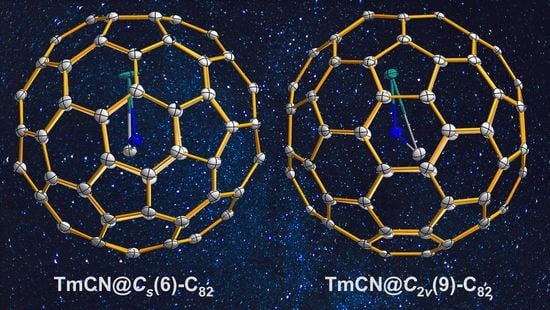
TmCN@C82: Monometallic Clusterfullerene Encapsulating a Tm3+ Ion
Abstract
1. Introduction
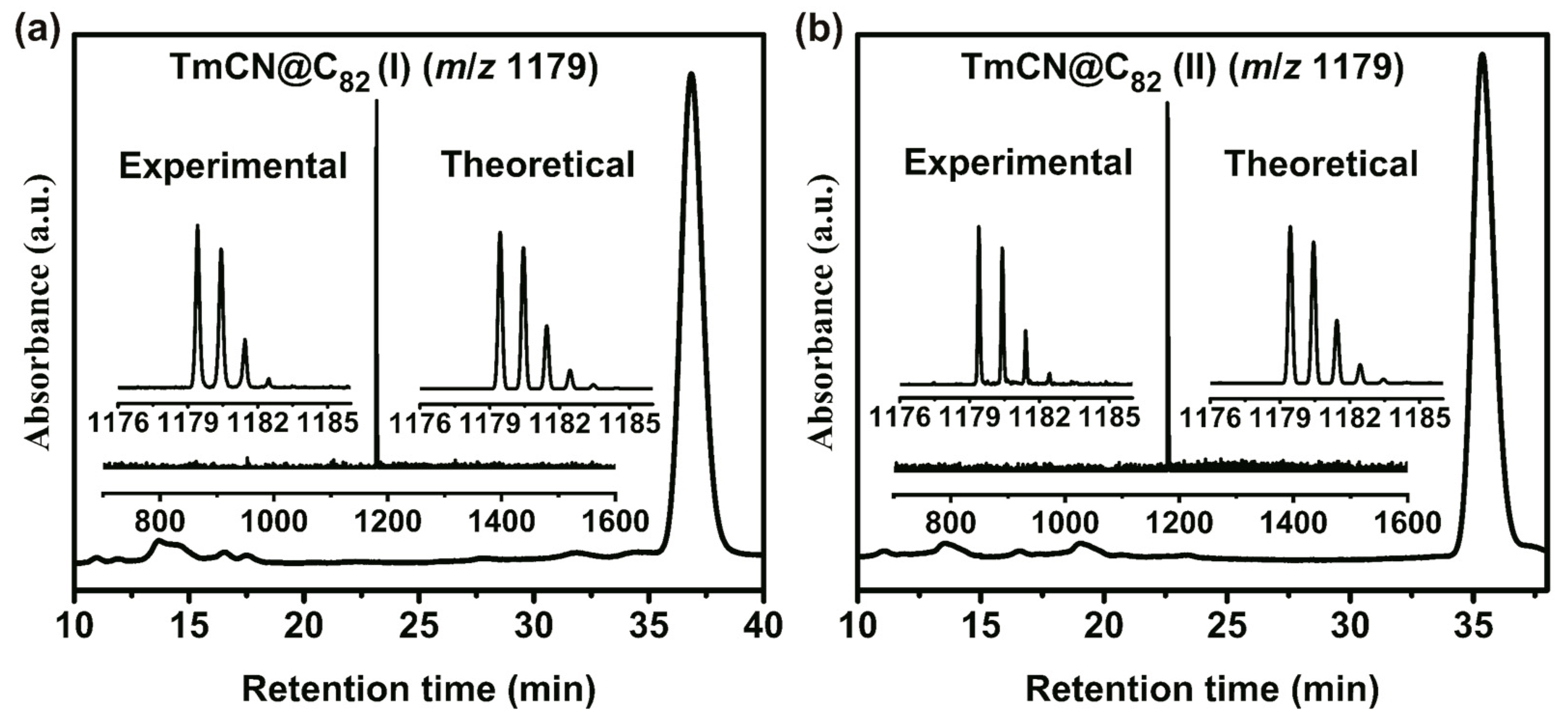
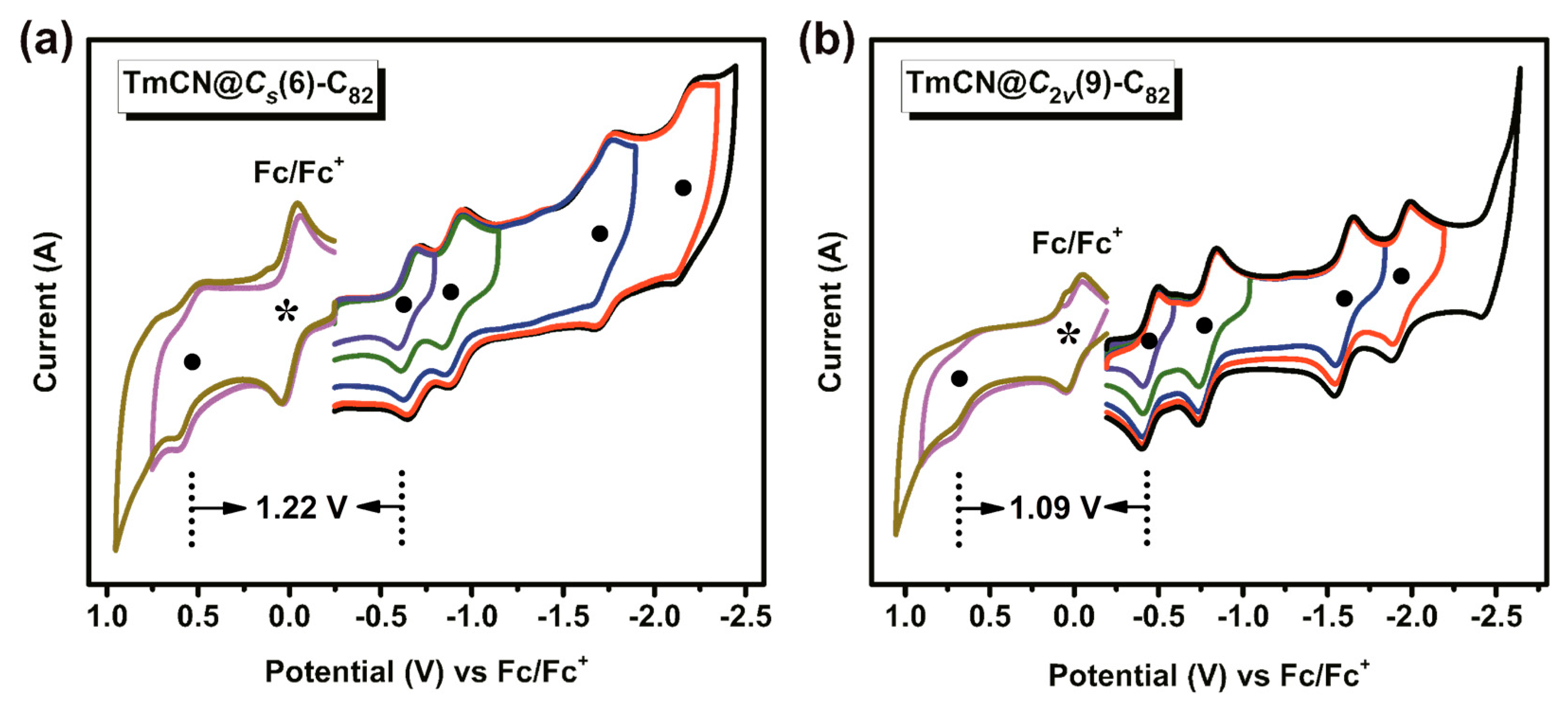
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Xie, S.-Y.; Gao, F.; Lu, X.; Huang, R.-B.; Wang, C.-R.; Zhang, X.; Liu, M.-L.; Deng, S.-L.; Zheng, L.-S. Capturing the Labile Fullerene[50] as C50Cl10. Science 2004, 304, 699. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-Z.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. The stabilization of fused-pentagon fullerene molecules. Nat. Chem. 2009, 1, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.-R.; Chen, M.-M.; Wang, K.; Chen, Z.-C.; Fu, C.-Y.; Zhang, Q.; Li, S.-H.; Deng, S.-L.; Yao, Y.-R.; Xie, S.-Y.; et al. An Unconventional Hydrofullerene C66H4 with Symmetric Heptagons Retrieved in Low-Pressure Combustion. J. Am. Chem. Soc. 2019, 141, 6651–6657. [Google Scholar] [CrossRef]
- Su, Y.; Chen, Z.-C.; Tian, H.-R.; Xu, Y.-Y.; Zhang, Q.; Xie, S.-Y.; Zheng, L.-S. Implications of Nitrogen Doping on Geometrical and Electronic Structure of the Fullerene Dimers. Chin. J. Chem. 2021, 39, 93–98. [Google Scholar] [CrossRef]
- Xiang, W.; Jiang, X.; Yao, Y.-R.; Xin, J.; Jin, H.; Guan, R.; Zhang, Q.; Chen, M.; Xie, S.-Y.; Popov, A.A.; et al. Monometallic Endohedral Azafullerene. J. Am. Chem. Soc. 2022, 144, 21587–21595. [Google Scholar] [CrossRef]
- Hu, Z.; Dong, B.-W.; Liu, Z.; Liu, J.-J.; Su, J.; Yu, C.; Xiong, J.; Shi, D.-E.; Wang, Y.; Wang, B.-W.; et al. Endohedral Metallofullerene as Molecular High Spin Qubit: Diverse Rabi Cycles in Gd2@C79N. J. Am. Chem. Soc. 2018, 140, 1123–1130. [Google Scholar] [CrossRef]
- Yao, Y.-R.; Chen, Z.-C.; Chen, L.; Zheng, S.-Y.; Yang, S.; Deng, S.-L.; Echegoyen, L.; Tan, Y.-Z.; Xie, S.-Y.; Zheng, L.-S. Two Metastable Endohedral Metallofullerenes Sc2C2@C1(39656)-C82 and Sc2C2@C1(51383)-C84: Direct-C2-Insertion Products from Their Most Stable Precursors. J. Am. Chem. Soc. 2023. [Google Scholar] [CrossRef]
- Yao, Y.-R.; Shi, X.-M.; Zheng, S.-Y.; Chen, Z.-C.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. Atomically Precise Insights into Metal-Metal Bonds Using Comparable Endo-Units of Sc2 and Sc2C2. CCS Chem. 2021, 3, 294–302. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, X.; Guo, M.; Yao, Y.-R.; Meng, Q.; Echegoyen, L.; Autschbach, J.; Chen, N. USc2C2 and USc2NC Clusters with U-C Triple Bond Character Stabilized Inside Fullerene Cages. J. Am. Chem. Soc. 2023, 145, 5645–5654. [Google Scholar] [CrossRef]
- Rodríguez-Fortea, A.; Balch, A.L.; Poblet, J.M. Endohedral metallofullerenes: A unique host–guest association. Chem. Soc. Rev. 2011, 40, 3551–3563. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.A.; Yang, S.; Dunsch, L. Endohedral Fullerenes. Chem. Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wei, T.; Jin, F. When metal clusters meet carbon cages: Endohedral clusterfullerenes. Chem. Soc. Rev. 2017, 46, 5005–5058. [Google Scholar] [CrossRef] [PubMed]
- Heath, J.R.; O’Brien, S.C.; Zhang, Q.; Liu, Y.; Curl, R.F.; Tittel, F.K.; Smalley, R.E. Lanthanum complexes of spheroidal carbon shells. J. Am. Chem. Soc. 1985, 107, 7779–7780. [Google Scholar] [CrossRef]
- Stevenson, S.; Rice, G.; Glass, T.; Harich, K.; Cromer, F.; Jordan, M.R.; Craft, J.; Hadju, E.; Bible, R.; Olmstead, M.M.; et al. Small-bandgap endohedral metallofullerenes in high yield and purity. Nature 1999, 401, 55–57. [Google Scholar] [CrossRef]
- Li, X.; Roselló, Y.; Yao, Y.-R.; Zhuang, J.; Zhang, X.; Rodríguez-Fortea, A.; de Graaf, C.; Echegoyen, L.; Poblet, J.M.; Chen, N. U2N@Ih(7)-C80: Fullerene cage encapsulating an unsymmetrical U(IV)=N=U(V) cluster. Chem. Sci. 2021, 12, 282–292. [Google Scholar] [CrossRef]
- Meng, Q.; Abella, L.; Yao, Y.-R.; Sergentu, D.-C.; Yang, W.; Liu, X.; Zhuang, J.; Echegoyen, L.; Autschbach, J.; Chen, N. A charged diatomic triple-bonded U≡N species trapped in C82 fullerene cages. Nat. Commun. 2022, 13, 7192. [Google Scholar] [CrossRef]
- Wang, C.-R.; Kai, T.; Tomiyama, T.; Yoshida, T.; Kobayashi, Y.; Nishibori, E.; Takata, M.; Sakata, M.; Shinohara, H. A Scandium Carbide Endohedral Metallofullerene: (Sc2C2)@C84. Angew. Chem. Int. Ed. 2001, 40, 397–399. [Google Scholar] [CrossRef]
- Wang, T.-S.; Chen, N.; Xiang, J.-F.; Li, B.; Wu, J.-Y.; Xu, W.; Jiang, L.; Tan, K.; Shu, C.-Y.; Lu, X.; et al. Russian-Doll-Type Metal Carbide Endofullerene: Synthesis, Isolation, and Characterization of Sc4C2@C80. J. Am. Chem. Soc. 2009, 131, 16646–16647. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Feng, L.; Chen, X.; Hansen, A.; Grimme, S.; Fortier, S.; Sergentu, D.-C.; Duignan, T.J.; Autschbach, J.; et al. A diuranium carbide cluster stabilized inside a C80 fullerene cage. Nat. Commun. 2018, 9, 2753. [Google Scholar] [CrossRef]
- Zhuang, J.; Abella, L.; Sergentu, D.-C.; Yao, Y.-R.; Jin, M.; Yang, W.; Zhang, X.; Li, X.; Zhang, D.; Zhao, Y.; et al. Diuranium(IV) Carbide Cluster U2C2 Stabilized Inside Fullerene Cages. J. Am. Chem. Soc. 2019, 141, 20249–20260. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, S.; Mackey, M.A.; Stuart, M.A.; Phillips, J.P.; Easterling, M.L.; Chancellor, C.J.; Olmstead, M.M.; Balch, A.L. A Distorted Tetrahedral Metal Oxide Cluster inside an Icosahedral Carbon Cage. Synthesis, Isolation, and Structural Characterization of Sc4(μ3-O)2@Ih-C80. J. Am. Chem. Soc. 2008, 130, 11844–11845. [Google Scholar] [CrossRef] [PubMed]
- Mercado, B.Q.; Olmstead, M.M.; Beavers, C.M.; Easterling, M.L.; Stevenson, S.; Mackey, M.A.; Coumbe, C.E.; Phillips, J.D.; Phillips, J.P.; Poblet, J.M.; et al. A seven atom cluster in a carbon cage, the crystallographically determined structure of Sc4(μ3-O)3@Ih-C80. Chem. Commun. 2010, 46, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Velkos, G.; Yang, W.; Yao, Y.-R.; Sudarkova, S.M.; Liu, X.; Büchner, B.; Avdoshenko, S.M.; Chen, N.; Popov, A.A. Shape-adaptive single-molecule magnetism and hysteresis up to 14 K in oxide clusterfullerenes Dy2O@C72 and Dy2O@C74 with fused pentagon pairs and flexible Dy–(μ2-O)–Dy angle. Chem. Sci. 2020, 11, 4766–4772. [Google Scholar] [CrossRef]
- Mercado, B.Q.; Stuart, M.A.; Mackey, M.A.; Pickens, J.E.; Confait, B.S.; Stevenson, S.; Easterling, M.L.; Valencia, R.; Rodríguez-Fortea, A.; Poblet, J.M.; et al. Sc2(μ2-O) Trapped in a Fullerene Cage: The Isolation and Structural Characterization of Sc2(μ2-O)@Cs(6)-C82 and the Relevance of the Thermal and Entropic Effects in Fullerene Isomer Selection. J. Am. Chem. Soc. 2010, 132, 12098–12105. [Google Scholar] [CrossRef]
- Dunsch, L.; Yang, S.; Zhang, L.; Svitova, A.; Oswald, S.; Popov, A.A. Metal Sulfide in a C82 Fullerene Cage: A New Form of Endohedral Clusterfullerenes. J. Am. Chem. Soc. 2010, 132, 5413–5421. [Google Scholar] [CrossRef]
- Chen, N.; Beavers, C.M.; Mulet-Gas, M.; Rodríguez-Fortea, A.; Munoz, E.J.; Li, Y.-Y.; Olmstead, M.M.; Balch, A.L.; Poblet, J.M.; Echegoyen, L. Sc2S@Cs(10528)-C72: A Dimetallic Sulfide Endohedral Fullerene with a Non Isolated Pentagon Rule Cage. J. Am. Chem. Soc. 2012, 134, 7851–7860. [Google Scholar] [CrossRef]
- Chen, N.; Mulet-Gas, M.; Li, Y.-Y.; Stene, R.E.; Atherton, C.W.; Rodríguez-Fortea, A.; Poblet, J.M.; Echegoyen, L. Sc2S@C2(7892)–C70: A metallic sulfide cluster inside a non-IPR C70 cage. Chem. Sci. 2013, 4, 180–186. [Google Scholar] [CrossRef]
- Mercado, B.Q.; Chen, N.; Rodríguez-Fortea, A.; Mackey, M.A.; Stevenson, S.; Echegoyen, L.; Poblet, J.M.; Olmstead, M.M.; Balch, A.L. The Shape of the Sc2(μ2-S) Unit Trapped in C82: Crystallographic, Computational, and Electrochemical Studies of the Isomers, Sc2(μ2-S)@Cs(6)-C82 and Sc2(μ2-S)@C3v(8)-C82. J. Am. Chem. Soc. 2011, 133, 6752–6760. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Gao, C.-L.; Deng, Q.; Zhu, X.; Kostanyan, A.; Westerström, R.; Jin, F.; Xie, S.-Y.; Popov, A.A.; et al. Mononuclear Clusterfullerene Single-Molecule Magnet Containing Strained Fused-Pentagons Stabilized by a Nearly Linear Metal Cyanide Cluster. Angew. Chem. Int. Ed. 2017, 56, 1830–1834. [Google Scholar] [CrossRef]
- Yang, S.; Chen, C.; Liu, F.; Xie, Y.; Li, F.; Jiao, M.; Suzuki, M.; Wei, T.; Wang, S.; Chen, Z.; et al. An Improbable Monometallic Cluster Entrapped in a Popular Fullerene Cage: YCN@Cs(6)-C82. Sci. Rep. 2013, 3, 1487. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Gao, C.-L.; Deng, Q.; Zhu, X.; Kostanyan, A.; Westerström, R.; Wang, S.; Tan, Y.-Z.; Tao, J.; Xie, S.-Y.; et al. Triangular Monometallic Cyanide Cluster Entrapped in Carbon Cage with Geometry-Dependent Molecular Magnetism. J. Am. Chem. Soc. 2016, 138, 14764–14771. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Jin, F.; Guan, R.; Chen, M.; Xie, X.-M.; Zhang, Q.; Xie, S.-Y.; Yang, S. Ancient pigment to treasure: Prussian blue as a cheap solid cyanide/nitrogen dual-source affording the high-yield syntheses of pricey endohedral clusterfullerenes. Inorg. Chem. Front. 2021, 8, 1719–1726. [Google Scholar] [CrossRef]
- Meng, Q.; Abella, L.; Yang, W.; Yao, Y.-R.; Liu, X.; Zhuang, J.; Li, X.; Echegoyen, L.; Autschbach, J.; Chen, N. UCN@Cs(6)-C82: An Encapsulated Triangular UCN Cluster with Ambiguous U Oxidation State [U(III) versus U(I)]. J. Am. Chem. Soc. 2021, 143, 16226–16234. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Hu, Z.; Yu, P.; Wei, Z.; Jin, P.; Shi, Z.; Lu, X. An experimental and theoretical study of LuNC@C76,82 revealing a cage-cluster selection rule. Inorg. Chem. Front. 2020, 7, 4563–4571. [Google Scholar] [CrossRef]
- Guan, R.; Chen, M.; Xin, J.; Xie, X.-M.; Jin, F.; Zhang, Q.; Xie, S.-Y.; Yang, S. Capturing the Missing Carbon Cage Isomer of C84 via Mutual Stabilization of a Triangular Monometallic Cyanide Cluster. J. Am. Chem. Soc. 2021, 143, 8078–8085. [Google Scholar] [CrossRef]
- Liu, F.; Wang, S.; Guan, J.; Wei, T.; Zeng, M.; Yang, S. Putting a Terbium-Monometallic Cyanide Cluster into the C82 Fullerene Cage: TbCN@C2(5)-C82. Inorg. Chem. 2014, 53, 5201–5205. [Google Scholar] [CrossRef]
- Jin, F.; Wang, S.; Yang, S.; Tamm, N.B.; Ioffe, I.N.; Troyanov, S.I. Trifluoromethyl Derivatives of a Monometallic Cyanide Cluster Fullerene, YCN@C82(6)(CF3)16/18. Inorg. Chem. 2016, 55, 12523–12526. [Google Scholar] [CrossRef]
- Jin, F.; Wang, S.; Tamm, N.B.; Yang, S.; Troyanov, S.I. Synthesis, Isolation, and Trifluoromethylation of Two Isomers of C84-Based Monometallic Cyanide Clusterfullerenes: Interplay between the Endohedral Cluster and the Exohedral Addends. Angew. Chem. Int. Ed. 2017, 56, 11990–11994. [Google Scholar] [CrossRef]
- Zuo, T.; Walker, K.; Olmstead, M.M.; Melin, F.; Holloway, B.C.; Echegoyen, L.; Dorn, H.C.; Chaur, M.N.; Chancellor, C.J.; Beavers, C.M.; et al. New egg-shaped fullerenes: Non-isolated pentagon structures of Tm3N@Cs(51 365)-C84 and Gd3N@Cs(51 365)-C84. Chem. Commun. 2008, 9, 1067–1069. [Google Scholar] [CrossRef][Green Version]
- Krause, M.; Wong, J.; Dunsch, L. Expanding the World of Endohedral Fullerenes—The Tm3N@C2n (39≤n≤43) Clusterfullerene Family. Chem. Eur. J. 2005, 11, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Liu, X.; Wong, J.; Pichler, T.; Knupfer, M.; Dunsch, L. The Electronic and Vibrational Structure of Endohedral Tm3N@C80 (I) Fullerene—Proof of an Encaged Tm3+. J. Phys. Chem. A 2005, 109, 7088–7093. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Olmstead, M.M.; Beavers, C.M.; Balch, A.L.; Wang, G.; Yee, G.T.; Shu, C.; Xu, L.; Elliott, B.; Echegoyen, L.; et al. Preparation and Structural Characterization of the Ih and the D5h Isomers of the Endohedral Fullerenes Tm3N@C80: Icosahedral C80 Cage Encapsulation of a Trimetallic Nitride Magnetic Cluster with Three Uncoupled Tm3+ Ions. Inorg. Chem. 2008, 47, 5234–5244. [Google Scholar] [CrossRef]
- Che, Y.; Yang, H.; Wang, Z.; Jin, H.; Liu, Z.; Lu, C.; Zuo, T.; Dorn, H.C.; Beavers, C.M.; Olmstead, M.M.; et al. Isolation and Structural Characterization of Two Very Large, and Largely Empty, Endohedral Fullerenes: Tm@C3v-C94 and Ca@C3v-C94. Inorg. Chem. 2009, 48, 6004–6010. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Z.; Izumi, N.; Nakanishi, Y.; Koyama, T.; Sugai, T.; Tange, M.; Okazaki, T.; Shinohara, H. Near-Infrared Photoluminescence Properties of Endohedral Mono- and Dithulium Metallofullerenes. ACS Nano 2016, 10, 4282–4287. [Google Scholar] [CrossRef] [PubMed]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Xu, Y.-Y.; Tian, H.-R.; Li, S.-H.; Chen, Z.-C.; Yao, Y.-R.; Wang, S.-S.; Zhang, X.; Zhu, Z.-Z.; Deng, S.-L.; Zhang, Q.; et al. Flexible decapyrrylcorannulene hosts. Nat. Commun. 2019, 10, 485. [Google Scholar] [CrossRef]
- Yao, Y.-R.; Roselló, Y.; Ma, L.; Puente Santiago, A.R.; Metta-Magaña, A.; Chen, N.; Rodríguez-Fortea, A.; Poblet, J.M.; Echegoyen, L. Crystallographic Characterization of U@C2n (2n = 82–86): Insights about Metal-Cage Interactions for Mono-metallofullerenes. J. Am. Chem. Soc. 2021, 143, 15309–15318. [Google Scholar] [CrossRef]
- Sabirov, D.S.; Ori, O.; László, I. Isomers of the C84 fullerene: A theoretical consideration within energetic, structural, and topological approaches. Fuller. Nanotub. Car. N. 2018, 26, 100–110. [Google Scholar] [CrossRef]
- Kirbach, U.; Dunsch, L. The Existence of Stable Tm@C82 Isomers. Angew. Chem. Int. Ed. 1996, 35, 2380–2383. [Google Scholar] [CrossRef]
- Sado, Y.; Aoyagi, S.; Kitaura, R.; Miyata, Y.; Nishibori, E.; Sawa, H.; Sugimoto, K.; Shinohara, H. Structure of Tm@C82(I) Metallofullerene by Single-Crystal X-ray Diffraction Using the 1:2 Co-Crystal with Octaethylporphyrin Nickel (Ni(OEP)). J. Phys. Chem. C 2013, 117, 6437–6442. [Google Scholar] [CrossRef]
- Kodama, T.; Ozawa, N.; Miyake, Y.; Sakaguchi, K.; Nishikawa, H.; Ikemoto, I.; Kikuchi, K.; Achiba, Y. Structural Study of Three Isomers of Tm@C82 by 13C NMR Spectroscopy. J. Am. Chem. Soc. 2002, 124, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Pichler, T.; Golden, M.S.; Knupfer, M.; Fink, J.; Kirbach, U.; Kuran, P.; Dunsch, L. Monometallofullerene Tm@C82: Proof of an Encapsulated Divalent Tm Ion by High-Energy Spectroscopy. Phys. Rev. Lett. 1997, 79, 3026–3029. [Google Scholar] [CrossRef]
- Pichler, T.; Knupfer, M.; Golden, M.S.; Böske, T.; Fink, J.; Kirbach, U.; Kuran, P.; Dunsch, L.; Jung, C. The metallofullerene Tm@C82: Isomer-selective electronic structure. Appl. Phys. A 1998, 66, 281–285. [Google Scholar] [CrossRef]
- Lu, X.; Slanina, Z.; Akasaka, T.; Tsuchiya, T.; Mizorogi, N.; Nagase, S. Yb@C2n (n = 40, 41, 42): New Fullerene Allotropes with Unexplored Electrochemical Properties. J. Am. Chem. Soc. 2010, 132, 5896–5905. [Google Scholar] [CrossRef]
- Bao, L.; Pan, C.; Slanina, Z.; Uhlik, F.; Akasaka, T.; Lu, X. Isolation and Crystallographic Characterization of the Labile Isomer of Y@C82 Cocrystallized with Ni(OEP): Unprecedented Dimerization of Pristine Metallofullerenes. Angew. Chem. Int. Ed. 2016, 55, 9234–9238. [Google Scholar] [CrossRef]
- Kikuchi, K.; Nakao, Y.; Suzuki, S.; Achiba, Y.; Suzuki, T.; Maruyama, Y. Characterization of the Isolated Y@C82. J. Am. Chem. Soc. 1994, 116, 9367–9368. [Google Scholar] [CrossRef]
- Suzuki, T.; Maruyama, Y.; Kato, T.; Kikuchi, K.; Achiba, Y. Electrochemical properties of La@C82. J. Am. Chem. Soc. 1993, 115, 11006–11007. [Google Scholar] [CrossRef]
- Akasaka, T.; Okubo, S.; Kondo, M.; Maeda, Y.; Wakahara, T.; Kato, T.; Suzuki, T.; Yamamoto, K.; Kobayashi, K.; Nagase, S. Isolation and characterization of two Pr@C82 isomers. Chem. Phys. Lett. 2000, 319, 153–156. [Google Scholar] [CrossRef]
- Hu, S.; Liu, T.; Shen, W.; Slanina, Z.; Akasaka, T.; Xie, Y.; Uhlik, F.; Huang, W.; Lu, X. Isolation and Structural Characterization of Er@C2v(9)-C82 and Er@Cs(6)-C82: Regioselective Dimerization of a Pristine Endohedral Metallofullerene Induced by Cage Symmetry. Inorg. Chem. 2019, 58, 2177–2182. [Google Scholar] [CrossRef]
- Cai, W.; Morales-Martínez, R.; Zhang, X.; Najera, D.; Romero, E.L.; Metta-Magaña, A.; Rodríguez-Fortea, A.; Fortier, S.; Chen, N.; Poblet, J.M.; et al. Single crystal structures and theoretical calculations of uranium endohedral metallofullerenes (U@C2n, 2n = 74, 82) show cage isomer dependent oxidation states for U. Chem. Sci. 2017, 8, 5282–5290. [Google Scholar] [CrossRef]
- Liu, X.; Zuo, T.; Dorn, H.C. Polarizability Effects Dominate the Chromatographic Retention Behavior of Spheroidal and Elipsoidal Metallofullerene Nanospheres. J. Phys. Chem. C 2017, 121, 4045–4049. [Google Scholar] [CrossRef]
- Liu, X.; Dorn, H.C. DFT prediction of chromatographic retention behavior for a trimetallic nitride metallofullerene series. Inorg. Chim. Acta 2017, 468, 316–320. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef]



| Compounds | Absorption Peaks (nm) | λonset (nm) | ΔEgap (eV) 1 | Note |
|---|---|---|---|---|
| TmCN@Cs(6)-C82 | 510, 758, 1016, 1134, 1334 | 1624 | 0.76 | this work |
| TbCN@Cs(6)-C82 | 491, 744, 1072, 1132, 1318 | 1620 | 0.77 | Ref [32] |
| DyCN@Cs(6)-C82 | 497, 744, 1024, 1140, 1339 | 1656 | 0.75 | Ref [33] |
| LuCN@Cs(6)-C82 | 494,596, 744, 1069, 1131, 1345 | 1638 | 0.76 | Ref [35] |
| YCN@Cs(6)-C82 | 491, 741,1064, 1123, 1318 | 1620 | 0.77 | Ref [31] |
| TmCN@C2v(9)-C82 | 524, 622, 706, 770, 1016, 1168, 1338 | 1740 | 0.71 | This work |
| TbCN@C2v(9)-C82 | 518, 618, 710, 772, 1144, 1320 | 1770 | 0.70 | Ref [32] |
| DyCN@C2v(9)-C82 | 536, 627, 1144, 1342 | 1785 | 0.69 | Ref [33] |
| LuCN@C2v(9)-C82 | 512, 621, 697, 782, 1096, 1296 | 1680 | 0.74 | Ref [35] |
| Compounds | oxE1 | redE1 | redE2 | redE3 | redE4 | ΔEgap (eV) | Note |
|---|---|---|---|---|---|---|---|
| TmCN@Cs(6)-C82 | 0.55 | −0.67 | −0.90 | −1.72 | −2.16 | 1.22 | This work |
| TbCN@Cs(6)-C82 | 0.55 | −0.59 | −0.84 | −1.77 | −1.92 | 1.14 | Ref [32] |
| DyCN@Cs(6)-C82 | 0.56 | −0.58 | −0.84 | 1.77 | −1.92 | 1.14 | Ref [33] |
| LuCN@Cs(6)-C82 | 0.52 | −0.58 | −0.90 | −1.69 | −1.86 | 1.10 | Ref [35] |
| YCN@Cs(6)-C82 | 0.56 | −0.59 | −0.84 | −1.76 | −1.92 | 1.15 | Ref [31] |
| La@Cs(6)-C82 | −0.07 | −0.47 | −1.40 | −2.01 | −2.40 | 0.40 | Ref [57] |
| Pr@Cs(6)-C82 | −0.07 | −0.48 | −1.39 | −1.99 | / | 0.41 | Ref [59] |
| Er@Cs(6)-C82 | −0.09 | −0.44 | −1.46 | −2.04 | −2.46 | 0.35 | Ref [60] |
| Y@Cs(6)-C82 | −0.07 | −0.43 | −1.43 | −2.05 | / | 0.36 | Ref [56] |
| Yb@Cs(6)-C82 | 0.34 | −0.62 | −0.92 | −1.81 | −2.01 | 0.94 | Ref [55] |
| TmCN@C2v(9)-C82 | 0.64 | −0.45 | −0.79 | −1.60 | −1.94 | 1.09 | This work |
| TbCN@C2v(9)-C82 | 0.55 | −0.46 | −0.81 | −1.78 | −1.96 | 1.01 | Ref [32] |
| DyCN@C2v(9)-C82 | 0.56 | −0.45 | −0.81 | −1.78 | −1.96 | 1.01 | Ref [33] |
| LuCN@C2v(9)-C82 | 0.50 | −0.54 | −0.96 | −1.71 | −1.93 | 1.04 | Ref [35] |
| La@C2v(9)-C82 | 0.07 | −0.42 | −1.37 | −1.53 | −2.26 | 0.49 | Ref [58] |
| Pr@C2v(9)-C82 | 0.07 | −0.39 | −1.35 | −1.46 | −2.21 | 0.46 | Ref [59] |
| Er@C2v(9)-C82 | 0.08 | −0.42 | −1.40 | −2.18 | / | 0.50 | Ref [60] |
| U@C2v(9)-C82 | 0.10 | −0.43 | −1.42 | −1.76 | −1.77 | 0.53 | Ref [61] |
| Y@C2v(9)-C82 | 0.10 | −0.34 | −1.34 | −2.22 | / | 0.44 | Ref [56] |
| Yb@C2v(9)-C82 | 0.61 | −0.46 | −0.78 | −1.60 | −1.90 | 1.07 | Ref [55] |
| Fraction | Subfraction | Major Component | Relative Abundance |
|---|---|---|---|
| A | A-1 | C84–86 | 46.1% |
| A-2 | Tm@C82/TmC77N | 3.4% | |
| A-3 | Tm@C80–84/TmC83N | 36.7% | |
| A-4 | Tm@C80,84/Ti2C80/TmCN@C82 | 13.8% | |
| A-4 | A-4-1 | Ti2C80 | 2.2% |
| A-4-2 | Tm@C80 | 6.5% | |
| A-4-3 | TmCN@C82 (Cs(6), C2v(9)) | 8.7% | |
| A-4-1 | Tm@C84 | 82.6% | |
| A-4-3 | A-4-3-1 | TmCN@Cs(6)-C82 | 52.1% |
| A-4-3-2 | TmCN@C2v(9)-C82 | 47.9% |
| Crystal | TmCN@Cs(6)-C82 | TmCN@C2v(9)-C82 |
|---|---|---|
| Empirical formula | C210H88N21Tm | C210H88N21Tm |
| Formula weight | 3073.94 | 3073.94 |
| Habit | Block | Block |
| Temperature, K | 100 | 100 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | P21/c |
| a, Å | 14.669 | 14.724 |
| b, Å | 32.146 | 32.032 |
| c, Å | 32.213 | 32.215 |
| α, deg | 90 | 90 |
| β, deg | 101.74 | 101.91 |
| γ, deg | 90 | 90 |
| Volume, Å3 | 14,872.3 | 14,866.8 |
| Z | 4 | 4 |
| Dx, g/cm3 | 1.373 | 1.373 |
| F(000) | 6256 | 6256 |
| Crystal Size, mm3 | 0.06 × 0.03 × 0.01 | 0.12 × 0.08 × 0.03 |
| 2θ max, ° | 20.499 | 24.712 |
| R1/wR2 (I > 2σ(I)) 1 | 0.1193/0.2831 | 0.0937/0.2531 |
| GOF | 1.116 | 1.052 |
| Completeness | 0.972 | 0.987 |
| Obs reflects | 5752 | 14,863 |
| Total reflects | 14,478 | 25,026 |
| Parameters | 2131 | 2162 |
| metal disorder | Tm1(0.60)/Tm2(0.08)/Tm3(0.17)/ Tm4(0.11)/Tm5(0.04) | Tm1(0.25)/Tm2(0.14)/Tm3(0.09)/Tm4(0.04)/Tm5(0.08)/Tm6(0.13)/ Tm7(0.20)/Tm8(0.07) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xin, J.; Jin, H.; Xiang, W.; Chen, M.; Yao, Y.-R.; Yang, S. TmCN@C82: Monometallic Clusterfullerene Encapsulating a Tm3+ Ion. Inorganics 2023, 11, 323. https://doi.org/10.3390/inorganics11080323
Zhang H, Xin J, Jin H, Xiang W, Chen M, Yao Y-R, Yang S. TmCN@C82: Monometallic Clusterfullerene Encapsulating a Tm3+ Ion. Inorganics. 2023; 11(8):323. https://doi.org/10.3390/inorganics11080323
Chicago/Turabian StyleZhang, Huichao, Jinpeng Xin, Huaimin Jin, Wenhao Xiang, Muqing Chen, Yang-Rong Yao, and Shangfeng Yang. 2023. "TmCN@C82: Monometallic Clusterfullerene Encapsulating a Tm3+ Ion" Inorganics 11, no. 8: 323. https://doi.org/10.3390/inorganics11080323
APA StyleZhang, H., Xin, J., Jin, H., Xiang, W., Chen, M., Yao, Y.-R., & Yang, S. (2023). TmCN@C82: Monometallic Clusterfullerene Encapsulating a Tm3+ Ion. Inorganics, 11(8), 323. https://doi.org/10.3390/inorganics11080323