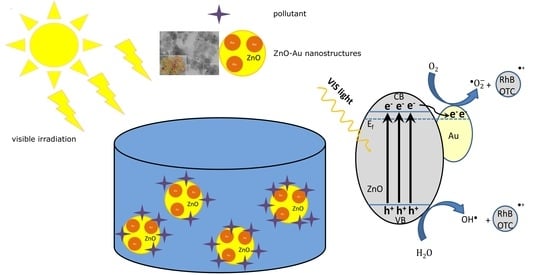
Enhanced Plasmonic Photocatalysis of Au-Decorated ZnO Nanocomposites
Abstract
1. Introduction
2. Results and Discussion
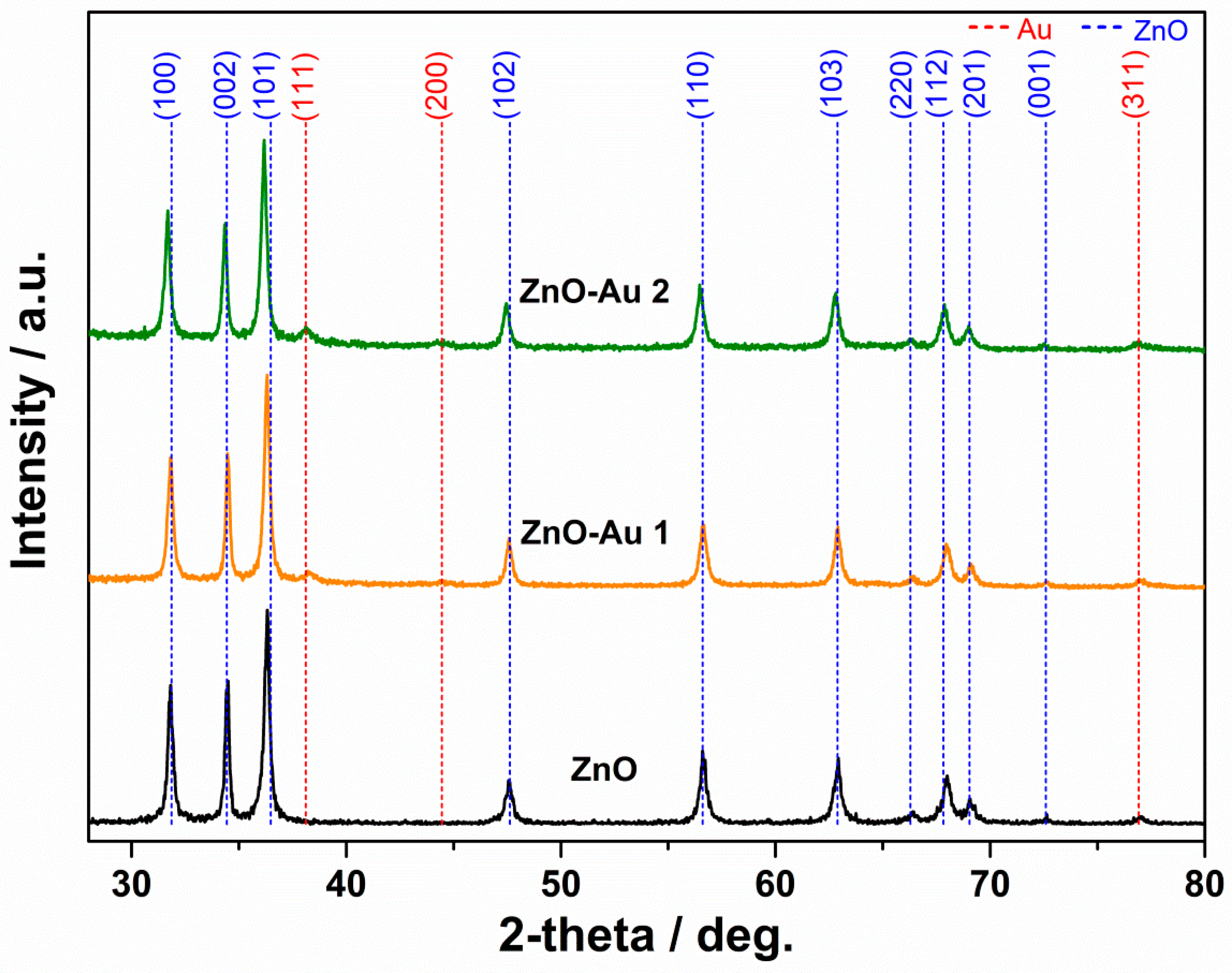
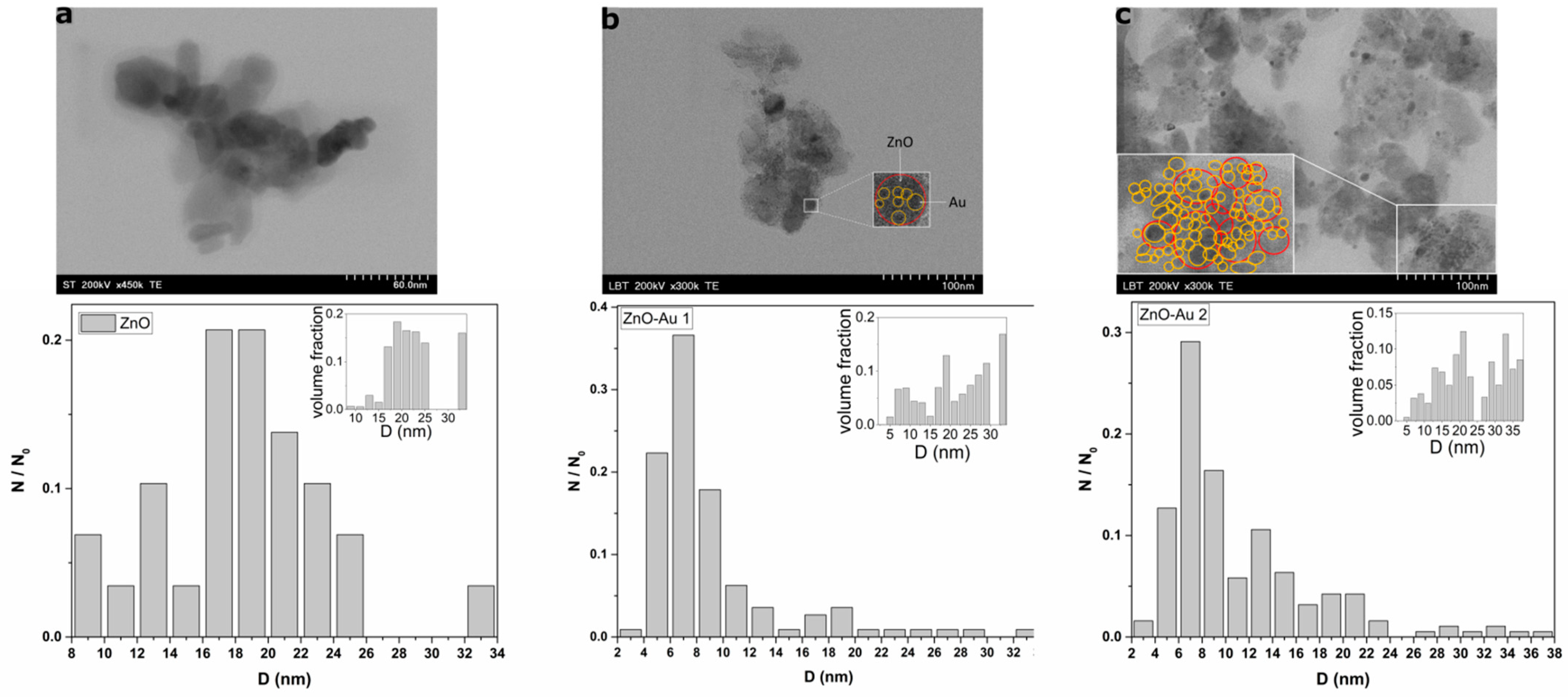
2.1. Structural, Morphological, and Thermal Properties
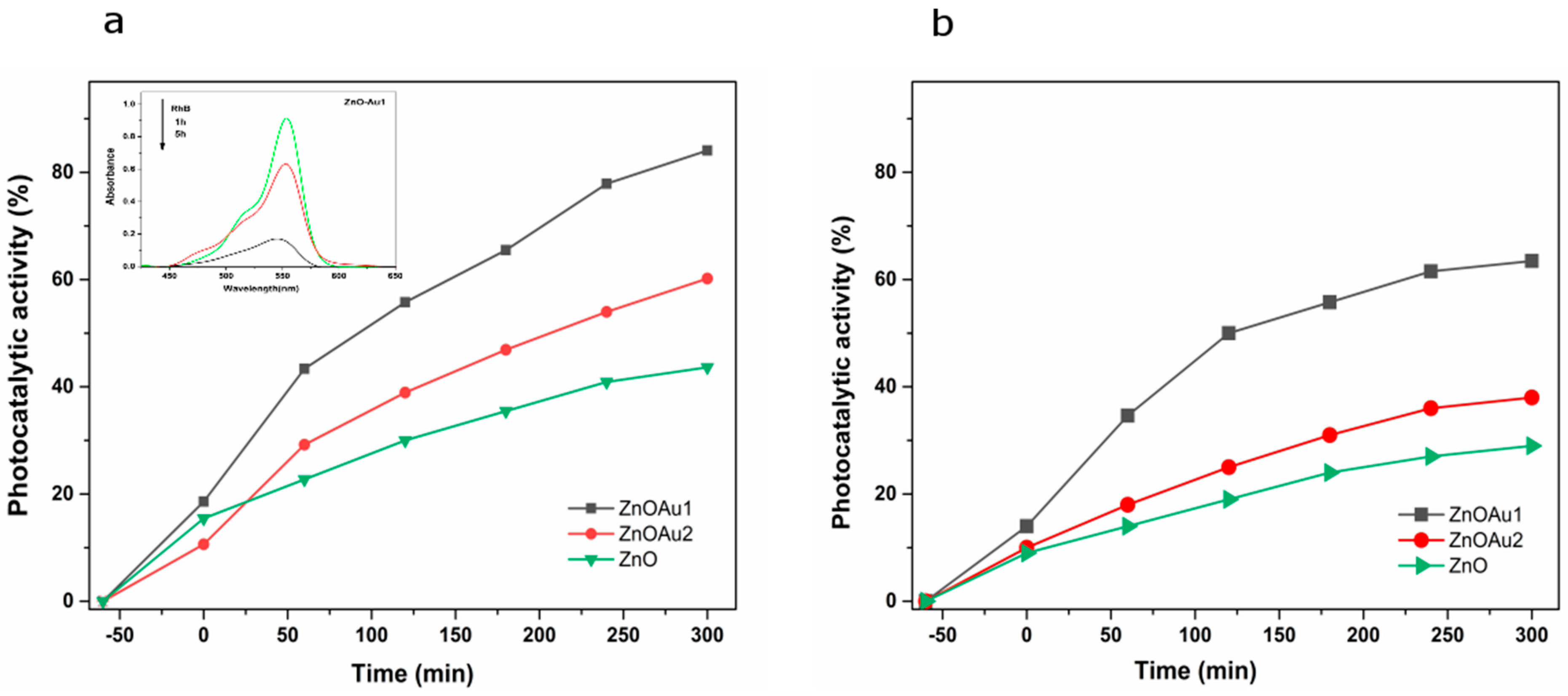
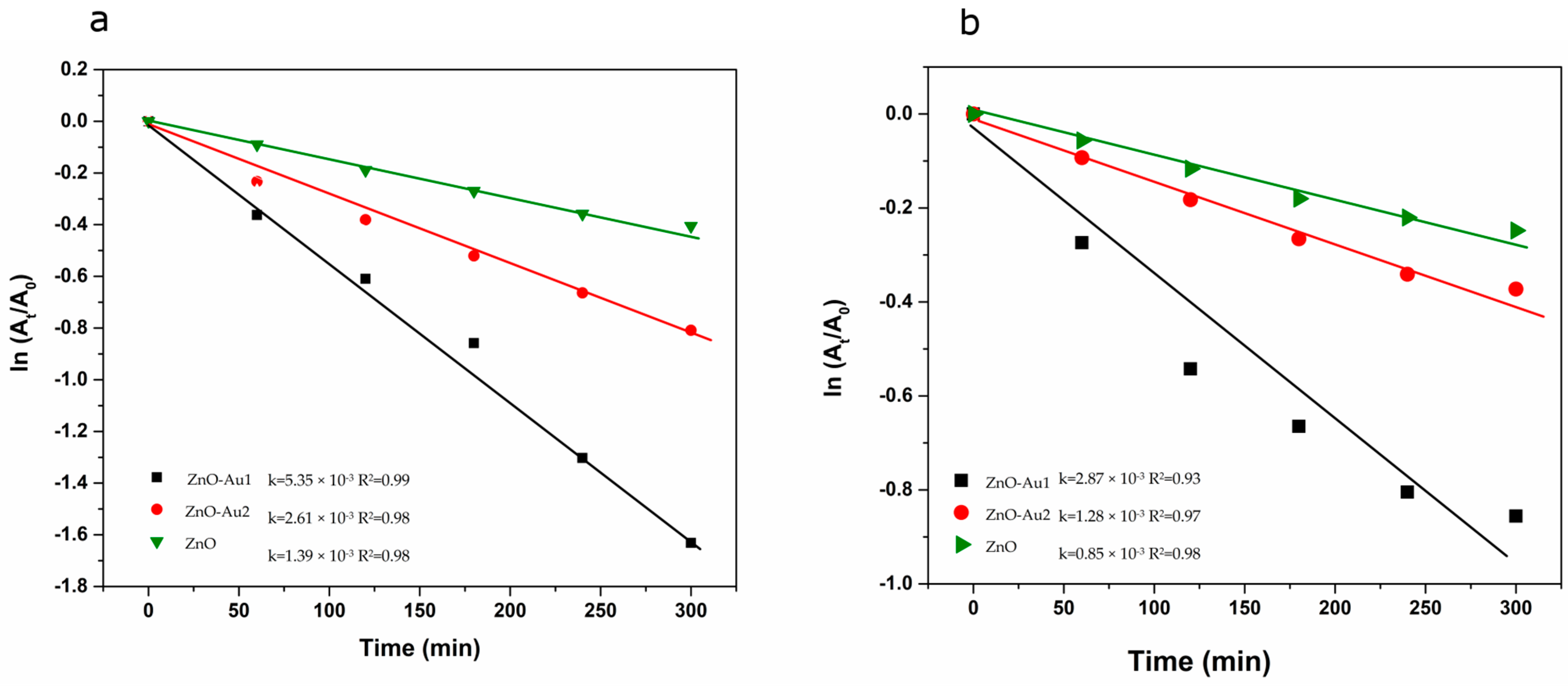
2.2. Photocatalytic Properties
2.3. Reutilization Tests
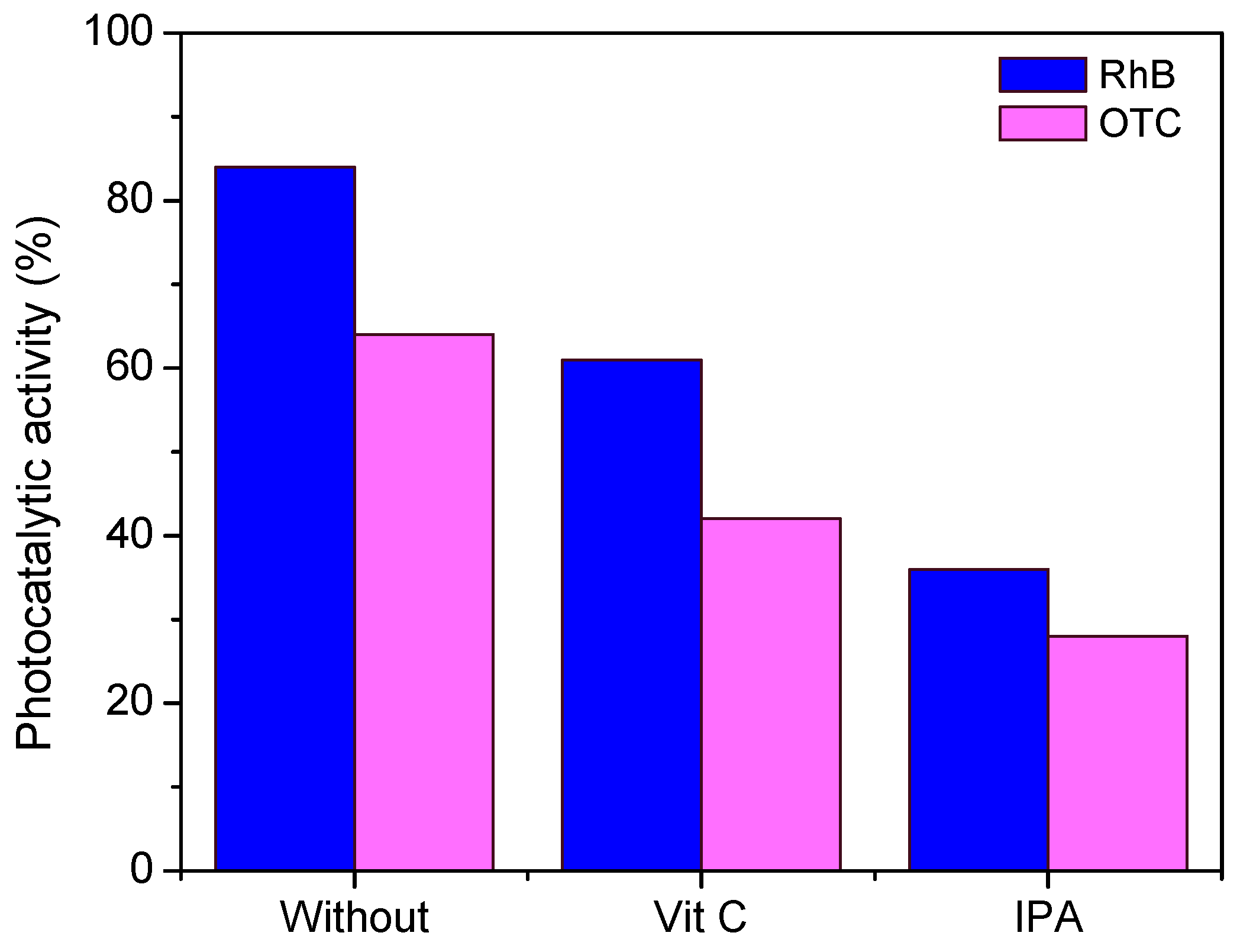
2.4. Generation of Reactive Oxygen Species
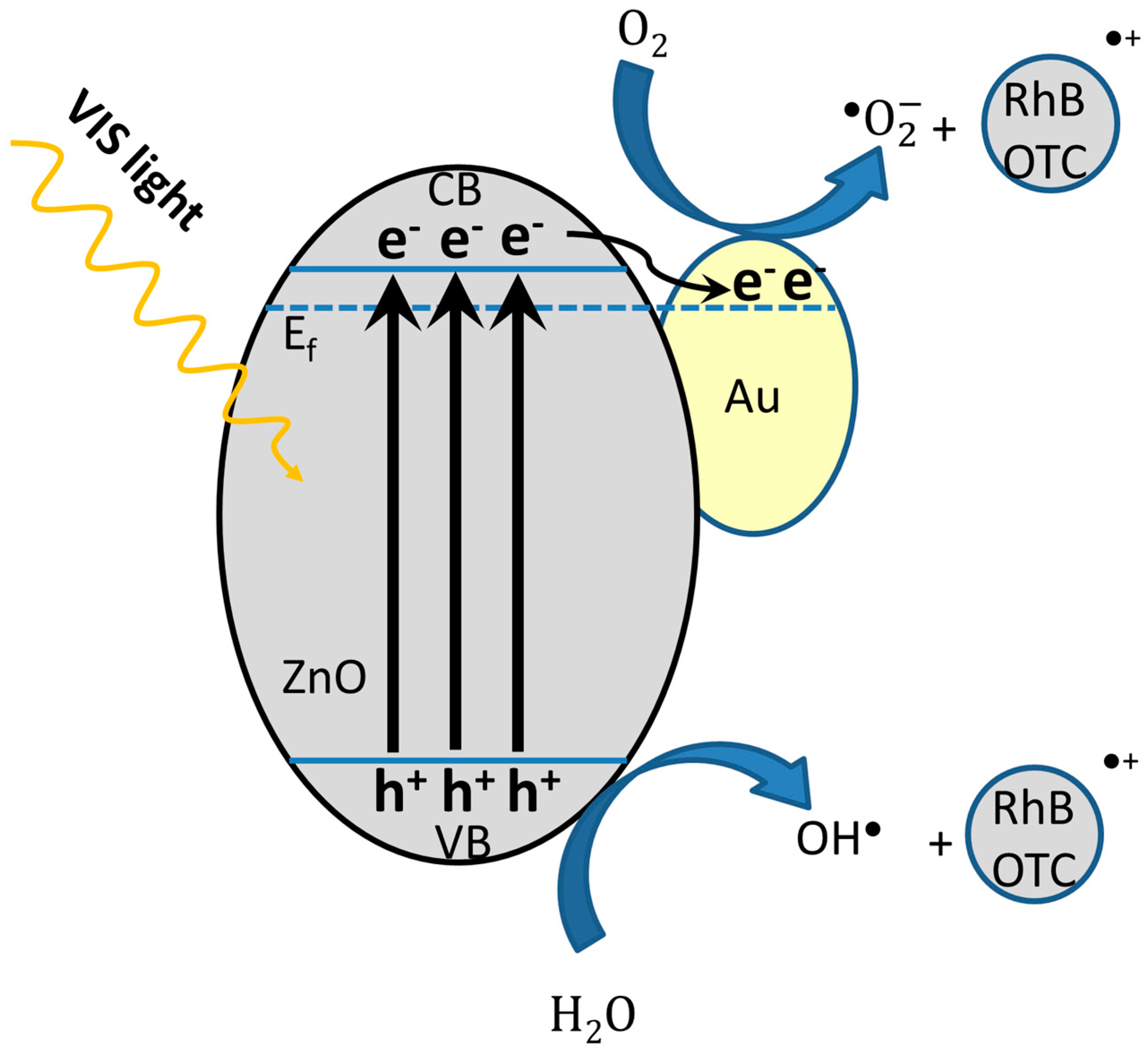
2.5. Photocatalytic Mechanism
3. Materials and Methods
3.1. Materials
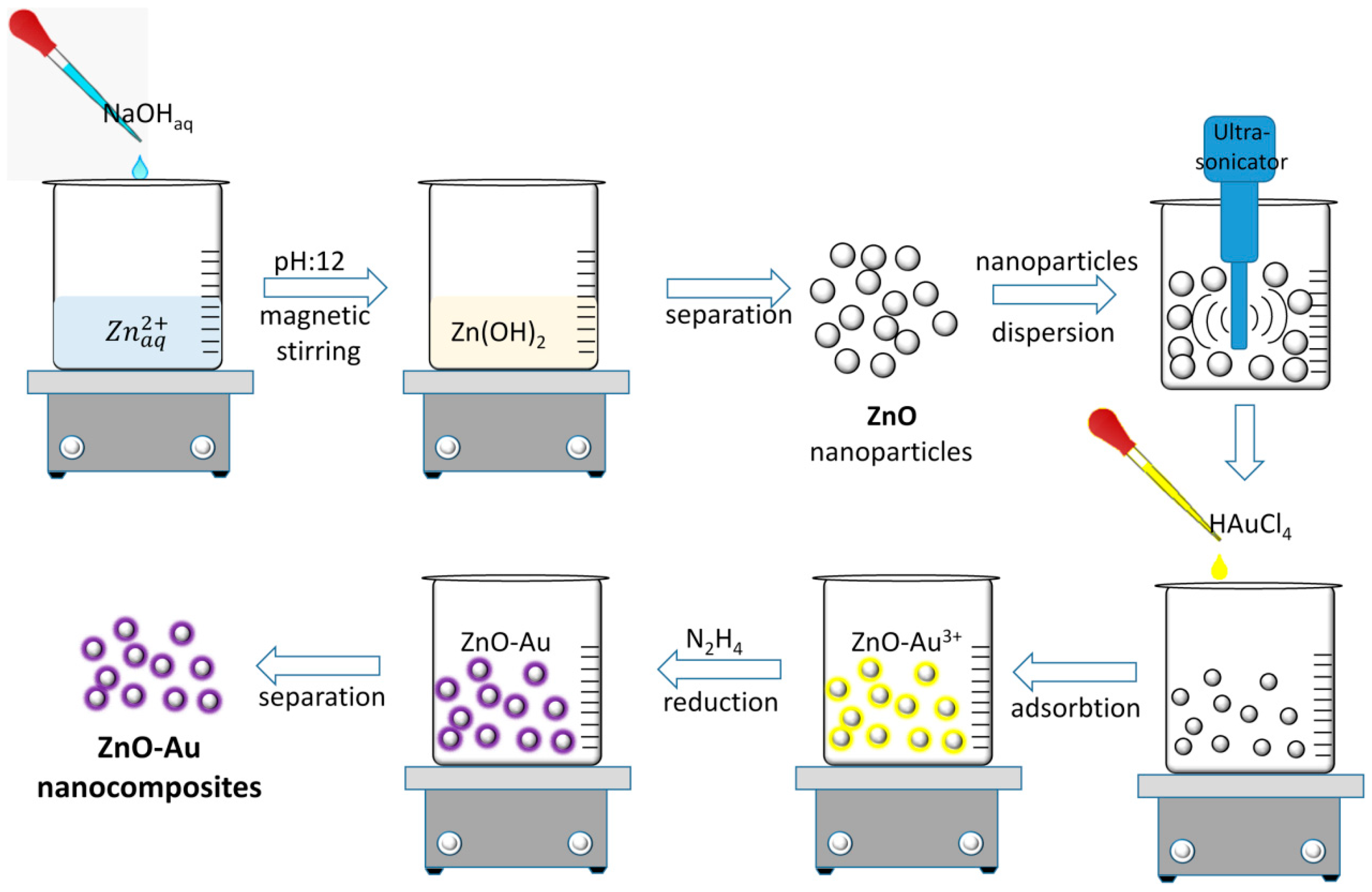
3.2. Synthesis of ZnO-Au Nanocomposites
3.3. Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, C.; Lin, J.; Li, L.; Jiang, K.; Hu, Z.; Xu, N.; Sun, J.; Wu, J. Au-Decorated ZnO Nanorod Powder and Its Application in Photodegradation of Organic Pollutants in the Visible Region. Phys. Status Solidi A Appl. Mat. Sci. 2021, 218, 2000737. [Google Scholar] [CrossRef]
- Yao, C.; Chen, W.; Li, L.; Jiang, K.; Hu, Z.; Lina, J.; Xu, N.; Sun, J.; Wu, J. ZnO:Au nanocomposites with high photocatalytic activity prepared by liquid-phase pulsed laser ablation. Opt. Laser Technol. 2021, 133, 106533. [Google Scholar] [CrossRef]
- Peralta, M.D.L.R.; Pal, U.; Zeferino, R.S. Photoluminescence (PL) Quenching and Enhanced Photocatalytic Activity of Au-Decorated ZnO Nanorods Fabricated through Microwave-Assisted Chemical Synthesis. ACS Appl. Mater. Interfaces 2012, 4, 4807–4816. [Google Scholar] [CrossRef] [PubMed]
- Das, T.K.; Remanan, S.; Ghosh, S.; Ghosh, S.K.; Das, N.C. Efficient synthesis of catalytic active silver nanoparticles illuminated cerium oxide nanotube: A mussel inspired approach. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100411. [Google Scholar] [CrossRef]
- Lux, K.C.; Hot, J.; Fau, P.; Bertron, A.; Kahn, M.L.; Ringot, E.; Fajerwerg, K. Nano-gold decorated ZnO: An alternative photocatalyst promising for NOx degradation. Chem. Eng. Sci. 2023, 267, 118377. [Google Scholar] [CrossRef]
- Zhu, Z.; Xia, H.; Li, H.; Han, S. Facile Construction of Bi2Sn2O7/g-C3N4 Heterojunction with Enhanced Photocatalytic Degradation of Norfloxacin. Inorganics 2022, 10, 131. [Google Scholar] [CrossRef]
- Das, T.K.; Ghosh, S.K.; Das, N.C. Green synthesis of a reduced graphene oxide/silver nanoparticles-based catalyst for degradation of a wide range of organic pollutants. Nano-Struct. Nano-Objects 2023, 34, 100960. [Google Scholar] [CrossRef]
- Sharma, A.; Ahmad, J.; Flora, S.J.S. Application of advanced oxidation processes and toxicity assessment of transformation products. Environ. Res. 2018, 167, 223–233. [Google Scholar] [CrossRef]
- Goktas, S.; Goktas, A. A comparative study on recent progress in efficient ZnO based nanocomposite and heterojunction photocatalysts: A review. J. Alloy. Compd. 2021, 863, 158734. [Google Scholar] [CrossRef]
- Mardosaitė, R.; Jurkevičiūtė, A.; Račkauskas, S. Superhydrophobic ZnO Nanowires: Wettability Mechanisms and Functional Applications. Cryst. Growth Des. 2021, 21, 4765–4779. [Google Scholar] [CrossRef]
- Manzano, C.V.; Philippe, L.; Serrà, A. Recent progress in the electrochemical deposition of ZnO nanowires: Synthesis approaches and applications. Crit. Rev. Solid State Mater. Sci. 2021, 47, 772–805. [Google Scholar] [CrossRef]
- Ajimsha, R.S.; Mahapatra, A.; Das, A.K.; Sahu, V.K.; Misra, P. High output power density owing to enhanced charge transfer in ZnO-based triboelectric nanogenerator. Energy 2023, 263, 125646. [Google Scholar] [CrossRef]
- Deckenbach, D.; Jörg, J.; Schneider, A. 3D hierarchically porous nanoscale ZnO anode for high-energy rechargeable zinc-air batteries. J. Power Sources 2021, 488, 229393. [Google Scholar] [CrossRef]
- Kang, Y.; Yu, F.; Zhang, L.; Wang, W.; Chen, L.; Li, Y. Review of ZnO-based nanomaterials in gas sensors. Solid State Ionics 2021, 360, 115544. [Google Scholar] [CrossRef]
- Vandarkuzhali, S.A.A.; Pugazhenthiran, N.; Mangalaraja, R.V.; Sathishkumar, P.; Viswanathan, B.; Anandan, S. Ultrasmall Plasmonic Nanoparticles Decorated Hierarchical Mesoporous TiO2 as an Efficient Photocatalyst for Photocatalytic Degradation of Textile Dyes. ACS Omega 2018, 3, 9834–9845. [Google Scholar] [CrossRef]
- Puspasari, V.; Ridhova, A.; Hermawan, A.; Amal, M.I.; Khan, M.M. ZnO-based antimicrobial coatings for biomedical applications. Bioprocess Biosyst. Eng. 2022, 45, 1421–1445. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, A.; Jackson, K.; Morgan, C.; Anderson, A.J.; Britt, D.W. A review of metal and metal-oxide nanoparticle coating technologies to inhibit agglomeration and increase bioactivity for agricultural applications. Agronomyc 2020, 10, 1018. [Google Scholar] [CrossRef]
- Van der Bruggen, B. Sustainable implementation of innovative technologies for water purification. Nat. Rev. Chem. 2021, 5, 217–218. [Google Scholar] [CrossRef]
- Allegretti, G.; Montoya, M.A.; Talamini, E. Assessing sectoral water stress states from the demand-side perspective through water footprint dimensions decomposition. Sci. Total. Environ. 2022, 809, 152216. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Li, C.; Tian, Q.; Zhang, Y.; Li, Y.; Yang, X.; Zheng, H.; Chen, L.; Li, F. Sequential combination of photocatalysis and microalgae technology for promoting the degradation and detoxification of typical antibiotics. Water Res. 2022, 210, 117985. [Google Scholar] [CrossRef]
- Cai, G.; Liu, T.; Zhang, J.; Song, H.; Jiang, Q.; Zhou, C. Control for chlorine resistant spore forming bacteria by the coupling of pre-oxidation and coagulation sedimentation, and UV-AOPs enhanced inactivation in drinking water treatment. Water Res. 2022, 219, 118540. [Google Scholar] [CrossRef]
- Yu, M.; Wang, J.; Tang, L.; Feng, C.; Liu, H.; Zhang, H.; Peng, B.; Chen, Z.; Xie, Q. Intimate coupling of photocatalysis and biodegradation for wastewater treatment: Mechanisms, recent advances and environmental applications. Water Res. 2020, 175, 115673. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Jiang, J.; Wan, Z.; Su, Y.; Tang, H. Insight into charge carrier separation and solar-light utilization: rGO decorated 3D ZnO hollow microspheres for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2020, 564, 322–332. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, F.M.; He, J. Controlled fabrication and photocatalytic properties of a three-dimensional ZnO nanowire/reduced graphene oxide/CdS heterostructure on carbon cloth. Nanoscale 2013, 5, 11291–11297. [Google Scholar] [CrossRef]
- Abarna, B.; Preethi, T.; Karunanithi, A.; Rajarajeswari, G.R. Influence of jute template on the surface, optical and photocatalytic properties of sol-gel derived mesoporous zinc oxide. Mater. Sci. Semicond. Process. 2016, 56, 243–250. [Google Scholar] [CrossRef]
- Sun, X.; Luo, X.; Zhang, X.; Xie, J.; Jin, S.; Wang, H.; Zheng, X.-S.; Wu, X.; Xie, Y. Enhanced Superoxide Generation on Defective Surfaces for Selective Photooxidation. J. Am. Chem. Soc. 2019, 141, 3797–3801. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Soni, R.K. Controlled synthesis of CuO decorated defect enriched ZnO nanoflakes for improved sunlight-induced photocatalytic degradation of organic pollutants. Appl. Surf. Sci. 2020, 521, 146420. [Google Scholar] [CrossRef]
- Toe, M.Z.; Pung, S.-Y.; Le, A.T.; Yaccob, K.A.B.; Matsuda, A.; Tan, W.K.; Han, S.S. Morphology and optical properties of ZnO nanorods coupled with metal oxides of various bandgaps by photo-oxidation. J. Lumin 2021, 229, 117649. [Google Scholar] [CrossRef]
- Azar, B.E.; Ramazani, A.; Fardood, S.T.; Morsali, A. Green synthesis and characterization of ZnAl2O4@ZnO nanocomposite and its environmental applications in rapid dye degradation. Optik 2020, 208, 164129. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Abou-Gamra, Z.M.; Alshakhanbeh, M.A.; Medien, H. Control Synthesis of Metallic Gold Nanoparticles Homogeneously Distributed on Hexagonal ZnO Nanoparticles for Pho-tocatalytic Degradation of Methylene Blue Dye. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100217. [Google Scholar] [CrossRef]
- He, W.; Kim, H.-K.; Wamer, W.G.; Melka, D.; Callahan, J.H.; Yin, J.-J. Photogenerated charge carriers and reactive oxygen species in ZnO/Au hybrid nanostructures with enhanced photo-catalytic and antibacterial activity. J. Am. Chem. Soc. 2014, 136, 750–757. [Google Scholar] [CrossRef]
- Macias-Montero, M.; Pelaez, R.J.; Rico, V.J.; Saghi, Z.; Midgley, P.; Afonso, C.N.; Gonzalez-Elipe, A.R.; Borras, A. Laser treatment of Ag@ZnO nanorods as longlife-span SERS surfaces. ACS Appl. Mater. Interfaces 2015, 7, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Chan, C.-H.; Chien, C.-H.; Yaseen, M.T.; Liang, C.-T.; Chang, Y.-C. Enhanced Emission and Photoconductivity Due to Photo-Induced Charge Transfer from Au Nanoislands to ZnO. Appl. Phys. Lett. 2016, 108, 041104. [Google Scholar] [CrossRef]
- Chamorro, W.; Ghanbaja, J.; Battie, Y.; Naciri, A.E.; Soldera, F.; Mücklich, F.; Horwat, D. Local Structure-Driven Localized Surface Plasmon Absorption and Enhanced Photoluminescence in ZnO-Au Thin Films. J. Phys. Chem. C 2016, 120, 29405–29413. [Google Scholar] [CrossRef]
- Viter, R.; Balevicius, Z.; Abou Chaaya, A.; Baleviciute, I.; Tumenas, S.; Mikoliunaite, L.; Ramanavicius, A.; Gertnere, Z.; Zalesska, A.; Vataman, V.; et al. The Influence of Localized Plasmons on the Optical Properties of Au/ZnO Nanostructures. J. Mat. Chem. C 2015, 3, 6815–6821. [Google Scholar] [CrossRef]
- Fageria, P.; Gangopadhyay, S.; Pande, S. Synthesis of ZnO/Au and ZnO/Ag nanoparticles and their photocatalytic application using UV and visible light. RSC Adv. 2014, 4, 294262. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Yan, Y.; Yang, L.; Li, X.; Lu, Z.; Zhai, H.; Han, D.; Huo, P. Two Hybrid Au-ZnO Heterostructures with Different Hierarchical Structures: Towards Highly Efficient Photocatalysts. Sci. Rep. 2019, 9, 16863. [Google Scholar] [CrossRef]
- Liu, L.; Yang, H.; Ren, X.; Tang, J.; Li, Y.; Zhang, X. Au-ZnO Janus Nanoparticles Exhibiting Strong Charge-Transfer-Induced SERS for Recyclable SERS-Active Substrates. Nanoscale 2015, 7, 5147–5151. [Google Scholar] [CrossRef]
- Raji, R.; Gopchandran, K.G. Plasmonic photocatalytic activity of ZnO:Au nanostructures: Tailoring the plasmon absorption and interfacial charge transfer mechanism. J. Hazard. Mater. 2019, 368, 345–357. [Google Scholar] [CrossRef]
- Georgiev, P.; Kaneva, N.; Bojinova, A.; Papazova, K.; Mircheva, K.; Balashev, K. Effect of gold nanoparticles on the photocatalytic efficiency of ZnO films. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 240–247. [Google Scholar] [CrossRef]
- Gebreslassie, G.; Gebrezgiabher, M.; Lin, B.; Thomas, M.; Linert, W. Direct Z-Scheme CoFe2O4-Loaded g-C3N4 Photocatalyst with High Degradation Efficiency of Methylene Blue under Visible-Light Irradiation. Inorganics 2023, 11, 119. [Google Scholar] [CrossRef]
- Chebanenko, M.I.; Tikhanova, S.M.; Nevedomskiy, V.N.; Popkov, V.I. Synthesis and Structure of ZnO-Decorated Graphitic Carbon Nitride (g-C3N4) with Improved Photocatalytic Activity under Visible Light. Inorganics 2022, 10, 249. [Google Scholar] [CrossRef]
- Gupta, N.K.; Gha, Y.; Kim, S.; Bae, J.; Kim, K.S. Photocatalytic Degradation of Organic Pollutants over MFe2O4 (M = Co, Ni, Cu, Zn) Nanoparticles at Neutral pH. Sci. Rep. 2020, 10, 4942. [Google Scholar] [CrossRef] [PubMed]
- Toloman, D.; Stefan, M.; Macavei, S.; Barbu-Tudoran, L.; Popa, A. Photocatalytic Self-Cleaning PVDF Membrane Blended with MWCNT-ZnO Nanocomposites for RhB Removal. Coatings 2023, 13, 594. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray Line Broadening from Filed Aluminium and Wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Pana, O.; Teodorescu, C.M.; Chauvet, O.; Payen, C.; Macovei, D.; Turcu, R.; Soran, M.L.; Aldea, N.; Barbu, L. Structure, morphology and magnetic properties of Fe–Au core-shell nanoparticles. Surf. Sci. 2007, 601, 4352–4357. [Google Scholar] [CrossRef]
- Gogurla, N.; Sinha, A.K.; Santra, S.; Manna, S.; Ray, S.K. Multifunctional Au-ZnO plasmonic nanostructures for enhanced UV photodetector and room temperature NO sensing devices. Sci. Rep. 2014, 4, 6483. [Google Scholar] [CrossRef]
- Yao, C.; Lin, J.; Wu, L.; Li, L.; Xu, N.; Sun, J.; Wu, J. High-Visible-Light Photocatalytic Activity of ZnO–Au Nanocomposites Synthesized by a Controlled Hydrothermal Method. Phys. Status Solidi A 2021, 218, 2100150. [Google Scholar] [CrossRef]
- Shan, G.; Zhong, M.; Wang, S.; Li, Y.; Liu, Y. The synthesis and optical properties of the heterostructured ZnO/Au nanocomposites. J. Colloid Interface Sci. 2008, 326, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, X.; Shan, X.; Fan, H.; Yang, L.; Zhang, Y.; Li, X. Blue-shift of UV emission in ZnO/graphene composites. J. Alloy. Compd. 2013, 556, 1–5. [Google Scholar] [CrossRef]
- Vanheusden, K.; Warren, W.L.; Seager, C.H.; Tallant, D.R.; Voigt, J.A.; Gnade, B.E. Mechanisms behind green photoluminescence in ZnO phosphor powders. J. Appl. Phys. 1996, 79, 7983–7990. [Google Scholar] [CrossRef]
- Zhou, H.; Hofstaetter, A.; Hofmann, D.M.; Meyer, B.K. Magnetic resonance studies on ZnO nanocrystals. Microelectron. Eng. 2003, 66, 59–64. [Google Scholar] [CrossRef]
- Li, D.; Leung, Y.H.; Djurišić, A.B.; Liu, Z.T.; Xie, M.H.; Shi, S.L.; Xu, S.; Chan, W.K. Different origins of visible luminescence in ZnO nanostructures fabricated by the chemical and evaporation methods. Appl. Phys. Lett. 2004, 85, 1601–1603. [Google Scholar] [CrossRef]
- Kaftelen, H.; Ocakoglu, K.; Thomann, R.; Tu, S.; Weber, S.; Erdem, E. EPR and photoluminescence spectroscopy studies on the defect structure of ZnO nanocrystals. Phys. Rev. B 2012, 86, 014113. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Choy, W.C.H.; Roy, V.A.L.; Leung, Y.H.; Kwong, C.Y.; Cheah, K.W.; Rao, T.K.G.; Chan, W.K.; Lui, H.F.; Surya, C. Photoluminescence and Electron Paramagnetic Resonance of ZnO Tetrapod Structures. Adv. Funct. Mater. 2004, 14, 856–864. [Google Scholar] [CrossRef]
- Toloman, D.; Pana, O.; Stefan, M.; Popa, A.; Leostean, C.; Macavei, S.; Silipas, D.; Perhaita, I.; Lazar, M.D.; Barbu-Tudoran, L. Photocatalytic activity of SnO2-TiO2 composite nanoparticles modified with PVP. J. Colloid Interface Sci. 2019, 542, 296–307. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Yu, J.; Li, C.; Xu, S.; Li, F.; Zhang, C.; Man, B.; Zhang, C. Plasmonic and bi-piezoelectric enhanced photocatalysis using PVDF/ZnO/Au nanobrush. Nanophotonics 2022, 11, 3339–3349. [Google Scholar] [CrossRef]
- Lu, J.; Wang, H.; Peng, D.; Chen, T.; Dong, S.; Chang, Y. Synthesis and properties of Au/ZnO nanorods as a plasmonic photocatalyst. Phys. E Low-Dimens. Syst. Nanostructures 2016, 78, 41–48. [Google Scholar] [CrossRef]
- She, P.; Liu, Z.; Sun, H.; Shang, Y.; Li, Z.; Qin, Z.; Xu, K.; Yu, Z. Bio-inspired Spin-ach-leaf-based Au/ZnO Nanocomposites as Photocatalyst. J. Bionic Eng. 2019, 16, 1080–1091. [Google Scholar] [CrossRef]
- Diaz-Uribe, C.E.; Daza, M.C.; Martínez, F.; Páez-Mozo, E.A.; Guedes, C.L.B.; Di Mauro, E. Visible light superoxide radical anion generation by tetra(4-carboxyphenyl) porphyrin /TiO2: EPR characterization. J. Photochem. Photobiol. A 2010, 215, 172. [Google Scholar] [CrossRef]
- Lee, Y.; Fujimoto, T.; Yamanaka, S.; Kuga, Y. Evaluation of photocatalysis of Au supported ZnO prepared by the spray pyrolysis method. Adv. Powder Technol. 2021, 32, 1619–1626. [Google Scholar] [CrossRef]
- Rapa, M.; Stefan, M.; Popa, A.; Toloman, D.; Leostean, C.; Borodi, G.; Vodnar, D.C.; Wrona, M.; Salafranca, J.; Nerin, C.; et al. Electrospun Nanosystems Based on PHBV and ZnO for Ecological Food Packaging. Polymers 2021, 13, 2123. [Google Scholar] [CrossRef]
- Falamas, A.; Marica, I.; Popa, A.; Toloman, D.; Pruneanu, S.; Pogacean, F.; Nekvapil, F.; Silipas, T.D.; Stefan, M. Size-dependent spectroscopic insight into the steady-state and time-resolved optical properties of ZnO photocatalysts. Mater. Sci. Semicond. Process. 2022, 145, 106644. [Google Scholar] [CrossRef]
- Madanu, T.L.; Mouchet, S.R.; Deparis, O.; Liu, J.; Li, Y.; Su, B.L. Tuning and transferring slow photons from TiO2 photonic crystals to BiVO4 nanoparticles for unprecedented visible light photoca-talysis. J. Colloid Interf. Sci. 2023, 634, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhu, H.; Li, Q. Visible-light-driven photocatalytic properties of ZnO/ZnFe2O4 core/shell nanocable arrays. Appl. Catal. B Environ. 2014, 160–161, 408–414. [Google Scholar] [CrossRef]
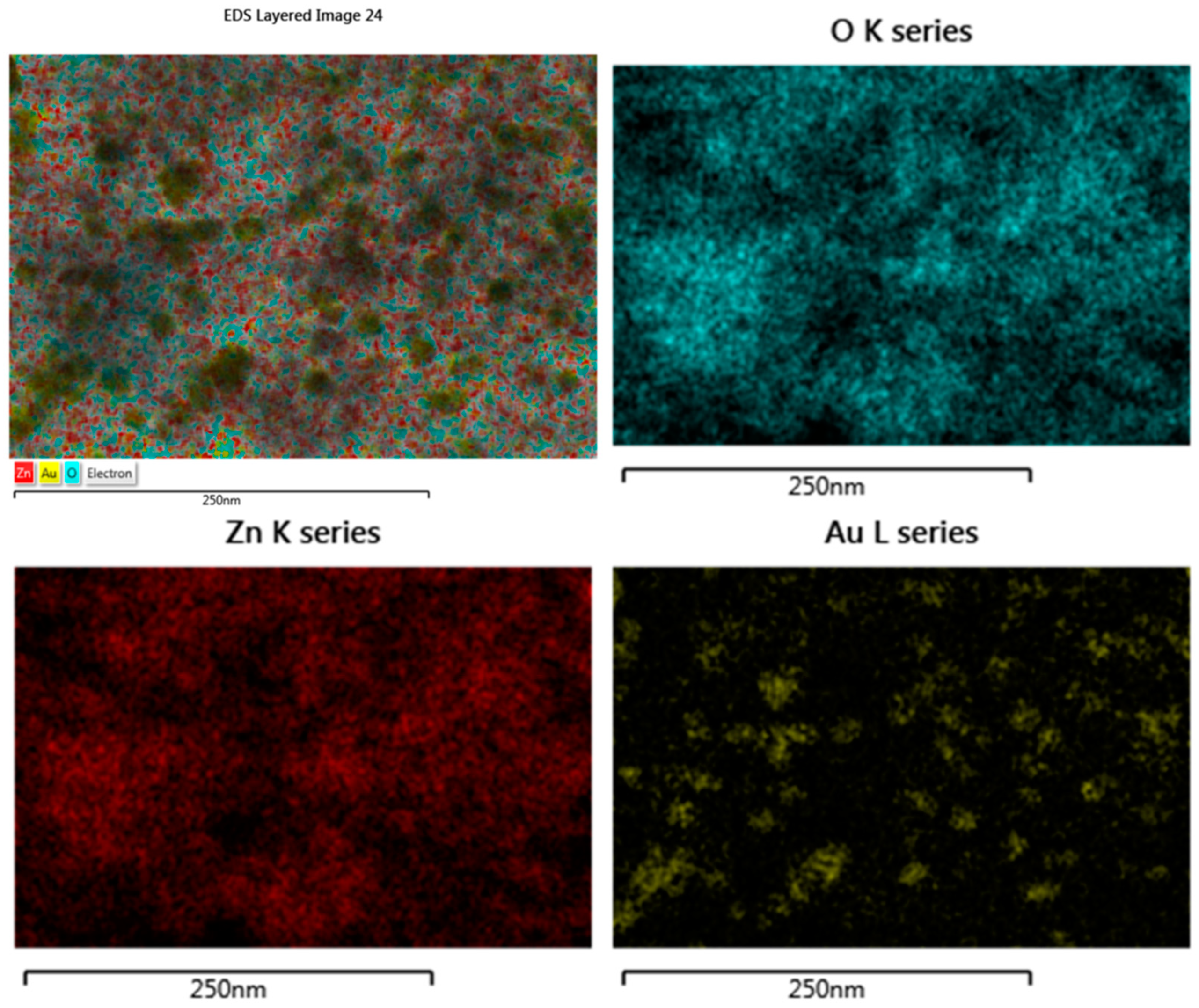

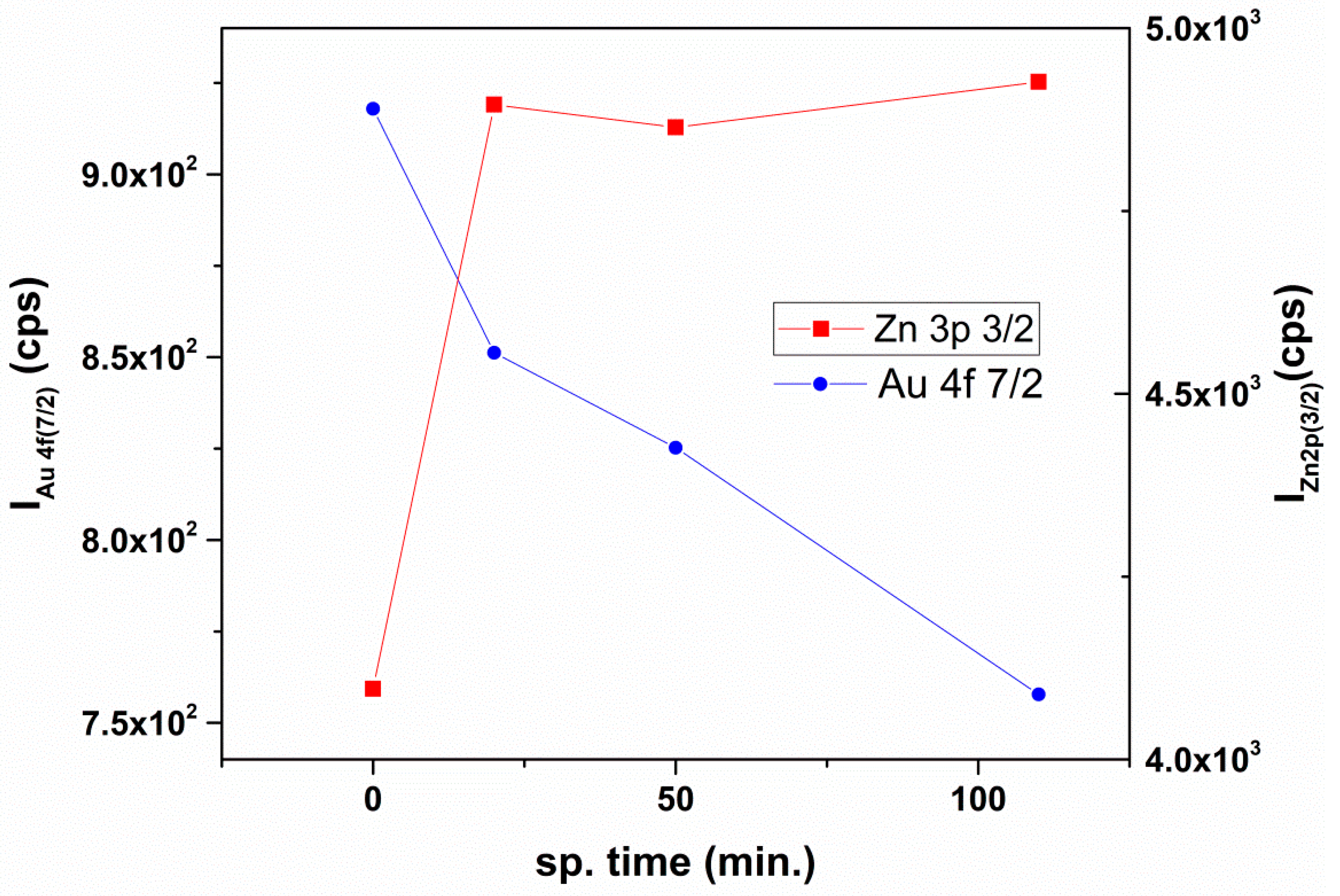
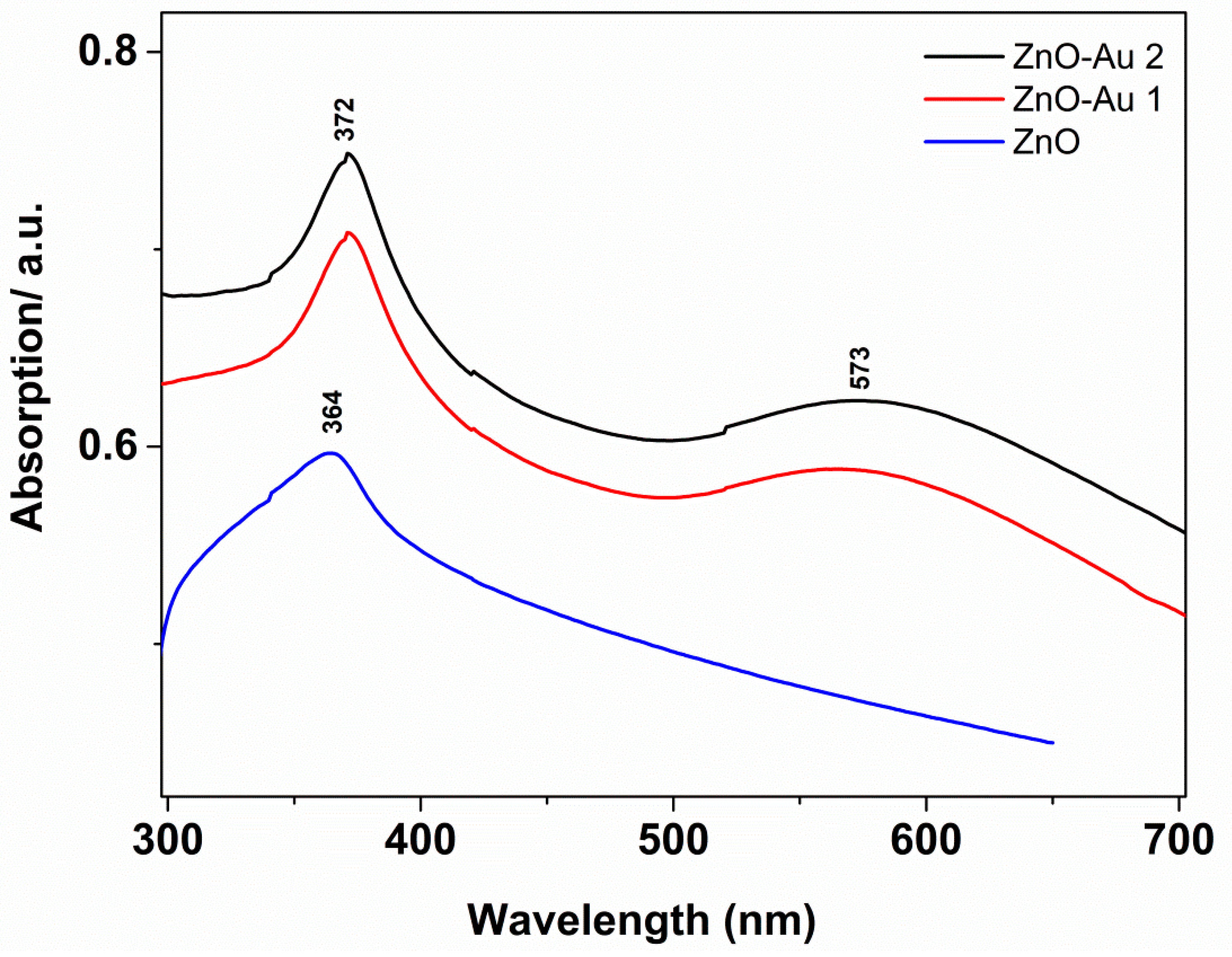


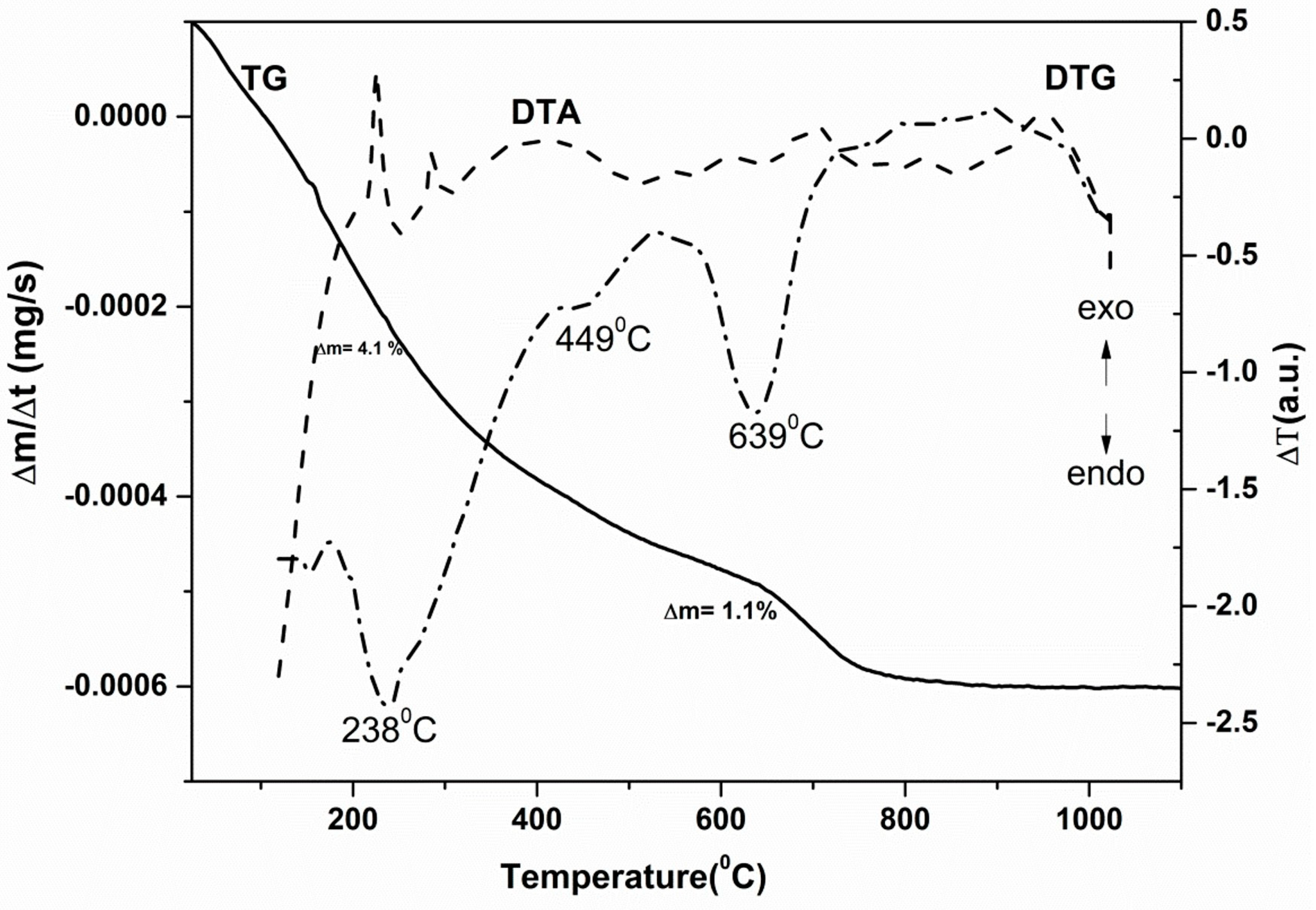


| Sample | Molar Ratio ZnO:Au | Crystallites Dimension (nm) | |
|---|---|---|---|
| ZnO | Au | ||
| ZnO | 1:0 | 21.8 | - |
| ZnO-Au1 | 1:3 | 35.0 | 14.5 |
| ZnO-Au2 | 1:4 | 32.1 | 19.2 |
| Sample | Zn (wt%) | O (wt%) | Au (wt%) |
|---|---|---|---|
| ZnO | 49.2 | 50.8 | - |
| ZnO-Au1 | 52.4 | 45.8 | 1.8 |
| ZnO-Au2 | 51.9 | 45.6 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefan, M.; Popa, A.; Toloman, D.; Leostean, C.; Barbu-Tudoran, L.; Falamas, A. Enhanced Plasmonic Photocatalysis of Au-Decorated ZnO Nanocomposites. Inorganics 2023, 11, 157. https://doi.org/10.3390/inorganics11040157
Stefan M, Popa A, Toloman D, Leostean C, Barbu-Tudoran L, Falamas A. Enhanced Plasmonic Photocatalysis of Au-Decorated ZnO Nanocomposites. Inorganics. 2023; 11(4):157. https://doi.org/10.3390/inorganics11040157
Chicago/Turabian StyleStefan, Maria, Adriana Popa, Dana Toloman, Cristian Leostean, Lucian Barbu-Tudoran, and Alexandra Falamas. 2023. "Enhanced Plasmonic Photocatalysis of Au-Decorated ZnO Nanocomposites" Inorganics 11, no. 4: 157. https://doi.org/10.3390/inorganics11040157
APA StyleStefan, M., Popa, A., Toloman, D., Leostean, C., Barbu-Tudoran, L., & Falamas, A. (2023). Enhanced Plasmonic Photocatalysis of Au-Decorated ZnO Nanocomposites. Inorganics, 11(4), 157. https://doi.org/10.3390/inorganics11040157