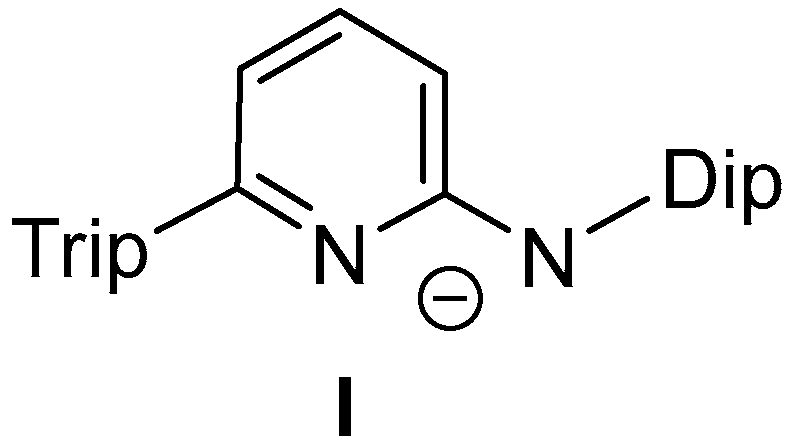
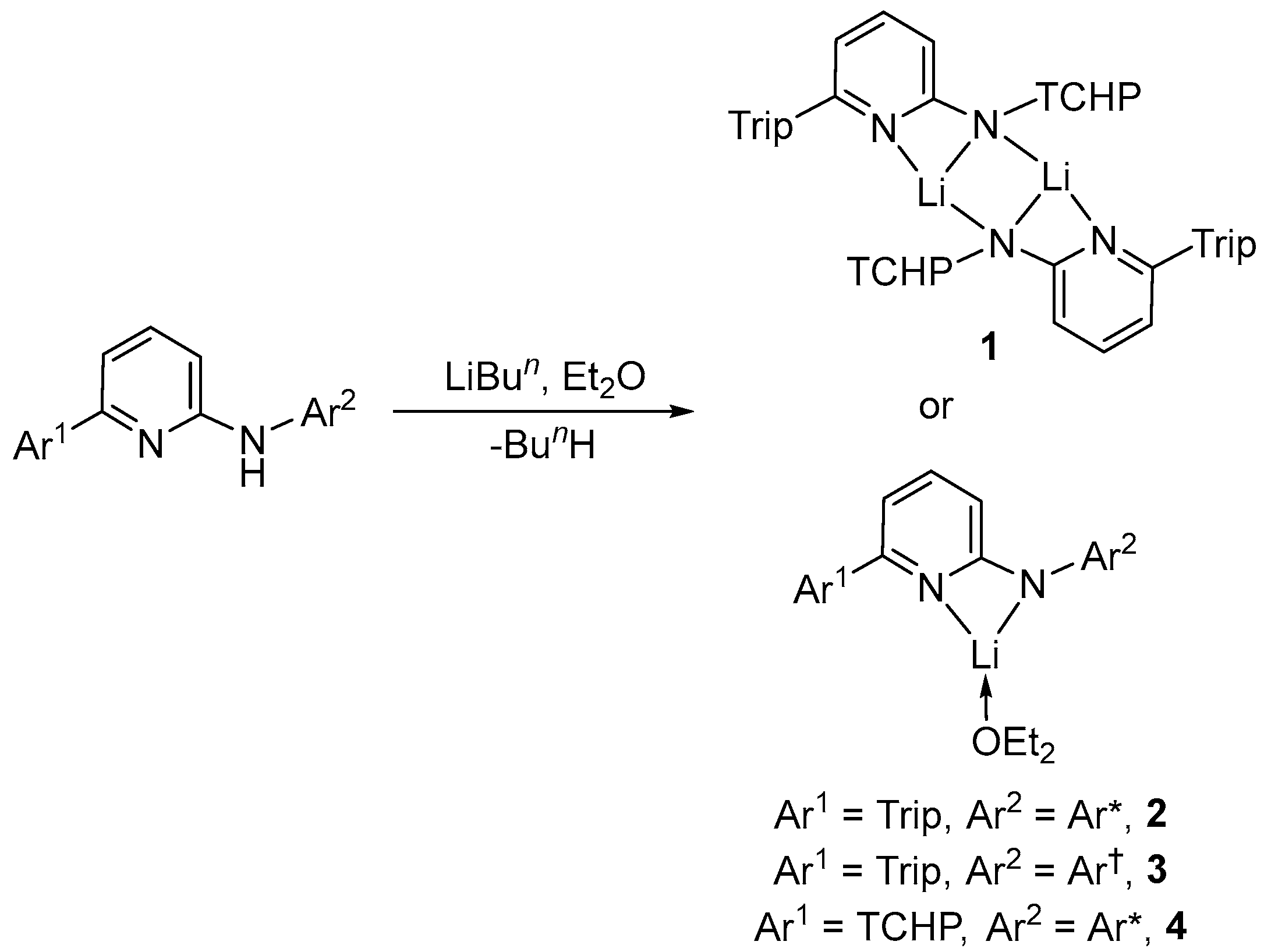
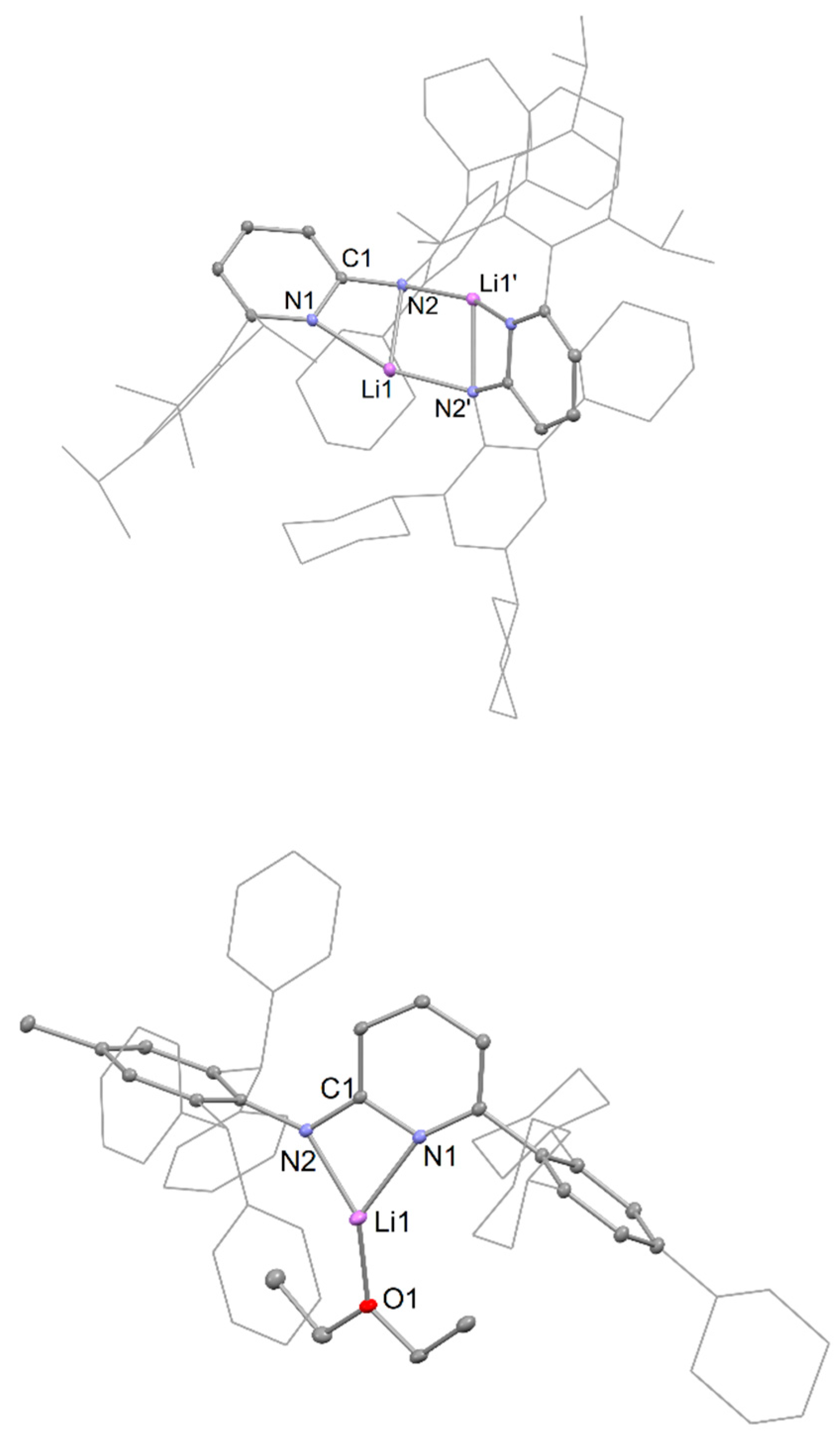
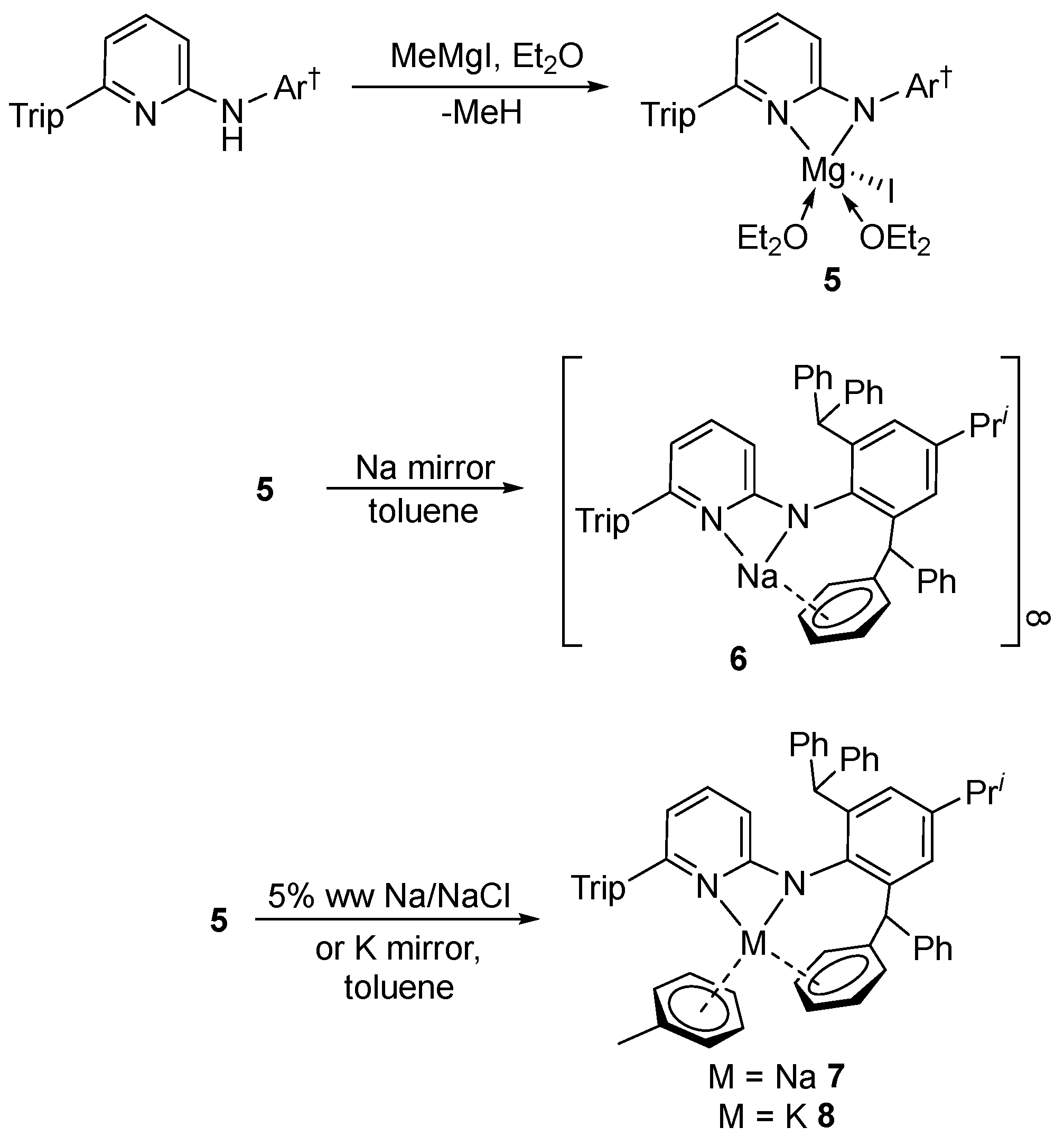
3.2. Syntheses of New Complexes
Preparation of 6-TCHP-2-bromopyridine, II (Ar1 = TCHP). 2,6-Dibromopyridine (7.91 g, 33.4 mmol), THF (35 mL), tricyclohexylphosphine (0.021 g, 0.075 mmol), and [(DME)NiBr2] (0.012 g, 0.0375 mmol) were added to a Schlenk flask under dinitrogen. A diethyl ether solution of (TCHP) MgBr (35.2 mmol) was then added to the stirred suspension, resulting in a beige precipitate. The reaction mixture was then heated at reflux for three days. Water (50 mL) and CHCl3 (50 mL) were then added carefully to the reaction mixture, and the resultant suspension transferred to a separating funnel. The organic phase was collected, and the residue was extracted with CHCl3 (80 mL). The combined organic phases were washed with brine (50 mL) and dried over Na2SO4. Volatiles were removed in vacuo to afford a white precipitate, which was recrystallised from hexane at –30 °C to give II as a colourless crystalline solid. Yield, 9.62 g (60%). M.p.: >260 °C; 1H NMR (benzene-d6, 600 MHz, 298 K): δ 1.01–2.57 (m, 33H, Cy-H), 6.73 (t, 1H, J = 7.6 Hz, pyAr-H), 6.90 (dd, 1H, J = 7.4, 0.8 Hz, pyAr-H), 6.98 (dd, 1H, J = 7.8, 0.8 Hz, pyAr-H), 7.22 (s, 2H, Ar-H); 13C{1H} NMR (benzene-d6, 151 MHz, 298 K): δ 26.3, 26.4, 27.0, 27.2, 34.6, 34.7, 34.9, 41.9, 45.3 (Cy-C), 120.2, 123.8, 125.7, 135.7, 137.5, 141.8, 145.7, 148.4, 148.4, 161.8 (pyAr-C, Ar-C); IR (ATR) ν (cm−1) = 1035 (m), 986 (s), 953 (m), 893 (m), 863 (s), 846 (m), 809 (vs), 782 (vs), 769 (vs), 752 (s), 677 (s); Acc. mass calc. for C29H38BrN (MH+): 480.2188; found: 480.2262.
Preparation of HAmPy1. Compound II (Ar1 = Trip) (2.75 g, 7.63 mmol), 1,3-bis(diphenylphosphino)propane (0.12 g, 0.28 mmol), [Pd2(dba)3] (0.13 g, 0.14 mmol), and sodium tert-butoxide (0.830 g, 8.6 mmol) were added to a Schlenk flask. After the addition of a toluene (30 mL) solution of (TCHP)NH2 (2.606 g, 7.63 mmol) to the flask, the resultant mixture was heated at 95 °C for 72 h. The reaction mixture was then cooled to room temperature, and water (50 mL) and diethyl ether (50 mL) were added. The organic phase was separated, and the remaining residue was extracted with diethyl ether (60 mL). The combined organic phases were washed with brine (50 mL) and dried over Na2SO4. Volatiles were removed under reduced pressure. The resulting red solid was purified by column chromatography (SiO2/CH2Cl2) to give the title compound as a colourless solid. Yield, 3.00 g (65%). M.p.: 230–233 °C; 1H NMR (benzene-d6, 600 MHz, 298 K): δ 1.16–1.26 (m, 5H, Cy-H), 1.27 (d, 6H, J = 7.0 Hz, CH(CH3)2), 1.31 (d, 6H, J = 7.0 Hz, CH(CH3)2), 1.32–1.37 (m, 5H, Cy-H), 1.40 (d, 6H, J = 7.0 Hz, CH(CH3)2), 1.51–1.85 (m, 18H, Cy-H), 1.98–2.01 (m, 2H, Cy-H), 2.53–2.58 (m, 1H, Cy-H), 2.90 (sept, 1H, J = 7.0 Hz, CH(CH3)2), 3.02–3.06 (m, 2H, Cy-H), 3.10 (sept, 2H, J = 7.0 Hz, CH(CH3)2), 5.88–5.90 (m, 1H, pyAr-H), 5.98 (br s, 1H, NH), 6.54 (dd, 1H, J = 7.2, 0.8 Hz, pyAr-H), 6.93–6.97 (m, 1H, pyAr-H), 7.21 (s, 2H, Ar-H), 7.27 (s, 2H, Ar-H); 13C{1H} NMR (benzene-d6, 151 MHz, 298 K): δ 24.5, 24.5, 24.8, 24.9 (CH(CH3)2), 26.6, 26.7, 27.4 (Cy-C), 30.9 (CH(CH3)2), 30.9 (Cy-C), 35.0 (CH(CH3)2), 35.1, 39.9, 39.9, 45.3 (Cy-C), 103.5, 115.1, 115.1, 120.8, 123.5, 128.3, 132.8, 137.4, 138.0, 146.7, 147.2, 147.6, 148.6, 159.4, 160.0 (pyAr-C, Ar-C); IR (ATR) ν (cm−1) = 3398 (w, NH), 1584 (s), 1571 (vs), 1065 (m), 985 (m), 879 (s), 865 (m), 804 (s), 753 (m), 733 (s), 697 (m), 662 (m); Acc. mass calc. for C44H62N2 (MH+): 619.4913; found: 619.4977.
Preparation of HAmPy2. The compound was prepared by a similar procedure used for HAmPy1, but using II (Ar1 = Trip) (2.75 g, 7.63 mmol) and Ar*NH2 (3.35 g, 7.63 mmol). Yield, 2.97 g (55%). M.p.: >260 °C; 1H NMR (benzene-d6, 600 MHz, 298 K): δ 1.25 (d, 6H, J = 7.0 Hz, CH(CH3)2), 1.27–1.28 (m, 6H, CH(CH3)2), 1.31 (d, 6H, J = 7.0 Hz, CH(CH3)2), 1.89 (s, 3H, CH3), 2.90 (sept, 1H, J = 7.0 Hz, CH(CH3)2), 3.02 (sept, 2H, J = 7.0 Hz, CH(CH3)2), 5.55 (br s, 1H, NH), 5.80 (d, 1H, J = 8.3 Hz, pyAr-H), 5.92 (s, 2H, CHPh2), 6.51–6.53 (m, 1H, pyAr-H), 6.92–7.23 (m, 25H, Ar-H, pyAr-H); 13C{1H} NMR (benzene-d6, 151 MHz, 298 K): δ 21.4 (CH3), 24.4, 24.5, 24.8 (CH(CH3)2), 30.9, 35.0 (CH(CH3)2), 52.9 (CHPh2), 103.1, 115.2, 116.0, 120.8, 126.6, 128.0, 128.3, 128.6, 130.0, 134.7, 137.1, 138.0, 143.6, 145.2, 146.7, 148.6, 158.0, 159.4 (pyAr-C, Ar-C); IR (ATR) ν (cm−1) = 3403 (w, NH), 1078 (m), 1032 (m), 986 (m), 879 (m), 796 (m), 765 (m), 742 (s), 697 (vs); Acc. mass calc. for C53H54N2 (MH+): 719.4287; found: 719.4358.
Preparation of HAmPy3. The compound was prepared by a similar procedure used for HAmPy1, but using II (Ar1 = Trip) (2.75 g, 7.63 mmol) and Ar†NH2 (3.56 g, 7.63 mmol). Yield, 3.42 g (60%). M.p.: 250–255 °C; 1H NMR (benzene-d6, 600 MHz, 298 K): δ 1.26 (d, 6H, J = 7.0 Hz, CH(CH3)2), 1.31 (d, 6H, J = 7.0 Hz, CH(CH3)2), 2.49 (sept, 1H, J = 7.1 Hz, CH(CH3)2), 2.90 (sept, 1H, J = 7.0 Hz, CH(CH3)2), 3.02 (sept, 2H, J = 7.1 Hz, CH(CH3)2), 5.56 (br s, 1H, NH), 5.80–5.82 (m, 1H, pyAr-H), 5.94 (s, 2H, CHPh2), 6.51–6.54 (m, 1H, pyAr-H), 6.90–6.93 (m, 1H, pyAr-H), 6.98–7.23 (m, 24H, Ar-H); 13C{1H} NMR (benzene-d6, 151 MHz, 298 K): δ 24.0, 24.4, 24.5, 24.8 (CH(CH3)2), 30.9, 34.2, 35.0 (CH(CH3)2), 53.1 (CHPh2), 103.2, 115.2, 120.8, 126.6, 127.3, 128.6, 129.9, 135.0, 137.1, 137.8, 143.7, 145.0, 146.7, 147.9, 148.6, 157.9, 159.3 (pyAr-C, Ar-C); IR (ATR) ν (cm−1) = 3396 (w, NH), 1099 (m), 1072 (m), 1030 (m), 984 (m), 876 (m), 796 (s), 760 (m), 741 (s), 696 (vs); acc. mass calc. for C55H58N2 (MH+): 747.4600; found: 747.4657.
Preparation of HAmPy4. The compound was prepared by a similar procedure used for HAmPy1, but using II (Ar1 = TCHP) (3.66 g, 7.63 mmol) and Ar*NH2 (3.35 g, 7.63 mmol). Yield, 3.20 g (50%).
M.p.: 250–260 °C; 1H NMR (benzene-d6, 600 MHz, 298 K): δ 1.17–2.64 (m, 33H, Cy-H), 1.91 (s, 3H, CH3), 5.67 (br s, 1H, NH), 5.87 (d, 1H, J = 9.0 Hz, pyAr-H), 5.99 (s, 2H, CHPh2), 6.58 (d, 1H, J = 7.2 Hz, pyAr-H), 6.93–6.98 (m, 1H, pyAr-H), 6.99–7.25 (m, 24H, Ar-H); 13C{1H} NMR (benzene-d6, 151 MHz, 298 K): δ 21.4 (CH3), 26.7, 27.5, 27.6, 27.7, 35.0, 35.0, 35.2, 41.9, 45.6 (Cy-C), 52.8 (CHPh2), 115.5, 122.1, 126.7, 128.7, 129.9, 135.1, 137.3, 138.3, 143.6, 145.3, 145.7, 147.6, 158.6, 159.8 (pyAr-C, Ar-C); IR (ATR) ν (cm−1) = 3393 (w, NH), 1076 (m), 1031 (m), 860 (m), 800 (m), 745 (s), 698 (vs); Acc. mass calc. for C62H66N2 (MH+): 839.5226; found: 839.5302.
Preparation of HAmPy5. The compound was prepared by a similar procedure used for HAmPy1, but using II (Ar1 = TCHP) (3.66 g, 7.63 mmol) and Ar†NH2 (3.56 g, 7.63 mmol). Yield, 3.30 g (50%).
M.p.: 250–260 °C; 1H NMR (benzene-d6, 600 MHz, 298 K): δ 0.99 (d, 6H, J = 7.0 Hz, CH(CH3)2), 1.17–2.62 (m, 33H, Cy-H), 2.52 (sept, 1H, J = 6.8 Hz, CH(CH3)2), 5.69 (br s, 1H, NH), 5.89 (d, 1H, J = 8.2 Hz, pyAr-H), 6.01 (s, 2H, CHPh2), 6.57 (d, 1H, J = 7.0 Hz, pyAr-H), 6.96 (m, 1H, pyAr-H), 7.00–7.25 (m, 24H, Ar-H); 13C{1H} NMR (benzene-d6, 151 MHz, 298 K): δ 24.1 (CH(CH3)2), 26.7, 27.5, 27.6, 27.7 (Cy-C), 34.2 (CH(CH3)2), 34.9, 35.0, 35.2, 41.9, 45.6 (Cy-C), 53.0 (CHPh2), 103.4, 115.5, 122.1, 126.7, 127.3, 128.4, 128.6, 128.7, 129.8, 130.0, 135.3, 137.3, 138.3, 143.5, 143.7, 145.2, 145.7, 147.5, 148.1, 158.6, 159.8 (pyAr-C, Ar-C); IR (ATR) ν (cm−1) = 3396 (w, NH), 1033 (m), 985 (m), 862 (m), 800 (m), 746 (m), 699 (vs); Acc. mass calc. for C64H70N2 (MH+): 867.5539; found: 867.5603.
Preparation of [{Li(AmPy1)}2], 1. LiBun in n-hexane (0.63 mL, 1.6 M, 1.0 mmol) was slowly added to a stirred solution of HAmPy1 (0.62 g, 1.0 mmol) in diethyl ether (30 mL) at 0 °C. After complete addition, the mixture was stirred and warmed slowly to room temperature over 2 h. The reaction mixture was then reduced to dryness under vacuum and the residue was extracted with hexane (20 mL). The solvent volume was then reduced to 10 mL in vacuo. Upon standing overnight, colourless crystals of 1 were obtained. Yield, 0.56 g (90%). M.p.: 250–255 °C; 1H NMR (toluene-d8, 600 MHz, 298 K): δ 1.22–2.98 (m, 33H, Cy-H), 1.25 (d, 6H, J = 7.0 Hz, CH(CH3)2), 1.30 (d, 6H, J = 7.0 Hz, CH(CH3)2), 1.37 (d, 6H, J = 7.0 Hz, CH(CH3)2), 2.89 (sept, 1H, J = 7.0 Hz, CH(CH3)2), 3.04 (sept, 2H, J = 7.0 Hz, CH(CH3)2), 5.80–5.84 (m, 1H, pyAr-H), 5.86 (d, 1H, J = 8.0 Hz, pyAr-H), 6.49 (d, 1H, J = 7.0 Hz, pyAr-H), 7.17 (s, 2H, Ar-H), 7.21 (s, 2H, Ar-H); 13C{1H} NMR (toluene-d8, 151 MHz, 298 K): δ 24.4, 24.5, 24.8 (CH(CH3)2), 26.6, 26.7, 27.4, 27.5 (Cy-C), 30.9 (CH(CH3)2), 35.0 (Cy-C), 35.1 (CH(CH3)2), 39.9, 45.3 (Cy-C), 103.4, 115.0, 120.7, 123.3, 124.7, 127.6, 128.3, 128.6, 132.7, 137.3, 138.1, 146.6, 147.0, 147.5, 148.4, 159.4, 160.0 (pyAr-C, Ar-C); IR (ATR) ν (cm−1) = 1154 (m), 1117 (m), 1075 (m), 1031 (m), 984 (m), 876 (m), 799 (m), 747 (m), 698 (vs). N.B. A reproducible microanalysis could not be obtained for this compound due to its high air and moisture sensitivity.
Preparation of [Li(AmPy2)(OEt2)], 2. LiBun in n-hexane (0.63 mL, 1.6 M, 1.0 mmol) was slowly added to a stirred solution of HAmPy2 (0.72 g, 1.0 mmol) in diethyl ether (30 mL) at 0 °C. After complete addition, the mixture was stirred and warmed slowly to room temperature over 2 h. The reaction mixture was reduced to dryness under vacuum and the residue was extracted with hexane (20 mL). The solvent volume was reduced to 10 mL. Upon standing overnight, colourless crystals of 2 were obtained. Yield, 0.68 g (85%). M.p.: 220–225 °C; 1H NMR (benzene-d6, 600 MHz, 298 K): δ 0.43 (t, 6H, J = 7.0 Hz, OCH2CH3), 1.25–1.28 (m, 18H, CH(CH3)2), 2.08 (s, 3H, CH3), 2.64 (q, 4H, J = 7.0 Hz, OCH2CH3), 2.84 (sept, 1H, J = 7.0 Hz, CH(CH3)2), 3.25 (sept, 2H, J = 7.0 Hz, CH(CH3)2), 5.74 (dd, 1H, J = 8.6 Hz, 0.8 Hz, pyAr-H), 5.96 (dd, 1H, J = 6.8 Hz, 0.8 Hz, pyAr-H), 6.05 (s, 2H, CHPh2), 6.92–7.31 (m, 25H, pyAr-H, Ar-H); 13C{1H} NMR (benzene-d6, 151 MHz, 298 K): δ 14.1 (OCH2CH3), 21.5 (CH3), 24.6, 24.8, 25.0 (CH(CH3)2), 30.6, 34.9 (CH(CH3)2), 53.0 (CH(Ph)2), 65.9 (OCH2CH3), 103.4, 105.3, 120.4, 125.7, 126.1, 127.9, 128.1, 128.2, 128.4, 128.4, 129.5, 129.6, 129.9, 130.5, 137.6, 139.2, 140.1, 145.2, 146.1, 146.7, 147.8, 147.9, 156.8, 167.6 (pyAr-C, Ar-C); 7Li NMR (benzene-d6, 155 MHz, 298 K): δ 0.70; IR (ATR) ν (cm−1) = 1155 (m), 1067 (m), 1031 (m), 990 (m), 786 (m), 764 (m), 740 (m), 698 (vs). N.B. A reproducible microanalysis could not be obtained for this compound due to its high air and moisture sensitivity.
Preparation of [Li(AmPy3)(OEt2)], 3. This compound was prepared by a similar procedure used for 2, but using HAmPy3 (0.75 g, 1.0 mmol). Yield, 0.78 g (95%). M.p.: 235–240 °C; 1H NMR (benzene-d6, 600 MHz, 298 K): δ 0.46 (t, 6H, J = 7.2 Hz, OCH2CH3), 1.10 (d, 6H, J = 7.0 Hz, CH(CH3)2), 1.25–1.29 (m, 18H, CH(CH3)2), 2.64 (sept, 1H, J = 7.0 Hz, CH(CH3)2), 2.67 (q, 4H, J = 7.2 Hz, OCH2CH3), 2.84 (sept, 1H, J = 7.0 Hz, CH(CH3)2), 3.25 (sept, 2H, J = 7.0 Hz, CH(CH3)2), 5.67 (dd, 1H, J = 8.6 Hz, 0.9 Hz, pyAr-H), 5.95 (dd, 1H, J = 7.0 Hz, 0.9 Hz, pyAr-H), 6.06 (s, 2H, CHPh2), 6.88 (dd, 1H, J = 9.7 Hz, 6.8 Hz, pyAr-H), 6.94–7.35 (m, 24H, Ar-H); 13C{1H} NMR (benzene-d6, 151 MHz, 298K): δ 14.2 (OCH2CH3), 24.6, 24.6, 24.8, 25.0 (CH(CH3)2), 30.6, 34.1, 34.9 (CH(CH3)2), 53.2 (CHPh2), 65.9 (OCH2CH3), 103.6, 105.3, 120.3, 125.6, 126.1, 126.7, 128.3, 128.4, 128.4, 128.7, 129.6, 130.0, 130.5, 137.5, 139.2, 139.9, 140.9, 143.5, 145.3, 146.3, 146.7, 147.9, 148.2, 156.8, 167.6 (pyAr-C, Ar-C). 7Li NMR (benzene-d6, 155 MHz, 298 K): δ 0.79; IR (ATR) ν (cm−1) = 1154 (m), 1076 (m), 1031 (m), 991 (m), 784 (m), 737 (m), 699 (vs). N.B. A reproducible microanalysis could not be obtained for this compound due to its high air and moisture sensitivity.
Preparation of [Li(AmPy4)(OEt2)], 4. This compound was prepared by a similar procedure used for 2, but using HAmPy4 (0.84 g, 1.0 mmol). Yield, 0.73 g (80%). M.p.: 240–242 °C; 1H NMR (benzene-d6, 600 MHz, 298 K): δ 0.60 (br t, 6H, OCH2CH3), 1.18–2.82 (m, 33H, Cy-H), 2.08 (s, 3H, CH3), 2.81 (br q, 4H, OCH2CH3), 5.89 (d, 1H, J = 8.6 Hz, pyAr-H), 6.03 (d, 1H, J = 6.8 Hz, pyAr-H), 6.13 (s, 2H, CH(Ph)2), 6.93–7.13 (m, 25H, pyAr-H, Ar-H); 13C{1H} NMR (benzene-d6, 151 MHz, 298 K): δ 14.5 (OCH2CH3), 21.5 (CH3), 26.6, 26.7, 27.5, 27.8, 35.3, 35.3, 35.6, 41.5, 45.5 (Cy-C), 52.6 (CHPh2), 65.9 (OCH2CH3), 103.8, 105.6, 121.8, 125.6, 126.2, 128.3, 128.5, 129.5, 129.6, 130.5, 137.8, 139.6, 139.7, 145.7, 145.7, 146.4, 146.8, 147.9, 157.3, 168.0 (pyAr-C, Ar-C). 7Li NMR (benzene-d6, 155 MHz, 298 K): δ 0.82; IR (ATR) ν (cm−1) = 1154 (m), 1076 (m), 1032 (m), 990 (m), 863 (m), 787 (m), 699 (vs). N.B. A reproducible microanalysis could not be obtained for this compound due to its high air and moisture sensitivity.
Preparation of [Mg(AmPy3)I(OEt2)2], 5. A 1M solution of MeMgI in diethyl ether (0.5 mL, 0.5 mmol) was added to a stirred solution of HAmPy3 (373 mg, 0.50 mmol) in diethyl ether (10 mL) at –78 °C over a period of 5 min. A colourless precipitate immediately formed. The resultant suspension was warmed to room temperature and stirred for 2 h. The white precipitate of 5 was collected by filtration. The supernatant solution was concentrated and then cooled to –30 °C overnight to give a second crop of 5. Yield, 432 mg (85%). M.p. 250–252 °C; 1H NMR (600 MHz, benzene-d6, 298 K): δ 0.90 (br t, 6H, OCH2CH3), 1.09 (d, 6H, J = 6.8 Hz, CH(CH3)2), 1.18 (d, 6H, J = 6.8 Hz, CH(CH3)2), 1.23 (d, 6H, J = 6.8 Hz, CH(CH3)2), 1.46 (d, 6H, J = 6.8 Hz, CH(CH3)2), 2.63 (sept, 1H, J = 6.8 Hz, CH(CH3)2), 2.81 (sept, 1H, J = 6.8 Hz, CH(CH3)2), 3.06 (sept, 2H, J = 6.8, CH(CH3)2), 3.17 (br q, 4H, OCH2CH3), 4.67 (d, 1H, J = 8.6 Hz, PyAr-H), 5.72 (d, 1H, J = 6.9 Hz, pyAr-H), 6.12–6.17 (m, 1H, pyAr-H), 6.61 (s, 2H, CHPh2) 6.94–7.44 (m, 24H, Ar-H); 13C{1H} NMR (151 MHz, benzene-d6, 298 K): δ 14.7 (OCH2CH3), 24.1, 24.4, 24.4, 26.3 (CH(CH3)2), 30.4, 34.1, 34.9 (CH(CH3)2), 52.4 (CHPh2), 66.8 (OCH2CH3), 106.7, 109.0, 120.8, 126.1, 126.1, 130.2, 130.4, 136.4, 136.9, 141.6, 144.2, 145.8, 147.0, 149.3, 153.9, 168.9 (pyAr-C, Ar-C). ATR-IR: ν = 1031 (m), 798 (m), 743 (m), 695 (vs); MS (EI, 70 eV): m/z (%, fragment) = 746.6 (35.77, [HAmPy3]+). A reproducible microanalysis could not be obtained for this compound due to its air and moisture sensitivity, and the fact that it readily loses diethyl ether of crystallization.
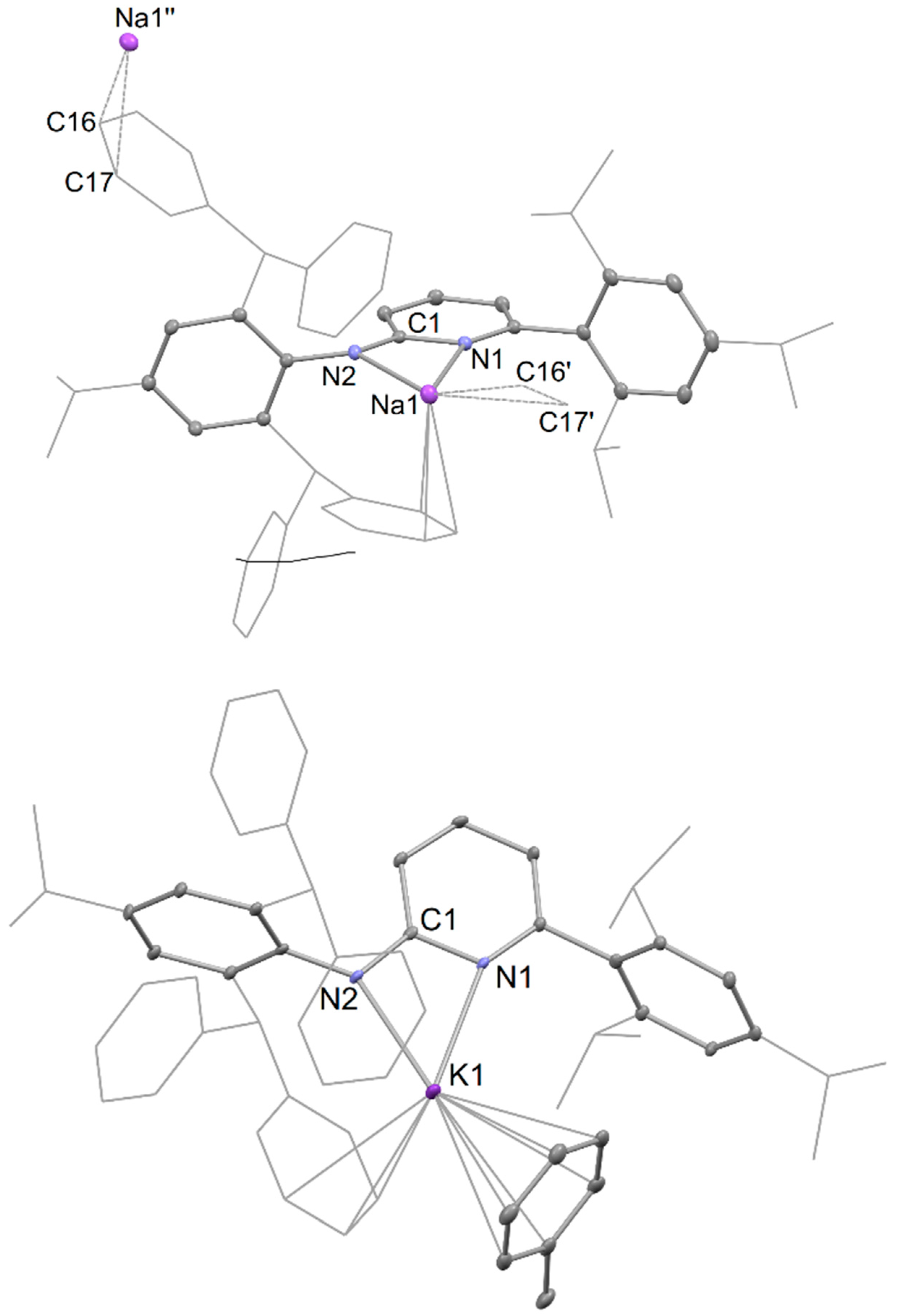
Preparation of [{Na(AmPy3)}∞], 6. A solution of 5 (1.0 g, 1 mmol) in toluene (20 mL) and diethyl ether (1 mL) was stirred vigorously for 3 days over a sodium mirror (230 mg, 10 mmol) at room temperature. The resultant yellowish suspension was filtered, and the filtrate was concentrated under reduced pressure. Subsequent slow cooling to –30 °C over a period of 24 h provided 6 as colourless crystals. Yield, 653 mg (86%). M.p. 245–250 °C; 1H NMR (600 MHz, benzene-d6, 298 K): δ 0.96 (d, 6H, J = 6.8 Hz, CH(CH3)2), 1.25 (d, 12H, J = 6.8 Hz, CH(CH3)2), 1.31 (d, 6H, J = 6.8 Hz, CH(CH3)2), 2.49 (sept, 1H, J = 7.0 Hz, CH(CH3)2), 2.90 (sept, 1H, J = 7.0 Hz, CH(CH3)2), 3.01 (sept, 2H, J = 7.0 Hz, CH(CH3)2), 5.81 (d, 1H, J = 8.2 Hz, pyAr-H), 5.94 (s, 2H, CHPh2), 6.52 (d, 1H, J = 6.8 Hz, pyAr-H), 6.91 (t, 1H, J = 7.4 Hz, pyAr-H) 6.98–7.23 (m, 24H, Ar-H); 13C{1H} NMR (151 MHz, benzene-d6, 298K): δ 24.1, 24.4, 24.5, 24.8 (CH(CH3)2), 30.9, 34.2, 35.0 (CH(CH3)2), 53.1 (CHPh2), 103.2, 115.2, 120.8, 125.7, 126.6, 127.3, 128.6, 128.6, 129.9, 135.1, 137.1, 137.8, 143.7, 145.1, 146.7, 147.9, 148.6, 157.9, 159.4 (pyAr-C, Ar-C). ATR-IR: ν = 1097 (m), 1072 (m), 1030 (m), 876 (m), 834 (m), 796 (s), 741 (m), 696 (vs); MS (EI, 70 eV): m/z (%, fragment) = 746.6 (30.66, [HAmPy3]+). A reproducible microanalysis could not be obtained for this compound due to its air and moisture sensitivity, and the fact that it readily loses toluene of crystallization.
Preparation of [Na(AmPy3)(toluene)], 7. A solution of 5 (1.0 g, 1 mmol) in toluene (20 mL) and diethyl ether (1 mL) was transferred to a Schlenk flask containing a suspension of 5% w/w Na/NaCl (4.60 g, 10 mmol Na) in 10 mL of toluene, then the mixture was stirred for 3 days. The resultant yellowish suspension was filtered, and the filtrate was concentrated under reduced pressure. Subsequent slow cooling to –30 °C over 24 h provided compound 7 as colourless crystals. Yield, 727 mg (84%). M.p. 247–250 °C; 1H NMR (600 MHz, benzene-d6, 298 K): δ 1.08 (d, 6H, J = 6.8 Hz, CH(CH3)2), 1.24–1.26 (m, 12H, CH(CH3)2), 1.27 (d, 6H, J = 6.8 Hz, CH(CH3)2), 2.11 (s, 6H, toluene-CH3), 2.62 (sept, 1H, J = 7.0 Hz, CH(CH3)2), 2.85 (sept, 1H, J = 7.0 Hz, CH(CH3)2), 3.26 (sept, 2H, J = 7.0 Hz, CH(CH3)2), 5.77 (s, 2H, CHPh2), 6.00–6.05 (m, 2H, pyAr-H), 6.68 (t, 1H, J = 7.2 Hz, pyAr-H), 6.86–7.27 (m, 34H, Ar-H); 13C{1H} NMR (151 MHz, benzene-d6, 298 K): δ 21.5 (toluene-CH3), 24.6, 24.7, 25.0, 25.2 (CH(CH3)2), 30.5, 34.2, 35.0 (CH(CH3)2), 53.8 (CHPh2), 103.1, 104.7, 120.5, 124.8, 125.7, 126.2, 126.6, 127.4, 128.3, 128.4, 128.6, 129.3, 129.5, 130.6, 137.1, 137.9, 138.8, 139.8, 140.7, 144.9, 146.7, 147.3, 147.9, 149.0, 158.1, 163.3 (pyAr-C, Ar-C). ATR-IR: ν = 1076 (m), 1030 (m), 984 (m), 875 (m), 767 (m), 742 (s), 697 (vs); MS (EI, 70 eV): m/z (%, fragment) = 746.7 (33.52, [HAmPy3]+). A reproducible microanalysis could not be obtained for this compound due to its air and moisture sensitivity, and the fact that it appears to readily loose toluene of coordination.
Preparation of [K(AmPy3)(toluene)], 8. A solution of 5 (1.0 g, 1 mmol) in toluene (20 mL) and diethyl ether (1 mL) was stirred vigorously for 3 days over a potassium mirror (390 mg, 10 mmol) at room temperature. The resultant reddish suspension was filtered, and the filtrate was concentrated under reduced pressure. Placement at –30 °C for 2 days provided compound 8 as colourless crystals. Yield, 654 mg (75%). M.p. 215–220 °C; 1H NMR (600 MHz, benzene-d6, 298 K) δ = 1.10 (d, 6H, J = 6.8 Hz, CH(CH3)2), 1.25–1.29 (m, 18H, CH(CH3)2), 2.11 (s, 3H, toluene-CH3), 2.64 (sept, 1H, J = 7.0 Hz, CH(CH3)2), 2.87 (sept, 1H, J = 7.0 Hz, CH(CH3)2), 3.32 (sept, 2H, J = 7.0 Hz, CH(CH3)2), 5.81 (s, 2H, CHPh2), 6.00 (d, 1H, J = 6.8 Hz, pyAr-H), 6.13 (d, 1H, J = 8.2 Hz, pyAr-H), 6.61 (t, 1H, J = 7.3 Hz, pyAr-H), 7.01–7.25 (m, 29H, Ar-H); 13C{1H} NMR (151 MHz, benzene-d6, 298K) δ 21.4 (toluene-CH3), 24.7, 24.7, 24.8, 25.6 (CH(CH3)2), 30.6, 34.2, 35.0 (CH(CH3)2), 53.9 (CHPh2), 103.2, 103.7, 120.4, 124.3, 125.7, 126.2, 126.3, 127.2, 128.3, 128.4, 128.6, 129.3, 129.9, 130.6, 136.7, 137.9, 138.2, 138.5, 141.5, 144.8, 146.8, 147.6, 149.3, 149.9, 158.2, 162.6 (pyAr-C, Ar-C); ATR-IR: ν = 1153 (m), 1073 (m), 1031 (m), 983 (m), 875 (m), 797 (m), 741 (s), 696 (vs); MS (EI, 70 eV): m/z (%, fragment) = 746.7 (39.23, [HAmPy3]+). A reproducible microanalysis could not be obtained for this compound due to its air and moisture sensitivity, and the fact that it appears to readily loose toluene of coordination.
Preparation of [(AmPy3)Mg(μ-I)2Mg(AmPy3)(OEt2)], 9. A solution of 5 (500 mg, 0.5 mmol) and [{(MesNacNac)Mg}2] (182 mg, 0.25 mmol) in toluene (20 mL) was stirred overnight at 70 °C, during which time, the initial yellow solution turned red. The reaction mixture was then filtered, and the filtrate was concentrated under reduced pressure. Placement at –30 °C overnight yielded a few colourless crystals of 9. Due to the very low yield of the compound, no spectroscopic data could be obtained.