Abstract
Oxidative stress is the cause of various pathologies and disorders of cellular functions. Substances that reduce the pathological effect of oxidative stress on homeostasis include organoselenium compounds of natural and synthetic origin. Depending on the structure, organoselenium compounds can exhibit different biological activities, for example, reducing oxidative stress, participating in the regulation of signaling systems, influencing the synthesis of cytokines, etc. This makes them promising products for the development of new means of metabolic correction and drugs with enzyme-like activity. This study is aimed at developing an effective method for the synthesis of functional organoselenium compounds and studying their antioxidant effect in vivo under stress conditions. A one-pot catalyst-free method of transannular addition-functionalization of cis,cis-1,5-cyclooctadiene with selenium dihalides in the presence of nucleophiles was developed. For the first time, the antioxidant activity of functionalized 9-selenabicyclo[3.3.1]nonanes was studied in vivo. Quantitative characteristics of the effect on the level of lipid peroxidation and the activity of glutathione peroxidase and glutathione reductase under stress conditions were obtained. The effect of substituents in the selenium-containing scaffold on the biological activity of the compounds was studied. The water-soluble 9-selenabicyclo[3.3.1]nonane derivatives, containing hydroxyl and 2-hydroxyethoxy groups, which increased the activity of both glutathione peroxidase and glutathione reductase, were discovered.
1. Introduction
In modern medicine, attention is increasingly focused on post-therapeutic reactions of the organism, such as allergies, intoxication, drug hepatitis, etc. Among the biochemical processes associated with these reactions is oxidative stress, which is developed due to the increased cell-generated reactive oxygen species (ROS). Peroxide derivatives of unsaturated fatty acids in cell membranes are involved in the biosynthesis of prostaglandins and leukotrienes (mediators of inflammation and allergic reactions). As ROS is accumulated in the macroorganism, many mechanisms mobilize, including enzymes that utilize peroxide compounds. With insufficient capacity for compensatory resources, activation of lipid peroxidation (LPO) can destabilize cell membranes with subsequent complications [1]. Intoxications of various nature activate LPO against the background of inhibition of cell antioxidant protection as well as the accumulation of immunosuppressive factors in the blood (acylhydroperoxides, diene conjugates of fatty acids, MDA, abnormal lipid metabolites). Disturbances in metabolic status and decreased energy supply to cells elicit immunodeficiency states [2]. In this case, it is advisable to use metabolic correction agents that increase antioxidant potential.
Selenium is recognized as an important micronutrient for humans [3,4,5,6]. This element is an important part of glutathione peroxidase, a selenium-containing enzyme whose biological role is to protect the body from oxidative damage. A number of organoselenium compounds exhibit high glutathione peroxidase-like activity [3,4,5,6,7,8,9,10] including ebselen, a well-known selenium heterocycle, which is undergoing evaluation as a therapeutic agent in clinical trials [11,12].
Natural and synthetic organoselenium compounds can reduce the pathological effect of oxidative stress on homeostasis [13,14] and prove to be antioxidants [15,16,17]. Meanwhile, it becomes clear that an uncontrolled and unreasonable increase in the antioxidant profile of the organism has a negative effect on the immune system and a number of other body functions. Note that enhancement of the catalytic activity of organoselenium compounds is also not a priority, since it leads to lower selectivity and higher toxicity of the studied compounds. This, together with a decrease in biostability, negatively affects the possibilities of using organoselenium compounds as bioactive substances for drug design. For the time being, a standing challenge for medicine remains the development of mild drugs of metabolic correction for use in vaccination processes, including as hepatoprotectors or general protectors of immunogenesis organs, antiviral therapy, and diseases associated with immunodeficiency states.
Important electrophilic reagents, selenium dichloride and dibromide, were recently introduced in organic synthesis [18,19,20,21]. The use of these reagents opened up new possibilities for the synthesis of new classes of organoselenium compounds. Novel approaches, including transannular selenocyclization, annulation/selenofunctionalization, and selenobisfunctionalization, were developed [19,20,21,22,23,24,25,26,27].
Selenofunctionalization reactions have found useful applications in modern organic synthesis, allowing the simultaneous introduction of the selenium atom and a functional group into the double bonds [28]. Seleniranium cations are postulated as intermediates in these reactions. Recently, we developed one-pot bis-alkoxyselenenylation of alkenes with selenium dibromide and alcohols [29]. This approach allows to realize one-pot selenobisfunctionalization reactions under mild conditions and to increase yields of the target products, avoiding the stage of the formation of the intermediate bis(β-halogenorganyl) selenides [29].
Recently, 2,6-dichloro-9-selenabicyclo[3.3.1]nonane and its sulfur analogue [30] were involved in nucleophilic substitution reactions to evaluate a relative anchimeric assistance effect [31]. The anchimeric assistance effect of the selenium atom was found to be considerably higher than that of the sulfur atom [31].
We developed a method for the synthesis of 2,6-dichloro- and 2,6-dibromo-9-selenabicyclo[3.3.1]nonanes based on selenium dihalides and cis,cis-1,5-cyclooctadiene (COD) [31,32]. A number of 2,6-bifunctional derivatives were obtained based on 2,6-dibromo-9-selenabicyclo[3.3.1]nonane [33,34,35,36]. It was found that the 2,6-dipyridinium salt of 9-selenabicyclo[3.3.1]nonane with high glutathione peroxidase-like activity is a promising drug for metabolic correction during vaccination [35]. The introduction of this compound into the bodies of experimental animals significantly decreases the development of pathological reactions under the influence of the tularemia and brucellosis vaccines [35]. This compound potentiates the proliferative activity of immune cells and shows a high anti-inflammatory effect [36]. It also enhances the immune response against Yersinia pestis, thereby increasing resistance to the plague pathogen.
2. Results and Discussion
2.1. Chemistry
This study is aimed at developing an effective method for the synthesis of functional organoselenium compounds and studying their antioxidant effect in vivo under stress conditions.
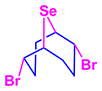
Previously, 2,6-dibromo-9-selenabicyclo[3.3.1]nonane (1) was obtained by the reaction of selenium dibromide with COD in carbon tetrachloride (Scheme 1) [32].
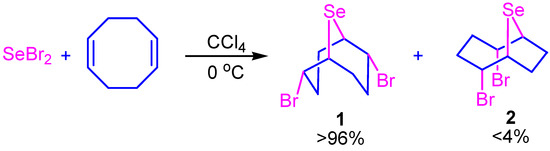
Scheme 1.
Reaction selenium dibromide with cis,cis-1,5-cyclooctadiene.
However, the target product contained some amounts of the isomeric compound 2,5-dibromo-9-selenabicyclo[4.2.1]nonane (2) as an impurity.
In this work, we propose a new one-pot catalyst-free method of transannular addition-functionalization of COD with selenium dibromide in the presence of nucleophiles. This approach makes it possible to avoid the stage of isolation of the intermediate dibromo adduct 1 with the use of highly toxic and carcinogenic CCl4 and to increase the yields of target 9-selenabicyclo[3.3.1]nonanes. The proposed reaction conditions make it possible to obtain only 2,6-bifunctional 9-selenabicyclo[3.3.1]nonanes without traces of isomeric 9-selenabicyclo[4.2.1]nonanes and to simplify the preparation of high-purity products.
It was found that carrying out the reaction in more polar solvents (chloroform, methylene chloride, acetonitrile, etc.) decreased the content of compound 2. Also, the latter can be isomerized into a thermodynamically more stable compound 1 by refluxing the reaction mixture in chloroform (Scheme 2). It was established that the isomerization of compound 2 did not lead to the formation of other products except for nonane 1.
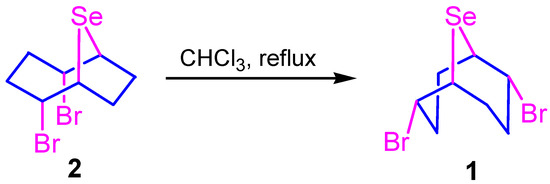
Scheme 2.
Isomerization of 2,5-dibromo-9-selenabicyclo[4.2.1]nonane.
It was shown that the isomeric composition of the substitution products did not depend on the isomeric ratio of compounds 1 and 2, but was determined by the conditions of the substitution reaction.
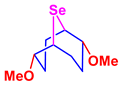
For example, methoxylation of compound 1 in a mixture of carbon tetrachloride and methanol (20:1) at 0 °C delivered a mixture of isomeric 2,6-dimethoxy-9-selenabicyclo[3.3.1]nonane (3) and 2,5-dimethoxy-9-selenabicyclo[4.2.1]nonane (4) in a 93:7 ratio, whereas this reaction in methylene chloride-methanol (5:1) proceeded at room temperature, affording exclusively compound 3. These trends indicate that the nucleophilic substitution reactions proceed via seleniranium intermediates.
We have recently demonstrated the first examples of selenomethoxylation and annulation-selenomethoxylation reactions of selenium dihalides with alkenes [22,29]. The experimental conditions were found to allow the use of selenium dihalides in the methoxyselenation reaction in the presence of alcohols (methanol, ethanol, isopropanol, mercaptoethanol, etc.) and not involve alcoholysis of the selenium-halogen bond in selenium dihalides.
A one-pot transannular selenomethoxylation of COD was developed. The target compound 3 was synthesized in high yield (96%) by a one-pot atom-economic reaction of selenium dibromide with COD in the presence of methanol under mild conditions at room temperature (Scheme 3).
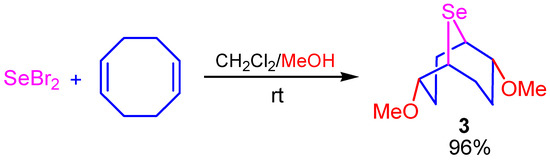
Scheme 3.
Selenomethoxylation of COD by SeBr2.
The experimental conditions for the acetoxyselenation reaction of selenium dihalides with COD were found. The target 2,6-diacetoxy-9-selenabicyclo[3.3.1]nonane (3) was synthesized in almost quantitative yield (98%) by a one-pot atom-economic reaction of selenium dibromide with COD in a chloroform-acetic acid (5:1) mixture (Scheme 4).
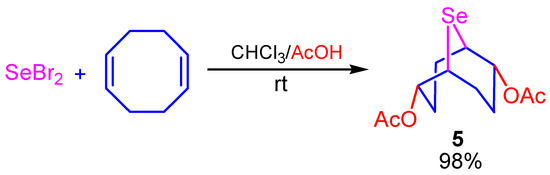
Scheme 4.
Selenoacetoxylation of COD by SeBr2.
Using the reaction of selenium dibromide with COD in chloroform or methylene chloride in the presence of acetic acid, we showed the possibility of an acetoxyselenation reaction (Scheme 5). The reaction proceeded regio- and stereoselectively and led to the formation of 2,6-diacetoxy-9-selenabicyclo[3.3.1]nonane (5).
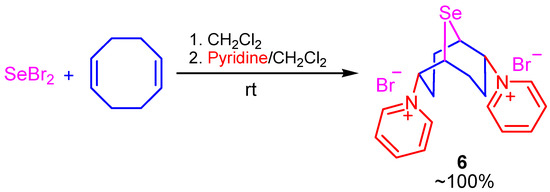
Scheme 5.
One-pot synthesis of 2,6-dipyridinium 9-selenabicyclo[3.3.1]nonane dibromide (6).
The structure of compound 5 was proven by single-crystal X-ray diffraction analysis (Figure 1). These data indicate that the bis-functionalization reaction proceeds as an anti-addition to the double bond, giving the product a trans-disposition of the selenium atom and two functional (acetoxy) groups.
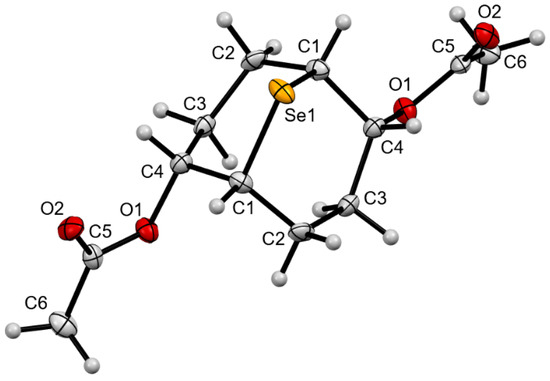
Figure 1.
ORTEP molecular structure of compound 5 at 50% thermal ellipsoid probability.
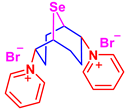
The one-pot, two-stage synthesis of 2,6-dipyridinium 9-selenabicyclo[3.3.1]nonane dibromide (6) in quantitative yield was developed based on the addition of selenium dibromide to COD followed by nucleophilic substitution of intermediate compound 1 with pyridine (two equivalents) in methylene chloride (Scheme 5).
When the reaction of selenium dibromide with COD was carried out in the presence of pyridine, mainly halogenated products of double bonds were formed.
In this case, the reaction was realized as a one-pot, two-stage synthesis, with the addition of selenium dihalide to COD in methylene chloride followed by the addition of two equivalents of pyridine to a solution of intermediate compound 1 (Scheme 6). The reaction proceeds in a chemo- and regioselective fashion to produce 2,6-dipyridinium 9-selenabicyclo[3.3.1]nonane dibromide (6) in quantitative yield.
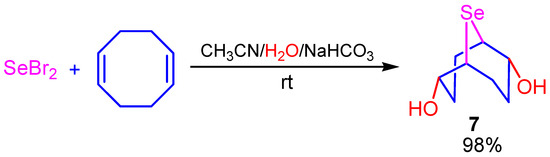
Scheme 6.
Hydroxyselenation of COD by SeBr2.
We found that the hydroxyselenation reaction of selenium dichloride and selenium dibromide with COD can be realized in the system acetonitrile/water/NaHCO3 (Na2CO3, K2CO3) at room temperature, affording 2,6-dihydroxy-9-selenabicyclo[3.3.1]nonane (7) in almost quantitative yield (98%). The reaction occurred with high chemo- and regioselectivity, and the formation of 2,5-dihydroxy-9-selenabicyclo[4.2.1]nonane as a possible by-product was not observed under these conditions (Scheme 6).
The reaction of selenium dibromide with COD and the excess ethylene glycol in acetonitrile afforded 2,6-bis(2-hydroxyethoxy)-9-selenabicyclo[3.3.1]nonane (8) in high yield (Scheme 7).
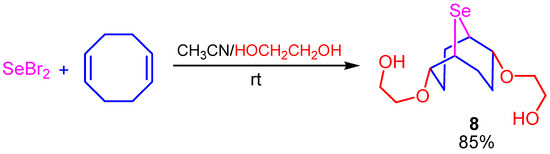
Scheme 7.
Synthesis of 2,6-bis(2-hydroxyethoxy)-9-selenabicyclo[3.3.1]nonane (8).
When ethylene glycol was used as a solvent for the reaction of selenium dihalides with COD, the formation of a mixture of oligomeric products was observed.
2.2. Biological Evaluation
In the present work, the effect of functionalized 2,6-disubstituted selenabicyclo[3.3.1]nonanes on the main characteristics of the antioxidant profile—the level of lipid peroxidation (LPO), glutathione peroxidase (GPx), and glutathione reductase (GR) activities—was examined. The tested compounds were divided into two groups: hydrophobic compounds 1, 3, and 5, and water-soluble derivatives 6–8 (Table 1).

Table 1.
First group—hydrophobic compounds 1, 3, and 5 with Ph2Se2 as reference. Second group—hydrophilic water-soluble compounds 6, 7, and 8 with Na2SeO3 as reference.
Increasing salinity was chosen as a method for causing stress in plant cells. The salinity was created using NaCl at a concentration of 200 mM [37]. In fact, such a concentration generates stress since it almost doubles the level of lipid peroxidation as compared with the control. Selenium is known to play a major role in the antioxidant protection of plants [38]. In this line, we commenced our study with the determination of the diene conjugate content.
In the series of water-soluble derivatives 7 and 8, a significant decrease in lipid peroxidation was observed under stress conditions at both low and high concentrations of compounds (Figure 2). In contrast, derivative 6 at a 1000 μM concentration showed an increase in lipid peroxidation, thus indicating toxicity. However, the increase in lipid peroxidation by compound 6 was about twice lower compared with the reference compound sodium selenite (Figure 1). Taking into account the low lipid peroxidation levels observed for compounds 7 and 8, the toxicity of compound 6 may be attributed to the blocking of ion channels, which is characteristic for pyridinium and ammonium salts.
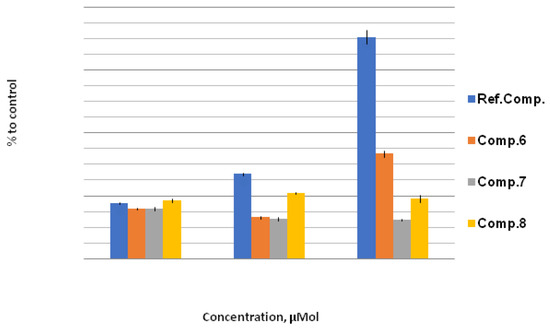
Figure 2.
LPO level, % to control. The effect of compounds 6, 7, and 8 on LPO level under stress conditions relative to control (H2O + 200 mmol NaCl; taken as 100%) at concentrations of 10 μM, 100 μM, and 1000 μM. Quantitative values are given in Electronic Supplementary Information.
Previously, a toxic effect of sulfur analogs of compounds 6 and 7 (2,6-dipyridyl- and 2,6-dihydroxy substituted 9-thiabicyclo[3.3.1]nonanes) was noted when fragmentable oligo- and polycations derived from 2,6-dichloro-9-thiabicyclo[3.3.1]nonane were studied [39].
For lipophilic derivatives 1, 3, and 5, the level of lipid peroxidation at a 1000 μM concentration was significantly decreased with respect to the reference substance (Ph2Se2). Compound 1, despite the highly active bromine atom in the β-position relative to the selenium atom, did not exhibit high toxicity (Figure 3).
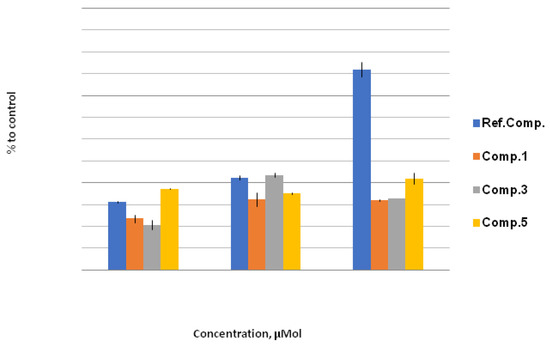
Figure 3.
LPO level, % to control. The effect of compounds 1, 3, and 5 on LPO level under stress conditions relative to control (H2O/1% EtOH + 200 mmol NaCl; taken as 100%) at concentrations of 10 μM, 100 μM, and 1000 μM. Quantitative values are given in Electronic Supplementary Information.
Glutathione peroxidase is a key enzyme in protecting cells from oxidative damage. Glutathione peroxidase activity is a direct characteristic of the antioxidant system’s capacity. We have studied the effect of various concentrations of 9-selenabicyclo[3.3.1]nonane derivatives on glutathione peroxidase activity under stress conditions.
In the series of water-soluble derivatives 6–8, a multidirectional tendency was found (Figure 4). The hydroxyethoxy compound 8 at a 10 μM concentration showed a significant increase in GPx activity, with a tendency to decrease, with the elevation of concentration.
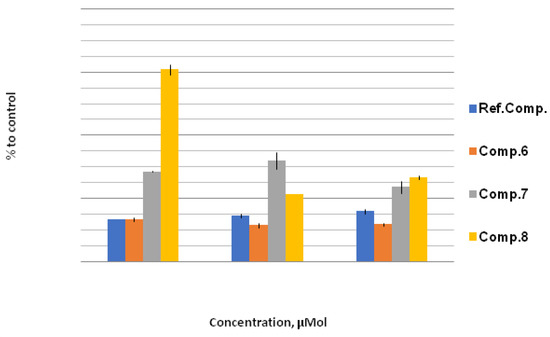
Figure 4.
GPx activity, % to control. The effect of compounds 6, 7, and 8 on GPx activity under stress conditions relative to control (H2O + 200 mmol NaCl; taken as 100%) at concentrations of 10 μM, 100 μM, and 1000 μM. Quantitative values are given in Electronic Supplementary Information.
The pyridine derivative 6 demonstrated a result close to the inorganic source of selenium, sodium selenite, which is characterized by reduced GPx activity in all the concentrations studied, thus contrasting with the previously demonstrated decrease in the lipid peroxidation level (Figure 4).
In the case of lipophilic derivatives 1, 3, and 5, a noticeable effect on the activity of GPx was not observed (Figure 5). A gradual lowering of GPx activity with the growth of the concentration of the acetoxy compound 5 may indicate a weak inhibitory effect. The increase in GPx activity in the case of bromine derivative 1 may be attributed to the accumulation of the metabolites, including hydrolysis products such as hydroxy derivative 7, which enhanced the activity of GPx.
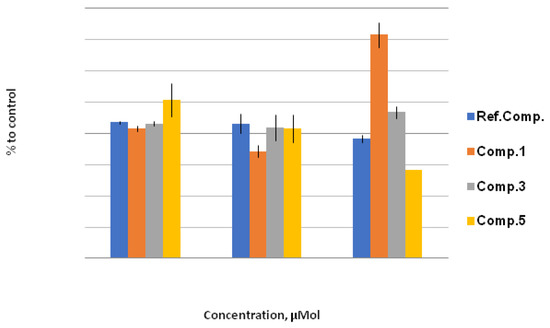
Figure 5.
GPx activity, % to control. The effect of compounds 1, 3, and 5 on GPx activity under stress conditions relative to control (H2O/1% EtOH + 200 mmol NaCl; taken as 100%) at concentrations of 10 μM, 100 μM, and 1000 μM. Quantitative values are given in Electronic Supplementary Information.
The key parameter of the antioxidant protective potential is the redox status of glutathione, which is significantly biased towards the reduced form under normal conditions. The oxidation-reduction status of glutathione is supported by a number of enzymes, the main of which is glutathione reductase (GR). Glutathione reductase is an important component of the ROS detoxification system in plants and catalyzes the conversion of oxidized glutathione (GSSG) to its reduced form (GSH). It is known that selenium affects the activity of enzymes in the glutathione plant protection system [40]. Following this line, we have investigated the effect of compounds 1, 3, 5, and 6–8 on the activity of glutathione reductase (GR) in the present work.
In contrast to the inhibitory effect of compound 6 toward GPx, a significant increase in GR activity with concentration growth was found. These results may indicate the promotion of the GSSG reduction reaction. However, this effect of a multiple increase in GR activity may be a combination of the GPx inhibitory effect of compound 6 and an increase in cellular antioxidant potential under stress, which can also lead to an increase in GR activity.
An increase in GR activity by compounds 7 and 8 was observed, but already against the background of a substantial growth of GPx activity, thus evidencing a drastic augmentation of the antioxidant profile even at concentrations of 10 μM (Figure 6). A sharp increase in the antioxidant profile even at 10 μM concentration is noteworthy, taking into account the significant increase in GPx activity by these compounds.
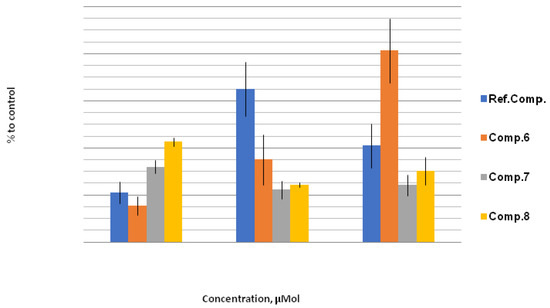
Figure 6.
GR activity, % to control. The effect of compounds 6, 7, and 8 on GR activity under stress conditions relative to control (H2O + 200 mmol NaCl; taken as 100%) at concentrations of 10 μM, 100 μM, and 1000 μM. Quantitative values are given in Electronic Supplementary Information.
Lipophilic derivatives of 9-selenabicyclo[3.3.1]nonane demonstrated a considerable drop in GR activity, which, together with a slight increase in GPx activity, allows us to consider these compounds as potential medicines for mild metabolic correction. Furthermore, it is worthwhile to note a significant GR-inhibitory effect of the bromo derivative 1, which in this case is characterized by a general decrease in lipid peroxidation (Figure 7).
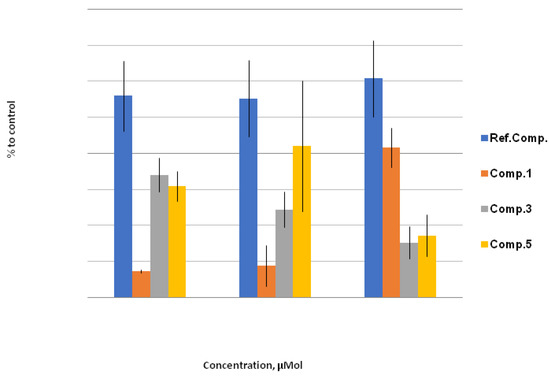
Figure 7.
GR activity, % to control. The effect of compounds 1, 3, and 5 on GR activity under stress conditions relative to control (H2O/1% EtOH + 200 mmol NaCl; taken as 100%) at concentrations of 10 μM, 100 μM, and 1000 μM. Quantitative values are given in Electronic Supplementary Information.
3. Materials and Methods
3.1. General Information
X-ray diffraction experiments were carried out on a Bruker D8 Venture Photon 100 CMOS diffractometer (Bruker BioSpin GmbH, Rheinstetten, Germany) with Mo-Kα radiation (λ = 0.71073 Å). X-ray crystallographic data for compound 5 (CCDC 2277477) are shown in Electronic Supplementary Information. These data can be obtained free of charge at http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 28 June 2023).
1H (400.1 MHz) and 13C (100.6 MHz) NMR spectra were recorded on a Bruker DPX-400 spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany) in 5–10% solution in CDCl3. 1H and 13C chemical shifts (δ) are reported in parts per million (ppm), relative to the residual solvent peak of CDCl3 (δ = 7.27 and 77.16 ppm in 1H and 13C-NMR, respectively).
The mass spectra were recorded on a Shimadzu GCMS-QP5050A (Shimadzu Corporation, Kyoto, Japan) with electron impact (EI) ionization (70 eV). Elemental analysis was performed on a Thermo Scientific Flash 2000 Elemental Analyzer (Thermo Fisher Scientific Inc., Milan, Italy).
The organic solvents were dried and distilled according to standard procedures.
3.2. The Synthesis of Compound 3
2,6-Dimethoxy-9-selenabicyclo[3.3.1]nonane (3). A solution of SeBr2 (4.80 g, 20 mmol) in CH2Cl2 (50 mL) was added dropwise to a solution of 1,5-cyclooctadiene (2.16 g, 20 mmol) in CH2Cl2 (35 mL) and MeOH (15 mL). The reaction mixture was stirred for 15 h at room temperature. The solvent was removed on a rotary evaporator, and the residue was washed with C6H14 (2 mL) at 0 °C and dried in vacuum to constant mass. Product compound 3 (4.8 g, 96% yield) was colorless or light-yellow liquid.
1H NMR (400 MHz, CDCl3) δ 1.78–1.82 (m, 2H, SeCHCH2), 2.00–2.04 (m, 2H, SeCHCH), 2.17–2.19 (m, 2H, OCHCH), 2.60–2.64 (m, 2H, OCHCH), 3.02–3.03 (m, 2H, SeCH), 3.36 (s, 6H, CH3), 3.88–3.92 (m, 2H, MeOCH). 13C NMR (100 MHz, CDCl3) δ 27.87, 28.05, 28.87, 55.86, 81.04. Found: C, 47.96; H, 7.45; Se, 31.93. Calc. for C10H18O2Se: C, 48.20; H, 7.28; Se, 31.68. MS (EI): m/z (%) = 250 (30, M+), 218 (10), 179 (18), 137 (64), 105 (50), 79 (100), 71 (89), 45 (78), 41 (90). IR (film): 1086, 1153, 1186, 2817, 2922, 2977 cm−1.
3.3. The Synthesis of Compound 5
2,6-Diacetoxy-9-selenabicyclo[3.3.1]nonane (5). A solution of SeBr2 (4.80 g, 20 mmol) in CH2Cl2 (30 mL) was added dropwise to a solution of cis,cis-1,5-cyclooctadiene (2.16 g, 20 mmol) in a mixture of CH2Cl2 (20 mL) and AcOH (10 mL). The reaction mixture was stirred for 16 h at room temperature. Then the mixture was washed with water (3 × 20 mL), dried with CaCl2, filtrated, and the solvent removed on a rotary evaporator. The residue was washed with C6H14 (2 mL) and dried in vacuum. Compound 5 (6.0 g, 98% yield) was obtained as colorless crystals, mp 108–109 °C.
1H NMR (400 MHz, CDCl3) δ 1.96–2.05 (m, 10H, SeCHCH2, CH3), 2.28–2.33 (m, 2H, OCHCH2), 2.62–2.66 (m, 2H, OCHCH2), 2.94–2.96 (m, 2H, SeCH), 5.46–5.48 (m, 2H, OCH). 13C NMR (100 MHz, CDCl3) δ 20.97, 27.37, 27.80, 28.00, 74.07, 169.85. Found: C, 46.98; H, 6.09; Se, 26.11. Calc. for C12H18O4Se: C, 47.22; H, 5.94; Se, 25.87. IR: 2922, 1729, 1364, 1231, 1021, 960, 870 cm−1. MS (EI): m/z (%) = 306.00 (9, M+) 186.00 (18), 123.15 (10), 105.10 (59), 95.10 (11), 93.10 (11), 79.10 (28), 67.10 (10), 43.05 (100), 41.10 (17), 39.10 (10).
3.4. The Synthesis of Compound 6 (Modified Procedure)
2,6-Dipyridinium-9-selenabicyclo[3.3.1]nonane dibromide (6). A solution of SeBr2 (2.4 g, 10 mmol) in methylene chloride (20 mL) was added dropwise to a solution of cis,cis-1,5-cyclooctadiene (1.08 g, 10 mmol) in methylene chloride (20 mL). The reaction mixture was stirred for 8 h at room temperature. A solution of pyridine (1 g, 1.25 mmol) in methylene chloride (5 mL) was added dropwise to the reaction mixture. The reaction mixture was stirred overnight (16 h) at room temperature. The precipitate was filtered, washed with cold methylene chloride, and dried in vacuum. Compound 6 (5.05 g, ~100% yield) was obtained as a beige powder, mp 177–178 °C.
1H NMR (400 MHz, D2O) δ 2.49–2.55 (m, 2H, SeCHCH2), 2.69–2.73 (m, 4H, NCHCH2, SeCHCH2), 3.21–3.33 (m, 2H, NCHCH2), 3.57–3.59 (m, 2H, SeCH), 5.99–6.04 (m, 2H, NCH), 8.24 (t, 4H, m-Hpyr), 8.69 (t, 2H, p-Hpyr), 9.17 (d, 4H, o-Hpyr). 13C NMR (100 MHz, D2O) δ 28.39, 31.01, 32.19, 77.43, 131.52, 145.99, 149.29. Found: C, 43.08; H, 4.58; Br, 31.48; N, 5.73; Se, 15.76. Calc. for C18H22Br2N2Se: C, 42.80; H, 4.39; Br, 31.64; N, 5.55; Se, 15.63. MS (EI): m/z (%) = 345.80 (0.36, M+-2Br), 187.05 (14.20), 105.15 (19.59), 91.05 (9.20), 80.00 (17.64), 79.05 (100.00), 78.10 (20.72), 77.05 (10.78), 52.05 (56.84), 51.10 (24.21), 39.05 (19.49). IR: 3404, 3044, 1629, 1488, 1134, 769, 690 cm−1.
3.5. The Synthesis of Compound 7
2,6-Dihydroxy-9-selenabicyclo[3.3.1]nonane (7). A solution of SeBr2 (4.80 g, 20 mmol) in CH3CN (30 mL) was added dropwise to a mixture of cis,cis-1,5-cyclooctadiene (2.16 g, 20 mmol) in CH3CN (35 mL), H2O (5 mL), and NaHCO3 (3.5 g, 42 mmol). The reaction mixture was stirred for 14 h at room temperature. The solvent was removed on a rotary evaporator, and the residue was washed with cold C6H14 (5 mL) and dried in vacuum. Compound 7 (4.35 g, 98% yield) was obtained as white crystals, mp 249–250 °C.
1H NMR (400 MHz, DMSO-D6/CDCl3) δ 1.73–1.81 (m, 2H, SeCHCH2), 1.85–1.92 (m, 2H, SeCHCH2), 2.04–2.14 (m, 2H, OCHCH2), 2.62–2.70 (m, 4H, OCHCH2, OH), 3.53–3.57 (m, 2H, SeCH), 4.24–4.27 (m, 2H, OCH). 13C NMR (100 MHz, DMSO-D6/CDCl3) δ 25.91, 29.50, 30.45, 69.43. Found: C, 43.74; H, 6.56; Se, 35.49. Calc. for C8H14O2Se: C, 43.45; H, 6.38; Se, 35.70. MS (EI): m/z (%) = 222 (52, M+), 205 (5), 178 (20), 149 (15), 133 (17), 123 (27), 95 (58), 79 (69), 71 (49), 67 (41), 57 (31), 55 (58), 41 (100), 39 (51). IR: 873, 982, 1017, 2899, 2932, 3340 cm−1.
3.6. The Synthesis of Compound 8
2,6-Di(2-hydroxyethoxy)-9-selenabicyclo[3.3.1]nonane (8). A solution of SeBr2 (4.80 g, 20 mmol) in CH3CN (30 mL) was added dropwise to a solution of cis,cis-1,5-cyclooctadiene (2.16 g, 20 mmol) in a mixture of CH3CN (30 mL) and ethylene glycol (10 mL). The reaction mixture was stirred for 14 h at room temperature. The solvents were removed in vacuum, and water (10 mL) was added to the residue. The product was extracted with CH2Cl2 (4 × 20 mL), the combined organic phase was washed with 1M NaHCO3 solution (3 × 20 mL), dried with CaCl2, and filtered. The solvent was removed on a rotary evaporator, and the residue was dried in vacuum. Compound 8 (5.27 g, 85% yield) was obtained as a colorless oil.
1H NMR (400 MHz, D2O) δ 1.84–1.91 (m, 2H, SeCHCH2), 2.11–2.16 (m, 2H, SeCHCH2), 2.27–2.31 (m, 4H, OCHCH2, OH), 2.63–2.67 (m, 2H, OCHCH2), 3.20–3.22 (m, 2H, SeCH), 3.63–3.68 (m, 2H, CH2OCH), 3.70–3.83 (m, 6H, OCH2CH2O), 4.19–4.22 (m, 2H, OCH). 13C NMR (100 MHz, D2O) δ 30.28, 30.57, 31.18, 63.45, 71.73, 82.96. Found: C, 46.37; H, 7.03; Se, 25.82. Calc. for C12H22O4Se: C, 46.60; H, 7.17; Se, 25.53. IR: 3392, 2919, 1649, 1432, 1362, 1089, 888, 653 cm−1. MS (EI): m/z (%) = 310.10 (15, M+), 186.00 (13), 167.15 (27), 123.05 (15), 105.10 (53), 101.15 (21), 95.10 (20), 91.15 (23), 80.05(29), 79.10 (89), 73.10 (42), 57.05 (53), 45.10 (100), 41.10 (59), 39.10 (30)
3.7. Plant Material
Studies were carried out on oilseed radish seeds (Raphanus sativus L. var. oleiferus Metzg.) of lines of Irkutsk State Agricultural Academy with laboratory germinability of 80–98% and weighing 1000 seeds 9.5 g. Seeds were germinated on wet filter paper in Petri dishes at a constant 23 °C temperature in the dark for 4 days after wetting with the test solutions. The number of seeds in one cup was 30 pcs. The experiment was repeated 3 times.
3.8. Evaluation of Germinability and Mass of Seedlings
Germinability was analyzed according to the All-Union State Standard 10-14-86, “Oilseed Radish Seeds. Varietal and sowing qualities”. These indicators were determined in accordance with All-Union State Standard 12038-84, “Seeds of agricultural crops. Methods for determining germinability” [41]. The mass of shoots and roots was determined using gravimetric analysis.
3.9. Determination of Protein Content
Protein content was determined by the degree of binding to the Coomassie blue dye (CBB G250 “Sigma”) according to the Bradford method [42].
3.10. Determination of Glutathion Reductase Activity
Glutathione reductase activity (EC 1.6.4.2) was measured according to the method described by Nigmatullina et al. [43]. The activity of glutathione reductase was determined by the change in absorption at 340 nm caused by the oxidation of NADPH in 3.5 min with an interval of 1 s on the spectrophotometer. The enzyme activity was calculated using the extinction coefficient for NADP+ at a wavelength of 340 nm, which is equal to 6.22 mmol−1cm−1.
3.11. Evaluation of Diene Conjugates
Analysis of the content of the primary products of lipid peroxidation—diene conjugates (DC)—was carried out according to the method [44] in our modification. The measurement was performed on a spectrophotometer at a wavelength of 203 nm. The obtained optical density (D) was used to calculate the concentration of diene conjugates (recalculated per 1 g wet mass) using an extinction coefficient equal to 2.2 × 105 mol−1cm−1. Salinization was chosen as a stress, which was created with NaCl, a concentration of 200 mmol was taken from literature data [40]. This concentration causes stress since it significantly increases the level of lipid peroxidation by almost two times compared with the control.
3.12. Statistics
The results obtained are given as relative values in comparison with the control (taken as 100%). Electronic supplementary information contains the data, which are presented as arithmetic mean values and their standard deviations, which were obtained in three independent experiments and calculated using Microsoft Excel. The statistical significance of the differences between the compared mean values was assessed using the Mann–Whitney U-test.
3.13. X-ray Analysis
Data were collected on a BRUKER D8 VENTURE PHOTON 100 CMOS diffractometer with MoKα radiation (α = 0.71073 Å) using the φ and ω scans technique. Using Olex2 [45], the structure was solved with the ShelXS [46] structure solution program using Direct Methods and refined with the XL refinement package using Least Squares minimization. Data were corrected for absorption effects using the multi-scan method (SADABS). All non-hydrogen atoms were refined anisotropically using SHELX [46]. The coordinates of the hydrogen atoms were calculated from geometrical positions.
Crystallographic data for compound 5 and experimental details are given in Tables 1ESM and S1ESM (Electronic Supplementary Information). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk.
4. Conclusions
A number of functional selenium-containing compounds were synthesized and isolated. The fundamental possibility of the synthesis of selenium-containing heterocycles via one-pot selenofunctionalization of dienes by selenium dihalides in the presence of nucleophiles was shown and realized for the first time. The synthesized compounds showed promising enzyme-like activity. The effect of 9-selenabicyclo[3.3.1]nonanes on the following major characteristics of the antioxidant profile was first studied: the level of lipid peroxidation, the activity of glutathione peroxidase, and the activity of glutathione reductase. A significant effect of the nature of the substituent in the polycyclic selenium-containing core on the biological activity of the compounds was shown. All studied compounds drastically reduced the level of lipid peroxidation both under normal conditions and under stress at concentrations of up to 100 μm. Unexpectedly, pyridinium compound 6 and hydrophobic derivatives 1, 3, and 5 displayed an inhibitory effect towards glutathione reductase activity with a decrease in LPO levels, while hydroxy and hydroxyethoxy derivatives showed an increase in the activity of both glutathione peroxidase and glutathione reductase.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics11070304/s1, 1H and 13C NMR spectra of the obtained compounds, X-ray crystallographic data including bond lengths, bond angles and torsion angles for compound 5 (Tables 1ESM and S1ESM), experimental data of evaluation of diene conjugates, glutathione reductase and glutathione peroxidase activities.
Author Contributions
Methodology, M.V.M. and I.S.K.; Validation, N.V.O. and V.A.P.; Formal analysis, T.N.B.; Investi-gation, M.V.M., I.S.K. and E.V.S.; Data curation, N.V.O., S.V.A. and V.A.P.; Writing—original draft preparation, M.V.M. and I.S.K.; Writing—review and editing, M.V.M. and V.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
The synthesis of 9-selenabicyclo[3.3.1]nonane derivatives was developed under project No. 22-13-00339 of the Russian Science Foundation.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Baikal Analytical Center SB RAS for providing the instrumental equipment for structural investigations. The authors express their deep gratitude to the research member of the laboratory of physiological and biochemical adaptation of SIPPB SB RAS, Natalya Bairovna Katysheva, for providing oilseed radish seeds (Raphanus sativus L. var. oleiferus Metzg.) of the line of Irkutsk State Agricultural Academy.
Conflicts of Interest
There are no conflict to declare.
References
- Serban, C.; Dragan, S. The relationship between inflammatory and oxidative stress biomarkers, atherosclerosis and rheumatic diseases. Curr. Pharm. Des. 2014, 20, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Afanasieva, G.A.; Chesnokova, N.P. On pathogenetic significance of activation of lipid peroxidation in the mechanisms of disturbance of blood rheologic properties in experimental intoxication induced by fraction FII of vaccinal EV strain of Y. pestis. Bull. Russ. Akad. Med. Sci. 2009, 2, 14–17. [Google Scholar]
- Lenardao, E.J.; Santi, C.; Sancineto, L. New Frontiers in Organoselenium Compounds; Springer International Publishing AG: Cham, Switzerland, 2018; 189p. [Google Scholar]
- Santi, C. Organoselenium Chemistry: Between Synthesis and Biochemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; 563p. [Google Scholar]
- Gandhil, U.H.; Nagaraja, T.P.; Prabhu, K.S. Selenoproteins and their role in oxidative stress and inflammation. Curr. Chem. Biol. 2013, 7, 65–73. [Google Scholar] [CrossRef]
- Mamgain, R.; Kostic, M.; Singh, F.V. Synthesis and Antioxidant Properties of Organoselenium Compounds. Curr. Med. Chem. 2023, 30, 2421–2448. [Google Scholar] [PubMed]
- Selvakumar, K.; Shah, P.; Singh, H.B.; Butcher, R.J. Synthesis, Structure, and Glutathione Peroxidase-Like Activity of Amino Acid Containing Ebselen Analogues and Diaryl Diselenides. Chem. Eur. J. 2011, 17, 12741–12755. [Google Scholar] [CrossRef]
- McNeil, N.M.R.; Press, D.J.; Mayder, D.M.; Garnica, P.; Doyle, L.M.; Back, T.G. Enhanced Glutathione Peroxidase Activity of Water-Soluble and Polyethylene Glycol-Supported Selenides, Related Spirodioxyselenuranes, and Pincer Selenuranes. J. Org. Chem. 2016, 81, 7884–7897. [Google Scholar] [CrossRef]
- Kumakura, F.; Mishra, B.; Priyadarsini, K.I.; Iwaoka, M. A Water-Soluble Cyclic Selenide with Enhanced Glutathione Peroxidase-Like Catalytic Activities. Eur. J. Org. Chem. 2010, 2010, 440–445. [Google Scholar] [CrossRef]
- Refaay, D.A.; Ahmed, D.M.; Mowafy, A.M.; Shaaban, S. Evaluation of novel multifunctional organoselenium compounds as potential cholinesterase inhibitors against Alzheimer’s disease. Med. Chem. Res. 2022, 31, 894–904. [Google Scholar] [CrossRef]
- Obieziurska-Fabisiak, M.; Pacuła-Miszewska, A.J.; Laskowska, A.; Ścianowski, J. Organoselenium compounds as antioxidants. Arkivoc 2023, 2023, 69–92. [Google Scholar] [CrossRef]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef]
- Plemenkov, V.V. Natural organic compounds of selenium. Butlerov Commun. 2006, 8, 1–5. [Google Scholar]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealized. Dalton Trans. 2012, 41, 6390–6395. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.; Koketsu, M. Recent developments in the synthesis of biologically relevant selenium-containing scaffolds. Coord. Chem. Rev. 2017, 339, 104–127. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V. New Methods for Preparation of Organoselenium and Organotellurium Compounds from Elemental Chalcogens. Russ. J. Org. Chem. 2003, 39, 1373–1380. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Amosova, S.V. Recent Advances in Organochalcogen Synthesis Based on Reactions of Chalcogen Halides with Alkynes and Alkenes. Curr. Org. Chem. 2016, 20, 136–145. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A. Selenium dihalides: New possibilities for the synthesis of selenium-containing heterocycles. Chem. Heterocycl. Compd. 2017, 53, 150–152. [Google Scholar] [CrossRef]
- Abakumov, G.A.; Piskunov, A.V.; Cherkasov, V.K.; Fedushkin, I.L.; Ananikov, V.P.; Eremin, D.B.; Gordeev, E.G.; Beletskaya, I.P.; Averin, A.D.; Bochkarev, M.N.; et al. Organoelement chemistry: Promising growth areas and challenges. Russ. Chem. Rev. 2018, 87, 393–507. [Google Scholar] [CrossRef]
- Musalov, M.V.; Yakimov, V.A.; Potapov, V.A.; Amosova, S.V.; Borodina, T.N.; Zinchenko, S.V. A novel methodology for the synthesis of condensed selenium heterocycles based on the annulation and annulation–methoxylation reactions of selenium dihalides. New J. Chem. 2019, 43, 18476–18483. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Maylyan, A.A.; Khabibulina, A.G.; Zinchenko, S.V.; Amosova, S.V. Selenium Dihalides Click Chemistry: Highly Efficient Stereoselective Addition to Alkynes and Evaluation of Glutathione Peroxidase-Like Activity of Bis(E-2-halovinyl) Selenides. Molecules 2022, 27, 1050. [Google Scholar] [CrossRef] [PubMed]
- Musalov, M.V.; Potapov, V.A.; Yakimov, V.A.; Musalova, M.V.; Maylyan, A.A.; Zinchenko, S.V.; Amosova, S.V. A Regioselective Synthesis of Novel Functionalized Organochalcogen Compounds by Chalcogenocyclofunctionalization Reactions Based on Chalcogen Halides and Natural Products. Molecules 2021, 26, 3729. [Google Scholar] [CrossRef] [PubMed]
- Potapov, V.A.; Musalov, M.V.; Amosova, S.V. Reactions of selenium dichloride and dibromide with unsaturated ethers. Annulation of 2,3-dihydro-1,4-oxaselenine to the benzene ring. Tetrahedron Lett. 2011, 52, 4606–4610. [Google Scholar] [CrossRef]
- Abramova, E.V.; Sterkhova, I.V.; Molokeev, M.S.; Potapov, V.A.; Amosova, S.V. First coordination compounds of SeBr2 with selenium ligands: X-ray structural determination. Mendeleev Commun. 2016, 26, 532–534. [Google Scholar] [CrossRef]
- Amosova, S.V.; Shagun, V.A.; Makhaeva, N.A.; Novokshonova, A.I.; Potapov, V.A. Quantum Chemical and Experimental Studies of an Unprecedented Reaction Pathway of Nucleophilic Substitution of 2-Bromomethyl-1,3-thiaselenole with 1,3-Benzothiazole-2-thiol Proceeding Stepwise at Three Different Centers of Seleniranium Intermediates. Molecules 2021, 26, 6685. [Google Scholar] [CrossRef]
- Wirth, T. Organoselenium Chemistry; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Potapov, V.A.; Musalov, M.V.; Khabibulina, A.G.; Maylyan, A.A.; Borodina, T.N.; Zinchenko, S.V.; Amosova, S.V. Regioselective One-Pot Synthesis of Novel Functionalized Organoselenium Compound by Bis-Alkoxyselenenylation of Alkenes with Selenium Dibromide and Alcohols. Inorganics 2022, 10, 239. [Google Scholar] [CrossRef]
- Converso, A.; Burow, K.; Marzinzik, A.; Sharpless, K.B.; Finn, M.G. 2,6-Dichloro-9-thiabicyclo[3.3.1]nonane: A privileged, bivalent scaffold for the display of nucleophilic components. J. Org. Chem. 2001, 66, 4386–4392. [Google Scholar] [CrossRef]
- Accurso, A.A.; Cho, S.-H.; Amin, A.; Potapov, V.A.; Amosova, S.V.; Finn, M.G. Thia-, Aza-, and Selena[3.3.1]bicyclononane Dichlorides: Rates vs. Internal Nucleophile in Anchimeric Assistance. J. Org. Chem. 2011, 76, 4392–4395. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Abramova, E.V.; Musalov, M.V.; Lyssenko, K.A.; Finn, M.G. 2,6-Dihalo-9-selenabicyclo[3.3.1]nonanes and their complexes with selenium dihalides: Synthesis and structural characterization. New J. Chem. 2015, 39, 8055–8059. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Amosova, S.V. Efficient Synthesis of a New Family of 2,6-Disulfanyl-9-selenabicyclo[3.3.1]nonanes. Molecules 2021, 26, 2849. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V. Triple-Click Chemistry of Selenium Dihalides: Catalytic Regioselective and Highly Efficient Synthesis of Bis-1,2,3-Triazole Derivatives of 9-Selenabicyclo[3.3.1]nonane. Catalysts 2022, 12, 1032. [Google Scholar] [CrossRef]
- Yurieva, O.V.; Dubrovina, V.I.; Potapov, V.A.; Musalov, M.V.; Starovoitova, T.P.; Ivanova, T.A.; Gromova, A.V.; Shkaruba, T.T.; Balakhonov, S.V. Effect of Synthetic Organoselenium Drug on the Degree of Pathological Changes in the Organs of White Mice Immunized with Tularemia and Brucellosis Vaccines. Bull. Exp. Biol. Med. 2019, 168, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Dubrovina, V.I.; Starovoitova, T.P.; Yur’eva, O.V.; Pyatidesyatnikova, A.B.; Potapov, V.A.; Musalov, M.V.; Balakhonov, S.V. Effect of a Synthetic Organoselenium Compound on Post-Vaccination Immunopoiesis in the Red Bone Marrow and Cell Composition of Peripheral Blood of White Mice Vaccinated with Yersinia pestis EV. Bull. Exp. Biol. Med. 2021, 171, 651–655. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868–883. [Google Scholar] [CrossRef]
- Mugesh, G.; Panda, A.; Singh, H.B.; Punekar, N.S.; Butcher, R.J. Glutathione Peroxidase-like Antioxidant Activity of Diaryl Diselenides: A Mechanistic Study. J. Am. Chem. Soc. 2001, 123, 839–850. [Google Scholar] [CrossRef]
- Geng, Z.; Finn, M.G. Fragmentable Polycationic Materials Based on Anchimeric Assistance. Chem. Mater. 2016, 28, 146–152. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione reductase and glutathione: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Dorofeev, N.V.; Bojarkin, E.V.; Peshkova, A.A. Factors Defining Field Germination of Oilseed Radish Seeds. J. Stress Phys. Biochem. 2013, 9, 159–168. [Google Scholar]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nigmatullina, L.R.; Rumyantseva, N.I.; Kostyukova, Y.A. The effect of D,L-buthionine-S,R-sulfoximine on the ratio of glutathione forms and the growth of Tatar buckwheat calli. Ontogenesis 2014, 45, 50–62. [Google Scholar] [CrossRef]
- Placer, Z. Lip peroxidation systeme im biologischen material. Nahrung 1968, 12, 679–684. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).