Photocatalytic Reduction of Cr(VI) to Cr(III) and Photocatalytic Degradation of Methylene Blue and Antifungal Activity of Ag/TiO2 Composites Synthesized via the Template Induced Route
Abstract
1. Introduction
2. Experimental Section
2.1. Material and Method
2.2. Preparation of Ag/TiO2 Composites
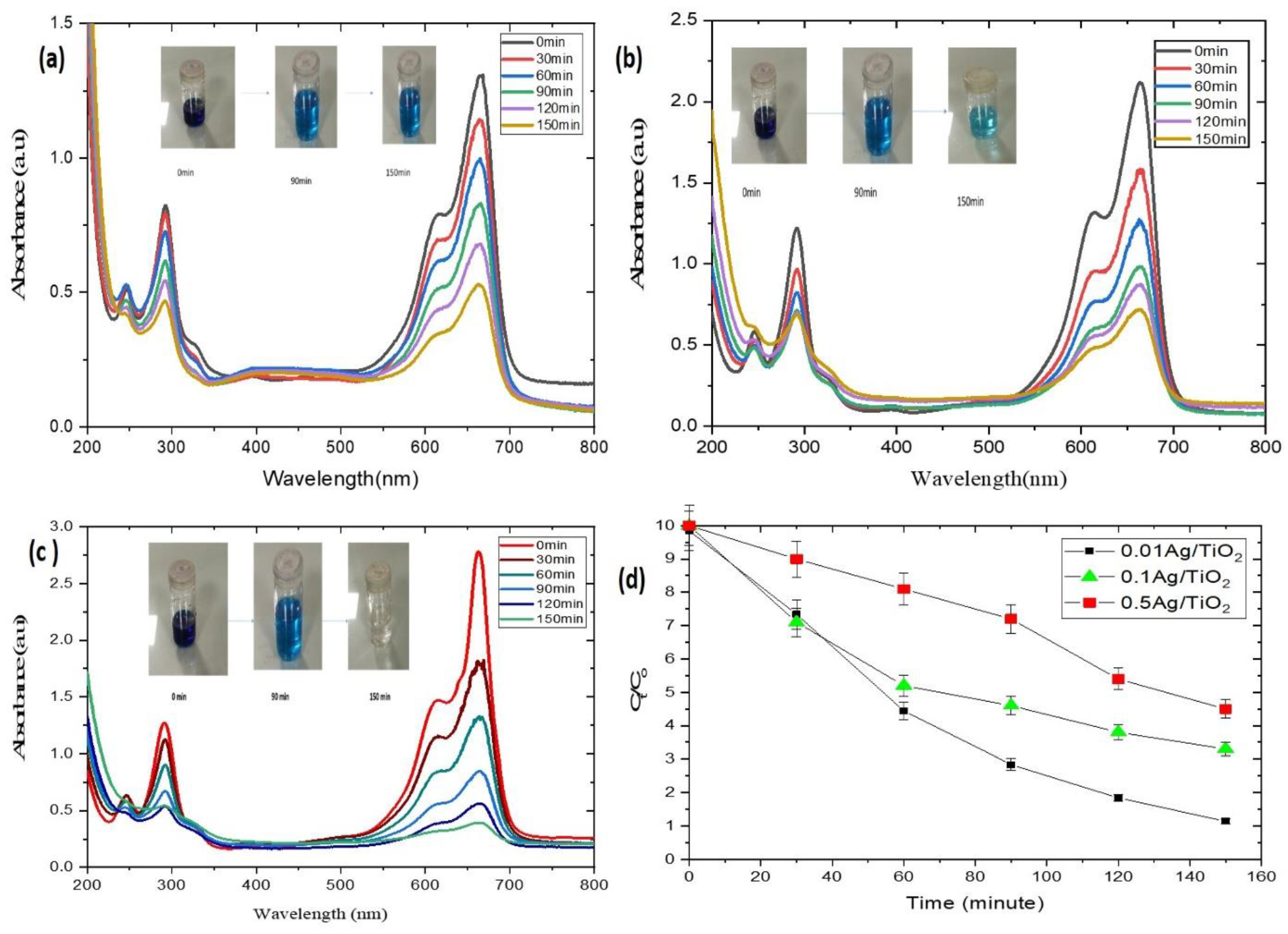
2.3. Photocatalytic Degradation Experiment
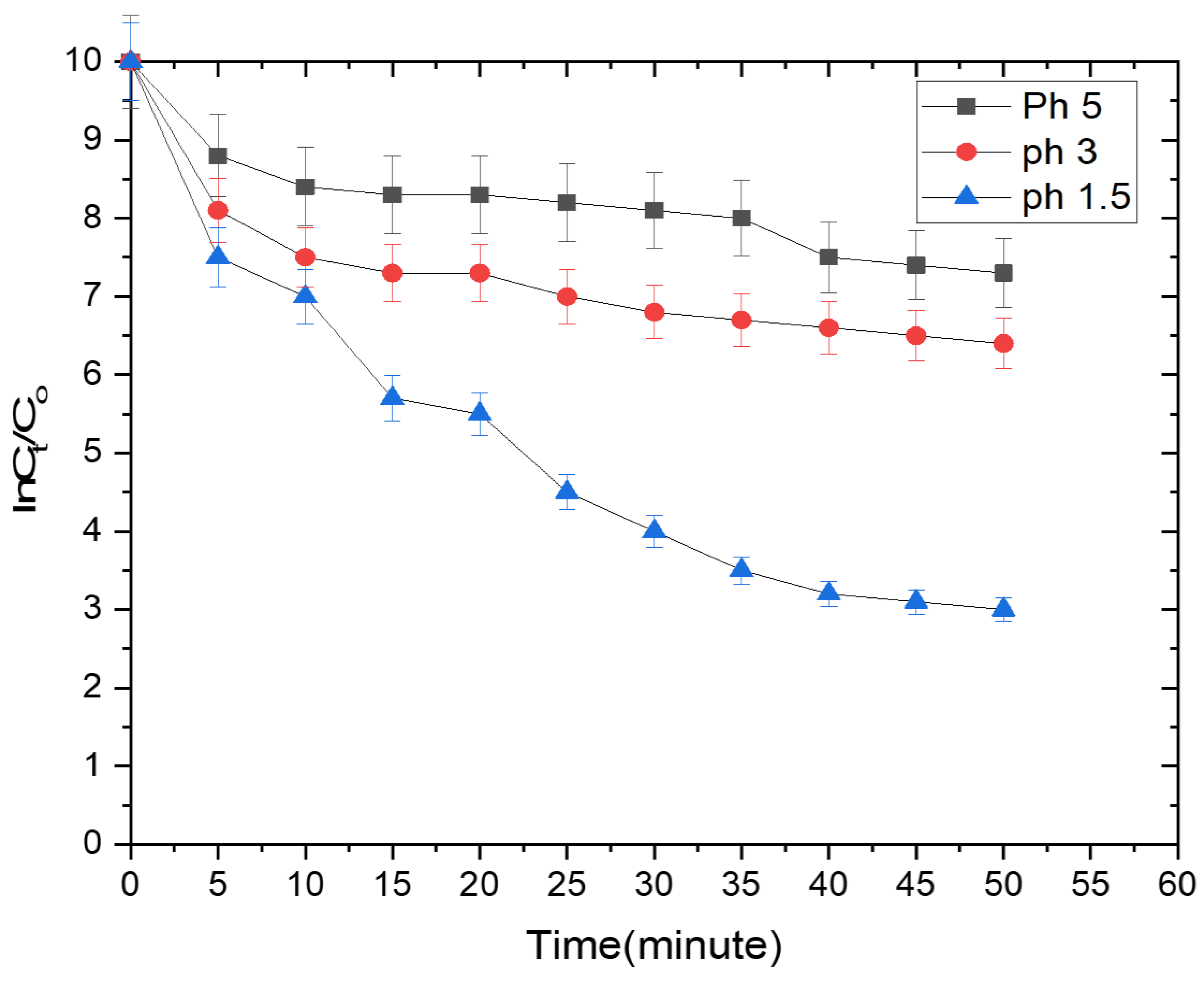
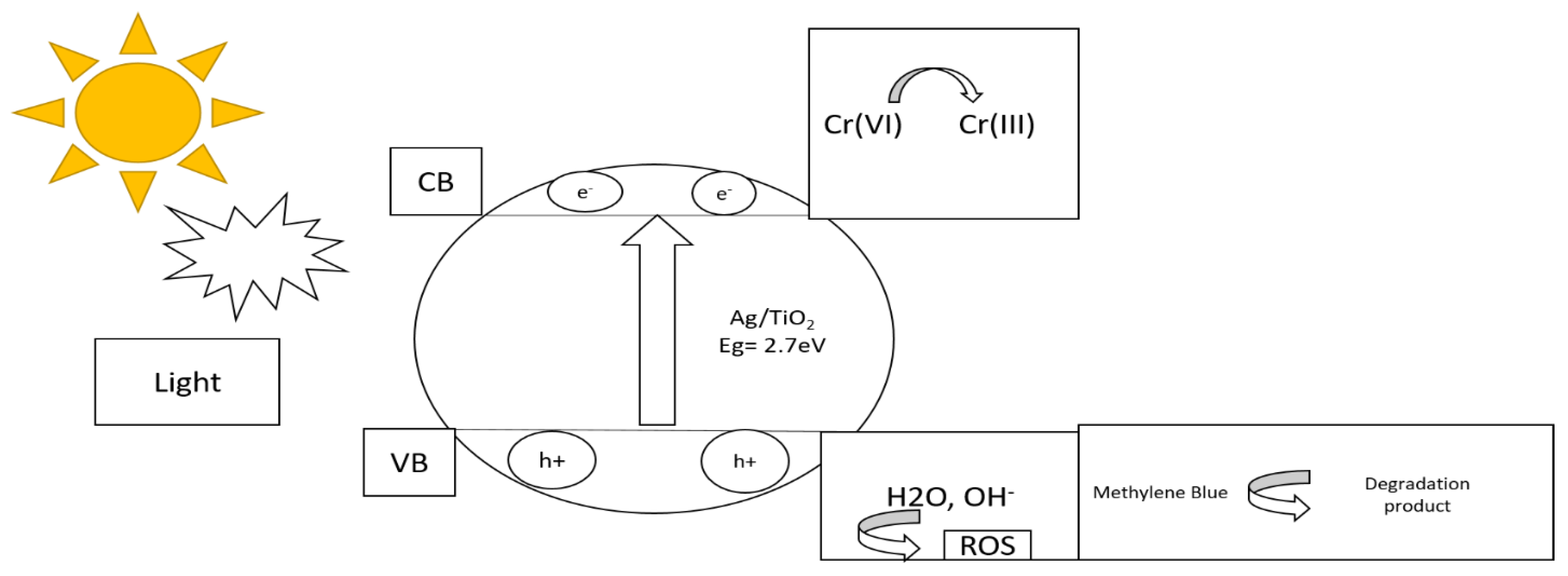
2.4. Photocatalytic Reduction Experiment
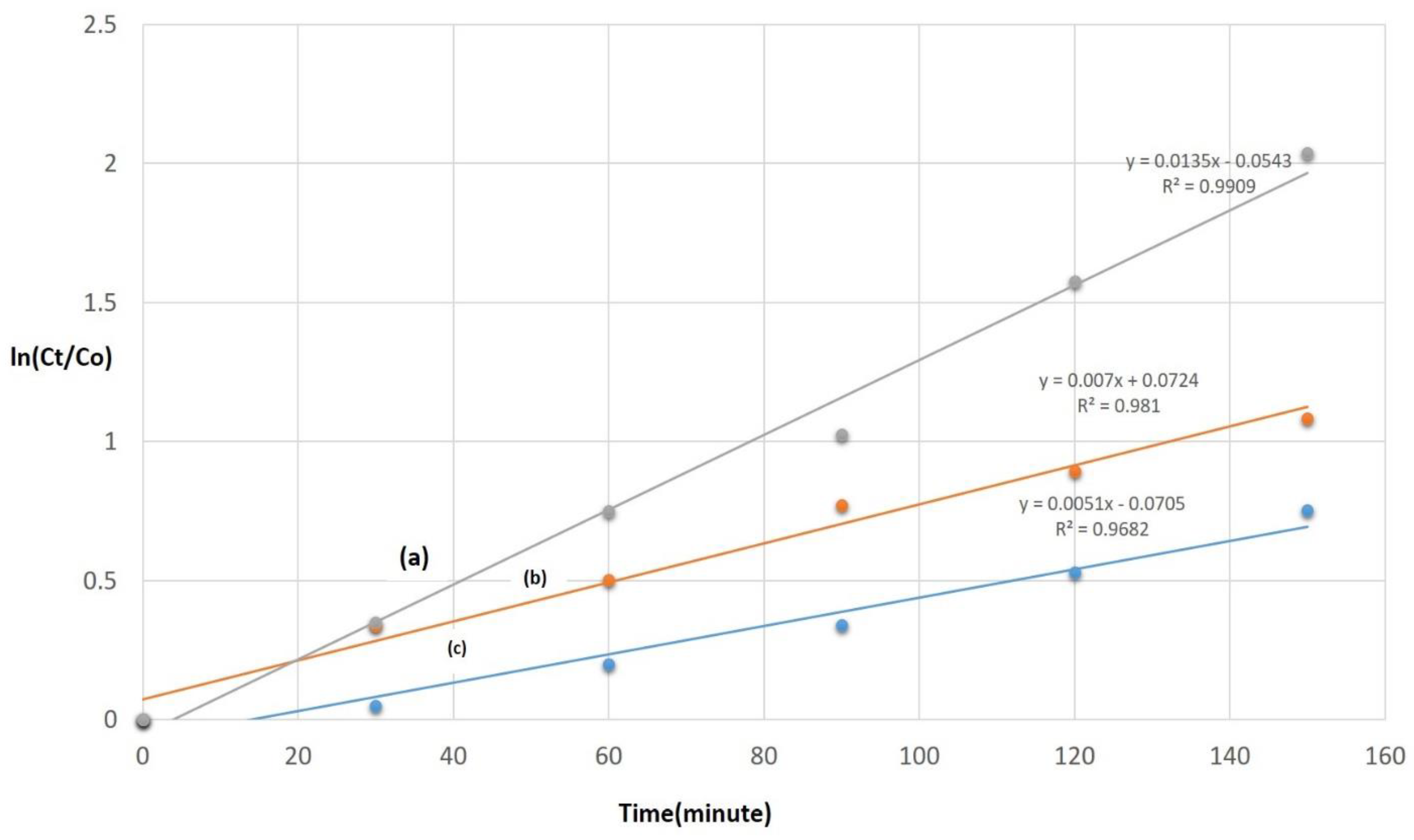
3. Result and Discussion
 e−cb + h+vb
e−cb + h+vb
 HO + H+
HO + H+
 degradation product
degradation product
 Oxidation Product
Oxidation Product
 O.2
O.2
 degradation product
degradation product
 reduction product
reduction product
3.1. Effect of Methanol
 CH2OH
CH2OH
 CH2O + H+ + e−
CH2O + H+ + e−
 CH2OH• + H2O
CH2OH• + H2O
3.2. Antifungal Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ridha, N.; Alosfur, F.; Kadhim, H.; Aboud, L.; Al-Dahan, N. Novel method to synthesis ZnO nanostructures via irradiation zinc acetate with a nanosecond laser for photocatalytic applications. J. Mater. Sci. Mater. Electron. 2020, 31, 9835–9845. [Google Scholar] [CrossRef]
- Sher, M.; Javed, M.; Shahid, S.; Hakami, O.; Qamar, M.; Iqbal, S.; AL-Anazy, M.; Baghdadi, H.; Chemistry, P. Designing of highly active g-C3N4/Sn doped ZnO heterostructure as a photocatalyst for the disinfection and degradation of the organic pollutants under visible light irradiation. J. Photochem. Photobiol. 2021, 418, 113393. [Google Scholar] [CrossRef]
- Iqbal, S.; Javed, M.; Hassan, S.; Nadeem, S.; Akbar, A.; Alotaibi, M.; Alzhrani, R.; Awwad, N.; Ibrahium, H.; Mohyuddin, A.; et al. Binary Co@ ZF/S@ GCN S-scheme heterojunction enriching spatial charge carrier separation for efficient removal of organic pollutants under sunlight irradiation. Colloids Surf. A Physicochem. Eng. Asp 2022, 636, 128177. [Google Scholar] [CrossRef]
- Owa, F. Water pollution: Sources, effects, control and management. Mediterr. J. Soc. Sci. 2013, 4, 65. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and health impacts of air pollution: A review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K. Environmental contamination by heavy metals. Heavy Met. 2018, 10, 115–132. [Google Scholar]
- Ridha, N.; Alosfur, F.; Kadhim, H.; Ahmed, L. Synthesis of Ag decorated TiO2 nanoneedles for photocatalytic degradation of methylene blue dye. Mater. Res. Express 2021, 8, 125013. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, C.; Cui, Y.; Li, D.; Zhang, Y.; Xu, J.; Li, C.; Iqbal, S.; Cao, M.; Physicochemical, S.; et al. Blue-emitting carbon quantum dots: Ultrafast microwave synthesis, purification and strong fluorescence in organic solvents. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126673. [Google Scholar] [CrossRef]
- Iqbal, M.; Iqbal, S. Synthesis of stable and highly luminescent beryllium and magnesium doped ZnS quantum dots suitable for design of photonic and sensor material. J. Lumin 2013, 134, 739–746. [Google Scholar] [CrossRef]
- Bahadur, A.; Saeed, A.; Shoaib, M.; Iqbal, S.; Anwer, S. Modulating the burst drug release effect of waterborne polyurethane matrix by modifying with polymethylmethacrylate. J. Appl. Polym. Sci. 2019, 136, 47253. [Google Scholar] [CrossRef]
- Azam, M.; Alam, M.; Hafeez, M. Effect of tourism on environmental pollution: Further evidence from Malaysia, Singapore and Thailand. J. Clean. Prod. 2018, 190, 330–338. [Google Scholar] [CrossRef]
- Amalanathan, M.; Parvathiraja, C.; Alothman, A.; Wabaidur, S.; Islam, M. Enhanced photocatalytic and biological observations of green synthesized AC, AC/Ag and AC/Ag/TiO2 nanocomposites. Res. Sq. 2021, 739706. [Google Scholar]
- Šuljagić, M.; Milenković, M.; Uskoković, V.; Mirković, M.; Vrbica, B.; Pavlović, V.; Živković-Radovanović, V.; Stanković, D.; Andjelković, L. Silver distribution and binding mode as key determinants of the antimicrobial performance of iron oxide/silver nanocomposites. Mater. Today Commun. 2022, 32, 104157. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- BinSabt, M.; Sagar, V.; Singh, J.; Rawat, M.; Shaban, M. Green synthesis of CS-TiO2 NPs for efficient photocatalytic degradation of methylene blue dye. Polymers 2022, 14, 2677. [Google Scholar] [CrossRef]
- Bai, L.; Liu, L.; Esquivel, M.; Tardy, B.; Huan, S.; Niu, X.; Liu, S.; Yang, G.; Fan, Y.; Rojas, O. Nanochitin: Chemistry, Structure. Assem. Appl. Chem. Rev. 2022, 122, 11604–11674. [Google Scholar]
- Sun, L.; Hu, D.; Zhang, Z.; Deng, X. Oxidative degradation of methylene blue via PDS-based advanced oxidation process using natural pyrite. Int. J. Environ. Res. Public Health 2019, 16, 4773. [Google Scholar] [CrossRef]
- Qamar, M.; Javed, M.; Shahid, S.; Iqbal, S.; Abubshait, S.; Abubshait, H.; Ramay, S.; Mahmood, A.; Ghaithan, H. Designing of highly active g-C3N4/Co@ ZnO ternary nanocomposites for the disinfection of pathogens and degradation of the organic pollutants from wastewater under visible light. J. Environ. Chem. Eng. 2021, 9, 105534. [Google Scholar] [CrossRef]
- Bahadur, A.; Shoaib, M.; Iqbal, S.; Saeed, A.; Rahman, M.; Channar, P.; Polymers, F. Regulating the anticancer drug release rate by controlling the composition of waterborne polyurethane. React. Funct. Polym. 2018, 131, 134–141. [Google Scholar] [CrossRef]
- Alsukaibi, A. Various approaches for the detoxification of toxic dyes in wastewater. Processes 2022, 10, 1968. [Google Scholar] [CrossRef]
- Gücek, A.; Şener, S.; Bilgen, S.; Mazmancı, M. Adsorption and kinetic studies of cationic and anionic dyes on pyrophyllite from aqueous solutions. J. Colloid Interface Sci. 2005, 286, 53–60. [Google Scholar] [CrossRef]
- Gürses, A.; Karaca, S.; Doğar, Ç.; Bayrak, R.; Açıkyıldız, M.; Yalçın, M. Determination of adsorptive properties of clay/water system: Methylene blue sorption. J. Colloid Interface Sci. 2004, 269, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Shawabkeh, R.; Tutunji, M. Experimental study and modeling of basic dye sorption by diatomaceous clay. Appl. Clay Sci. 2003, 24, 111–120. [Google Scholar] [CrossRef]
- Duruibe, J.; Ogwuegbu, M.; Egwurugwu, J. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Noreen, U.; Ahmed, Z.; Khalid, A.; Di Serafino, A.; Habiba, U.; Ali, F.; Hussain, M. Water pollution and occupational health hazards caused by the marble industries in district Mardan, Pakistan. Environ. Technol. Innov. 2019, 16, 100470. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, S.; Parakh, S.; Tong, Y. Health hazards of hexavalent chromium (Cr (VI)) and its microbial reduction. Bioengineered 2022, 13, 4923–4938. [Google Scholar] [CrossRef] [PubMed]
- Shaibur, M. Heavy metals in chrome-tanned shaving of the tannery industry are a potential hazard to the environment of Bangladesh. Case Stud. Chem. Environ. Eng. 2022, 7, 100281. [Google Scholar] [CrossRef]
- Cappelletti, G.; Bianchi, C.; Ardizzone, S. Nano-titania assisted photoreduction of Cr (VI): The role of the different TiO2 polymorphs. Appl. Catal. B Environ. 2008, 78, 193–201. [Google Scholar] [CrossRef]
- Boughriet, A.; Deram, L.; Wartel, M. Determination of dissolved chromium (III) and chromium (VI) in sea-water by electrothermal atomic absorption spectrometry. J. Anal. At. Spectrom. 1994, 9, 1135–1142. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.; Beeregowda, K. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef]
- Wen, J.; Xue, Z.; Yin, X.; Wang, X. Insights into aqueous reduction of Cr (VI) by biochar and its iron-modified counterpart in the presence of organic acids. Chemosphere 2022, 286, 131918. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, J.; Dai, D.; Zhou, Y.; Liu, X.; Yao, J. Cr-metal-organic framework coordination with ZnIn2S4 nanosheets for photocatalytic reduction of Cr (VI). J. Clean. Prod. 2022, 341, 130891. [Google Scholar] [CrossRef]
- Bradberry, S.; Vale, J. Therapeutic review: Is ascorbic acid of value in chromium poisoning and chromium dermatitis? J. Toxicol. Clin. Toxicol. 1999, 37, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, R.; Selvam, K.; Krishnakumar, B.; Swaminathan, M. An efficient reusable and antiphotocorrosive nano ZnO for the mineralization of Reactive Orange 4 under UV-A light. Sep. Purif. Technol. 2011, 80, 119–124. [Google Scholar] [CrossRef]
- Ma, C.-M.; Hong, G.-B.; Chen, H.-W.; Hang, N.-T.; Shen, Y.-S. Photooxidation contribution study on the decomposition of azo dyes in aqueous solutions by VUV-based AOPs. Int. J. Photoenergy 2011, 2011, 1–78. [Google Scholar] [CrossRef]
- Bahadur, A.; Iqbal, S.; Shoaib, M.; Saeed, A. Electrochemical study of specially designed graphene-Fe3O4-polyaniline nanocomposite as a high-performance anode for lithium-ion battery. Dalton Trans. 2018, 47, 15031–15037. [Google Scholar] [CrossRef]
- Koutavarapu, R.; Reddy, C.; Syed, K.; Reddy, K.; Saleh, T.; Lee, D.-Y.; Shim, J.; Aminabhavi, T. Novel Z-scheme binary zinc tungsten oxide/nickel ferrite nanohybrids for photocatalytic reduction of chromium (Cr (VI)), photoelectrochemical water splitting and degradation of toxic organic pollutants. J. Hazard. Mater. 2022, 423, 127044. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Chaudhuri, B.; Bhattacharjee, S.; Ray, A.; Dutta, B. Photo-reduction of hexavalent chromium in aqueous solution in the presence of zinc oxide as semiconductor catalyst. Chem. Eng. J. 2009, 153, 86–93. [Google Scholar] [CrossRef]
- Al-Hamoud, K.; Shaik, M.; Khan, M.; Alkhathlan, H.; Adil, S.; Kuniyil, M.; Assal, M.; Al-Warthan, A.; Siddiqui, M.; Tahir, M. Pulicaria undulata Extract-Mediated Eco-Friendly Preparation of TiO2 Nanoparticles for Photocatalytic Degradation of Methylene Blue and Methyl Orange. ACS Omega 2022, 7, 4812–4820. [Google Scholar] [CrossRef]
- Gaya, U.; Abdullah, A. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Mishra, S.; Chakinala, N.; Chakinala, A.; Surolia, P. Photocatalytic degradation of methylene blue using monometallic and bimetallic Bi-Fe doped TiO2. Catal. Commun. 2022, 171, 106518. [Google Scholar] [CrossRef]
- Ayed, S.B.; Sbihi, H.; Azam, M.; Al-Resayes, S.; Ayadi, M.; Ayari, F. Local iron ore identification: Comparison to synthesized Fe3O4 nanoparticles obtained by ultrasonic assisted reverse co-precipitation method for Auramine O dye adsorption. Desalination Water Treat. 2021, 220, 446–458. [Google Scholar] [CrossRef]
- Ridha, N.; Kadhim, H.; Alosfur, F.; Ahmed, R. ZnO nanofluids prepared by laser ablation in various solvents. Mater. Res. Express. 2018, 5, 125008. [Google Scholar] [CrossRef]
- Ridha, N.; Kadhim, H.; Alosfur, F.; Abood, L.; Madlol, R. Synthesis ZnO nanoparticles by LAL method using different energies of Nd-YAG laser. In AIP Conference Proceedings; AIP Publishing LLC: New York, NY, USA, 2019; p. 030026. [Google Scholar]
- Jumali, M.H.; Noor, J.; Umar, A.; Yahya, M.; Salleh, M. Characterization of SnO2 nanoparticles prepared by two different wet chemistry methods. Adv. Mater. Res. 2012, 364, 322–326. [Google Scholar] [CrossRef]
- Ulhaq, I.; Ahmad, W.; Ahmad, I.; Yaseen, M.; Ilyas, M. Engineering TiO2 supported CTAB modified bentonite for treatment of refinery wastewater through simultaneous photocatalytic oxidation and adsorption. J. Water Process Eng. 2021, 43, 102239. [Google Scholar] [CrossRef]
- Shrestha, K.; Sorensen, C.; Klabunde, K. Synthesis of CuO nanorods, reduction of CuO into Cu nanorods, and diffuse reflectance measurements of CuO and Cu nanomaterials in the near infrared region. J. Phys. Chem. C 2010, 114, 14368–14376. [Google Scholar] [CrossRef]
- Irimpan, L.; Krishnan, B.; Nampoori, V.; Radhakrishnan, P. Luminescence tuning and enhanced nonlinear optical properties of nanocomposites of ZnO–TiO2. J. Colloid Interface Sci. 2008, 324, 99–104. [Google Scholar] [CrossRef]
- Likodimos, V. Photonic crystal-assisted visible light activated TiO2 photocatalysis. Appl. Catal. B Environ. 2018, 230, 269–303. [Google Scholar] [CrossRef]
- Lee, J.-K.; Lee, Y.-J.; Chae, W.-S.; Sung, Y.-M. Enhanced ionic conductivity in PEO-LiClO 4 hybrid electrolytes by structural modification. J. Electroceramics 2006, 17, 941–944. [Google Scholar] [CrossRef]
- Fadillah, G.; Wahyuningsih, S.; Ramelan, A. Enhanced photovoltaic performance by surface modification of TiO2 nanorods with aminopropyltrimethoxysilane (APTMS). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; p. 012005. [Google Scholar]
- Lorenzetti, M.; Biglino, D.; Novak, S.; Kobe, S. Photoinduced properties of nanocrystalline TiO2-anatase coating on Ti-based bone implants. Mater. Sci. Eng. C 2014, 37, 390–398. [Google Scholar] [CrossRef]
- Thu, P.; Thinh, V.; Lam, V.; Bach, T.; Phong, L.; Tung, D.; Manh, D.; Van Khien, N.; Anh, T.; Le, N.T.H. Decorating of Ag and CuO on ZnO Nanowires by Plasma Electrolyte Oxidation Method for Enhanced Photocatalytic Efficiency. Catalysts 2022, 12, 801. [Google Scholar] [CrossRef]
- Nalbandian, M.; Zhang, M.; Sanchez, J.; Kim, S.; Choa, Y.-H.; Cwiertny, D.; Myung, N. Synthesis and optimization of Ag–TiO2 composite nanofibers for photocatalytic treatment of impaired water sources. J. Hazard. Mater. 2015, 299, 141–148. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Basnet, P.; Murph, S.; Zhao, Y. Ag nanoparticle embedded TiO2 composite nanorod arrays fabricated by oblique angle deposition: Toward plasmonic photocatalysis. ACS Appl. Mater. Interfaces 2013, 5, 11818–11827. [Google Scholar] [CrossRef]
- ZMa; Guo, Q.; Mao, X.; Ren, Z.; Wang, X.; Xu, C.; Yang, W.; Dai, D.; Zhou, C.; Fan, H. Photocatalytic dissociation of ethanol on TiO2 (110) by near-band-gap excitation. J. Phys. Chem. C 2013, 117, 10336–10344. [Google Scholar]
- Wu, L.; Pei, X.; Mei, M.; Li, Z.; Lu, S. Study on Photocatalytic Performance of Ag/TiO2 Modified Cement Mortar. Materials 2022, 15, 4031. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Feng, Y.; Li, H.; Chen, Y.; Wen, J.; Zhu, J.; Bian, Z. Microwave induced surface enhanced pollutant adsorption and photocatalytic degradation on Ag/TiO2. Appl. Surf. Sci. 2019, 483, 772–778. [Google Scholar] [CrossRef]
- Chakhtouna, H.; Benzeid, H.; Zari, N.; Qaiss, A.; Bouhfid, R. Recent progress on Ag/TiO2 photocatalysts: Photocatalytic and bactericidal behaviors. Environ. Sci. Pollut. Res. 2021, 28, 44638–44666. [Google Scholar] [CrossRef]
- Shan, R.; Lu, L.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y.; Luo, B. Photocatalytic degradation of methyl orange by Ag/TiO2/biochar composite catalysts in aqueous solutions. Mater. Sci. Semicond. Process. 2020, 114, 105088. [Google Scholar] [CrossRef]
- Jiang, L.; Zhou, G.; Mi, J.; Wu, Z. Fabrication of visible-light-driven one-dimensional anatase TiO2/Ag heterojunction plasmonic photocatalyst. Catal. Commun. 2012, 24, 48–51. [Google Scholar] [CrossRef]
- Albiter, E.; Valenzuela, M.; Alfaro, S.; Valverde-Aguilar, G.; Martínez-Pallares, F. Photocatalytic deposition of Ag nanoparticles on TiO2: Metal precursor effect on the structural and photoactivity properties. J. Saudi Chem. Soc. 2015, 19, 563–573. [Google Scholar] [CrossRef]
- Avciata, O.; Benli, Y.; Gorduk, S.; Koyun, O. Ag doped TiO2 nanoparticles prepared by hydrothermal method and coating of the nanoparticles on the ceramic pellets for photocatalytic study: Surface properties and photoactivity. J. Eng. Technol. Appl. Sci. 2016, 1, 34–50. [Google Scholar] [CrossRef]
- Al-Mamun, M.; Karim, M.; Nitun, N.; Kader, S.; Islam, M.; Khan, M. Photocatalytic performance assessment of GO and Ag co-synthesized TiO2 nanocomposite for the removal of methyl orange dye under solar irradiation. Environ. Technol. Innov. 2021, 22, 101537. [Google Scholar] [CrossRef]
- Desai, M.; Patil, R.; Pawar, K. Selective and sensitive colorimetric detection of platinum using Pseudomonas stutzeri mediated optimally synthesized antibacterial silver nanoparticles. Biotechnol. Rep. 2020, 25, e00404. [Google Scholar] [CrossRef] [PubMed]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase characterization of TiO2 powder by XRD and TEM. Agric. Nat. Resour. 2008, 42, 357–361. [Google Scholar]
- Ghanbari, S.; Givianrad, M.; Azar, P.A. Simultaneous reduction of Cr (VI) and degradation of azo dyes by F-Fe-codoped TiO2/SiO2 photocatalysts under visible and solar irradiation. Can. J. Chem. 2019, 97, 659–671. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Banerjee, P.; Mitra, P. Solar photocatalytic reduction of hexavalent chromium in wastewater using zinc oxide semiconductor catalyst: A comparison of performances between micro and nanoparticles. In Physical Chemical and Biological Treatment Processes for Water and Wastewater; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; p. 95À111. [Google Scholar]
- Tan, T.; Beydoun, D.; Amal, R. Effects of organic hole scavengers on the photocatalytic reduction of selenium anions. J. Photochem. Photobiol. A Chem. 2003, 159, 273–280. [Google Scholar] [CrossRef]
- Qamar, M.; Gondal, M.; Yamani, Z. Laser-induced efficient reduction of Cr (VI) catalyzed by ZnO nanoparticles. J. Hazard. Mater. 2011, 187, 258–263. [Google Scholar] [CrossRef]
- Guzman, F.; Chuang, S.; Yang, C. Role of methanol sacrificing reagent in the photocatalytic evolution of hydrogen. Ind. Eng. Chem. Res. 2013, 52, 61–65. [Google Scholar] [CrossRef]

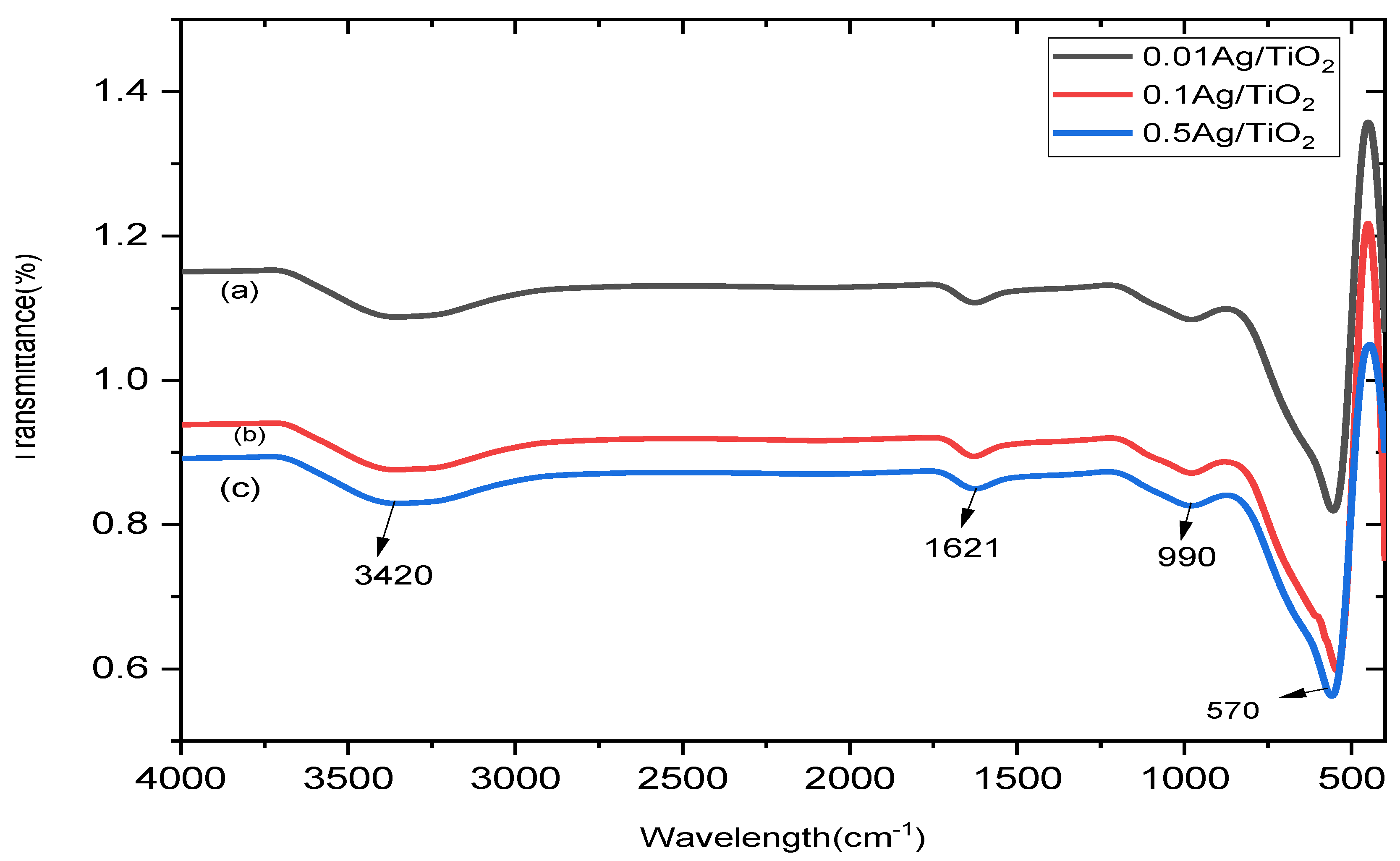
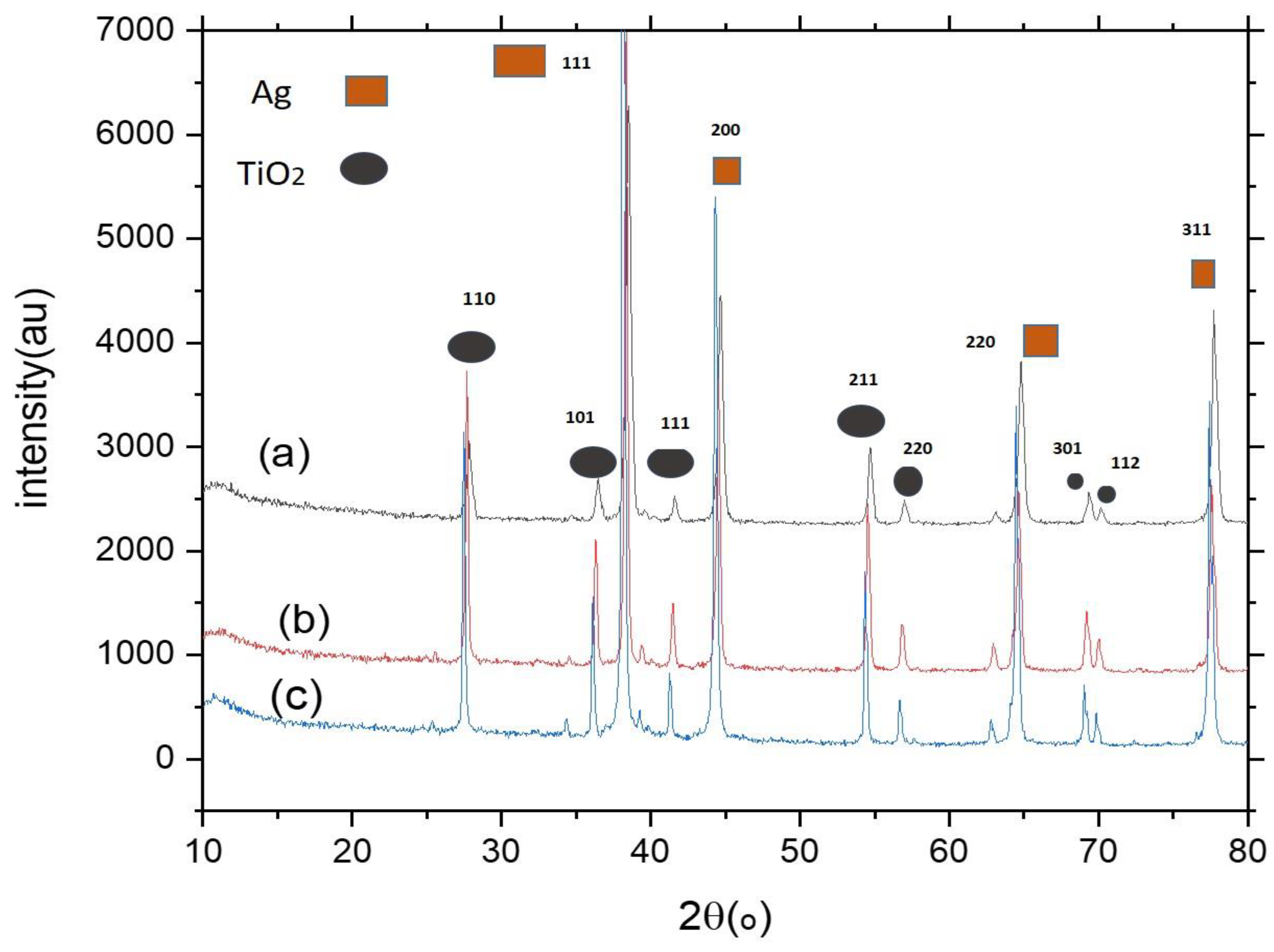
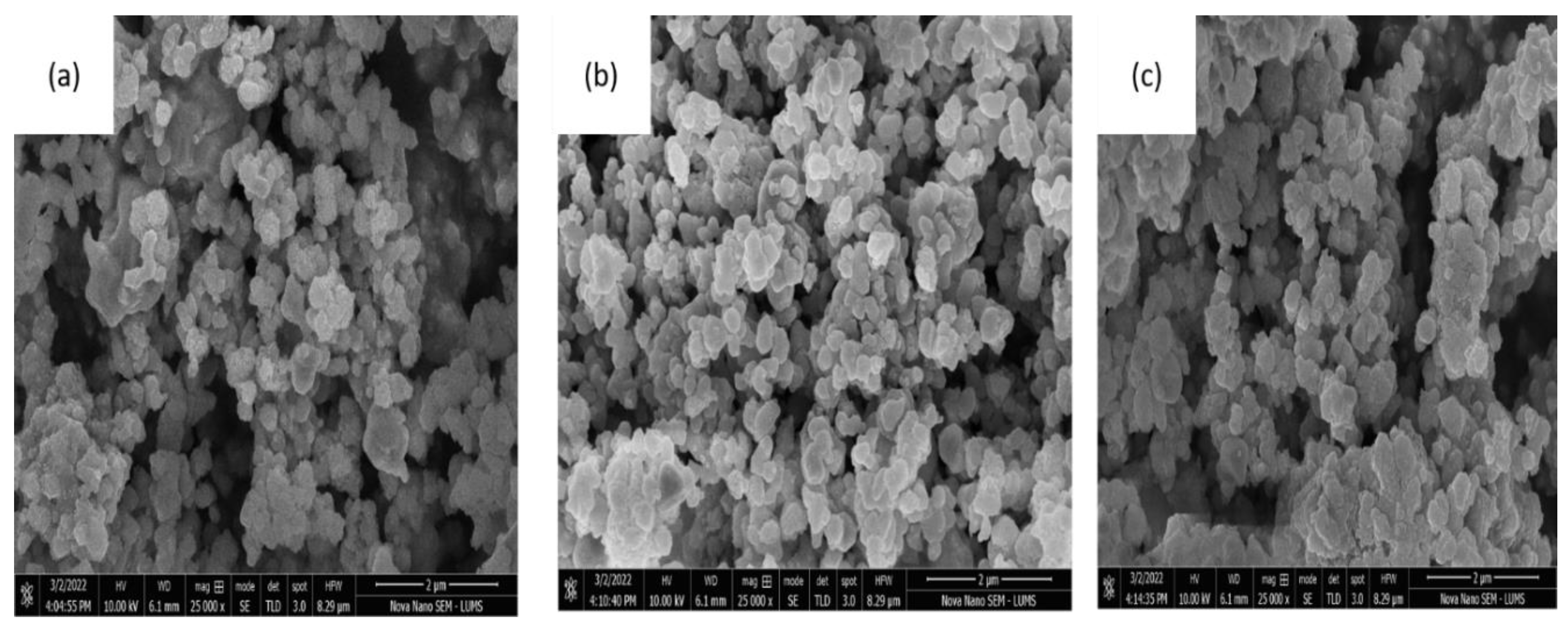

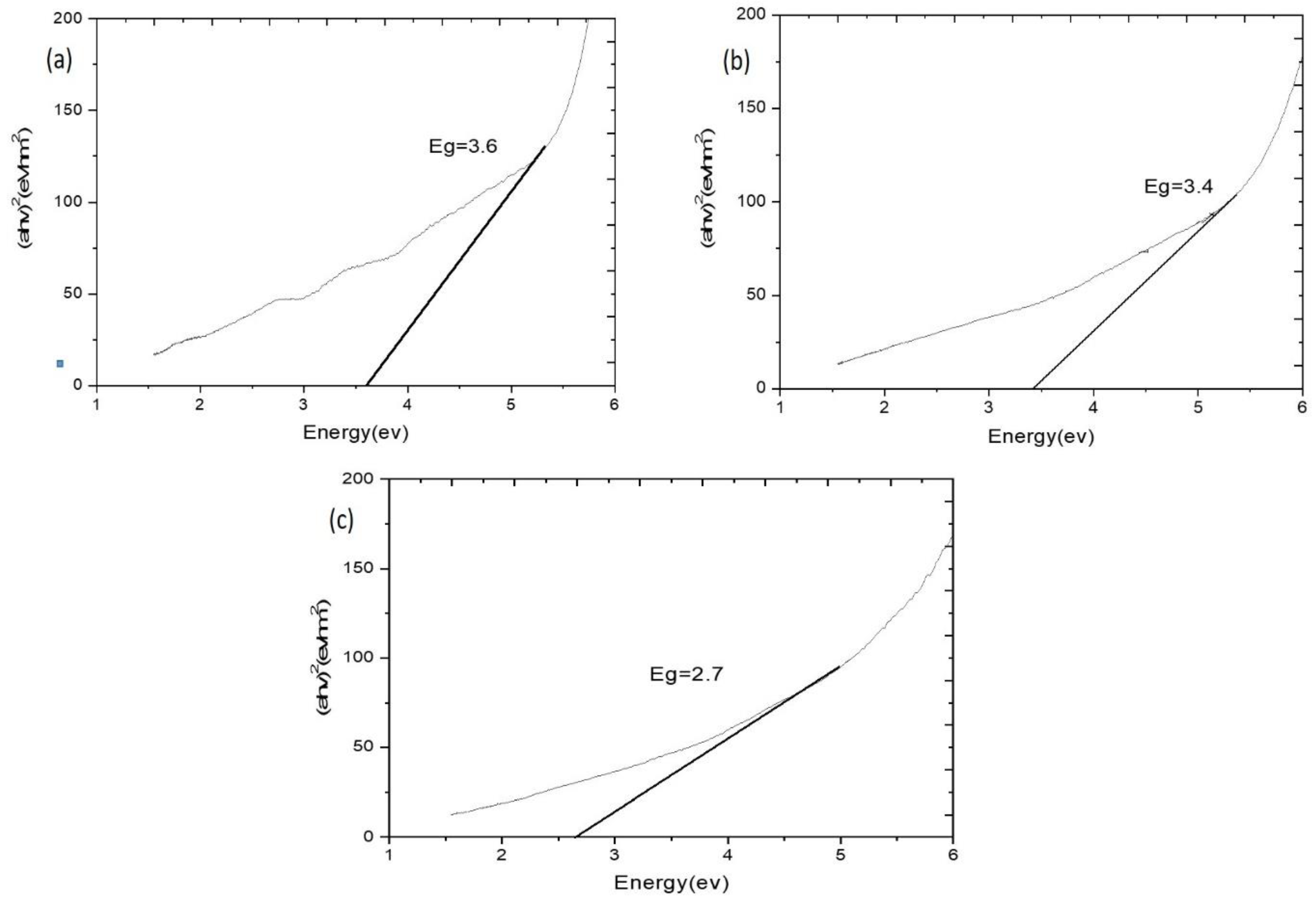


| Composite | Element | Line Type | Apparent Concentration | k Ratio | Wt% | Wt% Sigma | Standard Lable | Factory Standard |
|---|---|---|---|---|---|---|---|---|
| (a) | O | K series | 10.63 | 0.0357 | 25.65 | 0.52 | O2 | Yes |
| Ti | K series | 14.32 | 0.1432 | 21.19 | 0.32 | Ti | Yes | |
| Ag | L series | 25.29 | 0.2529 | 37.96 | 0.53 | Ag | Yes | |
| Au | M series | 2.42 | 0.0242 | 4.03 | 0.24 | Au | Yes | |
| (b) | O | K series | 10.69 | 0.0359 | 26.53 | 0.55 | O2 | Yes |
| Ti | K series | 17.00 | 0.1700 | 25.58 | 0.33 | Ti | Yes | |
| Ag | L series | 22.69 | 0.2268 | 34.87 | 0.46 | Ag | Yes | |
| Au | M series | 2.75 | 0.0275 | 4.69 | 0.28 | Au | Yes | |
| (c) | O | K series | 11.58 | 0.0389 | 27.23 | 0.46 | O2 | Yes |
| Ti | K series | 14.57 | 0.1456 | 21.34 | 0.28 | Ti | Yes | |
| Ag | L series | 24.79 | 0.2479 | 36.89 | 0.46 | Ag | Yes | |
| Au | M series | 2.43 | 0.0242 | 4.01 | 0.21 | Au | Yes |
| Ag/TiO2 Composites | R2 | Kapp | Degradation |
|---|---|---|---|
| 0.01 Ag/TiO2 | 0.0090 | 0.0135 | 90% |
| 0.1 Ag/TiO2 | 0.981 | 0.007 | 68% |
| 0.5 Ag/TiO2 | 0.9682 | 0.0051 | 54% |
| Ag/TiO2 Composites | R2 | Kapp | Reduction |
|---|---|---|---|
| 0.01 Ag/TiO2 | 0.8965 | 0.0259 | 75% |
| 0.1 Ag/TiO2 | 0.8708 | 0.014 | 54% |
| 0.5 Ag/TiO2 | 0.9243 | 0.0166 | 50% |
| Antifungal Performance | |||
|---|---|---|---|
| Bacterial Strains | Samples | Blank | Zone of Inhibition (mm) |
| S. macrospora | 0.01 Ag/TiO2 | 0 | 38.4 |
| 0.1 Ag/TiO2 | 0 | 30.6 | |
| 0.5 Ag/TiO2 | 0 | 27.8 | |
| S. maydis | 0.01 Ag/TiO2 | 0 | 34.3 |
| 0.1 Ag/TiO2 | 0 | 29.4 | |
| 0.5 Ag/TiO2 | 0 | 27.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahid, Z.; Rauf, A.; Javed, M.; Alhujaily, A.; Iqbal, S.; Amjad, A.; Arif, M.; Hussain, S.; Bahadur, A.; Awwad, N.S.; et al. Photocatalytic Reduction of Cr(VI) to Cr(III) and Photocatalytic Degradation of Methylene Blue and Antifungal Activity of Ag/TiO2 Composites Synthesized via the Template Induced Route. Inorganics 2023, 11, 133. https://doi.org/10.3390/inorganics11030133
Zahid Z, Rauf A, Javed M, Alhujaily A, Iqbal S, Amjad A, Arif M, Hussain S, Bahadur A, Awwad NS, et al. Photocatalytic Reduction of Cr(VI) to Cr(III) and Photocatalytic Degradation of Methylene Blue and Antifungal Activity of Ag/TiO2 Composites Synthesized via the Template Induced Route. Inorganics. 2023; 11(3):133. https://doi.org/10.3390/inorganics11030133
Chicago/Turabian StyleZahid, Zunaira, Abdul Rauf, Mohsin Javed, Ahmad Alhujaily, Shahid Iqbal, Adnan Amjad, Muhammad Arif, Sajjad Hussain, Ali Bahadur, Nasser S. Awwad, and et al. 2023. "Photocatalytic Reduction of Cr(VI) to Cr(III) and Photocatalytic Degradation of Methylene Blue and Antifungal Activity of Ag/TiO2 Composites Synthesized via the Template Induced Route" Inorganics 11, no. 3: 133. https://doi.org/10.3390/inorganics11030133
APA StyleZahid, Z., Rauf, A., Javed, M., Alhujaily, A., Iqbal, S., Amjad, A., Arif, M., Hussain, S., Bahadur, A., Awwad, N. S., Ibrahium, H. A., Al-Fawzan, F. F., & Elkaeed, E. B. (2023). Photocatalytic Reduction of Cr(VI) to Cr(III) and Photocatalytic Degradation of Methylene Blue and Antifungal Activity of Ag/TiO2 Composites Synthesized via the Template Induced Route. Inorganics, 11(3), 133. https://doi.org/10.3390/inorganics11030133









