0 |
| KTK-Al2O3-7.5 | 15.6 | 13.2 | 7.5 | 5.3 | 3.3 | 3.3 | 3.2 | 3.1 | 3.0 |
| KTK-Al2O3-10.0 | 15.6 | 13.8 | 8.1 | 5.2 | 3.2 | 3.2 | 3.1 | 3.1 | 3.0 |
| KTK-Al(OH)3-2.5 | 15.6 | 14.6 | 11.1 | 6.0 | 3.7 | 3.7 | 3.5 | 3.4 | 3.4 |
| KTK-Al(OH)3-5.0 | 15.6 | 12.9 | 6.8 | 4.5 | 3.0 | 2.9 | 2.9 | 2.9 | 2.9 |
| KTK-Al(OH)3-7.5 | 15.6 | 12.7 | 6.9 | 4.3 | 2.9 | 2.7 | 2.7 | 2.7 | 2.7 |
| KTK-Al(OH)3-10.0 | 15.6 | 13.5 | 7.9 | 5.1 | 3.2 | 3.1 | 3.0 | 3.0 | 2.9 |
| PAX-XL-100 | 15.6 | 15.1 | 14.2 | 9.6 | 7.4 | 5.2 | 4.6 | 4.5 | 4.5 |
| ALS-50 | 15.6 | 15.5 | 15.2 | 10.3 | 8.6 | 6.3 | 5.2 | 5.1 | 5.1 |
| Coagulant | Zeta Potential, mV | Surface Area, m2/g |
|---|---|---|
| KTK-Al2O3-5.0 | +7 | 82.3 |
| KTK-Al(OH)3-5.0 | +4 | 84.5 |
| PAX-XL-100 | +17 | 69.3 |
| ALS-50 | +12 | 51.5 |
| PIX-111 | −13 | 89.4 |
| Particle Size Range, µm | Sedimentation Rate, min | Filtration Rate, mL/min | |
|---|---|---|---|
| KTK-Al2O3-5.0 | 360–420 | 3.5 | 67 |
| KTK-Al(OH)3-5.0 | 440–490 | 3.0 | 70 |
| PAX-XL-100 | 280–370 | 4.5 | 55 |
| ALS-50 | 210–310 | 6.0 | 51 |
| PIX-111 | 150–400 | 4.0 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzin, E. Synthesis and Use of Complex Titanium-Containing Coagulant in Water Purification Processes. Inorganics 2025, 13, 9. https://doi.org/10.3390/inorganics13010009
Kuzin E. Synthesis and Use of Complex Titanium-Containing Coagulant in Water Purification Processes. Inorganics. 2025; 13(1):9. https://doi.org/10.3390/inorganics13010009
Chicago/Turabian StyleKuzin, Evgenii. 2025. "Synthesis and Use of Complex Titanium-Containing Coagulant in Water Purification Processes" Inorganics 13, no. 1: 9. https://doi.org/10.3390/inorganics13010009
APA StyleKuzin, E. (2025). Synthesis and Use of Complex Titanium-Containing Coagulant in Water Purification Processes. Inorganics, 13(1), 9. https://doi.org/10.3390/inorganics13010009






