Structural Evolution of Olivine during Mechanochemically Assisted Mineral Carbonation under CO2 Flow
Abstract
1. Introduction
2. Results and Discussion
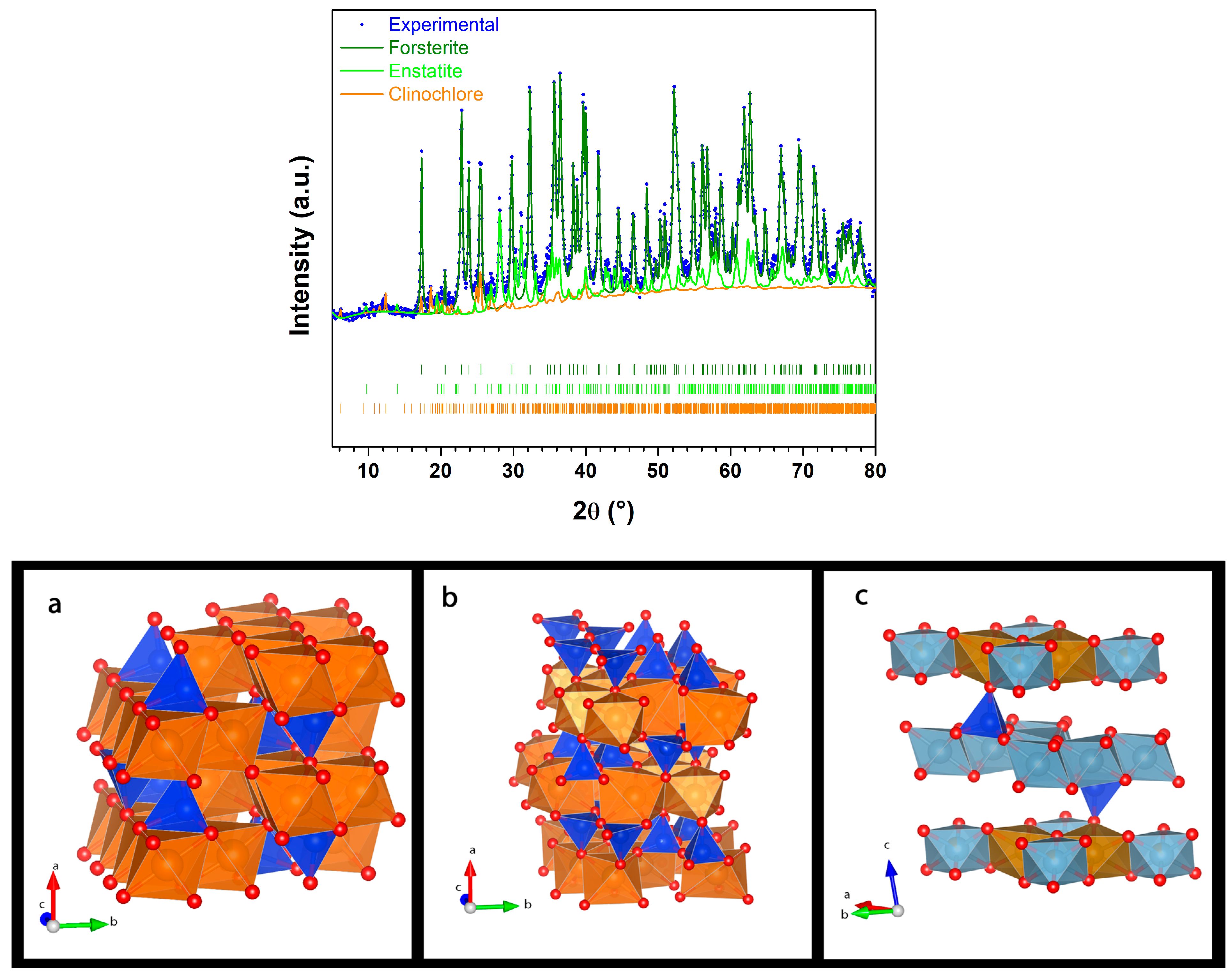
| ← Sample | Phase name → | Forsterite Ferroan | Enstatite Ferrous | Clinochlore | Nesquehonite | Magnesite | Iron | ||
| Formula → | Mg1.8Fe0.2SiO4 | Mg0.8Fe0.2SiO3 | (Mg,Fe(II))5Al(Si3Al) O10(OH)8 | MgCO3·3H2O | MgCO3 | α-Fe | |||
| Sp. Group→ | Pbnm | Pbca | P | P21c | |||||
| Pristine | a (Å) | 4.76 | 18.26 | 5.15 | α = 93.95 | – | – | – | |
| b (Å) | 10.22 | 8.83 | 9.58 | β = 95.60 | – | – | – | ||
| c (Å) | 5.99 | 5.20 | 14.42 | γ = 89.58 | – | – | – | ||
| Weight % | 92.5 | 5.0 | 2.5 | – | – | – | |||
| Rwp % | 11.43 | ||||||||
| 2 min BM | a (Å) | 4.76 | 18.25 | 5.38 | α = 91.55 | 7.70 | – | – | |
| b (Å) | 10.22 | 8.83 | 9.52 | β = 102.24 | 5.37 | β = 89.99 | – | – | |
| c (Å) | 5.99 | 5.19 | 14.63 | γ = 88.67 | 12.11 | – | – | ||
| Weight % | 91.0 | 4.4 | 3.4 | 1.1 | – | – | |||
| Rwp % | 11.59 | ||||||||
| 20 min BM | a (Å) | 4.76 | 18.26 | 5.40 | α = 92.21 | 7.70 | 5.03 | ||
| b (Å) | 10.22 | 8.83 | 9.52 | β = 101.71 | 5.38 | β = 90.01 | |||
| c (Å) | 5.99 | 5.19 | 14.61 | γ = 88.91 | 12.09 | 17.95 | |||
| Weight % | 91.1 | 5.2 | 2.9 | 0.4 | 0.2 | 0.2 | |||
| Rwp % | 11.57 | ||||||||
| 60 min BM | a (Å) | 4.76 | 18.26 | 5.32 | α = 93.24 | 7.71 | 5.05 | 2.86 | |
| b (Å) | 10.22 | 8.84 | 9.54 | β = 99.99 | 5.36 | β = 90.31 | |||
| c (Å) | 5.99 | 5.19 | 14.55 | γ = 91.47 | 12.10 | 17.85 | |||
| Weight % | 90.4 | 6.0 | 1.8 | 0.9 | 0.2 | 0.6 | |||
| Rwp % | 11.34 | ||||||||
| 90 min BM | a (Å) | 4.76 | 18.26 | 5.34 | α = 90.68 | 7.74 | 5.05 | 2.86 | |
| b (Å) | 10.22 | 8.84 | 9.39 | β = 98.11 | 5.36 | β = 90.71 | |||
| c (Å) | 5.99 | 5.19 | 14.47 | γ = 92.01 | 12.10 | 17.85 | |||
| Weight % | 90.6 | 6.5 | 1.8 | 0.3 | 0.2 | 0.6 | |||
| Rwp % | 9.66 | ||||||||
| 140 min BM | a (Å) | 4.76 | 18.26 | 5.42 | α = 91.67 | 7.73 | 5.43 | 2.87 | |
| b (Å) | 10.22 | 8.82 | 9.43 | β = 101.79 | 5.36 | β = 89.64 | |||
| c (Å) | 5.99 | 5.21 | 14.60 | γ = 88.37 | 12.02 | 16.62 | |||
| Weight % | 90.7 | 5.6 | 2.3 | 0.3 | 0.1 | 0.9 | |||
| Rwp % | 11.90 | ||||||||
| 180 min BM | a (Å) | 4.76 | 18.26 | 5.42 | α = 91.69 | 7.36 | 5.41 | 2.86 | |
| b (Å) | 10.22 | 8.82 | 9.42 | β = 101.75 | 5.52 | β = 90.78 | |||
| c (Å) | 5.99 | 5.25 | 14.60 | γ = 88.35 | 11.7 | 16.79 | |||
| Weight % | 88.8 | 6.3 | 2.4 | 0.4 | 0.2 | 1.8 | |||
| Rwp % | 10.62 | ||||||||
3. Materials and Methods
3.1. Synthesis
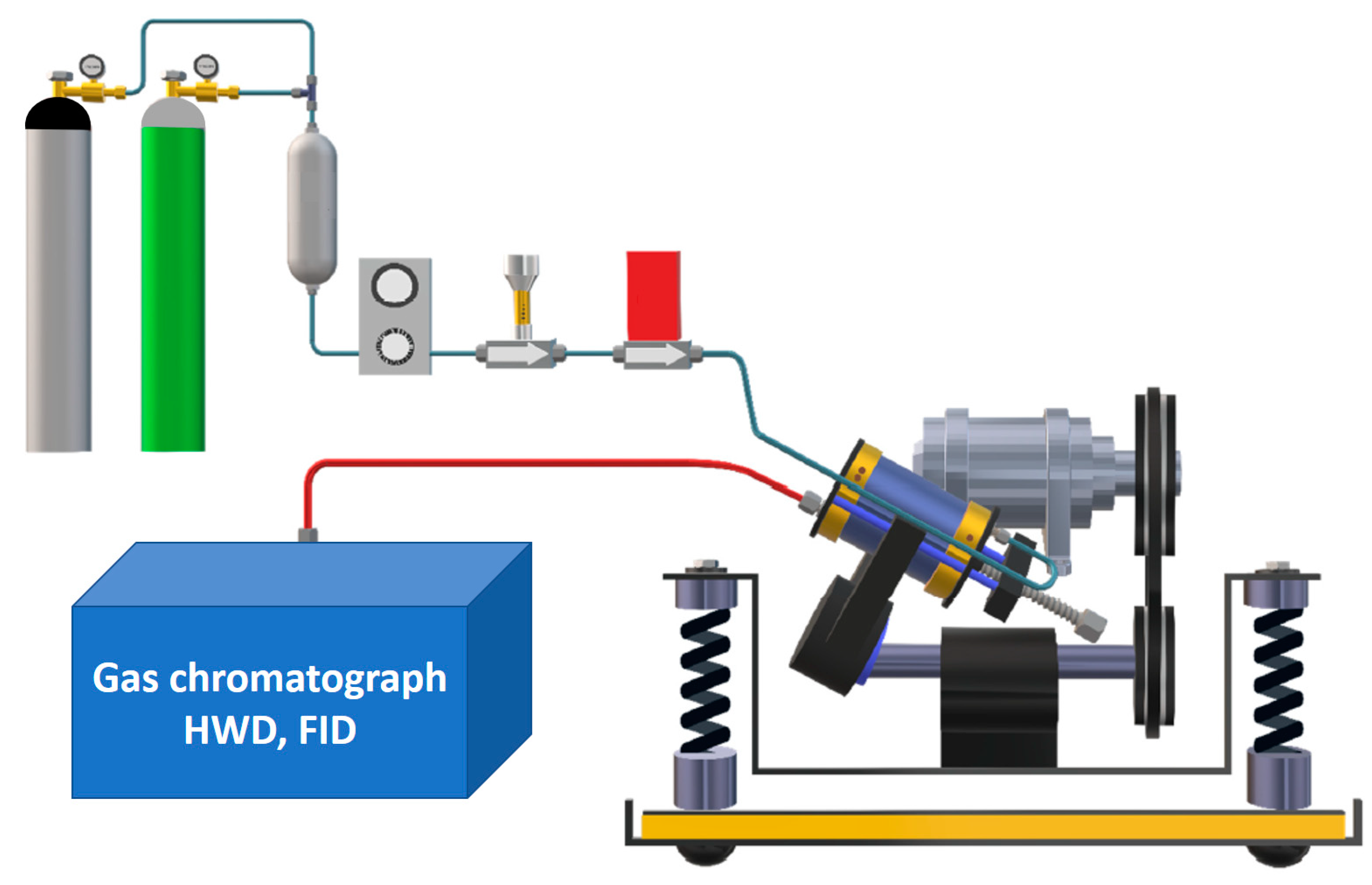
3.2. Reactant Gas Mixture: Setup
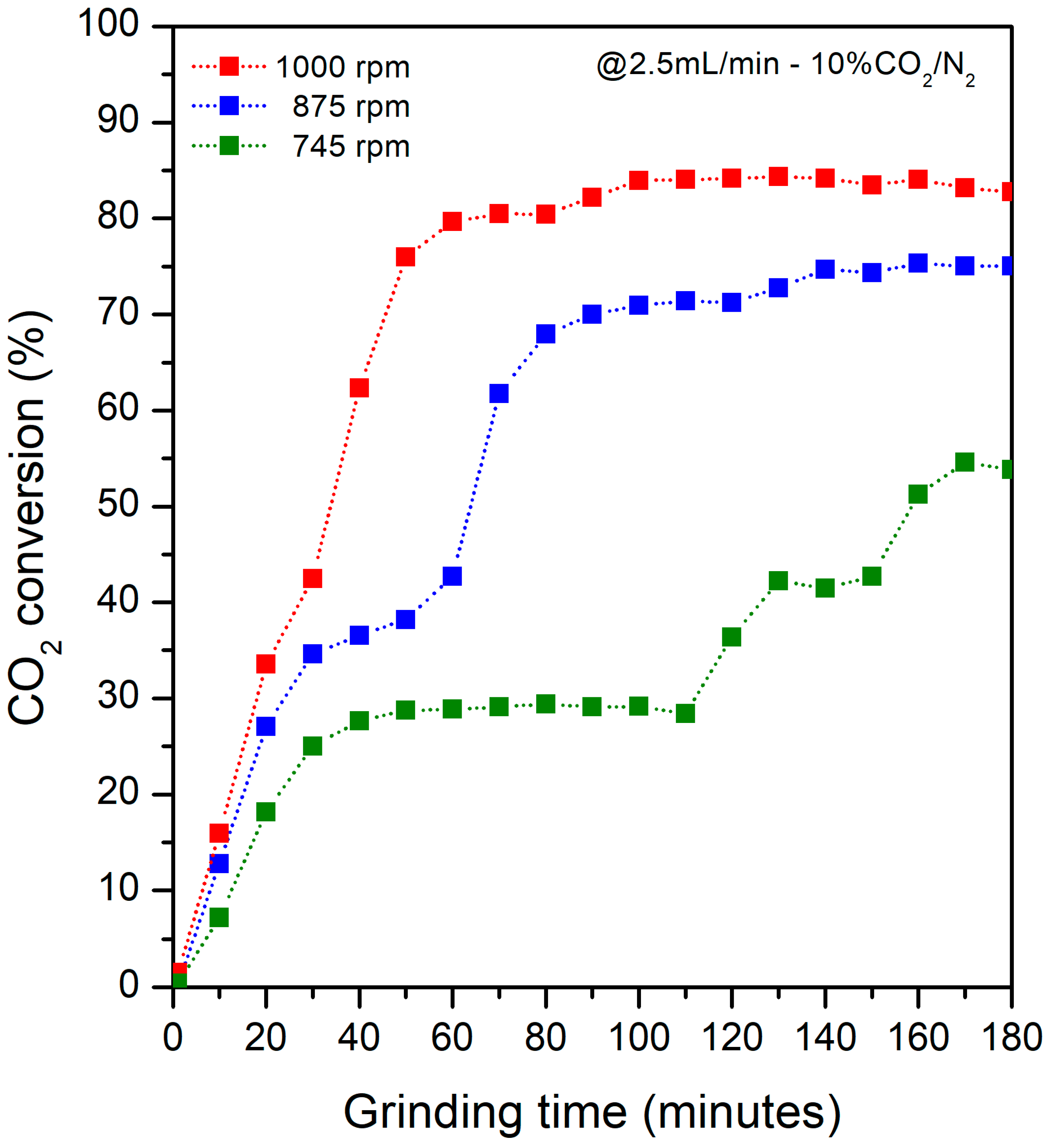
3.3. Percentage Conversion of CO2
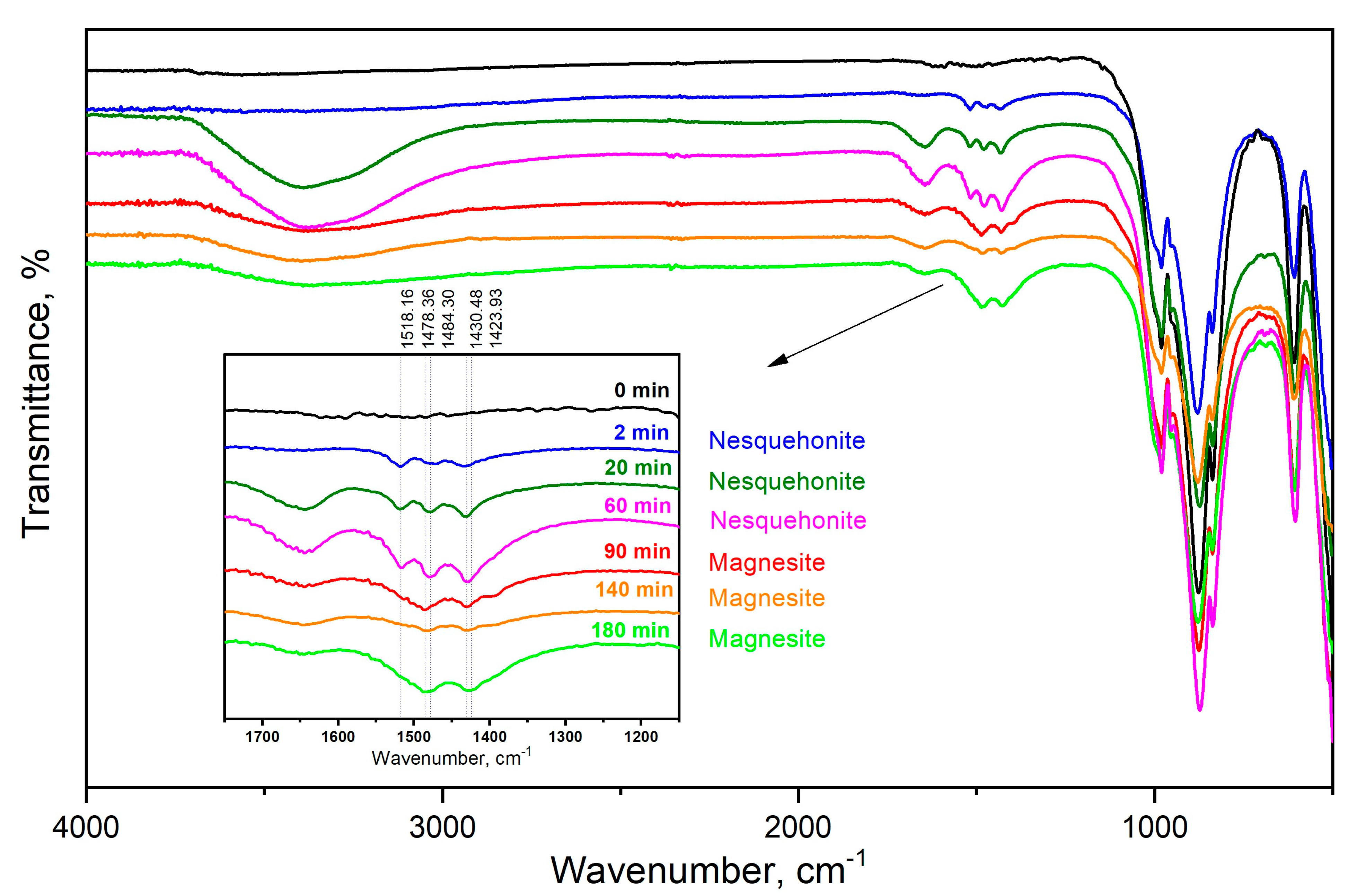
3.4. Assessment of Phase Evolution during Reactive Milling
4. Conclusions
5. Patents
- Method for Converting Carbon Dioxide into High Added Value Chemical Compounds through a Mechanochemical Process under Continuous Gas Flow Conditions Int. Patent WO/2022200941 A1 2022
- Process for the Conversion of Carbon Dioxide into Value-Added Products by Means of a Process of Mechanochemical Activation of Industrial Processing Scraps Int. Patent WO/2023199254 A9 2023
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busca, G. Critical Aspects of Energetic Transition Technologies and the Roles of Materials Chemistry and Engineering. Energies 2024, 17, 3565. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Blunt, M.J.; Anthony, E.J.; Park, A.H.A.; Hughes, R.W.; Webley, P.A.; Yan, J. Advances in Carbon Capture, Utilization and Storage. Appl. Energy 2020, 278, 115627. [Google Scholar] [CrossRef]
- Wei, R.; Alshahrani, T.; Chen, B.; Ibragimov, A.B.; Xu, H.; Gao, J. Advances in Porous Materials for Efficient Separation and Purification of Flue Gas. Sep. Purif. Technol. 2024, 352, 128238. [Google Scholar] [CrossRef]
- Dubey, A.; Arora, A. Advancements in Carbon Capture Technologies: A Review. J. Clean. Prod. 2022, 373, 133932. [Google Scholar] [CrossRef]
- Liu, W.; Ji, Y.; Huang, Y.; Zhang, X.J.; Wang, T.; Fang, M.X.; Jiang, L. Adsorption-Based Post-Combustion Carbon Capture Assisted by Synergetic Heating and Cooling. Renew. Sustain. Energy Rev. 2024, 191, 114141. [Google Scholar] [CrossRef]
- Bukar, A.M.; Asif, M. Technology Readiness Level Assessment of Carbon Capture and Storage Technologies. Renew. Sustain. Energy Rev. 2024, 200, 114578. [Google Scholar] [CrossRef]
- Songolzadeh, M.; Soleimani, M.; Takht Ravanchi, M.; Songolzadeh, R. Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014, 2014, 828131. [Google Scholar] [CrossRef]
- Dziejarski, B.; Krzyżyńska, R.; Andersson, K. Current Status of Carbon Capture, Utilization, and Storage Technologies in the Global Economy: A Survey of Technical Assessment. Fuel 2023, 342, 127776. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A Review of Mineral Carbonation Technologies to Sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, Q.; Xu, L.; Tan, Y. A Review of in Situ Carbon Mineralization in Basalt. J. Rock Mech. Geotech. Eng. 2024, 16, 1467–1485. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Structural and Chemical Changes in Mine Waste Mechanically-Activated in Various Milling Environments. Powder Technol. 2017, 308, 13–19. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 Disposal by Means of Silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L.; Sharp, D.H. Carbon Dioxide Disposal in Carbonate Minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Vega, L.F.; Bahamon, D.; Alkhatib, I.I.I. Perspectives on Advancing Sustainable CO2 Conversion Processes: Trinomial Technology, Environment, and Economy. ACS Sustain. Chem. Eng. 2024, 12, 5357–5382. [Google Scholar] [CrossRef]
- McKelvy, M.J.; Chizmeshya, A.V.G.; Diefenbacher, J.; Béarat, H.; Wolf, G. Exploration of the Role of Heat Activation in Enhancing Serpentine Carbon Sequestration Reactions. Environ. Sci. Technol. 2004, 38, 6897–6903. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, W.K.; Dahlin, D.C.; Rush, G.E.; Dahlin, C.L.; Collins, W.K. Carbon Dioxide Sequestration by Direct Mineral Carbonation: Process Mineralogy of Feed and Products. Min. Metall. Explor. 2002, 19, 95–101. [Google Scholar] [CrossRef]
- Haug, T.A.; Kleiv, R.A.; Munz, I.A. Investigating Dissolution of Mechanically Activated Olivine for Carbonation Purposes. Appl. Geochem. 2010, 25, 1547–1563. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Hamidi, H.; Junin, R.; Karaei, M.A. A Review on Carbon Dioxide Mineral Carbonation through PH-Swing Process. Chem. Eng. J. 2015, 279, 615–630. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Economic Analysis on the Application of Mechanical Activation in an Integrated Mineral Carbonation Process. Int. Biodeterior. Biodegrad. 2018, 128, 63–71. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Ultra-Fine Grinding and Mechanical Activation of Mine Waste Rock Using a Planetary Mill for Mineral Carbonation. Int. J. Miner. Process. 2017, 158, 18–26. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Mechanical Activation of Magnesium Silicates for Mineral Carbonation, a Review. Miner. Eng. 2018, 128, 69–83. [Google Scholar] [CrossRef]
- Baláž, P.; Turianicová, E.; Fabián, M.; Kleiv, R.A.; Briančin, J.; Obut, A. Structural Changes in Olivine (Mg, Fe)2SiO4 Mechanically Activated in High-Energy Mills. Int. J. Miner. Process. 2008, 88, 1–6. [Google Scholar] [CrossRef]
- Zhang, Q.; Sugiyama, K.; Saito, F. Enhancement of Acid Extraction of Magnesium and Silicon from Serpentine by Mechanochemical Treatment. Hydrometallurgy 1997, 45, 323–331. [Google Scholar] [CrossRef]
- Kalinkina, E.V.; Kalinkin, A.M.; Forsling, W.; Makarov, V.N. Sorption of Atmospheric Carbon Dioxide and Structural Changes of Ca and Mg Silicate Minerals during Grinding I. Diopside. Int. J. Miner. Process. 2001, 61, 273–288. [Google Scholar] [CrossRef]
- Kalinkin, A.M.; Kalinkina, E.V.; Politov, A.A.; Makarov, V.N.; Boldyrev, V.V. Mechanochemical Interaction of Ca Silicate and Aluminosilicate Minerals with Carbon Dioxide. J. Mater. Sci. 2004, 39, 5393–5398. [Google Scholar] [CrossRef]
- Fabian, M.; Shopska, M.; Paneva, D.; Kadinov, G.; Kostova, N.; Turianicová, E.; Briančin, J.; Mitov, I.; Kleiv, R.A.; Baláž, P. The Influence of Attrition Milling on Carbon Dioxide Sequestration on Magnesium-Iron Silicate. Miner. Eng. 2010, 23, 616–620. [Google Scholar] [CrossRef]
- Bolm, C.; Hernández, J.G. Mechanochemistry of Gaseous Reactants. Angew. Chem. Int. Ed. 2019, 58, 3285–3299. [Google Scholar] [CrossRef]
- Farina, V.; Gamba, N.S.; Gennari, F.; Garroni, S.; Torre, F.; Taras, A.; Enzo, S.; Mulas, G. CO2 Hydrogenation Induced by Mechanochemical Activation of Olivine with Water Under CO2 Atmosphere. Front. Energy Res. 2019, 7, 107. [Google Scholar] [CrossRef]
- Torre, F.; Farina, V.; Taras, A.; Pistidda, C.; Santoru, A.; Bednarcik, J.; Mulas, G.; Enzo, S.; Garroni, S. Room Temperature Hydrocarbon Generation in Olivine Powders: Effect of Mechanical Processing under CO2 Atmosphere. Powder Technol. 2020, 364, 915–923. [Google Scholar] [CrossRef]
- Yang, M.; Ye, M.; Han, H.; Ren, G.; Han, L.; Zhang, Z. Near-Infrared Spectroscopic Study of Chlorite Minerals. J. Spectrosc. 2018, 2018, 6958260. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 8th ed.; Wiley: Hoboken, NJ, USA, 2010; ISBN 0470419970. [Google Scholar]
- Delogu, F.; Monagheddu, M.; Mulas, G.; Schiffini, L.; Cocco, G. Impact Characteristics and Mechanical Alloying Processes by Ball Milling: Experimental Evaluation and Modelling Outcomes. Int. J. Non-Equilib. Process. 2000, 11, 235–269. [Google Scholar]
- Napolitano, E.; Mulas, G.; Enzo, S.; Delogu, F. Kinetics of Mechanically Induced Anatase-to-Rutile Phase Transformations under Inelastic Impact Conditions. Acta Mater. 2010, 58, 3798–3804. [Google Scholar] [CrossRef]
- Galwey, A.K.; Bettany, D.G.; Mortimer, M. Kinetic Compensation Effects Observed During Oxidation of Carbon Monoxide on γ-Alumina Supported Palladium, Platinum, and Rhodium Metal Catalysts: Toward a Mechanistic Explanation. Int. J. Chem. Kinet. 2006, 38, 689–702. [Google Scholar] [CrossRef]
- Galwey, A.K. Solid State Reaction Kinetics, Mechanisms and Catalysis: A Retrospective Rational Review. React. Kinet. Mech. Catal. 2015, 114, 1–29. [Google Scholar] [CrossRef]
- Firsova, A.A.; Morozova, O.S.; Leonov, A.V.; Streletskii, A.N.; Korchak, V.N. Mechanochemical Synthesis of CuO-CeO2 Catalysts for the Preferential Oxidation of CO in the Presence of H2. Kinet. Catal. 2014, 55, 783–791. [Google Scholar] [CrossRef]
- Li, K.; Chen, J.G. CO2 Hydrogenation to Methanol over ZrO2-Containing Catalysts: Insights into ZrO2 Induced Synergy. ACS Catal. 2019, 9, 7840–7861. [Google Scholar] [CrossRef]
- Streletskii, A.N.; Morozova, O.S.; Berestetskaya, I.V.; Borunova, A.B.; Butyagin, P.J. Mechanochemical Reactions in Gas (H2, Co)/Solid (Zr, a-NiZr) Systems. Mater. Sci. Forum 1996, 225–227, 539–544. [Google Scholar] [CrossRef]
- Kalinkin, A.M.; Politov, A.A.; Boldyrev, V.V.; Kalinkina, E.V.; Makarov, V.N.; Kalinnikov, V.T. Mechanical Activation of Diopside in CO2. Inorg. Mater. 2002, 38, 163–167. [Google Scholar] [CrossRef]
- Mateti, S.; Chen, Y.; Sathikumar, G.; Han, Q.; Prasad, S.; Ferdowsi, R.G.; Battacharjee, A. A Mechanochemical Process to Capture and Separate Carbon Dioxide from Natural Gas Using Boron Nitride Nanosheets. Mater. Horizons 2024, 11, 2950–2956. [Google Scholar] [CrossRef]
- Farina, V.; Simula, M.D.; Taras, A.; Cappai, L.; Sougrati, M.T.; Mulas, G.; Garroni, S.; Enzo, S.; Stievano, L. Unveiling Redox Mechanism at the Iron Centers in the Mechanochemically Activated Conversion of CO2 in the Presence of Olivine. J. Mater. Sci. 2022, 57, 10017–10027. [Google Scholar] [CrossRef]
- Ugapeva, S.S.; Oleinikov, O.B.; Zayakina, N.V. Rare Hydrated Magnesium Carbonate Minerals Nesquehonite and Dypingite of the Obnazhennaya Kimberlite Pipe, in the Yakutian Kimberlite Province. Minerals 2023, 13, 1363. [Google Scholar] [CrossRef]
- Ballirano, P.; De Vito, C.; Mignardi, S.; Ferrini, V. Phase Transitions in the Mg-CO2-H2O System and the Thermal Decomposition of Dypingite, Mg5(CO3)4(OH)25H2O: Implications for Geosequestration of Carbon Dioxide. Chem. Geol. 2013, 340, 59–67. [Google Scholar] [CrossRef]
- Enzo, S.; Mulas, G.; Frattini, R. The Structure of Mechanically Alloyed AlxFe(1-x) End-Products after Annealing. Mater. Sci. Forum 1998, 269–272, 385–390. [Google Scholar] [CrossRef]
- Masci, L.; Dubacq, B.; Verlaguet, A.; Chopin, C.; De Andrade, V.; Herviou, C. A XANES and EPMA Study of Fe3+ in Chlorite: Importance of Oxychlorite and Implications for Cation Site Distribution and Thermobarometry. Am. Mineral. 2019, 104, 403–417. [Google Scholar] [CrossRef]
- Toby, B.H. R Factors in Rietveld Analysis: How Good Is Good Enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Turianicová, E.; Baláž, P.; Tuček, Ľ.; Zorkovská, A.; Zeleňák, V.; Németh, Z.; Šatka, A.; Kováč, J. A Comparison of the Reactivity of Activated and Non-Activated Olivine with CO2. Int. J. Miner. Process. 2013, 123, 73–77. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S.J. Infrared and Infrared Emission Spectroscopy of Nesquehonite Mg(OH)(HCO3)·2H2O-Implications for the Formula of Nesquehonite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1255–1260. [Google Scholar] [CrossRef]
- Taras, A.; Farina, V.; Cappai, L.; Enzo, S.; Garroni, S.; Mulas, G. Method for Converting Carbon Dioxide into High Added Value Chemical Compounds Through a Mechanochemical Process under Continuous Gas Flow Conditions. U.S. Patent Application No. 18/551,953, 23 May 2024. [Google Scholar]
- Graulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An Open-Access Collection of Crystal Structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Lutterotti, L. Total Pattern Fitting for the Combined Size-Strain-Stress-Texture Determination in Thin Film Diffraction. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 334–340. [Google Scholar] [CrossRef]


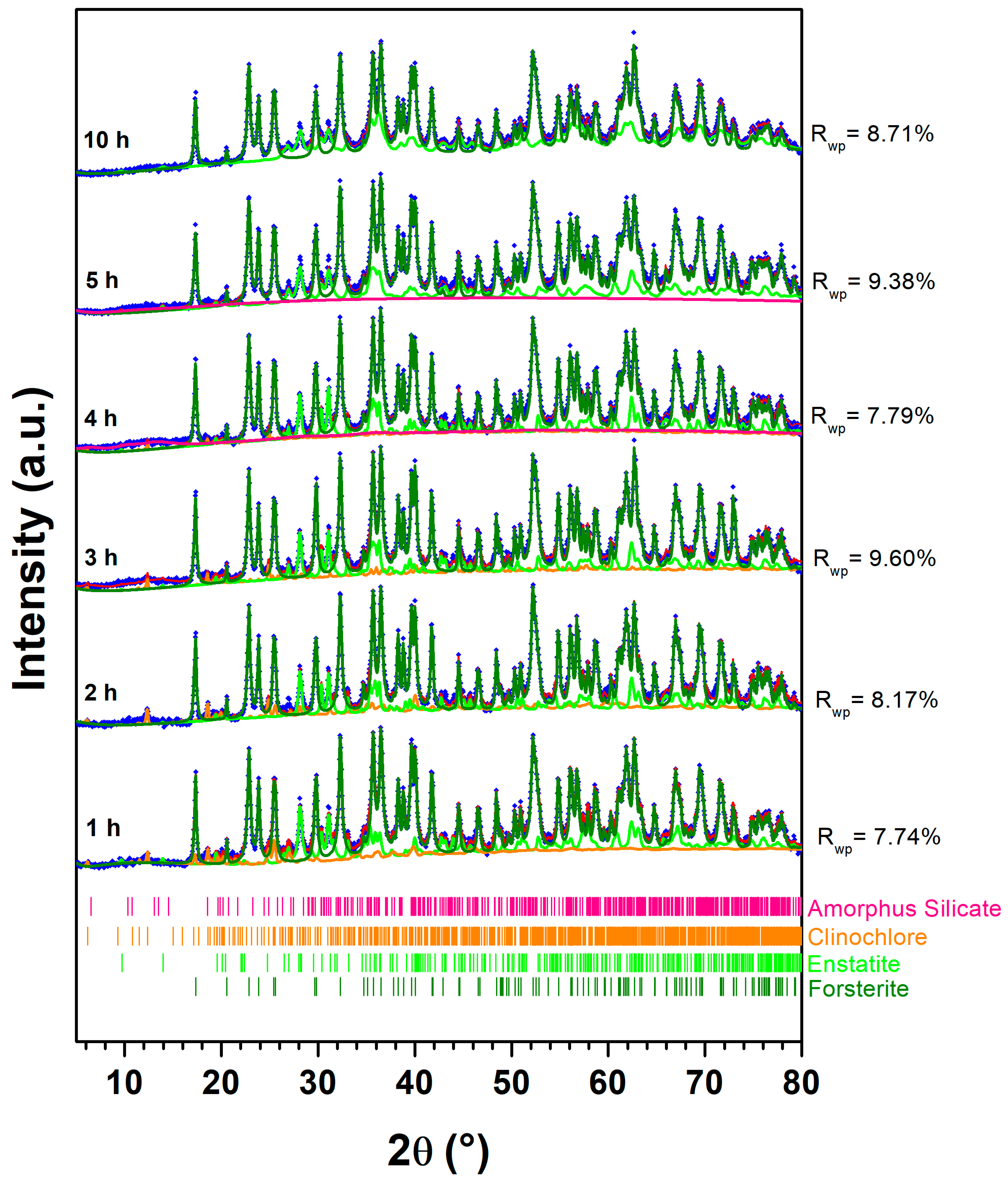
| ← Sample | Phase Name → | Forsterite Ferroan | Enstatite Ferrous | Clinochlore |
| Chemical formula → | Mg1.8Fe0.2SiO4 | Mg0.8Fe0.2SiO3 | (Mg,Fe(II))5Al(Si3Al)O10(OH)8 | |
| Space group → | Pbnm | Pbca | P | |
| 1 h BM | a (Å) | 4.76 | 18.25 | 5.15 |
| b (Å) | 10.22 | 8.83 | 9.58 | |
| c (Å) | 5.99 | 5.20 | 14.42 | |
| Grain size (nm) | 105 | 54 | 123 | |
| Weight % | 93.7 | 4.0 | 2.3 | |
| Rwp % | 7.74 | |||
| 2 h BM | a (Å) | 4.76 | 18.25 | 5.13 |
| b (Å) | 1.23 | 8.83 | 9.55 | |
| c (Å) | 5.99 | 5.20 | 14.45 | |
| Grain size (nm) | 134 | 69 | 134 | |
| Weight % | 88.9 | 7.7 | 3.4 | |
| Rwp % | 8.17 | |||
| 3 h BM | a (Å) | 4.76 | 18.26 | 5.11 |
| b (Å) | 10.22 | 8.83 | 9.59 | |
| c (Å) | 5.99 | 5.20 | 14.47 | |
| Grain size (nm) | 124 | 78 | 121.15 | |
| Weight % | 88.8 | 8.3 | 1.7 | |
| Rwp % | 9.60 | |||
| 4 h BM | a (Å) | 4.76 | 18.26 | 5.10 |
| b (Å) | 10.22 | 8.83 | 9.59 | |
| c (Å) | 5.99 | 5.20 | 14.46 | |
| Grain size (nm) | 109 | 79 | 124 | |
| Weight % | 89.9 | 8.0 | 0.9 | |
| Rwp % | 7.79 | |||
| 5 h BM | a (Å) | 4.76 | 18.26 | - |
| b (Å) | 10.23 | 8.82 | - | |
| c (Å) | 6.00 | 5.20 | - | |
| Grain size (nm) | 88 | 29 | - | |
| Weight % | 88.7 | 10.3 | Not found | |
| Rwp % | 9.38 | |||
| 10 h BM | a (Å) | 4.76 | 18.29 | - |
| b (Å) | 10.23 | 8.78 | - | |
| c (Å) | 6.00 | 5.28 | - | |
| Grain size (nm) | 78 | 21 | - | |
| Weight % | 85.4 | 14.6 | Not found | |
| Rwp % | 8.71 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cau, C.; Taras, A.; Masia, G.; Caggiu, L.; Enzo, S.; Garroni, S.; Murgia, F.; Mulas, G. Structural Evolution of Olivine during Mechanochemically Assisted Mineral Carbonation under CO2 Flow. Inorganics 2024, 12, 269. https://doi.org/10.3390/inorganics12100269
Cau C, Taras A, Masia G, Caggiu L, Enzo S, Garroni S, Murgia F, Mulas G. Structural Evolution of Olivine during Mechanochemically Assisted Mineral Carbonation under CO2 Flow. Inorganics. 2024; 12(10):269. https://doi.org/10.3390/inorganics12100269
Chicago/Turabian StyleCau, Costantino, Alessandro Taras, Gabriele Masia, Laura Caggiu, Stefano Enzo, Sebastiano Garroni, Fabrizio Murgia, and Gabriele Mulas. 2024. "Structural Evolution of Olivine during Mechanochemically Assisted Mineral Carbonation under CO2 Flow" Inorganics 12, no. 10: 269. https://doi.org/10.3390/inorganics12100269
APA StyleCau, C., Taras, A., Masia, G., Caggiu, L., Enzo, S., Garroni, S., Murgia, F., & Mulas, G. (2024). Structural Evolution of Olivine during Mechanochemically Assisted Mineral Carbonation under CO2 Flow. Inorganics, 12(10), 269. https://doi.org/10.3390/inorganics12100269










