Emerging Carbon-Based Catalysts for the Oxygen Reduction Reaction: Insights into Mechanisms and Applications
Abstract
1. Introduction
2. The Mechanisms and Influencing Factors for ORR
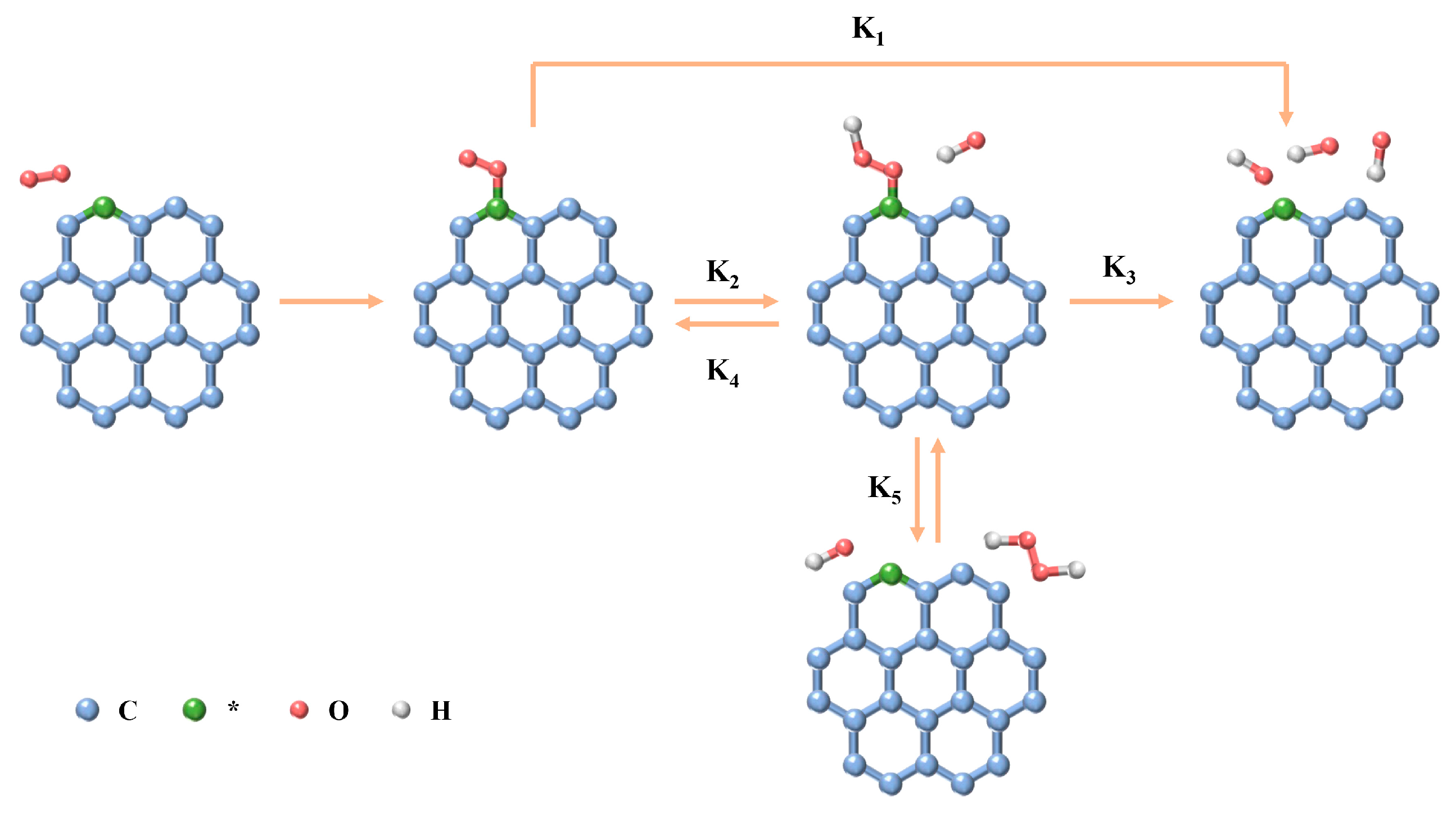
2.1. Mechanisms for ORR
2.2. Influencing Factors for ORR
2.3. Key Metrics for ORR
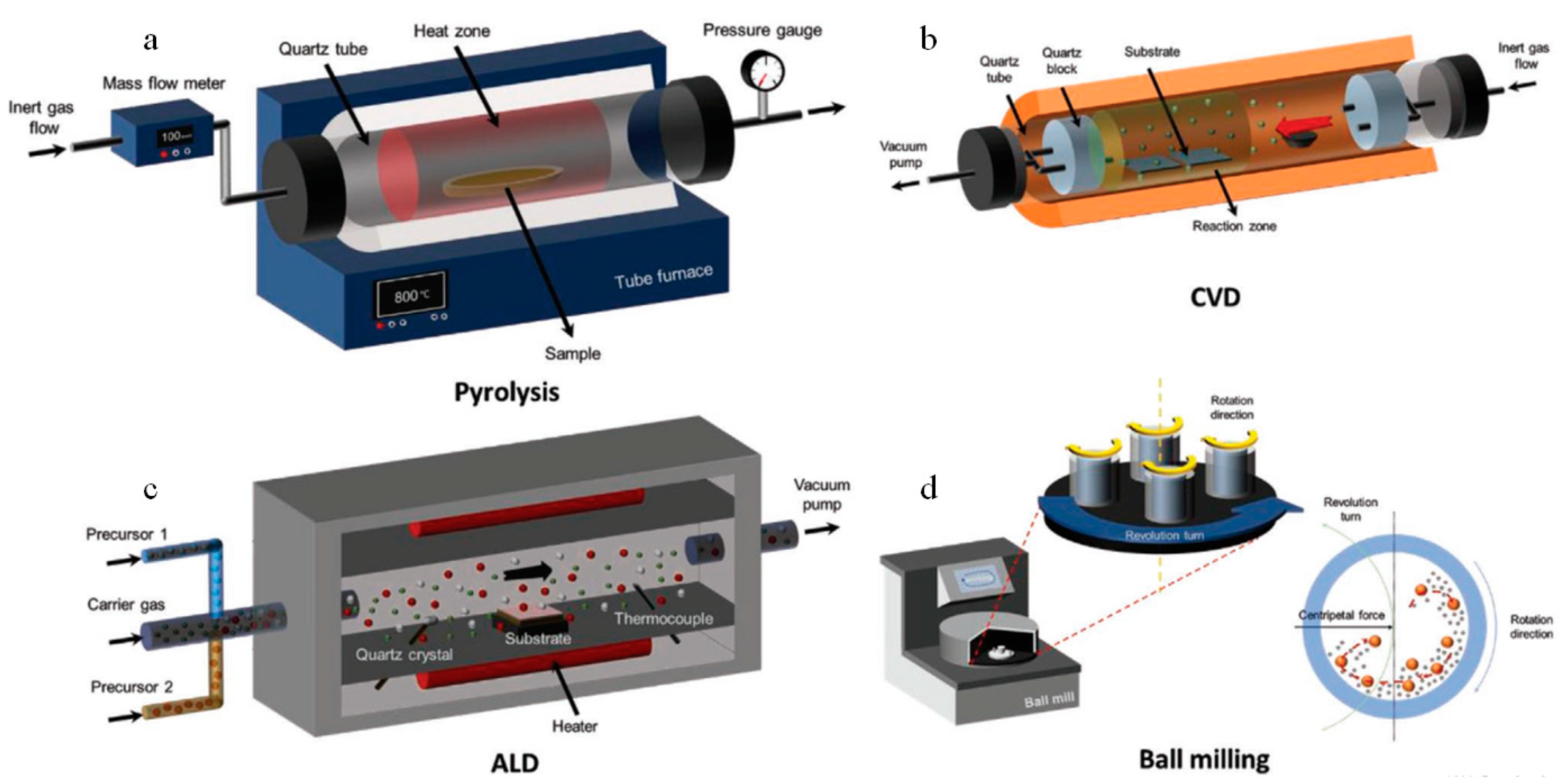
3. Synthesis Methods of Carbon-Based Catalysts
3.1. Pyrolysis
3.2. Deposition
3.3. Ball Milling
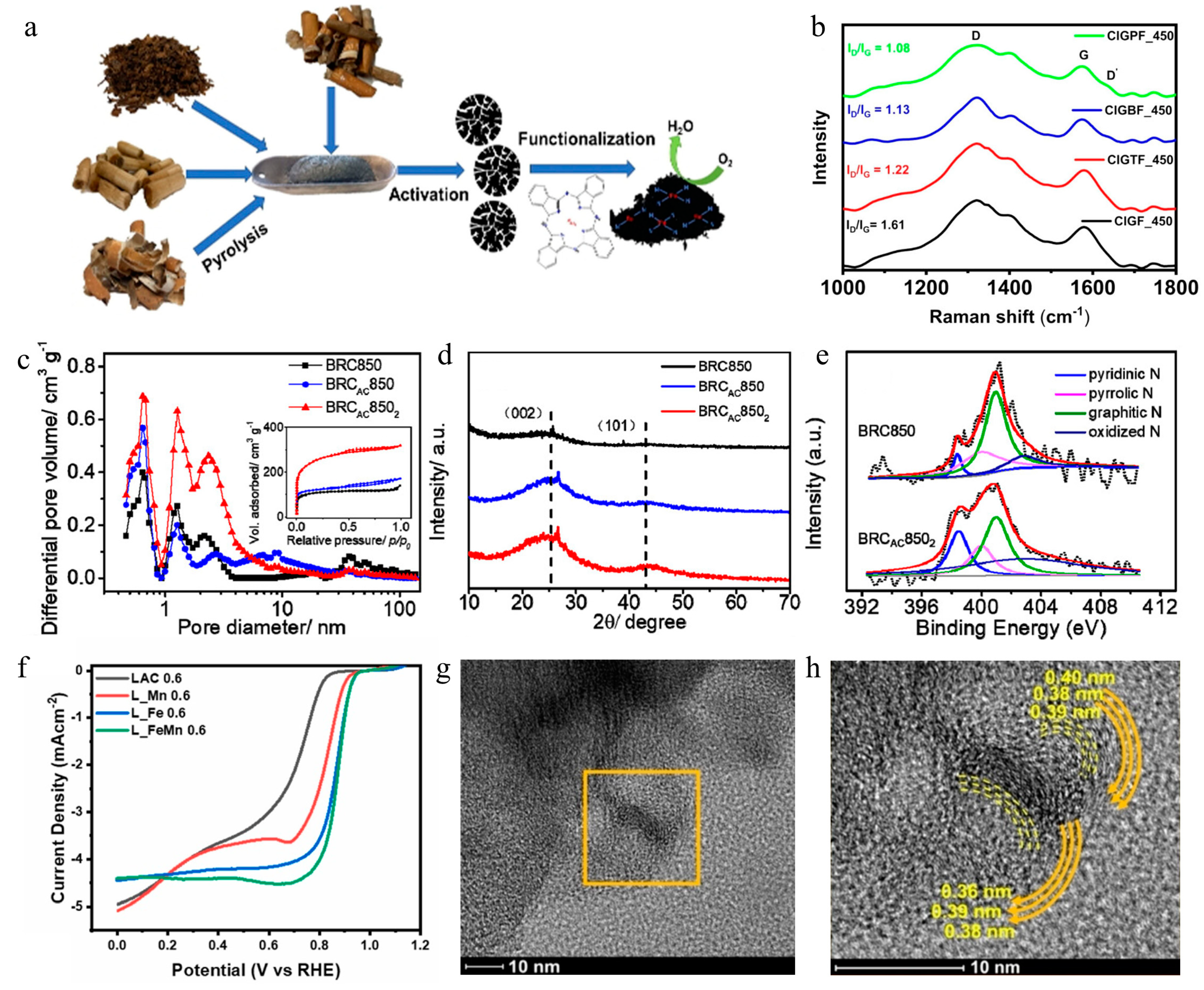
3.4. Waste-Derived Carbon
| Catalysts | EOnset | E1/2 | pH | Methods | Reference |
|---|---|---|---|---|---|
| FeSA/AC@HNC | 0.88 V | 0.78 V 0.90 V | 0.5 M H2SO4 0.1 M KOH | Pyrolysis | [73] |
| Zr-N/O-C | 1.000 V | 0.910 V | 0.1 M KOH | Pyrolysis | [74] |
| MnNC-CVD | ~0.9 V | — | 0.5 M H2SO4 | CVD | [79] |
| N-DC/G | 0.78 V | — | 0.1 M KOH | waste-derived carbon | [94] |
| N-PCN | — | −0.154 V | 0.1 M KOH | waste-derived carbon | [95] |
| CIGPF_450 | 0.77 V 0.95 V | 0.63 V 0.89 V | 0.5 M H2SO4 0.1 M KOH | waste-derived carbon | [96] |
| Fe-Co 600 | ~0.9 V | ~0.81 V | 0.1 M KOH | waste-derived carbon | [98] |
| L_Fe | 0.94 V 0.84 V | 0.87 V 0.77 V | 0.1 M KOH 0.5 M H2SO4 | waste-derived carbon | [99] |
| CC1U | 0.97 V | 0.70 V | 0.1 M KOH | waste-derived carbon | [100] |
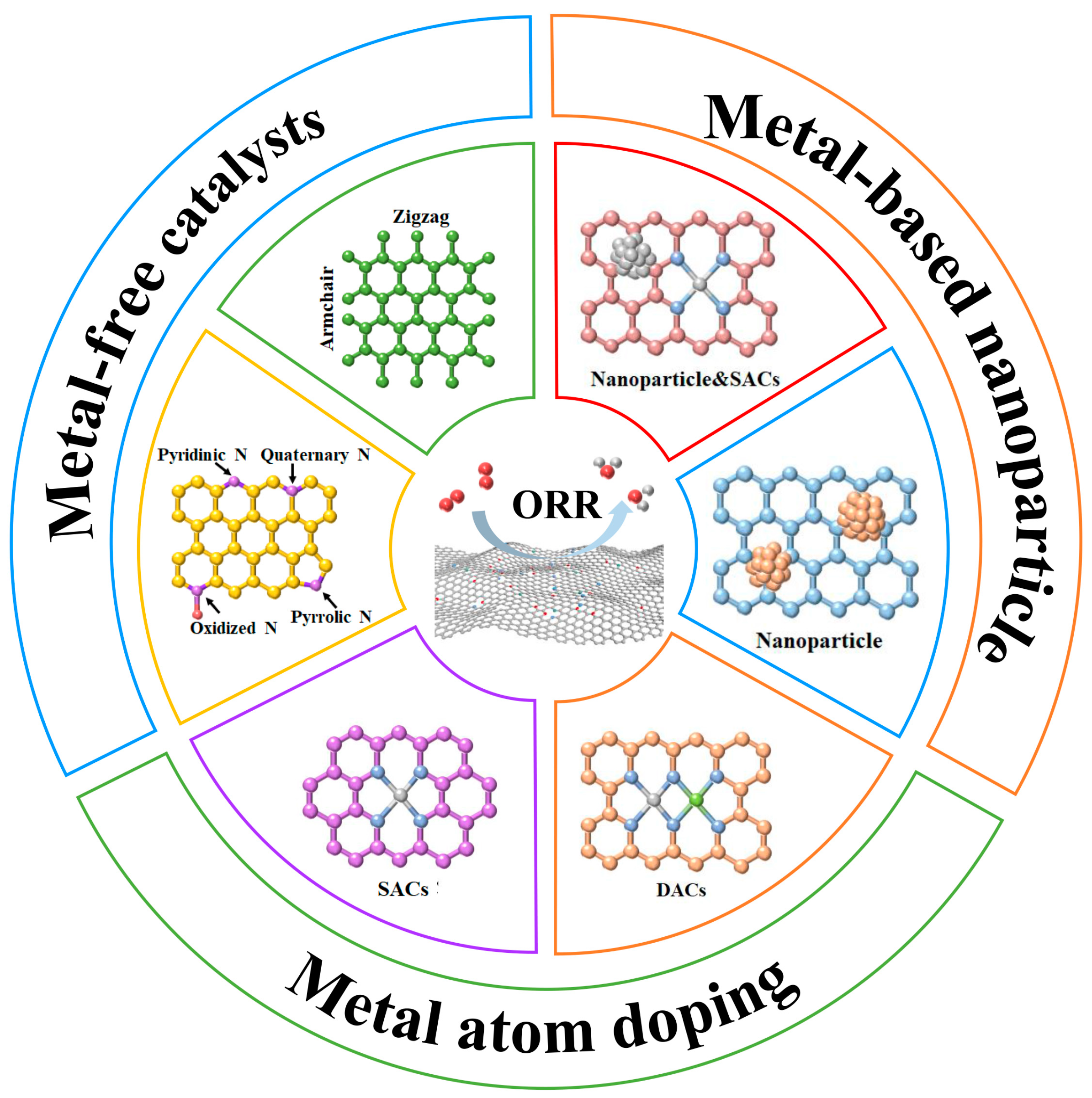
4. Carbon-Based ORR Catalysts
4.1. Metal-Free Catalysts
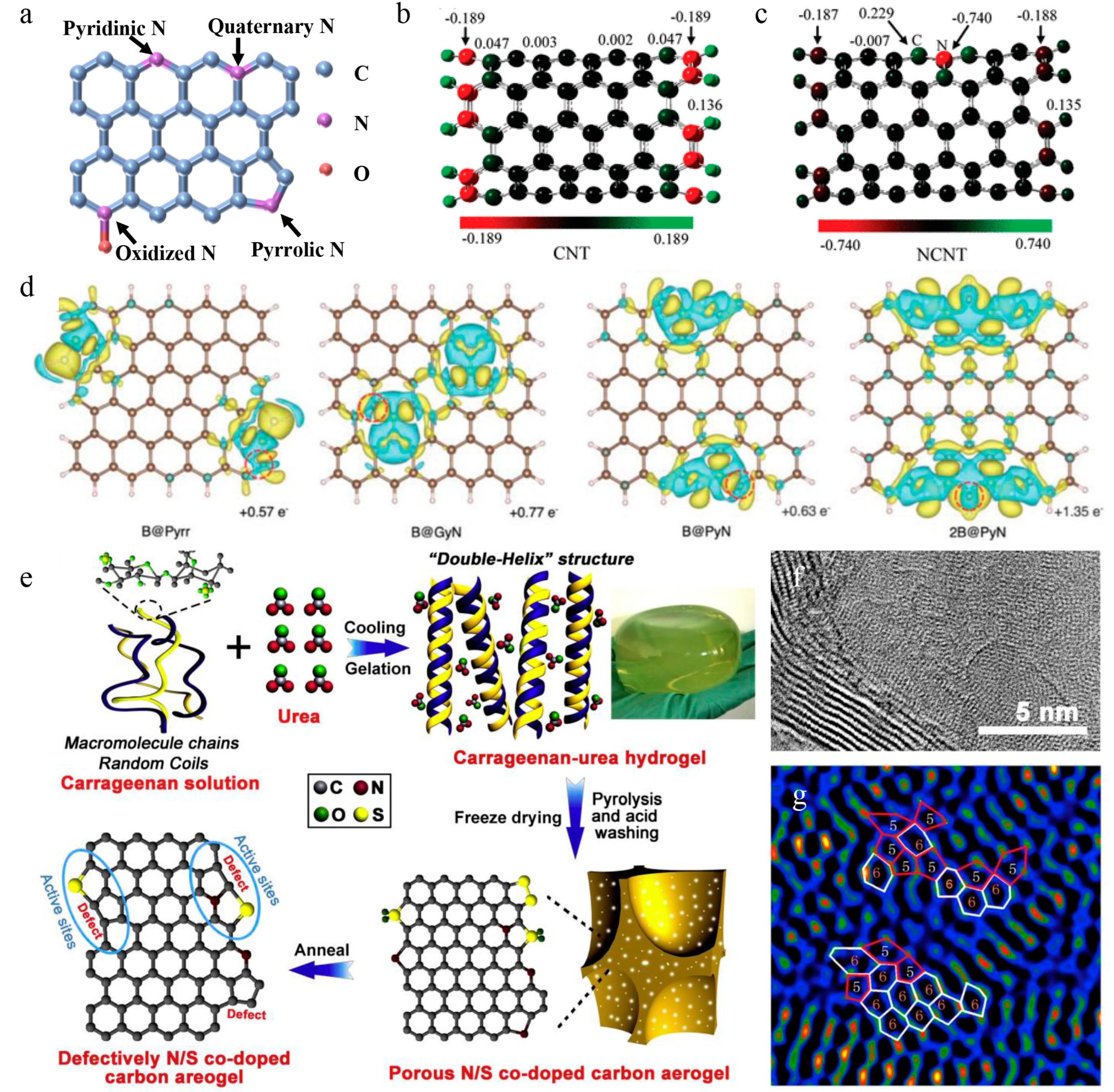
4.1.1. Defect Doping
4.1.2. Heteroatom Doping
4.2. Metal Single-Atom/Dual-Atom Catalysts
4.2.1. Single-Atom Catalysts
4.2.2. Dual-Atom Catalysts
4.3. Metal-Based Nanoparticle Catalysts
4.3.1. Carbon-Supported Metal Nanoparticles
4.3.2. Synergistic Effect of Metal Single Atom and Nanoparticle
| Catalysts | EOnset | E1/2 | pH | Reference |
|---|---|---|---|---|
| SGnP | 0.81 V | — | 0.1 M KOH | [105] |
| GQD-GNR | — | ~0.805 V | 0.1 M KOH | [106] |
| BNC-1 | 0.876 V | 0.812 V | 0.1 M KOH | [103] |
| NSCA-700-1000 | — | 0.85 V 0.76 V 0.76 V | 0.1 M KOH 0.5 M H2SO4 0.1 M HClO4 | [116] |
| s-Hf-N/O-C | 1.050 V | 0.920 V | 0.1 M KOH | [64] |
| Co-N-C@F127 | 0.93 V | 0.84 V | 0.5 M H2SO4 | [119] |
| Fe2N6 | — | 0.84 V | 0.5 M H2SO4 | [122] |
| FeCo-N3O3@C | — | 0.936 V | 0.1 M KOH | [123] |
| Fe1Se1-NC | 1.0 V 0.88 V | 0.88 V 0.74 V | 0.1 M KOH 0.5 M H2SO4 | [124] |
| PtCu/C | ~0.90 V ~0.87 V | — | 0.1 M KOH 0.1 M HClO4 | [125] |
| Pd/N-HsGY | 0.96 V | 0.849 V | 0.1 M KOH | [126] |
| 37 wt%-FePt/rGO | — | ~0.92 V | 0.1 M HClO4 | [127] |
| Co3O4/N-rmGO | 0.88 V | 0.83 V | 0.1 M KOH | [128] |
| NC-CO3O4-90 | 0.91 V | 0.87 V | 1 M KOH | [129] |
| Fe-P-900 | 0.95 V 0.84 V | — | 0.1 M KOH 0.1 M HClO4 | [130] |
| Fe@C-FeNC | ~1.025 V | 0.917 V | 0.1 M KOH | [131] |
| FePNC | — | 0.76 V 0.90 V | 0.5 M H2SO4 0.1 M KOH | [132] |
| Pt@MnSA-NC | — | 0.915 V | 0.1 M HClO4 | [133] |
| FeSAs+NPsCeSAs+Fe−ONPs/NC | — | 0.948 V | 0.1 M KOH | [134] |
5. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Zhao, X.; Zou, G.; Zhang, L.; Han, S.; Li, Y.; Liu, D.; Fernandez, C.; Li, L.; Ren, L. Crystal-defect engineering of electrode materials for energy storage and conversion. Mater. Today Nano 2023, 22, 100336. [Google Scholar] [CrossRef]
- Cui, P.; Zhao, L.; Long, Y.; Dai, L.; Hu, C. Carbon-based electrocatalysts for acidic oxygen reduction reaction. Angew. Chem. Int. Ed. 2023, 62, e202218269. [Google Scholar] [CrossRef]
- Song, K.; Yang, B.; Zou, X.; Zhang, W.; Zheng, W.T. Unified ORR mechanism criteria via charge-spin-coordination of Fe functional units. Energy Environ. Sci. 2023, 17, 27–48. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Z.; Duan, X.; Huang, Y. Nanoscale structure design for high-performance Pt-based ORR catalysts. Adv. Mater. 2018, 31, e1802234. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Y.; Liu, A.; Zhang, Z.; Lv, Q.; Liu, B. Current progress and performance improvement of Pt/C catalysts for fuel cells. J. Mater. Chem. A 2020, 8, 24284–24306. [Google Scholar] [CrossRef]
- Khezri, R.; Motlagh, S.R.; Etesami, M.; Mohamad, A.A.; Mahlendorf, F.; Somwangthanaroj, A.; Kheawhom, S. Stabilizing zinc anodes for different configurations of rechargeable zinc-air batteries. Chem. Eng. J. 2022, 449, 137796. [Google Scholar] [CrossRef]
- Liu, X.; Fan, X.; Liu, B.; Ding, J.; Deng, Y.; Han, X.; Zhong, C.; Hu, W. Mapping the design of electrolyte materials for electrically rechargeable zinc-air batteries. Adv. Mater. 2021, 33, 2006461. [Google Scholar] [CrossRef]
- Wang, N.; Ma, S.; Zuo, P.; Duan, J.; Hou, B. Recent progress of electrochemical production of hydrogen peroxide by two-electron oxygen reduction reaction. Adv. Sci. 2021, 8, 2100076. [Google Scholar] [CrossRef]
- Byeon, A.; Yun, W.C.; Kim, J.M.; Lee, J.W. Recent progress in heteroatom-doped carbon electrocatalysts for the two-electron oxygen reduction reaction. Chem. Eng. J. 2022, 456, 141042. [Google Scholar] [CrossRef]
- Hu, J.; Liu, W.; Xin, C.; Guo, J.; Cheng, X.; Wei, J.; Hao, C.; Zhang, G.; Shi, Y. Carbon-based single atom catalysts for tailoring the ORR pathway: A concise review. J. Mater. Chem. A 2021, 9, 24803–24829. [Google Scholar] [CrossRef]
- Lin, L.; Miao, N.; Wallace, G.G.; Chen, J.; Allwood, D.A. Engineering carbon materials for electrochemical oxygen reduction reactions. Adv. Energy Mater. 2021, 11, 2100695. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, Y.; He, P.; Zhang, Z. Recent advances in revealing the electrocatalytic mechanism for hydrogen energy conversion system. Small 2024, 20, 2405008. [Google Scholar] [CrossRef] [PubMed]
- Xue, S.; Yang, R.; Lei, C.; Zhao, J.; Sun, S.; Zhao, M.; Wang, Z.; Huang, Q.; Wu, Y. Application of vertically ordered polyaniline nanofibers in enhancing the ORR activity of Pt catalysis. Chem. Eng. J. 2024, 485, 149891. [Google Scholar] [CrossRef]
- Shi, W.; Park, A.-H.; Kwon, Y.-U. Scalable synthesis of (Pd, Cu)@ Pt core-shell catalyst with high ORR activity and durability. J. Electroanal. Chem. 2022, 918, 116451. [Google Scholar] [CrossRef]
- Zhu, J.; Xie, M.; Chen, Z.; Lyu, Z.; Chi, M.; Jin, W.; Xia, Y. Pt-Ir-Pd trimetallic nanocages as a dual catalyst for efficient oxygen reduction and evolution reactions in acidic media. Adv. Energy Mater. 2020, 10, 1904114. [Google Scholar] [CrossRef]
- Zhao, X.; Hao, Z.; Zhang, X.; Li, L.; Gao, Y.; Liu, L. Alkaline oxygen reduction/evolution reaction electrocatalysis: A critical review focus on orbital structure, non-noble metal catalysts, and descriptors. Chem. Eng. J. 2024, 497, 155005. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, M.; Zhao, X.; Cai, J.; Yan, W.; Yen, J.C.; Chen, S.; Yu, Y.; Zhang, J. Advanced noncarbon materials as catalyst supports and non-noble electrocatalysts for fuel cells and metal-air batteries. Electrochem. Energy Rev. 2021, 4, 336–381. [Google Scholar] [CrossRef]
- Li, J.; Banis, M.N.; Ren, Z.; Adair, K.R.; Doyle-Davis, K.; Meira, D.M.; Finfrock, Y.Z.; Zhang, L.; Kong, F.; Sham, T.; et al. Unveiling the nature of Pt single-atom catalyst during electrocatalytic hydrogen evolution and oxygen reduction reactions. Small 2021, 17, 2007245. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Song, S.; Cai, W.; Luo, X.; Jiao, L.; Fang, Q.; Wang, X.; Wu, N.; Luo, Z.; Wang, H.; et al. Tuning the spin state of Fe single atoms by Pd nanoclusters enables robust oxygen reduction with dissociative pathway. Chem 2022, 9, 181–197. [Google Scholar] [CrossRef]
- Tian, H.; Song, A.; Zhang, P.; Sun, K.; Wang, J.; Sun, B.; Fan, Q.; Shao, G.; Chen, C.; Liu, H.; et al. High durability of Fe-N-C single-atom catalysts with carbon vacancies toward the oxygen reduction reaction in alkaline media. Adv. Mater. 2023, 35, 2210714. [Google Scholar] [CrossRef]
- Hong, J.; Chen, M.; Zhang, L.; Qin, L.; Hu, J.; Huang, X.; Zhou, C.; Zhou, Y.; Wågberg, T.; Hu, G. Asymmetrically coupled Co Single-atom and Co nanoparticle in Double-shelled Carbon-based nanoreactor for enhanced reversible oxygen catalysis. Chem. Eng. J. 2022, 455, 140401. [Google Scholar] [CrossRef]
- Tsai, J.-E.; Hong, W.-X.; Pourzolfaghar, H.; Wang, W.-H.; Li, Y.-Y. Fe-Ni-Zn triple single-atom catalyst for efficient oxygen reduction and oxygen evolution reaction in rechargeable Zn-air batteries. Chem. Eng. J. 2023, 460, 141868. [Google Scholar] [CrossRef]
- Ma, J.; Habrioux, A.; Luo, Y.; Ramos-Sanchez, G.; Calvillo, L.; Granozzi, G.; Balbuena, P.B.; Alonso-Vante, N. Electronic interaction between platinum nanoparticles and nitrogen-doped reduced graphene oxide: Effect on the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 11891–11904. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, K.; Wang, H.; Yao, X. The role of defect sites in nanomaterials for electrocatalytic energy conversion. Chem 2019, 5, 1371–1397. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; García-Dalí, S.; Daouli, A.; Zitolo, A.; Canevesi, R.L.; Emo, M.; Izquierdo, M.T.; Badawi, M.; Celzard, A.; Fierro, V. Advanced design of metal nanoclusters and single atoms embedded in C1N1-derived carbon materials for ORR, HER, and OER. Adv. Funct. Mater. 2023, 33, 2300405. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, J.; Li, Y.; Lv, E.; Khan, U.; Yang, X.; Yao, J.; Nairan, A.; Zhang, Q. Constructing nitrogen-doped carbon hierarchy structure derived from metal-organic framework as high-performance ORR cathode material for Zn-air battery. Small 2023, 20, 2304594. [Google Scholar] [CrossRef]
- Nasim, F.; Nadeem, M.A. Understanding the mechanism and synergistic interaction of cobalt-based electrocatalysts containing nitrogen-doped carbon for 4 e− ORR. J. Mater. Chem. A 2023, 11, 10095–10124. [Google Scholar] [CrossRef]
- Yang, L.; Shui, J.; Du, L.; Shao, Y.; Liu, J.; Dai, L.; Hu, Z. Carbon-based metal-free ORR electrocatalysts for fuel cells: Past, present, and future. Adv. Mater. 2019, 31, 1804799. [Google Scholar] [CrossRef]
- Huang, B.; Peng, L.; Yang, F.; Liu, Y.; Xie, Z. Improving ORR activity of carbon nanotubes by hydrothermal carbon deposition method. J. Energy Chem. 2017, 26, 712–718. [Google Scholar] [CrossRef]
- Feng, X.; Bai, Y.; Liu, M.; Li, Y.; Yang, H.; Wang, X.; Wu, C. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci. 2021, 14, 2036–2089. [Google Scholar] [CrossRef]
- Ni, B.; Ouyang, C.; Xu, X.; Zhuang, J.; Wang, X. Modifying commercial carbon with trace amounts of ZIF to prepare derivatives with superior ORR activities. Adv. Mater. 2017, 29, 1701354. [Google Scholar] [CrossRef] [PubMed]
- Mamtani, K.; Jain, D.; Dogu, D.; Gustin, V.; Gunduz, S.; Co, A.C.; Ozkan, U.S. Insights into oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) active sites for nitrogen-doped carbon nanostructures (CNx) in acidic media. Appl. Catal. B Environ. 2018, 220, 88–97. [Google Scholar] [CrossRef]
- Kong, F.; Cui, X.; Huang, Y.; Yao, H.; Chen, Y.; Tian, H.; Meng, G.; Chen, C.; Chang, Z.; Shi, J. N-doped carbon electrocatalyst: Marked ORR activity in acidic media without the contribution from metal sites? Angew. Chem. Int. Ed. 2022, 61, e202116290. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.-B.; Li, F.; Cao, Q.-C.; Wu, H.; Qin, Y.-H.; Yang, L.; Wang, T.; Zheng, X.; Wang, C.-W. Core-shell S-doped g-C3N4@ P123 derived N and S co-doped carbon as metal-free electrocatalysts highly efficient for oxygen reduction reaction. Chem. Eng. J. 2022, 429, 132469. [Google Scholar] [CrossRef]
- Li, Y.; Han, J.; Xu, Z.; Zhao, R.; Wang, Y.; Guo, R. N-, P-, and O-tridoped carbon hollow nanospheres with openings in the shell surfaces: A highly efficient electrocatalyst toward the ORR. Langmuir 2021, 37, 2001–2010. [Google Scholar] [CrossRef]
- Wei, P.; Li, X.; He, Z.; Sun, X.; Liang, Q.; Wang, Z.; Fang, C.; Li, Q.; Yang, H.; Han, J.; et al. Porous N, B co-doped carbon nanotubes as efficient metal-free electrocatalysts for ORR and Zn-air batteries. Chem. Eng. J. 2021, 422, 130134. [Google Scholar] [CrossRef]
- Feng, X.; Chen, G.; Cui, Z.; Qin, R.; Jiao, W.; Huang, Z.; Shang, Z.; Ma, C.; Zheng, X.; Han, Y.; et al. Engineering electronic structure of nitrogen-carbon sites by sp3-hybridized carbon and incorporating chlorine to boost oxygen reduction activity. Angew. Chem. Int. Ed. 2023, 63, e202316314. [Google Scholar] [CrossRef]
- Jasinski, R. A new fuel cell cathode catalyst. Nature 1964, 201, 1212–1213. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, C.; Luo, M.; Wang, Q.; Wang, J.; Liao, Z.; Chen, Z.; Chen, Z. Understanding the synergetic effect from foreign metals in bimetallic oxides for PMS activation: A common strategy to increase the stoichiometric efficiency of oxidants. Chem. Eng. J. 2019, 381, 122587. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, F.; Hu, H.; Li, J.; Sun, Y.; Wang, J.; Zou, G.; Chen, X.; Wang, Y.; Fernandez, C.; et al. N-decorated main-group MgAl2O4 spinel: Unlocking exceptional oxygen reduction activity for Zn-air batteries. Small 2024, 20, 2311268. [Google Scholar] [CrossRef]
- Sari, F.N.I.; Lai, Y.; Huang, Y.; Wei, X.; Pourzolfaghar, H.; Chang, Y.; Ghufron, M.; Li, Y.; Su, Y.; Clemens, O.; et al. Electronic structure engineering in NiFe sulfide via a third metal doping as efficient bifunctional OER/ORR electrocatalyst for rechargeable zinc-air battery. Adv. Funct. Mater. 2024, 34, 2310181. [Google Scholar] [CrossRef]
- Parra-Puerto, A.; Ng, K.L.; Fahy, K.; Goode, A.E.; Ryan, M.P.; Kucernak, A. Supported transition metal phosphides: Activity survey for HER, ORR, OER, and corrosion resistance in acid and alkaline electrolytes. ACS Catal. 2019, 9, 11515–11529. [Google Scholar] [CrossRef]
- Doherty, T.; Sunderland, J.; Roberts, E.; Pickett, D. An improved model of potential and current distribution within a flow-through porous electrode. Electrochim. Acta 1996, 41, 519–526. [Google Scholar] [CrossRef]
- Lee, D.U.; Xu, P.; Cano, Z.P.; Kashkooli, A.G.; Park, M.G.; Chen, Z. Recent progress and perspectives on bi-functional oxygen electrocatalysts for advanced rechargeable metal-air batteries. J. Mater. Chem. A 2016, 4, 7107–7134. [Google Scholar] [CrossRef]
- Cao, R.G.; Lee, J.S.; Liu, M.L. Recent progress in non-precious catalysts for metal-air batteries. Adv. Energy Mater. 2012, 2, 816–829. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Xu, D.; Xu, J.-J.; Zhang, X.-B. Oxygen electrocatalysts in metal-air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2013, 43, 7746–7786. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Jin, Y.; Qiao, S.Z. Nanostructured metal-free electrochemical catalysts for highly efficient oxygen reduction. Small 2012, 8, 3550–3566. [Google Scholar] [CrossRef]
- Zhang, J.; Sasaki, K.; Sutter, E.; Adzic, R.R. Stabilization of platinum oxygen-reduction electrocatalysts using gold clusters. Science 2007, 315, 220–222. [Google Scholar] [CrossRef]
- Yu, D.; Nagelli, E.; Du, F.; Dai, L. Metal-free carbon nanomaterials become more active than metal catalysts and last longer. J. Phys. Chem. Lett. 2010, 1, 2165–2173. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Xia, C.; Xia, Y.; Zhu, P.; Fan, L.; Wang, H. Direct electrosynthesis of pure aqueous H2O2 solutions up to 20% by weight using a solid electrolyte. Science 2019, 366, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Siahrostami, S.; Verdaguer-Casadevall, A.; Karamad, M.; Deiana, D.; Malacrida, P.; Wickman, B.; Escudero-Escribano, M.; Paoli, E.A.; Frydendal, R.; Hansen, T.W.; et al. Enabling direct H2O2 production through rational electrocatalyst design. Nat. Mater. 2013, 12, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gennari, M.; Reinhard, F.G.C.; Gutiérrez, J.; Morozan, A.; Philouze, C.; Demeshko, S.; Artero, V.; Meyer, F.; de Visser, S.P.; et al. A non-heme diiron complex for (electro) catalytic reduction of dioxygen: Tuning the selectivity through electron delivery. J. Am. Chem. Soc. 2019, 141, 8244–8253. [Google Scholar] [CrossRef]
- Ortiz-Medina, J.; Wang, Z.; Cruz-Silva, R.; Morelos-Gomez, A.; Wang, F.; Yao, X.; Terrones, M.; Endo, M. Defect engineering and surface functionalization of nanocarbons for metal-free catalysis. Adv. Mater. 2019, 31, 1805717. [Google Scholar] [CrossRef]
- Chi, B.; Zhang, L.; Yang, X.; Zeng, Y.; Deng, Y.; Liu, M.; Huo, J.; Li, C.; Zhang, X.; Shi, X.; et al. Promoting ZIF-8-derived Fe-N-C oxygen reduction catalysts via Zr doping in proton exchange membrane fuel cells: Durability and activity enhancements. ACS Catal. 2023, 13, 4221–4230. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, P.; Wan, L.; Zheng, Y.; Qu, X.; Zhang, H.; Wang, Y.; Zaghib, K.; Yuan, J.; Sun, S.; et al. A general carboxylate-assisted approach to boost the ORR performance of ZIF-derived Fe/N/C catalysts for proton exchange membrane fuel cells. Adv. Funct. Mater. 2021, 31, 2009645. [Google Scholar] [CrossRef]
- Zhu, Z.; Yin, H.; Wang, Y.; Chuang, C.; Xing, L.; Dong, M.; Lu, Y.; Casillas-Garcia, G.; Zheng, Y.; Chen, S.; et al. Coexisting single-atomic Fe and Ni sites on hierarchically ordered porous carbon as a highly efficient ORR electrocatalyst. Adv. Mater. 2020, 32, 2004670. [Google Scholar] [CrossRef]
- Lan, B.; Zheng, X.; Cheng, G.; Han, J.; Li, W.; Sun, M.; Yu, L. The art of balance: Engineering of structure defects and electrical conductivity of α-MnO2 for oxygen reduction reaction. Electrochim. Acta 2018, 283, 459–466. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Huo, J.; Chen, R.; Dai, L.; Wang, S. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; He, F.; Chen, Y.; Zhu, J.; Wang, D.; Mu, S.; Yang, H.Y. Defect and doping co-engineered non-metal nanocarbon ORR electrocatalyst. Nano-Micro Lett. 2021, 13, 65. [Google Scholar] [CrossRef]
- Jiang, H.; Gu, J.; Zheng, X.; Liu, M.; Qiu, X.; Wang, L.; Li, W.; Chen, Z.; Ji, X.; Li, J. Defect-rich and ultrathin N doped carbon nanosheets as advanced trifunctional metal-free electrocatalysts for the ORR, OER and HER. Energy Environ. Sci. 2018, 12, 322–333. [Google Scholar] [CrossRef]
- Cheng, W.; Yuan, P.; Lv, Z.; Guo, Y.; Qiao, Y.; Xue, X.; Liu, X.; Bai, W.; Wang, K.; Xu, Q.; et al. Boosting defective carbon by anchoring well-defined atomically dispersed metal-N4 sites for ORR, OER, and Zn-air batteries. Appl. Catal. B Environ. 2019, 260, 118198. [Google Scholar] [CrossRef]
- Hu, H.; Wang, J.; Tao, P.; Song, C.; Shang, W.; Deng, T.; Wu, J. Stability of single-atom catalysts for electrocatalysis. J. Mater. Chem. A 2021, 10, 5835–5849. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Y.; Wang, J.; Nie, A.; Zou, G.; Ren, L.; Wang, J.; Wang, Y.; Fernandez, C.; Peng, Q. Regulating d-Orbital Hybridization of Subgroup-IVB Single Atoms for Efficient Oxygen Reduction Reaction. Adv. Mater. 2024, 36, 2312117. [Google Scholar] [CrossRef]
- Singh, H.; Zhuang, S.; Ingis, B.; Nunna, B.B.; Lee, E.S. Carbon-based catalysts for oxygen reduction reaction: A review on degradation mechanisms. Carbon 2019, 151, 160–174. [Google Scholar] [CrossRef]
- Qu, Y.; Li, Z.; Chen, W.; Lin, Y.; Yuan, T.; Yang, Z.; Zhao, C.; Wang, J.; Zhao, C.; Wang, X.; et al. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms. Nat. Catal. 2018, 1, 781–786. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, W.; Zhao, L.; Jiang, Z.; Tian, X.; Jiang, Z. Ar/NH3 plasma etching of cobalt-nickel selenide microspheres rich in selenium vacancies wrapped with nitrogen doped carbon nanotubes as highly efficient air cathode catalysts for zinc-air batteries. Small Methods 2024, 2400565. [Google Scholar] [CrossRef]
- Ahmed, I.; Lee, H.J.; Jhung, S.H. Porous carbon derived from covalent organic frameworks and relevant porous polymers: Preparation and application in adsorption and catalysis. Chem. Eng. J. 2024, 499, 156148. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Ahmad, M.W.; Khan, M.S.; Imran, M.; Siyal, S.H.; Javed, M.S. Synthetic methodologies and energy storage/conversion applications of porous carbon nanosheets: A systematic review. Energy Fuels 2022, 36, 3420–3442. [Google Scholar] [CrossRef]
- Xiong, C.; Zheng, C.; Jiang, X.; Xiao, X.; Wei, H.; Zhou, Q.; Ni, Y. Recent progress of green biomass based composite materials applied in supercapacitors, sensors, and electrocatalysis. J. Energy Storage 2023, 72, 108633. [Google Scholar] [CrossRef]
- Singh, S.K.; Takeyasu, K.; Nakamura, J. Active sites and mechanism of oxygen reduction reaction electrocatalysis on nitrogen-doped carbon materials. Adv. Mater. 2018, 31, 1804297. [Google Scholar] [CrossRef]
- Ren, Q.; Wang, H.; Lu, X.-F.; Tong, Y.-X.; Li, G.-R. Recent progress on MOF-derived heteroatom-doped carbon-based electrocatalysts for oxygen reduction reaction. Adv. Sci. 2018, 5, 1700515. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, H.; Feizpoor, S.; Li, L.; Zhang, X.; Xu, X.; Zhuang, Z.; Li, Z.; Hu, W.; Snyders, R.; et al. Tailoring oxygen reduction reaction kinetics of Fe-N-C catalyst via spin manipulation for efficient zinc-air batteries. Adv. Mater. 2024, 36, 2400523. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Wang, J.; Yang, M.; Yan, C.; Zou, G.; Tse, J.S.; Fernandez, C.; Peng, Q. Atomically dispersed quintuple nitrogen and oxygen co-coordinated zirconium on graphene-type substrate for highly efficient oxygen reduction reaction. Cell Rep. Phys. Sci. 2022, 3, 100773. [Google Scholar] [CrossRef]
- Yin, S.; Yi, H.; Liu, M.; Yang, J.; Yang, S.; Zhang, B.-W.; Chen, L.; Cheng, X.; Huang, H.; Huang, R.; et al. An in situ exploration of how Fe/N/C oxygen reduction catalysts evolve during synthesis under pyrolytic conditions. Nat. Commun. 2024, 15, 6229. [Google Scholar] [CrossRef]
- Muhyuddin, M.; Berretti, E.; Mirshokraee, S.A.; Orsilli, J.; Lorenzi, R.; Capozzoli, L.; D’acapito, F.; Murphy, E.; Guo, S.; Atanassov, P.; et al. Formation of the active site structures during pyrolysis transformation of Fe-phthalocyanine into Fe-Nx-C electrocatalysts for the oxygen reduction reaction. Appl. Catal. B Environ. 2023, 343, 123515. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Xu, M.; Asset, T.; Tieu, P.; Gili, A.; Kulkarni, D.; De Andrade, V.; De Carlo, F.; Barnard, H.S.; et al. Catalysts by pyrolysis: Direct observation of chemical and morphological transformations leading to transition metal-nitrogen-carbon materials. Mater. Today 2021, 47, 53–68. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Xu, M.; Asset, T.; Yan, X.; Artyushkova, K.; Kodali, M.; Murphy, E.; Ly, A.; Pan, X.; et al. Catalysts by pyrolysis: Direct observation of transformations during re-pyrolysis of transition metal-nitrogen-carbon materials leading to state-of-the-art platinum group metal-free electrocatalyst. Mater. Today 2022, 53, 58–70. [Google Scholar] [CrossRef]
- Stracensky, T.; Jiao, L.; Sun, Q.; Liu, E.; Yang, F.; Zhong, S.; Cullen, D.A.; Myers, D.J.; Kropf, A.J.; Jia, Q.; et al. Bypassing formation of oxide intermediate via chemical vapor deposition for the synthesis of an Mn-NC catalyst with improved ORR activity. ACS Catal. 2023, 13, 14782–14791. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.-J.; Baek, J.-B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, L.; Guo, D.; Xi, B. Efficient modulation of electrocatalyst interfaces by atomic layer deposition: Fundamentals to application. Adv. Energy Sustain. Res. 2022, 3, 2200026. [Google Scholar] [CrossRef]
- Cheng, N.; Shao, Y.; Liu, J.; Sun, X. Electrocatalysts by atomic layer deposition for fuel cell applications. Nano Energy 2016, 29, 220–242. [Google Scholar] [CrossRef]
- Guan, Q.; Zhu, C.; Lin, Y.; Vovk, E.I.; Zhou, X.; Yang, Y.; Yu, H.; Cao, L.; Wang, H.; Zhang, X.; et al. Bimetallic monolayer catalyst breaks the activity-selectivity trade-off on metal particle size for efficient chemoselective hydrogenations. Nat. Catal. 2021, 4, 840–849. [Google Scholar] [CrossRef]
- Zhuang, S.; Lee, E.S.; Lei, L.; Nunna, B.B.; Kuang, L.; Zhang, W. Synthesis of nitrogen-doped graphene catalyst by high-energy wet ball milling for electrochemical systems. Int. J. Energy Res. 2016, 40, 2136–2149. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Pan, J.; Zhang, L.; Zhao, M.; Xu, J.; Liu, B.; Shi, W.; Song, S.; Zhang, H. Ball-milling induced debonding of surface atoms from metal bulk for construing high-performance dual-site single-atom catalysts. Angew. Chem. 2021, 133, 23338–23342. [Google Scholar] [CrossRef]
- Soares, O.; Rocha, R.; Gonçalves, A.; Figueiredo, J.; Órfão, J.; Pereira, M. Highly active N-doped carbon nanotubes prepared by an easy ball milling method for advanced oxidation processes. Appl. Catal. B Environ. 2016, 192, 296–303. [Google Scholar] [CrossRef]
- Karuppiah, C.; Thirumalraj, B.; Alagar, S.; Piraman, S.; Li, Y.-J.J.; Yang, C.-C. Solid-state ball-milling of Co3O4 nano/microspheres and carbon black endorsed LaMnO3 perovskite catalyst for bifunctional oxygen electrocatalysis. Catalysts 2021, 11, 76. [Google Scholar] [CrossRef]
- Hu, Y.; Li, B.; Yu, C.; Fang, H.; Li, Z. Mechanochemical preparation of single atom catalysts for versatile catalytic applications: A perspective review. Mater. Today 2023, 63, 288–312. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Choi, H.-J.; Ju, M.J.; Choi, I.T.; Lim, K.; Ko, J.; Kim, H.K.; Kim, J.C.; Lee, J.-J.; Shin, D.; et al. Direct nitrogen fixation at the edges of graphene nanoplatelets as efficient electrocatalysts for energy conversion. Sci. Rep. 2013, 3, 2260. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; He, Q.; Zhang, H.; Xiao, H.; Liu, Y.; Zhang, Y.; He, X.; Ji, H. Unveiling the kilogram-scale gold single-atom catalysts via ball milling for preferential oxidation of CO in excess hydrogen. Chem. Eng. J. 2020, 389, 124490. [Google Scholar] [CrossRef]
- Wang, J.; Kim, J.; Choi, S.; Wang, H.; Lim, J. A review of carbon-supported nonprecious metals as energy-related electrocatalysts. Small Methods 2020, 4, 2000621. [Google Scholar] [CrossRef]
- Borghei, M.; Lehtonen, J.; Liu, L.; Rojas, O.J. Advanced biomass-derived electrocatalysts for the oxygen reduction reaction. Adv. Mater. 2017, 30, 1703691. [Google Scholar] [CrossRef] [PubMed]
- Matsagar, B.M.; Yang, R.-X.; Dutta, S.; Ok, Y.S.; Wu, K.C.-W. Recent progress in the development of biomass-derived nitrogen-doped porous carbon. J. Mater. Chem. A 2020, 9, 3703–3728. [Google Scholar] [CrossRef]
- Wang, B.; Li, S.; Wu, X.; Liu, J.; Chen, J. Biomass chitin-derived honeycomb-like nitrogen-doped carbon/graphene nanosheet networks for applications in efficient oxygen reduction and robust lithium storage. J. Mater. Chem. A 2016, 4, 11789–11799. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Park, J.-S. Biomass-derived N-doped porous carbon nanosheets for energy technologies. Chem. Eng. J. 2021, 425, 129017. [Google Scholar] [CrossRef]
- Zuccante, G.; Muhyuddin, M.; Ficca, V.C.A.; Placidi, E.; Acciarri, M.; Lamanna, N.; Franzetti, A.; Zoia, L.; Bellini, M.; Berretti, E.; et al. Transforming Cigarette Wastes into Oxygen Reduction Reaction Electrocatalyst: Does Each Component Behave Differently? An Experimental Evaluation. ChemElectroChem 2024, 11, e202300725. [Google Scholar] [CrossRef]
- Li, Q.; He, T.; Zhang, Y.-Q.; Wu, H.; Liu, J.; Qi, Y.; Lei, Y.; Chen, H.; Sun, Z.; Peng, C.; et al. Biomass waste-derived 3D metal-free porous carbon as a bifunctional electrocatalyst for rechargeable zinc-air batteries. ACS Sustain. Chem. Eng. 2019, 7, 17039–17046. [Google Scholar] [CrossRef]
- Mirshokraee, S.A.; Muhyuddin, M.; Lorenzi, R.; Tseberlidis, G.; Vecchio, C.L.; Baglio, V.; Berretti, E.; Lavacchi, A.; Santoro, C. Litchi-derived platinum group metal-free electrocatalysts for oxygen reduction reaction and hydrogen evolution reaction in alkaline media. Susmat 2023, 3, 248–262. [Google Scholar] [CrossRef]
- Muhyuddin, M.; Friedman, A.; Poli, F.; Petri, E.; Honig, H.; Basile, F.; Fasolini, A.; Lorenzi, R.; Berretti, E.; Bellini, M.; et al. Lignin-derived bimetallic platinum group metal-free oxygen reduction reaction electrocatalysts for acid and alkaline fuel cells. J. Power Sources 2022, 556, 232416. [Google Scholar] [CrossRef]
- Martínez-Loyola, J.; Carrasco-Cordero, M.; Alonso-Lemus, I.; Rodríguez-Varela, F.; Bartolo-Pérez, P.; Escobar-Morales, B.; Vega-Cantú, Y.; Rodríguez-Macías, F. Systematic study of the N concentration effects on metal-free ORR electrocatalysts derived from corncob: Less is more. Electrochem. Commun. 2024, 166, 107792. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sun, C.; Jin, Z.; Wang, D.-W.; Yan, X.; Chen, Z.; Zhu, G.; Yao, X. Carbon for the oxygen reduction reaction: A defect mechanism. J. Mater. Chem. A 2015, 3, 11736–11739. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, L.; Sun, T.; Zhao, J.; Lyu, Z.; Zhuo, O.; Wang, X.; Wu, Q.; Ma, J.; Hu, Z. Significant contribution of intrinsic carbon defects to oxygen reduction activity. ACS Catal. 2015, 5, 6707–6712. [Google Scholar] [CrossRef]
- Deng, D.; Yu, L.; Pan, X.; Wang, S.; Chen, X.; Hu, P.; Sun, L.; Bao, X. Size effect of graphene on electrocatalytic activation of oxygen. Chem. Commun. 2011, 47, 10016–10018. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Choi, H.-J.; Jung, S.-M.; Seo, J.-M.; Kim, M.-J.; Dai, L.; Baek, J.-B. Large-scale production of edge-selectively functionalized graphene nanoplatelets via ball milling and their use as metal-free electrocatalysts for oxygen reduction reaction. J. Am. Chem. Soc. 2012, 135, 1386–1393. [Google Scholar] [CrossRef]
- Jin, H.; Huang, H.; He, Y.; Feng, X.; Wang, S.; Dai, L.; Wang, J. Graphene quantum dots supported by graphene nanoribbons with ultrahigh electrocatalytic performance for oxygen reduction. J. Am. Chem. Soc. 2015, 137, 7588–7591. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Metal-free heteroatom-doped carbon-based catalysts for ORR: A critical assessment about the role of heteroatoms. Carbon 2020, 165, 434–454. [Google Scholar] [CrossRef]
- Wang, D.-W.; Su, D. Heterogeneous nanocarbon materials for oxygen reduction reaction. Energy Environ. Sci. 2013, 7, 576–591. [Google Scholar] [CrossRef]
- Luo, X.; Zheng, H.; Lai, W.; Yuan, P.; Li, S.; Li, D.; Chen, Y. Defect engineering of carbons for energy conversion and storage applications. Energy Environ. Mater. 2023, 6, e12402. [Google Scholar] [CrossRef]
- Wu, B.; Meng, H.; Morales, D.M.; Zeng, F.; Zhu, J.; Wang, B.; Risch, M.; Xu, Z.J.; Petit, T. Nitrogen-rich carbonaceous materials for advanced oxygen electrocatalysis: Synthesis, characterization, and activity of nitrogen sites. Adv. Funct. Mater. 2022, 32, 2204137. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Wang, L.; Fu, H. Structural design strategy and active site regulation of high-efficient bifunctional oxygen reaction electrocatalysts for Zn-air battery. Small 2021, 17, 2006766. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, S.; Duraivel, M.; Hira, S.A.; Prabakar, K.; Ha, C.-S.; Joo, S.H.; Nam, K.M.; Park, K.H. Heteroatom-doped nanomaterials/core-shell nanostructure based electrocatalysts for the oxygen reduction reaction. J. Mater. Chem. A 2021, 10, 987–1021. [Google Scholar] [CrossRef]
- Hu, X.; Wu, Y.; Li, H.; Zhang, Z. Adsorption and activation of O2 on nitrogen-doped carbon nanotubes. J. Phys. Chem. C 2010, 114, 9603–9607. [Google Scholar] [CrossRef]
- Vineesh, T.V.; Kumar, M.P.; Takahashi, C.; Kalita, G.; Alwarappan, S.; Pattanayak, D.K.; Narayanan, T.N. Bifunctional electrocatalytic activity of boron-doped graphene derived from boron carbide. Adv. Energy Mater. 2015, 5, 1500658. [Google Scholar] [CrossRef]
- Fan, M.; Yuan, Q.; Zhao, Y.; Wang, Z.; Wang, A.; Liu, Y.; Sun, K.; Wu, J.; Wang, L.; Jiang, J. A facile “double-catalysts” approach to directionally fabricate pyridinic N B-pair-doped crystal graphene nanoribbons/amorphous carbon hybrid electrocatalysts for efficient oxygen reduction reaction. Adv. Mater. 2022, 34, 2107040. [Google Scholar] [CrossRef]
- Li, D.; Jia, Y.; Chang, G.; Chen, J.; Liu, H.; Wang, J.; Hu, Y.; Xia, Y.; Yang, D.; Yao, X. A defect-driven metal-free electrocatalyst for oxygen reduction in acidic electrolyte. Chem 2018, 4, 2345–2356. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, H.; Liu, B. Coordination engineering of single-atom catalysts for the oxygen reduction reaction: A review. Adv. Energy Mater. 2020, 11, 2002473. [Google Scholar] [CrossRef]
- Gawande, M.B.; Ariga, K.; Yamauchi, Y. Single-atom catalysts. Small 2021, 17, 2101584. [Google Scholar] [CrossRef]
- He, Y.; Hwang, S.; Cullen, D.A.; Uddin, M.A.; Langhorst, L.; Li, B.; Karakalos, S.; Kropf, A.J.; Wegener, E.C.; Sokolowski, J.; et al. Highly active atomically dispersed CoN4 fuel cell cathode catalysts derived from surfactant-assisted MOFs: Carbon-shell confinement strategy. Energy Environ. Sci. 2018, 12, 250–260. [Google Scholar] [CrossRef]
- He, Y.; Zhou, X.; Jia, Y.; Li, H.; Wang, Y.; Liu, Y.; Tan, Q. Advances in transition-metal-based dual-atom oxygen electrocatalysts. Small 2023, 19, 2206477. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Y.; Zhang, C.; Ma, X.; Wu, J.; Liu, Y.; Lu, B.; Zhang, H.; Ming, C.; Xiang, J. Insights into the electronic structure coupling effect of dual-metal atomic electrocatalytic platform for efficient clean energy conversion. Chem. Eng. J. 2023, 461, 141911. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, T.; Ge, J.; Lin, Y.; Du, Z.; Zhong, C.; Wang, W.; Jiao, Q.; Yuan, R.; Tian, Y.; et al. High-density planar-like Fe2N6 structure catalyzes efficient oxygen reduction. Matter 2020, 3, 509–521. [Google Scholar] [CrossRef]
- Tang, B.; Zhou, Y.; Ji, Q.; Zhuang, Z.; Zhang, L.; Wang, C.; Hu, H.; Wang, H.; Mei, B.; Song, F.; et al. A Janus dual-atom catalyst for electrocatalytic oxygen reduction and evolution. Nat. Synth. 2024, 3, 878–890. [Google Scholar] [CrossRef]
- Chen, Z.; Su, X.; Ding, J.; Yang, N.; Zuo, W.; He, Q.; Wei, Z.; Zhang, Q.; Huang, J.; Zhai, Y. Boosting oxygen reduction reaction with Fe and Se dual-atom sites supported by nitrogen-doped porous carbon. Appl. Catal. B Environ. 2022, 308, 121206. [Google Scholar] [CrossRef]
- Coleman, E.J.; Chowdhury, M.H.; Co, A.C. Insights into the oxygen reduction reaction activity of Pt/C and PtCu/C catalysts. ACS Catal. 2015, 5, 1245–1253. [Google Scholar] [CrossRef]
- Si, W.; Yang, Z.; Hu, X.; Lv, Q.; Li, X.; Zhao, F.; He, J.; Huang, C. Preparation of zero valence Pd nanoparticles with ultra-efficient electrocatalytic activity for ORR. J. Mater. Chem. A 2021, 9, 14507–14514. [Google Scholar] [CrossRef]
- Yoo, T.Y.; Yoo, J.M.; Sinha, A.K.; Bootharaju, M.S.; Jung, E.; Lee, H.S.; Lee, B.-H.; Kim, J.; Antink, W.H.; Kim, Y.M.; et al. Direct synthesis of intermetallic platinum-alloy nanoparticles highly loaded on carbon supports for efficient electrocatalysis. J. Am. Chem. Soc. 2020, 142, 14190–14200. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef]
- Guan, C.; Sumboja, A.; Wu, H.; Ren, W.; Liu, X.; Zhang, H.; Liu, Z.; Cheng, C.; Pennycook, S.J.; Wang, J. Hollow Co3O4 nanosphere embedded in carbon arrays for stable and flexible solid-state zinc-air batteries. Adv. Mater. 2017, 29, 1704117. [Google Scholar] [CrossRef]
- Singh, K.P.; Bae, E.J.; Yu, J.-S. Fe-P: A new class of electroactive catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 3165–3168. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Hu, R.; Yao, L.; Sui, L.; Liu, Y.; Abdelkader, A.; Li, Y.; Ren, X.; Deng, L. Mutual self-regulation of d-electrons of single atoms and adjacent nanoparticles for bifunctional oxygen electrocatalysis and rechargeable zinc-air batteries. Nano-Micro Lett. 2023, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, L.; Sun, Y.; Khan, J.; Feng, B.; Xiao, J.; Zhang, H.; Xie, H.; Li, L.; Wang, S.; et al. Asymmetric N, P-coordinated single-atomic Fe sites with Fe2P nanoclusters/nanoparticles on porous carbon nanosheets for highly efficient oxygen electroreduction. Adv. Energy Mater. 2023, 13, 2301223. [Google Scholar] [CrossRef]
- Zeng, Y.; Liang, J.; Li, C.; Qiao, Z.; Li, B.; Hwang, S.; Kariuki, N.N.; Chang, C.-W.; Wang, M.; Lyons, M.; et al. Regulating catalytic properties and thermal stability of Pt and PtCo intermetallic fuel-cell catalysts via strong coupling effects between single-metal site-rich carbon and Pt. J. Am. Chem. Soc. 2023, 145, 17643–17655. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, X.; Lu, W.; Sun, X.; Huang, H.; Cui, X.; Li, L.; Zou, X.; Zheng, W.; Zhao, X. Collective effect in a multicomponent ensemble combining single atoms and nanoparticles for efficient and durable oxygen reduction. Angew. Chem. Int. Ed. 2024, 63, e202400765. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Yao, Y.; Yan, X.; Meng, X.; Wang, Q.; Zhang, Y.; Yan, S.; Zhao, X.; Luo, S. Emerging Carbon-Based Catalysts for the Oxygen Reduction Reaction: Insights into Mechanisms and Applications. Inorganics 2024, 12, 303. https://doi.org/10.3390/inorganics12120303
Guo J, Yao Y, Yan X, Meng X, Wang Q, Zhang Y, Yan S, Zhao X, Luo S. Emerging Carbon-Based Catalysts for the Oxygen Reduction Reaction: Insights into Mechanisms and Applications. Inorganics. 2024; 12(12):303. https://doi.org/10.3390/inorganics12120303
Chicago/Turabian StyleGuo, Jing, Yuqi Yao, Xin Yan, Xue Meng, Qing Wang, Yahui Zhang, Shengxue Yan, Xue Zhao, and Shaohua Luo. 2024. "Emerging Carbon-Based Catalysts for the Oxygen Reduction Reaction: Insights into Mechanisms and Applications" Inorganics 12, no. 12: 303. https://doi.org/10.3390/inorganics12120303
APA StyleGuo, J., Yao, Y., Yan, X., Meng, X., Wang, Q., Zhang, Y., Yan, S., Zhao, X., & Luo, S. (2024). Emerging Carbon-Based Catalysts for the Oxygen Reduction Reaction: Insights into Mechanisms and Applications. Inorganics, 12(12), 303. https://doi.org/10.3390/inorganics12120303






