Synthesis of Nanostructured Alumina from Byproduct Aluminum Filings: Production and Characterization
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Equipment
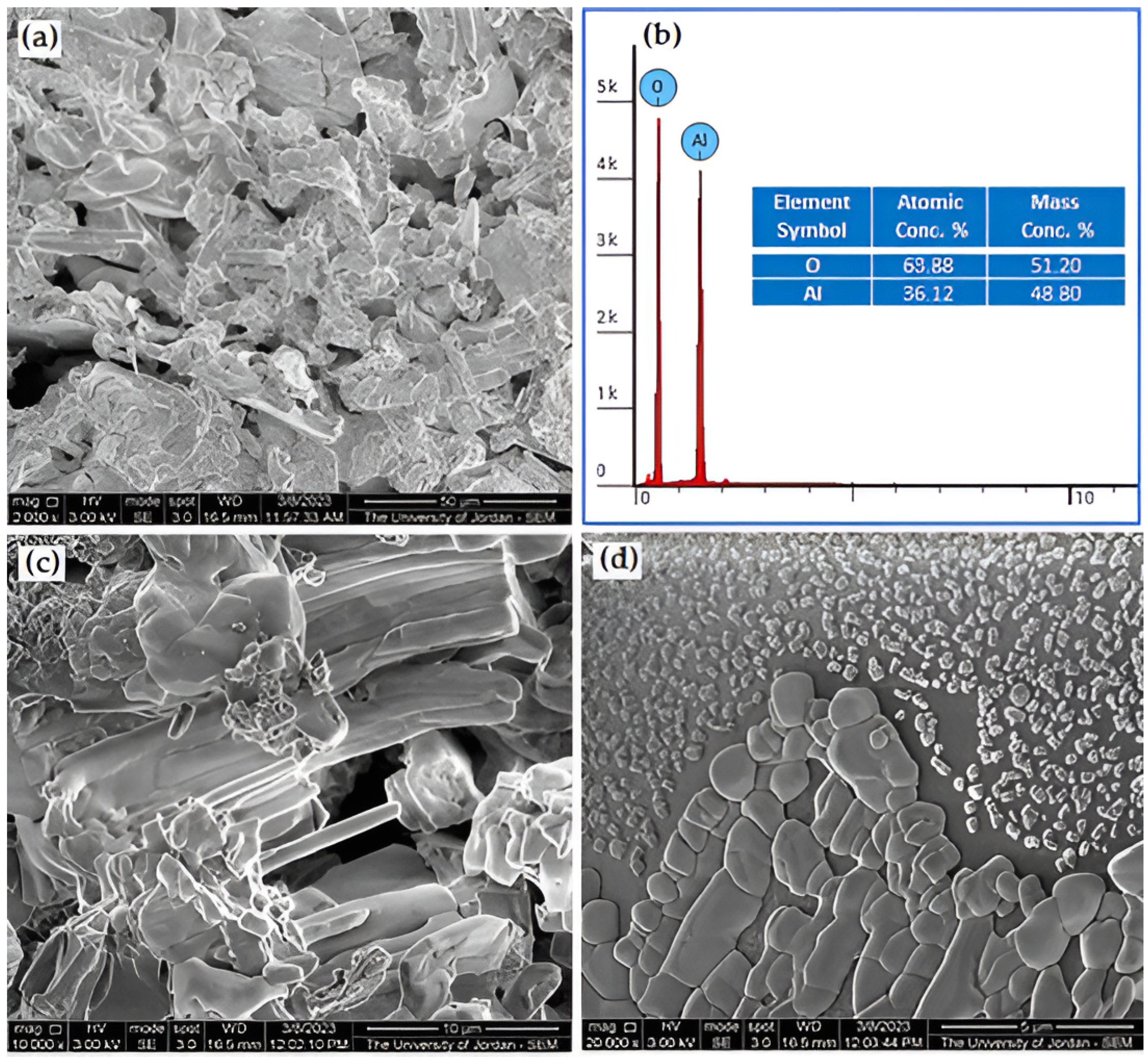
2.2. Starting Material and Scanning Electron Microscope Examination
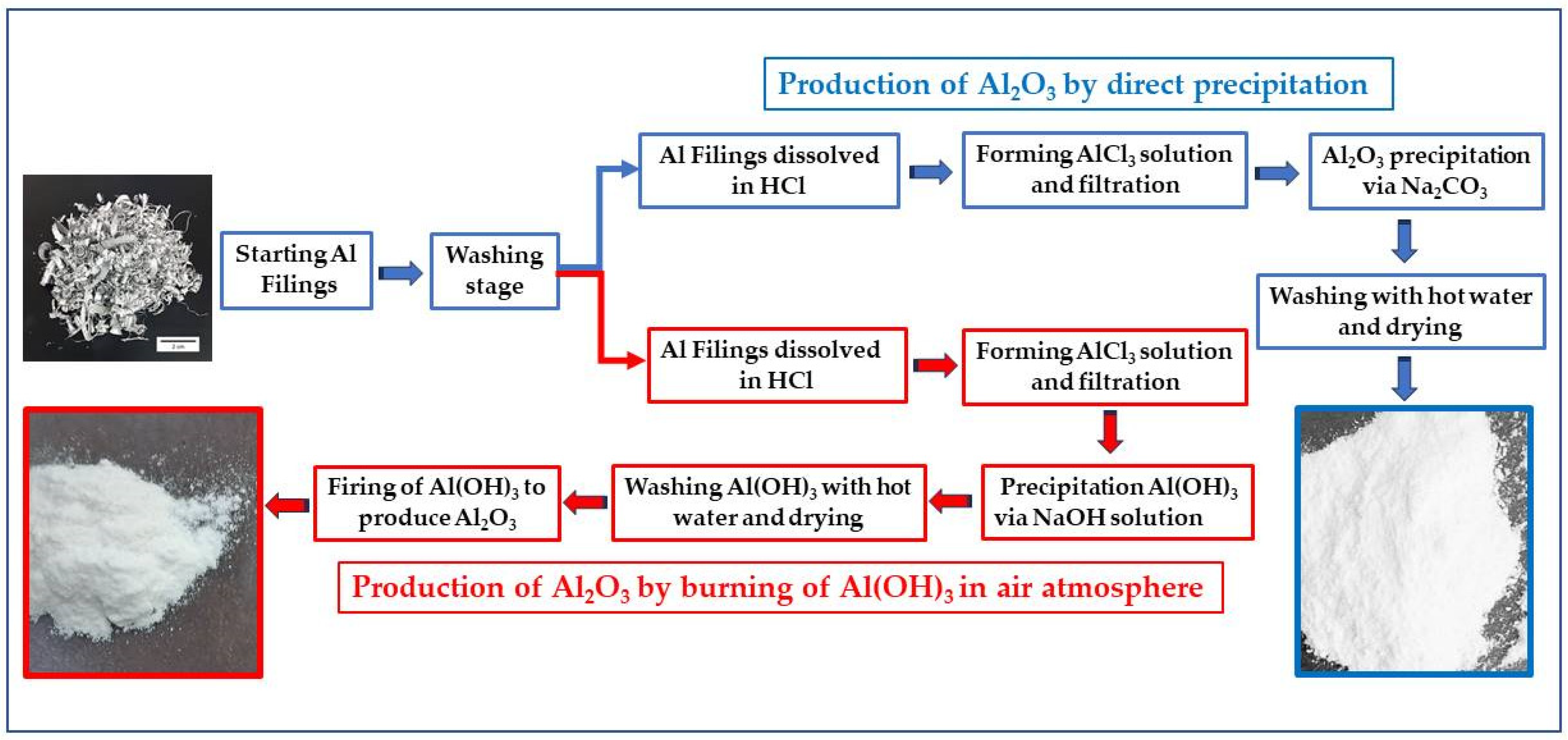
2.3. Production of Alumina
2.4. X-ray Diffraction (XRD) Test
3. Results and Discussion
3.1. The Microstructure and Chemical Composition Examinations of the As-Received Al
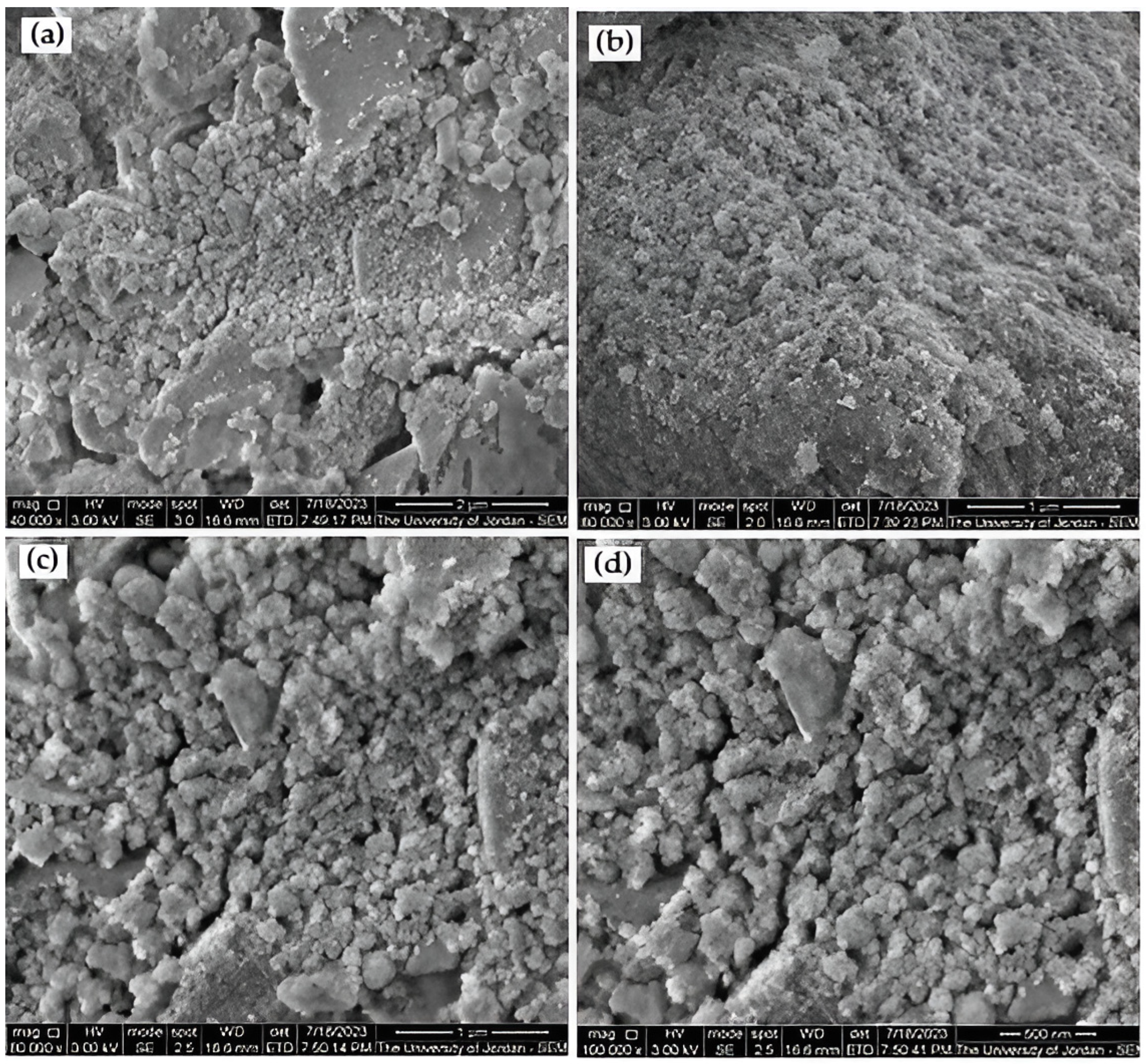
3.2. Inspection of the Produced Al2O3
3.3. XRD Investigation
4. Conclusions
- (i).
- Firing precipitated Al(OH)3 at 800 °C, 1000 °C, and 1200 °C after 1 h and 4 h, and the obtained Al2O3 was then well washed with hot distilled water at approximately 80 °C to produce Al2O3 with no salt residues.
- (ii).
- A chemical reaction of an AlCl3 solution with Na2CO3 solution was used to directly precipitate Al2O3 powder, which was also well washed with hot distilled water.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padamata, S.K.; Yasinskiy, A.; Polyakov, P. A Review of Secondary Aluminum Production and Its Byproducts. JOM 2021, 73, 2603–2614. [Google Scholar] [CrossRef]
- Ahmad, Z. Aluminium Alloys: New Trends in Fabrication and Applications; IntechOpen: London, UK, 2012; Available online: https://www.intechopen.com/books/3053 (accessed on 10 April 2023).
- Løvik, A.N.; Modaresi, R.; Müller, D.B. Long-Term Strategies for Increased Recycling of Automotive Aluminum and Its Alloying Element. Environ. Sci. Technol. 2014, 48, 4257–4265. [Google Scholar] [CrossRef] [PubMed]
- Capuzzi, S.; Timelli, G. Preparation and Melting of Scrap in Aluminum Recycling: A Review. Metals 2018, 8, 249. [Google Scholar] [CrossRef]
- Risonarta, V.Y.; Anggono, J.; Suhendra, Y.M.; Nugrowibowo, S.; Jani, Y. Strategy to Improve Recycling Yield of Aluminium Cans. E3S Web Conf. 2019, 130, 01033. [Google Scholar] [CrossRef]
- Srivastava, A.; Meshram, A. On trending technologies of aluminium dross recycling: A review. Process Saf. Environ. Prot. 2023, 171, 38–54. [Google Scholar] [CrossRef]
- AlShamaileh, E.; Altwaiq, A.M.; Esaifan, M.; Al-Fayyad, H.; Shraideh, Z.; Moosa, I.S.; Hamadneh, I. Study of the Microstructure, Corrosion and Optical Properties of Anodized Aluminum for Solar Heating Applications. Metals 2022, 12, 1635. [Google Scholar] [CrossRef]
- Adans, Y.F.; Martins, A.R.; Coelho, R.E.; Das Virgens, C.F.; Ballarini, A.D.; Carvalho, L.S. A Simple Way to Produce γ-Alumina from Aluminum Cans by Precipitation Reactions. Mater. Res. 2016, 19, 977–982. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; McLaren, M.; Laffir, F.; Nockemann, P.; Rooney, D. A Facile Green Synthetic Route for the Preparation of Highly Active γ-Al2O3 from Aluminum Foil Waste. Sci. Rep. 2017, 7, 3593. [Google Scholar] [CrossRef]
- Kazantsev, S.; Lozhkomoev, A.; Glazkova, E.; Gotman, I.; Gutmanas, E.; Lerner, M.; Psakhie, S. Preparation of aluminum hydroxide and oxide nanostructures with controllable morphology by wet oxidation of AlN/Al nanoparticles. Mater. Res. Bull. 2018, 104, 97–103. [Google Scholar] [CrossRef]
- Ghulam, N.A.; Abbas, M.N.; Sachit, D.E. Preparing of Alumina from Aluminum Waste. Int. J. Innov. Sci. Res. Technol. 2019, 4, 326–331. [Google Scholar]
- El Sheltawy, S.; Salam, N.A.; Barakat, F. Acidic Reaction of Waste Aluminum Foil for Alumina Production. J. Environ. Sci. Pollut. Res. 2019, 5, 325–327. [Google Scholar] [CrossRef]
- Ghulam, N.A.; Abbas, M.N.; Sachit, D.E. Preparation of synthetic alumina from Aluminium foil waste and investigation of its performance in the removal of RG-19 dye from its aqueous solution. Indian Chem. Eng. 2019, 62, 301–313. [Google Scholar] [CrossRef]
- Stippich, F.; Vera, E.; Scheerer, H.; Wolf, G.; Xue, J.-M. Corrosion properties of alumina coatings on steel and aluminum deposited by ion beam assisted deposition. Surf. Coat. Technol. 1998, 98, 997–1001. [Google Scholar] [CrossRef]
- Lin, W.-C.; Tsai, C.-H.; Zhang, D.-N.; Syu, S.-S.; Kuo, Y.-M. Recycling of aluminum dross for producing calcinated alumina by microwave plasma. Sustain. Environ. Res. 2022, 32, 50. [Google Scholar] [CrossRef]
- Joy, J.; Krishnamoorthy, A.; Tanna, A.; Kamathe, V.; Nagar, R.; Srinivasan, S. Recent Developments on the Synthesis of Nanocomposite Materials via Ball Milling Approach for Energy Storage Applications. Appl. Sci. 2022, 12, 9312. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.; Sopian, K.; Kazem, H.A.; Chaichan, M.T. Photovoltaic/Thermal (PV/T) systems: Status and future prospects. Renew. Sustain. Energy Rev. 2017, 77, 109–130. [Google Scholar] [CrossRef]
- Al-Waeli, A.H.; Chaichan, M.T.; Kazem, H.A.; Sopian, K. Comparative study to use nano-(Al2O3, CuO, and SiC) with water to enhance photovoltaic thermal PV/T collectors. Energy Convers. Manag. 2017, 148, 963–973. [Google Scholar] [CrossRef]
- Bandaru, S.H.; Becerra, V.; Khanna, S.; Radulovic, J.; Hutchinson, D.; Khusainov, R. A Review of Photovoltaic Thermal (PVT) Technology for Residential Applications: Performance Indicators, Progress, and Opportunities. Energies 2021, 14, 3853. [Google Scholar] [CrossRef]
- Hajjaj, S.S.H.; Aqeel, A.A.K.A.; Sultan, M.T.H.; Shahar, F.S.; Shah, A.U.M. Review of Recent Efforts in Cooling Photovoltaic Panels (PVs) for Enhanced Performance and Better Impact on the Environment. Nanomaterials 2022, 12, 1664. [Google Scholar] [CrossRef]
- Hamdan, M.A.; Al Momani, A.M.; Ayadi, O.; Sakhrieh, A.H.; Manzano-Agugliaro, F. Enhancement of Solar Water Desalination Using Copper and Aluminum Oxide Nanoparticles. Water 2021, 13, 1914. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Khodair, Z.T.; Khadom, A.A. Preparation, characterization and application of Al2O3 nanoparticles for the protection of boiler steel tubes from high temperature corrosion. Ceram. Int. 2020, 46, 26945–26955. [Google Scholar] [CrossRef]
- Wang, N.; Fu, Y.; Liu, Y.; Yu, H.; Liu, Y. Synthesis of aluminum hydroxide thin coating and its influence on the thermomechanical and fire-resistant properties of wood. Holzforschung 2014, 68, 781–789. [Google Scholar] [CrossRef]
- Souza, A.D.; Salomão, R. Evaluation of the porogenic behavior of aluminum hydroxide particles of different size distributions in castable high-alumina. J. Eur. Ceram. Soc. 2016, 36, 885–897. [Google Scholar] [CrossRef]
- Mwase, J.M.; Vafeias, M.; Marinos, D.; Dimitrios, P.; Safarian, J. Investigating Aluminum Tri-Hydroxide Production from Sodium Aluminate Solutions in the Pedersen Process. Processes 2022, 10, 1370. [Google Scholar] [CrossRef]
- Kamel, B.A.F.; Jaber, S.H.; Mahdi, A.S.; Alsouz, M.A.K.; Dawood, K.M. Synthesis and Characterization of Nano Aluminum Oxide Via Biological and Electrochemical Methods. Al-Mustansiriyah J. Sci. 2018, 29, 67–72. [Google Scholar] [CrossRef]
- Nduni, M.N.; Osano, A.M.; Chaka, B. Synthesis and characterization of Aluminium oxide nanoparticles from waste Aluminium foil and potential application in Aluminium-ion cell. Clean. Eng. Technol. 2021, 3, 100108. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; McLaren, M.; Laffir, F.; Rooney, D.W. Characterisation of Robust Combustion Catalyst from Aluminium Foil Waste. Chem. Sel. 2018, 3, 1545–1550. [Google Scholar] [CrossRef]
- Osman, A.I.; Skillen, N.C.; Robertson, P.K.J.; Rooney, D.W.; Morgan, K. Exploring the photocatalytic hydrogen production potential of titania doped with alumina derived from foil waste. Int. J. Hydrogen Energy 2020, 45, 34494–34502. [Google Scholar] [CrossRef]
- Fedoročková, A.; Sučik, G.; Pleŝingerová, B.; Popoviŝ, L.; Kova, M.; Vavra, M. Simplified waste-free process for synthesis of nanoporous compact alumina under technologically advantageous conditions. RSC Adv. 2020, 10, 32423–32435. [Google Scholar] [CrossRef] [PubMed]
- García, L.; Dietz, C.; Criado, A.J.; Martínez, J.A. Colour Metallography of Cast Aluminium Alloys. Pract. Met. 2014, 51, 514–529. [Google Scholar] [CrossRef]
- Krane, J.; Kevorkijan, V.; Godec, M.; Paulin, I. Metallographic methods for determining the quality of Aluminium alloys. Mater. Technol. 2021, 55, 541–547. [Google Scholar] [CrossRef]
- Serway, R.A.; Vuille, C. College Physics, 9th ed.; Library of Congress Control Number: 2010930876; Cengage Learning: Boston, MA, USA, 2012; p. 309. Available online: https://www.bau.edu.jo/UserPortal/UserProfile/PostsAttach/57751_5218_1.pdf (accessed on 1 February 2023).
- Shardt, N.; Wang, Y.; Jin, Z.; Elliott, J.A. Surface tension as a function of temperature and composition for a broad range of mixtures. Chem. Eng. Sci. 2021, 230, 116095. [Google Scholar] [CrossRef]
- Samain, L.; Jaworski, A.; Edén, M.; Ladd, D.M.; Seo, D.-K.; Garcia-Garcia, F.J.; Häussermann, U. Structure analysis of highly porous γ-Al2O3. J. Solid State Chem. 2014, 217, 1–8. [Google Scholar] [CrossRef]
- Matori, K.A.; Wah, L.C.; Hashim, M.; Ismail, I.; Zaid, M.H.M. Phase Transformations of α-Alumina Made from Waste Aluminum via a Precipitation Technique. Int. J. Mol. Sci. 2012, 13, 16813–16821. [Google Scholar] [CrossRef] [PubMed]
- Omer, A.H.; Bin Mokaizh, A.A.; Shariffuddin, J.H.B.H. Low-Calcination Temperature to Synthesize A-Alumina From Aluminium Waste Can Using Sol-Gel Method. Earth Environ. Sci. 2021, 641, 012023. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esaifan, M.; Al-Mobydeen, A.; Al-Masri, A.N.; Altwaiq, A.M.; Al-Saqarat, B.S.; Mahmoud, W.; Hamaideh, A.; Moosa, I.S.; Hamadneh, I.; AlShamaileh, E. Synthesis of Nanostructured Alumina from Byproduct Aluminum Filings: Production and Characterization. Inorganics 2023, 11, 355. https://doi.org/10.3390/inorganics11090355
Esaifan M, Al-Mobydeen A, Al-Masri AN, Altwaiq AM, Al-Saqarat BS, Mahmoud W, Hamaideh A, Moosa IS, Hamadneh I, AlShamaileh E. Synthesis of Nanostructured Alumina from Byproduct Aluminum Filings: Production and Characterization. Inorganics. 2023; 11(9):355. https://doi.org/10.3390/inorganics11090355
Chicago/Turabian StyleEsaifan, Muayad, Ahmed Al-Mobydeen, Ahmed N. Al-Masri, Abdelmnim M. Altwaiq, Bety S. Al-Saqarat, Wadah Mahmoud, Arwa Hamaideh, Iessa Sabbe Moosa, Imad Hamadneh, and Ehab AlShamaileh. 2023. "Synthesis of Nanostructured Alumina from Byproduct Aluminum Filings: Production and Characterization" Inorganics 11, no. 9: 355. https://doi.org/10.3390/inorganics11090355
APA StyleEsaifan, M., Al-Mobydeen, A., Al-Masri, A. N., Altwaiq, A. M., Al-Saqarat, B. S., Mahmoud, W., Hamaideh, A., Moosa, I. S., Hamadneh, I., & AlShamaileh, E. (2023). Synthesis of Nanostructured Alumina from Byproduct Aluminum Filings: Production and Characterization. Inorganics, 11(9), 355. https://doi.org/10.3390/inorganics11090355









