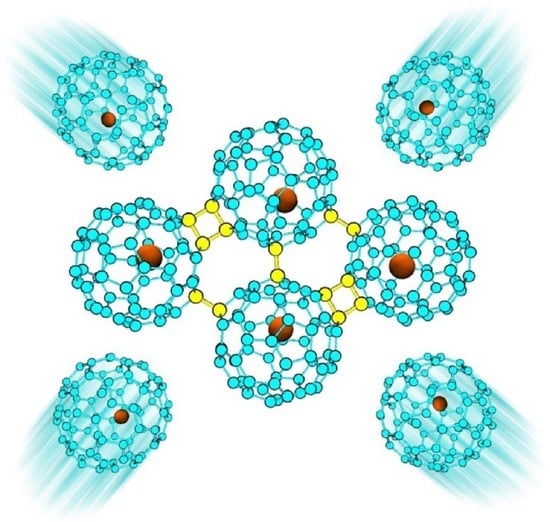
Stability and Electronic Properties of 1D and 2D Ca@C60 Oligomers and Polymers
Abstract
1. Introduction
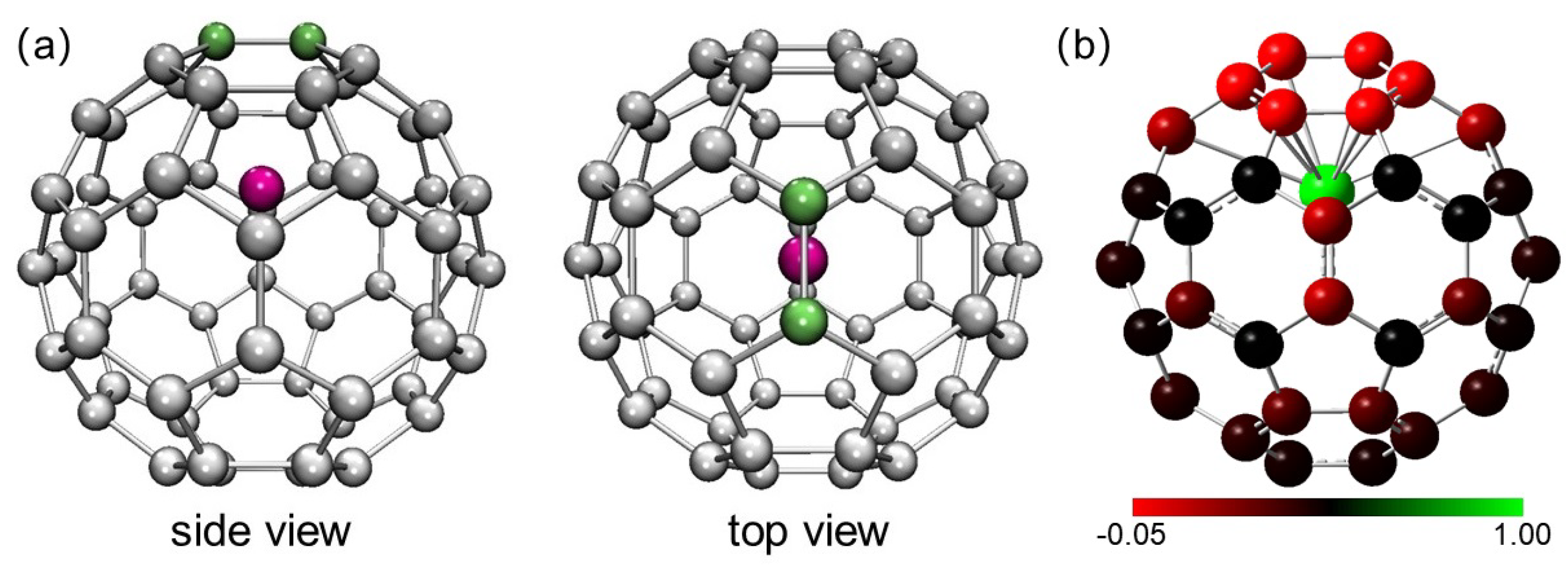
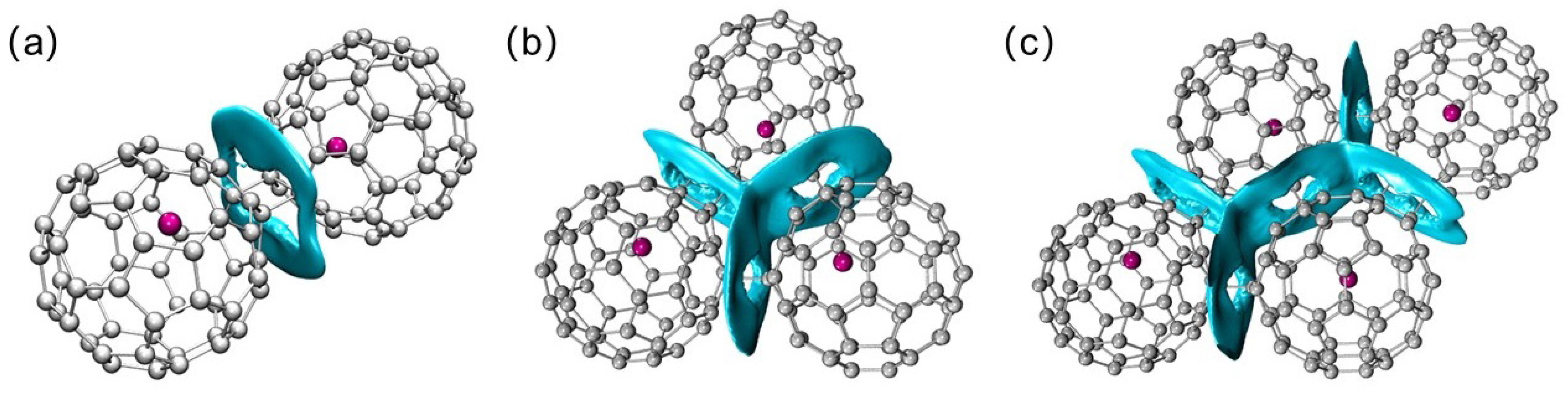
2. Results and Discussion
3. Computational Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lindle, J.; Pong, R.; Bartoli, F.; Kafafi, Z. Nonlinear optical properties of the fullerenes C60 and C70 at 1.064 μm. Phys. Rev. B 1993, 48, 9447. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.P.; Lawson, G.E.; Riggs, J.E.; Ma, B.; Wang, N.; Moton, D.K. Photophysical and nonlinear optical properties of [60] fullerene derivatives. J. Phys. Chem. A 1998, 102, 5520–5528. [Google Scholar] [CrossRef]
- Makarova, T. Electrical and optical properties of pristine and polymerized fullerenes. Semiconductors 2001, 35, 243–278. [Google Scholar] [CrossRef]
- Sachdeva, S.; Singh, D.; Tripathi, S. Optical and electrical properties of fullerene C70 for solar cell applications. Opt. Mater. 2020, 101, 109717. [Google Scholar] [CrossRef]
- Guo, K.; Li, N.; Bao, L.; Lu, X. Fullerenes and derivatives as electrocatalysts: Promises and challenges. Green Energy Environ. 2022, 9, 7–27. [Google Scholar] [CrossRef]
- Huang, C.; Yang, Y.; Li, M.; Qi, X.; Pan, C.; Guo, K.; Bao, L.; Lu, X. Ultrahigh Capacity from Complexation-Enabled Aluminum-Ion Batteries with C70 as the Cathode. Adv. Mater. 2023, 2306244. [Google Scholar] [CrossRef]
- Li, C.Z.; Yip, H.L.; Jen, A.K.Y. Functional fullerenes for organic photovoltaics. J. Mater. Chem. 2012, 22, 4161–4177. [Google Scholar] [CrossRef]
- Collavini, S.; Delgado, J.L. Fullerenes: The stars of photovoltaics. Sustain. Energy Fuels 2018, 2, 2480–2493. [Google Scholar] [CrossRef]
- Castro, E.; Garcia, A.H.; Zavala, G.; Echegoyen, L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523–6535. [Google Scholar] [CrossRef]
- Su, S.; Zhen, M.; Zhou, C.; Cao, X.; Sun, Z.; Xu, Y.; Li, L.; Jia, W.; Wu, Z.; Wang, C. Efficiently Inhibiting Systemic Inflammatory Cascades by Fullerenes for Retarding HFD-Fueled Atherosclerosis. Adv. Healthc. Mater. 2023, 12, 2202161. [Google Scholar] [CrossRef]
- Burger, B.; Winter, J.; Kuzmany, H. Dimer and cluster formation in C60 photoreaction. Z. Phys. B Condens. Matter 1996, 101, 227–233. [Google Scholar] [CrossRef]
- Blank, V.D.; Buga, S.G.; Dubitsky, G.A.; Serebryanaya, N.R.; Popov, M.Y.; Sundqvist, B. High-pressure polymerized phases of C60. Carbon 1998, 36, 319–343. [Google Scholar] [CrossRef]
- Okada, S.; Saito, S. Electronic structure and energetics of pressure-induced two-dimensional C60 polymers. Phys. Rev. B 1999, 59, 1930. [Google Scholar] [CrossRef]
- Xu, C.H.; Scuseria, G.E. Theoretical predictions for a two-dimensional rhombohedral phase of solid C60. Phys. Rev. Lett. 1995, 74, 274. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Eklund, P.; Venkateswaran, U.; Tucker, J.; Duncan, M.; Bendele, G.; Stephens, P.; Hodeau, J.L.; Marques, L.; Nunez-Regueiro, M.; et al. Properties of C60 polymerized under high pressure and temperature. Appl. Phys. A 1997, 64, 231–2239. [Google Scholar] [CrossRef]
- Giacalone, F.; Martin, N. Fullerene polymers: Synthesis and properties. Chem. Rev. 2006, 106, 5136–5190. [Google Scholar] [CrossRef]
- Blank, V.; Buga, S.; Serebryanaya, N.; Denisov, V.; Dubitsky, G.; Ivlev, A.; Mavrin, B.; Popov, M.Y. Ultrahard and superhard carbon phases produced from C60 by heating at high pressure: Structural and Raman studies. Phys. Lett. A 1995, 205, 208–216. [Google Scholar] [CrossRef]
- Sabirov, D.S. Polarizability of C60 fullerene dimer and oligomers: The unexpected enhancement and its use for rational design of fullerene-based nanostructures with adjustable properties. RSC Adv. 2013, 3, 19430–19439. [Google Scholar] [CrossRef]
- Sabirov, D.S.; Ori, O.; Tukhbatullina, A.A.; Shepelevich, I.S. Covalently bonded fullerene nano-aggregates (C60)n: Digitalizing their energy–topology–symmetry. Symmetry 2021, 13, 1899. [Google Scholar] [CrossRef]
- Hou, L.; Cui, X.; Guan, B.; Wang, S.; Li, R.; Liu, Y.; Zhu, D.; Zheng, J. Synthesis of a monolayer fullerene network. Nature 2022, 606, 507–510. [Google Scholar] [CrossRef]
- Meirzadeh, E.; Evans, A.M.; Rezaee, M.; Milich, M.; Dionne, C.J.; Darlington, T.P.; Bao, S.T.; Bartholomew, A.K.; Handa, T.; Rizzo, D.J.; et al. A few-layer covalent network of fullerenes. Nature 2023, 613, 71–76. [Google Scholar] [CrossRef]
- Argaman, U.; Makov, G. Structure and properties of graphullerene: A semiconducting two-dimensional C60 crystal. Npj Comput. Mater. 2023, 9, 211. [Google Scholar] [CrossRef]
- Shinohara, H. Endohedral metallofullerenes. Rep. Prog. Phys. 2000, 63, 843. [Google Scholar] [CrossRef]
- Chaur, M.N.; Melin, F.; Ortiz, A.L.; Echegoyen, L. Chemical, electrochemical, and structural properties of endohedral metallofullerenes. Angew. Chem. Int. Ed. 2009, 48, 7514–7538. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Yu, B.; Akasaka, T.; Lu, X. Endohedral metallofullerenes: An unconventional core–shell coordination union. Coord. Chem. Rev. 2013, 257, 2880–2898. [Google Scholar] [CrossRef]
- Popov, A.A.; Yang, S.; Dunsch, L. Endohedral fullerenes. Chem. Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, C. Endohedral Metallofullerenes Based on Spherical Ih-C80 Cage: Molecular Structures and Paramagnetic Properties. Acc. Chem. Res. 2014, 47, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wei, T.; Jin, F. When metal clusters meet carbon cages: Endohedral clusterfullerenes. Chem. Soc. Rev. 2017, 46, 5005–5058. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Li, Y.; Magagula, S.; Chen, Z. Exohedral functionalization of endohedral metallofullerenes: Interplay between inside and outside. Coord. Chem. Rev. 2019, 388, 406–439. [Google Scholar] [CrossRef]
- Shen, W.; Hu, S.; Lu, X. Endohedral metallofullerenes: New structures and unseen phenomena. Chem.-Eur. J. 2020, 26, 5748–5757. [Google Scholar] [CrossRef]
- Li, M.; Zhao, R.; Dang, J.; Zhao, X. Theoretical study on the stabilities, electronic structures, and reaction and formation mechanisms of fullerenes and endohedral metallofullerenes. Coord. Chem. Rev. 2022, 471, 214762. [Google Scholar] [CrossRef]
- Shen, W.; Bao, L.; Lu, X. Endohedral Metallofullerenes: An Ideal Platform of Sub-Nano Chemistry. Chin. J. Chem. 2022, 40, 275–284. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Ullah, A.; Gutiérrez-Finol, G.M.; Bedoya-Pinto, A.; Gargiani, P.; Shi, D.; Yang, S.; Shi, Z.; Gaita-Ariño, A.; et al. High-temperature magnetic blocking in a monometallic dysprosium azafullerene single-molecule magnet. Chem 2023, 9, 3613–3622. [Google Scholar] [CrossRef]
- Kubozono, Y.; Noto, T.; Ohta, T.; Maeda, H.; Kashino, S.; Emura, S.; Ukita, S.; Sogabe, T. Extractions of Ca@C60 and Sr@C60 with aniline. Chem. Lett. 1996, 25, 453–454. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Y.; Deng, J.; Wang, Z. Capturing unconventional metallofullerene M@C60 through activation of the unreactive [5,6] bond toward Diels–Alder reaction. Phys. Chem. Chem. Phys. 2020, 22, 24249–24256. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Aoyagi, S.; Yamazaki, Y.; Ohkubo, K.; Ikuma, N.; Okada, H.; Kato, T.; Matsuo, Y.; Fukuzumi, S.; Kokubo, K. Electrochemical reduction of cationic Li+@C60 to neutral Li+@C60˙−: Isolation and characterisation of endohedral [60]fulleride. Chem. Sci. 2016, 7, 5770–5774. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully Optimized Contracted Gaussian Basis Sets of Triple Zeta Valence Quality for Atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.C.; Contreras-García, J.; Hénon, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Delley, B. An all-electron numerical method for solving the local density functional for polyatomic molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol 3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Hamann, D.; Schlüter, M.; Chiang, C. Norm-conserving pseudopotentials. Phys. Rev. Lett. 1979, 43, 1494. [Google Scholar] [CrossRef]
- Tkatchenko, A.; Scheffler, M. Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data. Phys. Rev. Lett. 2009, 102, 073005. [Google Scholar] [CrossRef]







| HOMO-LUMO Gap | |
|---|---|
| Ca@C | 1.14 |
| 2-1 | 0.50 |
| 2-2 | 1.07 |
| 2-3 | 1.36 |
| 2-4 | 0.94 |
| 3-1 | 0.22 |
| 3-2 | 1.02 |
| 3-3 | 1.21 |
| 3-4 | 0.79 |
| 3-5 | 0.60 |
| 4-1 | 0.14 |
| 4-2 | 1.05 |
| 4-3 | 1.14 |
| 4-4 | 0.80 |
| 4-5 | 0.38 |
| 4-6 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhou, Z.; Wang, Z. Stability and Electronic Properties of 1D and 2D Ca@C60 Oligomers and Polymers. Inorganics 2024, 12, 45. https://doi.org/10.3390/inorganics12020045
Wu Y, Zhou Z, Wang Z. Stability and Electronic Properties of 1D and 2D Ca@C60 Oligomers and Polymers. Inorganics. 2024; 12(2):45. https://doi.org/10.3390/inorganics12020045
Chicago/Turabian StyleWu, Yabei, Zhonghao Zhou, and Zhiyong Wang. 2024. "Stability and Electronic Properties of 1D and 2D Ca@C60 Oligomers and Polymers" Inorganics 12, no. 2: 45. https://doi.org/10.3390/inorganics12020045
APA StyleWu, Y., Zhou, Z., & Wang, Z. (2024). Stability and Electronic Properties of 1D and 2D Ca@C60 Oligomers and Polymers. Inorganics, 12(2), 45. https://doi.org/10.3390/inorganics12020045