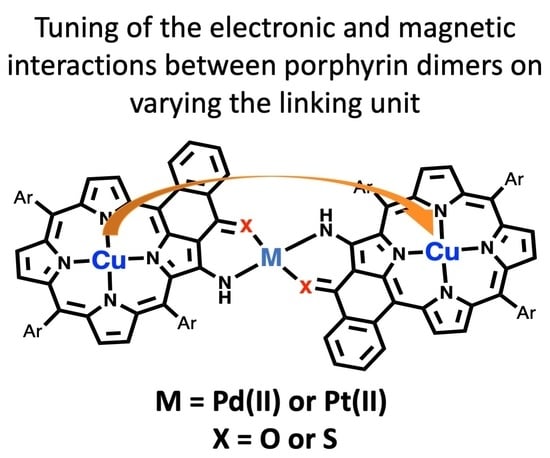
The Effect of the Linking Unit on the Electronic and Magnetic Interactions in Copper(II) Porphyrin Dimers Linked by Metal Ions
Abstract
1. Introduction
2. Results and Discussion
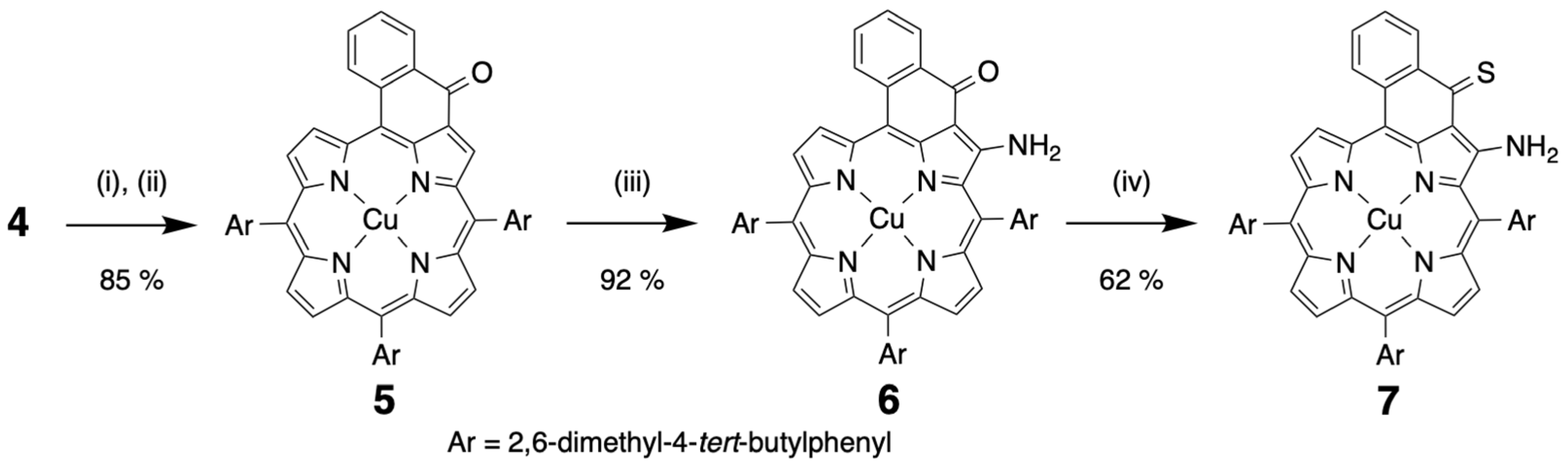
2.1. Syntheses of the Compounds
2.2. Electronic Interactions—Electrochemical Studies
2.3. EPR Studies
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kadish, K.M.; Smith, K.M.; Guilard, R. (Eds.) The Porphyrin Handbook; Academic Press: San Diego, CA, USA, 2000; Volumes 1–7. [Google Scholar]
- Zhang, Y.; Lovell, J.F. Porphyrins as theranostic agents from prehistoric to modern times. Theranostics 2012, 2, 905–915. [Google Scholar] [CrossRef]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef]
- Li, L.-L.; Diau, E.W.-G. Porphyrin-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 291–304. [Google Scholar] [CrossRef]
- Higashino, T.; Imahori, H. Porphyrins as excellent dyes for dye-sensitized solar cells: Recent developments and insights. Dalton Trans. 2015, 44, 448–463. [Google Scholar] [CrossRef]
- Urbani, M.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-substituted porphyrins for dye-sensitized solar cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O. Stirring the porphyrin alphabet soup—Functionalization reactions for porphyrins. Chem. Commun. 2011, 47, 1943–1960. [Google Scholar] [CrossRef] [PubMed]
- Hiroto, S.; Miyake, Y.; Shinokubo, H. Synthesis and functionalization of porphyrins through organometallic methodologies. Chem. Rev. 2017, 117, 2910–3043. [Google Scholar] [CrossRef] [PubMed]
- Harriman, A.; Sauvage, J.-P. A strategy for constructing photosynthetic models: Porphyrin-containing modules assembled around transition metals. Chem. Soc. Rev. 1996, 25, 41–48. [Google Scholar] [CrossRef]
- Crossley, M.J.; Burn, P.L. Rigid, laterally-bridged bis-porphyrin system. J. Chem. Soc. Chem. Commun. 1987, 39–40. [Google Scholar] [CrossRef]
- Anderson, H.L. Conjugated porphyrin ladders. Inorg. Chem. 1994, 33, 972–981. [Google Scholar] [CrossRef]
- Lin, V.S.-Y.; DiMagno, S.G.; Therien, M.J. Highly conjugated, acetylenyl bridged porphyrins: New models for light-harvesting antenna systems. Science 1994, 264, 1105–1111. [Google Scholar] [CrossRef]
- Jaquinod, L.; Siri, O.; Khoury, R.G.; Smith, K.M. Linear fused oligoporphyrins: Potential molecular wires with enhanced electronic communication between bridged metal ions. Chem. Commun. 1998, 1261–1262. [Google Scholar] [CrossRef]
- Mori, H.; Tanaka, T.; Osuka, A. Fused porphyrinoids as promising near-infrared absorbing dyes. J. Mater. Chem. C 2013, 1, 2500–2519. [Google Scholar] [CrossRef]
- Tanaka, T.; Osuka, A. Conjugated porphyrin arrays: Synthesis, properties and applications for functional materials. Chem. Soc. Rev. 2015, 44, 943–969. [Google Scholar] [CrossRef]
- Tsuda, A.; Osuka, A. Fully conjugated porphyrin tapes with electronic absorption bands that reach into infrared. Science 2001, 293, 79–82. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH: New York, NY, USA, 1993. [Google Scholar]
- Kobayashi, N.; Numao, N.; Kondo, R.; Nakajima, S.; Osa, T. A planar binuclear tetrabenzoporphyrin and its dicopper derivative. Inorg. Chem. 1991, 30, 2241–2244. [Google Scholar] [CrossRef]
- Ikeue, T.; Furukawa, K.; Hata, H.; Aratani, N.; Shinokubo, H.; Kato, T.; Osuka, A. The importance of a β−β bond for long-range antiferromagnetic coupling in directly linked copper(II) and silver(II) diporphyrins. Angew. Chem. Int. Ed. 2005, 44, 6899–6901. [Google Scholar] [CrossRef]
- Toganoh, M.; Furuta, H. Blooming of confused porphyrinoids- fusion, expansion, contraction and more confusion. Chem. Commun. 2012, 48, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Brugh, A.M.; Rawson, J.; Therien, M.J.; Forbes, M.D.E. Alkyne-bridged multi[copper(II) porphyrin] structures: Nuances of orbital symmetry in long-range, through-bond mediated, isotropic spin exchange interactions. J. Am. Chem. Soc. 2017, 139, 9759–9762. [Google Scholar] [CrossRef]
- Wang, R.; Ko, C.-H.; Brugh, A.M.; Bai, Y.; Forbes, M.D.E.; Therien, M.J. Topology, distance, and orbital symmetry effects on electronic spin-spin couplings in rigid molecular systems: Implications for long-distance spin-spin interactions. J. Phys. Chem. A 2020, 124, 7411–7415. [Google Scholar] [CrossRef]
- Brugh, A.M.; Wang, R.; Therien, M.J.; Forbes, M.D.E. Spinning molecules, spinning spins: Modulation of an electron spin exchange interaction in a highly anisotropic hyperfine field. ACS Omega 2021, 6, 27865–27873. [Google Scholar] [CrossRef] [PubMed]
- Richert, S.; Kuprov, I.; Peeks, M.D.; Suturina, E.A.; Cremers, J.; Anderson, H.L.; Timmel, C.R. Quantifying the exchange coupling in linear copper porphyrin oligomers. Phys. Chem. Chem. Phys. 2017, 19, 16057–16061. [Google Scholar] [CrossRef]
- Leznoff, C.C.; Lam, H.; Marcuccio, S.M.; Nevin, W.A.; Janda, P.; Kobayashi, N.; Lever, A.B.P. A planar binuclear phthalocyanine and its dicobalt derivatives. J. Chem. Soc. Chem. Commun. 1987, 699–701. [Google Scholar] [CrossRef]
- Lelièvre, D.; Bosio, L.; Simon, J.; André, J.-J.; Bensebaa, F. Dimeric substituted copper phthalocyanine liquid crystals. Synthesis, characterization and magnetic properties. J. Am. Chem. Soc. 1992, 114, 4475–4479. [Google Scholar] [CrossRef]
- Zhao, M.; Stern, C.; Barrett, A.G.M.; Hoffman, B.M. Porphyrazines as molecular scaffolds: Periphery-core spin coupling between metal ions of a Schiff base porphyrazine. Angew. Chem. Int. Ed. 2003, 42, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Matano, Y.; Fujii, D.; Shibano, T.; Furukawa, K.; Higashino, T.; Nakano, H.; Imahori, H. Covalently linked 5,15-diazaporphyrin dimers: Promising scaffolds for a highly conjugated azaporphyrin π system. Chem. Eur. J. 2014, 20, 3342–3349. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, P.J. Lucky seven: Characterization of stable T- shaped copper(II) complexes of [32]heptaphyrins. Angew. Chem. Int. Ed. 2010, 49, 1359–1361. [Google Scholar] [CrossRef]
- Gaita-Arino, A.; Luis, F.; Hill, S.; Coronado, E. Molecular spins for quantum computation. Nat. Chem. 2019, 11, 301–309. [Google Scholar] [CrossRef]
- Atzori, M.; Tesi, L.; Morra, E.; Chiesa, M.; Sorace, L.; Sessoli, R. Room-temperature quantum coherence and Rabi oscillations in vanadyl phthalocyanine: Toward multifunctional molecular spin qubits. J. Am. Chem. Soc. 2016, 138, 2154–2157. [Google Scholar] [CrossRef]
- Von Kugelgen, S.; Krzyaniak, M.D.; Gu, M.; Puggioni, D.; Rondinelli, J.M.; Wasielewski, M.R.; Freedman, D.E. Spectral Addressability in a Modular Two Qubit System. J. Am. Chem. Soc. 2021, 143, 8069–8077. [Google Scholar] [CrossRef]
- Ranieri, D.; Santanni, F.; Privitera, A.; Albino, A.; Salvadori, E.; Chiesa, M.; Totti, F.; Sorace, L.; Sessoli, R. An exchange coupled meso-meso linked vanadyl porphyrin dimer for quantum information processing. Chem. Sci. 2023, 14, 61–69. [Google Scholar] [CrossRef]
- Pozo, I.; Huang, Z.; Lombardi, F.; Alexandropoulos, D.I.; Kong, F.; Slota, M.; Tkach, I.; Bennati, M.; Deng, J.-R.; Stawski, W.; et al. Enhanced coherence by coupling spins through a delocalized-system: Vanadyl porphyrin dimers. Chem 2024, 10, 299–316. [Google Scholar] [CrossRef]
- Ranieri, D.; Privitera, A.; Santanni, F.; Urbanska, K.; Strachan, G.J.; Twanley, B.; Salvadori, E.; Liao, Y.-K.; Chiesa, M.; Senge, M.O.; et al. A heterometallic porphyrin dimer as a potential quantum gate: Magneto-structural correlations and spin coherence properties. Angew. Chem. Int. Ed. 2023, 62, e202312936. [Google Scholar] [CrossRef]
- Richeter, S.; Jeandon, C.; Gisselbrecht, J.-P.; Ruppert, R.; Callot, H.J. Syntheses and optical and electrochemical properties of porphyrin dimers linked by metal ions. J. Am. Chem. Soc. 2002, 124, 6168–6179. [Google Scholar] [CrossRef] [PubMed]
- Wytko, J.A.; Ruppert, R.; Jeandon, C.; Weiss, J. Metal-mediated linear self-assembly of porphyrins. Chem. Commun. 2018, 54, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.-A.; Dekkiche, H.; Karmazin, L.; Sanchez, F.; Vincent, B.; Kanesato, M.; Kikkawa, Y.; Ruppert, R. Synthesis and study at a solid/liquid interface of porphyrin dimers linked by metal ions. Inorg. Chem. 2017, 56, 15081–15090. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.-A.; Dekkiche, H.; Nagasaki, M.; Kikkawa, Y.; Ruppert, R. Coordination-driven construction of porphyrin nanorib- bons at a highly oriented pyrolytic graphite (HOPG)/liquid interface. J. Am. Chem. Soc. 2019, 141, 10137–10141. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.-A.; Dekkiche, H.; Richeter, S.; Bailly, C.; Karmazin, L.; McKearney, D.; Leznoff, D.B.; Rogez, G.; Vileno, B.; Choua, S.; et al. Antiferromagnetic coupling in copper(II)- porphyrin dimers linked by copper(II) or palladium(II) ion. J. Porphyr. Phthalocyanines 2020, 24, 238–246. [Google Scholar] [CrossRef]
- Appleton, J.L.; Le Breton, N.; Carvalho, M.-A.; Weiss, J.; Boudalis, A.K.; Gourlaouen, C.; Choua, S.; Ruppert, R. Vanadyl(IV) porphyrin dimers with palladium(II) and platinum(II) linkages: Syntheses, electronic properties, and magnetic interactions between the two moieties. Cryst. Growth Des. 2023, 23, 1689–1696. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Schreiman, I.C.; Hsu, H.C.; Kearney, P.C.; Marguerettaz, A.M. Rothemund and Adler-Longo reactions revisited: Synthesis of tetraphenylporphyrins under equilibrium conditions. J. Org. Chem. 1987, 52, 827–836. [Google Scholar] [CrossRef]
- Dekkiche, H.; Carvalho, M.-A.; Jeandon, C.; Karmazin, L.; Boudon, C.; Ruhlmann, L.; Ruppert, R. Synthesis and electrochemistry of nickel(II)porphyrins bearing external palladium(II) or platinum(II) complexes. J. Porphyr. Phthalocyanines 2021, 25, 1133–1142. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Laurenzo, K.S. Direct amination of nitrobenzenes by vicarious nucleophilic substitution. J. Org. Chem. 1986, 51, 5039–5040. [Google Scholar] [CrossRef]
- Pedersen, B.S.; Scheibye, S.; Nilsson, N.H.; Lawesson, S.-O. Studies on organophosphorus compounds XX. Syntheses of thioketones. Bull. Soc. Chim. Belg. 1978, 87, 223–228. [Google Scholar] [CrossRef]
- Scheibye, S.; Pedersen, B.S.; Lawesson, S.-O. Studies on organophosphorus compounds XXI. The dimer of p-methoxyphenylthionophosphine sulfide as thiation reagent. A new route to thiocarboxamides. Bull. Soc. Chim. Belg. 1978, 87, 229–238. [Google Scholar] [CrossRef]
- Battersby, A.R. Tetrapyrroles: The pigments of life. Nat. Prod. Rep. 2000, 17, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.L. Building molecular wires from the colours of life: Conjugated porphyrin oligomers. Chem. Commun. 1999, 2323–2330. [Google Scholar] [CrossRef]
- Kadish, K.M.; Royal, G.; Van Caemelbecke, E.; Gueletti, L. The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 9, pp. 1–219. [Google Scholar]
- Kadish, K.M.; Van Caemelbecke, E.; Royal, G. The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 8, pp. 1–114. [Google Scholar]
- Lin, V.S.-Y.; Therien, M.J. The role of porphyrin-to-porphyrin linkage topology in the extensive modulation of the absorptive and emissive properties of a series of ethynyl- and butadiynyl-bridged bis- and tris(porphinato)zinc chromophores. Chem. Eur. J. 1995, 1, 645–651. [Google Scholar] [CrossRef]
- Tsuda, A.; Furuta, H.; Osuka, A. Syntheses, structural characterizations, and optical and electrochemical properties of directly fused diporphyrins. J. Am. Chem. Soc. 2001, 123, 10304–10321. [Google Scholar] [CrossRef] [PubMed]
- Dekkiche, H.; Buisson, A.; Langlois, A.; Karsenti, P.-L.; Ruhlmann, L.; Harvey, P.D.; Ruppert, R. Ultrafast singlet energy transfer in porphyrin dyads. Inorg. Chem. 2016, 55, 10329–10336. [Google Scholar] [CrossRef]
- Senge, M.O. X-ray data of porphyrins, see for example. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000; Volume 10, pp. 1–218. [Google Scholar]
- Zhao, M.; Zhong, C.; Stern, C.; Barrett, A.G.M.; Hoffman, B.M. Synthesis and Magnetic Properties Comparison of M-Cu(II) and M-VO(II) Schiff Base-Porphyrazine Complexes: What Is the Mechanism for Spin-Coupling? J. Am. Chem. Soc. 2005, 127, 9769–9775. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Field, J.E.; Hill, T.J.; Venkataraman, D. Bridged Triarylamines: A New Class of Heterohelicenes. J. Org. Chem. 2003, 68, 6071–6078. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Hiroto, S.; Lee, S.; Son, M.; Hisaki, I.; Yoshida, T.; Kim, D.; Kobayashi, N.; Shinokubo, H. Synthesis of highly twisted and fully p-conjugated porphyrinic oligomers. J. Am. Chem. Soc. 2015, 137, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Osuka, A.; Nakajima, S.; Maruyama, K. Synthesis of a 1,2-phenylene-bridged triporphyrin. J. Org. Chem. 1992, 57, 7355–7359. [Google Scholar] [CrossRef]




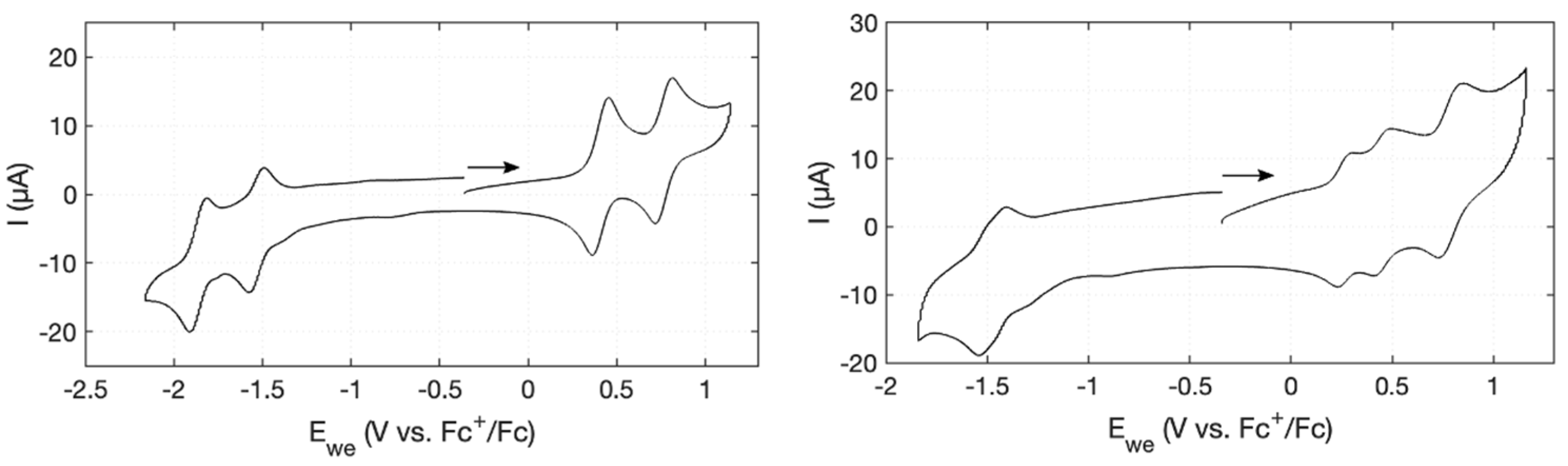
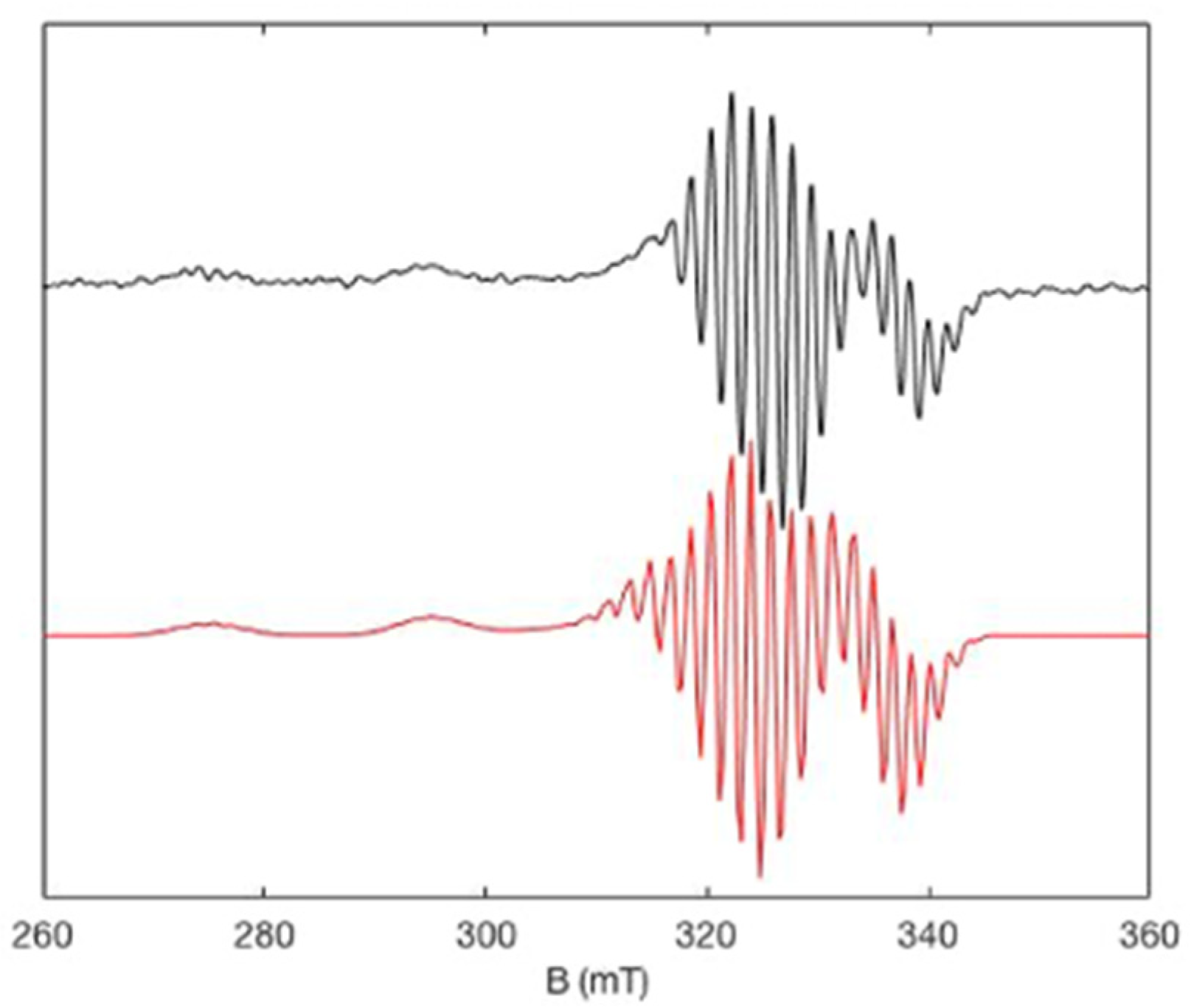


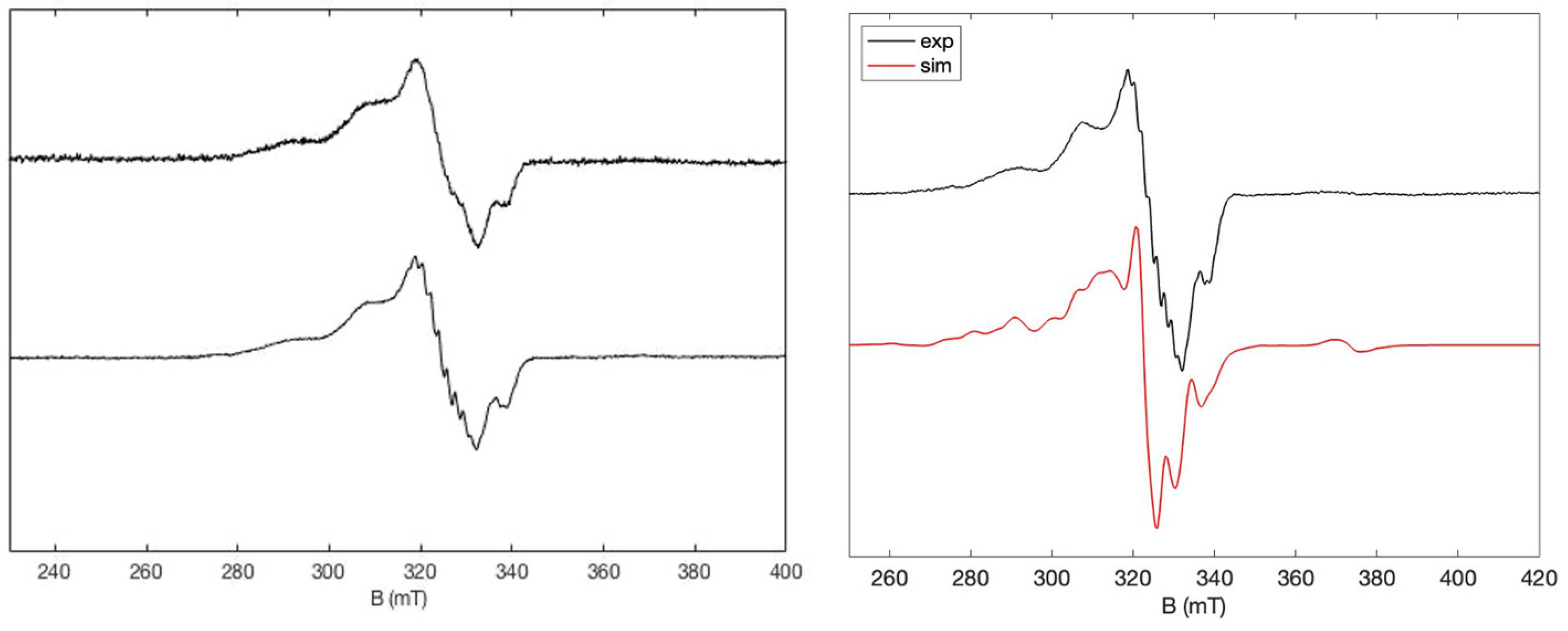
| Compound | EOx1 (Volt) | EOx2 (Volt) | EOx3 (Volt) | EOx2–EOx1 (mV) |
|---|---|---|---|---|
| 6 | 0.47 | 0.82 | - | - |
| 8 | 0.29 | 0.43 | 0.77 | 140 |
| 10 | 0.28 | 0.49 | 0.85 | 209 |
| 7 | - | - | - | - |
| 9 | 0.26 | 0.50 | 0.83 | 237 |
| 11 | 0.23 | 0.54 | 0.90 | 310 |
| Compound 6 | Dimer 8 PdNO | Dimer 10 PtNO | Dimer 9 PdNS | Dimer 11 PtNS | |
|---|---|---|---|---|---|
| gx (g strain) | 2.061 (0.003) | 2.061 (0.04) | 2.061 (0.04) | 2.061 (0.03) | 2.061 (0.03) |
| gy (g strain) | 2.051 (0.0075) | 2.061 (0.04) | 2.061 (0.04) | 2.061 (0.03) | 2.061 (0.03) |
| gz (g strain) | 2.180 (0.001) | 2.180 (0.07) | 2.180 (0.07) | 2.180 (0.05) | 2.180 (0.05) |
| ACu (MHz) | [135; 125; 600] | [130; 130; 600] | [130; 130; 600] | [130; 130; 600] | [130; 130; 600] |
| AN (MHz) | 49 | - | - | - | - |
| |J| (MHz) | - | 700 ± 50 | 700 ± 50 | 1500 ± 200 | 1500 ± 200 |
| D (MHz) | - | 42 ± 5 | 42 ± 5 | 60 ± 5 | 60 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appleton, J.L.; Le Breton, N.; Choua, S.; Ruppert, R. The Effect of the Linking Unit on the Electronic and Magnetic Interactions in Copper(II) Porphyrin Dimers Linked by Metal Ions. Inorganics 2024, 12, 44. https://doi.org/10.3390/inorganics12020044
Appleton JL, Le Breton N, Choua S, Ruppert R. The Effect of the Linking Unit on the Electronic and Magnetic Interactions in Copper(II) Porphyrin Dimers Linked by Metal Ions. Inorganics. 2024; 12(2):44. https://doi.org/10.3390/inorganics12020044
Chicago/Turabian StyleAppleton, Jordan L., Nolwenn Le Breton, Sylvie Choua, and Romain Ruppert. 2024. "The Effect of the Linking Unit on the Electronic and Magnetic Interactions in Copper(II) Porphyrin Dimers Linked by Metal Ions" Inorganics 12, no. 2: 44. https://doi.org/10.3390/inorganics12020044
APA StyleAppleton, J. L., Le Breton, N., Choua, S., & Ruppert, R. (2024). The Effect of the Linking Unit on the Electronic and Magnetic Interactions in Copper(II) Porphyrin Dimers Linked by Metal Ions. Inorganics, 12(2), 44. https://doi.org/10.3390/inorganics12020044