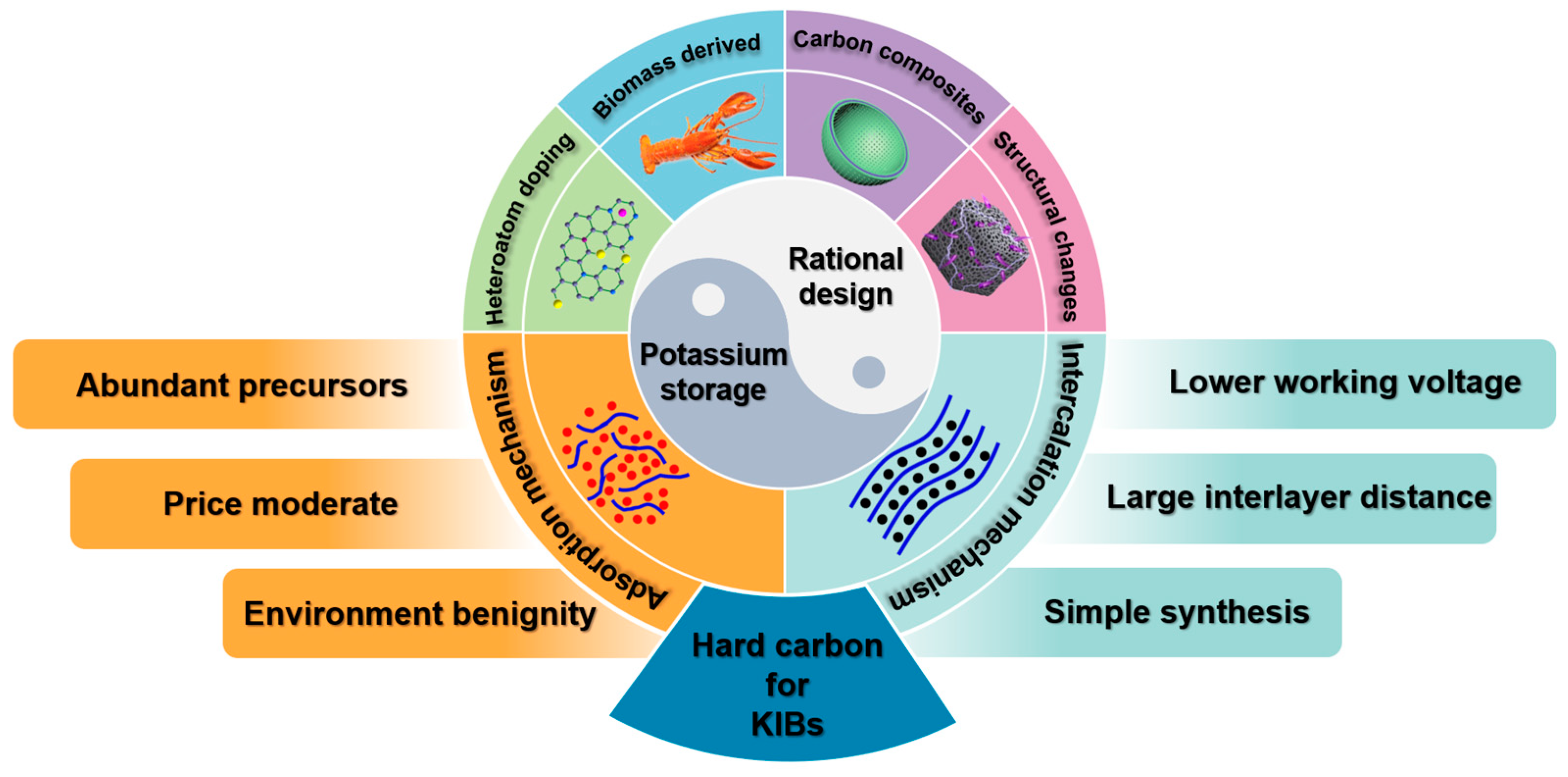
Hard Carbon as Anodes for Potassium-Ion Batteries: Developments and Prospects
Abstract
1. Introduction
2. Potassium Storage Mechanism
2.1. Interpolation Mechanism
2.2. Adsorption Mechanism
3. Rational Design
3.1. Heteroatom Doping
3.1.1. Single-Atom Doping
3.1.2. Dual-Atom Doping
3.2. Biomass-Derived Carbon
3.3. Carbon Composite Materials
3.4. Structural Engineering
4. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, H.; Kim, J.C.; Bianchini, M.; Seo, D.H.; Rodriguez-Garcia, J.; Ceder, G. Recent progress and perspective in electrode materials for K-ion batteries. Adv. Energy Mater. 2018, 8, 1702384. [Google Scholar] [CrossRef]
- Wu, X.; Leonard, D.P.; Ji, X. Emerging non-aqueous potassium-ion batteries: Challenges and opportunities. Chem. Mater. 2017, 29, 5031–5042. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Wang, D.; Li, S.-Z.; Lai, Q.-S.; Yang, D.-R.; Zhao, L.-K.; Mu, J.-J.; Wang, X.-C.; Gao, X.-W.; Luo, W.-B. An Ultrafast Rechargeable Hybrid Potassium Dual-Ion Capacitor Based on Carbon Quantum Dot@Ultrathin Carbon Film Cathode. Rare Met. 2024, 43, 5070–5081. [Google Scholar] [CrossRef]
- Veerasubramani, G.K.; Park, M.-S.; Woo, H.-S.; Sun, Y.-K.; Kim, D.-W. Closely coupled binary metal sulfide nanosheets shielded molybdenum sulfide nanorod hierarchical structure via eco-benign surface exfoliation strategy towards efficient lithium and sodium-ion batteries. Energy Storage Mater. 2021, 38, 344–353. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, X.W.; Liu, X.; Mu, J.J.; Gu, Q.; Liu, Z.; Luo, W.B. Flexible Wearable Energy Storage Devices: Materials, Structures, and Applications. Battery Energy 2024, 3, 20230061. [Google Scholar] [CrossRef]
- Zhao, L.-K.; Gao, X.-W.; Ren, T.-Z.; Wang, D.; Wang, D.-W.; Liu, Z.-M.; Chen, H.; Luo, W.-B. Regulating Ion Transport Behaviors toward Dendrite-Free Potassium Metal Batteries: Recent Advances and Perspectives. Rare Met. 2024, 43, 1435–1460. [Google Scholar] [CrossRef]
- Zhou, J.-L.; Zhao, L.-K.; Lu, Y.; Gao, X.-W.; Chen, Y.; Liu, Z.-M.; Gu, Q.-F.; Luo, W.-B. Bark-Inspired Functional-Carbon/Potassium Composite Electrode with Fast Ion Transport Channels for Dendrite-Free Potassium Metal Batteries. Adv. Funct. Mater. 2024, 34, 2403754. [Google Scholar] [CrossRef]
- Ma, P.; Zhang, Y.; Li, W.; Luo, J.; Wen, L.; Tang, G.; Gai, J.; Wang, Q.; Zhao, L.; Ge, J. Tailoring alloy-reaction-induced semi-coherent interface to guide sodium nucleation and growth for long-term anode-less sodium-metal batteries. Sci. China Mater. 2024, 67, 3648–3657. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Tang, Y.; Ji, X.; Jia, C.; Wang, H. Advancements and challenges in potassium ion batteries: A comprehensive review. Adv. Funct. Mater. 2020, 30, 1909486. [Google Scholar] [CrossRef]
- Ren, H.; Gao, X.-W.; Yong, D.; Liu, Z.; Wang, X.; Gao, G.; Chen, H.; Gu, Q.; Luo, W.-B. Multiphase Riveting Structure Constructed by Slight Molybdenum for Enhanced P3/O3 Layer-Structured Oxide Cathode Material. Chem. Eng. J. 2024, 494, 152787. [Google Scholar] [CrossRef]
- Shi, H.; Gao, X.-W.; Wang, X.; Chen, H.; Han, W.; Gu, Q.; Liu, Z.; Luo, W.-B. Surface Residual Alkali Reverse Utilization: Stabilizing the Lay-Structured Oxide Cathode for High Stability Potassium Ion Batteries. Chem. Eng. J. 2024, 484, 149574. [Google Scholar] [CrossRef]
- Yang, D.; Gao, X.W.; Gao, G.; Lai, Q.; Ren, T.; Gu, Q.; Liu, Z.; Luo, W.B. Local Electronic Structure Constructing of Layer-Structured Oxide Cathode Material for High-Voltage Sodium-Ion Batteries. Carbon Energy 2024, 6, e574. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Hara, T.; Janek, J.; Adelhelm, P. Room-temperature sodium-ion batteries: Improving the rate capability of carbon anode materials by templating strategies. Energy Environ. Sci. 2011, 4, 3342–3345. [Google Scholar] [CrossRef]
- Mu, J.; Wang, D.; Zhou, S.; Jia, X.; Gao, X.-W.; Liu, Z.; Luo, W.-B. MAX-Derived B-doped Mo1.33C MXene for Ambient Electrocatalytic Conversion of Nitrate to Ammonia. J. Mater. Chem. A 2024, 12, 18082–18088. [Google Scholar] [CrossRef]
- Liu, Z.; Li, S.; Mu, J.; Zhao, L.-K.; Gao, X.-W.; Gu, Q.; Wang, X.-C.; Chen, H.; Luo, W.-B. Element-Tailored Quenching Methods: Phase-Defective K0.5Mn1−xCrxO2 Cathode Materials for Potassium Ion Batteries. Mater. Today Chem. 2024, 40, 102251. [Google Scholar] [CrossRef]
- Liu, Z.; Song, Y.; Fu, S.; An, P.; Dong, M.; Wang, S.; Lai, Q.; Gao, X.-W.; Luo, W.-B. Multiphase manganese-based layered oxide for sodium-ion batteries: Structural change and phase transition. Microstructures 2024, 4, 2024036. [Google Scholar] [CrossRef]
- Mu, J.; Gao, X.-W.; Yu, T.; Zhao, L.-K.; Luo, W.-B.; Yang, H.; Liu, Z.-M.; Sun, Z.; Gu, Q.-F.; Li, F. Ambient Electrochemical Ammonia Synthesis: From Theoretical Guidance to Catalyst Design. Adv. Sci. 2024, 11, 2308979. [Google Scholar] [CrossRef]
- Hu, Z.; Hao, J.; Shen, D.; Gao, C.; Liu, Z.; Zhao, J.; Lu, B. Electro-Spraying/Spinning: A Novel Battery Manufacturing Technology. Green Energy Environ. 2024, 9, 81–88. [Google Scholar] [CrossRef]
- Li, S.; Zhao, L.-K.; Bian, Y.-H.; Chen, H.; Gao, X.-W.; Song, Y.; Liu, Z.; Luo, W.-B. Protective Layer Constructed by Liquid Phase Quenching for Long Lifespan Potassium Ion Batteries. Chem. Eng. J. 2024, 496, 154382. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, S.; Wang, S.; An, P.; Dong, M.; Wang, Z.; Yang, H.; Zhang, Y.; Gong, Z.; He, K. Ultrathin Carbon Film as Ultrafast Rechargeable Cathode for Hybrid Sodium Dual-Ion Capacitor. Nanotechnology 2024, 35, 375601. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, D.; Zhang, Y.; Gong, Z.; Lv, X.; Qin, Q.; Gong, Y. Low-Strain KVPO4F@C as Hyperstable Anode for Potassium-Ion Batteries. Metals 2023, 13, 1038. [Google Scholar] [CrossRef]
- Zhao, L.K.; Gao, X.W.; Mu, J.; Luo, W.B.; Liu, Z.; Sun, Z.; Gu, Q.F.; Li, F. Durable Integrated K-Metal Anode with Enhanced Mass Transport through Potassiphilic Porous Interconnected Mediator. Adv. Funct. Mater. 2023, 33, 2304292. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Lu, Y.; Gao, X.-W.; Zhou, J.-L.; Wang, X.-C.; Gu, Q.-F.; Luo, W.-B. Uniform Potassium Metal Deposition under Fast Ion Transportation Kinetic: Porous Carbon Nanotube/Metal–Organic Frameworks Composite Host. J. Power Sources 2024, 613, 234909. [Google Scholar] [CrossRef]
- Gong, Z.; Liu, Z.; Gao, X.-W.; Chen, N.; Song, Y.; Wu, X.; Hu, A. Constructing Cyclic Hydrogen Bonding to Suppress Side Reactions and Dendrite Formation on Zinc Anodes. Chem. Eur. J. 2024, e202402558. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Z.; Gao, X.-W.; Gu, Q.; Zhao, L.; Luo, W.-B. Massive Anionic Fluorine Substitution Two-Dimensional δ-MnO2 Nanosheets for High-Performance Aqueous Zinc-Ion Battery. J. Energy Storage 2023, 72, 108740. [Google Scholar] [CrossRef]
- Wang, S.-S.; Liu, Z.-M.; Gao, X.-W.; Wang, X.-C.; Chen, H.; Luo, W.-B. Layer-Structured Multitransition-Metal Oxide Cathode Materials for Potassium-Ion Batteries with Long Cycling Lifespan and Superior Rate Capability. ACS Appl. Mater. Interfaces 2023, 15, 57165–57173. [Google Scholar] [CrossRef]
- Zhao, L.-K.; Gao, X.-W.; Gu, Q.; Ge, X.; Ding, Z.; Liu, Z.; Luo, W.-B. Realizing a Dendrite-Free Metallic-Potassium Anode using Reactive Prewetting Chemistry. eScience 2023, 4, 100201. [Google Scholar] [CrossRef]
- Dhir, S.; Wheeler, S.; Capone, I.; Pasta, M. Outlook on K-Ion Batteries. Chem 2020, 6, 2442–2460. [Google Scholar] [CrossRef]
- Fan, S.-S.; Liu, H.-P.; Liu, Q.; Ma, C.-S.; Yi, T.-F. Comprehensive insights and perspectives into the recent progress of electrode materials for non-aqueous K-ion battery. J. Mater. 2020, 6, 431–454. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Li, J.; Yan, S.; Zhang, X.; Wei, D.; Xing, Z.; Zhuang, Q.; Ju, Z. Nitrogen/sulphur co-doped porous carbon derived from wasted wet wipes as promising anode material for high performance capacitive potassium-ion storage. Mater. Today Energy 2019, 13, 195–204. [Google Scholar] [CrossRef]
- Komaba, S.; Hasegawa, T.; Dahbi, M.; Kubota, K. Potassium intercalation into graphite to realize high-voltage/high-power potassium-ion batteries and potassium-ion capacitors. Electrochem. Commun. 2015, 60, 172–175. [Google Scholar] [CrossRef]
- Okoshi, M.; Yamada, Y.; Komaba, S.; Yamada, A.; Nakai, H. Theoretical analysis of interactions between potassium ions and organic electrolyte solvents: A comparison with lithium, sodium, and magnesium ions. J. Electrochem. Soc. 2016, 164, A54. [Google Scholar] [CrossRef]
- Chang, X.; Zhou, X.; Ou, X.; Lee, C.S.; Zhou, J.; Tang, Y. Ultrahigh nitrogen doping of carbon nanosheets for high capacity and long cycling potassium ion storage. Adv. Energy Mater. 2019, 9, 1902672. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, C.; Nie, L.; Chen, H.; Zang, S.; Ma, M.; Lai, Q. Recent Advances in Potassium-Ion Batteries: From Material Design to Electrolyte Engineering. Adv. Mater. Technol. 2023, 8, 2201591. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Myung, S.T.; Sun, Y.K. Recent progress in rechargeable potassium batteries. Adv. Funct. Mater. 2018, 28, 1802938. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, M.; Zhang, W.; Li, G.; Soomro, R.A.; Sun, N.; Xu, B. Advancements and Prospects of Graphite Anode for Potassium-Ion Batteries. Small Methods 2023, 7, 2300708. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Zhang, B.; Miao, L.; Cai, J.; Peng, L.; Huang, Y.; Jiang, J.; Huang, Y.; Zhang, L. Nitrogen-rich hard carbon as a highly durable anode for high-power potassium-ion batteries. Energy Storage Mater. 2017, 8, 161–168. [Google Scholar] [CrossRef]
- Chen, M.; Wang, W.; Liang, X.; Gong, S.; Liu, J.; Wang, Q.; Guo, S.; Yang, H. Sulfur/oxygen codoped porous hard carbon microspheres for high-performance potassium-ion batteries. Adv. Energy Mater. 2018, 8, 1800171. [Google Scholar] [CrossRef]
- Jian, Z.; Xing, Z.; Bommier, C.; Li, Z.; Ji, X. Hard carbon microspheres: Potassium-ion anode versus sodium-ion anode. Adv. Energy Mater. 2016, 6, 1501874. [Google Scholar] [CrossRef]
- Wang, X.; Han, K.; Qin, D.; Li, Q.; Wang, C.; Niu, C.; Mai, L. Polycrystalline soft carbon semi-hollow microrods as anode for advanced K-ion full batteries. Nanoscale 2017, 9, 18216–18222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, J.; Jia, X.; Li, W.; Zhang, Q.; Fan, L.; Ding, H.; Yang, H.; Yu, X.; Li, X.; et al. Graphene armored with a crystal carbon shell for ultrahigh-performance potassium ion batteries and aluminum batteries. ACS Nano 2019, 13, 10631–10642. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Ding, H.; Chen, S.; Yu, X.; Lu, B. Carbon Nanoscrolls for Aluminum Battery. ACS Nano 2018, 12, 8456–8466. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Luo, W.; Ji, X. Carbon electrodes for K-ion batteries. J. Am. Chem. Soc. 2015, 137, 11566–11569. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gong, Z.; He, K.; Qiu, P.; Wang, X.-C.; Zhao, L.-K.; Gu, Q.-F.; Gao, X.-W.; Luo, W.-B. Developments and Prospects of Carbon Anode Materials in Potassium-Ion Batteries. Sci. China Mater. 2024, 1–16. [Google Scholar] [CrossRef]
- Fan, L.; Chen, S.; Ma, R.; Wang, J.; Wang, L.; Zhang, Q.; Zhang, E.; Liu, Z.; Lu, B. Ultrastable Potassium Storage Performance Realized by Highly Effective Solid Electrolyte Interphase Layer. Small 2018, 14, 1801806. [Google Scholar] [CrossRef]
- Ge, J.; Fan, L.; Wang, J.; Zhang, Q.; Liu, Z.; Zhang, E.; Liu, Q.; Yu, X.; Lu, B. MoSe2/N-Doped Carbon as Anodes for Potassium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1801477. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Liu, Z.; Li, H.; Liu, Y.; Wang, Z.; Li, X.; Shih, K.; Mai, L. Li3V(MoO4)3 as a Novel Electrode Material with Good Lithium Storage Properties and Improved Initial Coulombic Efficiency. Nano Energy 2018, 44, 272–278. [Google Scholar] [CrossRef]
- Cheng, N.; Zhao, J.; Fan, L.; Liu, Z.; Chen, S.; Ding, H.; Yu, X.; Liu, Z.; Lu, B. Sb-MOFs Derived Sb Nanoparticles@Porous Carbon for High Performance Potassium-Ion Batteries Anode. Chem. Commun. 2019, 55, 12511–12514. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, H.; Bai, Y.; Liu, Z.; Chen, S.; Wu, Y.; Yu, X.; Fan, L.; Lu, B. Rational Design of a Polyimide Cathode for a Stable and High-Rate Potassium-Ion Battery. ACS Appl. Mater. Interfaces 2019, 11, 42078–42085. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Ge, J.; Hu, Y.; Shen, D.; Luo, W.; Chen, S.; Wu, L.; Liu, Z.; Zhou, J.; Yang, H.; et al. Hybrid High-Performance Aqueous Batteries with Potassium-Based Cathode||Zinc Metal Anode. Sci. China Mater. 2022, 66, 923–931. [Google Scholar] [CrossRef]
- Chen, H.; Mu, J.-J.; Bian, Y.-H.; Gao, X.-W.; Wang, D.; Liu, Z.-M.; Luo, W.-B. A Bimetallic Sulfide Co9S8/MoS2/C Heterojunction in a Three-Dimensional Carbon Structure for Increasing Sodium Ion Storage. New Carbon Mater. 2023, 38, 510–519. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, L.-K.; Zhou, J.-L.; Bian, Y.-H.; Gao, X.-W.; Chen, H.; Liu, Z.-M.; Luo, W.-B. Advances in the Use of Carbonaceous Scaffolds for Constructing Stable Composite Li Metal Anodes. New Carbon Mater. 2023, 38, 698–718. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, W.; Xu, Z.; Shih, K.; Wang, J.; Wang, Z.; Lv, X.; Chen, J.; Li, X. Molybdenum Disulfide-Coated Lithium Vanadium Fluorophosphate Anode: Experiments and First-Principles Calculations. ChemSusChem 2016, 9, 2122–2128. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, L.; Wang, J.; Xing, C.; Ge, J.; Fan, L.; Liu, Z.; Lu, X.; Wu, M.; Yu, X.; et al. Low-Temperature Synthesis of Edge-Rich Graphene Paper for High-Performance Aluminum Batteries. Energy Storage Mater. 2018, 15, 361–367. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Liu, Z.; Fan, L.; Zhang, Q.; Ding, H.; Wang, L.; Yang, H.; Yu, X.; Lu, B. Nature of Bimetallic Oxide Sb2MoO6/rGO Anode for High-Performance Potassium-Ion Batteries. Adv. Sci. 2019, 6, 1900904. [Google Scholar] [CrossRef]
- Wang, P.; Gong, Z.; Ye, K.; Gao, Y.; Zhu, K.; Yan, J.; Wang, G.; Cao, D. N-rich biomass carbon derived from hemp as a full carbon-based potassium ion hybrid capacitor anode. Appl. Surf. Sci. 2021, 553, 149569. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Q.; Liu, Z.; Yang, H.; Lu, B. Solvothermal Synthesis of Graphene Encapsulated Selenium/Carboxylated Carbon Nanotubes Electrode for Lithium–Selenium Battery. J. Alloys Compd. 2019, 810, 151894. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, J.; Wang, Z.; Zhao, L.; Li, P.; Yang, Y.; Yang, C.; Huang, H.; Guo, S. Short-Range Order in Mesoporous Carbon Boosts Potassium-Ion Battery Performance. Adv. Energy Mater. 2018, 8, 1701648. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Lu, B. Plum Pudding Model Inspired KVPO4F@3DC as High-Voltage and Hyperstable Cathode for Potassium Ion Batteries. Sci. Bull. 2020, 65, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Liu, Z.; Zhao, J.; Yu, X.; Lu, B. Rose-Petals-Derived Hemispherical Micropapillae Carbon with Cuticular Folds for Super Potassium Storage. Electrochim. Acta 2021, 368, 137629. [Google Scholar] [CrossRef]
- Yu, W.; Liu, Z.; Yu, X.; Lu, B. Balsa-Wood-Derived Binder–Free Freestanding Carbon Foam as High-Performance Potassium Anode. Adv. Energy Sustain. Res. 2021, 2, 2100018. [Google Scholar] [CrossRef]
- Mu, J.; Gao, X.-W.; Liu, Z.; Luo, W.-B.; Sun, Z.; Gu, Q.; Li, F. Boosting nitrogen electrocatalytic fixation by three-dimensional TiO2-δNδ nanowire arrays. J. Energy Chem. 2022, 75, 293–300. [Google Scholar] [CrossRef]
- Mu, J.-J.; Liu, Z.-M.; Lai, Q.-S.; Wang, D.; Gao, X.-W.; Yang, D.-R.; Chen, H.; Luo, W.-B. An Industrial Pathway to Emerging Presodiation Strategies for Increasing the Reversible Ions in Sodium-Ion Batteries and Capacitors. Energy Mater. 2022, 2, 200043. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Z.; Hu, Q.; Li, X.; Wang, Z.; Guo, H.; Wang, J. Synthesis and Performance of xLiVPO4F–yLi3V2(PO4)3 Composites as Cathode Materials for Lithium Ion Batteries. Ceram. Int. 2015, 41, 13891–13895. [Google Scholar] [CrossRef]
- Liu, Z.; Fan, Y.; Peng, W.; Wang, Z.; Guo, H.; Li, X.; Wang, J. Mechanical Activation Assisted Soft Chemical Synthesis of Na-Doped Lithium Vanadium Fluorophosphates with Improved Lithium Storage Properties. Ceram. Int. 2015, 41, 4267–4271. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, W.; Fan, Y.; Li, X.; Wang, Z.; Guo, H.; Wang, J. One-Step Facile Synthesis of Graphene-Decorated LiVPO4F/C Nanocomposite as Cathode for High-Performance Lithium Ion Battery. Ceram. Int. 2015, 41, 9188–9192. [Google Scholar] [CrossRef]
- Saju, S.K.; Chattopadhyay, S.; Xu, J.; Alhashim, S.; Pramanik, A.; Ajayan, P.M. Hard carbon anode for lithium-, sodium-, and potassium-ion batteries: Advancement and future perspective. Cell Rep. Phys. Sci. 2024, 5, 101851. [Google Scholar] [CrossRef]
- Abramova, E.N.; Bobyleva, Z.V.; Drozhzhin, O.A.; Abakumov, A.M.; Antipov, E.V. Hard carbon—Anode material for metal-ion batteries. Russ. Chem. Rev. 2024, 93, 2. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.; Tan, H.; Zhang, B. Advanced lignin-derived hard carbon for Na-ion batteries and a comparison with Li and K ion storage. Carbon 2020, 157, 316–323. [Google Scholar] [CrossRef]
- Alvin, S.; Cahyadi, H.S.; Hwang, J.; Chang, W.; Kwak, S.K.; Kim, J. Revealing the intercalation mechanisms of lithium, sodium, and potassium in hard carbon. Adv. Energy Mater. 2020, 10, 2000283. [Google Scholar] [CrossRef]
- Guo, Z.; Xu, Z.; Xie, F.; Jiang, J.; Zheng, K.; Alabidun, S.; Crespo-Ribadeneyra, M.; Hu, Y.S.; Au, H.; Titirici, M.M. Investigating the Superior Performance of Hard Carbon Anodes in Sodium-Ion Compared With Lithium-and Potassium-Ion Batteries. Adv. Mater. 2023, 35, 2304091. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fan, C.; Ou, M.; Sun, S.; Xu, Y.; Liu, Y.; Wang, X.; Li, Q.; Fang, C.; Han, J. Correlation between potassium-ion storage mechanism and local structural evolution in hard carbon materials. Chem. Mater. 2022, 34, 4202–4211. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhong, W.; Zhang, Y.; Dong, P.; Sun, S.; Zhang, Y.; Li, X. Elucidating Electrochemical Intercalation Mechanisms of Biomass-Derived Hard Carbon in Sodium-/Potassium-Ion Batteries. Carbon Energy 2021, 3, 541–553. [Google Scholar] [CrossRef]
- Kim, H.; Hyun, J.C.; Jung, J.I.; Lee, J.B.; Choi, J.; Cho, S.Y.; Jin, H.-J.; Yun, Y.S. Potassium-ion storage behavior of microstructure-engineered hard carbons. J. Mater. Chem. A 2022, 10, 2055–2063. [Google Scholar] [CrossRef]
- Maák, M.; Vyroubal, P.; Kazda, T.; Jao, K. Numerical investigation of lithium-sulfur batteries by cyclic voltammetry. J. Energy Storage 2020, 27, 101158. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Z.; Knibbe, R.; Luo, B.; Ahad, S.A.; Sun, D.; Wang, L. Cyclic Voltammetry in Lithium-Sulfur Batteries-Challenges and Opportunities. Energy Technol. Gener. Convers. Storage Distrib. 2019, 7, 1801001. [Google Scholar] [CrossRef]
- Chen, C.; Wu, M.; Wang, Y.; Zaghib, K. Insights into pseudographite-structured hard carbon with stabilized performance for high energy K-ion storage. J. Power Sources 2019, 444, 227310. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, J.; Huang, L.; Peng, H.; Xu, J.; Liu, G.; Shi, C.; Sun, Z. Low-Temperature Carbonized N/O/S-Tri-Doped Hard Carbon for Fast and Stable K-Ions Storage. Adv. Energy Mater. 2024, 14, 2303081. [Google Scholar] [CrossRef]
- Wahid, M.; Puthusseri, D.; Gawli, Y.; Sharma, N.; Ogale, S. Hard carbons for sodium-ion battery anodes: Synthetic strategies, material properties, and storage mechanisms. ChemSusChem 2018, 11, 506–526. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Pan, Z.; Zhang, Y.; Wang, B.; Peng, H. The recent progress of nitrogen-doped carbon nanomaterials for electrochemical batteries. J. Mater. Chem. A 2018, 6, 12932–12944. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zheng, F.; Ou, X.; Pan, Q.; Liu, Y.; Wang, G. High pyridine N-doped porous carbon derived from metal–organic frameworks for boosting potassium-ion storage. J. Mater. Chem. A 2018, 6, 17959–17966. [Google Scholar] [CrossRef]
- Ju, Z.; Li, P.; Ma, G.; Xing, Z.; Zhuang, Q.; Qian, Y. Few layer nitrogen-doped graphene with highly reversible potassium storage. Energy Storage Mater. 2018, 11, 38–46. [Google Scholar] [CrossRef]
- Li, D.; Ren, X.; Ai, Q.; Sun, Q.; Zhu, L.; Liu, Y.; Liang, Z.; Peng, R.; Si, P.; Lou, J. Facile fabrication of nitrogen-doped porous carbon as superior anode material for potassium-ion batteries. Adv. Energy Mater. 2018, 8, 1802386. [Google Scholar] [CrossRef]
- Chu, K.; Zhang, X.; Yang, Y.; Li, Z.; Wei, L.; Yao, G.; Zheng, F.; Chen, Q. Edge-nitrogen enriched carbon nanosheets for potassium-ion battery anodes with an ultrastable cycling stability. Carbon 2021, 184, 277–286. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, J.; Ma, Q.; Sun, H.; Li, Z.; Zhang, D.; Wang, Q.; Wu, Y.; Li, W.; Wang, B. Edge-nitrogen synergize with micropores to realize fast and durable potassium storage for carbon anode. Carbon 2023, 213, 118291. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, X.B.; Liu, Z. High sulfur-doped hard carbon anode from polystyrene with enhanced capacity and stability for potassium-ion storage. J. Energy Chem. 2022, 68, 688–698. [Google Scholar] [CrossRef]
- Alvin, S.; Chandra, C.; Kim, J. Extended plateau capacity of phosphorus-doped hard carbon used as an anode in Na-and K-ion batteries. Chem. Eng. J. 2020, 391, 123576. [Google Scholar] [CrossRef]
- Li, P.; Hwang, J.-Y.; Sun, Y.-K. Highly wrinkled carbon tubes as an advanced anode for K-ion full batteries. J. Mater. Chem. A 2019, 7, 20675–20682. [Google Scholar] [CrossRef]
- Ruan, J.; Zhao, Y.; Luo, S.; Yuan, T.; Yang, J.; Sun, D.; Zheng, S. Fast and stable potassium-ion storage achieved by in situ molecular self-assembling N/O dual-doped carbon network. Energy Storage Mater. 2019, 23, 46–54. [Google Scholar] [CrossRef]
- Cui, R.C.; Xu, B.; Dong, H.J.; Yang, C.C.; Jiang, Q. N/O dual-doped environment-friendly hard carbon as advanced anode for potassium-ion batteries. Adv. Sci. 2020, 7, 1902547. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, H.; Wu, L.; Zhou, W.; He, L.; Wang, W.; Yan, W.; Huang, Q.; Fu, L.; Wu, Y. A large scalable and low-cost sulfur/nitrogen dual-doped hard carbon as the negative electrode material for high-performance potassium-ion batteries. Adv. Energy Mater. 2019, 9, 1901379. [Google Scholar] [CrossRef]
- Qiu, W.; Xiao, H.; Li, Y.; Lu, X.; Tong, Y. Nitrogen and phosphorus codoped vertical graphene/carbon cloth as a binder-free anode for flexible advanced potassium ion full batteries. Small 2019, 15, 1901285. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Yuan, L.; Li, T.; Shu, C.; Qiao, S.; Dong, S.; Liu, Z.; Yang, J.; Liu, H.K.; Dou, S.X. Nitrogen and oxygen Co-doped porous hard carbon nanospheres with core-shell architecture as anode materials for superior potassium-ion storage. Small 2022, 18, 2104296. [Google Scholar] [CrossRef]
- Tao, L.; Yang, Y.; Wang, H.; Zheng, Y.; Hao, H.; Song, W.; Shi, J.; Huang, M.; Mitlin, D. Sulfur-nitrogen rich carbon as stable high capacity potassium ion battery anode: Performance and storage mechanisms. Energy Storage Mater. 2020, 27, 212–225. [Google Scholar] [CrossRef]
- Qiao, Y.; Ma, M.; Liu, Y.; Li, S.; Lu, Z.; Yue, H.; Dong, H.; Cao, Z.; Yin, Y.; Yang, S. First-principles and experimental study of nitrogen/sulfur co-doped carbon nanosheets as anodes for rechargeable sodium ion batteries. J. Mater. Chem. A 2016, 4, 15565–15574. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Gong, Z.; Guo, X.; Liu, J.; Zhang, Z.; Li, G. Superior potassium-ion storage properties by engineering pseudocapacitive sulfur/nitrogen-containing species within three-dimensional flower-like hard carbon architectures. Carbon 2020, 161, 97–107. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, Y.; Zhang, Q.; Luo, C.; Li, H.Y.; Wu, Y.; Zhang, H.; Wang, X.; Liu, H.; He, X. Designing and understanding the superior potassium storage performance of nitrogen/phosphorus co-doped hollow porous bowl-like carbon anodes. Adv. Funct. Mater. 2021, 31, 2007158. [Google Scholar] [CrossRef]
- Jin, S.; Liang, P.; Jiang, Y.; Min, H.; Niu, M.; Yang, H.; Zhang, R.; Yan, J.; Shen, X.; Wang, J. Preferentially engineering edge–nitrogen sites in porous hollow spheres for ultra–fast and reversible potassium storage. Chem. Eng. J. 2022, 435, 134821. [Google Scholar] [CrossRef]
- Lu, J.; Wang, C.; Yu, H.; Gong, S.; Xia, G.; Jiang, P.; Xu, P.; Yang, K.; Chen, Q. Oxygen/fluorine dual-doped porous carbon nanopolyhedra enabled ultrafast and highly stable potassium storage. Adv. Funct. Mater. 2019, 29, 1906126. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Huang, C.; Fan, H.; Yuan, D.; Luo, W.-B.; Hu, A.; Tang, Q.; Chen, X. Understanding the effect of I/N dual-doped hard carbon for high performance K-ion storage. Electrochim. Acta 2021, 394, 139146. [Google Scholar] [CrossRef]
- Jiang, Y.; Xiao, N.; Song, X.; Xiao, J.; Yu, K.; Dai, X.; Lv, Z.; Qiu, J. Coal Tar Pitch Derived sp2 Configuration-Dominated Vacancy-Rich Carbon with Expand Interlayer Spacing for Low-Voltage, Durable, and Fast Potassium Storage. Adv. Funct. Mater. 2024, 34, 2316207. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, K.; La, M.; Yang, C. Insight into a Nitrogen-Doping mechanism in a Hard-Carbon-Microsphere anode material for the Long-Term cycling of Potassium-Ion batteries. Materials 2022, 15, 4249. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yu, K.; Wang, X.; Liang, J.; Liang, C. Nitrogen self-doped porous carbon based on sunflower seed hulls as excellent double anodes for potassium/sodium ion batteries. Diam. Relat. Mater. 2023, 131, 109593. [Google Scholar] [CrossRef]
- Deng, W.-J.; He, X.-D.; Zhang, L.-M.; Wang, J.-R.; Chen, C.-H. Highly Graphitic N-Doped Biomass-Derived Hard Carbon with a Low Operating Potential for Potassium-Ion Batteries. Energy Technol. 2021, 9, 2100644. [Google Scholar] [CrossRef]
- Li, W.; Zhang, R.; Chen, Z.; Fan, B.; Xiao, K.; Liu, H.; Gao, P.; Wu, J.; Tu, C.; Liu, J. Microstructure-dependent K+ storage in porous hard carbon. Small 2021, 17, 2100397. [Google Scholar] [CrossRef]
- Zhang, D.; Niu, B.; Li, Y.; Li, Z.; Wang, H.; Wang, Q.; Yuan, F.; Hu, Z.; Wang, B. Sulfur-grafted hard carbon with expanded interlayer spacing and increased defects for high stability potassium-ion batteries. Solid State Ion. 2023, 393, 116172. [Google Scholar] [CrossRef]
- Sun, Q.; Mu, J.; Ma, F.; Li, Y.; Zhou, P.; Zhou, T.; Wu, X.; Zhou, J. Sulfur-doped hollow porous carbon spheres as high-performance anode materials for potassium ion batteries. J. Energy Storage 2023, 72, 108297. [Google Scholar] [CrossRef]
- Qiu, D.; Zhang, B.; Zhang, T.; Shen, T.; Zhao, Z.; Hou, Y. Sulfur-doped carbon for potassium-ion battery anode: Insight into the doping and potassium storage mechanism of sulfur. ACS Nano 2022, 16, 21443–21451. [Google Scholar] [CrossRef]
- Chi, C.; Liu, Z.; Lu, X.; Meng, Y.; Huangfu, C.; Yan, Y.; Qiu, Z.; Qi, B.; Wang, G.; Pang, H. Balance of sulfur doping content and conductivity of hard carbon anode for high-performance K-ion storage. Energy Storage Mater. 2023, 54, 668–679. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, Q.; Xu, W.; Zhao, R.; Zhang, C.; Guo, J.; Zhu, H.; Yuan, G.; Lv, W.; Li, X. Edge-enriched and S-doped carbon nanorods to accelerate electrochemical kinetics of sodium/potassium storage. Carbon 2023, 201, 776–784. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, X.; Qu, Z.; Zhang, X.; Lin, J.; Wu, F.; Chen, R.; Li, L. Defects and sulfur-doping design of porous carbon spheres for high-capacity potassium-ion storage. J. Mater. Chem. A 2022, 10, 682–689. [Google Scholar] [CrossRef]
- Yang, M.; Kong, Q.; Feng, W.; Yao, W. N/O double-doped biomass hard carbon material realizes fast and stable potassium ion storage. Carbon 2021, 176, 71–82. [Google Scholar] [CrossRef]
- Zhang, K.; He, Q.; Xiong, F.; Zhou, J.; Zhao, Y.; Mai, L.; Zhang, L. Active Sites Enriched Hard Carbon Porous Nanobelts for Stable and High-Capacity Potassium-Ion Storage. Nano Energy 2020, 77, 105018. [Google Scholar] [CrossRef]
- Liu, Y.; Ru, Q.; Gao, Y.; An, Q.; Chen, F.; Shi, Z.; Zheng, M.; Pan, Z. Constructing volcanic-like mesoporous hard carbon with fast electrochemical kinetics for potassium-ion batteries and hybrid capacitors. Appl. Surf. Sci. 2020, 525, 146563. [Google Scholar] [CrossRef]
- Tao, S.; Xu, W.; Zheng, J.; Kong, F.; Cui, P.; Wu, D.; Qian, B.; Chen, S.; Song, L. Soybean roots-derived N, P Co-doped mesoporous hard carbon for boosting sodium and potassium-ion batteries. Carbon 2021, 178, 233–242. [Google Scholar] [CrossRef]
- Huang, S.; Lv, Y.; Wen, W.; Xue, T.; Jia, P.; Wang, J.; Zhang, J.; Zhao, Y. Three-dimensional hierarchical porous hard carbon for excellent sodium/potassium storage and mechanism investigation. Mater. Today Energy 2021, 20, 100673. [Google Scholar] [CrossRef]
- Gong, J.; Zhao, G.; Feng, J.; An, Y.; Li, T.; Zhang, L.; Li, B.; Qian, Z. Controllable phosphorylation strategy for free-standing phosphorus/nitrogen cofunctionalized porous carbon monoliths as high-performance potassium ion battery anodes. ACS Nano 2020, 14, 14057–14069. [Google Scholar] [CrossRef]
- Kim, M.; Ma, L.; Li, Z.; Mai, W.; Amiralian, N.; Rowan, A.E.; Yamauchi, Y.; Qin, A.; Afzal, R.A.; Martin, D. N and S co-doped nanosheet-like porous carbon derived from sorghum biomass: Mechanical nanoarchitecturing for upgraded potassium ion batteries. J. Mater. Chem. A 2023, 11, 16626–16635. [Google Scholar] [CrossRef]
- Yan, F.; Yang, Q.; Li, M.; Chen, G.; Zhang, W.; Chen, Y. Facile synthesis of hollow stalagmite-like N, S-doped C and its capacity attenuation mechanism as anodes in K-ion batteries. Carbon 2022, 200, 56–62. [Google Scholar] [CrossRef]
- Qian, Y.; Li, Y.; Pan, Z.; Tian, J.; Lin, N.; Qian, Y. Hydrothermal “disproportionation” of biomass into oriented carbon microsphere anode and 3D porous carbon cathode for potassium ion hybrid capacitor. Adv. Funct. Mater. 2021, 31, 2103115. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, R.; Wu, F.; Chen, R.; Li, L. Mixed-biomass engineering achieves multi-doped highly-disordered hierarchical flower-like hard carbon for advanced potassium-ion battery. Nano Energy 2023, 117, 108913. [Google Scholar] [CrossRef]
- Wang, D.; Du, G.; Han, D.; Su, Q.; Ding, S.; Zhang, M.; Zhao, W.; Xu, B. Porous Flexible Nitrogen-Rich Carbon Membranes Derived from Chitosan as Free-Standing Anodes for Potassium-Ion and Sodium-Ion Batteries. Carbon 2021, 181, 1–8. [Google Scholar] [CrossRef]
- Jian, Z.; Hwang, S.; Li, Z.; Hernandez, A.S.; Wang, X.; Xing, Z.; Su, D.; Ji, X. Hard–soft composite carbon as a long-cycling and high-rate anode for potassium-ion batteries. Adv. Funct. Mater. 2017, 27, 1700324. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, B.; Du, Q.; Zhang, Y.; Duan, H.; Egun, I.L.; Yin, B.; He, H. Strengthening synergistic effects between hard carbon and soft carbon enabled by connecting precursors at molecular level towards high-performance potassium ion batteries. Nano Res. 2023, 16, 10985–10991. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Tong, Y.; Li, H. Unique Spindle-Like Bismuth-Based Composite toward Ultrafast Potassium Storage. Small 2022, 18, 2204045. [Google Scholar] [CrossRef]
- Li, D.; Sun, Q.; Zhang, Y.; Dai, X.; Ji, F.; Li, K.; Yuan, Q.; Liu, X.; Ci, L. Fast and stable K-ion storage enabled by synergistic interlayer and pore-structure engineering. Nano Res. 2021, 14, 4502–4511. [Google Scholar] [CrossRef]
- Yuan, F.; Zhang, D.; Li, Z.; Sun, H.; Yu, Q.; Wang, Q.; Zhang, J.; Wu, Y.; Xi, K.; Wang, B. Unraveling the intercorrelation between micro/mesopores and K migration behavior in hard carbon. Small 2022, 18, 2107113. [Google Scholar] [CrossRef]
- Park, J.; Kim, K.; Lim, E.; Hwang, J. Tuning Internal Accessibility via Nanochannel Orientation of Mesoporous Carbon Spheres for High-Rate Potassium-Ion Storage in Hybrid Supercapacitors. Adv. Funct. Mater. 2024, 2410010. [Google Scholar] [CrossRef]
- Guo, R.; Liu, X.; Wen, B.; Liu, F.; Mai, L. Engineering Mesoporous Structure in Amorphous Carbon Boosts Potassium Storage with High Initial Coulombic Efficiency. Nano-Micro Lett. 2020, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Chen, J.; Wang, L.; Li, N.; Yang, Y.; Chen, Y.; Guo, L.; Ji, X.; Zhu, Y. High-Coulombic-Efficiency Hard Carbon Anode Material for Practical Potassium-Ion Batteries. Batter. Supercaps 2024, 7, e202400010. [Google Scholar] [CrossRef]
- Nam, K.H.; Ganesan, V.; Chae, K.H.; Park, C.M. High-performance carbon by amorphization and prepotassiation for potassium-ion battery anodes. Carbon Int. J. Spons. Am. Carbon Soc. 2021, 181, 290–299. [Google Scholar] [CrossRef]








| Materials | ICE (%) | Rate Capability (mAh g −1) | Cycling Stability [Specific Capacity (mAh g−1) (Cycle Number) @Current Density (A g−1)] | Reference |
|---|---|---|---|---|
| Single-atom doping for carbon materials | ||||
| High N-doped carbon | 35.7 | 111.8 at 10 A g −1 | 203.9 (8500) @1 | [103] |
| Edge-N doped carbon | 46.8 | 217.9 at 2 A g −1 | 189.9 (2000) @2 | [87] |
| N-doped hard carbon microspheres | 53 | 93 at 2 A g −1 | 201.6 (600) @0.2 | [104] |
| N-doped porous hard carbon | 20.04 | 146 at 1.4 A g −1 | 193.1 (1000) @0.28 | [105] |
| Highly graphitic nitrogen-doped hard carbon | 65 | 118 at 2 A g −1 | 176 (260) @1 | [106] |
| P-doped hard carbon | 44.4 | 81.6 at 2 A g −1 | 72.6 (1000) @1 | [107] |
| S-doped hard carbon | 35.12 | 229 at 2 A g −1 | 220 (5000) @2 | [108] |
| S-doped hollow porous carbon spheres | 36.25 | 210 at 2 A g −1 | 211.4 (1000) @1 | [109] |
| S-doped hard carbon | 48.2 | 86.1 at 10 A g −1 | 128.2 (1500) @2 | [110] |
| High S-doped hard carbon | 42 | 162 at 10 A g −1 | 159 (1500) @2 | [111] |
| S-doped carbon nanorods | — | 43 at 5 A g −1 | 81 (1000) @1 | [112] |
| High S-doped hard carbon | 47.3 | 216 at 1 A g −1 | 188.9 (1000) @0.5 | [113] |
| Dual-atom doping for carbon materials | ||||
| N/O-co-doped yolk–shell carbon spheres | 31 | 183.3 at 1 A g −1 | 189.2 (2500) @0.5 | [95] |
| N/O-co-doped biomass carbon | 77.74 | 220.5 at 5 A g −1 | 124.19 (5000) @10 | [114] |
| N/O dual-doped hard carbon | 50.7 | 178.9 at 5 A g −1 | 189.5 (5000) @10 | [92] |
| N/O-co-doped porous hard carbon nanobelts | 49 | 235 at 1.6 A g −1 | 277 (1600) @1 | [115] |
| N/O-co-doped volcanic rock-like carbon | — | 103 at 4 A g −1 | 81 (4000) @2 | [116] |
| Hollow porous N/P-co-doped carbon spheres | 20 | 193 at 4 A g −1 | 137.6 (1500) @2 | [100] |
| N/P-co-doped hard carbon | 44 | 179 at 5 A g −1 | 138 (2000) @2 | [117] |
| N/P-co-doped hard carbon | — | 98 at 2 A g −1 | 154.2 (200) @0.1 | [118] |
| N/P dual-doped hollow porous bowl-like hard carbon | 58.3 | 213.6 at 4 A g −1 | 205.2 (1000) @2 | [99] |
| N/P-cofunctionalized porous carbon monoliths | 63.6 | 168 at 5 A g −1 | 218 (3000) @1 | [119] |
| N/S-co-doped hard carbon | 60 | 90 at 2 A g −1 | 268 (2400) @0.1 | [120] |
| Hollow stalagmite-like N/S-doped carbon | 50 | 256 at 1 A g −1 | 148 (1000) @1 | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, P.; Chen, H.; Zhang, H.; Wang, H.; Wang, L.; Guo, Y.; Qi, J.; Yi, Y.; Zhang, G. Hard Carbon as Anodes for Potassium-Ion Batteries: Developments and Prospects. Inorganics 2024, 12, 302. https://doi.org/10.3390/inorganics12120302
Qiu P, Chen H, Zhang H, Wang H, Wang L, Guo Y, Qi J, Yi Y, Zhang G. Hard Carbon as Anodes for Potassium-Ion Batteries: Developments and Prospects. Inorganics. 2024; 12(12):302. https://doi.org/10.3390/inorganics12120302
Chicago/Turabian StyleQiu, Peng, Haohong Chen, Hanzhi Zhang, Han Wang, Lianhao Wang, Yingying Guo, Ji Qi, Yong Yi, and Guobin Zhang. 2024. "Hard Carbon as Anodes for Potassium-Ion Batteries: Developments and Prospects" Inorganics 12, no. 12: 302. https://doi.org/10.3390/inorganics12120302
APA StyleQiu, P., Chen, H., Zhang, H., Wang, H., Wang, L., Guo, Y., Qi, J., Yi, Y., & Zhang, G. (2024). Hard Carbon as Anodes for Potassium-Ion Batteries: Developments and Prospects. Inorganics, 12(12), 302. https://doi.org/10.3390/inorganics12120302







