Hydrogen Release and Uptake of MgH2 Modified by Ti3CN MXene
Abstract
1. Introduction
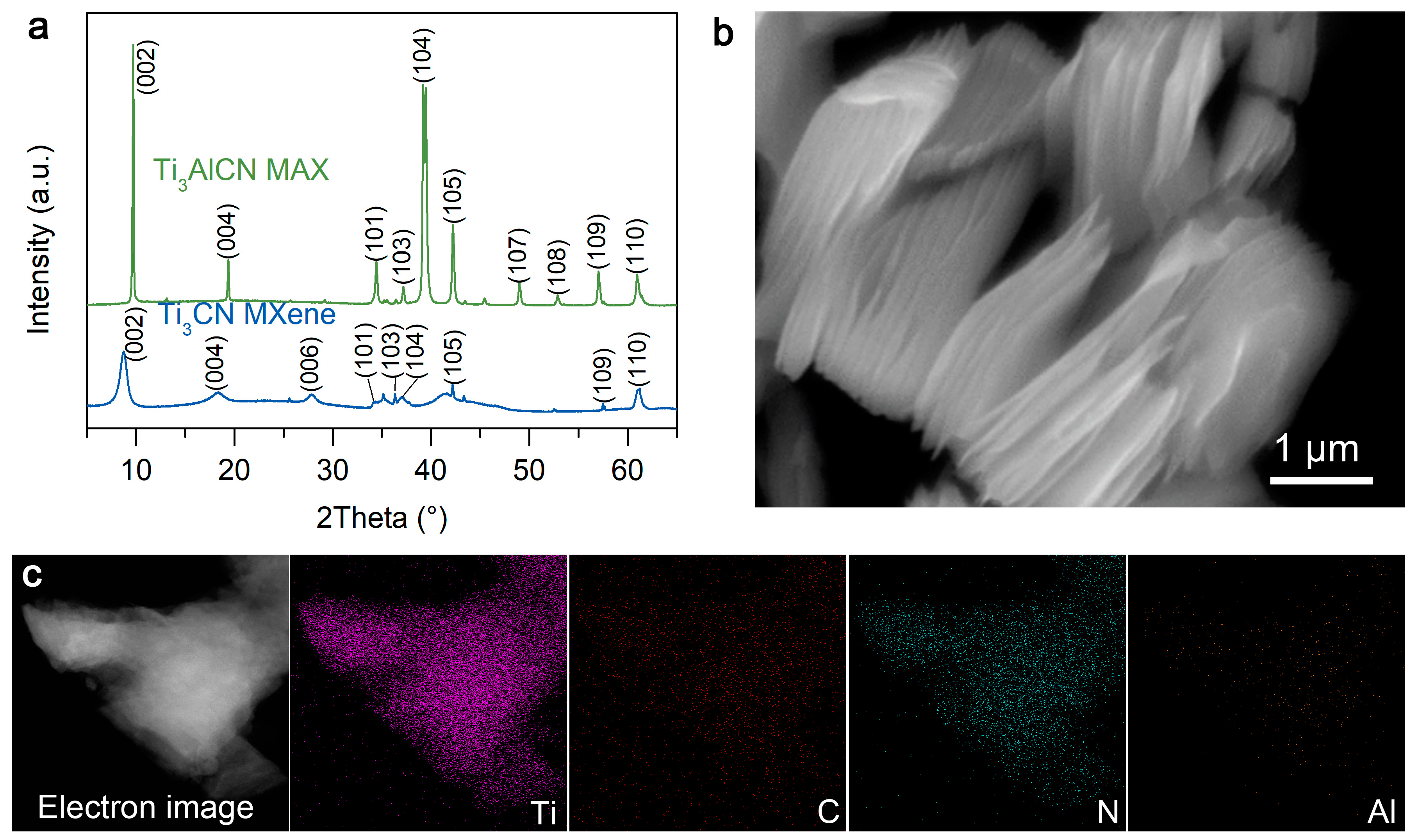
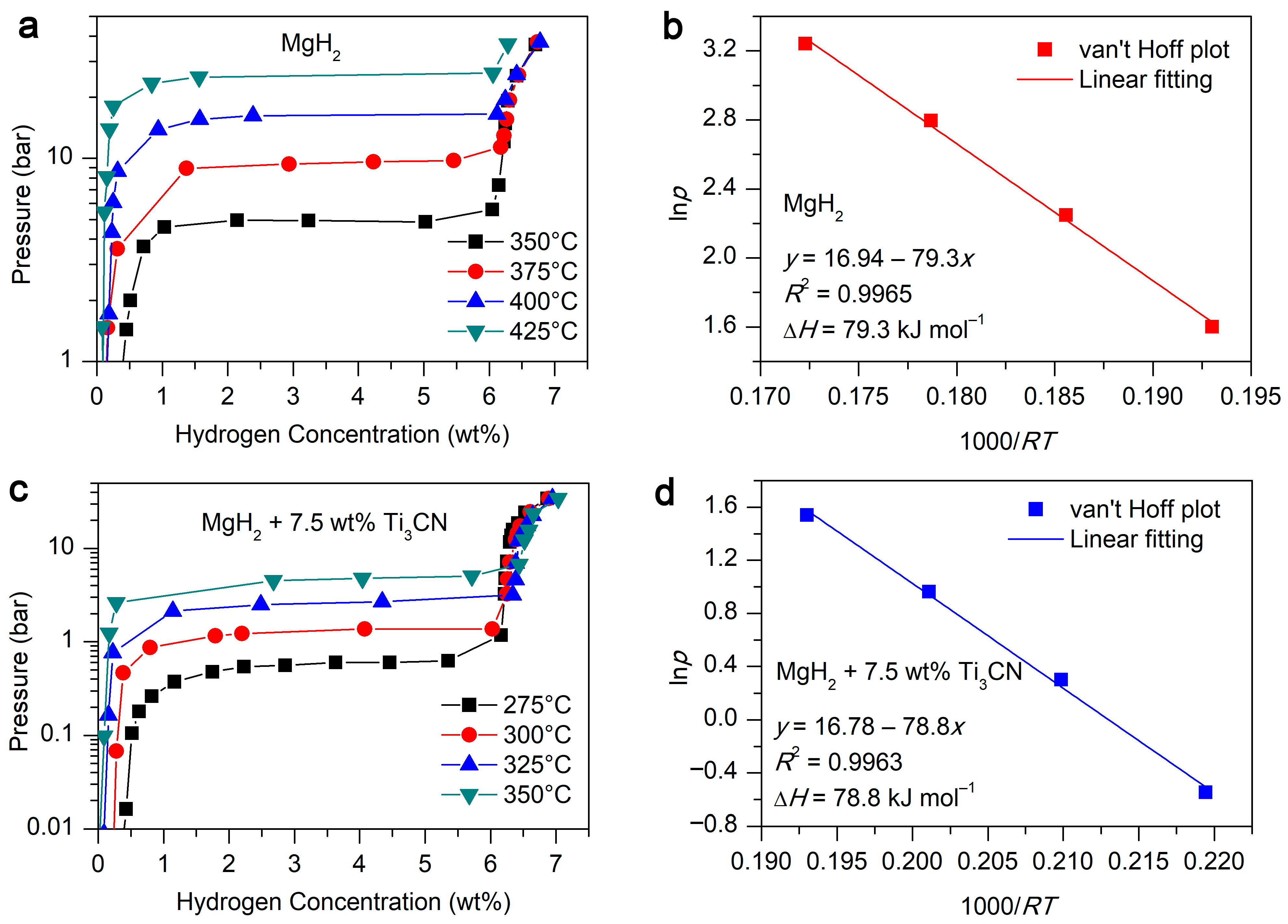
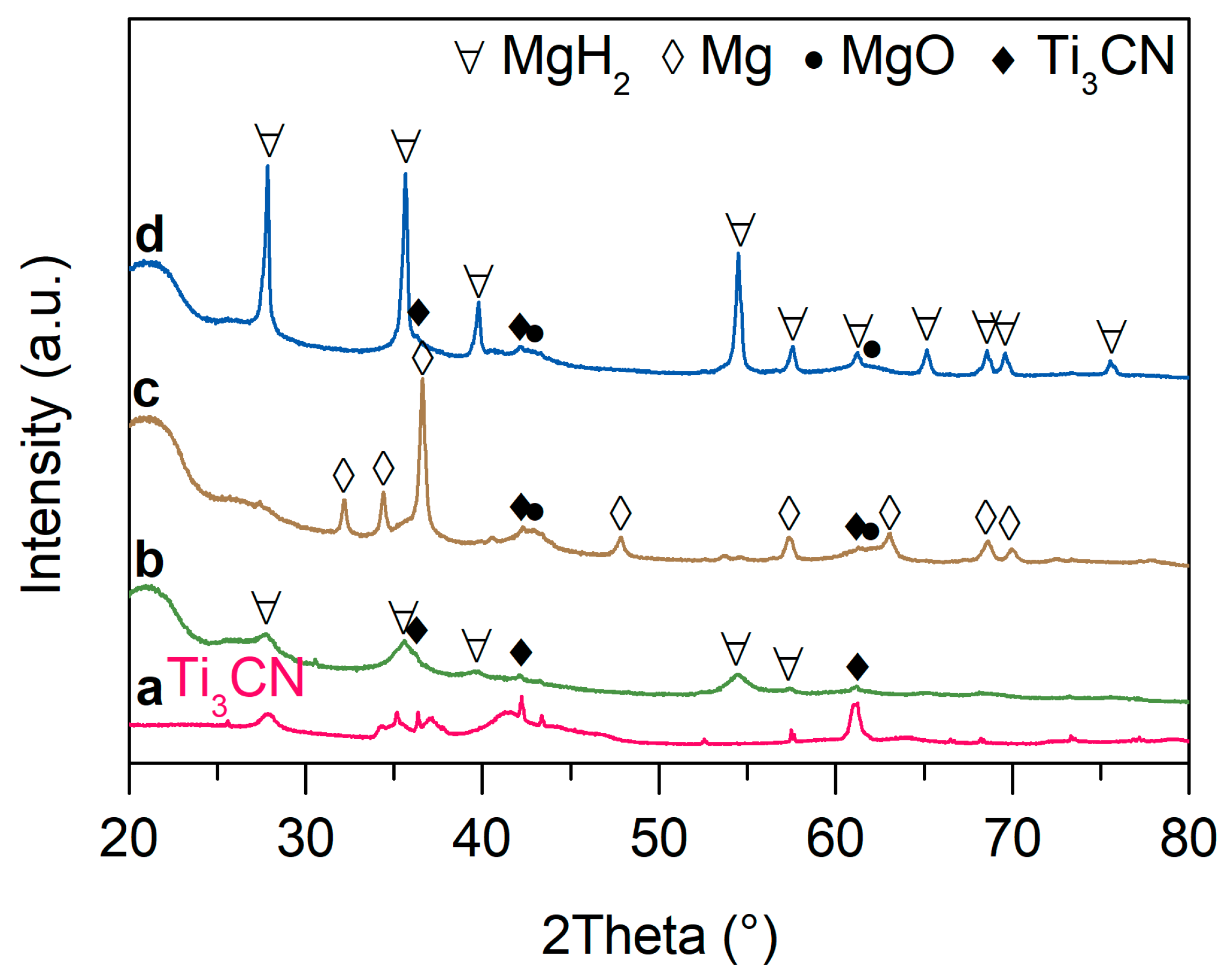
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.-X.; Li, X.-G.; Yao, Q.-L.; Lu, Z.-H.; Zhang, N.; Xia, J.; Yang, K.; Wang, Y.-Q.; Zhang, K.; Liu, H.-Z.; et al. 2022 roadmap on hydrogen energy from production to utilizations. Rare Met. 2022, 41, 3251–3267. [Google Scholar] [CrossRef]
- Schlapbach, L.; Zuttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Berstad, D.; Gardarsdottir, S.; Roussanaly, S.; Voldsund, M.; Ishimoto, Y.; Nekså, P. Liquid hydrogen as prospective energy carrier: A brief review and discussion of underlying assumptions applied in value chain analysis. Renew. Sust. Energy Rev. 2022, 154, 111772. [Google Scholar] [CrossRef]
- Hassan, I.A.; Ramadan, H.S.; Saleh, M.A.; Hissel, D. Hydrogen storage technologies for stationary and mobile applications: Review, analysis and perspectives. Renew. Sust. Energy Rev. 2021, 149, 111311. [Google Scholar] [CrossRef]
- Liu, L.; Ilyushechkin, A.; Liang, D.; Cousins, A.; Tian, W.; Chen, C.; Yin, J.; Schoeman, L. Metal Hydride Composite Structures for Improved Heat Transfer and Stability for Hydrogen Storage and Compression Applications. Inorganics 2023, 11, 181. [Google Scholar] [CrossRef]
- Yao, J.; Wu, Z.; Wang, H.; Yang, F.; Ren, J.; Zhang, Z. Application-oriented hydrolysis reaction system of solid-state hydrogen storage materials for high energy density target: A review. J. Energy Chem. 2022, 74, 218–238. [Google Scholar] [CrossRef]
- Simanullang, M.; Prost, L. Nanomaterials for on-board solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2022, 47, 29808–29846. [Google Scholar] [CrossRef]
- Lin, H.J.; Lu, Y.S.; Zhang, L.T.; Liu, H.Z.; Edalati, K.; Révész, Á. Recent advances in metastable alloys for hydrogen storage: A review. Rare Met. 2022, 41, 1797–1817. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, F.; Zhang, C.; Wang, Z.; Ju, H.; Gao, X.; Zhang, X.; Sun, L.; Liu, Z. Enhanced hydrogen storage of alanates: Recent progress and future perspectives. Prog. Nat. Sci. Mater. Int. 2021, 31, 165–179. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Ma, H.; Lu, C.; Luo, H.; Wang, X.; Huang, X.; Lan, Z.; Guo, J. Aluminum hydride for solid-state hydrogen storage: Structure, synthesis, thermodynamics, kinetics, and regeneration. J. Energy Chem. 2021, 52, 428–440. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, H.; Zhu, M. AlH3 as a hydrogen storage material: Recent advances, prospects and challenges. Rare Met. 2021, 40, 3337–3356. [Google Scholar] [CrossRef]
- Sui, Y.; Yuan, Z.; Zhou, D.; Zhai, T.; Li, X.; Feng, D.; Li, Y.; Zhang, Y. Recent progress of nanotechnology in enhancing hydrogen storage performance of magnesium-based materials: A review. Int. J. Hydrogen Energy 2022, 47, 30546–30566. [Google Scholar] [CrossRef]
- Shang, Y.; Pistidda, C.; Gizer, G.; Klassen, T.; Dornheim, M. Mg-based materials for hydrogen storage. J. Magnes. Alloy. 2021, 9, 1837–1860. [Google Scholar] [CrossRef]
- Grigorova, E.; Nihtianova, D.; Tsyntsarski, B.; Stoycheva, I. Investigation of Hydrogen Storage Characteristics of MgH2 Based Materials with Addition of Ni and Activated Carbon. Inorganics 2020, 8, 12. [Google Scholar] [CrossRef]
- Ren, L.; Zhu, W.; Zhang, Q.; Lu, C.; Sun, F.; Lin, X.; Zou, J. MgH2 confinement in MOF-derived N-doped porous carbon nanofibers for enhanced hydrogen storage. Chem. Eng. J. 2022, 434, 134701. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Ren, Z.; Zhang, X.; Hu, J.; Huang, Z.; Lu, Y.; Gao, M.; Pan, H. Realizing 6.7 wt% reversible storage of hydrogen at ambient temperature with non-confined ultrafine magnesium hydrides. Energy Environ. Sci. 2021, 14, 2302–2313. [Google Scholar] [CrossRef]
- Yan, N.; Lu, X.; Lu, Z.; Yu, H.; Wu, F.; Zheng, J.; Wang, X.; Zhang, L. Enhanced hydrogen storage properties of Mg by the synergistic effect of grain refinement and NiTiO3 nanoparticles. J. Magnes. Alloy. 2022, 10, 3542–3552. [Google Scholar] [CrossRef]
- Si, T.-Z.; Zhang, X.-Y.; Feng, J.-J.; Ding, X.-L.; Li, Y.-T. Enhancing hydrogen sorption in MgH2 by controlling particle size and contact of Ni catalysts. Rare Met. 2021, 40, 995–1002. [Google Scholar] [CrossRef]
- Le, T.T.; Pistidda, C.; Nguyen, V.H.; Singh, P.; Raizada, P.; Klassen, T.; Dornheim, M. Nanoconfinement effects on hydrogen storage properties of MgH2 and LiBH4. Int. J. Hydrogen Energy 2021, 46, 23723–23736. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, Y.F.; Zhang, X.; Hu, J.J.; Gao, M.X.; Pan, H.G. Empowering hydrogen storage performance of MgH2 by nanoengineering and nanocatalysis. Mater. Today Nano 2020, 9, 100064. [Google Scholar] [CrossRef]
- Pang, X.; Ran, L.; Chen, Y.A.; Luo, Y.; Pan, F. Enhancing hydrogen storage performance via optimizing Y and Ni element in magnesium alloy. J. Magnes. Alloy. 2022, 10, 821–835. [Google Scholar] [CrossRef]
- Liu, P.; Lian, J.; Chen, H.; Liu, B.; Zhou, S. In situ formation of Mg2Ni on magnesium surface via hydrogen activation for improving hydrogen sorption performance. ACS Appl. Energy Mater. 2022, 5, 6043–6049. [Google Scholar] [CrossRef]
- Ali, N.A.; Ismail, M. Advanced hydrogen storage of the Mg-Na-Al system: A review. J. Magnes. Alloy. 2021, 9, 1111–1122. [Google Scholar] [CrossRef]
- Yong, H.; Guo, S.; Yuan, Z.; Qi, Y.; Zhao, D.; Zhang, Y. Phase transformation, thermodynamics and kinetics property of Mg90Ce5RE5 (RE = La, Ce, Nd) hydrogen storage alloys. J. Mater. Sci. Technol. 2020, 51, 84–93. [Google Scholar] [CrossRef]
- Tian, G.; Wu, F.; Zhang, H.; Wei, J.; Zhao, H.; Zhang, L. Boosting the hydrogen storage performance of MgH2 by Vanadium based complex oxides. J. Phys. Chem. Solids 2023, 174, 111187. [Google Scholar] [CrossRef]
- Lu, Z.Y.; He, J.H.; Song, M.C.; Zhang, Y.; Wu, F.Y.; Zheng, J.G.; Zhang, L.T.; Chen, L.X. Bullet-like vanadium-based MOFs as a highly active catalyst for promoting the hydrogen storage property in MgH2. Int. J. Min. Met. Mater. 2023, 30, 44–53. [Google Scholar] [CrossRef]
- Duan, X.-Q.; Li, G.-X.; Zhang, W.-H.; Luo, H.; Tang, H.-M.; Xu, L.; Sheng, P.; Wang, X.-H.; Huang, X.-T.; Huang, C.-K.; et al. Ti3AlCN MAX for tailoring MgH2 hydrogen storage material: From performance to mechanism. Rare Met. 2023; in press. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, S.; Wang, K.; Xu, N.; Sun, W.; Sun, L.; Cao, H.; Lin, H.; Zhu, Y.; Zhang, Y. In-situ formed Pt nano-clusters serving as destabilization-catalysis bi-functional additive for MgH2. Chem. Eng. J. 2022, 435, 135050. [Google Scholar] [CrossRef]
- Shao, Y.; Gao, H.; Tang, Q.; Liu, Y.; Liu, J.; Zhu, Y.; Zhang, J.; Li, L.; Hu, X.; Ba, Z. Ultra-fine TiO2 nanoparticles supported on three-dimensionally ordered macroporous structure for improving the hydrogen storage performance of MgH2. Appl. Surf. Sci. 2022, 585, 152561. [Google Scholar] [CrossRef]
- Pukazhselvan, D.; Sandhya, K.S.; Ramasamy, D.; Shaula, A.; Bdikin, I.; Fagg, D.P. Active catalytic species generated in situ in zirconia incorporated hydrogen storage material magnesium hydride. J. Magnes. Alloy. 2022, 10, 786–796. [Google Scholar] [CrossRef]
- Lu, C.; Liu, H.; Xu, L.; Luo, H.; He, S.; Duan, X.; Huang, X.; Wang, X.; Lan, Z.; Guo, J. Two-dimensional vanadium carbide for simultaneously tailoring the hydrogen sorption thermodynamics and kinetics of magnesium hydride. J. Magnes. Alloy. 2022, 10, 1051–1065. [Google Scholar] [CrossRef]
- Lan, Z.; Fu, H.; Zhao, R.; Liu, H.; Zhou, W.; Ning, H.; Guo, J. Roles of in situ-formed NbN and Nb2O5 from N-doped Nb2C MXene in regulating the re/hydrogenation and cycling performance of magnesium hydride. Chem. Eng. J. 2022, 431, 133985. [Google Scholar] [CrossRef]
- Dan, L.; Wang, H.; Liu, J.; Ouyang, L.; Zhu, M. H2 plasma reducing Ni nanoparticles for superior catalysis on hydrogen sorption of MgH2. ACS Appl. Energy Mater. 2022, 5, 4976–4984. [Google Scholar] [CrossRef]
- Dai, M.; Lei, G.T.; Zhang, Z.; Li, Z.; Cao, H.J.; Chen, P. Room temperature hydrogen absorption of V2O5 catalyzed MgH2/Mg. Acta Chim. Sin. 2022, 80, 303–309. [Google Scholar] [CrossRef]
- Bolarin, J.A.; Zou, R.; Li, Z.; Zhang, Z.; Cao, H. MXenes for magnesium-based hydrides: A review. Appl. Mater. Today 2022, 29, 101570. [Google Scholar] [CrossRef]
- Lu, Z.-Y.; Yu, H.-J.; Lu, X.; Song, M.-C.; Wu, F.-Y.; Zheng, J.-G.; Yuan, Z.-F.; Zhang, L.-T. Two-dimensional vanadium nanosheets as a remarkably effective catalyst for hydrogen storage in MgH2. Rare Met. 2021, 40, 3195–3204. [Google Scholar] [CrossRef]
- Liu, X.-S.; Liu, H.-Z.; Qiu, N.; Zhang, Y.-B.; Zhao, G.-Y.; Xu, L.; Lan, Z.-Q.; Guo, J. Cycling hydrogen desorption properties and microstructures of MgH2-AlH3-NbF5 hydrogen storage materials. Rare Met. 2021, 40, 1003–1007. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Bowman, R.C.; Fang, Z.Z. Roles of Ti-Based Catalysts on Magnesium Hydride and Its Hydrogen Storage Properties. Inorganics 2021, 9, 36. [Google Scholar] [CrossRef]
- Gao, H.; Shi, R.; Liu, Y.; Zhu, Y.; Zhang, J.; Hu, X.; Li, L. Enhanced hydrogen storage performance of magnesium hydride with incompletely etched Ti3C2Tx: The nonnegligible role of Al. Appl. Surf. Sci. 2022, 600, 154140. [Google Scholar] [CrossRef]
- Liu, H.; Lu, C.; Wang, X.; Xu, L.; Huang, X.; Wang, X.; Ning, H.; Lan, Z.; Guo, J. Combinations of V2C and Ti3C2 MXenes for boosting the hydrogen storage performances of MgH2. ACS Appl. Mater. Interfaces 2021, 13, 13235–13247. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, H.; Yuan, Z.; Liu, J.; Li, L.; Fan, Y.; Fan, G.; Liu, B. Hamamelis-like K2Ti6O13 Synthesized by Alkali Treatment of Ti3C2 MXene: Catalysis for Hydrogen Storage in MgH2. ACS Sust. Chem. Eng. 2020, 8, 4755–4763. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zhang, X.L.; Ren, Z.H.; Liu, Y.; Hu, J.J.; Li, H.W.; Gao, M.X.; Pan, H.G.; Liu, Y.F. In situ formed ultrafine NbTi nanocrystals from a NbTiC solid-solution MXene for hydrogen storage in MgH2. J. Mater. Chem. A 2019, 7, 14244–14252. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, H.; Zhu, Y.; Li, S.; Zhang, J.; Li, L. Excellent catalytic activity of a two-dimensional Nb4C3Tx (MXene) on hydrogen storage of MgH2. Appl. Surf. Sci. 2019, 493, 431–440. [Google Scholar] [CrossRef]
- Li, J.X.; Wang, S.; Du, Y.L.; Liao, W.H. Catalytic effect of Ti2C MXene on the dehydrogenation of MgH2. Int. J. Hydrogen Energy 2019, 44, 6787–6794. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Y.; Zhu, Y.; Zhang, J.; Li, L. Catalytic Effect of Sandwich-Like Ti3C2/TiO2(A)-C on Hydrogen Storage Performance of MgH2. Nanotechnology 2019, 31, 115404. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Z.; Zhang, M.; Gao, M.; Hu, J.; Du, F.; Liu, Y.; Pan, H. A novel solid-solution MXene (Ti0.5V0.5)3C2 with high catalytic activity for hydrogen storage in MgH2. Materialia 2018, 1, 114–120. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H.; Zhang, X.; Yang, Y.; Gao, M.; Pan, H. Superior catalytic activity derived from a two-dimensional Ti3C2 precursor towards the hydrogen storage reaction of magnesium hydride. Chem. Commun. 2016, 52, 705–708. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Lu, C.; Li, Y.; Tang, H.; Duan, X.; Wang, K.; Liu, H. Hydrogen Release and Uptake of MgH2 Modified by Ti3CN MXene. Inorganics 2023, 11, 243. https://doi.org/10.3390/inorganics11060243
Huang X, Lu C, Li Y, Tang H, Duan X, Wang K, Liu H. Hydrogen Release and Uptake of MgH2 Modified by Ti3CN MXene. Inorganics. 2023; 11(6):243. https://doi.org/10.3390/inorganics11060243
Chicago/Turabian StyleHuang, Xiantun, Chenglin Lu, Yun Li, Haimei Tang, Xingqing Duan, Kuikui Wang, and Haizhen Liu. 2023. "Hydrogen Release and Uptake of MgH2 Modified by Ti3CN MXene" Inorganics 11, no. 6: 243. https://doi.org/10.3390/inorganics11060243
APA StyleHuang, X., Lu, C., Li, Y., Tang, H., Duan, X., Wang, K., & Liu, H. (2023). Hydrogen Release and Uptake of MgH2 Modified by Ti3CN MXene. Inorganics, 11(6), 243. https://doi.org/10.3390/inorganics11060243









