Encapsulating Ultrafine In2O3 Particles in Carbon Nanofiber Framework as Superior Electrode for Lithium-Ion Batteries
Abstract
1. Introduction
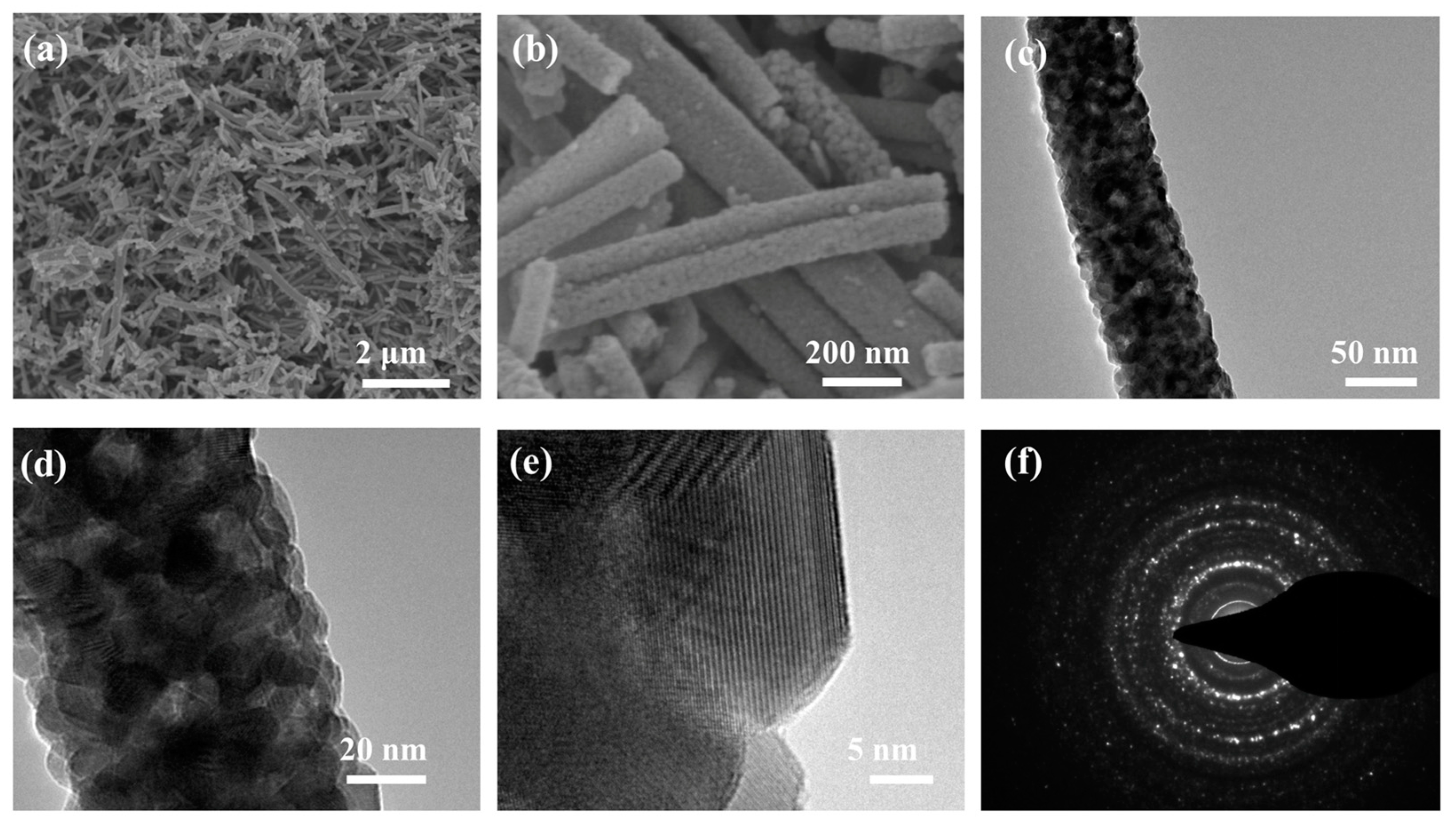
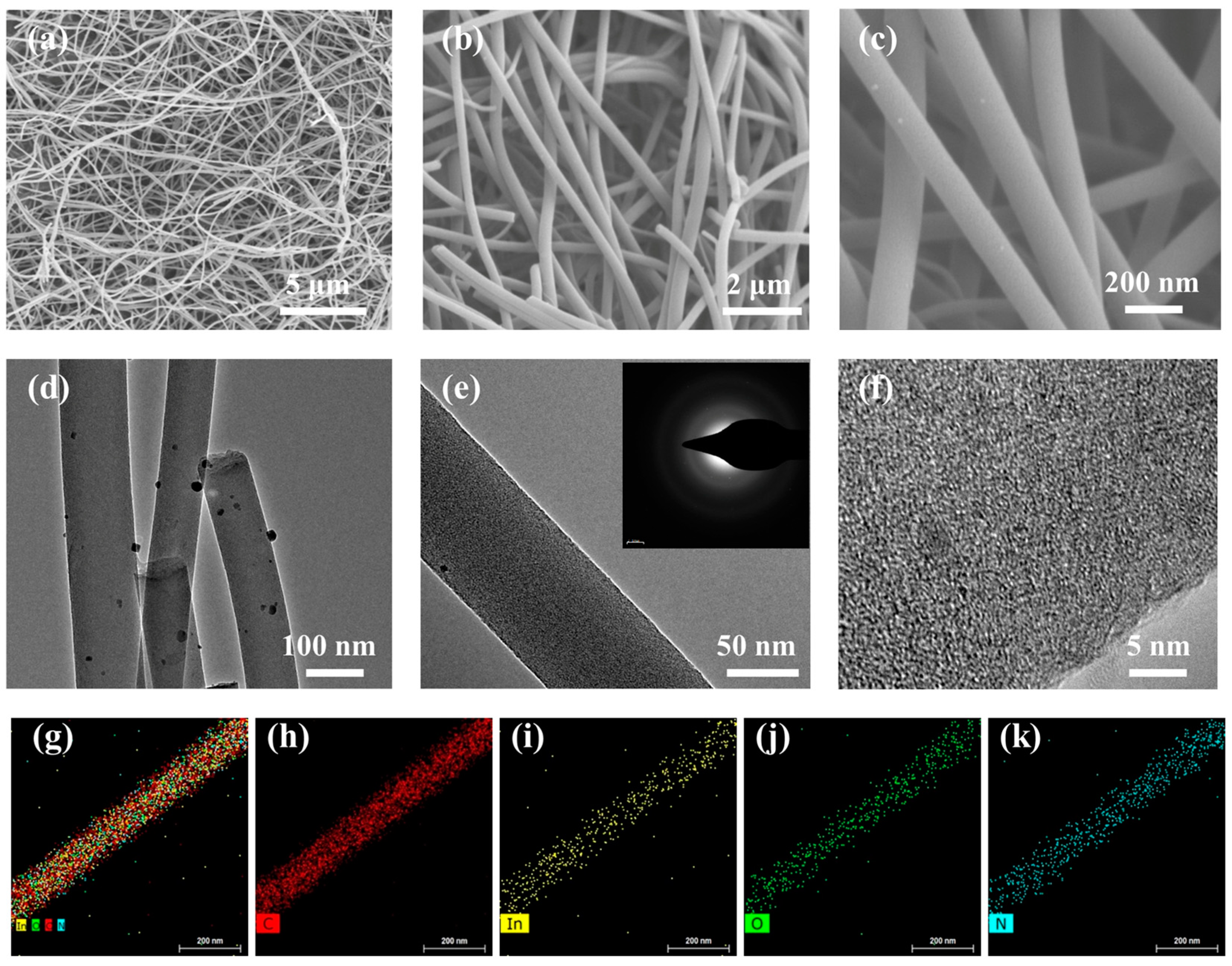
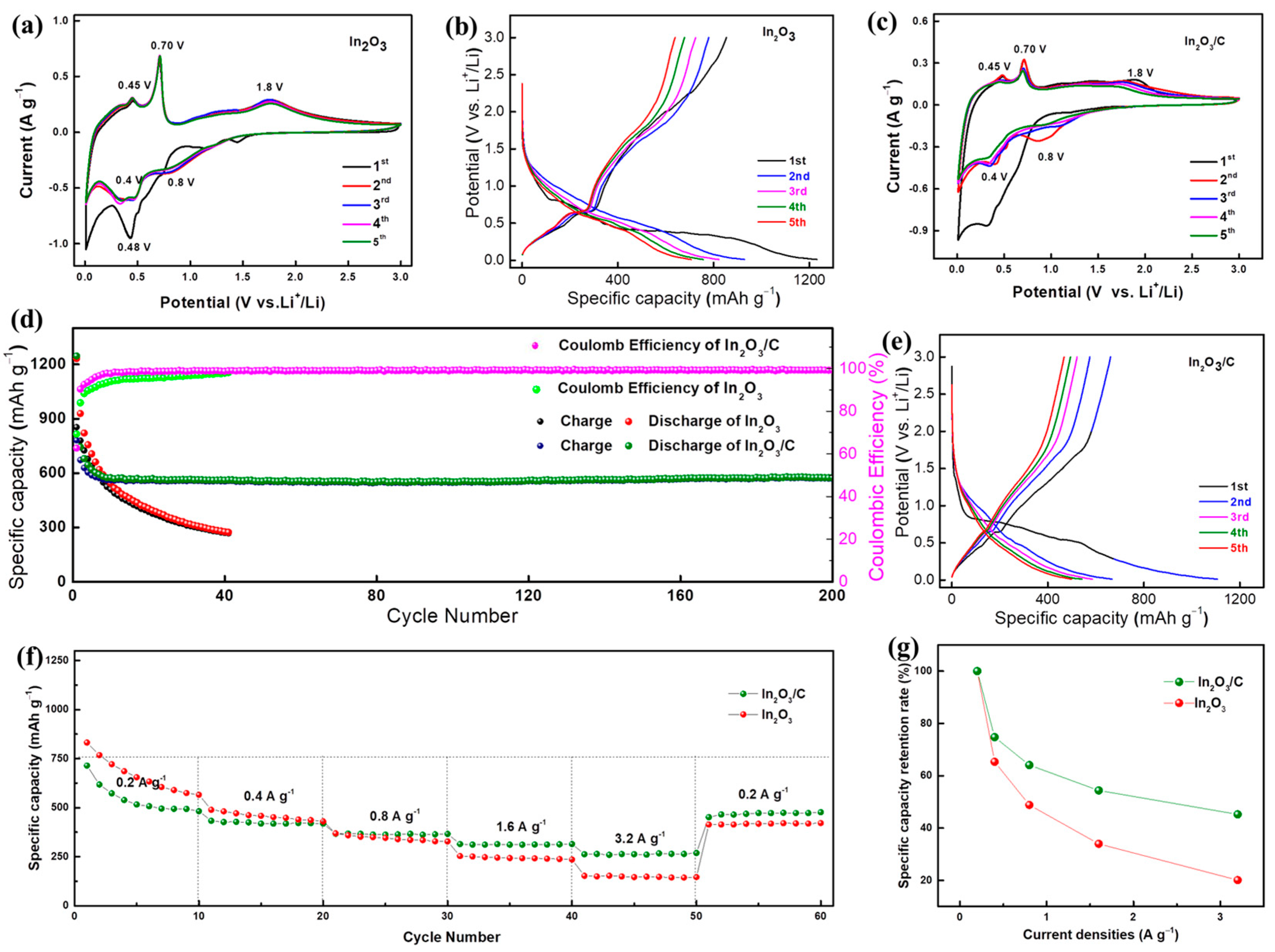
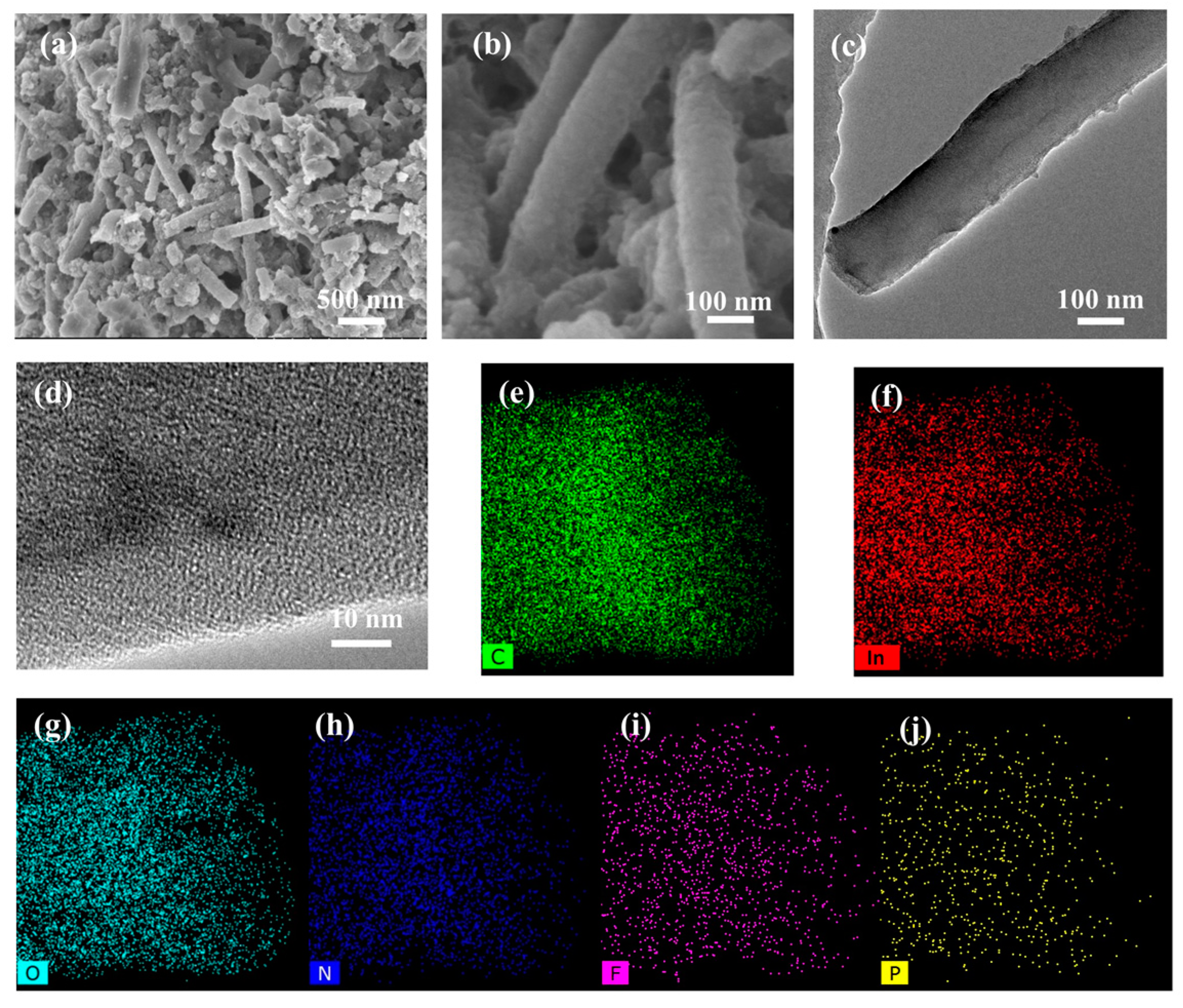
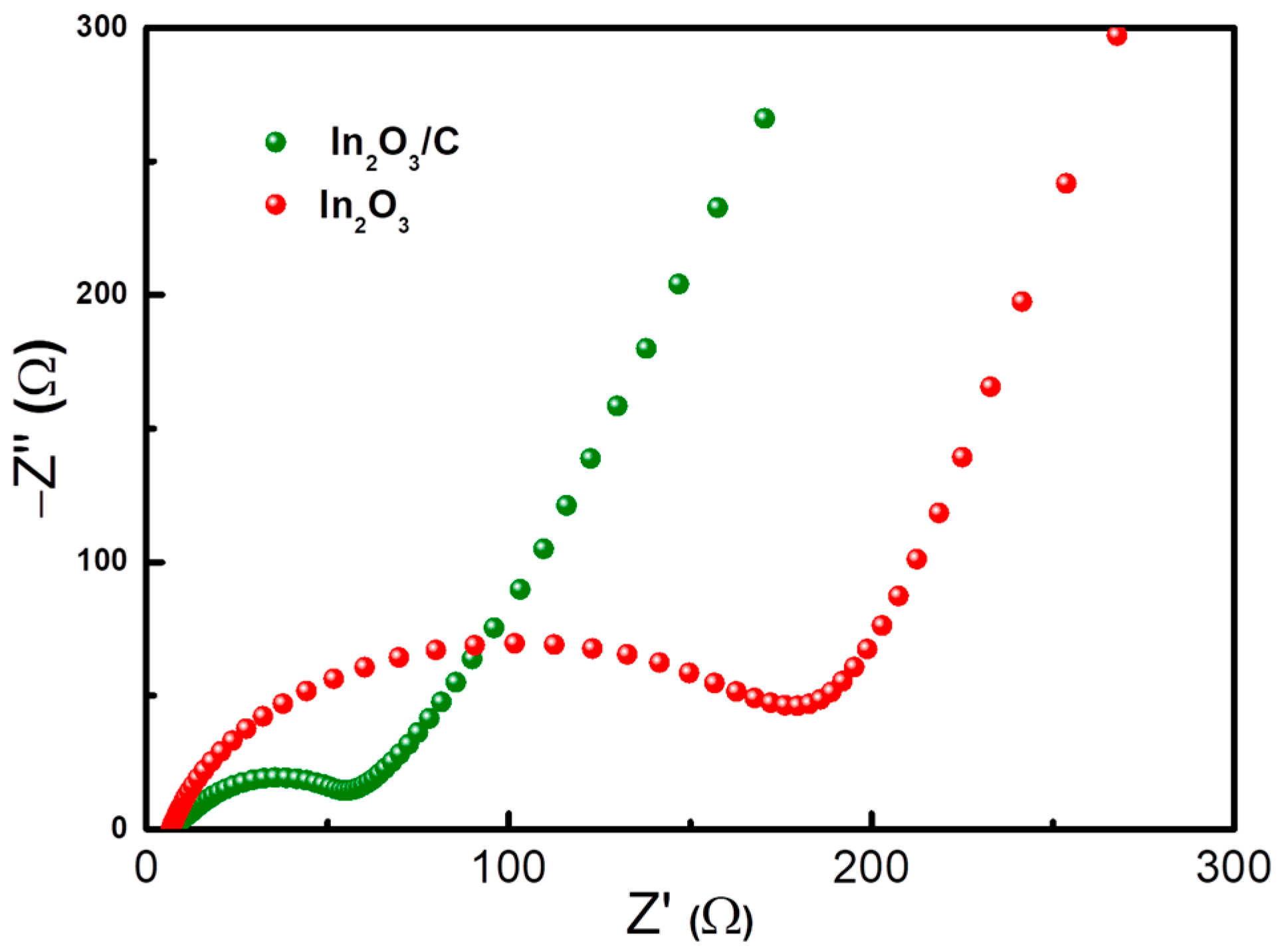
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of Sample
3.2. Material Characterization
3.3. Cell Preparation and Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Sun, X.; Yang, J.; Chen, Y.; Luo, F. Interconnected MoO2/MoS2@NC nanosheets as anodes with high-rate and long-life for lithium-ion and sodium-ion batteries. Chem. Eng. J. 2024, 495, 153418. [Google Scholar] [CrossRef]
- Sun, X.; Jing, M.; Dong, H.; Xie, W.; Luo, F. CuO-ZnO submicroflakes with nanolayered Al2O3 coatings as high performance anode materials in lithium-ion batteries. J. Alloys Compd. 2023, 953, 170137. [Google Scholar] [CrossRef]
- Kulova, T.L.; Fateev, V.N.; Seregina, E.A.; Grigoriev, A.S. A Brief Review of Post-Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2020, 15, 7242–7259. [Google Scholar] [CrossRef]
- Bai, X.; Li, D.; Zhang, D.; Yang, S.; Pei, C.; Sun, B.; Ni, S. Boosting high-rate lithium storage in Li3VO4 via a honeycomb structure design and electrochemical reconstruction. J. Mater. Chem. A 2023, 11, 12164–12175. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, C.; Wu, S.; Wang, A.; Zhao, L.; Zhai, D.; Ren, B.; Cao, K.; Zhou, Z. Exploiting Synergistic Effect by Integrating Ruthenium–Copper Nanoparticles Highly Co-Dispersed on Graphene as Efficient Air Cathodes for Li–CO2 Batteries. Adv. Energy Mater. 2019, 9, 1802805. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, B.; Peng, Y.; Li, Q.; Chen, M.; Liu, X.; Wang, Z.; Yang, J.; Chen, J.; Gu, D. Dual-confined growth of mesoporous molybdenum nitride@carbon arrays for fast pseudocapacitive lithium storage. J. Alloys Compd. 2024, 1003, 175521. [Google Scholar] [CrossRef]
- Manthiram, A.; Goodenough, J.B. Lithium-based polyanion oxide cathodes. Nat. Energy 2021, 6, 844–845. [Google Scholar] [CrossRef]
- Du, J.; Zhang, C.; Li, S.; Zhang, L.; Zhang, W. Two-stage prediction method for capacity aging trajectories of lithium-ion batteries based on Siamese-convolutional neural network. Energy 2024, 295, 130947. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Zhao, C.-Z.; Huang, J.-Q.; Zhang, Q. Recent Advances in Energy Chemical Engineering of Next-Generation Lithium Batteries. Engineering 2018, 4, 831–847. [Google Scholar] [CrossRef]
- Gao, S.; Tang, Y.; Zhao, H.; Liu, L.; Gu, Y.; Sheng, R. MoO/C hybrid synthesized by a facile molten-salt-assisted approach for high-performance lithium-ion batteries. Int. J. Energy Res. 2021, 45, 6418–6425. [Google Scholar] [CrossRef]
- Chen, C.; Hu, Q.; Xue, H.; Li, H.; Li, W.; Cao, S.; Peng, T.; Yang, Y.; Luo, Y. Ultrafast and ultrastable FeSe2 embedded in nitrogen-doped carbon nanofibers anode for sodium-ion half/full batteries. Nanotechnology 2024, 35, 055404. [Google Scholar] [CrossRef] [PubMed]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Gao, S.; Liu, L.; Mao, F.; Zhang, Z.; Pan, K.; Zhou, Z. Coal-based ultrathin N-doped carbon nanosheets synthesized by molten-salt method for high-performance lithium-ion batteries. Nanotechnology 2022, 33, 425401. [Google Scholar] [CrossRef]
- Becker, D.; Haberkorn, R.; Kickelbick, G. Mechanochemical Induced Structure Transformations in Lithium Titanates: A Detailed PXRD and 6Li MAS NMR Study. Inorganics 2018, 6, 117. [Google Scholar] [CrossRef]
- Opra, D.P.; Sokolov, A.A.; Sinebryukhov, S.L.; Tkachenko, I.A.; Ziatdinov, A.M.; Gnedenkov, S.V. Electronic Structure, Optical and Magnetic Properties of Oxygen-Deficient Gray TiO2–δ(B). Inorganics 2022, 10, 184. [Google Scholar] [CrossRef]
- Opra, D.P.; Sinebryukhov, S.L.; Modin, E.B.; Sokolov, A.A.; Podgorbunsky, A.B.; Ziatdinov, A.M.; Ustinov, A.Y.; Mayorov, V.Y.; Gnedenkov, S.V. Manganese, Fluorine, and Nitrogen Co-Doped Bronze Titanium Dioxide Nanotubes with Improved Lithium-Ion Storage Properties. Batteries 2023, 9, 229. [Google Scholar] [CrossRef]
- Chen, M.; Liu, F.-M.; Zhao, H.; Chen, S.-S.; Qian, X.; Yuan, Z.-Y.; Wan, R. In situ encapsulation of iron oxide nanoparticles into nitrogen-doped carbon nanotubes as anodic electrode materials of lithium ion batteries. Phys. Chem. Chem. Phys. 2022, 24, 27114–27120. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, M.-Y.; Liu, F.-M.; Li, M.-T.; Zhang, M.-L.; Qian, X.; Yuan, Z.-Y.; Li, C.-S.; Wan, R. Self-Catalyzed Synthesis of Length-Controlled One-Dimensional Nickel Oxide@N-Doped Porous Carbon Nanostructures from Metal Ion Modified Nitrogen Heterocycles for Efficient Lithium Storage. Langmuir 2024, 40, 4852–4859. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, D.; Bai, Z.; Yang, Y.; Wang, Y.; Cheng, J.; Chu, P.K.; Luo, Y. MXene nanofibers confining MnOx nanoparticles: A flexible anode for high-speed lithium ion storage networks. Dalton Tran. 2022, 51, 1423–1433. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, D.; Yang, Y.; Wang, Y.; Bai, Z.; Chu, P.K.; Luo, Y. MXene-encapsulated hollow Fe3O4 nanochains embedded in N-doped carbon nanofibers with dual electronic pathways as flexible anodes for high-performance Li-ion batteries. Nanoscale 2021, 13, 4624–4633. [Google Scholar] [CrossRef]
- Cao, K.; Jia, Y.; Wang, S.; Huang, K.-J.; Liu, H. Mn3O4 nanoparticles anchored on carbon nanotubes as anode material with enhanced lithium storage. J. Alloys Compd. 2021, 854, 157179. [Google Scholar] [CrossRef]
- Cao, K.; Zheng, R.; Wang, S.; Shu, J.; Liu, X.; Liu, H.; Huang, K.-J.; Jing, Q.-S.; Jiao, L. Boosting Coulombic Efficiency of Conversion-Reaction Anodes for Potassium-Ion Batteries via Confinement Effect. Adv. Funct. Mater. 2020, 30, 2007712. [Google Scholar] [CrossRef]
- Sun, X.; Chen, Y.; Luo, F. Nanoporous ALD-modified oxygen-deficient NiO flakes as anodes for lithium-ion batteries. Ceram. Int. 2024, 50, 3480–3490. [Google Scholar] [CrossRef]
- Li, C.; Ke, S.; Liu, S.; Wu, G.; Li, Q.; Zhang, Y.; Cao, K. Heterostructured Mn–Sn Bimetallic Sulfide Nanocubes Confined in N, S-co-Doped Carbon Framework as High-Performance Anodes for Sodium-Ion Batteries. Langmuir 2024, 40, 15815–15823. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, F.-M.; Chen, S.-S.; Qian, X.; Zhao, Y.-J.; Sun, Y.; Li, C.-S.; Wan, R.; Yuan, Z.-Y. Low-temperature metal-catalyzed synthesis of encapsulated metal oxide nanoparticles in nitrogen-doped carbon nanotubes from carbon nitride as anodic materials of high-performance lithium-ion batteries. New J. Chem. 2023, 47, 3215–3221. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Zhang, D.; Wang, Y.; Luo, X.; Liu, X.; Kim, J.-K.; Luo, Y. Inter-overlapped MoS2/C composites with large-interlayer-spacing for high-performance sodium-ion batteries. Nanoscale Horiz. 2020, 5, 1127–1135. [Google Scholar] [CrossRef]
- Liu, H.; He, Y.; Zhang, H.; Cao, K.; Wang, S.; Jiang, Y.; Jing, Q.-S.; Jiao, L. Lowering the voltage-hysteresis of CuS anode for Li-ion batteries via constructing heterostructure. Chem. Eng. J. 2021, 425, 130548. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Q.; Dong, Z.; Wang, L.; Xie, X.; Jiang, Y.; Wei, Z.; Gao, Y.; Zhang, Y.; Huang, K. Interconnected MoS2 on 2D Graphdiyne for Reversible Sodium Storage. ACS Appl. Mater. Interfaces 2021, 13, 54974–54980. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Wang, Y.; Zhang, D.; Lu, Y.; Luo, R.; Wang, Y.; Liu, X.; Kim, J.-K.; Luo, Y. Vertically aligned ultrathin MoS2 nanosheets grown on graphene-wrapped hollow carbon microtubes derived from loofah sponge as advanced anodes for highly reversible lithium storage. Electrochim. Acta 2019, 296, 989–998. [Google Scholar] [CrossRef]
- Liu, H.; He, Y.; Zhang, H.; Cao, K.; Wang, S.; Jiang, Y.; Jing, Q.-S.; Jiao, L. Bi-continuous ion/electron transfer avenues enhancing the rate capability of SnS2 anode for potassium-ion batteries. J. Power Sources 2021, 506, 230160. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Yang, Y.; Guo, Y.; Bai, Z.; Chu, P.K.; Luo, Y. Three-dimensional nano/micro-structured porous MoP/CNTs microspheres as high-capacity anode for lithium-ion batteries. J. Alloys Compd. 2021, 872, 159608. [Google Scholar] [CrossRef]
- Guo, W.; Mei, L.; Li, X.; Mao, M.; Ma, J. Electrospun In@C nanofibers as a superior Li-ion battery anode. RSC Adv. 2015, 5, 92522–92525. [Google Scholar] [CrossRef]
- Na, Z.; Yao, R.; Yan, Q.; Wang, X.; Sun, X. Metal-organic frameworks derived In-based nanoparticles encapsulated by carbonaceous matrix for highly efficient energy storage. Appl. Surf. Sci. 2020, 513, 145894. [Google Scholar] [CrossRef]
- Zeng, Q.; Dong, Y.; Chen, Y.; Yue, X.; Liang, Z. Recent advancements and perspectives of fast-charging composite anodes for lithium-ion batteries. Sci. Chin.-Chem. 2024, 67, 3952–3963. [Google Scholar] [CrossRef]
- Pendashteh, A.; Tomey, R.; Vilatela, J.J. Nanotextile 100% Si Anodes for the Next Generation Energy-Dense Li-ion Batteries. Adv. Energy Mater. 2024, 14, 2304018. [Google Scholar] [CrossRef]
- Du, X.; Zhang, H.; Lan, X.; Yuan, B.; Hu, R. Sn Alloy and Graphite Addition to Enhance Initial Coulombic Efficiency and Cycling Stability of SiO Anodes for Li-Ion Batteries. Energy Environ. Mater. 2022, 5, 353–359. [Google Scholar] [CrossRef]
- Pham, H.T.; Lee, D.-S.; Dao, T.D.; Jeong, H.-D. In2O3 nanocrystal–π conjugated molecule hybrid materials for high-capacity anode in lithium ion battery. J. Ind. Eng. Chem. 2018, 57, 22–27. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Shen, G.; Guo, Y.; Zhao, X.; Xia, Y.; Sun, H.; Hou, P.; Xie, W.; Xu, X. Large-scale carbon framework microbelts anchoring ultrafine SnO2 nanoparticles with enhanced lithium storage properties. Electrochim. Acta 2019, 297, 879–887. [Google Scholar] [CrossRef]
- Yang, H.; Song, T.; Lee, S.; Han, H.; Xia, F.; Devadoss, A.; Sigmund, W.; Paik, U. Tin indium oxide/graphene nanosheet nanocomposite as an anode material for lithium ion batteries with enhanced lithium storage capacity and rate capability. Electrochim. Acta 2013, 91, 275–281. [Google Scholar] [CrossRef]
- Zhao, H.; Yin, H.; Yu, X.-X.; Zhang, W.; Li, C.; Zhu, M.-Q. In2O3 nanoparticles/carbon fiber hybrid mat as free-standing anode for lithium-ion batteries with enhanced electrochemical performance. J. Alloys Compd. 2018, 735, 319–326. [Google Scholar] [CrossRef]
- Yue, L.; Pan, X.; Chen, S.; Song, J.; Liu, C.; Luo, G.; Guan, R.; Zhang, W. High lithium storage capacity achieved by regulating monodisperse C/In2O3 nanosheet composite with double phases. Mater. Chem. Phys. 2017, 193, 89–98. [Google Scholar] [CrossRef]
- Yue, L.; Zhang, W.; Zhang, W.; Zhang, Q.; Guan, R.; Hou, G.; Xu, N. One-step solvothermal process of In2O3/C nanosheet composite with double phases as high-performance lithium-ion battery anode. Electrochim. Acta 2015, 160, 123–130. [Google Scholar] [CrossRef]
- Xu, H.; Wang, L.; Zhong, J.; Wang, T.; Cao, J.; Wang, Y.; Li, X.; Fei, H.; Zhu, J.; Duan, X. Ultra-stable and High-rate Lithium Ion Batteries Based on Metal–organic Framework-derived In2O3 Nanocrystals/Hierarchically Porous Nitrogen-doped Carbon Anode. Energy Environ. Mater. 2020, 3, 177–185. [Google Scholar] [CrossRef]
- Kim, M.; Park, C.; Jung, W.; Hur, J. Enabling high capacity and reversible Li storage in indium (III) oxide anode surrounded by carbon nanotube matrix. J. Alloys Compd. 2024, 996, 174796. [Google Scholar] [CrossRef]
- Xue, Y.; Xu, T.; Guo, Y.; Song, H.; Wang, Y.; Guo, Z.; Li, J.; Zhao, H.; Bai, X.; Lai, C. Amorphous/crystalline heterostructured indium (III) sulfide/carbon with favorable kinetics and high capacity for lithium storage. Adv. Compos. Hybrid Mater. 2024, 7, 213. [Google Scholar] [CrossRef]
- Jin, L.; Zhao, X.; Qian, X.; Wang, S.; Shen, X.; Dong, M. Synthesis of porous In2O3 /carbon composites derived from metal-organic frameworks for high performance Li-ion batteries. Mater. Lett. 2017, 199, 176–179. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Gong, L.; Zhang, Z.; Liu, G.; Tan, P. Revealing the mechanism of stress rebound during discharging in lithium-ion batteries. J. Energy Storage 2023, 58, 106454. [Google Scholar] [CrossRef]
- Li, R.; Li, W.; Singh, A.; Ren, D.; Hou, Z.; Ouyang, M. Effect of external pressure and internal stress on battery performance and lifespan. Energy Storage Mater. 2022, 52, 395–429. [Google Scholar] [CrossRef]

| Electrode Description | Current Density (mA g−1 ) | Cycle Number | Reversible Capacity (mAh g−1 ) | High Rate Capability | Reference |
|---|---|---|---|---|---|
| In2O3/C composite nanofibers | 300 | 200 | 571 | 264 mAh g−1 at 3.2 A g−1 | This work |
| SnO2–In2O3/GNS | 60 | 50 | 969 | 263 mAh g−1 at 0.6 A g−1 | [40] |
| C/In2O3 nanosheets | 100 | 100 | 893 | 379 mAh g−1 at 2 A g−1 | [43] |
| In2O3/HPNC | 1000 | 2000 | 623 | 139 mAh g−1 at 20 A g−1 | [44] |
| In2O3/C fibers | 100 | 500 | 435 | 190 mAh g−1 at 1.5 A g−1 | [41] |
| In2O3 NC-EBA | 200 | 100 | 910 | 316 mAh g−1 at 20 A g−1 | [38] |
| In2O3/carbon | 100 | 150 | 720 | 140 mAh g−1 at 1.0 A g−1 | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.; An, Z.; Li, X.; Wang, Q.; Hu, C.; Ma, Y.; Liu, S.; Sun, H.; Sun, X. Encapsulating Ultrafine In2O3 Particles in Carbon Nanofiber Framework as Superior Electrode for Lithium-Ion Batteries. Inorganics 2024, 12, 336. https://doi.org/10.3390/inorganics12120336
Xie W, An Z, Li X, Wang Q, Hu C, Ma Y, Liu S, Sun H, Sun X. Encapsulating Ultrafine In2O3 Particles in Carbon Nanofiber Framework as Superior Electrode for Lithium-Ion Batteries. Inorganics. 2024; 12(12):336. https://doi.org/10.3390/inorganics12120336
Chicago/Turabian StyleXie, Wenhe, Zhe An, Xuefeng Li, Qian Wang, Chen Hu, Yuanxiao Ma, Shenghong Liu, Haibin Sun, and Xiaolei Sun. 2024. "Encapsulating Ultrafine In2O3 Particles in Carbon Nanofiber Framework as Superior Electrode for Lithium-Ion Batteries" Inorganics 12, no. 12: 336. https://doi.org/10.3390/inorganics12120336
APA StyleXie, W., An, Z., Li, X., Wang, Q., Hu, C., Ma, Y., Liu, S., Sun, H., & Sun, X. (2024). Encapsulating Ultrafine In2O3 Particles in Carbon Nanofiber Framework as Superior Electrode for Lithium-Ion Batteries. Inorganics, 12(12), 336. https://doi.org/10.3390/inorganics12120336






