Recent Advances in Anode Materials for Sodium-Ion Batteries
Abstract
1. Introduction
2. Anode Materials
2.1. Carbon
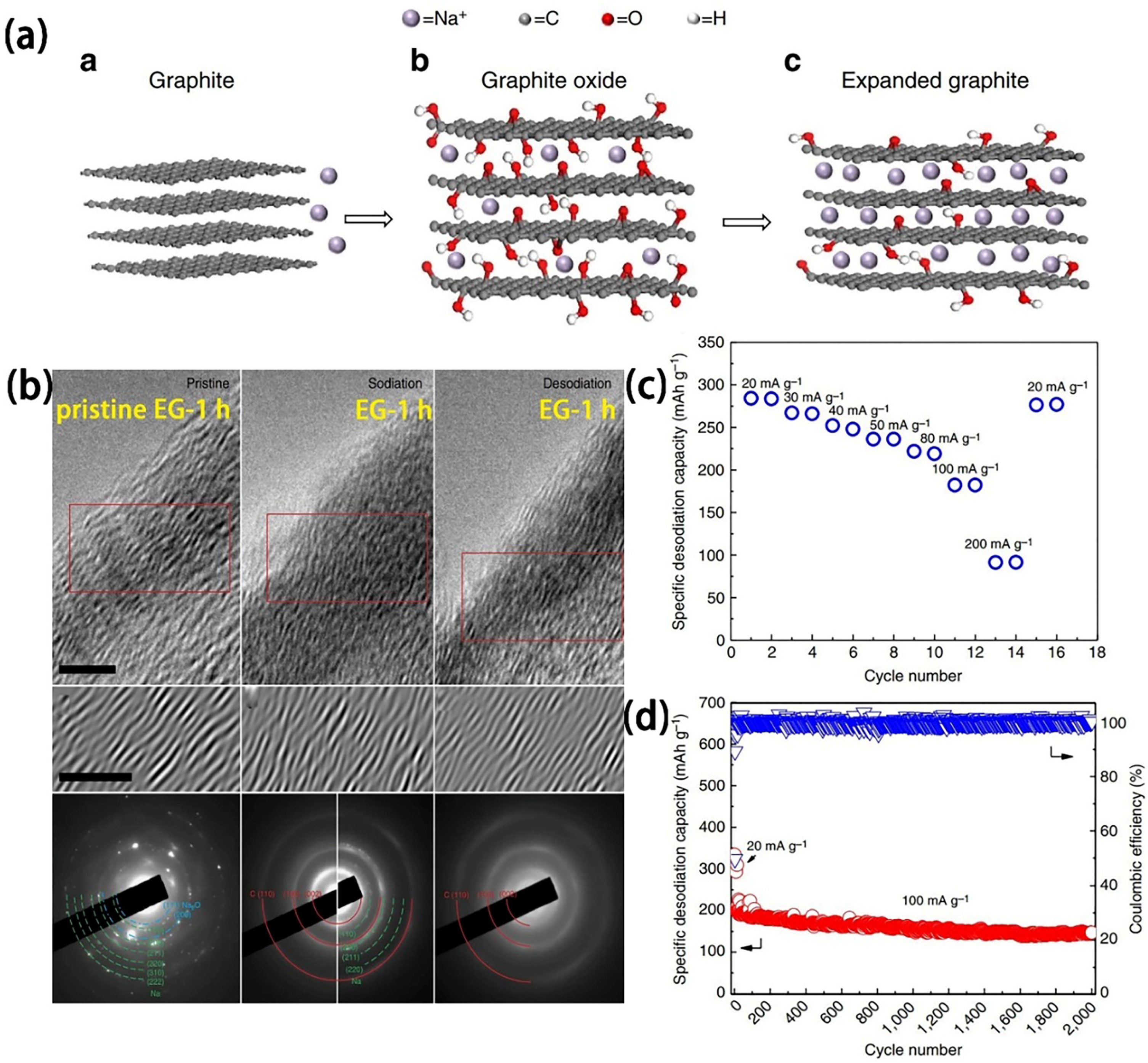
2.1.1. Graphite
2.1.2. Non-Graphite Carbon
2.2. Metal Oxides
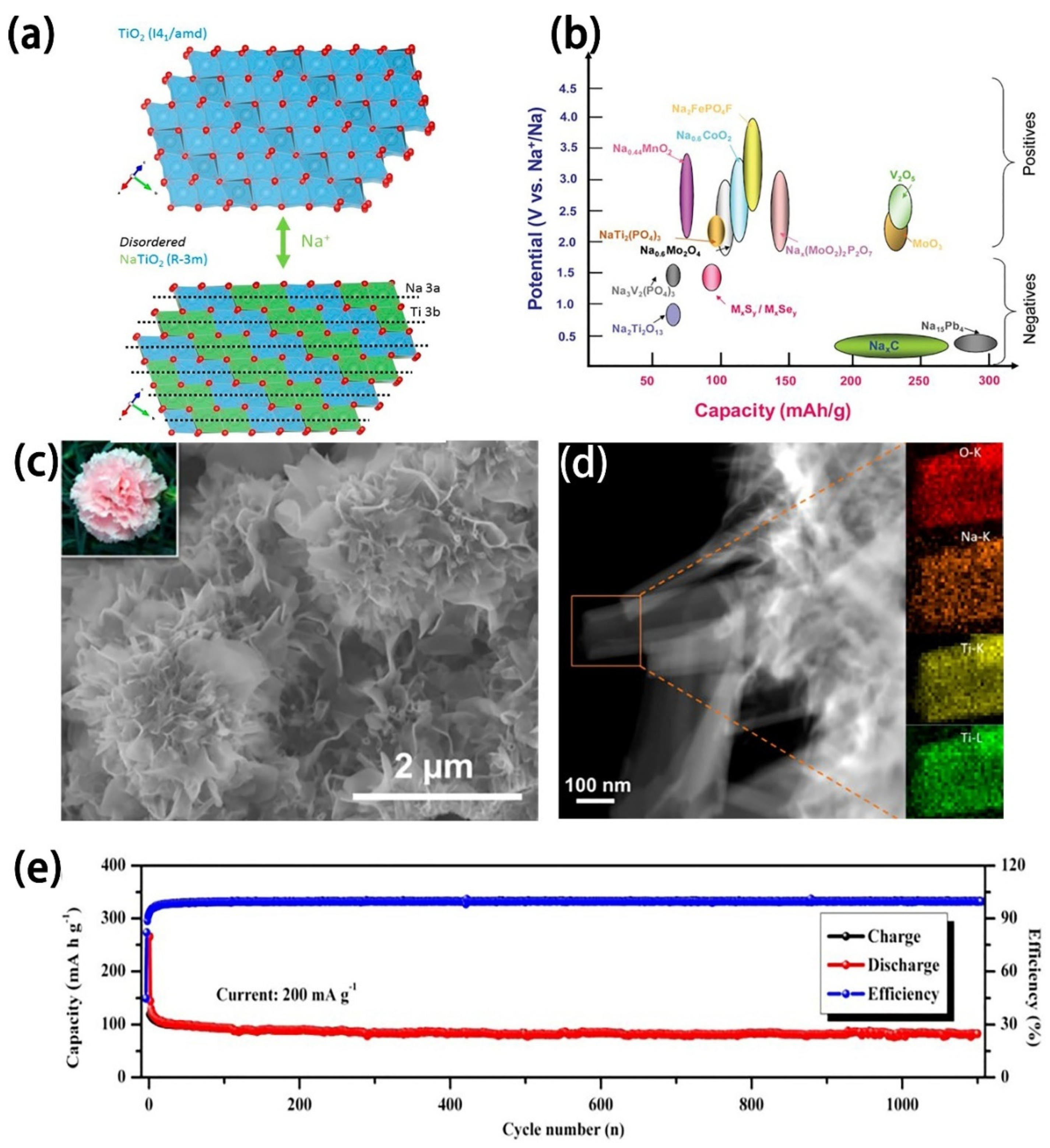
2.2.1. Titanium-Based Oxides
2.2.2. Other Conversion-Type Oxides
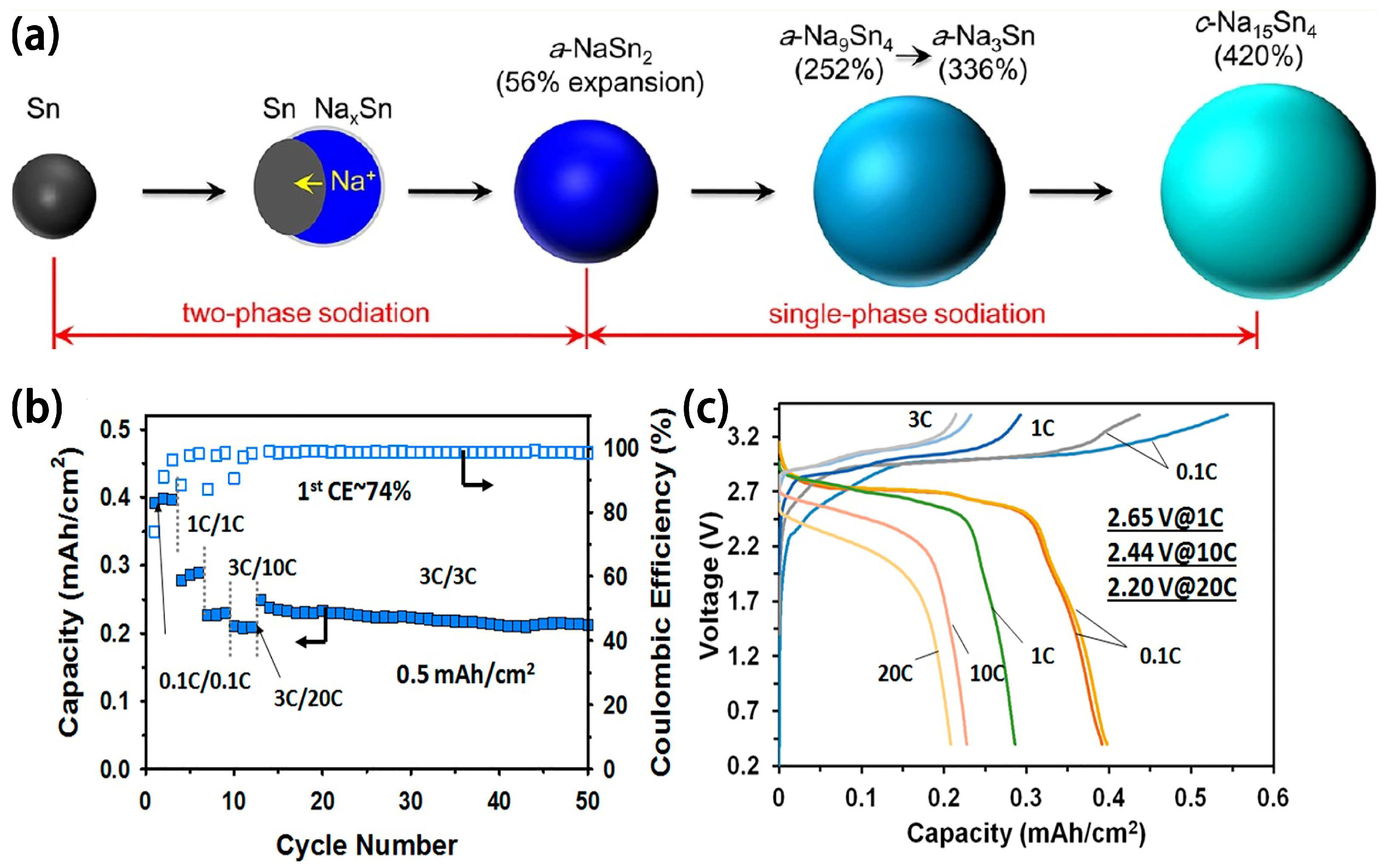
2.3. Intermetallic Compounds
2.4. Sulfides and Phosphates
2.5. Organic Compounds
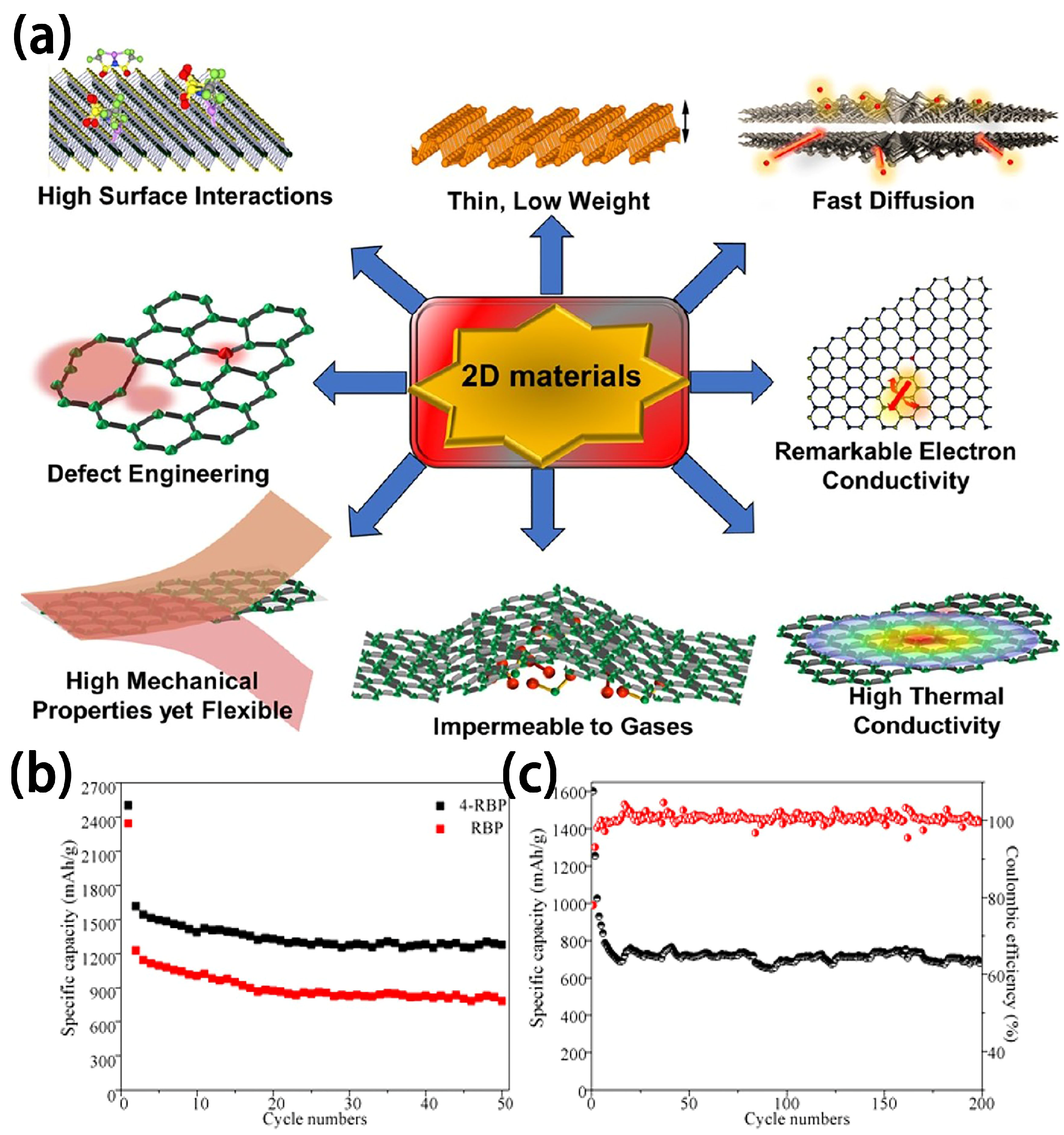
2.6. 2D Materials
3. Prospect
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuo, W.; Innocenti, A.; Zarrabeitia, M.; Bresser, D.; Yang, Y.; Passerini, S. Layered Oxide Cathodes for Sodium-Ion Batteries: Storage Mechanism, Electrochemistry, and Techno-Economics. Acc. Chem. Res. 2023, 56, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Li, Q.; Li, C.; Yang, Z.; Yu, G.; Bai, X.; Li, T. Synchronous Modification to Realize Micron-Siox Anode with Durable and Superior Electrochemical Performance for Lithium-Ion Batteries. Appl. Surf. Sci. 2023, 627, 157293. [Google Scholar] [CrossRef]
- Li, T.; Huang, M.; Bai, X.; Wang, Y.-X. Metal–Air Batteries: A Review on Current Status and Future Applications. Prog. Nat. Sci. Mater. Int. 2023, 33, 151–171. [Google Scholar] [CrossRef]
- Yu, G.; Jing, J.; Li, C.; Li, Q.; Yang, Z.; Yao, S.; Li, T.; Bai, X. A Review on Recent Progress of Non-Stoichiometric Siox Anodes Based on Lithium Ion Batteries. Prog. Nat. Sci. Mater. Int. 2023, 33, 47–54. [Google Scholar] [CrossRef]
- Fang, Y.; Luan, D.; Lou, X.W. Recent Advances on mixed metal sulfides for advanced sodium-ion batteries. Adv. Mater. 2020, 32, 2002976. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Yu, H.; Hu, F.; Liu, T.; Cheng, X.; Zheng, R.; Bai, Y.; Shui, M.; Shu, J. Metal Selenides for High Performance Sodium Ion Batteries. Chem. Eng. J. 2020, 380, 122557. [Google Scholar]
- Vaalma, C.; Buchholz, D.; Weil, M.; Passerini, S. A Cost and resource analysis of sodium-ion batteries. Nat. Rev. Mater. 2018, 3, 18013. [Google Scholar] [CrossRef]
- Song, K.; Liu, C.; Mi, L.; Chou, S.; Chen, W.; Shen, C. Recent progress on the alloy-based anode for sodium-ion batteries and potassium-ion batteries. Small 2021, 17, 1903194. [Google Scholar] [CrossRef]
- Li, L.; Zheng, Y.; Zhang, S.; Yang, J.; Shao, Z.; Guo, Z. Recent progress on sodium ion batteries: Potential high-performance anodes. Energy Environ. Sci. 2018, 11, 2310–2340. [Google Scholar] [CrossRef]
- Wasalathilake, K.C.; Li, H.; Xu, L.; Yan, C. Recent Advances in Graphene Based Materials as Anode Materials in Sodium-Ion Batteries. J. Energy Chem. 2020, 42, 91–107. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium ion insertion in hollow carbon nanowires for battery applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; He, K.; Zhu, Y.; Han, F.; Xu, Y.; Matsuda, I.; Ishii, Y.; Cumings, J.; Wang, C. Expanded graphite as superior anode for sodium-ion batteries. Nat. Commun. 2014, 5, 4033. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Ma, L.; Yu, C.; Ji, Z.; Pan, L.; Mai, W. Graphite Anode for Potassium Ion Batteries: Current Status and Perspective. Energy Environ. Mater. 2022, 5, 458–469. [Google Scholar] [CrossRef]
- Subramanyan, K.; Lee, Y.-S.; Aravindan, V. Highly Promoted Solvent-Co-Intercalation Process in Pencil Graphite Anode and Na3V2(PO4)3 Cathode in Full-Cell Na-Ion Battery. J. Colloid Interface Sci. 2023, 632, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Jache, B.; Adelhelm, P. Use of Graphite as a Highly Reversible Electrode with Superior Cycle Life for Sodium-Ion Batteries by Making Use of Co-Intercalation Phenomena. Angew. Chem. Int. Ed. 2014, 53, 10169–10173. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.; Kim, H.; Park, I.; Kang, K. Conditions for Reversible Na Intercalation in Graphite: Theoretical Studies on the Interplay among Guest Ions, Solvent, and Graphite Host. Adv. Energy Mater. 2017, 7, 1601519. [Google Scholar] [CrossRef]
- Kim, H.; Yoon, G.; Lim, K.; Kang, K. A Comparative Study of Graphite Electrodes Using the Co-Intercalation Phenomenon for Rechargeable Li, Na and K Batteries. Chem. Commun. 2016, 52, 12618–12621. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, K.; Maruyama, H.; Miyatou, T.; Mizuno, M.; Urita, K.; Ishida, H. Structure and Dynamic Behavior of Sodium–Diglyme Complex in the Graphite Anode of Sodium Ion Battery by 2H Nuclear Magnetic Resonance. J. Phys. Chem. C 2016, 120, 28152–28156. [Google Scholar] [CrossRef]
- Jung, S.C.; Kang, Y.-J.; Han, Y.-K. Origin of Excellent Rate and Cycle Performance of Na+-Solvent Cointercalated Graphite vs. Poor Performance of Li+-Solvent Case. Nano Energy 2017, 34, 456–462. [Google Scholar] [CrossRef]
- Kim, H.; Hong, J.; Park, Y.U.; Kim, J.; Hwang, I.; Kang, K. Sodium Storage Behavior in Natural Graphite Using Ether-Based Electrolyte Systems. Adv. Funct. Mater. 2015, 25, 534–541. [Google Scholar] [CrossRef]
- Hasa, I.; Dou, X.; Buchholz, D.; Shao-Horn, Y.; Hassoun, J.; Passerini, S.; Scrosati, B. A Sodium-Ion Battery Exploiting Layered Oxide Cathode, Graphite Anode and Glyme-Based Electrolyte. J. Power Sources 2016, 310, 26–31. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, J.; Zhang, Y.; Li, Q.; Jia, Y.; Dong, X.; Xiao, J.; Tao, Y.; Yang, Q.H. Reconfiguring Hard Carbons with Emerging Sodium-Ion Batteries: A Perspective. Adv. Mater. 2023, 2212186. [Google Scholar] [CrossRef]
- Stevens, D.; Dahn, J. High Capacity Anode Materials for Rechargeable Sodium-Ion Batteries. J. Electrochem. Soc. 2000, 147, 1271. [Google Scholar] [CrossRef]
- Gan, Q.; Qin, N.; Gu, S.; Wang, Z.; Li, Z.; Liao, K.; Zhang, K.; Lu, L.; Xu, Z.; Lu, Z. Extra Sodiation Sites in Hard Carbon for High Performance Sodium Ion Batteries. Small Methods 2021, 5, 2100580. [Google Scholar] [CrossRef]
- Jin, Q.; Wang, K.; Feng, P.; Zhang, Z.; Cheng, S.; Jiang, K. Surface-Dominated Storage of Heteroatoms-Doping Hard Carbon for Sodium-Ion Batteries. Energy Storage Mater. 2020, 27, 43–50. [Google Scholar] [CrossRef]
- Wu, S.; Lu, X.; Zhang, K.; Xu, J.; Sun, Z. Nitrogen/Phosphorus Dual-Doped Hard Carbon Anode with High Initial Coulombic Efficiency for Superior Sodium Storage. Batter. Supercaps 2023, 6, e202200427. [Google Scholar] [CrossRef]
- Aristote, N.T.; Liu, C.; Deng, X.; Liu, H.; Gao, J.; Deng, W.; Hou, H.; Ji, X. Sulfur-Doping Biomass Based Hard Carbon as High Performance Anode Material for Sodium-Ion Batteries. J. Electroanal. Chem. 2022, 923, 116769. [Google Scholar] [CrossRef]
- Zhang, Q.; Zeng, Y.; Ling, C.; Wang, L.; Wang, Z.; Fan, T.E.; Wang, H.; Xiao, J.; Li, X.; Qu, B. Boosting Fast Sodium Ion Storage by Synergistic Effect of Heterointerface Engineering and Nitrogen Doping Porous Carbon Nanofibers. Small 2022, 18, 2107514. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.-Y.; Zhang, J.; Li, Q.; Wang, C.-Y. Electrochemical Performance of Fulvic Acid-Based Electrospun Hard Carbon Nanofibers as Promising Anodes for Sodium-Ion Batteries. J. Power Sources 2016, 334, 170–178. [Google Scholar] [CrossRef]
- Zhao, P.-Y.; Yu, B.-J.; Sun, S.; Guo, Y.; Chang, Z.-Z.; Li, Q.; Wang, C.-Y. High-Performance Anode of Sodium Ion Battery from Polyacrylonitrile/Humic Acid Composite Electrospun Carbon Fibers. Electrochim. Acta 2017, 232, 348–356. [Google Scholar] [CrossRef]
- Ye, J.; Zang, J.; Tian, Z.; Zheng, M.; Dong, Q. Sulfur and Nitrogen Co-Doped Hollow Carbon Spheres for Sodium-Ion Batteries with Superior Cyclic and Rate Performance. J. Mater. Chem. A 2016, 4, 13223–13227. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Park, J.-S.; Jung, H.-G.; Chung, K.-Y.; Aurbach, D.; Sun, Y.-K.; Myung, S.-T. NaCrO2 Cathode for High-Rate Sodium-Ion Batteries. Energy Environ. Sci. 2015, 8, 2019–2026. [Google Scholar] [CrossRef]
- Dugas, R.; Zhang, B.; Rozier, P.; Tarascon, J. Optimization of Na-Ion Battery Systems Based on Polyanionic or Layered Positive Electrodes and Carbon Anodes. J. Electrochem. Soc. 2016, 163, A867–A874. [Google Scholar] [CrossRef]
- De la Llave, E.; Borgel, V.; Park, K.-J.; Hwang, J.-Y.; Sun, Y.-K.; Hartmann, P.; Chesneau, F.-F.; Aurbach, D. Comparison between Na-Ion and Li-Ion Cells: Understanding the Critical Role of the Cathodes Stability and the Anodes Pretreatment on the Cells Behavior. ACS Appl. Mater. Interfaces 2016, 8, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Vaalma, C.; Buchholz, D.; Passerini, S. Development and Characterization of High-Performance Sodium-Ion Cells Based on Layered Oxide and Hard Carbon. ChemElectroChem 2016, 3, 1124–1132. [Google Scholar] [CrossRef]
- Yu, P.; Tang, W.; Wu, F.-F.; Zhang, C.; Luo, H.-Y.; Liu, H.; Wang, Z.-G. Recent Progress in Plant-Derived Hard Carbon Anode Materials for Sodium-Ion Batteries: A Review. Rare Met. 2020, 39, 1019–1033. [Google Scholar] [CrossRef]
- Xie, L.; Tang, C.; Bi, Z.; Song, M.; Fan, Y.; Yan, C.; Li, X.; Su, F.; Zhang, Q.; Chen, C. Hard Carbon Anodes for Next-Generation Li-Ion Batteries: Review and Perspective. Adv. Energy Mater. 2021, 11, 2101650. [Google Scholar] [CrossRef]
- Xiang, J.; Lv, W.; Mu, C.; Zhao, J.; Wang, B. Activated Hard Carbon from Orange Peel for Lithium/Sodium Ion Battery Anode with Long Cycle Life. J. Alloys Compd. 2017, 701, 870–874. [Google Scholar] [CrossRef]
- Cao, L.; Hui, W.; Xu, Z.; Huang, J.; Zheng, P.; Li, J.; Sun, Q. Rape Seed Shuck Derived-Lamellar Hard Carbon as Anodes for Sodium-Ion Batteries. J. Alloys Compd. 2017, 695, 632–637. [Google Scholar] [CrossRef]
- Lv, W.; Wen, F.; Xiang, J.; Zhao, J.; Li, L.; Wang, L.; Liu, Z.; Tian, Y. Peanut Shell Derived Hard Carbon as Ultralong Cycling Anodes for Lithium and Sodium Batteries. Electrochim. Acta 2015, 176, 533–541. [Google Scholar] [CrossRef]
- Selvamani, V.; Ravikumar, R.; Suryanarayanan, V.; Velayutham, D.; Gopukumar, S. Garlic Peel Derived High Capacity Hierarchical N-Doped Porous Carbon Anode for Sodium/Lithium Ion Cell. Electrochim. Acta 2016, 190, 337–345. [Google Scholar] [CrossRef]
- Elizabeth, I.; Singh, B.P.; Trikha, S.; Gopukumar, S. Bio-Derived Hierarchically Macro-Meso-Micro Porous Carbon Anode for Lithium/Sodium Ion Batteries. J. Power Sources 2016, 329, 412–421. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.S.; Titirici, M.M.; Chen, L.; Huang, X. Hard Carbon Microtubes Made from Renewable Cotton as High-Performance Anode Material for Sodium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1600659. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Yang, D.; Narayan, R.; Raju, K.; Kumar, N.A.; Zhao, X. Biomass Derived Carbon Nanoparticle as Anodes for High Performance Sodium and Lithium Ion Batteries. Nano Energy 2016, 26, 346–352. [Google Scholar] [CrossRef]
- Yan, D.; Yu, C.; Zhang, X.; Qin, W.; Lu, T.; Hu, B.; Li, H.; Pan, L. Nitrogen-Doped Carbon Microspheres Derived from Oatmeal as High Capacity and Superior Long Life Anode Material for Sodium Ion Battery. Electrochim. Acta 2016, 191, 385–391. [Google Scholar] [CrossRef]
- Wu, L.; Buchholz, D.; Vaalma, C.; Giffin, G.A.; Passerini, S. Apple-Biowaste-Derived Hard Carbon as a Powerful Anode Material for Na-Ion Batteries. ChemElectroChem 2016, 3, 292–298. [Google Scholar] [CrossRef]
- Chen, H.; Sun, N.; Wang, Y.; Soomro, R.A.; Xu, B. One Stone Two Birds: Pitch Assisted Microcrystalline Regulation and Defect Engineering in Coal-Based Carbon Anodes for Sodium-Ion Batteries. Energy Storage Mater. 2023, 56, 532–541. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, Y.; Kang, W.; Xing, B.; Jiang, H.; Huang, G.; Zhang, C.; Cao, Y. Anthracite-Based Reduced Graphene Oxide/Antimony Composites as Anode Materials for High Performance Sodium Ion Batteries. J. Alloys Compd. 2022, 925, 166631. [Google Scholar] [CrossRef]
- Li, J.; Chang, X.; Huang, T.; Wang, B.; Zheng, H.; Luo, Q.; Peng, D.-L.; Wei, Q. Surface-Controlled Sodium-Ion Storage Mechanism of Li4Ti5O12 Anode. Energy Storage Mater. 2023, 54, 724–731. [Google Scholar] [CrossRef]
- Ezhyeh, Z.N.; Khodaei, M.; Torabi, F. Review on Doping Strategy in Li4Ti5O12 as an Anode Material for Lithium-Ion Batteries. Ceram. Int. 2023, 49, 7105–7141. [Google Scholar] [CrossRef]
- Xiong, H.; Slater, M.D.; Balasubramanian, M.; Johnson, C.S.; Rajh, T. Amorphous TiO2 Nanotube Anode for Rechargeable Sodium Ion Batteries. J. Phys. Chem. Lett. 2011, 2, 2560–2565. [Google Scholar] [CrossRef]
- Zou, D.; Wang, W.; Liu, J.; Weng, J.; Duan, J.; Zhou, J.; Zhou, P. Insights into the Storage Mechanism of Novel Mesoporous Hollow TiO2−x/C Nanofibers as a High-Performance Anode Material for Sodium-Ion Batteries. Carbon 2022, 194, 248–256. [Google Scholar] [CrossRef]
- Lv, D.; Wang, D.; Wang, N.; Liu, H.; Zhang, S.; Zhu, Y.; Song, K.; Yang, J.; Qian, Y. Nitrogen and Fluorine Co-Doped TiO2/Carbon Microspheres for Advanced Anodes in Sodium-Ion Batteries: High Volumetric Capacity, Superior Power Density and Large Areal Capacity. J. Energy Chem. 2022, 68, 104–112. [Google Scholar] [CrossRef]
- Qiu, S.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. Yolk-Shell TiO2@C Nanocomposite as High-Performance Anode Material for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Usui, H.; Yoshioka, S.; Wasada, K.; Shimizu, M.; Sakaguchi, H. Nb-Doped Rutile TiO2: A Potential Anode Material for Na-Ion Battery. ACS Appl. Mater. Interfaces 2015, 7, 6567–6573. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Sun, D.; Zhang, Q.; Fu, F.; Tang, Y.; Guo, J.; Shao, M.; Wang, H. Iron-Doped Cauliflower-Like Rutile TiO2 with Superior Sodium Storage Properties. ACS Appl. Mater. Interfaces 2017, 9, 6093–6103. [Google Scholar] [CrossRef]
- Li, W.; Fukunishi, M.; Morgan, B.J.; Borkiewicz, O.J.; Chapman, K.W.; Pralong, V.; Maignan, A.; Lebedev, O.I.; Ma, J.; Groult, H. A Reversible Phase Transition for Sodium Insertion in Anatase TiO2. Chem. Mater. 2017, 29, 1836–1844. [Google Scholar] [CrossRef]
- Delmas, C.; Carlier, D.; Guignard, M. The Layered Oxides in Lithium and Sodium-Ion Batteries: A Solid-State Chemistry Approach. Adv. Energy Mater. 2021, 11, 2001201. [Google Scholar] [CrossRef]
- Pak, Y.-C.; Rim, C.-H.; Hwang, S.-G.; Ri, K.-C.; Yu, C.-J. Defect Formation and Ambivalent Effects on Electrochemical Performance in Layered Sodium Titanate Na2Ti3O7. Phys. Chem. Chem. Phys. 2023, 25, 3420–3431. [Google Scholar] [CrossRef]
- Kumari, P.; Li, Y.; Boston, R. An Ionic Liquid Synthesis Route for Mixed-Phase Sodium Titanate (Na2Ti3O7 and Na2Ti6O13) Rods as an Anode for Sodium-Ion Batteries. Nanoscale 2023. [Google Scholar] [CrossRef]
- Lai, Q.S.; Mu, J.J.; Liu, Z.M.; Zhao, L.K.; Gao, X.W.; Yang, D.R.; Chen, H.; Luo, W.B. Tunnel-Type Na2Ti6O13@Carbon Nanowires as Anode Materials for Low-Temperature Sodium-Ion Batteries. Batter. Supercaps 2023, 6, e202200549. [Google Scholar] [CrossRef]
- Senguttuvan, P.; Rousse, G.; Seznec, V.; Tarascon, J.-M.; Palacin, M.R. Na2Ti3O7: Lowest Voltage Ever Reported Oxide Insertion Electrode for Sodium Ion Batteries. Chem. Mater. 2011, 23, 4109–4111. [Google Scholar] [CrossRef]
- Nava-Avendaño, J.; Morales-García, A.; Ponrouch, A.; Rousse, G.; Frontera, C.; Senguttuvan, P.; Tarascon, J.-M.; Arroyo-de Dompablo, M.; Palacín, M. Taking Steps Forward in Understanding the Electrochemical Behavior of Na2Ti3O7. J. Mater. Chem. A 2015, 3, 22280–22286. [Google Scholar] [CrossRef]
- Costa, S.I.; Choi, Y.S.; Fielding, A.J.; Naylor, A.J.; Griffin, J.M.; Sofer, Z.; Scanlon, D.O.; Tapia-Ruiz, N. Surface Engineering Strategy Using Urea to Improve the Rate Performance of Na2Ti3O7 in Na-Ion Batteries. Chem. Eur. J. 2021, 27, 3875–3886. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, D.; Qiu, R.; Tang, S.; Li, S.; Wang, R.; He, B.; Gong, Y.; Fan, H.J. Aligned Arrays of Na2Ti3O7 Nanobelts and Nanowires on Carbon Nanofiber as High-Rate and Long-Cycling Anodes for Sodium-Ion Hybrid Capacitors. Small Struct. 2021, 2, 2000073. [Google Scholar] [CrossRef]
- Mukherjee, A.; Das, D.; Banerjee, S.; Majumder, S.B. Synthesis and Electrochemical Performance of in-Situ and Ex-Situ Carbon-Coated Na2Ti3O7, as a Promising Anode for Sodium-Ion Batteries. Electrochem. Sci. Adv. 2022, e2100118. [Google Scholar] [CrossRef]
- Anwer, S.; Huang, Y.; Liu, J.; Liu, J.; Xu, M.; Wang, Z.; Chen, R.; Zhang, J.; Wu, F. Nature-Inspired Na2Ti3O7 Nanosheets-Formed Three-Dimensional Microflowers Architecture as a High-Performance Anode Material for Rechargeable Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 11669–11677. [Google Scholar] [CrossRef]
- Rudola, A.; Sharma, N.; Balaya, P. Introducing a 0.2 V Sodium-Ion Battery Anode: The Na2Ti3O7 to Na3−xTi3O7 Pathway. Electrochem. Commun. 2015, 61, 10–13. [Google Scholar] [CrossRef]
- Hariharan, S.; Saravanan, K.; Balaya, P. A-MoO3: A High Performance Anode Material for Sodium-Ion Batteries. Electrochem. Commun. 2013, 31, 5–9. [Google Scholar] [CrossRef]
- Moretti, A.; Secchiaroli, M.; Buchholz, D.; Giuli, G.; Marassi, R.; Passerini, S. Exploring the Low Voltage Behavior of V2O5 Aerogel as Intercalation Host for Sodium Ion Battery. J. Electrochem. Soc. 2015, 162, A2723–A2728. [Google Scholar] [CrossRef]
- Ming, J.; Ming, H.; Yang, W.; Kwak, W.-J.; Park, J.-B.; Zheng, J.; Sun, Y.-K. A Sustainable Iron-Based Sodium Ion Battery of Porous Carbon–Fe3O4/Na2FeP2O7 with High Performance. RSC Adv. 2015, 5, 8793–8800. [Google Scholar] [CrossRef]
- Kumar, P.R.; Jung, Y.H.; Bharathi, K.K.; Lim, C.H.; Kim, D.K. High Capacity and Low Cost Spinel Fe3O4 for the Na-Ion Battery Negative Electrode Materials. Electrochim. Acta 2014, 146, 503–510. [Google Scholar] [CrossRef]
- Oh, S.-M.; Myung, S.-T.; Yoon, C.S.; Lu, J.; Hassoun, J.; Scrosati, B.; Amine, K.; Sun, Y.-K. Advanced Na [Ni0.25Fe0.5Mn0.25]O2/C–Fe3O4 Sodium-Ion Batteries Using Ems Electrolyte for Energy Storage. Nano Lett. 2014, 14, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Han, S.; Li, Z.; Cui, H.; Lei, D.; Wang, C. Compact Sn/C Composite Realizes Long-Life Sodium-Ion Batteries. Nano Res. 2023, 16, 3804–3813. [Google Scholar] [CrossRef]
- Qiao, S.; Zhou, Q.; Ma, M.; Liu, H.K.; Dou, S.X.; Chong, S. Advanced Anode Materials for Rechargeable Sodium-Ion Batteries. ACS Nano 2023, 17, 11220–11252. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Liu, X.H.; Mao, S.X.; Huang, J.Y. Microstructural Evolution of Tin Nanoparticles During in Situ Sodium Insertion and Extraction. Nano Lett. 2012, 12, 5897–5902. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, A.; Wang, Q.; Xu, Y. MOF-Derived Porous Carbon Nanofibers Wrapping Sn Nanoparticles as Flexible Anodes for Lithium/Sodium Ion Batteries. Nanotechnology 2021, 32, 165401. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, S.; Meng, Z.; Sun, Z.; Han, W.-Q. Ultrasmall Sn Nanodots Embedded inside N-Doped Carbon Microcages as High-Performance Lithium and Sodium Ion Battery Anodes. J. Mater. Chem. A 2017, 5, 8334–8342. [Google Scholar] [CrossRef]
- Oh, S.-M.; Myung, S.-T.; Jang, M.-W.; Scrosati, B.; Hassoun, J.; Sun, Y.-K. An Advanced Sodium-Ion Rechargeable Battery Based on a Tin–Carbon Anode and a Layered Oxide Framework Cathode. Phys. Chem. Chem. Phys. 2013, 15, 3827–3833. [Google Scholar] [CrossRef]
- Chen, S.; Ao, Z.; Sun, B.; Xie, X.; Wang, G. Porous Carbon Nanocages Encapsulated with Tin Nanoparticles for High Performance Sodium-Ion Batteries. Energy Storage Mater. 2016, 5, 180–190. [Google Scholar] [CrossRef]
- Kim, C.; Lee, K.-Y.; Kim, I.; Park, J.; Cho, G.; Kim, K.-W.; Ahn, J.-H.; Ahn, H.-J. Long-Term Cycling Stability of Porous Sn Anode for Sodium-Ion Batteries. J. Power Sources 2016, 317, 153–158. [Google Scholar] [CrossRef]
- Zhang, B.; Rousse, G.; Foix, D.; Dugas, R.; Corte, D.A.D.; Tarascon, J.M. Microsized Sn as Advanced Anodes in Glyme-Based Electrolyte for Na-Ion Batteries. Adv. Mater. 2016, 28, 9824–9830. [Google Scholar] [CrossRef]
- Yang, J.; Li, J.; Wang, T.; Notten, P.H.; Ma, H.; Liu, Z.; Wang, C.; Wang, G. Novel Hybrid of Amorphous Sb/N-Doped Layered Carbon for High-Performance Sodium-Ion Batteries. Chem. Eng. J. 2021, 407, 127169. [Google Scholar] [CrossRef]
- Zhao, X.; Vail, S.A.; Lu, Y.; Song, J.; Pan, W.; Evans, D.R.; Lee, J.-J. Antimony/Graphitic Carbon Composite Anode for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 13871–13878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Chen, C.; Li, Z.; Huang, Y.; Hu, X. Flexible and Binder-Free Electrodes of Sb/rGO and Na3V2 (PO4)3/rGO Nanocomposites for Sodium-Ion Batteries. Small 2015, 11, 3822–3829. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Bai, Z.; Qian, Y.; Yang, J. Double-Walled Sb@TiO2−x Nanotubes as a Superior High-Rate and Ultralong-Lifespan Anode Material for Na-Ion and Li-Ion Batteries. Adv. Mater. 2016, 28, 4126–4133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, H.; Wei, H.; Ang, E.H.; Yang, J.; Miao, X.; Geng, H.; Zuo, X. Interface and Structure Engineering of Bimetallic Selenides toward High-Performance Sodium-Ion Half/Full Batteries. J. Power Sources 2021, 506, 230216. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, X.; Liu, T.; Zhang, L.; Yu, J. Nickel-Cobalt Selenide@N-Doped Carbon Towards High-Performance Anode Materials for Sodium-Ion Batteries. J. Energy Storage 2022, 51, 104522. [Google Scholar] [CrossRef]
- Hu, X.; Qiu, M.; Liu, Y.; Yuan, J.; Chen, J.; Zhan, H.; Wen, Z. Interface and Structure Engineering of Tin-Based Chalcogenide Anodes for Durable and Fast-Charging Sodium Ion Batteries. Adv. Energy Mate. 2022, 12, 2202318. [Google Scholar] [CrossRef]
- He, X.; Wang, R.; Yin, H.; Zhang, Y.; Chen, W.; Huang, S. 1T-MoS2 Monolayer as a Promising Anode Material for (Li/Na/Mg)-Ion Batteries. Appl. Surf. Sci. 2022, 584, 152537. [Google Scholar] [CrossRef]
- Lim, H.; Yu, S.; Choi, W.; Kim, S.-O. Hierarchically Designed Nitrogen-Doped MoS2/Silicon Oxycarbide Nanoscale Heterostructure as High-Performance Sodium-Ion Battery Anode. ACS Nano 2021, 15, 7409–7420. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Yang, Y.; Lv, W.; Lian, G.; Golberg, D.; Wang, X.; Zhao, X.; Ding, Y. A MoS2/Carbon Hybrid Anode for High-Performance Li-Ion Batteries at Low Temperature. Nano Energy 2020, 70, 104550. [Google Scholar] [CrossRef]
- Shi, Z.-T.; Kang, W.; Xu, J.; Sun, Y.-W.; Jiang, M.; Ng, T.-W.; Xue, H.-T.; Denis, Y.; Zhang, W.; Lee, C.-S. Hierarchical Nanotubes Assembled from MoS2-Carbon Monolayer Sandwiched Superstructure Nanosheets for High-Performance Sodium Ion Batteries. Nano Energy 2016, 22, 27–37. [Google Scholar] [CrossRef]
- Jacobson, A.; Chianelli, R.; Rich, S.; Whittingham, M. Amorphous Molybdenum Trisulfide: A New Lithium Battery Cathode. Mater. Res. Bull. 1979, 14, 1437–1448. [Google Scholar] [CrossRef]
- Ye, H.; Wang, L.; Deng, S.; Zeng, X.; Nie, K.; Duchesne, P.N.; Wang, B.; Liu, S.; Zhou, J.; Zhao, F. Amorphous MoS3 Infiltrated with Carbon Nanotubes as an Advanced Anode Material of Sodium-Ion Batteries with Large Gravimetric, Areal, and Volumetric Capacities. Adv. Energy Mater. 2017, 7, 1601602. [Google Scholar] [CrossRef]
- Hibble, S.J.; Wood, G.B. Modeling the Structure of Amorphous MoS3: A Neutron Diffraction and Reverse Monte Carlo Study. J. Am. Chem. Soc. 2004, 126, 959–965. [Google Scholar] [CrossRef]
- Dogrusoz, M.; Devic, T.; Ahsen, A.Ş.; Demir-Cakan, R. A Gallic Acid Based Metal Organic Framework Derived Nis/C Anode for Sodium Ion Batteries. Sustain. Energy Fuels 2021, 5, 3363–3372. [Google Scholar] [CrossRef]
- Fan, M.-P.; Chen, Y.-C.; Chen, Y.-M.; Huang, Z.-X.; Wu, W.-L.; Wang, P.; Ke, X.; Sun, S.-H.; Shi, Z.-C. NiS2 Nanosheet Arrays on Stainless Steel Foil as Binder-Free Anode for High-Power Sodium-Ion Batteries. Rare Met. 2022, 41, 1294–1303. [Google Scholar] [CrossRef]
- Sadan, M.K.; Jeon, M.; Yun, J.; Song, E.; Cho, K.-K.; Ahn, J.-H.; Ahn, H.-J. Ultrafast Sodium-Ion Storage in an Interconnected Ni/Ni3S2 Nanocomposite with Long-Term Cycling Performance. J. Alloys Compd. 2022, 909, 164705. [Google Scholar] [CrossRef]
- Cai, J.; Chen, X.; Duan, X.; Yang, G.; Zhang, Q.; Fan, H.; Liu, Z.; Peng, F. Porous NiS2 Nanosheets Anchored on Reduced Graphene Oxide as High-Rate and Long-Life Anode Materials for Sodium-Ion Batteries. Electrochim. Acta. 2023, 462, 142705. [Google Scholar] [CrossRef]
- Qin, W.; Chen, T.; Lu, T.; Chua, D.H.; Pan, L. Layered Nickel Sulfide-Reduced Graphene Oxide Composites Synthesized Via Microwave-Assisted Method as High Performance Anode Materials of Sodium-Ion Batteries. J. Power Sources 2016, 302, 202–209. [Google Scholar] [CrossRef]
- Zhao, W.; Li, M.; Qi, Y.; Tao, Y.; Shi, Z.; Liu, Y.; Cheng, J. Ultrasound Sonochemical Synthesis of Amorphous Sb2S3-Graphene Composites for Sodium-Ion Batteries. J. Colloid Interface Sci. 2021, 586, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, J.; Han, D.; Liu, A.; Zhou, M.; Huang, J.; Zhu, Y.; Hou, Z.; Yin, H. Layer-by-Layer Hetero-Carbon Modifying Zns Nanocubes Anode with Improved Long-Term Life for Sodium-Ion Batteries. Ceram. Int. 2023, 49, 18421–18431. [Google Scholar] [CrossRef]
- Tang, L.B.; Li, P.Y.; Cui, R.D.; Peng, T.; Wei, H.X.; Wang, Z.Y.; Wang, H.Y.; Yan, C.; Mao, J.; Dai, K.H. Adjusting Crystal Orientation to Promote Sodium-Ion Transport in V5S8@Graphene Anode Materials for High-Performance Sodium-Ion Batteries. Small Methods 2023, 7, 2201387. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, H.; Wang, Y.; Zhang, Y.; Hou, L.; Jiang, R.; Yuan, C. Boosting Sodium-Storage Behaviors of Nasicon-Type NaTi2(PO4)3 Anode by Synergistic Modulations in Both Materials and Electrolytes Towards Aqueous Na-Ion Batteries. Electrochim. Acta 2023, 447, 142128. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Q.; Chen, C.; Li, M.; Meng, X.; Bie, X.; Wei, Y.; Huang, Y.; Du, F.; Wang, C. Nasicon-Structured NaTi2(PO4)3@C Nanocomposite as the Low Operation-Voltage Anode Material for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 2238–2246. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, J.; Wang, M.; Zeng, L.; Gu, L.; Yu, Y. Highly Reversible and Ultrafast Sodium Storage in NaTi2(PO4)3 Nanoparticles Embedded in Nanocarbon Networks. ACS Appl. Mater. Interfaces 2015, 8, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xiao, L.; Qian, J.; Cao, Y.; Ai, X.; Huang, Y.; Yang, H. 3D Graphene Decorated NaTi2(PO4)3 Microspheres as a Superior High-Rate and Ultracycle-Stable Anode Material for Sodium Ion Batteries. Adv. Energy Mater. 2016, 6, 1502197. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, M.; Lei, Y. Organic Materials for Rechargeable Sodium-Ion Batteries. Mater. Today 2018, 21, 60–78. [Google Scholar] [CrossRef]
- Lin, X.-M.; Han, C.; Yang, X.-T.; Lin, J.-S.; Yang, W.-Q.; Guo, H.-X.; Wang, Y.-H.; Dong, J.-C.; Li, J.-F. In Situ Tracking of the Lithiation and Sodiation Process of Disodium Terephthalate as Anodes for Rechargeable Batteries by Raman Spectroscopy. Nano Res. 2023. [Google Scholar] [CrossRef]
- Zhang, J.; Mu, Y. A Schiff Based P-Phenylenediimine Polymer as High Capacity Anode Materials for Stable Lithium Ion Batteries. Electrochim. Acta 2023, 450, 142276. [Google Scholar] [CrossRef]
- Armand, M.; Grugeon, S.; Vezin, H.; Laruelle, S.; Ribière, P.; Poizot, P.; Tarascon, J.-M. Conjugated Dicarboxylate Anodes for Li-Ion Batteries. Nat. Mater. 2009, 8, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, H.; Liang, J.; Tao, Z.; Chen, J. The Disodium Salt of 2,5-Dihydroxy-1,4-Benzoquinone as Anode Material for Rechargeable Sodium Ion Batteries. Chem. Commun. 2015, 51, 1446–1448. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jin, S.; Zhang, Z.; Jiang, L.; Mu, L.; Hu, Y.-S.; Li, H.; Chen, X.; Armand, M.; Chen, L. Unraveling the Storage Mechanism in Organic Carbonyl Electrodes for Sodium-Ion Batteries. Sci. Adv. 2015, 1, e1500330. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, J.; Hu, Y.S.; Li, H.; Zhou, Z.; Armand, M.; Chen, L. Disodium Terephthalate (Na2C8H4O4) as High Performance Anode Material for Low-Cost Room-Temperature Sodium-Ion Battery. Adv. Energy Mater. 2012, 2, 962–965. [Google Scholar] [CrossRef]
- Wang, H.; Hu, P.; Yang, J.; Gong, G.; Guo, L.; Chen, X. Renewable-Juglone-Based High-Performance Sodium-Ion Batteries. Adv. Mater. 2015, 27, 2348–2354. [Google Scholar] [CrossRef] [PubMed]
- Rojaee, R.; Shahbazian-Yassar, R. Two-Dimensional Materials to Address the Lithium Battery Challenges. ACS Nano 2020, 14, 2628–2658. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, R.; Jiang, D.; Qian, Z.; Li, Y.; Yang, K.; Sun, Y.; Zeng, Z.; Wu, F. Recent Developments of Two-Dimensional Anode Materials and Their Composites in Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 7440–7461. [Google Scholar] [CrossRef]
- Yuan, M.; Zheng, X.; Xu, J.; Ni, Q.; Luo, L.; Cai, Z.; Sun, Z.; Lin, L.; Sun, G. A Review of the Structural Design of Anode Materials in Sodium-Ion Batteries Based on MXenes and Their Composites. Batteries 2023, 9, 48. [Google Scholar] [CrossRef]
- Aslam, M.K.; AlGarni, T.S.; Javed, M.S.; Shah, S.S.A.; Hussain, S.; Xu, M. 2D MXene Materials for Sodium Ion Batteries: A Review on Energy Storage. J. Energy Storage 2021, 37, 102478. [Google Scholar] [CrossRef]
- Arnold, S.; Gentile, A.; Li, Y.; Wang, Q.; Marchionna, S.; Ruffo, R.; Presser, V. Design of High-Performance Antimony/Mxene Hybrid Electrodes for Sodium-Ion Batteries. J. Mater. Chem. A 2022, 10, 10569–10585. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Lang, P.; Yuan, N.; Tang, J. Fabrication and Applications of 2D Black Phosphorus in Catalyst, Sensing and Electrochemical Energy Storage. J. Alloys Compd. 2021, 850, 156580. [Google Scholar] [CrossRef]
- Ma, X.; Ji, C.; Li, X.; Liu, Y.; Xiong, X. Red@Black Phosphorus Core–Shell Heterostructure with Superior Air Stability for High-Rate and Durable Sodium-Ion Battery. Mater. Today 2022, 59, 36–45. [Google Scholar] [CrossRef]
- Liu, H.; Tao, L.; Zhang, Y.; Xie, C.; Zhou, P.; Liu, H.; Chen, R.; Wang, S. Bridging Covalently Functionalized Black Phosphorus on Graphene for High-Performance Sodium-Ion Battery. ACS Appl. Mater. Interfaces 2017, 9, 36849–36856. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Huang, J.; Feng, Y.; Zu, L.; Peng, C.; Zheng, L.; Zheng, L.; Chen, Z.; Liu, G.; Chen, B.; et al. Black Phosphorus Quantum Dot/Ti3C2 Mxene Nanosheet Composites for Efficient Electrochemical Lithium/Sodium-Ion Storage. Adv. Energy Mater. 2018, 8, 1801514. [Google Scholar] [CrossRef]
- Wang, T.; Yao, K.; Hua, Y.; Shankar, E.G.; Shanthappa, R.; Yu, J.S. Rational Design of Mxene-MoS2 Heterostructure with Rapid Ion Transport Rate as an Advanced Anode for Sodium-Ion Batteries. Chem. Eng. J. 2023, 457, 141363. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Wu, N.; Yu, G.; Li, T. Recent Advances in Anode Materials for Sodium-Ion Batteries. Inorganics 2023, 11, 289. https://doi.org/10.3390/inorganics11070289
Bai X, Wu N, Yu G, Li T. Recent Advances in Anode Materials for Sodium-Ion Batteries. Inorganics. 2023; 11(7):289. https://doi.org/10.3390/inorganics11070289
Chicago/Turabian StyleBai, Xue, Nannan Wu, Gengchen Yu, and Tao Li. 2023. "Recent Advances in Anode Materials for Sodium-Ion Batteries" Inorganics 11, no. 7: 289. https://doi.org/10.3390/inorganics11070289
APA StyleBai, X., Wu, N., Yu, G., & Li, T. (2023). Recent Advances in Anode Materials for Sodium-Ion Batteries. Inorganics, 11(7), 289. https://doi.org/10.3390/inorganics11070289








