Dibromo- and Dichlorotriphenylphosphino N-Acyclic Carbene Complexes of Platinum(II)—Synthesis and Cytotoxicity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Complexes [PtX2(PPh3)(Et2NCN(H)C6H11)]
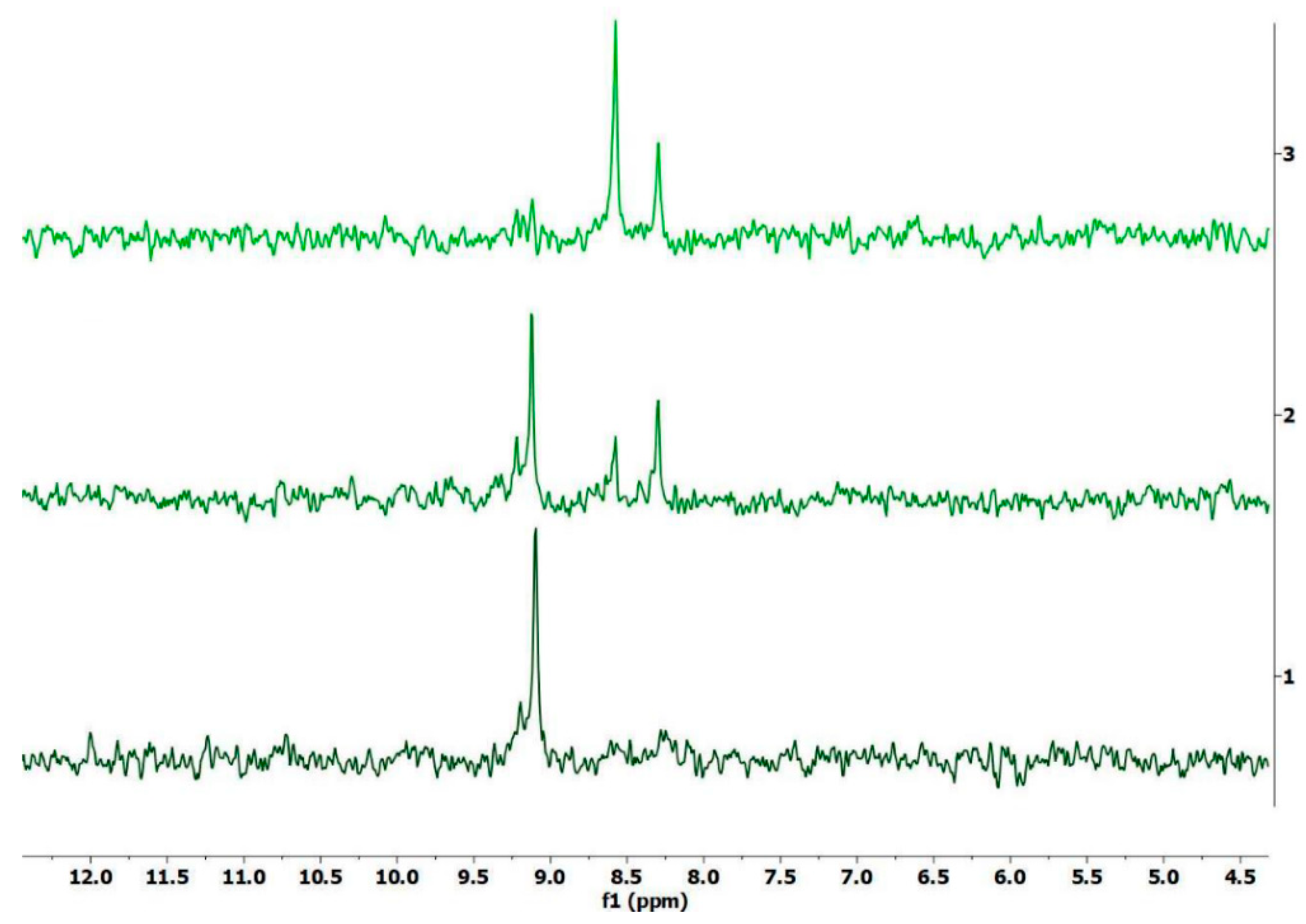
2.2. Stability of Complexes
2.3. Cytotoxic Effect
3. Conclusions
4. Materials and Methods
4.1. Synthesis of [PtX2(PPh3)(CN C6H11)] (X = Cl, Br)
4.2. Synthesis of [PtX2(PPh3)(Et2NCN(H)C6H11)] (X = Cl, Br)
4.3. Biological Evaluation
4.3.1. Cell Cultures
4.3.2. Inhibition Growth Assay
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of Cell Division in Escherichia Coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; Van Camp, L.; Grimley, E.B.; Thomson, A.J. The Inhibition of Growth or Cell Division in Escherichia coli by Different Ionic Species of Platinum(IV) Complexes. J. Biol. Chem. 1967, 242, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; VanCamp, L. The Successful Regression of Large Solid Sarcoma 180 Tumors by Platinum Compounds. Cancer Res. 1970, 30, 1799–1802. [Google Scholar] [PubMed]
- Prestayko, A.; D’Aoust, J.; Issell, B.; Crooke, S. Cisplatin (cis-diamminedichloroplatinum II). Cancer Treat. Rev. 1979, 6, 17–39. [Google Scholar] [CrossRef]
- Lebwohl, D.; Canetta, R. Clinical development of platinum complexes in cancer therapy: An historical perspective and an update. Eur. J. Cancer 1998, 34, 1522–1534. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Makrilia, N.; Syrigou, E.; Kaklamanos, I.; Manolopoulos, L.; Saif, M.W. Hypersensitivity Reactions Associated with Platinum Antineoplastic Agents: A Systematic Review. Met. Drugs 2010, 2010, 207084. [Google Scholar] [CrossRef]
- Rottenberg, S.; Disler, C.; Perego, P. The Rediscovery of Platinum-Based Cancer Therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular Mechanisms of Cisplatin Resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef]
- Fulda, S.; Galluzzi, L.; Kroemer, G. Targeting Mitochondria for Cancer Therapy. Nat. Rev. Drug Discov. 2010, 9, 447–464. [Google Scholar] [CrossRef] [PubMed]
- Costantini, P.; Jacotot, E.; Decaudin, D.; Kroemer, G. Mitochondrion as a Novel Target of Anticancer Chemotherapy. J. Natl. Cancer Inst. 2000, 92, 1042–1053. [Google Scholar] [CrossRef] [PubMed]
- Pathania, D.; Millard, M.; Neamati, N. Opportunities in discovery and delivery of anticancer drugs targeting mitochondria and cancer cell metabolism. Adv. Drug Deliv. Rev. 2009, 61, 1250–1275. [Google Scholar] [CrossRef]
- Wagstaff, A.J.; Ward, A.; Benfield, P.; Heel, R.C. Carboplatin. A Preliminary Review of Its Pharmacodynamic and Pharmacokinetic Properties and Therapeutic Efficacy in the Treatment of Cancer. Drugs 1989, 37, 162–190. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Hirschfeld, S.; Cohen, M.H.; Griebel, D.J.; Williams, G.A.; Pazdur, R. FDA Drug Approval Summaries: Oxaliplatin. Oncologist 2004, 9, 8–12. [Google Scholar] [CrossRef]
- Dilruba, S.; Kalayda, G.V. Platinum-Based Drugs: Past, Present and Future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef]
- Stordal, B.; Pavlakis, N.; Davey, R. Oxaliplatin for the Treatment of Cisplatin-Resistant Cancer: A Systematic Review. Cancer Treat. Rev. 2007, 33, 347–357. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Hartinger, C.G.; Nazarov, A.A.; Ashraf, S.M.; Dyson, P.J.; Keppler, B.K. Carbohydrate-Metal Complexes and Their Potential as Anticancer Agents. Curr. Med. Chem. 2008, 15, 2574–2591. [Google Scholar] [CrossRef]
- Gust, R.; Beck, W.; Jaouen, G.; Schönenberger, H. Optimization of Cisplatin for the Treatment of Hormone Dependent Tumoral Diseases: Part 1: Use of Steroidal Ligands. Coord. Chem. Rev. 2009, 253, 2742–2759. [Google Scholar] [CrossRef]
- Vitols, K.; Montejano, Y.; Duffy, T.; Pope, L.; Grundler, G.; Huennekens, F. Platinum-Folate Compounds: Synthesis, Properties and Biological Activity. Adv. Enzym. Reg. 1987, 26, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Simpson, J.E.; Burns, A.; Kupchinsky, S.; Brooks, N.; Hartley, J.A.; Kelland, L. Novel Platinum (II) Derivatives of Analogues of Netropsin and Distamycin: Synthesis, DNA Binding and Cytotoxic Properties. Med. Chem. Res. 1996, 6, 365–371. [Google Scholar]
- Farrell, N.; Ha, T.T.B.; Souchard, J.P.; Wimmer, F.L.; Cros, S.; Johnson, N.P. Cytostatic Trans-Platinum (II) Complexes. J. Med. Chem. 1989, 32, 2240–2241. [Google Scholar] [CrossRef]
- Farrell, N.; Kelland, L.R.; Roberts, J.D.; Van Beusichem, M. Activation of the Trans Geometry in Platinum Antitumor Complexes: A Survey of the Cytotoxicity of Trans Complexes Containing Planar Ligands in Murine L1210 and Human Tumor Panels and Studies on Their Mechanism of Action. Cancer Res. 1992, 52, 5065–5072. [Google Scholar]
- Intini, F.P.; Boccarelli, A.; Francia, V.C.; Pacifico, C.; Sivo, M.F.; Natile, G.; Giordano, D.; De Rinaldis, P.; Coluccia, M. Platinum Complexes with Imino Ethers or Cyclic Ligands Mimicking Imino Ethers: Synthesis, in Vitro Antitumour Activity, and DNA Interaction Properties. J. Biol. Inorg. Chem. 2004, 9, 768–780. [Google Scholar] [CrossRef]
- Montero, E.I.; Díaz, S.; González-Vadillo, A.M.; Pérez, J.M.; Alonso, C.; Navarro-Ranninger, C. Preparation and Characterization of Novel Trans-[PtCl2(Amine)(Isopropylamine)] Compounds: Cytotoxic Activity and Apoptosis Induction in ras-Transformed Cells. J. Med. Chem. 1999, 42, 4264–4268. [Google Scholar] [CrossRef]
- Billecke, C.; Finniss, S.; Tahash, L.; Miller, C.; Mikkelsen, T.; Farrell, N.P.; Bögler, O. Polynuclear Platinum Anticancer Drugs Are More Potent than Cisplatin and Induce Cell Cycle Arrest in Glioma. Neurooncology 2006, 8, 215–226. [Google Scholar] [CrossRef]
- Kemp, S.; Wheate, N.J.; Buck, D.P.; Nikac, M.; Collins, J.G.; Aldrich-Wright, J.R. The Effect of Ancillary Ligand Chirality and Phenanthroline Functional Group Substitution on the Cytotoxicity of Platinum (II)-Based Metallointercalators. J. Inorg. Biochem. 2007, 101, 1049–1058. [Google Scholar] [CrossRef]
- Cleare, M.J.; Hoeschele, J.D. Studies on the Antitumor Activity of Group VIII Transition Metal Complexes. Part I. Platinum (II) Complexes. Bioinorg. Chem. 1973, 2, 187–210. [Google Scholar] [CrossRef]
- Iafisco, M.; Margiotta, N. Silica Xerogels and Hydroxyapatite Nanocrystals for the Local Delivery of Platinum–Bisphosphonate Complexes in the Treatment of Bone Tumors: A Mini-Review. J. Inorg. Biochem. 2012, 117, 237–247. [Google Scholar] [CrossRef]
- Bhargava, A.; Vaishampayan, U.N. Satraplatin: Leading the New Generation of Oral Platinum Agents. Expert Opin. Investig. Drugs 2009, 18, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, A.; Ramos-Lima, F.; Alvarez-Valdés, A.; Font-Bardía, M.; Bergamo, A.; Sava, G.; Navarro-Ranninger, C. Synthesis, Characterization and Tumor Cell Growth Inhibition of New Trans Platinum Complexes with Phosphane Derivatives. Polyhedron 2011, 30, 1646–1650. [Google Scholar] [CrossRef]
- Ramos-Lima, F.J.; Quiroga, A.G.; García-Serrelde, B.; Blanco, F.; Carnero, A.; Navarro-Ranninger, C. New Trans-Platinum Drugs with Phosphines and Amines as Carrier Ligands Induce Apoptosis in Tumor Cells Resistant to Cisplatin. J. Med. Chem. 2007, 50, 2194–2199. [Google Scholar] [CrossRef] [PubMed]
- Farasat, A.; Nerli, F.; Labella, L.; Taddei, M.; Samaritani, S. Dibromo–Isonitrile and N-acyclic Carbene Complexes of Platinum(II): Synthesis and Reactivity. Inorganics 2023, 11, 137. [Google Scholar] [CrossRef]
- Farasat, A.; Labella, L.; Marchetti, F.; Samaritani, S. Addition of Aliphatic, Secondary Amines to Coordinated Isonitriles. N-Acyclic Carbene (NAC) Platinum(II) Complexes from Trans-[Pt(μ-Cl)Cl(PPh3)]2. Inorg. Chim. Acta 2022, 540, 121062. [Google Scholar] [CrossRef]
- Hyeraci, M.; Agnarelli, L.; Labella, L.; Marchetti, F.; Di Paolo, M.L.; Samaritani, S.; Dalla Via, L. trans-Dichloro(triphenylarsino)(N,N-dialkylamino)platinum(II) Complexes: In Search of New Scaffolds to Circumvent Cisplatin Resistance. Molecules 2022, 27, 644. [Google Scholar] [CrossRef]
- Hyeraci, M.; Scalcon, V.; Folda, A.; Labella, L.; Marchetti, F.; Samaritani, S.; Rigobello, M.P.; Dalla Via, L. New Platinum(II) Complexes Affecting Different Biomolecular Targets in Resistant Ovarian Carcinoma Cells. ChemMedChem 2021, 16, 1956–1966. [Google Scholar] [CrossRef]
- Hyeraci, M.; Colalillo, M.; Labella, L.; Marchetti, F.; Samaritani, S.; Scalcon, V.; Rigobello, M.P.; Dalla Via, L. Platinum(II) Complexes Bearing Triphenylphosphine and Chelating Oximes: Antiproliferative Effect and Biological Profile in Resistant Cells. ChemMedChem 2020, 15, 1464–1472. [Google Scholar] [CrossRef]
- Bondi, R.; Dalla Via, L.; Hyeraci, M.; Pagot, G.; Labella, L.; Marchetti, F.; Samaritani, S. Cytotoxicity and DNA Interaction in a Series of Aryl Terminated Iminopyridine Pt(II) Complexes. J. Inorg. Biochem. 2021, 216, 111335. [Google Scholar] [CrossRef]
- Bondi, R.; Biver, T.; Dalla Via, L.; Guarra, F.; Hyeraci, M.; Sissi, C.; Labella, L.; Marchetti, F.; Samaritani, S. DNA Interaction of a Fluorescent, Cytotoxic Pyridinimino Platinum(II) Complex. J. Inorg. Biochem. 2020, 202, 7. [Google Scholar] [CrossRef]
- Belli Dell’Amico, D.; Colalillo, M.; Dalla Via, L.; Dell’Acqua, M.; García-Argáez, A.N.; Hyeraci, M.; Labella, L.; Marchetti, F.; Samaritani, S. Synthesis and Reactivity of Cytotoxic Platinum(II) Complexes of Bidentate Oximes: A Step towards the Functionalization of Bioactive Complexes. Eur. J. Inorg. Chem. 2018, 2018, 1589–1594. [Google Scholar] [CrossRef]
- Dalla Via, L.; García-Argáez, A.N.; Agostinelli, E.; Belli Dell’amico, D.; Labella, L.; Samaritani, S. New Trans Dichloro (Triphenylphosphine)Platinum(II) Complexes Containing N-(Butyl),N-(Arylmethyl)Amino Ligands: Synthesis, Cytotoxicity and Mechanism of Action. Bioorg. Med. Chem. 2016, 24, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Erxleben, A. Mitochondria-Targeting Anticancer Metal Complexes. Curr. Med. Chem. 2019, 26, 694–728. [Google Scholar] [CrossRef]
- Sun, R.W.-Y.; Chow, A.L.-F.; Li, X.-H.; Yan, J.J.; Chui, S.S.-Y.; Che, C.-M. Luminescent cyclometalated platinum(II) complexes containing N-heterocyclic carbene ligands with potent in vitro and in vivo anti-cancer properties accumulate in cytoplasmic structures of cancer cells. Chem. Sci. 2011, 2, 728–736. [Google Scholar] [CrossRef]
- Bellemin-Laponnaz, S. N-Heterocyclic Carbene Platinum Complexes: A Big Step Forward for Effective Antitumor Compounds. Eur. J. Inorg. Chem. 2020, 2020, 10–20. [Google Scholar] [CrossRef]
- Zhao, S.; Yang, Z.; Jiang, G.; Huang, S.; Bian, M.; Lu, Y.; Liu, W. An Overview of Anticancer Platinum N-Heterocyclic Carbene Complexes. Coord. Chem. Rev. 2021, 449, 214217. [Google Scholar] [CrossRef]
- Porchia, M.; Pellei, M.; Marinelli, M.; Tisato, F.; Del Bello, F.; Santini, C. New Insights in Au-NHCs Complexes as Anticancer Agents. Eur. J. Med. Chem. 2018, 146, 709–746. [Google Scholar] [CrossRef]
- Marzo, T.; Bartoli, G.; Gabbiani, C.; Pescitelli, G.; Severi, M.; Pillozzi, S.; Michelucci, E.; Fiorini, B.; Arcangeli, A.; Quiroga, A.G.; et al. Cisplatin and Its Dibromido Analogue: A Comparison of Chemical and Biological Profiles. BioMetals 2016, 29, 535–542. [Google Scholar] [CrossRef]
- Tolbatov, I.; Marzo, T.; Cirri, D.; Gabbiani, C.; Coletti, C.; Marrone, A.; Paciotti, R.; Messori, L.; Re, N. Reactions of Cisplatin and Cis-[PtI2(NH3)2] with Molecular Models of Relevant Protein Sidechains: A Comparative Analysis. J. Inorg. Biochem. 2020, 209, 111096. [Google Scholar] [CrossRef]
- Fioco, D.; Belli Dell’Amico, D.; Labella, L.; Marchetti, F.; Samaritani, S. A Sound Sequence to Triphenylphosphino Dibromoplatinum(II) Complexes—Solvothermal Preparation of trans -[PtBr(µ-Br)(PPh3)]2. Eur. J. Inorg. Chem. 2019, 2019, 3970–3974. [Google Scholar] [CrossRef]
- Priqueler, J.R.L.; Butler, I.S.; Rochon, F.D. An Overview of 195Pt Nuclear Magnetic Resonance Spectroscopy. App. Spectr. Rev. 2006, 41, 185–226. [Google Scholar] [CrossRef]
- Pregosin, P.S. Platinum-195 Nuclear Magnetic Resonance. Coord. Chem. Rev. 1982, 44, 247–291. [Google Scholar] [CrossRef]
- Hall, M.D.; Telma, K.A.; Chang, K.-E.; Lee, T.D.; Madigan, J.P.; Lloyd, J.R.; Goldlust, I.S.; Hoeschele, J.D.; Gottesman, M.M. Say No to DMSO: Dimethylsulfoxide Inactivates Cisplatin, Carboplatin, and Other Platinum Complexes. Cancer Res. 2014, 74, 3913–3922. [Google Scholar] [CrossRef] [PubMed]
- Varbanov, H.P.; Ortiz, D.; Höfer, D.; Menin, L.; Galanski, M.S.; Keppler, B.K.; Dyson, P.J. Oxaliplatin Reacts with DMSO Only in the Presence of Water. Dalton Trans. 2017, 46, 8929–8932. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, E.Z.; Divsalar, A.; Saboury, A.A.; Khaleghizadeh, S.; Mansouri-Torshizi, H.; Kostova, I. Palladium Complexes: New Candidates for Anti-Cancer Drugs. J. Iran. Chem. Soc. 2016, 13, 967–989. [Google Scholar] [CrossRef]
- Goetzfried, S.K.; Gallati, C.M.; Cziferszky, M.; Talmazan, R.A.; Wurst, K.; Liedl, K.R.; Podewitz, M.; Gust, R. N-Heterocyclic Carbene Gold(I) Complexes: Mechanism of the Ligand Scrambling Reaction and Their Oxidation to Gold(III) in Aqueous Solutions. Inorg. Chem. 2020, 59, 15312–15323. [Google Scholar] [CrossRef]
- Kapitza, P.; Scherfler, A.; Salcher, S.; Sopper, S.; Cziferszky, M.; Wurst, K.; Gust, R. Reaction Behavior of [1,3-Diethyl-4,5-Diphenyl-1H-Imidazol-2-Ylidene] Containing Gold(I/III) Complexes against Ingredients of the Cell Culture Medium and the Meaning on the Potential Use for Cancer Eradication Therapy. J. Med. Chem. 2023, 66, 8238–8250. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C. Chapter 4—Purification of Organic Chemicals. In Purification of Laboratory Chemicals, 7th ed.; Armarego, W.L.F., Chai, C., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2013; pp. 103–554. ISBN 978-0-12-382161-4. [Google Scholar]
- Armarego, W.L.F.; Chai, C. Chapter 5—Purification of Inorganic and Metal-Organic Chemicals. In Purification of Laboratory Chemicals, 7th ed.; Armarego, W.L.F., Chai, C., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2013; pp. 555–661. ISBN 978-0-12-382161-4. [Google Scholar]



| Complex | 31P NMR δ ppm (1JP-Pt, Hz) | 195Pt NMR δ ppm (1JP-Pt, Hz) |
|---|---|---|
| 1 | 8.4 (3410) | −4120 (3410) |
| 2 | 9.0 (3346) | −4402 (3346) |
| 3 | 8.3 (4092) | −4126 (4092) |
| 4 | 9.3 (4020) | −4187 (4020) |
| Compound | Cell Viability (% at 20 μM) a | |
|---|---|---|
| HepG2 | MSTO-211H | |
| 1 | 99 ± 1 | 54 ± 6 |
| 3 | 57 ± 12 | 2.6 ± 1.8 (4.0 ± 0.9) b |
| 5 | 93 ± 6 | 87 ± 11 |
| 6 | 79 ± 15 | 24 ± 4 (9.4 ± 1.5) b |
| 7 | 64 ± 9 | 43 ± 1 |
| 8 | 59 ± 7 | 38 ± 10 |
| cisplatin | (0.6 ± 0.1) b | (1.4 ± 0.1) b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farasat, A.; Labella, L.; Di Paolo, M.L.; Dalla Via, L.; Samaritani, S. Dibromo- and Dichlorotriphenylphosphino N-Acyclic Carbene Complexes of Platinum(II)—Synthesis and Cytotoxicity. Inorganics 2023, 11, 365. https://doi.org/10.3390/inorganics11090365
Farasat A, Labella L, Di Paolo ML, Dalla Via L, Samaritani S. Dibromo- and Dichlorotriphenylphosphino N-Acyclic Carbene Complexes of Platinum(II)—Synthesis and Cytotoxicity. Inorganics. 2023; 11(9):365. https://doi.org/10.3390/inorganics11090365
Chicago/Turabian StyleFarasat, Anna, Luca Labella, Maria Luisa Di Paolo, Lisa Dalla Via, and Simona Samaritani. 2023. "Dibromo- and Dichlorotriphenylphosphino N-Acyclic Carbene Complexes of Platinum(II)—Synthesis and Cytotoxicity" Inorganics 11, no. 9: 365. https://doi.org/10.3390/inorganics11090365
APA StyleFarasat, A., Labella, L., Di Paolo, M. L., Dalla Via, L., & Samaritani, S. (2023). Dibromo- and Dichlorotriphenylphosphino N-Acyclic Carbene Complexes of Platinum(II)—Synthesis and Cytotoxicity. Inorganics, 11(9), 365. https://doi.org/10.3390/inorganics11090365









