Based on a comparison of thermochemical and electrochemical multiphase reactions, we have investigated the use of liquid electrolytes in the form of eutectics, ionic liquids, or solvents containing dissolved salts, to facilitate the transport of atoms such as Li, Mg, B, and Al, between phases and thereby increase reaction rates (or reduce desorption temperatures and hydrogenation pressures) in complex and destabilized hydride materials. Here we further analyze previous results for MgH2/Sn and NaAlH4 and then present new results for the hydrogenation of MgB2.
3.1. Analysis of the Electrolyte-Assisted MgH2/Sn Hydrogen Storage Reaction Using ∆GK
The MgH
2/Sn destabilized hydride system was studied using a eutectic electrolyte with a composition of 0.725 LiBH
4/0.275 KBH
4 [
1]. This composition was chosen to have a melting point (~110 °C) [
18] well below the melting point of Sn (232 °C). Straightforward measurement of T
K during a heating ramp (as shown in
Figure 1a for MgH
2/Si) was not observed due to the extremely slow reaction rates even with the eutectic. Instead, rates were measured over extended times at fixed temperatures of 150 °C, 175 °C, and 200 °C. Although slow, the rate of dehydrogenation at 150 °C increased 12× from 0.0008 wt%-H
2/h without eutectic to 0.01 wt%-H
2/h with the eutectic. At 200 °C, the rate with eutectic is 0.23 wt%-H
2/h, which is similar to the rate described above for MgH
2/Si (without electrolyte). Thus, we chose
TK = 200 °C, which gives
ΔGK = 20 kJ/mol-H
2 (based on data in
Table 1). By using the measured rates and extrapolating, we estimate that the same rate (0.23 wt%-H
2/h) would occur without the eutectic at 215 °C. This gives
ΔGK = 22 kJ/mol-H
2, so there is a modest reduction of 2 kJ/mol-H
2 upon the addition of the eutectic electrolyte.
In addition to improving dehydrogenation, the 0.725 LiBH
4/0.275 KBH
4 electrolyte also enabled full (re)hydrogenation of Mg
2Sn back to MgH
2/Sn using 1000 bar H
2 at 215 °C−175 °C [
1]. Without the eutectic, essentially no hydrogenation was observed. This was the first time that significant hydrogenation was observed in the Mg
2Si or Mg
2Sn systems. A similar attempt using an eutectic electrolyte was made with Mg
2Si but no hydrogenation was seen. The thermodynamics of the two systems are similar. The difference may have been a more inert oxide layer on the Mg
2Si and/or more mobility in the Sn-based system due to the relatively low melting points for Sn (232 °C) and Mg
2Sn (778 °C) compared to Si (1400 °C) and Mg
2Si (1100 °C).
3.2. Analysis of Electrolyte-Assisted NaAlH4 Dehydrogenation Using the Avrami-Erofe’ev Model
The influence of electrolytes was also explored for the dehydrogenation of LiAlH
4 and NaAlH
4 [
2]. These hydrides dehydrogenate in two steps beginning from a single phase. Thus, the multiphase reaction transport limitations between reacting phases described for MgH
2 + Si or Sn might be considered to not apply. However, there are three solid phase products when MAlH
4 dehydrogenates, M
3AlH
6 + Al for the 1st step and MH + Al for the 2nd step. These products must form at 3-phase boundaries that could present transport limitations. Indeed, pure NaAlH
4 does not decompose until it melts at ~180 °C, despite a thermodynamic
T1bar = 33 °C (
Table 1) for the 1st step. This gives
ΔGK = 19 kJ/mol-H
2, which is somewhat lower than other hydrogen storage reactions but still much higher than the Mg/Sn battery reaction.
For NaAlH
4 catalyzed with 3 mol% TiCl
3, dehydrogenation was compared without an electrolyte and with 50 wt% diglyme [
2]. No additional electrolyte salt was included with the diglyme. Rather, the solubility of NaAlH
4 or NaCl (formed during milling with TiCl
3) was relied upon to possibly provide mobile [AlH
4]
− or Na
+ ions. The results are shown in
Figure 2 following the format in
Figure 1. The two dehydrogenation steps are clearly seen. Without electrolyte, the
TK for the 1st step is 70 °C, giving
ΔGK = 4.7 kJ/mol-H
2. This is a significant decrease from pure NaAlH
4 and similar to that for the Mg/Sn battery reaction. Thus, as is well known, catalyzed NaAlH
4 dehydrogenates with rates that are, or are close to, practical. Including diglyme further decreases T
K by 15 °C to 55 °C,
ΔGK = 2.8 kJ/mol-H
2, a decrease of ~2 kJ/mol-H
2. However, perhaps more significantly, is how the electrolyte influences the rates as the reactions proceed. For the 1st step, the rates at a given wt% with and without electrolytes are similar, except that the reaction occurs ~15 °C lower with electrolytes. A more distinct difference is seen in the 2nd step where the dependence of the rate on the extent of reaction is very different. Specifically, with diglyme the rate increased reaching ~3.5 wt%-H
2/h at 4 wt% desorbed hydrogen while without electrolyte the rate simply decreases starting from ~0.5 wt%-H
2/h at 3 wt%.
To understand this further, a full kinetic analysis was performed using the Avrami-Erofe’ev (A-E) model:
which is typical for systems with rates driven by nucleation and growth, where α is the extent of reaction,
k is a temperature-dependent rate constant, and
n is a growth parameter (with typical values between ~1 and 4) that is related to nucleation and growth of the product phases [
19]. A series of A-E calculations were performed at a fixed rate constant to illustrate how the growth parameter (
n) affects the shape of the desorption curve (
Figure 3). Simulated curves are shown in
Figure 3a at various values of
n (all other parameters were fixed). Similarly,
Figure 3b,c show the same data plotted as the rate vs. time and rate vs. fractional decomposition, respectively. At
n = 1, the A-E equation is equivalent to a traditional first-order (homogeneous) rate equation where the reaction starts at the highest rate and decays as the reaction proceeds. For systems with 2 ≤
n ≤ 4 the fractional decomposition curve takes on a sigmoidal shape with an initial induction period (nucleation), followed by an acceleratory period (growth of nuclei), and finally, a deceleration period (growth with overlap). For these systems, the
n value determines the growth geometry and the maximum rate (during the acceleratory period) increases with
n.
Using data from
Figure 2, the rate of dehydrogenation from the 2nd step (Na
3AlH
6 + Al → 3 NaH + Al + 3/2 H
2) with and without the electrolyte is shown in
Figure 3d where the fractional decomposition (extent of reaction) is plotted on the x-axis and the temperature is constant (150 °C). As previously mentioned, the first step (not shown) is first order (with
n ~ 1) with and without electrolytes. The 2nd desorption step without the electrolyte is clearly characteristic of a first-order reaction (
n ~ 1) with a continuously decreasing rate with the extent of the reaction (open circles in
Figure 3d). A very different shape is observed with the sample containing an electrolyte, where the rate increases initially, plateaus (constant rate), and then decreases at the very end of the reaction (filled circles). This behavior is consistent with A-E with
n = 3.5. The increase in the growth parameter from
n ~ 1 to
n = 3.5 coupled with a ~5× increase in the rate constant leads to the observed ~10× decrease in overall reaction time.
The unusual kinetic behavior observed in the 2nd desorption step of NaAlH
4 was also seen with an electrolyte in MgH
2/Sn [
1] and LiAlH
4 2nd step [
2]. In multiphase hydrogen storage reactions (and more generally, solid-state reactions) without an electrolyte, the majority of the reaction can be treated as the first order, where the rate continuously decreases or the temperature must be continuously increased to maintain a constant rate. The difference with an electrolyte suggests that in addition to improving
interparticle transport, the electrolyte also enables
intraparticle transport, presumably over the particle surface (assuming the electrolyte does not penetrate or break up a multiphase particle). For a solid-state reaction that generates two new solid phases (such as dehydrogenation of M
3AlH
6 to MH + Al), a reaction occurs along a triple-phase boundary (assuming the product phases are not continuously nucleated). These boundaries are spatially very confined; locally, they are one-dimensional. This confinement will favor possible alternative transport pathways such as surface transport linking the different phases. This transport could be facilitated by an electrolyte, which effectively increases the velocity of the advancing reactant-product interface, and decreases the activation energy. The concept of expanding the growth geometry (in addition to the rate constants) is supported by the kinetic analysis which shows an increase in the growth parameters from
n = 1 with no electrolyte to
n = 3 with electrolyte.
3.3. Influence of Electrolytes on MgB2 Hydrogenation
The hydrogenation of MgB
2 was also studied [
1]. Similar to the dehydrogenation of LiAlH
4 and NaAlH
4, an influence of an electrolyte might not be expected for MgB
2 because hydrogenation begins from a single phase (and for MgB
2 also ends in a single phase, Mg(BH
4)
2). However, it is thought that there are multiple intermediate phases including MgH
2, Mg, and MgB
12H
12 [
7] that perhaps could be contained within a single particle. As described above, the reaction at the confined multiphase boundaries could be improved by providing surface transport through an electrolyte.
Significant hydrogenation of MgB
2 was shown using a 0.33 LiI/0.33 KI/0.33 CsI eutectic electrolyte at 53 wt% with hydrogen treatment at 1000 bar and 320 °C for 50 h. [
1]. As shown in
Table 3, from a starting boron-based composition of 96% B in MgB
2 and 4% B in (impurity) boron oxides (
Table 3, row 1) using
11B nuclear magnetic resonance (NMR), the hydrogen treatment with eutectic resulted in a final composition with 72% of the boron as [BH
4]
− (
Table 3, row 3). In contrast, without the eutectic, only 3% of the B was hydrogenated to [BH
4]
− (
Table 3, row 2). The portion of the
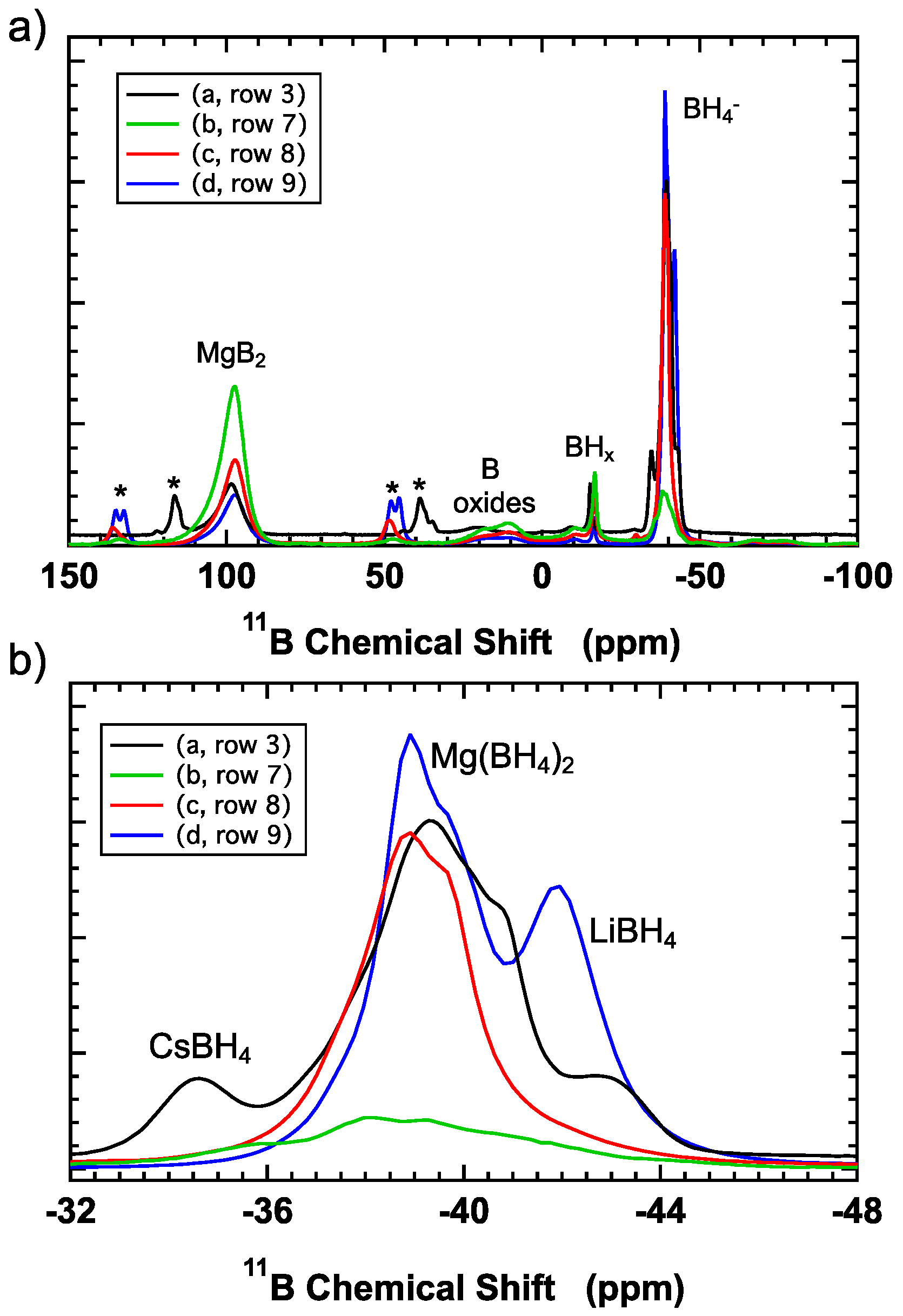
11B NMR spectrum focused on the borohydride region is shown in
Figure 4. Comparison with literature spectra indicates the formation of Mg(BH
4)
2 together with CsBH
4 and LiBH
4. Although the
11B chemical shift can be influenced by the local environment and mixed cation solid solution phases are possible, an approximate composition (based on areas between the minima between the peaks) is 12%BH
4 in CsBH
4; 74%BH
4 in Mg(BH
4)
2; 14%BH
4 in LiBH
4. The extremely high pressure used (1000 bar) clearly resulted in some hydrogenation of the eutectic.
In this work, we present new results where lower pressures were explored. Reducing the pressure to 700 bar and (unintentionally) the temperature to 300 °C (
Table 3, row 4) reduced the [BH
4]
− fraction drastically, from 72% to 13%. The inclusion of 3 at% TiF
3 as a catalyst (by milling) increased the [BH
4]
− fraction to 26% but also increased the fraction of non-[BH
4]
− boron hydride species (BH
x) from 4% to 9% (
Table 3, row 5). These results indicate that well-known catalysts for complex hydrides (such as TiF
3) can still operate in an electrolyte environment. This is not surprising if we consider that the catalyst facilitates reaction on the surface of and within individual powder particles, while the eutectic facilitates transport between particles.
Further reducing the pressure to 350 bar (at 320 °C) reduced the [BH
4]
− fraction to 16% but actually increased the BH
x fraction to 11% (
Table 3, row 6). Switching the catalyst from TiF
3 to TiCl
3 (and using a different high-pressure hydrogenation apparatus, at 310 °C) gave similar results with 14% [BH
4]
− and 13% BH
x (
Table 3, row 7). The increased BH
x fraction at lower pressure suggests that these species are intermediates on the way to formation of the fully hydrogenated [BH
4]
− anions and that lower pressure favors these partially hydrogenated species. While the yields are much greater than the yields possible without a eutectic, the total yield of hydrogenated MgB
2 was only ~30% with only ~50% of that yield being [BH
4]
−. Although the yield is low, the hydrogenation appears to give predominately Mg(BH
4)
2 as shown in
Figure 4, curve b.
Next, to try and improve the yields, the electrolyte was switched from the Li/K/Cs iodide-based eutectic to a Li/K/Cs chloride-based eutectic, with the specific composition of 0.575 LiCl/0.165 KCl/0.26 CsCl [
20,
21]. Significant improvement was seen with the [BH
4]
− fraction increasing from 0.14 (with the iodide) to 0.49 (with the chloride), while the BH
x fraction actually decreased slightly (
Table 3, row 8). The NMR spectrum (
Figure 4, curve c) suggests the formation of relatively pure Mg(BH
4)
2. Compared with the sample hydrogenated at 1000 bar, the peaks associated with CsBH
4 and LiBH
4 are not clearly visible.
The chloride eutectic has a melting point of ~266 °C (higher than the iodide, ~210 °C) but still below the temperature for these hydrogenations, >300 °C. During hydrogenation, the higher melting point may lead to greater viscosity and therefore reduced transport properties. However, counteracting the viscosity and perhaps more important, the molar mass of the chloride eutectic is lower; 80.4 g/mol-Cl compared to 186.5 g/mol-I. This difference means that at 50 wt%, the eutectic: MgB2 molar ratio increases from 0.25:1 for the iodide eutectic to 0.57:1 for the chloride eutectic, a factor of ~2.3×. Thus, during hydrogenation, there are 2.3× more liquid-state anions (Cl−) and total cations (individually 4 × Li+, 1.15 × K+, and 1.8 × Cs+) to possibly (depending on solubility) facilitate the transport of Mg2+ cations and [BH4]− or other intermediate anions within the reacting mixture. Testing other eutectic compositions with suitable melting temperatures may reveal the relative importance of the Cl− anions compared to the different cations in facilitating hydrogenation.
A final variation involved the addition of 0.2 LiH per mole of MgB
2. The 2 LiH + MgB
2 system is a well-known destabilized hydride system, which can be nearly completely hydrogenated to 2 LiBH
4 + MgH
2 at ~100 bar [
7]. Thus at 350 bar, the addition of 0.2 LiH should result in the facile formation of 0.2 LiBH
4, reacting 10% of the MgB
2 and leaving the remaining 90% for possible hydrogenation to Mg(BH
4)
2. As shown in
Table 3 (row 9), hydrogenation at 350 bar of a MgB
2 + 0.2 LiH + 3 at% TiCl
3 mixture with 50 wt% LiKCsCl eutectic resulted in 73% of the boron as [BH
4]
− with only 3% as BH
x. From the NMR spectrum (
Figure 4, curve d), the [BH
4]
− is a mixture of Mg(BH
4)
2 and LiBH
4. Using the area of the spectrum (divided using the minimum between the peaks) indicates that ~25% of the [BH
4]
− is present as LiBH
4. This is more than the expected amount of ~14%, assuming 10% of the [BH
4]
− boron was LiBH
4 and the remaining 63% was in Mg(BH
4)
2. The origin is this difference is not understood. One possibility is that the simple area estimate is inaccurate. Another possibility is that some of the Li from the chloride eutectic is forming LiBH
4 although, there was no clear indication of LiBH
4 in the sample without added LiH. Despite clearly being a mixture, the utility of this formulation lies in the dehydrogenation behavior, i.e., if it cycles hydrogen well with high capacity, it is not particularly important whether pure Mg(BH
4)
2 or a mixture with LiBH
4 is formed. Thus far, the dehydrogenation has not been tested. Overall, the total amount of hydrogenation (73% [BH
4]
−) in the MgB
2 + 0.2 LiH sample hydrogenated at 350 bar is comparable to that originally achieved at 1000 bar. Including the complete formulation with the 50 wt% electrolytes, the uptake is 5.6 wt% hydrogen. If similar uptake could be achieved with an electrolyte at 28 wt% (or 10.5 wt%), the uptake would be 8 (or 10) wt% hydrogen.