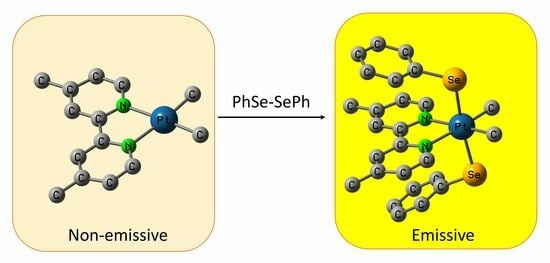
Luminescent Diimine-Pt(IV) Complexes with Axial Phenyl Selenide Ligands
Abstract
1. Introduction
2. Results and Discussion
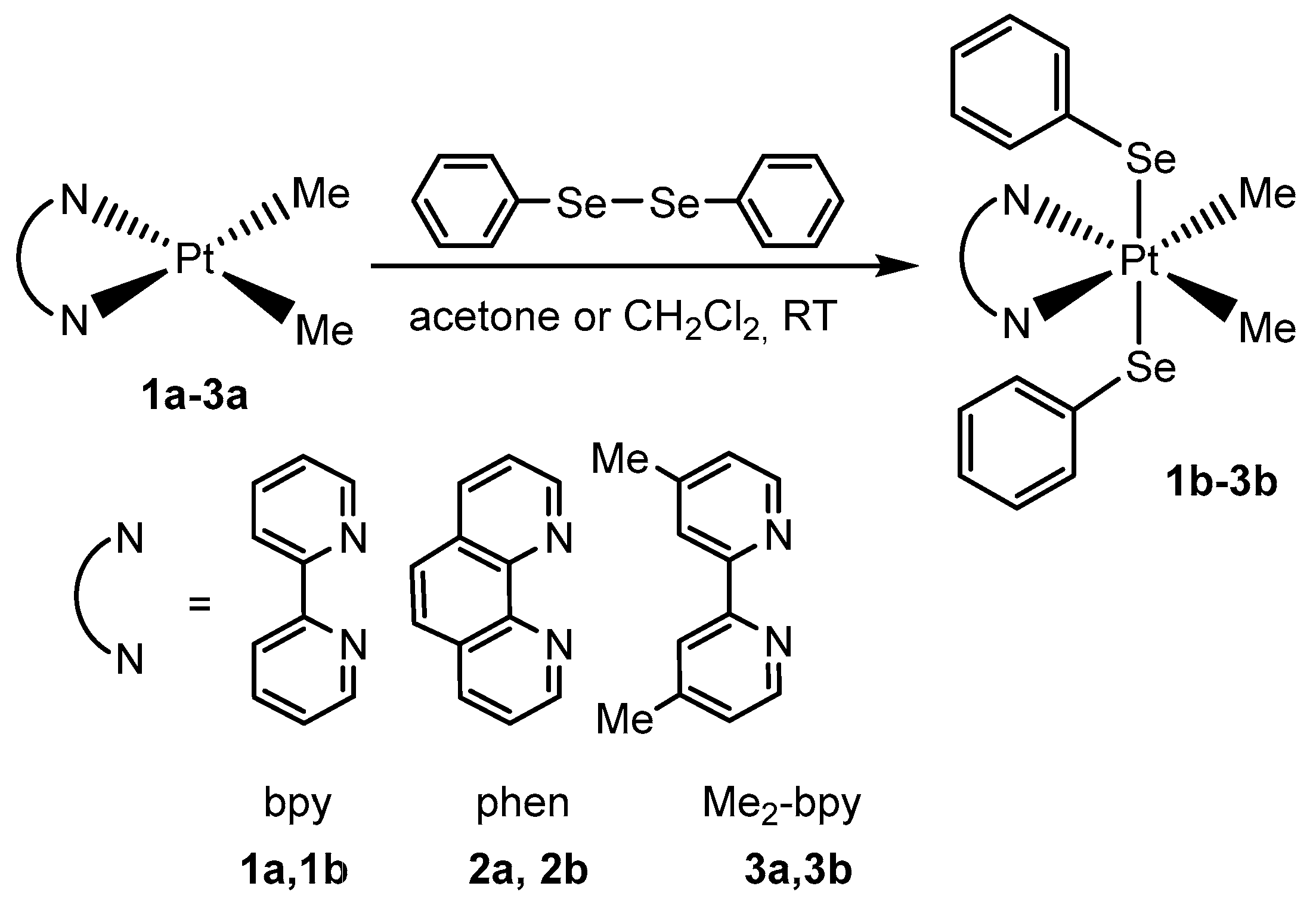
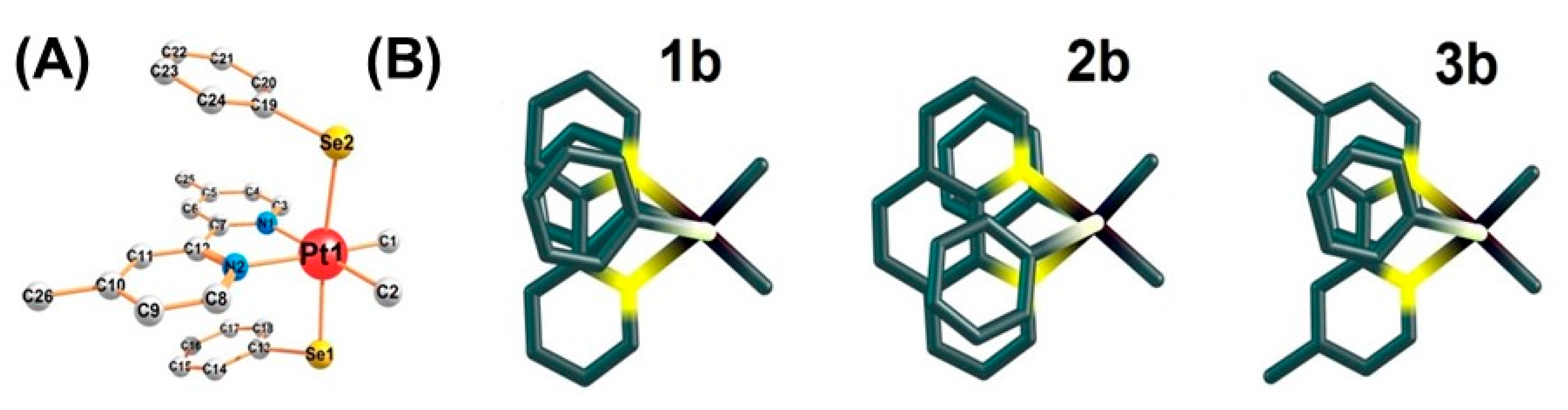
2.1. Synthesis, Analytical, and Structural Characterisation
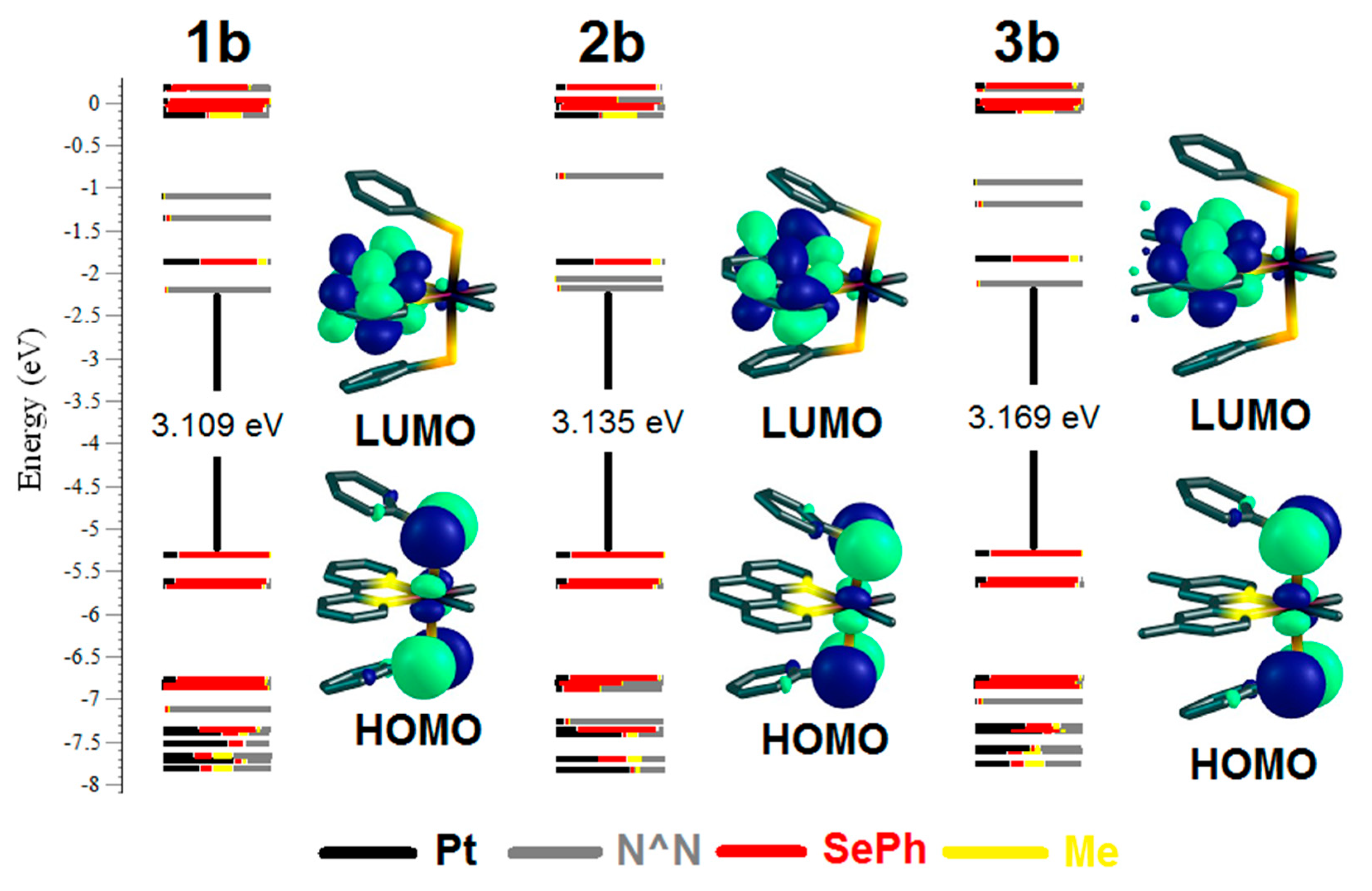
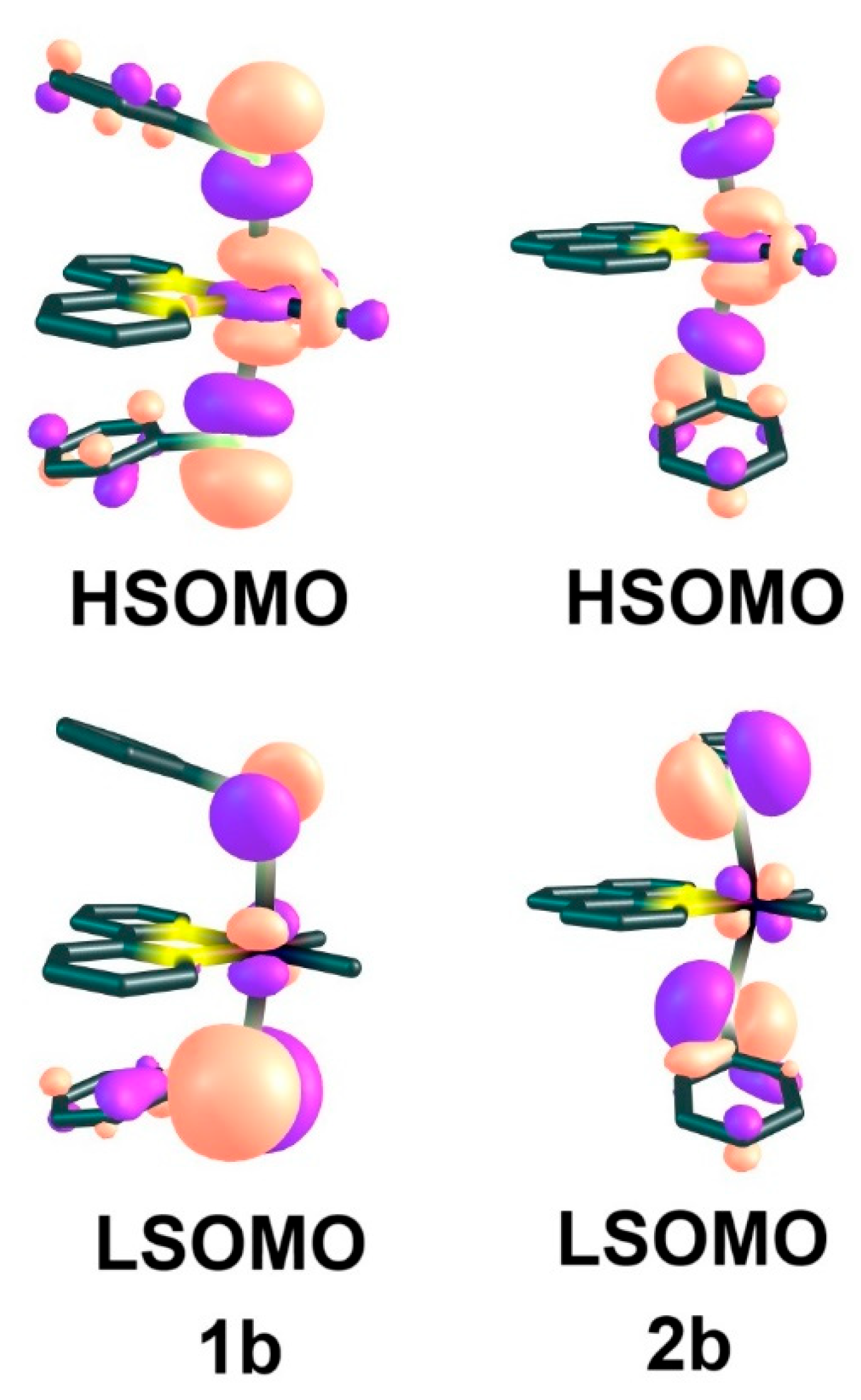
2.2. UV-Vis Absorption Spectra and TD-DFT Calculations
2.3. Photoluminescence Spectroscopy
3. Methods and Materials
3.1. General Procedures and Materials
3.2. Synthesis of [Pt(Me2bpy)(Me)2(PhSe)2] 3b
3.3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Y.-C.; Wang, S.F.; Hu, Y.; Liao, L.-S.; Chen, D.-G.; Chang, K.-H.; Wang, C.-W.; Liu, S.-H.; Chan, W.-H.; Liao, J.-L. Overcoming the energy gap law in near-infrared OLEDs by exciton-vibration decoupling. Nat. Photonics 2020, 14, 570–577. [Google Scholar] [CrossRef]
- Mills, I.N.; Porras, J.A.; Bernhard, S. Judicious design of cationic, cyclometalated Ir(III) complexes for photochemical energy conversion and optoelectronics. Acc. Chem. Res. 2018, 51, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Fleetham, T.; Li, G.; Li, J. Phosphorescent Pt(II) and Pd(II) complexes for efficient, high-color-quality, and stable OLEDs. Adv. Mater. 2017, 29, 1601861. [Google Scholar] [CrossRef] [PubMed]
- Prokhorov, A.M.; Hofbeck, T.; Czerwieniec, R.; Suleymanova, A.F.; Kozhevnikov, D.N.; Yersin, H. Brightly luminescent Pt(II) pincer complexes with a sterically demanding carboranyl-phenylpyridine ligand: A new material class for diverse optoelectronic applications. J. Am. Chem. Soc. 2014, 136, 9637–9642. [Google Scholar] [CrossRef] [PubMed]
- Büyükekşi, S.I.; Şengül, A.; Erdönmez, S.; Altındal, A.; Orman, E.B.; Özkaya, A.R. Spectroscopic, electrochemical and photovoltaic properties of Pt(II) and Pd(II) complexes of a chelating 1,10-phenanthroline appended perylene diimide. Dalton Trans. 2018, 47, 2549–2560. [Google Scholar] [CrossRef]
- Goswami, S.; Hernandez, J.L.; Gish, M.K.; Wang, J.; Kim, B.; Laudari, A.P.; Guha, S.; Papanikolas, J.M.; Reynolds, J.R.; Schanze, K.S. Cyclometalated platinum-containing diketopyrrolopyrrole complexes and polymers: Photophysics and photovoltaic applications. Chem. Mater. 2017, 29, 8449–8461. [Google Scholar] [CrossRef]
- Wenger, O. Vapochromism in organometallic and coordination complexes: Chemical sensors for volatile organic compounds. Chem. Rev. 2013, 113, 3686–3733. [Google Scholar] [CrossRef]
- De los Santos, Z.A.; Wolf, C. Optical Terpene and Terpenoid Sensing: Chiral Recognition, Determination of Enantiomeric Composition and Total Concentration Analysis with Late Transition Metal Complexes. J. Am. Chem. Soc. 2020, 142, 4121–4125. [Google Scholar] [CrossRef]
- Choi, W.J.; Choi, S.; Ohkubo, K.; Fukuzumi, S.; Cho, E.J.; You, Y. Mechanisms and applications of cyclometalated Pt(II) complexes in photoredox catalytic trifluoromethylation. Chem. Sci. 2015, 6, 1454–1464. [Google Scholar] [CrossRef]
- Lang, P.; Habermehl, J.; Troyanov, S.I.; Rau, S.; Schwalbe, M. Photocatalytic Generation of Hydrogen Using Dinuclear π-Extended Porphyrin–Platinum Compounds. Chem.-Eur. J. 2018, 24, 3225–3233. [Google Scholar] [CrossRef]
- Zhong, J.-J.; To, W.-P.; Liu, Y.; Lu, W.; Che, C.-M. Efficient acceptorless photo-dehydrogenation of alcohols and N-heterocycles with binuclear platinum(II) diphosphite complexes. Chem. Sci. 2019, 10, 4883–4889. [Google Scholar] [CrossRef] [PubMed]
- Martynova, E.A.; Voloshkin, V.A.; Guillet, S.G.; Bru, F.; Beliš, M.; Van Hecke, K.; Cazin, C.S.; Nolan, S.P. Energy transfer (EnT) photocatalysis enabled by gold-N-heterocyclic carbene (NHC) complexes. Chem. Sci. 2022, 13, 6852–6857. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-N.; Li, G.; Leung, C.-H.; Ma, D.-L. Dual function luminescent transition metal complexes for cancer theranostics: The combination of diagnosis and therapy. Coord. Chem. Rev. 2019, 381, 79–103. [Google Scholar] [CrossRef]
- Yip, A.M.-H.; Lo, K.K.-W. Luminescent rhenium(I), ruthenium(II), and iridium(III) polypyridine complexes containing a poly(ethylene glycol) pendant or bioorthogonal reaction group as biological probes and photocytotoxic agents. Coord. Chem. Rev. 2018, 361, 138–163. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, J. Responsive metal complex probes for time-gated luminescence biosensing and imaging. Acc. Chem. Res. 2020, 53, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.; Wang, Y.; Ur Rehman, F.; Jiang, H.; Wang, X. Phosphorescent Ir(III) complexes as cellular staining agents for biomedical molecular imaging. Coord. Chem. Rev. 2020, 416, 213344. [Google Scholar] [CrossRef]
- Fernández-Moreira, V.; Thorp-Greenwood, F.L.; Coogan, M.P. Application of d6 transition metal complexes in fluorescence cell imaging. Chem. Commun. 2010, 46, 186–202. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Yu, Q.; Wei, H.; Liu, S.; Zhao, Q.; Huang, W. Long-lived emissive probes for time-resolved photoluminescence bioimaging and biosensing. Chem. Rev. 2018, 118, 1770–1839. [Google Scholar] [CrossRef]
- Li, K.; Chen, Y.; Wang, J.; Yang, C. Diverse emission properties of transition metal complexes beyond exclusive single phosphorescence and their wide applications. Coord. Chem. Rev. 2021, 433, 213755. [Google Scholar] [CrossRef]
- Hu, J.; Nikravesh, M.; Shahsavari, H.R.; Babadi Aghakhanpour, R.; Rheingold, A.L.; Alshami, M.; Sakamaki, Y.; Beyzavi, H. A C^N Cycloplatinated(II) Fluoride Complex: Photophysical Studies and Csp3–F Bond Formation. Inorg. Chem. 2020, 59, 16319–16327. [Google Scholar] [CrossRef]
- Zanoni, K.P.S.; Coppo, R.L.; Amaral, R.C.; Iha, N.Y.M. Ir(III) complexes designed for light-emitting devices: Beyond the luminescence color array. Dalton Trans. 2015, 44, 14559–14573. [Google Scholar] [CrossRef] [PubMed]
- Buil, M.L.; Esteruelas, M.A.; López, A.M. Recent Advances in Synthesis of Molecular Heteroleptic Osmium and Iridium Phosphorescent Emitters. Eur. J. Inorg. Chem. 2021, 2021, 4731–4761. [Google Scholar] [CrossRef]
- Giménez, N.; Lalinde, E.; Lara, R.; Moreno, M.T. Design of luminescent, heteroleptic, cyclometalated PtII and PtIV complexes: Photophysics and effects of the cyclometalated ligands. Chem.-Eur. J. 2019, 25, 5514–5526. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.R.; Lalinde, E.; Moreno, M.T. Luminescent cyclometalated-pentafluorophenyl PtII, PtIV and heteropolynuclear complexes. Coord. Chem. Rev. 2018, 366, 69–90. [Google Scholar] [CrossRef]
- Kunkely, H.; Vogler, A. Electronic spectra and photochemistry of methyl platinum(IV) complexes. Coord. Chem. Rev. 1991, 111, 15–25. [Google Scholar] [CrossRef]
- Chassot, L.; Von Zelewsky, A.; Sandrini, D.; Maestri, M.; Balzani, V. Photochemical preparation of luminescent platinum(IV) complexes via oxidative addition on luminescent platinum (II) complexes. J. Am. Chem. Soc. 1986, 108, 6084–6085. [Google Scholar] [CrossRef]
- Jenkins, D.M.; Bernhard, S. Synthesis and Characterization of Luminescent Bis-Cyclometalated PlatinumIV Complexes. Inorg. Chem. 2010, 49, 11297–11308. [Google Scholar] [CrossRef]
- Katlenok, E.A.; Balashev, K.P. Complexes of Rh(III), Ir(III), and Pt(IV) with Metallated 2-(n-Tolyl)pyridine, Ethylendiamine, Acetate, and Diethyldithiocarbamate Ligands. Russ. J. Gen. Chem. 2014, 84, 927–933. [Google Scholar] [CrossRef]
- Juliá, F.; Bautista, D.; González-Herrero, P. Developing strongly luminescent platinum(IV) complexes: Facile synthesis of bis-cyclometalated neutral emitters. Chem. Commun. 2016, 52, 1657–1660. [Google Scholar] [CrossRef]
- Juliá, F.; García-Legaz, M.-D.; Bautista, D.; González-Herrero, P. Influence of Ancillary Ligands and Isomerism on the Luminescence of Bis-cyclometalated Platinum(IV) Complexes. Inorg. Chem. 2016, 55, 7647–7660. [Google Scholar] [CrossRef]
- Giménez, N.; Lara, R.; Moreno, M.T.; Lalinde, E. Facile Approaches to Phosphorescent Bis(cyclometalated) Pentafluorophenyl PtIV Complexes: Photophysics and Computational Studies. Chem.-Eur. J. 2017, 23, 5758–5771. [Google Scholar] [CrossRef]
- Vivancos, Á.; Poveda, D.; Muñoz, A.; Moreno, J.; Bautista, D.; González-Herrero, P. Selective synthesis, reactivity and luminescence of unsymmetrical bis-cyclometalated Pt(IV) complexes. Dalton Trans. 2019, 48, 14367–14382. [Google Scholar] [CrossRef] [PubMed]
- Juliá, F.; Bautista, D.; Fernández-Hernández, J.M.; González-Herrero, P. Homoleptic tris-cyclometalated platinum(IV) complexes: A new class of long-lived, highly efficient 3LC emitters. Chem. Sci. 2014, 5, 1875–1880. [Google Scholar] [CrossRef]
- Juliá, F.; Aullón, G.; Bautista, D.; González-Herrero, P. Exploring Excited-State Tunability in Luminescent Tris-cyclometalated Platinum(IV) Complexes: Synthesis of Heteroleptic Derivatives and Computational Calculations. Chem.-Eur. J. 2014, 20, 17346–17359. [Google Scholar] [CrossRef] [PubMed]
- Juliá, F.; González-Herrero, P. Spotlight on the ligand: Luminescent cyclometalated Pt(IV) complexes containing a fluorenyl moiety. Dalton Trans. 2016, 45, 10599–10608. [Google Scholar] [CrossRef] [PubMed]
- López-López, J.C.; Bautista, D.; González-Herrero, P. Stereoselective Formation of Facial Tris-Cyclometalated PtIV Complexes: Dual Phosphorescence from Heteroleptic Derivatives. Chem.-Eur. J. 2020, 26, 11307–11315. [Google Scholar] [CrossRef]
- Julia, F.; González-Herrero, P. Aromatic C–H activation in the triplet excited state of cyclometalated platinum(II) complexes using visible light. J. Am. Chem. Soc. 2016, 138, 5276–5282. [Google Scholar] [CrossRef]
- Poveda, D.; Vivancos, A.; Bautista, D.; Gonzalez-Herrero, P. Visible light driven generation and alkyne insertion reactions of stable bis-cyclometalated Pt(IV) hydrides. Chem. Sci. 2020, 11, 12095–12102. [Google Scholar] [CrossRef]
- Vivancos, A.; Jiménez-García, A.; Bautista, D.; González-Herrero, P. Strongly Luminescent Pt(IV) Complexes with a Mesoionic N-Heterocyclic Carbene Ligand: Tuning Their Photophysical Properties. Inorg. Chem. 2021, 60, 7900–7913. [Google Scholar] [CrossRef]
- López-López, J.-C.; Bautista, D.; González-Herrero, P. Phosphorescent biaryl platinum(IV) complexes obtained through double metalation of dibenzoiodolium ions. Chem. Commun. 2022, 58, 4532–4535. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, F.; You, C.; Yang, S.; Xiong, L.; Xiong, W.; Zhu, W.; Wang, Y.; Pei, Y.; Su, S. Efficient near-infrared emitting tetradentate bis-cyclometalated platinum(IV) complexes for solution-processed polymer light emitting diodes. Dyes Pigment. 2017, 142, 457–464. [Google Scholar] [CrossRef]
- Ionescu, A.; Godbert, N.; Aiello, I.; Ricciardi, L.; La Deda, M.; Crispini, A.; Sicilia, E.; Ghedini, M. Anionic cyclometalated Pt(II) and Pt(IV) complexes respectively bearing one or two 1,2-benzenedithiolate ligands. Dalton Trans. 2018, 47, 11645–11657. [Google Scholar] [CrossRef]
- Lalinde, E.; Lara, R.; López, I.P.; Moreno, M.T.; Alfaro-Arnedo, E.; Pichel, J.G.; Piñeiro-Hermida, S. Benzothiazole-Based Cycloplatinated Chromophores: Synthetic, Optical, and Biological Studies. Chem.-Eur. J. 2018, 24, 2440–2456. [Google Scholar] [CrossRef]
- Monsour, C.G.; Decosto, C.M.; Tafolla-Aguirre, B.J.; Morales, L.A.; Selke, M. Singlet Oxygen Generation, Quenching and Reactivity with Metal Thiolates. Photochem. Photobiol. 2021, 97, 1219–1240. [Google Scholar] [CrossRef] [PubMed]
- Mackay, F.S.; Farrer, N.J.; Salassa, L.; Tai, H.-C.; Deeth, R.J.; Moggach, S.A.; Wood, P.A.; Parsons, S.; Sadler, P.J. Synthesis, characterisation and photochemistry of PtIV pyridyl azido acetato complexes. Dalton Trans. 2009, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Harkins, S.B.; Peters, J.C. Unexpected Photoisomerization of a Pincer-type Amido Ligand Leads to Facial Coordination at Pt(IV). Inorg. Chem. 2006, 45, 4316–4318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van Slageren, J.; Klein, A.; Zalis, S. Ligand-to-ligand charge transfer states and photochemical bond homolysis in metal-carbon bonded platinum complexes. Coord. Chem. Rev. 2002, 230, 193–211. [Google Scholar] [CrossRef]
- Kaim, W.; Klein, A.; Hasenzahl, S.; Stoll, H.; Zalis, S.; Fiedler, J. Reactions of New Organoplatinum(II) and -(IV) Complexes of 1,4-Diaza-1,3-butadienes with Light and Electrons. Emission vs Photochemistry and the Electronic Structures of Ground, Reduced, Oxidized, and Low-Lying Charge-Transfer Excited States. Organometallics 1998, 17, 237–247. [Google Scholar] [CrossRef]
- Hux, J.E.; Puddephatt, R.J. Photochemistry of mononuclear and binuclear tetramethylplatinum(IV) complexes: Reactivity of an organometallic free radical. J. Organomet. Chem. 1992, 437, 251–263. [Google Scholar] [CrossRef]
- Hux, J.E.; Puddephatt, R.J. Photochemistry of tetramethyl(2,2′-bipyridine)platinum(IV). J. Organomet. Chem. 1988, 346, C31–C34. [Google Scholar] [CrossRef]
- Steel, H.L.; Allinson, S.L.; Andre, J.; Coogan, M.P.; Platts, J.A. Platinum trimethyl bipyridyl thiolates—New, tunable, red-to near IR emitting luminophores for bioimaging applications. Chem. Commun. 2015, 51, 11441–11444. [Google Scholar] [CrossRef][Green Version]
- Mala, B.; Murtagh, L.E.; Farrow, C.M.A.; Akien, G.R.; Halcovich, N.R.; Allinson, S.L.; Platts, J.A.; Coogan, M.P. Photochemical Oxidation of Pt(IV)Me3(1,2-diimine) Thiolates to Luminescent Pt(IV) Sulfinates. Inorg. Chem. 2021, 60, 7031–7043. [Google Scholar] [CrossRef]
- Taylor, S.D.; Shingade, V.M.; Muvirimi, R.; Hicks, S.D.; Krause, J.A.; Connick, W.B. Spectroscopic Characterization of Platinum(IV) Terpyridyl Complexes. Inorg. Chem. 2019, 58, 16364–16371. [Google Scholar] [CrossRef]
- Dogan, A.; Kavakli, C.; Sieger, M.; Niemeyer, M.; Sarkar, B.; Kaim, W. Structural and spectroscopic evidence for marginal metal-to-ligand charge transfer in complexes Pt(abpy)Me3X, abpy = 2,2′-azobispyridine, X = Cl, Br, I.Z. Anorg. Allgem. Chem. 2008, 634, 2527–2531. [Google Scholar] [CrossRef]
- Van Slageren, J.; Stufkens, D.J.; Zális, S.; Klein, A. Influence of the variation of the co-ligands on the electronic transitions and emission properties of [Pt(I)(CH3)3(iPr-DAB)], [Pt(CH3)4(α-diimine)] and [Pt(SnPh3)2(CH3)2(iPr-DAB)]: An experimental and theoretical study. J. Chem. Soc. Dalton Trans. 2002, 218–225. [Google Scholar] [CrossRef]
- Bonnington, K.J.; Jennings, M.C.; Puddephatt, R.J. Oxidative Addition of S–S Bonds to Dimethylplatinum(II) Complexes: Evidence for a Binuclear Mechanism. Organometallics 2008, 27, 6521–6530. [Google Scholar] [CrossRef]
- Panunzi, A.; Roviello, G.; Ruffo, F. Fine-Tuning the Basicity of Metal Complexes: Reversible Oxidative Addition of Se−Se Bonds to Platinum(II) Precursors. Organometallics 2002, 21, 3503–3505. [Google Scholar] [CrossRef]
- Canty, A.J.; Jin, H.; Skelton, B.W.; White, A.H. Oxidation of Complexes by (O2CPh)2 and (ER)2 (E = S, Se), Including Structures of Pd(CH2CH2CH2CH2)(SePh)2(bpy) (bpy = 2,2‘-Bipyridine) and MMe2(SePh)2(L2) (M = Pd, Pt; L2 = bpy, 1,10-Phenanthroline) and C...O and C...E Bond Formation at Palladium(IV). Inorg. Chem. 1998, 37, 3975–3981. [Google Scholar] [CrossRef]
- Aye, K.-T.; Vittal, J.J.; Puddephatt, R.J. Oxidative addition of E–E bonds (E = Group 16 element) to platinum(II): A route to platinum(IV) thiolate and selenolate complexes. J. Chem. Soc. Dalton Trans. 1993, 1835–1839. [Google Scholar] [CrossRef]
- Osakada, K. Chemistry of Transition Metal Complexes with Group 16 Elements: Transition Metal Complexes with Two Lone Pairs of Electrons on the Coordinating Atom. In Organometallic Chemistry; Nakazawa, H., Koe, J., Eds.; Royal Society of Chemistry: London, UK, 2021; Volume 11, pp. 203–227. [Google Scholar]
- He, M.; Ching, H.Y.V.; Policar, C.; Bertrand, H.C. Rhenium tricarbonyl complexes with arenethiolate axial ligands. New J. Chem. 2018, 42, 11312–11323. [Google Scholar] [CrossRef]
- Klein, A.; Van Slageren, J.; Zalis, S. Co-Ligand Involvement in Ground and Excited States of Electron-Rich (Polypyridyl)PtII Complexes. Eur. J. Inorg. Chem. 2003, 2003, 1927–1938. [Google Scholar] [CrossRef]
- Ishida, H.; Tobita, S.; Hasegawa, Y.; Katoh, R.; Nozaki, K. Recent advances in instrumentation for absolute emission quantum yield measurements. Coord. Chem. Rev. 2010, 254, 2449–2458. [Google Scholar] [CrossRef]
- Armarego, W.L. Purification of Laboratory Chemicals, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Becke, A. Density-functional thermochemistry. III The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. Gaussian 09, Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Roy, L.E.; Hay, P.J.; Martin, R.L. Revised basis sets for the LANL effective core potentials. J. Chem. Theory Comput. 2008, 4, 1029–1031. [Google Scholar] [CrossRef]
- Cossi, M.; Scalmani, G.; Rega, N.; Barone, V. New developments in the polarizable continuum model for quantum mechanical and classical calculations on molecules in solution. Chem. Phys. 2002, 117, 43–54. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. A new definition of cavities for the computation of solvation free energies by the polarizable continuum model. Chem. Phys. 1997, 107, 3210–3221. [Google Scholar] [CrossRef]
- Zhurko, G.; Zhurko, D. Chemcraft, Version 1.7; Informer Technologies, Inc.: Redwood City, CA, USA, 2013. [Google Scholar]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef]
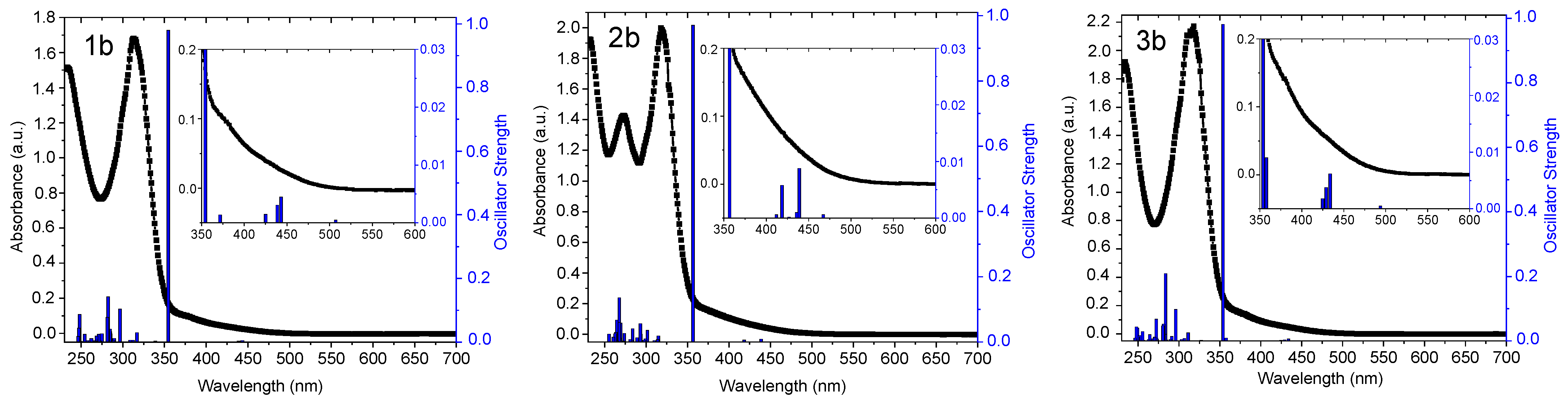
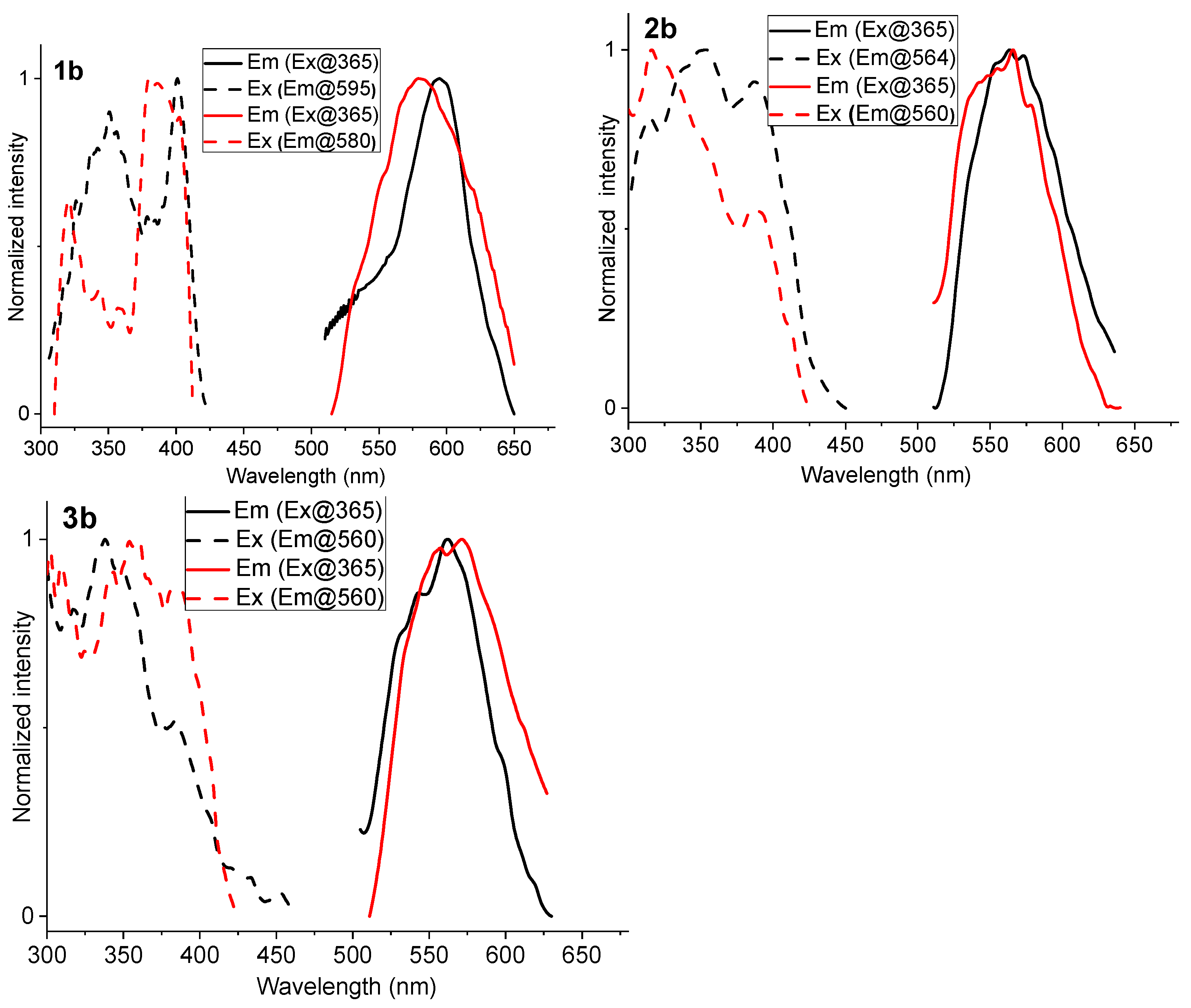
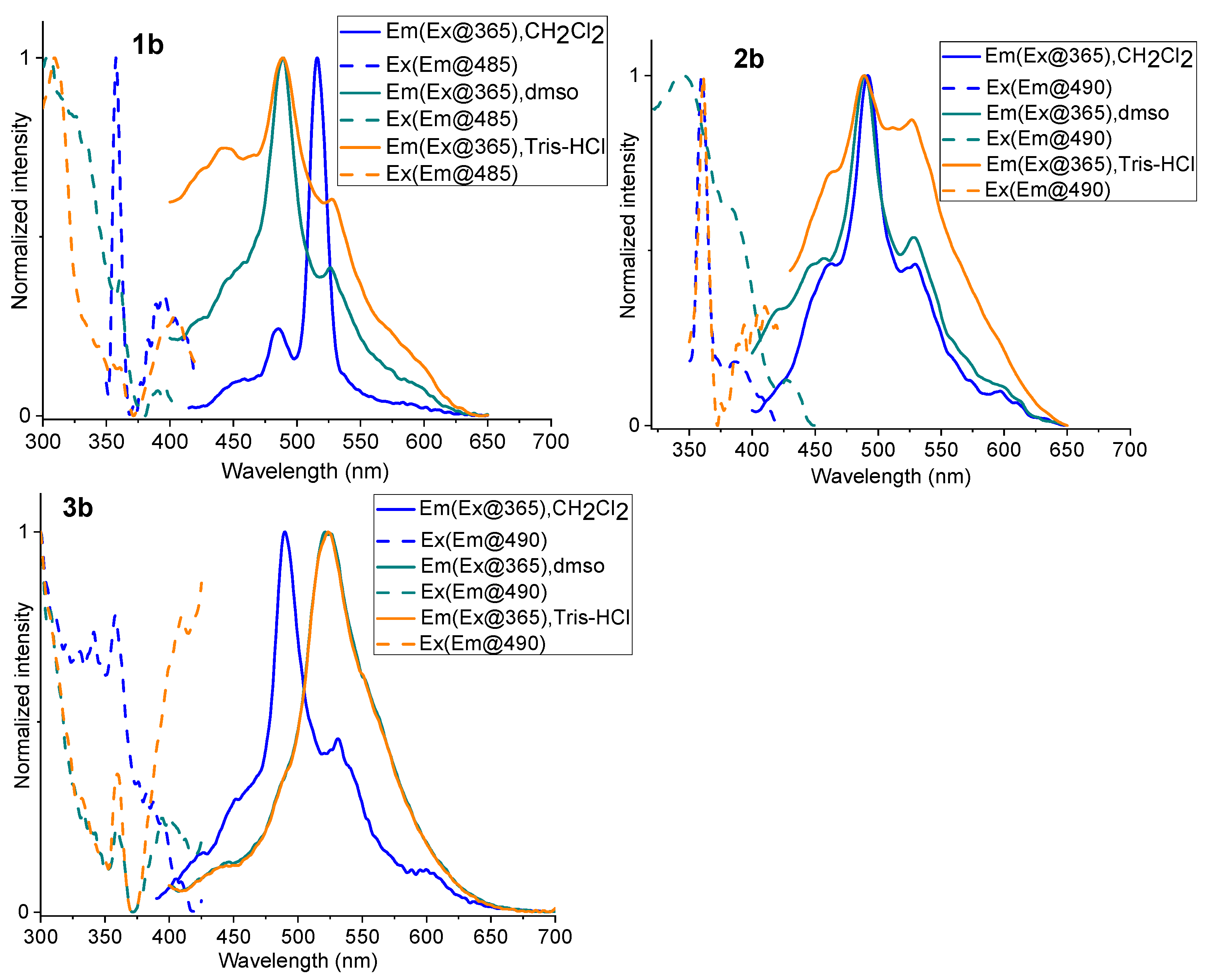
| Complex | λabs/nm (105 ε/M−1 cm−1) | State (T) | λem/nm | ΦL |
|---|---|---|---|---|
| 1b | 400 (0.0595), 313 (1.697) | solid (298 K) | 595 | 0.069 |
| solid (77 K) | 580 | 0.117 | ||
| CH2Cl2 (298 K) | 453, 484, 516 | 0.0065 | ||
| DMSO (298 K) | 450, 484, 526 | 0.0123 | ||
| tris-HCl (298 K) | 442, 484, 526 | 0.0093 | ||
| 2b | 400 (0.1167), 318 (2.003), 272 (1.451) | solid (298 K) | 564 | 0.081 |
| solid (77 K) | 560 | 0.140 | ||
| CH2Cl2 (298 K) | 463, 490, 529 | 0.0017 | ||
| DMSO (298 K) | 456, 490, 528 | 0.0032 | ||
| tris-HCl (298 K) | 462, 490, 526 | 0.0090 | ||
| 3b | 400 (0.1004), 314 (2.184) | solid (298 K) | 560 | 0.065 |
| solid (77 K) | 560 | 0.101 | ||
| CH2Cl2 (298 K) | 453, 490, 530 | 0.0042 | ||
| DMSO (298 K) | 447, 486, 522 | 0.0050 | ||
| tris-HCl (298 K) | 447, 486, 522 | 0.0053 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aseman, M.D.; Aghakhanpour, R.B.; Sharifioliaei, Z.; Klein, A.; Nabavizadeh, S.M. Luminescent Diimine-Pt(IV) Complexes with Axial Phenyl Selenide Ligands. Inorganics 2023, 11, 387. https://doi.org/10.3390/inorganics11100387
Aseman MD, Aghakhanpour RB, Sharifioliaei Z, Klein A, Nabavizadeh SM. Luminescent Diimine-Pt(IV) Complexes with Axial Phenyl Selenide Ligands. Inorganics. 2023; 11(10):387. https://doi.org/10.3390/inorganics11100387
Chicago/Turabian StyleAseman, Marzieh Dadkhah, Reza Babadi Aghakhanpour, Zohreh Sharifioliaei, Axel Klein, and S. Masoud Nabavizadeh. 2023. "Luminescent Diimine-Pt(IV) Complexes with Axial Phenyl Selenide Ligands" Inorganics 11, no. 10: 387. https://doi.org/10.3390/inorganics11100387
APA StyleAseman, M. D., Aghakhanpour, R. B., Sharifioliaei, Z., Klein, A., & Nabavizadeh, S. M. (2023). Luminescent Diimine-Pt(IV) Complexes with Axial Phenyl Selenide Ligands. Inorganics, 11(10), 387. https://doi.org/10.3390/inorganics11100387