Abstract
The surface modification of textile fabrics and therefore, the development of advanced textile materials featuring specific implemented and new properties, such as improved durability and resistance, is increasingly in demand from modern society and end-users. In this regard, the sol–gel technique has shown to be an innovative and convenient synthetic route for developing functional sol–gel coatings useful for the protection of textile materials. Compared with the conventional textile finishing process, this technique is characterized by several advantages, such as the environmentally friendly approaches based on one-step applications and low concentration of non-hazardous chemicals. The sol–gel method, starting from inorganic metal alkoxides or metal salts, leads to inorganic sols containing particles that enable a chemical or physical modification of fiber surfaces, giving rise to final multifunctional properties of treated textile fabrics. This review considered the recent developments in the synthesis of inorganic nanoparticles and nanosols by sol–gel approach for improving wear and UV resistance, as well as antibacterial or antimicrobial effects for textile applications.
1. Introduction
During the last decades, chemical finishing treatments for natural and synthetic fibers or fabrics have been widely used to improve their specific properties and confer particular functionalities. In this regard, textile materials are treated with various functional chemical finishes with antimicrobial, durable press, repellent, flame retardant, soil release and antistatic properties as well as many others. Furthermore, the interest in developing functional fabrics mainly arises from the consumers’ needs in terms of comfort, health, hygiene, protection against chemical, thermal or mechanical agents and easy care.
In this perspective, functional high-tech fabrics represent a challenge for the textile industry, with interesting implications in terms of future benefits and technological advancement, also related to the lengthening of the average life of textile products in compliance with the need for saving raw materials. Simultaneously, with the conferment of functional properties to the fabrics, the maintenance of textile properties, such as washing durability, feel and appearance should be guaranteed. Indeed, the employed finishing process (i.e., preparation method, if batch or continuous, dyes and dyeing methods, finishing agents and application techniques), mechanical and thermal combination treatments (sueding, sanding, calendering, brushing, sanforizing, etc.) have a relevant impact on the wearing and performance characteristics of textiles.
A quick look at recent literature [1] shows that various chemicals are frequently used as finishes to improve fabric performances. However, due to the intense pressure to ban harmful chemicals, such as halogen-containing flame retardants or chemical anti-microbial agents, different attempts have recently been made to reduce or replace questionable safety chemicals with new environmentally friendly solutions. Furthermore, the selection of the finishing process influences the industrial closing costs, making it another critical parameter to consider. To fulfil all these requirements, advanced and innovative technologies should be involved. In this regard, textile modifications through the creation of polymeric nanocomposites mainly based on metallic or inorganic nanostructured materials are constantly being developed due to their distinct and quite unique characteristics. Until now, surface modification of textiles has been accomplished using a variety of techniques, including (i) use of plasma pre-treatment [2,3]; (ii) ultraviolet irradiation of textiles [4]; (iii) sputtering of nano-particles during plasma polymerization [5]; (iv) in situ synthesis of metal nanoparticles [6] involving cellulose nanoporous structure; (v) use of ion-exchange functionalized surfactant during the polymerization process [7]; (vi) loading of nano-particles into liposomes [8]; and (vii) conventional pad-dry-cure systems.
Among these, most recently, the sol–gel process, which results in the formation of self-assembled (nano) layers on the fiber surface, has notably demonstrated its unique potential for the synthesis of new coatings with high molecular homogeneity and outstanding physical-chemical properties [9,10,11]. From 1984, more than 24,000 scientific papers reported sol–gel protective coatings and finishings. The sol–gel is a versatile synthetic route based on a two-step reaction (hydrolysis and condensation), generally starting from (semi) metal alkoxides (e.g., tetraethoxysilane (TEOS), tetramethoxysilane, titanium tetraisopropoxide), which conduct to the formation of completely inorganic or hybrid organic–inorganic coatings at, or near, room temperature [12,13]. These coatings can protect the polymer surface by acting as an insulator, thus improving the advanced performance of treated materials, such as flame retardancy [14,15,16], antimicrobial or UV radiation protection [16,17,18,19], anti-wrinkle finishing [20], washing fastness of dyes [21,22], bio-molecule immobilization [23] and super-hydrophobicity [24,25]. Moreover, the sol–gel technique has recently been investigated for novel applications, such as for imparting sensing [26,27,28,29] or self-cleaning [30] properties for fabrics, or for hydrogen production via water photo splitting [31]. This environmentally friendly method, in particular, has several advantages over other methods of film deposition, including the ability to develop a protective thin hybrid layer on the surface of a textile with well-defined physical characteristics, optical transparency and excellent chemical stability, as well as the ability to preserve the mechanical and chemical properties of the fibers, thereby introducing durability and highly performant functionalities [32,33,34,35,36,37]. Furthermore, the developed film, which is useful for the previously mentioned textile applications, does not have cytotoxic effects on human skin cells, providing additional benefits in the application of this technology [38]. An easy functionalization approach in order to improve the mechanical and thermal features, but also the chemical resistance and functional properties of the final coating, concerns the blend of the sols with proper hybrid modifiers. In this regard, a wide range of functional nanofillers can be employed as metals [39,40] and metal oxide nanoparticles [41], carbon-based nanomaterials [42,43] and silica-based nanofillers [44].
Although the majority of the literature data refers to sol–gel reactions for synthesizing hybrid organic–inorganic silane-based materials, on the other hand, this technology also contemplates the involvement of other inorganic precursors, such as Al, Ti, Zr, Sn, V salts or other alkoxides and organometallic substrates, in which the metal is linked to organic moieties able to hydrolyze and then condensed [45,46].
As already mentioned, the sol–gel process is based on the transition phase from a liquid (sol) into a dense system (gel), which is then dried and heated to form a hybrid organic–inorganic porous matrix. The involved organic and inorganic precursors can interact via weak bonds, such as ionic, hydrogen, or van der Waals (Class I) or covalent, coordination, or iono-covalent bonds (Class II) [33,47,48]. Therefore, the covalent interaction of organic and inorganic moieties results in final supramolecular Hybrid Organic-Inorganic Materials (HOIM), based on organofunctional alkoxysilane precursors (Class II) of the general chemical structure R′n-Si(OR)4-n (where R′ is an alkyl or organo-functional group and R is mainly methyl, propyl, or butyl group) useful in the chemical modification of textile fabrics. In particular, these three hydrolyzable and polymerizable R groups, together with a different functional group, such as an epoxy, vinyl, or methacryloxy group [33,47,49], are involved in forming well-oriented three-dimensional (3D) network structures.
As shown in Figure 1a–d, silica alkoxide precursors are hydrolyzed in water by acids (Figure 1a,b) or bases (Figure 1c,d), followed by parallel condensation reactions that result in the formation of a final 3D network bearing Si–O–Si bonds (Figure 2).
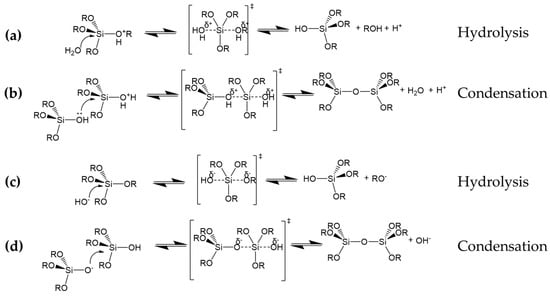
Figure 1.
Hydrolysis and condensation reactions of silica alkoxide precursors in acid (a,b) and alkaline (c,d) conditions.
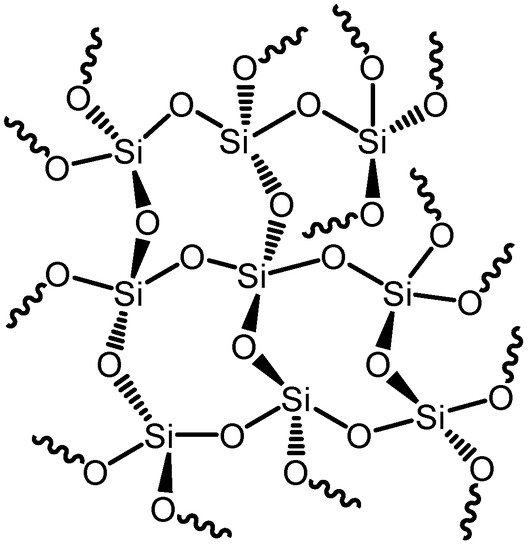
Figure 2.
The obtained 3D network after the hydrolysis and condensation reactions of silica precursors.
When compared with other techniques, the sol–gel approach has several advantages, including low-temperature conditions during the process, easily removable solvents during the handling period and a high purity of the obtained products. Furthermore, it is possible to control the physical properties of the final inorganic matrix to obtain several porous materials, such as glass, dry gel, polycrystalline powder or coating films by tailoring the synthesis conditions, such as concentrations, temperature, time, pH, reactant ratios, solvents and catalysts [50]. Accordingly, the addition of acidic or basic catalysts is normally used to achieve complete and rapid hydrolysis, and the polymerization process is also strongly pH-dependent. Moreover, the type of hydrolysis catalyst influences both the pH of the sols and the nature of the sol–gel polymer aggregates towards the fabrication of films, powders, or monoliths [50,51,52]. Acidic hydrolyzed nanosols may form small-particle aggregates with larger pores, whereas base-catalyzed sols form weakly cross-linked condensation products with a denser layer structure [53,54]. It should be noted that the hydrolysis and drying conditions govern the density, porosity and mechanical properties of the layers when coating textiles (and other material surfaces) and critical thickness for cracking. In particular, thermal post-coating treatment is required to achieve strongly adhesive and stable coatings on textiles, increasing the washing speed and mechanical properties of the obtained films.
Nonetheless, for most textiles, the temperature of thermal treatments must not exceed 180 °C to avoid the thermal destruction and degradation of polymeric fibers. The resulting xerogel layers are very interesting for functionalizing textiles because the coatings can be easily modified chemically or physically [55,56], allowing a broad range of changes in the textile properties by reacting with different opportune nanosol precursors. Moreover, because of the low temperature of sol–gel material processing and the porosity of the resulting 3D network, the inorganic matrix can be doped with various organic/inorganic functional molecules, resulting in advanced functional hybrid materials [57].
Furthermore, chemical modification is possible through co-condensation with additives that can form covalent bonds with the metal oxide matrix as co-reactants (Figure 3a) [46,58] or between different metal alkoxides (e.g., the reaction of tetraethoxysilane Si(OC2H5)4 (TEOS) with other metal alkoxides with generic formula M(OR)n (M = Al, Ti, Zr, Zn, etc.) [59] (see Figure 3b). When the hydrolysis rates of the precursors of mixed nanosols differ significantly, selective complexation of the more reactive component is necessary to coordinate the rate. Furthermore, trialkoxysilanes R-Si(OR)3 containing an organic substituent R that is chemically bonded to the silica matrix (according to hydrolysis and condensation) can also be used for functional chemical modifications (Figure 3c).
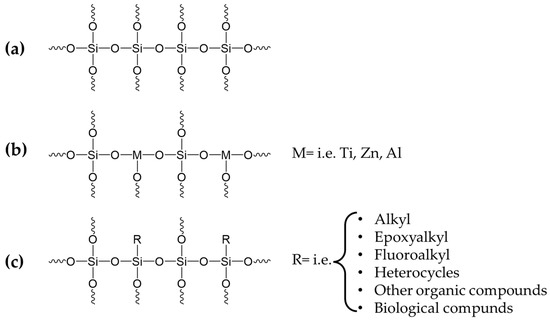
Figure 3.
Examples of the chemical functionalization of nanosols including the co-condensation with additives that can form covalent bonds with the metal oxide matrix as co-reactant (a), between different metal alkoxides (b) and other trialkoxysilanes containing an organic substituent (c).
The nanosol coatings can be modified in various ways using the incorporated substituent R. For example, alkyl or perfluoroalkyl containing trialkoxysilanes can be used to achieve hydrophobic or oleophobic properties [60,61,62,63]. On the other hand, modified nanosols with epoxy alkyl or acrylate R groups favor the thermal or photochemical coating crosslinking and linking to the textile surface, thus significantly improving the mechanical properties of the coating as well as its adhesion to the textile fiber [58]. Physical modification of the oxide matrix is a universal method for preparing coatings with immobilized additives (Ad), such as inorganic colloidal metals [64,65], dyes [66,67,68], oxides [69] and pigments [70], biomolecules [71,72,73] or organic polymers [74,75] and living cells [71,72,76]. The immobilization can be performed by adding the ingredients either before (Figure 4a) or after (Figure 4b) hydrolysis of the precursors. Since encapsulation occurs during the condensation step, both variants “a” and “b” have nearly identical composite structures and immobilization behavior. Therefore, it is very efficient to immobilize the additives within the inorganic matrix, and it can be controlled by several parameters, among which are the composition and structure of the oxide matrix, the Ad:oxide ratio and the addition of pore-forming agents [77,78].
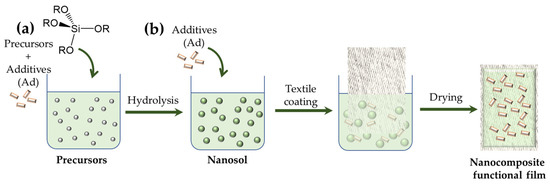
Figure 4.
Physical modification of nanosol coatings by doping the precursor solution (a) or the nanosol after the hydrolysis reaction (b).
As a result, combining chemical and physical modifications offers limitless potential for developing and applying inorganic nanosol coatings for textile functionalization (Figure 5) [79].
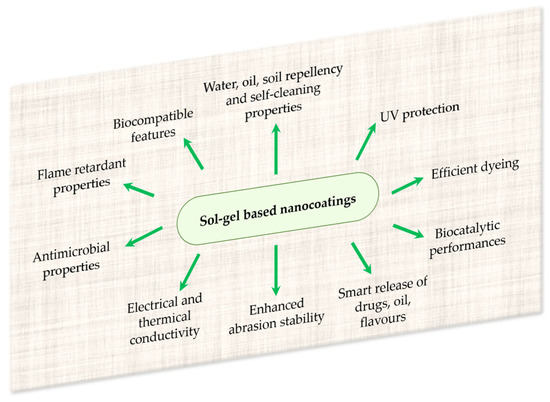
Figure 5.
Some possibilities of textile functionalizations by proper sol–gel-based nanocoatings.
In this wide panorama of sols, sols-modification techniques and functional applications for textile materials, the following review aimed to provide an overview of inorganic sols based on nanoparticular-modified silica and other metal oxides for textile finishing.
The relevance of inorganic sol coatings in the functionalization of textiles can be ascribed to the following [80]:
- 1.
- The formation of well-adhering transparent oxide layers on textiles due to particle-diameter dimensions (smaller than 50 nm) of the modified silica or other metal oxide-based nanosols;
- 2.
- High stability of these inorganic oxide layers against heat, light, microbial and chemical attacks;
- 3.
- The capability of these oxide coatings to improve the mechanical properties of the textiles (e.g., wear and abrasion resistance), thus further offering possible methods of varying the surface properties and high mechanical strength;
- 4.
- Layer porosity and the degree of immobilization of the embedded compounds (e.g., organic, or biological compounds, inorganic particles, and polymers) for whom oxide coatings can serve as carriers, can be easily controlled;
- 5.
- Common textile finishing processes (i.e., pad, exhaustion, dip-coating or spraying) can be used for coating application at room temperature and normal pressure.
The enormous interest in textile materials with even higher added values for application in several fields, such as industrial, workers’ protection or healthcare and medical uses continuously prompts scientific research towards innovative and efficient solutions for introducing specific functionalities in textile fibers. Particularly, to the best of our knowledge, this review considered the most relevant applications, as well as the most recent developments [81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98], of nanostructured materials for textile and polymeric material modifications to improve the wear- or UV-resistance properties or to introduce antibacterial effects. With this aim, the inorganic materials involved, as well as the textile material and functionalities achieved from the application of the inorganic coatings were described, taking into consideration the unique advantages and high potentialities of the nanostructured materials for producing high-performance textiles.
2. Inorganic-Based Materials for Improving Wear Resistance of Textile Fabrics
As commonly mentioned, textile fibers are very sensitive to wear generated from external mechanical effects, such as cellulose which, contrary to synthetic fibers, is less sensitive to such wear. In this regard, applying protective films on the fibers’ surfaces increases their durability against mechanical actions. For this purpose, sol–gel technology is very promising since it leads to the formation of surface ceramic-like inorganic nanocoatings characterized by high hardness and resistance to abrasion [99,100]. On the other hand, however, for producing inorganic protective coatings for fabrics, which allow them to improve their abrasion resistance, some fundamental aspects must be taken into account, meaning this application is not always easy to carry out. In particular, applying such coatings, also in several layers, must not significantly affect the natural soft, pleasant handle or other aspects relating to the “fibrous” nature of the fabric, and also must not increase its stiffness or negatively affect its aesthetic or hygienic properties.
This can be accomplished by applying inorganic xerogel coatings as thin as possible, as well as being elastic, transparent and having durable characteristics ensured by establishing chemical bonds between the coating and the fibers. Conversely, thick coating on textiles can deteriorate the abrasion resistance instead of improving it by accelerating the wear compared with untreated fabrics. Accordingly, inorganic sol–gel coatings with thicknesses in the range of 150–500 nm are already reported in the literature [13,53,101].
Moreover, these inorganic colloidal suspensions (i.e., in water, alcohol, water/alcohol medium) should have good stability, and, as a consequence, they should contain particles smaller than 50 nm. This condition is applied not only to inorganic sols but also to doping nanoparticles incorporated in the crosslinked xerogel for increasing the coating abrasion resistance (e.g., Al2O3 nanoparticles) [13,53,102].
When silica sol is applied to fabric, it forms an inorganic glass-like network that contains siloxane and silanol groups and is highly interconnected with fibers via stable covalent bonds as well as weak interactions, including dipolar–dipolar and hydrogen-bond interactions. In this regard, the greater the dipolar–dipolar and hydrogen-bond interaction, the stronger the coating adhesion must be. Additionally, the polar chain structure, combined with the former cotton-fiber chemical structure, is one of the primary factors for dipolar–dipolar and hydrogen bond interaction, so a high add-on percentage is expected. During the treatment, the diffusion of silica nanoparticles or nanosols from the surface into the fabric texture, particularly in the first layer, controls the contribution to adhesion [103]. Table 1 reports some systems and precursors involving the production of functional nanosols with the objective of improving the wear resistance of different fabrics.

Table 1.
Preparation and properties comparison of some inorganic nanosols employed as wear-resistant finishings for different textile substrates.
Anti-abrasion-coated textiles have relevant applications in several industries, such as polyester sieves in paper production, home textiles, carpets or furniture. For example, alkyl-modified silica nanosols coated on polyester sieves used in paper production can improve their abrasion properties due to the excellent smooth surface structure of the treated fibers and the homogeneous films that completely cover the fibers [80]. SEM characterizations of mixed-cotton textiles treated with different sol–gel coatings yielded similar results [80]. For this purpose, polyethylene terephthalate (PET) fabrics were also treated with methyltrimethoxysilane in an alkaline alcoholic solution containing ammonium hydroxide (Figure 6a) as the catalyst to achieve excellent resistance against different kinds of wear damage by mimicking superhydrophobic biological systems (Figure 6b,c) [104].
Indeed, several fiber materials, such as cellulose, polyamide and glass fibers have reported improved abrasion resistance and tensile strength when coated with inorganic nanosol [105,107]. However, as far as cotton is concerned, experimental findings demonstrated that the adding epoxy silanes, such as (3-Glycidyloxypropyl)trimethoxysilane (GPTMS), increases the adhesion of the organic-inorganic nanosol coating on the fibers, thereby improving their mechanical properties and the elasticity of the obtained coating.
However, in high concentrations, organic moieties can lead to adverse effects on the mechanical resistance of the final coating [13,53]. Certainly, the stiffness of nanosol-treated textiles is also affected by bridging or gel combinations between adjacent fibers that stabilize their mutual position and do not allow them to displace. Such bridging increases when excessively high concentrations of (or partially gelled) nanosols are applied on textile fibers, thus lowering the textile elasticity and increasing the coating thickness [100].
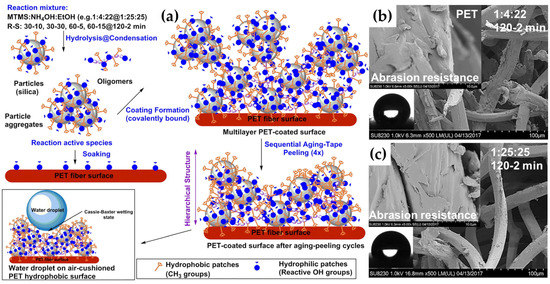
Figure 6.
Schematic representation of methyltrimethoxysilane coating preparation for PET fabrics (a) and relative SEM images describing the appearance of the nanocoatings after standard abrasion-resistance testing: 1:4:22 (120-2 min) (b) and 1:25:25 (120-2 min) (c). Reproduced with permission from ref. [104] Copyright 2018 Elsevier.
An attempt to slightly ameliorate the stiffness properties of textile materials treated by inorganic TEOS-based coatings can consist of the addition of positively charged cationic softeners and uncharged nonionic softeners separately to silica-based sols [92]. In this regard, the mechanical properties of commercial denim fabrics were improved, showing an increase in abrasion resistance and tear strength when using nonionic and cationic softener-doped silica coatings.
Generally, using inorganic nanoparticles for surface modification of textiles makes coatings impermanent, and susceptible to washing. The majority of the stabilization methods of inorganic nanostructured materials on textile surfaces necessitate several preparatory steps (e.g., functionalization, drying, curing, final treatment and other ones) that are expensive and time-consuming for large-scale manufacturing, and/or environmentally harmful due to the use of numerous chemicals or organic solvents. Most of them result in a decrease in tensile properties, abrasion resistance, softness, appearance and other properties of textiles, as well as color change. For example, UV irradiation was used for generating radical groups on the textile surface, able to bond nanoparticles [4] or reduce metallic salts to nanoparticles in a polymeric matrix. Besides economic disadvantages, such as being costly and time-consuming, the presence of free radicals on the textile surfaces that can migrate during clothing usage results in some health risks for people. For example, ZnO is used to improve wear resistance and anti-sliding properties in composites [108] and reinforce polymeric nano-composites [109] due to its high elastic modulus and strength. However, the use of acrylic binders for the fixation of ZnO-soluble starch nano-composite particles on cotton fabrics [109] not only reduces the abrasion resistance and the cloth’s comfort, but the possible decomposition of starch or acrylic binder during processing should also be of concern.
As an inorganic sol–gel precursor, TEOS, is widely used to prepare inorganic silica homogeneous films on textile materials due to its low temperature and cheap method of processing. Moreover, the TEOS gelation process through acid or alkaline catalysts [110,111] has been widely investigated by evaluating its viscoelastic behavior during the sol–gel transition.
In this regard, the influence of organic-tin compounds (i.e., DBTA-Dibutyltindiacetate) as polycondensation catalysts for TEOS was investigated in a multistep deposition process on cotton fabrics, thus leading to a coating well adhered to fabric surfaces [103]. The study was conducted by using two TEOS concentrations (0.03 M and 0.3 M) and evaluating the presence or not of the DBTA catalyst. In particular, the coating realized with the lowest TEOS concentration (0.03 M) showed the best mechanical properties when compared with untreated cotton or cotton treated with the highest TEOS concentration (0.3 M, Table 2). Although ensuring the highest thermal stability and washing fastness, this latter leads to surface coatings characterized by high rigidity that makes fiber movements difficult, thus lowering strength, yarn elongation and tensile strength (Table 1). On the other hand, DBTA was proven to increase the abrasion resistance, as well as the thermal and washing speed.

Table 2.
Tensile strength and elongation for untreated (CO_UT) and treated cotton fabrics in both warp and weft directions (standard deviation lower than ±3%; nL = number of layers, C = catalyst). Adapted with permission from ref. [103] Copyright 2013 Elsevier and ref. [106] Copyright 2020 IOP Science.
The efficiency of inorganic sols was also proven for the protection of cellulose-based polymers in cultural heritage textiles, thus representing a valid alternative to existing materials [106]. However, the use of the DBTA catalyst should be further investigated in terms of its mechanism and replaced with more environmentally friendly alternatives.
Recently, the development of sustainable coatings of SiO2 NPs-chitosan (with an average size of 150 nm) was proven to be efficient for multifunctional effects on polyester and viscose fabrics (both warp and braids structures) [91]. Concerning the mechanical properties, this nanocomposite led to an overall increase in both the elongation and tensile strength of the coated samples, thus measuring enhanced mechanical properties for increasing the SiO2 NPs-chitosan mass loading. However, the further increase of the nanocomposite on textiles resulted in the failure of both tensile strength and elongation due to the inclusion of nanoparticles in the fibers rather than on the fabric surfaces.
3. Inorganic-Based Materials for UV Protection Textile Finishing
Some textile polymers are slightly resistant to ultraviolet radiations (UV) and thus decompose, as is the case of cellulose, polyester, phenylenebenzobisoxazole (PBO) and p-aramide fibers. However, the textile kind of use could necessitate increased UV resistance relative with the intrinsic resistance of the fabrics themselves. For example, the light stability of polyester textiles is enough only for clothing applications; the fibers of a textile roofing exposed for several years to sunlight can decompose, showing decreased mechanical strength over the years.
In this regard, several textile polymer fibers can be modified before the spinning process with UV-absorbing pigments (e.g., titania) or using organic molecules acting as radical scavengers to improve their UV resistance [13].
Moreover, ultraviolet (UV) protection finishes (or UV shielding) are some of the most important groups of chemical finishing agents used on textile materials to protect people and fabrics from the harmful effects of UV radiation. Indeed, the energy of UV radiation is significantly higher than that of visible light, having the potential to cause a variety of chemical reactions that are hazardous to human health, besides deteriorating textile fibers. Even though moderate sun exposure has health benefits, excessive exposure to UV radiation can cause serious harm since both UVA (320–400 nm) and UVB (280–320 nm) rays induce different cellular responses that can generate skin aging, sunburn, pigmentation, skin cancer and DNA damage [112]. In addition, long-term UV weathering of textiles is associated with the cleavage of different chemical bonds by absorbed UV radiation, which results in the photochemical degradation of textile fibers, color fading, increased crystallinity and other chemical and physical changes [113]. As a result, UV protection finishes are used to manufacture functional textiles for sportswear, high-altitude clothing, covering materials, wearable sensors and other high-value technical textiles.
UV protection of fabrics varies depending on their type, porosity, thickness and color, with white summer clothes, in particular, providing only low UV protection. It is worth noting that textiles of darker shades provide relatively higher UV protection since the used dyestuffs can more readily absorb the ultraviolet radiation from the sun [13]. Moreover, the kind of textile material can affect UV protection: cotton fabrics are more sensitive to UV light than polyester materials because of their aromatic structure, which is responsible for higher UV absorption [13]. Furthermore, parameters such as thickness or the setting of threads strongly influence the UV-protection properties [114]. However, laundry detergents doped with optical-brightening agents can improve a textile material’s UV radiation blocking.
As a result, treating textiles to provide UV protection is a growing field of research. This can be accomplished through several approaches, such as the pigmentation (before the spinning) of the fibers with UV-absorbing inorganic pigments, such as titania nanoparticles (in rutile form) [13] or the covalent bonding of organic UV absorbers onto textile fibers. Recently, Attia et al. [91] synthesized a sustainable coating based on SiO2 NPs-chitosan for polyester and viscose fabrics (both warp and braids structure), observing an improvement of 260% in the UPF (UV protection factor) of the coated fabrics with respect to the uncoated ones and demonstrated that the textile-type structure has a fundamental role in the performance of UV-shielding properties. Liang et al. [97] proposed an ultra-thin amorphous TiO2 nano-film for silk fibers by the atomic layer deposition (ALD) method. The experimental findings revealed a decrease in the sample transmittance and an increase in the UPF values for increasing the film thickness, thus confirming the excellent UV-light protection of the TiO2 nanofilm toward silk and the maintenance of the tensile strength of the original fiber after exposure to UV light.
Nanosols and the sol–gel technique are promising approaches for obtaining highly effective UV-protective coatings for textiles (Figure 7a–c). For this purpose, the nanosols should contain inorganic UV-absorbers, such as ZnO [107,115] or titanium dioxide [116,117,118,119,120], that readily absorb UV radiation. These nanosols reveal strongly increased absorption in the radiation range between 200 and 400 nm since ZnO and TiO2 have extinction coefficients for ultraviolet radiation.
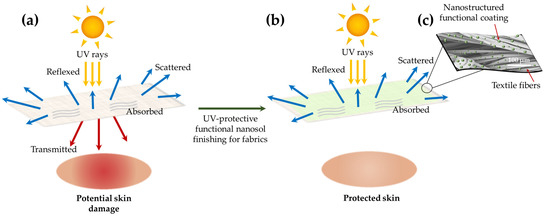
Figure 7.
Schematic representation of the comparison of untreated (a), treated (b) fabrics with sol–gel based functional nanosol finishings featuring UV-protection properties and details of the possible appearance of the final nanostructured coating at SEM analysis (c).
Moreover, at the transition from UV to visible light, the corresponding UV-Vis spectra reveal a steep drop in the absorption curves that ensure high protection against UV radiation and a colorless finish useful for maintaining the textile appearance [13]. Using TiO2 or ZnO with particles sizes no greater than 50 nm not only ensures the coating to be colourless, but also ensures its transparency (only towards visible radiation) since no light scattering occurs within the filled coatings [13].
Zhai et al. [98] developed an aramid fiber chemically coated with uniform and a thickness-controlled amorphous TiO2 coating by modified atomic layer deposition (mALD). The thickest coating (160 nm) achieved the highest mechanical performance and the best UV protection even after 10 accelerated washing cycles, thus confirming the UV-shielding effect and the good thermal stability of TiO2-based layers.
Moreover, a dual-layer ultrathin Al2O3–TiO2 coating with a thickness of 70–180 nm realized by a modified atomic-layer deposition (ALD) method on aramid fibers significantly improved their UV resistances, showing no yellowing effect after intense UV light for 90 min, and excellent washing durability with an ultraviolet protection factor higher than 70 after 50 commercial washing cycles [83].
Alternatively, the decomposition of gases [121], the effects of an electronic field on the optical properties of nano-TiO2 [122], their structural and optical properties [123], the effects of UV irradiation on the hydrophilicity of TiO2 treated fabric [124], cellulose decreasing with multi-functional organic TiO2 catalytic effects on acid (carboxylic acids) under UV irradiation [122] and the UV-protective effect of TiO2 on fabric [125] have been investigated.
ZnO has recently been discovered to be extremely appealing due to its significant potential applications in sensors, gas sensors, solar cells, varistors, displays, piezoelectric devices, electro-acoustic transducers, photo-diodes and UV light-emitting devices, sun-screens, anti-reflection coatings, catalysts, photo-catalysis and UV absorbers [126,127].
Behnajady et al. [128] demonstrated the ability of ZnO to remove dye from textile effluent under UVC light. Moreover, ZnO and TiO2 are n-type semiconductors. Only these two metal oxides in the 3D transition metal oxide semi-conductor series have sufficient photo-excitation-state stability. TiO2 and ZnO completely absorb light with energies greater than their gap energies (e.g., Egap/TiO2 3.0 eV; Egap/ZnO 3.2 eV), which correspond to wavelengths of 413 nm and 387 nm, respectively [13].
The combination of nanotechnology and textiles will enable fabrics to become more multifunctional and valuable, thus resulting in a significant economic impact. Nanotechnology is currently being used to develop UV light resistance, antistatic properties, antibacterial properties and water and oil repellency [129]. Table 3 reports some systems and precursors involving the production of functional nanosols with the objective to improve the UV-protective features of different fabrics.

Table 3.
Preparation and properties comparison of some inorganic nanosols employed as UV-protective finishings for different textile substrates.
Due to its intriguing properties as a photocatalyst [134,135] for the degradation reaction of environmental organic pollutants [136,137], crystalline TiO2 has received increasing attention in recent years, and many works have been devoted to the preparation and modification of this semiconductor. Additionally, TiO2 nanoparticles have received special attention due to their photo-catalytic activities [80,138]. The photoactivity property is associated with structure, microstructure and powder-purification properties [139,140].
The use of semiconductor systems to initiate and control various photo-catalytic processes has sparked interest in understanding the photophysical properties of semiconductor colloids, as well as the dynamics of interfacial processes at semiconductor/electrolyte interfaces [141,142]. The photo-catalytic process activates TiO2 by illuminating it with UV light with an energy greater than the band gap.
The photo-catalytic breakdown reaction of organic compounds proceeds in different steps, culminating in the mineralization of water, carbon dioxide and mineral acids. The formation of electron-hole pairs is the first step, followed by their separation. Electrons can be used in reduction processes, while holes can be used in oxidation processes.
Titania’s photo-catalytic activity varies depending on its crystal phase, particle size and crystallinity. Anatase and rutile are the most active phases of titania, as opposed to brookite [143]. A review of recent literature reveals that there are many studies on the photocatalytic activity of anatase and rutile crystalline forms [144,145] and that only a few studies on amorphous TiO2 thin films show rather low photo-catalytic activity, implying that crystallization treatment at high temperatures [146] or doping [147] is required to improve the photo-catalytic properties.
When exposed to UV light, TiO2 excites electrons from the valance band to the conduction band whenever the light energy exceeds the band gap energy (e.g., 3.2 eV). The resulting electrons and holes can be separated and used in a redox reaction with chemicals from the outside world. When water is added, oxygen is reduced to a superoxide radical, which reacts with water molecules to produce hydroxide ions and peroxide radicals. These lasts combine with H+ ions to form hydroxyl radicals. The combination of these radicals with highly oxidant species causes photocatalytic degradation of organic substrates as dyes (peroxide radicals). Because the resulting •OH radical is a strong oxidizing agent with a standard redox potential of +2.8 V, it can rapidly oxidize the majority of azo dyes to mineral end-products. In heterogeneous photocatalysis, reductive pathways have been observed to play a similar role in the degradation of several dyes, albeit with less importance than oxidation.
Furthermore, many factors influence titania crystallization, including annealing temperature, heating rate and atmosphere. These factors cannot be emphasized in the case of treatment to improve the amorphous-anatase transformation because they would destroy the textile polymer. From the standpoint of increasing the efficiency of the photo-catalytic process, it is clear that the tailoring and development of new and alternative photocatalysts are of great interest.
The photo-catalytic reactions activated by TiO2 nanoparticles not only ensure UV-protection properties, but also self-cleaning, antibacterial effects, as well as ecological and economical wastewater treatment [148,149].
As an example of self-cleaning properties, TiO2-SiO2 nanocomposite powder was synthesized through a sol–gel process from titanium isopropoxide and TEOS as precursors with different molar ratios, diethanol amine (DEA) as the stabilizer, isopropanol as the solvent and chitosan in acetic acid as the dispersing agent [130]. Therefore, an acrylic acid binder-coated fabric was immersed in a TiO2–SiO2 suspension containing a surfactant and isopropanol and finally coated with the TiO2–SiO2 suspension by spin-coating with the aim of obtaining a homogeneous finishing. Rilda et al. [130] demonstrated that fabrics treated with TiO2–SiO2 powders (1:2 molar ratios) were responsible for the absorbance falling of methylene blue when UV-irradiated for 120 min, thus providing self-cleaning properties. Similarly, cotton fabric treated with TiO2–SiO2 colloidal solution showed increased discoloration of a wine stain under a solar light simulator for increased exposure times 0, 4, 8 and 24 h [150].
Cotton fabric, treated through a dip–pad–steam process with acidic TiO2, prepared with titanium (IV) butoxide as the precursor, demonstrated excellent UV-protection properties, as well as a self-cleaning effect [131]. Moreover, Wang et al. [131] demonstrated that a higher photocatalytic activity of TiO2 was obtained when prepared by a low-temperature steaming process instead of TiO2 dried at 150 °C and that the higher the water amount in TiO2 hydrosol, the higher the crystallinity and photocatalytic activity of TiO2.
In another paper, a titania-based sol with the addition of PEG at acidic conditions was used for the TiO2 finishing of fabrics with high photocatalytic activity, useful for self-cleaning or environmental applications [30]. Accordingly, different TiO2 sols were synthesized from titanium tetraisopropoxide using three acidic solutions (nitric, hydrochloric and acetic acids) and two concentrations of PEG (0.025 M and 0.25 M). In particular, a titanium glycolate complex could be formed by adding PEG to the hydrolyzed precursor that is not present in the inorganic network of titania, as shown in Figure 8.
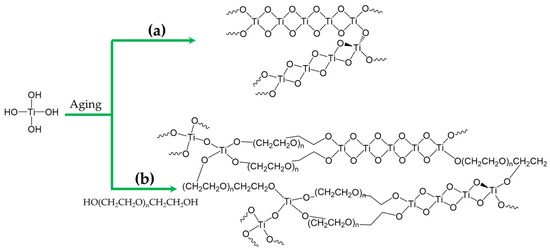
Figure 8.
Illustrative scheme of the TiO2 inorganic network (a) and possible titania glycolate cross-linking mechanism (b). Adapted with permission from ref. [30] Copyright 2012 Elsevier.
Indeed, as already demonstrated [151,152], PEGs are used as a template for obtaining high-porosity, crystalline TiO2 (after calcination), thus realizing the TiO2 cluster break from organic PEG structures instead of a complete inorganic matrix. This mechanism can explain the weak shift of the energy-gap value of amorphous titania. An optical analysis performed by the Tauc plot model showed an energy gap in a range of 3.3 eV to 3.5 eV, which is higher than the values for anatase (3.2 eV) [30]. It was demonstrated that the photocatalytic activity increased when the concentration of precursors increased, thus playing a fundamental role (Figure 9a–d).
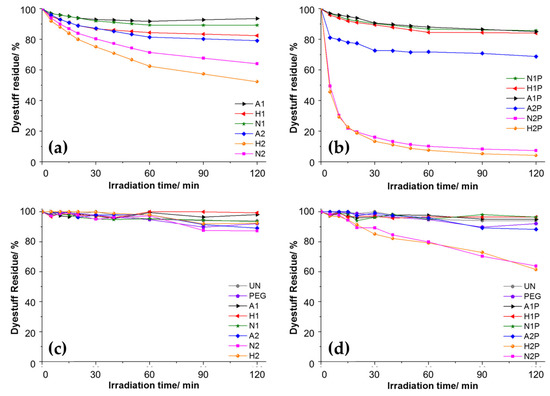
Figure 9.
The photocatalytic activity of treated samples by 0.025 M TiO2 (sample code 1) and 0.25 M TiO2 sols (sample code 2) by UV (a,b) and visible (c,d) source. (A = acetic acid, H = hydrochloric acid, N = nitric acid, P = PEG). Adapted with permission from ref. [30] Copyright 2012 Elsevier.
Moreover, increased photocatalytic activity was proved by moving from acetic to nitric and finally to chloride acid, as well as when PEG was added in the presence of mineral acids and using a UV source rather than visible light. Indeed, the dye degradation rate under UV exposure dramatically increased when PEG was added to the mineral sol–gel solution. However, while the decomposition trend was maintained, its rate slowing down at longer wavelengths was evident. On the other hand, in the absence of PEG, the TiO2 films revealed a rate of degradation linearly correlated with the pKa of the acid used. Shaban et al. [132] used a sol–gel spin-coating process to coat cotton fabrics with ZnO nanoparticles or ZnO solution containing zinc acetate dihydrate as a precursor, 2-methoxyethanol as a solvent and monoethanolamine as a stabilizer. Because of their high bandgap energy, ZnO nanoparticles had photocatalytic activity, especially under UV irradiation, and the methyl orange dye on cotton fabrics coated with ZnO nanoparticles or ZnO solution degraded under sunlight and 200 W lamp illumination [132]. In situ synthesis was used by Noorian et al. [133] to create precursor solutions based on copper sulfate and/or zinc chloride that contained folic acid, NaOH and water. Cotton fabric was immersed in the solutions, dried and finally washed. Authors stated that the combination of ZnO and Cu2O, and the addition of folic acid, improved the UV protection and anti-crease properties of the fabric samples, with 87.31% enhanced UVP and 100.75° of the crease recovery angle [133]. In another study, Noorian et al. [96] imparted UV protection to cotton fabrics involving a sonochemical modification process, according to which the ZnO nanoparticles were synthesized in situ on pre-oxidated cotton fabric and further treated with 4-aminobenzoic acid (PABA) (UV-blocking agent). The latter generates more sites for the ZnO-NP growth and allows cross-linking to occur between the nanoparticles and oxidized cellulosic fibers, thus improving the coating’s durability. In particular, the textile treated by the nanocomposite revealed an excellent level of UV protection factor percentage (EUPF %) of 62.19% and 61.92% after the washing and abrasion processes, with a decrease of 4.65% and 5.06%, respectively, with respect the unwashed samples. In a recent study, Sui et al. [84] proposed a nano-ZnO/fluorosiliconepolyacrylate emulsion finishing agent for the development of multifunctional linen fabric prepared by a semi-continuous seed emulsion polymerization method. The treated linen fabric showed excellent UV resistance, antibacterial properties and washing speed. In particular, by increasing the concentration of nano-ZnO, an increase in the UPF factor was also observed: the highest UV protection factor of 43.21 was measured for 1.0% of nano-ZnO, thus confirming the excellent UV protection performance. In another research, PET fabrics treated with ZnO-NPs revealed enhanced UPF as well as excellent protection for low concentrations of nanoparticles (0.5%) and nano-PU (50 g/L) [86]. In another experiment, Arik et al. [85] realized several sol–gel coatings with Zn salts (zinc acetate, zinc nitrate and zinc sulfate) and ZnO nanopowders dissolved in water and acetic acid/water [30:170], respectively. The sols were applied to linen fabrics using the pad–dry–cure method to improve the UV performance of textiles.
Otherwise, the effect of silver [153] and silver nano-particles (AgNPs) on the electrical conductivity of polymeric matrices [154,155], the effect of dyeing on the ultraviolet protection factor (UPF) [156] and the improvement of UV-protection properties [157] have also been reported. Babaahmadi et al. [93] developed a high-performance multi-layer nanocomposite coating for PET fabric by in situ synthesis of reduced graphene oxide-silver (rGO-Ag, average size of AgNPs lower than 100 nm) using ultrasonic and thermal treatment. The UV protection of this nanocoating on PET substrates was measured in terms of UPF by reaching 6145.66, up to 183-fold higher than uncoated PET fabric (UPF 33.63) thanks to the incorporation of the Ag-decorated rGO as a UV-blocking system. Porrawatkul et al. [94], for the first time, used Averrhoa carambola fruit extract as a reducing agent for the synthesis of Ag/ZnO composites using domestic microwave irradiation for imparting UV protection and antibacterial activity on cotton fabrics. According to the synthetic strategy, zinc acetate dihydrate (Zn(CH3CO2)2) was used as a ZnO nanopowder precursor in the presence of the crude star fruit extract. Moreover, different concentrations of silver nitrate hexahydrate (Ag(NO3)2·6H2O) were added to the zinc acetate solution, and the resulting Ag/ZnO NPs were dissolved in a chitosan solution for padding cotton fabric. Ag/ZnO-coated fabric revealed a UPF value of 69.67 ± 1.53, which indicated an excellent ability to block UV radiation
The unique properties of carbon nanotubes (CNTs) have consistently attracted the interest of researchers [158,159]. In this regard, UV protection [160], increasing garment comfort [160], high stiffness and high strength properties by using low CNT content in polymeric nano-composites are described in literature [158,161]. In addition, CNTs/TiO2 nanocomposite preparation has also been reported [162] with an expected synergistic photocatalytic effect. Additionally, the UV protection properties of a nonwoven polypropylene (PP) were improved using magnetron sputter deposition of Cu nanoparticles [163]. Good UV-protective properties were also reported for cotton fabrics treated by the pad–dry–cure method with a TEOS-based sol–gel coating containing CuO-NPs involving the chemical reaction between the –OH group of cotton and the Si–OMe group of TEOS [87].
Organic UV absorbers, such as phenyl-acrylates or benzotriazoles, can also be embedded in silica–sol coatings [164] and fixed on textiles, similarly to dyes. In fact, due to the typical absorption bands of molecular systems, organic UV absorbers absorb only UV light of specific wavelengths. As a result, in all of these cases, the UV protection of coatings containing only inorganic or organic UV absorbers is insufficient.
The sol–gel technique, which can embed both inorganic and organic UV absorbers in one and the same nanosol coating, can be used to create optimized UV-protective coatings with the full absorption of virtually all UV light, as demonstrated in principle for coatings on glass [123]. On textiles, analogous coatings can be prepared just as well.
Chemical bonding between the organic UV absorbers and the alkoxysilane compounds can be used to improve durability. The organic UV absorber can be directly covalently bonded to the inorganic matrix of the nanosol coatings using this method [13]. Moreover, nanosol coatings modified with both inorganic and organic UV absorbers improve UV absorption over a wide range [13]. Alternatively, a fluorescent whitening agent combined with a TiO2 sol can be used to increase UV absorption, particularly in the UVA region. The addition of such a fluorescent whitening agents reduces the slight yellowing of the textile that occurs when titania-based nanosols are used for UV protection [13].
4. Inorganic-Based Materials for Antimicrobial Textile Finishing
Thanks to their large specific surfaces with good adhesion and water-storage properties, textiles provide ideal conditions for the settling of micro-organisms and offer ideal temperature conditions for this purpose, especially in the case of worn apparel. As a result, there is a high demand for antimicrobial finishes to prevent microbial degradation of textile fibers, limit the incidence of bacteria, reduce odor formation (due to microbial degradation of sweat) and protect users by preventing pathogen transfer and spread [13].
Antimicrobial coatings, also known as self-sterilizing [165] or hygienic coatings [166], are currently the most commercially important bioactive nanosol systems. In 2003, it was estimated that the potential European market for biocide textiles amounted to around 28,000 tons, or 180 million m2 [80]. In the case of traditional clothing, sportswear, home textiles or outdoor textiles, such as tents or marquees, the antimicrobial property is critical for convenience and/or aesthetic reasons: a T-shirt should not have an odor, and a marquee should not be mildewed. On the other hand, the application and development of antimicrobial textiles that do not act selectively are especially important for medical applications.
Another important application of antimicrobial coatings is the use of antimicrobial wound dressing to prevent germ contamination of wounds [167]. In this regard, simple plasters or bandages could be modified with enough nanosols to aid the healing process. As an example, in innovative research, Maghimaa et al. [88] synthesized AgNPs from the aqueous extract of Curcuma longa leaf and the so-obtained AgNPs-coated cotton fabrics, revealing significant antimicrobial activity against Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pyogenes and Candida albicans as well as potent wound-healing activity in the fibroblast (L929) cells.
Existing commercial products are made of special fibers with embedded organic biocides, such as bisphenols, biguanides or incorporated silver. Other products include blended fabrics with silver-coated nylon (e.g., X-StaticTM) [168] or chitosan fibers [169]. Despite the wide range of specific applications mentioned, a finishing material that can be used for any common fiber while providing a defined antimicrobial efficiency combined with a low biocide consumption is highly desirable.
Because of the particular interest in coatings to prevent biocontamination, the following sections describe various strategies for realizing an antimicrobial textile through nanosol treatment. As examples, nanosol coatings containing non-diffusible antimicrobial additives, as well as those employing controlled release of embedded biocides, or photoactive coatings that kill germs by oxidizing them during light exposure, should be mentioned.
Biocer (biological ceramics) refers to inorganic nanosol materials that contain embedded biological components, such as enzymes, proteins or even entire cells; by embedding biological components into inorganic xerogels, new composite materials are created [72,76,170]. Inorganic bioactive components should also be included in this definition, such as silver-containing nanosol coatings that show antimicrobial properties [18], titania coatings that interact with biotin and protein [171] and aluminosilicate coatings that improve biocompatibility [172].
Various biocides, such as silver, triclosan, chitosan, quaternary ammonium salts, N-halamines, biguanide derivatives, synthetic dyes and peroxyacids, have been applied to the surface of textiles to serve as antibacterial and antimicrobial finishes [173,174,175]. The combination of antibacterial biopolymers with titania and silica matrices is a novel method that has been shown to provide ecological benefits as well as antibacterial activity in the presence or absence of light. Arik and Seventekin [176] investigated the antibacterial activity of chitosan/titania and chitosan/silica hybrid coatings. The sol–gel method was used to prepare coating solutions, which were then applied to cotton fabric. According to these findings, hybrid coatings outperformed chitosan, titania and silica coatings in terms of antibacterial activity and washing resistance. Recently, a chitosan nanocomposite containing ZnO, Ag and Ag:ZnO was prepared by sol–gel method using GPTMS and TEOS for antimicrobial textile finishing [177]. The findings revealed chemical interactions between dispersed Ag and ZnO nanoparticles, confirming the attachment of chitosan with siloxane moieties resulting in the formation of a siloxane-polymer network. Good antimicrobial activity was observed for all samples, with a reduction of up to 50%–95% in the viability of bacteria.
Because of many chemical antimicrobial agents’ potentially harmful or toxic effects, natural materials have recently been preferred for textile modification [178]. The use of inorganic nanoparticles and their nanocomposites would be a good substitute [179,180], potentially opening up a new alternative path for antimicrobial and multi-functional textile modification. The hydroxyapatite nano-ribbon spherites containing nano-silver were effectively synthesized by Liu et al. [181] due to the wide surface area and strong adsorption properties of hydroxyapatite nano-ribbon to adsorb bacteria and the high bioactivity of silver nano-particles. A composite coating of hydroxyapatite and silver was specifically developed to prevent bacterial infections following implant placement [182]. Magaña et al. created silver-modified montmorillonites through ion exchange with silver and tested the modified clay’s antibacterial properties [183]. Copper nanoparticles were embedded in submicron Sepiolite (Mg8Si12O30(OH)4(H2O)4.8H2O) particles, and their antibacterial properties were compared to those of triclosan, thus demonstrating that both these composites and triclosan have strong bactericidal properties [184]. However, Le Pape et al. confirmed that copper nanoparticles have significantly lower antibacterial activity than silver nanoparticles [185]. The combination of a bio-pretreatment using laccase from Cerrena unicolor and a further modification with CuO–SiO2 hybrid oxide microparticles by a dip-coating method of woven linen fabric results in strong antibacterial activity against Staphylococcus aureus, Escherichia coli and the fungus Candida albicans, besides good UV protection (UPF 40), thanks to the presence of CuO-SiO2 particles on the flax fiber surface [90]. Hu et al. [186] investigated the antibacterial activity of ion-exchanged montmorillonite with Cu ions. On the other hand, the antibacterial properties of a nano-layer structured organo-clay-loaded fiber matrix have not been reported. Moreover, Kang et al. reported the first evidence of the antimicrobial activity of carbon nanotubes (CNTs) against E. coli using single-walled carbon nanotubes (SWNTs). The CNT antibacterial mechanism is defined as cell membrane damage caused by direct contact between bacteria cells and CNTs [187]. The highly stronger antibacterial efficiency of SWNTs over multi-walled carbon nanotubes (MWNTs) against E. coli is emphasized based on their subsequent findings, which involved the flattening of bacterial cells and the comprising of their integrity [188].
As an alternative method, sol–gel technology can provide benefits, such as ecological treatment, less chemical use, low-temperature processing, low toxicity to human health, protection of inherent textile material properties and excellent washing and usage durability in this finishing process [80,173,189]. Indeed, various types of sol–gel systems feature antibacterial or antimicrobial properties. Photoactive titania coatings with anatase modification and sol–gel coatings with embedded colloidal metal or metal compounds, such as silver, silver salts, copper compounds, zinc or biocidal quaternary ammonium salts are examples of these systems [190,191,192]. On the other hand, they have several drawbacks, such as high operating temperatures to produce highly photoactive thin films and the use of strong acids to keep aqueous sols in the peptized state, which causes textile destruction. Furthermore, titania coatings require UV radiation to be antimicrobial or antibacterial [116,193]. In addition to titania-based sol–gel coatings, silica-based coatings are being investigated for antibacterial or antimicrobial effectiveness. Polycationic components are incorporated within the silica layer matrix to enable the antibacterial or antimicrobial effect, and positively charged polycationic components interact with negatively charged microbial cell membranes, damaging their cellular functions [18,80,190]. Overall, nano-structured materials based on inorganic active agents with good antimicrobial activity on textile materials (Figure 10) can be divided into two categories: inorganic nano-structured materials and their nanocomposites and inorganic nano-structured-loaded organic carriers.
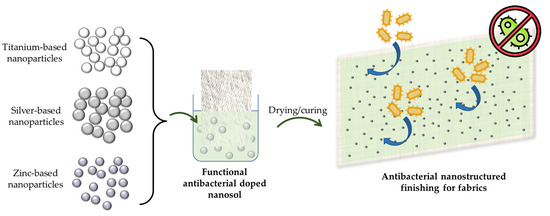
Figure 10.
Schematization of the procedure based on inorganic active agents for the preparation of textile antibacterial nanosols and coatings.
Mahltig et al. reported that sol–gel processing exhibited antimicrobial activity based on controlled release, contact action, or photocatalytic activity [189]. Rivero and Goicoechea presented a detailed review of the sol–gel process for improving antibacterial properties of textiles [194]. Finally, Zhang et al. classified the application methods on cotton for colloidal suspensions of metal nanoparticles and precursor solutions of metal ions, respectively, as sol and a solution to gain antimicrobial properties [173].
In the next paragraphs, an overview of the most relevant and most recent inorganic antimicrobial coatings for textile fabrics is provided to the best of our knowledge, as a function of the active metal nanoparticles involved. A comparison of these examples is given in Table 4.

Table 4.
Preparation and properties comparison of some inorganic nanosols employed as antibacterial finishings for different textile substrates.
4.1. Titanium-Based Antimicrobial Textile Finishing
Nowadays, TiO2 nanoparticles have been developed as a novel approach for exceptional applications as an appealing multifunctional material. Thus, TiO2 nanoparticles have been used to obtain self-cleaning [2], antibacterial [116], UV protection [125], hydrophilic [124] or ultra-hydrophobic properties [198]. Moreover, they are useful in a variety of fields, such as environmental purification [199], dye degradation in fabric effluent [200], water and air purifiers [201,202], gas sensors and high-efficiency solar cells [139,140], as well as it being used as a nano-catalyst for crosslinking cellulose with polycarboxylic acids [122,203]. As already reported, the sol–gel process is the most common among several methods available for synthesizing TiO2 nanoparticles [204]. As an example, textiles coated by TiO2 nanoparticles in combination with ZnO nanoparticles synthesized from titanyl sulphate and zinc nitrate hexahydrate by sol–gel technology revealed a suppression level of E. Coli of more than 99% [89]. Moreover, the method was tested on a semi-industrial scale in roll-to-roll experiments, thus confirming the stable antibacterial properties of the so-coated textile and the suitability of the method for upscale and industrial use.
Actually, when exposed to UV light, crystalline TiO2 coatings exhibit antimicrobial properties due to photo mineralisation processes taking place on the semiconductive TiO2 surface [80]. Furthermore, many studies have confirmed that the addition of noble metals, such as silver and gold, increases the photo-catalytic activity of titanium dioxide by extending the light absorption range of TiO2 from UV to visible light [6,205,206].
4.2. Silver-Based Antimicrobial Textile Finishing
Since olden times, silver has been the most extensively studied antimicrobial agent used to fight infections and prevent spoilage [207]. For this reason, many researchers are currently focusing on the antibacterial and multifunctional properties of silver nanoparticles [207,208].
For the manufacturing of nano-silver, several methods have been used, such as photo-catalytic reduction [206], matrix chemistry [209], chemical-reduction processes [210], photochemical or radiation-chemical reduction, metallic wire explosion, sono-chemical, polyols [211], photo-reduction [212], reverse micelle-based methods [213] and even biological synthesis [214,215].
With respect to other organic antimicrobial agents [216], which have been avoided since they are hazardous to the human body [178], silver is a safer antimicrobial agent. In this regard, it has long been known as ‘oligo-dynamic’ due to its ability to exert a bactericidal effect on silver-containing products, owing to its antimicrobial activities and low toxicity to human cells [217]. Its therapeutic property has been demonstrated against a wide range of microorganisms [218,219], including over 650 disease-causing organisms in the body at low concentrations [220]. Silver has also been shown to prevent the formation of biofilms [221]. Furthermore, silver ions and nanoparticles have been linked to a similar mechanism [218].
Silver nanoparticles are non-toxic and non-tolerant disinfectants [222,223], and their use may maximize antibacterial effects [224] by increasing the number of particles per unit area.
According to a general silver antimicrobial mechanism, metal ions destroy or pass through the cell membrane and bind to the -SH group of cellular enzymes [178]. The resulting critical decrease in enzymatic activity alters micro-organism metabolisms and inhibits cell growth, eventually leading to cell death. Metal ions also catalyze the formation of oxygen radicals, which oxidize bacteria’s molecular structure. A chemical reaction results in the formation of active oxygen (Equation (1)).
Since the active oxygen created by the antimicrobial agent diffuses from the fiber to the surrounding environment, a direct interaction between the antimicrobial agent and bacteria is not necessary for this method. As a result, metal ions prevent micro-organisms from multiplying. Since bacteria are not always exposed to oxygen radicals, the ionic addition does not appear to aid in the selection of resistant strains of bacteria [216,225].
Latterly, the antimicrobial mechanism of AgNPs, according to recent research by Kim et al. [225], is linked to the production of free radicals and subsequent free radical-induced membrane damage. They validated that the antimicrobial properties of silver nanoparticles and silver nitrate was influenced by NAC (N-acetylcysteine). They have also mentioned the possibility of free radicals that may have been produced from the AgNPs surface, which was responsible for antibacterial activity via ESR (electron spin resonance) [225]. According to research on the bio-innoxiousness of silver, smaller silver particles at the same concentration are less harmful to the skin than larger ones. The anti-fungal efficiency of polyamide fabrics treated with AgNPs, and retreated polyester, was described by Ilic et al. [226].
Scientists are still interested in silver and silver-based antibacterial compounds. Silk fibers were sequentially dipped in AgNO3 and NaCl to produce silver chloride nano-crystals. AgCl crystals have been pointed to exhibit antibacterial properties [227]. Wang et al. studied the antibacterial effectiveness of Ag/SiO2 grafted on wool [195], and Simoncic et al. prepared silver nanoparticles through in situ methods and colloidal solutions to attain antibacterial activity [228].
Mohamed et al. [229] prepared colloidal solutions of silver nanoparticles (10–25 nm) synthesized by a chemical reduction process using dextran as stabilizing and reducing agent and modified TEOS using ascorbic acid as a scavenging agent and (3-aminopropyl)trimethoxysilane (APTES) by using 1,2,3,4-butanetetracarboxylic acid (BTCA) or vinyltriethoxysilane (VTEOS) and applied it to cotton fabrics by the sol–gel process by dip coating. A photoinitiator was used to cure silver nanoparticles modified with TEOS and VTEOS. They demonstrated that the modified fabrics had antibacterial activity against S. aureus and E. coli, with nearly 90% bacterial reduction even after 20 washing cycles, as well as thermoregulating properties [229]. The novel Ag decorated conjugated PET-rGO hybrid materials developed by Babaahmadi et al. [93] were proven to show antibacterial activity toward Gram-positive and negative bacteria, thus revealing a good clear inhibition zone 1.33 and 2.16 mm against S. aureus and E. coli, respectively. However, the nanocomposite showed better antibacterial effects on E. coli maybe due to the thinner cell wall since AgNPs can penetrate inside the cell membrane by disrupting its structure and inactivating the bacteria. The innovative Ag/ZnO NPs modified cotton through the use of Averrhoa carambola fruit extract as a reducing agent for the synthesis of Ag/ZnO composites using domestic microwave showed better inhibition of Gram-positive bacteria (S. aureus) compared with Gram-negative bacteria (E. coli), thus revealing zone of inhibition with diameters of 55.48 ± 2.52 nm and 36.24 ± 2.08, respectively [94]. In another research, a highly durable antimicrobial coating for cellulose fibers through the in situ synthesis of Ag-NPs was developed using an extract of sumac leaves as the reducing agent, by observing a 99%–100% reduction of both E. Coli and S. aureus bacteria [95].
4.3. Zinc-Based Antibacterial Textile Finishing
ZnO was proven to have strong antibacterial effects on a broad bacterial spectrum [230,231], as well as on high-temperature- and high-pressure-resistant spores. Moreover, it was demonstrated that a decrease in the particle size (i.e., increasing specific surface area) with a rise in the powder concentration [232] leads to enhanced ZnO powder antibacterial activity. Indeed, as reported by Tam et al. [230], better antibacterial activity was provided by smaller ZnO particles, which were not affected by surface modifications (e.g., silane surfactant, gold nanoparticle attachments). Karunakaran et al. [233] demonstrated that sol–gel synthesized nanocrystalline ZnO has more extensive bactericidal activities against Escherichia coli than commercial ZnO nanoparticles. Furthermore, ZnO nanoparticles seem to be the most promising unconventional antibacterial agent that can fight methicillin-resistant Staphylococcus aureus (MRSA) and other drug-resistant bacteria [234]. However, thanks to its capability to degrade dirt, ZnO could also be used as a cheap self-cleaning substance [235]. Other advantages of this inorganic material are its no drug resistance generation, heat resistance and long-lasting, which make it of great interest [178]. Li and colleagues investigated the antibacterial activity and durability of nano-ZnO functionalized cotton fabric to sweat. They treated cotton fabrics at a concentration of 11 g/L ZnO and padded them to ensure 100% wet pick-up. Actually, the antibacterial activity of the finished fabric has been tested in alkaline, acidic and inorganic salt artificial sweat solutions. The treated fabrics demonstrated better salt and alkaline resistance than acid resistance [197]. ZnO nanoparticles have a negative surface charge and illumination can improve antibacterial performance compared with normal conditions [197]. Farouk et al. [236] demonstrated that the enhanced bioactivity of ZnO is attributed to the size of particles: the smaller their dimension, the higher the surface area to volume ratio, resulting in up to 98.8% of E. coli and 97.3% of M. luteus reduction within 5 h.
Recently, alcohol-based solutions applied at low temperatures on textile fabrics were investigated as alternative methods to overcome the harmful effects of strong acids and high temperatures on fabrics [189,190,196]. Accordingly, Poli et al. reported a less harmful method than conventional ones, as well as simple, reproducible and cheap, to prepare zinc-based coatings for cotton fabrics by sol–gel method in neutral hydro-alcoholic medium for obtaining antibacterial activity [190]. Additionally, also in this case, the one-step coating system used for preparing transparent antibacterial surfaces is simple and reproducible when compared with the conventional methods (by starting from zinc precursors in alkaline solution with reflux heating for obtaining ZnO powder). Moreover, this method highlights that the precipitation of ZnO is not necessary and other advantages are the low process temperature, the neutral media and the formation of the unique morphology on the treated textile fabrics. In particular, the acetate group of the Zn precursor plays a significant role in this innovative sol–gel finishing, in the absence of other nucleophilic species competing for the Zn2+ Lewis acid center (i.e., HO- or bidentate ligand). Furthermore, as reported in a previous study [237], colloids or precipitates are formed as a result of the progressive condensation of hydrolyzed moieties, thus resulting in this case in stable acetate-capped colloidal nano-sized to sub-micrometer-sized particles in dilute solutions (Figure 11).
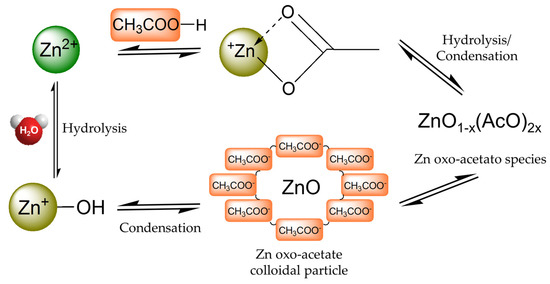
Figure 11.
Schematic representation of the chemical equilibria of Zn-acetate precursor in neutral hydro-alcoholic media during sol–gel reactions [190].
In this study, the nano-Zn acetate-based sol–gel finishing for cotton, in the presence or not of GPTMS, showed significant bactericidal and bacteriostatic activities against E. coli, S. aureus and K. pneumoniae bacteria, even after five cycles of washing in case of highest concentration (Figure 12).
In another study, the multifactional cotton treated with ZnO-NPs and PABA developed from Noorian et al. [96] reveals antibacterial activity against E. coli and S. aureus with a reduction bacteria percentage (R%) of more than 99.4% and 99.9%, respectively. Moreover, excellent antibacterial activities after the abrasion process were observed against E. coli and S. aureus bacteria with R % of 93.4% and 93.7%, respectively.
Literature data report inorganic antimicrobial finishing for substrates different from cellulose, such as linen and PET. Indeed, the previously described linen fabric treated with nano-ZnO/fluorosiliconepolyacrylate emulsion showed also antibacterial activity due to the inhibition zone against E. coli of about 7.4 mm [84]. PET fabrics treated with ZnO NPs and nano-PU revealed efficient antimicrobial reductions of Gram-positive bacteria, Gram-negative bacteria and yeast even after 25 washing cycles [86].
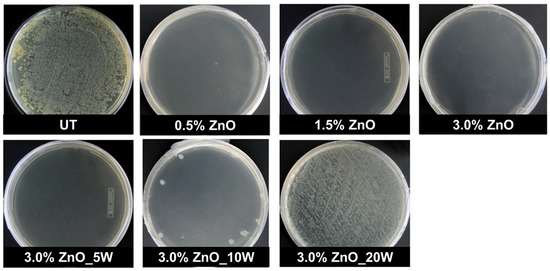
Figure 12.
Comparison of the bactericidal effects of untreated cotton (UT) and cotton treated with Zn-acetate, at different concentrations and after washing cycles (5, 10, 20 washing), on the number of colony-forming units (CFU) of Escherichia coli after 24 h. Adapted with permission from ref. [190] Copyright 2014 Springer Nature.
5. Conclusions and Future Perspectives
In recent decades, the growing demand from modern society for more sustainable production and long-lasting, high-performance textile products has consistently supported the research for innovative functional textile finishes. In this regard, different examples and methodologies based on sol–gel inorganic coatings for the development of advanced and functional textile finishings were evaluated in this review. In particular, different sol–gel-based formulations were explored, employing a wide range of inorganic precursors and functional additives to fabricate textiles featuring different implemented properties. As a result, the as-obtained functional nanosols intended for treating natural or synthetic fabrics represent a more eco-friendly and safe approach than the most commonly employed formulations containing harmful substances. Furthermore, different deposition approaches lead to the production of thin protective films on textile substrates, enhancing their durability and general wear and washing resistance.
Moreover, by a proper rational design and using different inorganic precursors and additives, it is possible to obtain UV-protective and photo-catalytic coatings, addressing the challenge of covering a wide spectral range of UV light and leading to efficient protection of the skin. Finally, the preparation and application of nanosols based on different inorganic nanoparticles were described, focusing on those containing titanium, silver and zinc for the development of antibacterial textile fabrics, able to prevent the microbial degradation of fibers, odor formation and protect users by avoiding pathogen transfer and spread. Therefore, it is possible to suggest that sol–gel synthesis is a key technique for developing simple, scalable and sustainable approaches for functional textile finishings.
The application fields involving the use of these functional and technical textiles concern not only the individuals but embrace a wide range of sectors from the industrial to the public. In particular, the innovative coatings described in this review aim to offer a contribution to more well-performing textiles intended for automotive, naval, aero spatial, furnishing, common clothes and workwear (i.e., protective suits) applications. The main advantage comes from the ease of nanosol functionalization, as performed to obtain hybrid and nanocomposite textile coatings with improved properties in terms of wear resistance, UV-protectivity and antimicrobial traits, aiming at high-performance textiles for better comfort in everyday life.
The scientific developments in the field of inorganic coatings for functional textile materials presented in this review highlight the great potential of inorganic coatings and nanotechnologies in developing increasingly high-added-value and competitive fabrics. Indeed, the current high demand for features such as comfort, safety, aesthetics and functionality as well as greener and more efficient products and processes push scientific research towards increasingly innovative, cutting-edge and sustainable solutions. However, the development of increasingly advanced materials capable of conferring long-lasting or permanent multifunctional properties to textiles still represents a challenge. Other future challenges concern the implementation of these inorganic finishings on an industrial scale since they have not yet reached the market for some application fields, and the reduction of the environmental impact of the finishing processes. Moreover, future developments can be focused on innovative strategies, already partially underway, for the design of functional textiles through inorganic materials, taking into account consumer and environmental needs.
Author Contributions
Conceptualization, V.T., M.H., S.S., G.R. (Giulia Rando), D.D., G.R. (Giuseppe Rosace) and M.R.P.; resources, G.R. (Giuseppe Rosace) and M.R.P.; data curation, V.T., M.H., S.S., G.R. (Giulia Rando), D.D., G.R. (Giuseppe Rosace) and M.R.P.; writing original draft preparation, V.T., M.H., S.S., G.R. (Giulia Rando), G.R. (Giuseppe Rosace) and M.R.P.; writing—review and editing, V.T., M.H., S.S., G.R. (Giulia Rando), D.D., G.R. (Giuseppe Rosace) and M.R.P.; supervision, G.R. (Giuseppe Rosace) and M.R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was also conducted within the framework of the doctoral program of Giulia Rando, as financed by PON-MUR “Ricerca e Innovazione 2014–2020” RESTART project; MUR and CNR are gratefully acknowledged. All authors wish to thank F. Giordano, S. Romeo and G. Napoli for their technical and informatic assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schindler, W.D.; Hauser, P.J. Chemical Finishing of Textiles; Woodhead Publishing Ltd.: Cambridge, UK; CRC Press LLC: Cambridge, UK, 2004. [Google Scholar]
- Bozzi, A.; Yuranova, T.; Kiwi, J. Self-cleaning of wool-polyamide and polyester textiles by TiO2-rutile modification under daylight irradiation at ambient temperature. J. Photochem. Photobiol. A Chem. 2005, 172, 27–34. [Google Scholar] [CrossRef]
- Gray, J.E.; Norton, P.R.; Alnouno, R.; Marold, C.L.; Valvano, M.A.; Griffiths, K. Biological efficacy of electroless-deposited silver on plasma activated polyurethane. Biomaterials 2003, 24, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Niu, M.; Wei, L.; Hou, W.; Liu, X. The structural analysis of biomacromolecule wool fiber with Ag-loading SiO2 nano-antibacterial agent by UV radiation. J. Photochem. Photobiol. A Chem. 2007, 188, 98–105. [Google Scholar] [CrossRef]
- Hegemann, D.; Hossain, M.M.; Balazs, D.J. Nanostructured plasma coatings to obtain multifunctional textile surfaces. Prog. Org. Coatings 2007, 58, 237–240. [Google Scholar] [CrossRef]
- He, J.; Kunitake, T.; Nakao, A. Facile In Situ Synthesis of Noble Metal Nanoparticles in Porous Cellulose Fibers. Chem. Mater. 2003, 15, 4401–4406. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, R.; Banerjee, U. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef]
- Park, S.-H.; Oh, S.-G.; Mun, J.-Y.; Han, S.-S. Loading of gold nanoparticles inside the DPPC bilayers of liposome and their effects on membrane fluidities. Colloids Surf. B Biointerfaces 2006, 48, 112–118. [Google Scholar] [CrossRef]
- Sfameni, S.; Rando, G.; Marchetta, A.; Scolaro, C.; Cappello, S.; Urzì, C.; Visco, A.; Plutino, M.R. Development of Eco-Friendly Hydrophobic and Fouling-Release Coatings for Blue-Growth Environmental Applications: Synthesis, Mechanical Characterization and Biological Activity. Gels 2022, 8, 528. [Google Scholar] [CrossRef]
- Sfameni, S.; Rando, G.; Galletta, M.; Ielo, I.; Brucale, M.; De Leo, F.; Cardiano, P.; Cappello, S.; Visco, A.; Trovato, V.; et al. Design and Development of Fluorinated and Biocide-Free Sol–Gel Based Hybrid Functional Coatings for Anti-Biofouling/Foul-Release Activity. Gels 2022, 8, 538. [Google Scholar]
- Trovato, V.; Colleoni, C.; Castellano, A.; Plutino, M.R. The key role of 3-glycidoxypropyltrimethoxysilane sol–gel precursor in the development of wearable sensors for health monitoring. J. Sol-Gel Sci. Technol. 2018, 87, 27–40. [Google Scholar] [CrossRef]
- Sakka, S. Sol-Gel Science and Technology: Topics and Fundamental Research and Applications; Sakka, S., Ed.; Kluwer Academic Publishers: Norwell, MA, USA, 2003; ISBN 978-1402072918. [Google Scholar]
- Mahltig, B.; Textor, T. Nanosols and Textiles; World Scientific: Singapore, 2008; ISBN 978-981-283-350-1. [Google Scholar]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Sol–gel treatments for enhancing flame retardancy and thermal stability of cotton fabrics: Optimisation of the process and evaluation of the durability. Cellulose 2011, 18, 167–177. [Google Scholar] [CrossRef]
- Brancatelli, G.; Colleoni, C.; Massafra, M.R.; Rosace, G. Effect of hybrid phosphorus-doped silica thin films produced by sol-gel method on the thermal behavior of cotton fabrics. Polym. Degrad. Stab. 2011, 96, 483–490. [Google Scholar] [CrossRef]
- Malucelli, G. Sol–Gel Flame Retardant and/or Antimicrobial Finishings for Cellulosic Textiles. In Handbook of Renewable Materials for Coloration and Finishing; Yusuf, M., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2018; pp. 501–520. [Google Scholar]
- Xing, Y.; Yang, X.; Dai, J. Antimicrobial finishing of cotton textile based on water glass by sol–gel method. J. Sol-Gel Sci. Technol. 2007, 43, 187–192. [Google Scholar] [CrossRef]
- Mahltig, B.; Fiedler, D.; Böttcher, H. Antimicrobial Sol?Gel Coatings. J. Sol-Gel Sci. Technol. 2004, 32, 219–222. [Google Scholar] [CrossRef]
- Mavrić, Z.; Tomšič, B.; Simončič, B. Recent advances in the ultraviolet protection finishing of textiles. Tekstilec 2018, 61, 201–220. [Google Scholar] [CrossRef]
- Huang, K.S.; Nien, Y.H.; Hsiao, K.C.; Chang, Y.S. Application of DMEU/SiO2 gel solution in the antiwrinkle finishing of cotton fabrics. J. Appl. Polym. Sci. 2006, 102, 4136–4143. [Google Scholar] [CrossRef]
- Aķ it, A.C.; Onar, N. Leaching and fastness behavior of cotton fabrics dyed with different type of dyes using sol-gel process. J. Appl. Polym. Sci. 2008, 109, 97–105. [Google Scholar] [CrossRef]
- Mahltig, B.; Textor, T. Combination of silica sol and dyes on textiles. J. Sol-Gel Sci. Technol. 2006, 39, 111–118. [Google Scholar] [CrossRef]
- Li, F.-Y.; Xing, Y.-J.; Ding, X. Immobilization of papain on cotton fabric by sol–gel method. Enzyme Microb. Technol. 2007, 40, 1692–1697. [Google Scholar] [CrossRef]
- Xue, C.-H.; Jia, S.-T.; Chen, H.-Z.; Wang, M. Superhydrophobic cotton fabrics prepared by sol–gel coating of TiO2 and surface hydrophobization. Sci. Technol. Adv. Mater. 2008, 9, 035001. [Google Scholar] [CrossRef]
- Xing, L.; Zhou, Q.; Chen, G.; Sun, G.; Xing, T. Recent developments in preparation, properties, and applications of superhydrophobic textiles. Text. Res. J. 2022, 92, 3857–3874. [Google Scholar] [CrossRef]
- Trovato, V.; Mezzi, A.; Brucale, M.; Rosace, G.; Rosaria Plutino, M. Alizarin-functionalized organic-inorganic silane coatings for the development of wearable textile sensors. J. Colloid Interface Sci. 2022, 617, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Trovato, V.; Teblum, E.; Kostikov, Y.; Pedrana, A.; Re, V.; Nessim, G.D.; Rosace, G. Sol-gel approach to incorporate millimeter-long carbon nanotubes into fabrics for the development of electrical-conductive textiles. Mater. Chem. Phys. 2020, 240, 122218. [Google Scholar] [CrossRef]
- Trovato, V.; Teblum, E.; Kostikov, Y.; Pedrana, A.; Re, V.; Nessim, G.D.; Rosace, G. Electrically conductive cotton fabric coatings developed by silica sol-gel precursors doped with surfactant-aided dispersion of vertically aligned carbon nanotubes fillers in organic solvent-free aqueous solution. J. Colloid Interface Sci. 2021, 586, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Libertino, S.; Plutino, M.R.; Rosace, G. Design and development of wearable sensing nanomaterials for smart textiles. AIP Conf. Proc. 2018, 1990, 020016. [Google Scholar]
- Colleoni, C.; Massafra, M.R.; Rosace, G. Photocatalytic properties and optical characterization of cotton fabric coated via sol–gel with non-crystalline TiO2 modified with poly(ethylene glycol). Surf. Coatings Technol. 2012, 207, 79–88. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Shende, R.V.; Puszynski, J.A. Thermochemical water-splitting for H2 generation using sol-gel derived Mn-ferrite in a packed bed reactor. Int. J. Hydrog. Energy 2012, 37, 2924–2934. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, C. Organic–inorganic hybrid silica film coated for improving resistance to capsicum oil on natural substances through sol–gel route. J. Sol-Gel Sci. Technol. 2012, 64, 743–749. [Google Scholar] [CrossRef]
- Vasiljević, J.; Tomšič, B.; Jerman, I.; Simončič, B. Organofunkcionalni trialkoksisilanski prekurzorji sol-gel za kemijsko modifikacijo tekstilnih vlaken. Tekstilec 2017, 60, 198–213. [Google Scholar] [CrossRef]
- Sfameni, S.; Del Tedesco, A.; Rando, G.; Truant, F.; Visco, A.; Plutino, M.R. Waterborne Eco-Sustainable Sol–Gel Coatings Based on Phytic Acid Intercalated Graphene Oxide for Corrosion Protection of Metallic Surfaces. Int. J. Mol. Sci. 2022, 23, 12021. [Google Scholar]
- Abu Bakar, N.H.; Yusup, H.M.; Ismail, W.N.W.; Zulkifli, N.F. Sol-Gel Finishing for Protective Fabrics. Biointerface Res. Appl. Chem. 2022, 13, 283. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Venkataraman, M.; Kremenakova, D.; Militky, J.; Zhou, Y. Progress in Sol-Gel Technology for the Coatings of Fabrics. Materials 2020, 13, 1838. [Google Scholar] [CrossRef]
- Krzak, J.; Szczurek, A.; Babiarczuk, B.; Gąsiorek, J.; Borak, B. Sol–gel surface functionalization regardless of form and type of substrate. In Handbook of Nanomaterials for Manufacturing Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 111–147. [Google Scholar]
- Plutino, M.R.; Colleoni, C.; Donelli, I.; Freddi, G.; Guido, E.; Maschi, O.; Mezzi, A.; Rosace, G. Sol-gel 3-glycidoxypropyltriethoxysilane finishing on different fabrics: The role of precursor concentration and catalyst on the textile performances and cytotoxic activity. J. Colloid Interface Sci. 2017, 506, 504–517. [Google Scholar] [CrossRef]
- Mohammed, M.K.A. Sol-gel synthesis of Au-doped TiO2 supported SWCNT nanohybrid with visible-light-driven photocatalytic for high degradation performance toward methylene blue dye. Optik 2020, 223, 165607. [Google Scholar] [CrossRef]
- Ramesan, M.T.; Varghese, M.P.J.; Periyat, P. Silver-Doped Zinc Oxide as a Nanofiller for Development of Poly(vinyl alcohol)/Poly(vinyl pyrrolidone) Blend Nanocomposites. Adv. Polym. Technol. 2018, 37, 137–143. [Google Scholar] [CrossRef]
- Sivasamy, R.; Venugopal, P.; Mosquera, E. Synthesis of Gd2O3/CdO composite by sol-gel method: Structural, morphological, optical, electrochemical and magnetic studies. Vacuum 2020, 175, 109255. [Google Scholar] [CrossRef]
- Rando, G.; Sfameni, S.; Plutino, M.R. Development of Functional Hybrid Polymers and Gel Materials for Sustainable Membrane-Based Water Treatment Technology: How to Combine Greener and Cleaner Approaches. Gels 2023, 9, 9. [Google Scholar] [CrossRef]
- Anand, S.; Pauline, S.; Prabagar, C.J. Zr doped Barium hexaferrite nanoplatelets and RGO fillers embedded Polyvinylidenefluoride composite films for electromagnetic interference shielding applications. Polym. Test. 2020, 86, 106504. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Sopchenski Santos, L.; Vitry, V.; Paint, Y.; Olivier, M.-G. Improvement of the corrosion performance of AA2024 alloy by a duplex PEO/clay modified sol-gel nanocomposite coating. Surf. Coatings Technol. 2022, 434, 128168. [Google Scholar] [CrossRef]
- Serra, A.; Ramis, X.; Fernández-Francos, X. Epoxy Sol-Gel Hybrid Thermosets. Coatings 2016, 6, 8. [Google Scholar] [CrossRef]
- Schubert, U.; Huesing, N.; Lorenz, A. Hybrid Inorganic-Organic Materials by Sol-Gel Processing of Organofunctional Metal Alkoxides. Chem. Mater. 1995, 7, 2010–2027. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, e3592. [Google Scholar] [CrossRef]
- Boury, B.; Corriu, R.J.P. Auto-organisation of hybrid organic–inorganic materials prepared by sol–gel chemistry. Chem. Commun. 2002, 8, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H. New type of non-crystalline solids between inorganic and organic materials. J. Non. Cryst. Solids 1985, 73, 681–691. [Google Scholar] [CrossRef]
- Sanchez, C.; Rozes, L.; Ribot, F.; Laberty-Robert, C.; Grosso, D.; Sassoye, C.; Boissiere, C.; Nicole, L. “Chimie douce”: A land of opportunities for the designed construction of functional inorganic and hybrid organic-inorganic nanomaterials. Comptes Rendus Chim. 2010, 13, 3–39. [Google Scholar] [CrossRef]
- Ismail, W.N.W. Sol–gel technology for innovative fabric finishing—A Review. J. Sol-Gel Sci. Technol. 2016, 78, 698–707. [Google Scholar] [CrossRef]
- Ielo, I.; Giacobello, F.; Sfameni, S.; Rando, G.; Galletta, M.; Trovato, V.; Rosace, G.; Plutino, M.R. Nanostructured Surface Finishing and Coatings: Functional Properties and Applications. Materials 2021, 14, 2733. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol–Gel Science: The Physics and Chemistry of Sol–Gel-Processing; A.P. Inc.: Boston, MA, USA, 1990. [Google Scholar]
- Reisfeld, R.; Jorgenson, C.K. (Eds.) Chemistry, Spectroscopy and Applications of Sol–Gel Glasses, Monograph Series Structure and Bonding; Springer: Berlin, Germany, 1992. [Google Scholar]
- Thim, G.P.; Oliveira, M.A.; Oliveira, E.D.; Melo, F.C. Sol–gel silica film preparation from aqueous solutions for corrosion protection. J. Non. Cryst. Solids 2000, 273, 124–128. [Google Scholar] [CrossRef]
- Sanchez, C.; Soler-Illia, G.J.D.A.; Ribot, F.; Grosso, D. Design of functional nano-structured materials through the use of controlled hybrid organic–inorganic interfaces. Comptes Rendus Chim. 2003, 6, 1131–1151. [Google Scholar] [CrossRef]
- Rando, G.; Sfameni, S.; Galletta, M.; Drommi, D.; Cappello, S.; Plutino, M.R. Functional Nanohybrids and Nanocomposites Development for the Removal of Environmental Pollutants and Bioremediation. Molecules 2022, 27, 4856. [Google Scholar] [CrossRef]
- Schottner, G. Hybrid Sol−Gel-Derived Polymers: Applications of Multifunctional Materials. Chem. Mater. 2001, 13, 3422–3435. [Google Scholar] [CrossRef]
- Francis, L.F. Sol-Gel Methods for Oxide Coatings. Mater. Manuf. Process. 1997, 12, 963–1015. [Google Scholar] [CrossRef]
- Monde, T.; Fukube, H.; Nemoto, F.; Yoko, T.; Konakahara, T. Preparation and surface properties of silica-gel coating films containing branched-polyfluoroalkylsilane. J. Non. Cryst. Solids 1999, 246, 54–64. [Google Scholar] [CrossRef]
- Haas, K.-H.; Amberg-Schwab, S.; Rose, K.; Schottner, G. Functionalized coatings based on inorganic–organic polymers (ORMOCER®s) and their combination with vapor deposited inorganic thin films. Surf. Coatings Technol. 1999, 111, 72–79. [Google Scholar] [CrossRef]
- Jung, J.-I.; Bae, J.Y.; Bae, B.-S. Characterization and mesostructure control of mesoporous fluorinated organosilicate films. J. Mater. Chem. 2004, 14, 1988–1994. [Google Scholar] [CrossRef]
- Sfameni, S.; Lawnick, T.; Rando, G.; Visco, A.; Textor, T.; Plutino, M.R. Functional Silane-Based Nanohybrid Materials for the Development of Hydrophobic and Water-Based Stain Resistant Cotton Fabrics Coatings. Nanomaterials 2022, 12, 3404. [Google Scholar] [CrossRef]
- Mennig, M.; Schmitt, M.; Schmidt, H. Synthesis of Ag-Colloids in Sol-Gel Derived SiO2-Coatings on Glass. J. Sol-Gel Sci. Technol. 1997, 8, 1035–1042. [Google Scholar] [CrossRef]
- Prokopenko, V.B.; Gurin, V.S.; Alexeenko, A.A.; Kulikauskas, V.S.; Kovalenko, D.L. Surface segregation of transition metals in sol-gel silica films. J. Phys. D Appl. Phys. 2000, 33, 3152–3155. [Google Scholar] [CrossRef]
- Dunn, B.; Zink, J.I. Optical properties of sol–gel glasses doped with organic molecules. J. Mater. Chem. 1991, 1, 903–913. [Google Scholar] [CrossRef]
- Reisfeld, R. Prospects of sol–gel technology towards luminescent materials. Opt. Mater. 2001, 16, 1–7. [Google Scholar] [CrossRef]
- Trovato, V.; Sfameni, S.; Rando, G.; Rosace, G.; Libertino, S.; Ferri, A.; Plutino, M.R. A Review of Stimuli-Responsive Smart Materials for Wearable Technology in Healthcare: Retrospective, Perspective, and Prospective. Molecules 2022, 27, 5709. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H. Nanoparticles by chemical synthesis, processing to materials and innovative applications. Appl. Organomet. Chem. 2001, 15, 331–343. [Google Scholar] [CrossRef]
- Böhmer, M.R.; Keursten, T.A.P.M. Incorporation of pigments in TEOS derived matrices. J. Sol-Gel Sci. Technol. 2000, 19, 361–364. [Google Scholar] [CrossRef]
- Carturan, G.; Campostrini, R.; Diré, S.; Scardi, V.; De Alteriis, E. Inorganic gels for immobilization of biocatalysts: Inclusion of invertase-active whole cells of yeast (saccharomyces cerevisiae) into thin layers of SiO2 gel deposited on glass sheets. J. Mol. Catal. 1989, 57, L13–L16. [Google Scholar] [CrossRef]
- Livage, J.; Coradin, T.; Roux, C. Encapsulation of biomolecules in silica gels. J. Phys. Condens. Matter 2001, 13, R673–R691. [Google Scholar] [CrossRef]
- Puoci, F.; Saturnino, C.; Trovato, V.; Iacopetta, D.; Piperopoulos, E.; Triolo, C.; Bonomo, M.G.; Drommi, D.; Parisi, O.I.; Milone, C.; et al. Sol–Gel Treatment of Textiles for the Entrapping of an Antioxidant/Anti-Inflammatory Molecule: Functional Coating Morphological Characterization and Drug Release Evaluation. Appl. Sci. 2020, 10, 2287. [Google Scholar] [CrossRef]
- Novak, B.M. Hybrid Nanocomposite Materials?between inorganic glasses and organic polymers. Adv. Mater. 1993, 5, 422–433. [Google Scholar] [CrossRef]
- Pomogailo, A.D. Hybrid polymer-inorganic nanocomposites. Russ. Chem. Rev. 2000, 69, 53–80. [Google Scholar] [CrossRef]
- Böttcher, H.; Soltmann, U.; Mertig, M.; Pompe, W. Biocers: Ceramics with incorporated microorganisms for biocatalytic, biosorptive and functional materials development. J. Mater. Chem. 2004, 14, 2176–2188. [Google Scholar] [CrossRef]
- Böttcher, H.; Kallies, K.-H.; Haufe, H. Model investigations of controlled release of bioactive compounds from thin metal oxide layers. J. Sol-Gel Sci. Technol. 1997, 8, 651–654. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, J.; Dong, H.; Dong, J.H.; Qiu, K.; Jansen-Varnum, S.A. Preparation and Physisorption Characterization of D-Glucose-Templated Mesoporous Silica Sol−Gel Materials. Chem. Mater. 1999, 11, 2023–2029. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; Sharaf, S.M. Functional Finishes for Cotton-Based Textiles: Current Situation and Future Trends. In Textiles and Clothing: Environmental Concerns and Solutions; Shabbir, M., Ed.; Scrivener Publishing LLC: Beverly, MA, USA, 2019; pp. 131–190. [Google Scholar]
- Mahltig, B.; Haufe, H.; Böttcher, H. Functionalisation of textiles by inorganic sol–gel coatings. J. Mater. Chem. 2005, 15, 4385–4398. [Google Scholar] [CrossRef]
- Attia, N.F.; Mohamed, A.; Hussein, A.; El-Demerdash, A.-G.M.; Kandil, S.H. Bio-inspired one-dimensional based textile fabric coating for integrating high flame retardancy, antibacterial, toxic gases suppression, antiviral and reinforcement properties. Polym. Degrad. Stab. 2022, 205, 110152. [Google Scholar] [CrossRef]
- Attia, N.F.; Osama, R.; Elashery, S.E.A.; Kalam, A.; Al-Sehemi, A.G.; Algarni, H. Recent Advances of Sustainable Textile Fabric Coatings for UV Protection Properties. Coatings 2022, 12, 1597. [Google Scholar] [CrossRef]
- Chen, F.; Zhai, L.; Yang, H.; Zhao, S.; Wang, Z.; Gao, C.; Zhou, J.; Liu, X.; Yu, Z.; Qin, Y.; et al. Unparalleled Armour for Aramid Fiber with Excellent UV Resistance in Extreme Environment. Adv. Sci. 2021, 8, 2004171. [Google Scholar] [CrossRef]
- Sui, Z.; Guo, Z.; Li, Y.; Zhang, Q.; Zu, B.; Zhao, X. Study on preparation and performance of multifunctional linen fabric finishing agent. Text. Res. J. 2022, 93. [Google Scholar] [CrossRef]
- Arik, B.; Karaman Atmaca, O.D. The effects of sol–gel coatings doped with zinc salts and zinc oxide nanopowders on multifunctional performance of linen fabric. Cellulose 2020, 27, 8385–8403. [Google Scholar] [CrossRef]
- Abo El-Ola, S.M.; El-Bendary, M.A.; Mohamed, N.H.; Kotb, R.M. Substantial Functional Finishing and Transfer Printing of Polyester Fabric Using Zinc Oxide/Polyurethane Nanocomposite. Fibers Polym. 2022, 23, 2798–2808. [Google Scholar] [CrossRef]
- Prilla, K.A.V.; Jacinto, J.M.; Ricardo, L.J.O.; Box, J.T.S.; Lim, A.B.C.; Francisco, E.F.; De Vera, G.I.N.; Yaya, J.A.T.; Natividad, V.V.M.; Awi, E.N.; et al. Flame retardant and uv-protective cotton fabrics functionalized with copper (II) oxide nanoparticles. ANTORCHA 2020, 7, 11–16. [Google Scholar]
- Maghimaa, M.; Alharbi, S.A. Green synthesis of silver nanoparticles from Curcuma longa L. and coating on the cotton fabrics for antimicrobial applications and wound healing activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111806. [Google Scholar] [CrossRef]
- Abramova, A.V.; Abramov, V.O.; Bayazitov, V.M.; Voitov, Y.; Straumal, E.A.; Lermontov, S.A.; Cherdyntseva, T.A.; Braeutigam, P.; Weiße, M.; Günther, K. A sol-gel method for applying nanosized antibacterial particles to the surface of textile materials in an ultrasonic field. Ultrason. Sonochem. 2020, 60, 104788. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, J.; Sójka-Ledakowicz, J.; Walawska, A.; Antecka, A.; Siwińska-Ciesielczyk, K.; Zdarta, J.; Jesionowski, T. Antimicrobial Activity and Barrier Properties against UV Radiation of Alkaline and Enzymatically Treated Linen Woven Fabrics Coated with Inorganic Hybrid Material. Molecules 2020, 25, 5701. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.F.; Ebissy, A.A.E.; Morsy, M.S.; Sadak, R.A.; Gamal, H. Influence of Textile Fabrics Structures on Thermal, UV Shielding, and Mechanical Properties of Textile Fabrics Coated with Sustainable Coating. J. Nat. Fibers 2021, 18, 2189–2196. [Google Scholar] [CrossRef]
- Sezgin Bozok, S.; Ogulata, R.T. Applying Softener Doped Silica Coating to Cotton Denim Fabrics. J. Nat. Fibers 2022, 19, 7566–7578. [Google Scholar] [CrossRef]
- Babaahmadi, V.; Abuzade, R.A.; Montazer, M. Enhanced ultraviolet-protective textiles based on reduced graphene oxide-silver nanocomposites on polyethylene terephthalate using ultrasonic-assisted in-situ thermal synthesis. J. Appl. Polym. Sci. 2022, 139, 52196. [Google Scholar] [CrossRef]
- Porrawatkul, P.; Pimsen, R.; Kuyyogsuy, A.; Teppaya, N.; Noypha, A.; Chanthai, S.; Nuengmatcha, P. Microwave-assisted synthesis of Ag/ZnO nanoparticles using Averrhoa carambola fruit extract as the reducing agent and their application in cotton fabrics with antibacterial and UV-protection properties. RSC Adv. 2022, 12, 15008–15019. [Google Scholar] [CrossRef]
- Filipič, J.; Glažar, D.; Jerebic, Š.; Kenda, D.; Modic, A.; Roškar, B.; Vrhovski, I.; Štular, D.; Golja, B.; Smolej, S.; et al. Tailoring of Antibacterial and UV-protective Cotton Fabric by an in situ Synthesis of Silver Particles in the Presence of a Sol-gel Matrix and Sumac Leaf Extract. Tekstilec 2020, 63, 4–13. [Google Scholar] [CrossRef]
- Noorian, S.A.; Hemmatinejad, N.; Navarro, J.A.R. Ligand modified cellulose fabrics as support of zinc oxide nanoparticles for UV protection and antimicrobial activities. Int. J. Biol. Macromol. 2020, 154, 1215–1226. [Google Scholar] [CrossRef]
- Liang, Z.; Zhou, Z.; Li, J.; Zhang, S.; Dong, B.; Zhao, L.; Wu, C.; Yang, H.; Chen, F.; Wang, S. Multi-functional silk fibers/fabrics with a negligible impact on comfortable and wearability properties for fiber bulk. Chem. Eng. J. 2021, 415, 128980. [Google Scholar] [CrossRef]
- Zhai, L.; Huang, Z.; Luo, Y.; Yang, H.; Xing, T.; He, A.; Yu, Z.; Liu, J.; Zhang, X.; Xu, W.; et al. Decorating aramid fibers with chemically-bonded amorphous TiO2 for improving UV resistance in the simulated extreme environment. Chem. Eng. J. 2022, 440, 135724. [Google Scholar] [CrossRef]
- Liu, H.-K. Investigation on the pressure infiltration of sol-gel processed textile ceramic matrix composites. J. Mater. Sci. 1996, 31, 5093–5099. [Google Scholar] [CrossRef]
- Brzeziński, S.; Kowalczyk, D.; Borak, B.; Jasiorski, M.; Tracz, A. Applying the sol-gel method to the deposition of nanocoats on textiles to improve their abrasion resistance. J. Appl. Polym. Sci. 2012, 125, 3058–3067. [Google Scholar] [CrossRef]
- Liu, J.; Berg, J.C. An aqueous sol–gel route to prepare organic–inorganic hybrid materials. J. Mater. Chem. 2007, 17, 4430–4435. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, F.; Wei, Q.; Wu, N. Surface modification of polyester nonwoven fabrics by Al2O3 sol–gel coating. J. Coatings Technol. Res. 2009, 6, 537–541. [Google Scholar] [CrossRef]
- Colleoni, C.; Donelli, I.; Freddi, G.; Guido, E.; Migani, V.; Rosace, G. A novel sol-gel multi-layer approach for cotton fabric finishing by tetraethoxysilane precursor. Surf. Coatings Technol. 2013, 235, 192–203. [Google Scholar] [CrossRef]
- Rosu, C.; Lin, H.; Jiang, L.; Breedveld, V.; Hess, D.W. Sustainable and long-time ‘rejuvenation’ of biomimetic water-repellent silica coating on polyester fabrics induced by rough mechanical abrasion. J. Colloid Interface Sci. 2018, 516, 202–214. [Google Scholar] [CrossRef]
- Schramm, C.; Binder, W.H.; Tessadri, R. Durable Press Finishing of Cotton Fabric with 1,2,3,4-Butanetetracarboxylic Acid and TEOS/GPTMS. J. Sol-Gel Sci. Technol. 2004, 29, 155–165. [Google Scholar] [CrossRef]
- Trovato, V.; Rosace, G.; Colleoni, C.; Sfameni, S.; Migani, V.; Plutino, M.R. Sol-gel based coatings for the protection of cultural heritage textiles. IOP Conf. Ser. Mater. Sci. Eng. 2020, 777, 012007. [Google Scholar] [CrossRef]
- Textor, T.; Bahners, T.; Schollmyer, E. Modern Approaches for Intelligent Surface Modification. J. Ind. Text. 2003, 32, 279–289. [Google Scholar] [CrossRef]
- Xu, T.; Xie, C.S. Tetrapod-like nano-particle ZnO/acrylic resin composite and its multi-function property. Prog. Org. Coatings 2003, 46, 297–301. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Kumar, S.; Kathe, A.A.; Varadarajan, P.V.; Prasad, V. Functional finishing of cotton fabrics using zinc oxide–soluble starch nanocomposites. Nanotechnology 2006, 17, 5087–5095. [Google Scholar] [CrossRef]
- Li, F.; Xing, Y.; Ding, X. Silica xerogel coating on the surface of natural and synthetic fabrics. Surf. Coatings Technol. 2008, 202, 4721–4727. [Google Scholar] [CrossRef]
- Sequeira, S.; Evtuguin, D.V.; Portugal, I.; Esculcas, A.P. Synthesis and characterisation of cellulose/silica hybrids obtained by heteropoly acid catalysed sol–gel process. Mater. Sci. Eng. C 2007, 27, 172–179. [Google Scholar] [CrossRef]
- GUGUMUS, F. Developments in the UV-Stabilisation of Polymers; Applied Science Publishers: London, UK, 1979. [Google Scholar]
- Biswa, R. Das UV Radiation Protective Clothing. Open Text. J. 2010, 3, 14–21. [Google Scholar]
- Osterwalder, U.; Schlenker, W.; Rohwer, H.; Martin, E.; Schuh, S. Facts and Ficton on Ultraviolet Protection by Clothing. Radiat. Prot. Dosim. 2000, 91, 255–259. [Google Scholar] [CrossRef]
- Wang, R.H.; Xin, J.H.; Tao, X.M. UV-Blocking Property of Dumbbell-Shaped ZnO Crystallites on Cotton Fabrics. Inorg. Chem. 2005, 44, 3926–3930. [Google Scholar] [CrossRef]
- Daoud, W.A.; Xin, J.H. Low Temperature Sol-Gel Processed Photocatalytic Titania Coating. J. Sol-Gel Sci. Technol. 2004, 29, 25–29. [Google Scholar] [CrossRef]
- Xin, J.H.; Daoud, W.A.; Kong, Y.Y. A New Approach to UV-Blocking Treatment for Cotton Fabrics. Text. Res. J. 2004, 74, 97–100. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E.; Tarimala, S.; Dai, L.L. Cotton fabric surface modification for improved UV radiation protection using sol–gel process. J. Appl. Polym. Sci. 2007, 104, 111–117. [Google Scholar] [CrossRef]
- Onar, N.; Ebeoglugil, M.F.; Kayatekin, I.; Celik, E. Low-temperature, sol–gel-synthesized, silver-doped titanium oxide coatings to improve ultraviolet-blocking properties for cotton fabrics. J. Appl. Polym. Sci. 2007, 106, 514–525. [Google Scholar] [CrossRef]
- Xing, Y.; Ding, X. UV photo-stabilization of tetrabutyl titanate for aramid fibers via sol–gel surface modification. J. Appl. Polym. Sci. 2007, 103, 3113–3119. [Google Scholar] [CrossRef]
- Dong, Y.; Bai, Z.; Zhang, L.; Liu, R.; Zhu, T. Finishing of cotton fabrics with aqueous nano-titanium dioxide dispersion and the decomposition of gaseous ammonia by ultraviolet irradiation. J. Appl. Polym. Sci. 2006, 99, 286–291. [Google Scholar] [CrossRef]
- Wang, C.-C.; Chen, C.-C. Physical properties of the crosslinked cellulose catalyzed with nanotitanium dioxide under UV irradiation and electronic field. Appl. Catal. A Gen. 2005, 293, 171–179. [Google Scholar] [CrossRef]
- Park, Y.R.; Kim, K.J. Structural and optical properties of rutile and anatase TiO2 thin films: Effects of Co doping. Thin Solid Films 2005, 484, 34–38. [Google Scholar] [CrossRef]
- Sawada, K.; Sugimoto, M.; Ueda, M. Chan Hun Park Hydrophilic Treatment of Polyester Surfaces Using TiO2 Photocatalytic Reactions. Text. Res. J. 2003, 73, 819–822. [Google Scholar] [CrossRef]
- Han, K.; Yu, M. Study of the preparation and properties of UV-blocking fabrics of a PET/TiO2 nanocomposite prepared by in situ polycondensation. J. Appl. Polym. Sci. 2006, 100, 1588–1593. [Google Scholar] [CrossRef]
- Pan, Z.W.; Dai, Z.R.; Wang, Z.L. Nanobelts of Semiconducting Oxides. Science 2001, 291, 1947–1949. [Google Scholar] [CrossRef]
- Pinnavaia, T.J.; Beall, G.W. (Eds.) Polymer–Clay Nanocomposites. John Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Behnajady, M.; Modirshahla, N.; Hamzavi, R. Kinetic study on photocatalytic degradation of C.I. Acid Yellow 23 by ZnO photocatalyst. J. Hazard. Mater. 2006, 133, 226–232. [Google Scholar] [CrossRef]
- Ahmed, N.S.E.; El-Shishtawy, R.M. The use of new technologies in coloration of textile fibers. J. Mater. Sci. 2010, 45, 1143–1153. [Google Scholar] [CrossRef]
- Rilda, Y.; Fadhli, F.; Syukri, S.; Alif, A.; Aziz, H.; Chandren, S.; Nur, H. Self-cleaning TiO2-SiO2 clusters on cotton textile prepared by dip-spin coating process. J. Teknol. 2016, 78. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Y.; Xu, L.; Cai, Z.; Zhang, H. Thermal crystallization of low-temperature prepared anatase nano-TiO2 and multifunctional finishing of cotton fabrics. J. Text. Inst. 2016, 107, 651–662. [Google Scholar] [CrossRef]
- Shaban, M.; Abdallah, S.; Khalek, A.A. Characterization and photocatalytic properties of cotton fibers modified with ZnO nanoparticles using sol–gel spin coating technique. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 277–283. [Google Scholar] [CrossRef]
- Noorian, S.A.; Hemmatinejad, N.; Bashari, A. One-Pot Synthesis of Cu 2 O/ZnO Nanoparticles at Present of Folic Acid to Improve UV-Protective Effect of Cotton Fabrics. Photochem. Photobiol. 2015, 91, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Lee, S.-K. A web-based overview of semiconductor photochemistry-based current commercial applications. J. Photochem. Photobiol. A Chem. 2002, 152, 233–247. [Google Scholar] [CrossRef]
- Langlet, M.; Kim, A.; Audier, M.; Herrmann, J.M. Sol-gel preparation of photocatalytic TiO2 films on polymer substrates. J. Sol-Gel Sci. Technol. 2002, 25, 223–234. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Karkmaz, M.; Puzenat, E.; Guillard, C.; Herrmann, J.M. Photocatalytic degradation of the alimentary azo dye amaranth. Appl. Catal. B Environ. 2004, 51, 183–194. [Google Scholar] [CrossRef]
- Lo, P.-H.; Kumar, S.A.; Chen, S.-M. Amperometric determination of H2O2 at nano-TiO2/DNA/thionin nanocomposite modified electrode. Colloids Surf. B Biointerfaces 2008, 66, 266–273. [Google Scholar] [CrossRef]
- Weibel, A.; Bouchet, R.; Knauth, P. Electrical properties and defect chemistry of anatase (TiO2). Solid State Ion. 2006, 177, 229–236. [Google Scholar] [CrossRef][Green Version]
- Verran, J.; Sandoval, G.; Allen, N.S.; Edge, M.; Stratton, J. Variables affecting the antibacterial properties of nano and pigmentary titania particles in suspension. Dye. Pigment. 2007, 73, 298–304. [Google Scholar] [CrossRef]
- Norris, J.R.; Meisel, D. Photochemical Energy Conversion; Elsevier: New York, NY, USA, 1989. [Google Scholar]
- Gratzel, M. Heterogenous Photochemical Electron Transfer; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351081658. [Google Scholar]
- Kontos, A.I.; Arabatzis, I.M.; Tsoukleris, D.S.; Kontos, A.G.; Bernard, M.C.; Petrakis, D.E.; Falaras, P. Efficient photocatalysts by hydrothermal treatment of TiO2. Catal. Today 2005, 101, 275–281. [Google Scholar] [CrossRef]
- Šegota, S.; Ćurković, L.; Ljubas, D.; Svetličić, V.; Houra, I.F.; Tomašić, N. Synthesis, characterization and photocatalytic properties of sol-gel TiO2 films. Ceram. Int. 2011, 37, 1153–1160. [Google Scholar] [CrossRef]
- Kho, Y.K.; Iwase, A.; Teoh, W.Y.; Mädler, L.; Kudo, A.; Amal, R. Photocatalytic H 2 Evolution over TiO2 Nanoparticles. The Synergistic Effect of Anatase and Rutile. J. Phys. Chem. C 2010, 114, 2821–2829. [Google Scholar] [CrossRef]
- Randorn, C.; Irvine, J.T.S.; Robertson, P. Synthesis of visible-light-activated yellow amorphous TiO2 photocatalyst. Int. J. Photoenergy 2008, 2008, 426872. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.; Zhao, G.; Cai, X. Photocatalytic degradation characteristic of amorphous TiO2-W thin films deposited by magnetron sputtering. Trans. Nonferrous Met. Soc. China 2006, 16, s280–s284. [Google Scholar] [CrossRef]
- Onar, N.; Aksit, A.C.; Sen, Y.; Mutlu, M. Antimicrobial, UV-protective and self-cleaning properties of cotton fabrics coated by dip-coating and solvothermal coating methods. Fibers Polym. 2011, 12, 461–470. [Google Scholar] [CrossRef]
- Fulekar, M.H. Environmental Biotechnology; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9780429065040. [Google Scholar]
- Yuranova, T.; Mosteo, R.; Bandara, J.; Laub, D.; Kiwi, J. Self-cleaning cotton textiles surfaces modified by photoactive SiO2/TiO2 coating. J. Mol. Catal. A Chem. 2006, 244, 160–167. [Google Scholar] [CrossRef]
- Bu, S.; Jin, Z.; Liu, X.; Yang, L.; Cheng, Z. Fabrication of TiO2 porous thin films using peg templates and chemistry of the process. Mater. Chem. Phys. 2004, 88, 273–279. [Google Scholar] [CrossRef]
- Zhang, W.J.; Yang, B.; Bai, J.W. Photocatalytic Activity of TiO2/Ti Film Prepared by Sol-Gel Method on Methyl Orange Degradation. Adv. Mater. Res. 2011, 214, 65–69. [Google Scholar] [CrossRef]
- Boiteux, G.; Boullanger, C.; Cassagnau, P.; Fulchiron, R.; Seytre, G. Influence of Morphology on PTC in Conducting Polypropylene-Silver Composites. Macromol. Symp. 2006, 233, 246–253. [Google Scholar] [CrossRef]
- Lee, H.-H.; Chou, K.-S.; Shih, Z.-W. Effect of nano-sized silver particles on the resistivity of polymeric conductive adhesives. Int. J. Adhes. Adhes. 2005, 25, 437–441. [Google Scholar] [CrossRef]
- Xu, M.; Feng, J.Q.; Cao, X.L. Electrical properties of nano-silver/polyacrylamide/ethylene vinyl acetate composite. J. Shanghai Univ. 2008, 12, 85–90. [Google Scholar] [CrossRef]
- Gorenšek, M.; Recelj, P. Nanosilver Functionalized Cotton Fabric. Text. Res. J. 2007, 77, 138–141. [Google Scholar] [CrossRef]
- Jiang, S.; Newton, E.; Yuen, C.-W.M.; Kan, C.-W.; Jiang, S.-X.K. Application of Chemical Silver Plating on Polyester and Cotton Blended Fabric. Text. Res. J. 2007, 77, 85–91. [Google Scholar] [CrossRef]
- Schartel, B.; Pötschke, P.; Knoll, U.; Abdel-Goad, M. Fire behaviour of polyamide 6/multiwall carbon nanotube nanocomposites. Eur. Polym. J. 2005, 41, 1061–1070. [Google Scholar] [CrossRef]
- Xue, P.; Park, K.H.; Tao, X.M.; Chen, W.; Cheng, X.Y. Electrically conductive yarns based on PVA/carbon nanotubes. Compos. Struct. 2007, 78, 271–277. [Google Scholar] [CrossRef]
- Mondal, S.; Hu, J.L. A novel approach to excellent UV protecting cotton fabric with functionalized MWNT containing water vapor permeable PU coating. J. Appl. Polym. Sci. 2007, 103, 3370–3376. [Google Scholar] [CrossRef]
- Kim, H.-S.; Park, B.H.; Yoon, J.-S.; Jin, H.-J. Preparation and characterization of poly[(butylene succinate)-co-(butylene adipate)]/carbon nanotube-coated silk fiber composites. Polym. Int. 2007, 56, 1035–1039. [Google Scholar] [CrossRef]
- Yen, C.-Y.; Lin, Y.-F.; Hung, C.-H.; Tseng, Y.-H.; Ma, C.-C.M.; Chang, M.-C.; Shao, H. The effects of synthesis procedures on the morphology and photocatalytic activity of multi-walled carbon nanotubes/TiO2 nanocomposites. Nanotechnology 2008, 19, 045604. [Google Scholar] [CrossRef]
- Wei, Q.; Yu, L.; Wu, N.; Hong, S. Preparation and Characterization of Copper Nanocomposite Textiles. J. Ind. Text. 2008, 37, 275–283. [Google Scholar] [CrossRef]
- Dong, W.; Zhu, C.; Bongard, H.-J. Preparation and optical properties of UV dye DMT-doped silica films. J. Phys. Chem. Solids 2003, 64, 399–404. [Google Scholar] [CrossRef]
- Tiller, J. Selbststerilisierende Oberflächen. Nachr. Aus Der Chem. 2007, 55, 499–502. [Google Scholar] [CrossRef]
- Johns, K. Hygienic coatings: The next generation. Surf. Coatings Int. Part B Coatings Trans. 2003, 86, 101–110. [Google Scholar] [CrossRef]
- Ovington, L.G. Battling Bacteria in Wound Care. Home Healthc. Nurse J. Home Care Hosp. Prof. 2001, 19, 622–630. [Google Scholar] [CrossRef]
- MacKeen, P.C.; Person, S.; Warner, S.C.; Snipes, W.; Stevens, S.E. Silver-coated nylon fiber as an antibacterial agent. Antimicrob. Agents Chemother. 1987, 31, 93–99. [Google Scholar] [CrossRef]
- Knittel, D.; Schollmeyer, E. Chitosan and its derivatives for textile finishing Part 4: Permanent finishing of cotton with ionic carbohydrates and analysis of thin layers obtained. Melliand Textilb. Int. 2002, 83, 58–61. [Google Scholar]
- Gadre, S.Y.; Gouma, P.I. Biodoped Ceramics: Synthesis, Properties, and Applications. J. Am. Ceram. Soc. 2006, 89, 2987–3002. [Google Scholar] [CrossRef]
- Huang, J.; Ichinose, I.; Kunitake, T. Biomolecular Modification of Hierarchical Cellulose Fibers through Titania Nanocoating. Angew. Chemie Int. Ed. 2006, 45, 2883–2886. [Google Scholar] [CrossRef]
- Leivo, J.; Meretoja, V.; Vippola, M.; Levänen, E.; Vallittu, P.; Mäntylä, T.A. Sol–gel derived aluminosilicate coatings on alumina as substrate for osteoblasts. Acta Biomater. 2006, 2, 659–668. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Q.; Fu, F.; Liu, X. Durable antimicrobial cotton textiles modified with inorganic nanoparticles. Cellulose 2016, 23, 2791–2808. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, X.; Liang, J. Antimicrobial modification review. BioResources 2015, 10, 1964–1985. [Google Scholar]
- Ielo, I.; Giacobello, F.; Castellano, A.; Sfameni, S.; Rando, G.; Plutino, M.R. Development of Antibacterial and Antifouling Innovative and Eco-Sustainable Sol–Gel Based Materials: From Marine Areas Protection to Healthcare Applications. Gels 2022, 8, 26. [Google Scholar]
- Buket, A.R.I.K.; Seventekin, N. Evaluation of Antibacterial and Structural Properties of Cotton Fabric Coated By Chitosan/Titania and Chitosan/Silica Hybrid Sol-Gel Coatings. Text. Appar. 2011, 21, 107–115. [Google Scholar]
- Buşilă, M.; Muşat, V.; Textor, T.; Mahltig, B. Synthesis and characterization of antimicrobial textile finishing based on Ag:ZnO nanoparticles/chitosan biocomposites. RSC Adv. 2015, 5, 21562–21571. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Montazer, M. A review on the application of inorganic nano-structured materials in the modification of textiles: Focus on anti-microbial properties. Colloids Surf. B Biointerfaces 2010, 79, 5–18. [Google Scholar] [CrossRef]
- Chen, Q.; Shen, X.; Gao, H. One-step synthesis of silver-poly(4-vinylpyridine) hybrid microgels by γ-irradiation and surfactant-free emulsion polymerisation. The photoluminescence characteristics. Colloids Surf. A Physicochem. Eng. Asp. 2006, 275, 45–49. [Google Scholar] [CrossRef]
- Dimitrov, D.S. Interactions of antibody-conjugated nanoparticles with biological surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2006, 282–283, 8–10. [Google Scholar] [CrossRef]
- Liu, J.-K.; Yang, X.-H.; Tian, X.-G. Preparation of silver/hydroxyapatite nanocomposite spheres. Powder Technol. 2008, 184, 21–24. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Courtney, H.; Bettenga, M.; Agrawal, C.M.; Bumgardner, J.D.; Ong, J.L. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 2006, 27, 5512–5517. [Google Scholar] [CrossRef]
- Magaña, S.M.; Quintana, P.; Aguilar, D.H.; Toledo, J.A.; Ángeles-Chávez, C.; Cortés, M.A.; León, L.; Freile-Pelegrín, Y.; López, T.; Sánchez, R.M.T. Antibacterial activity of montmorillonites modified with silver. J. Mol. Catal. A Chem. 2008, 281, 192–199. [Google Scholar] [CrossRef]
- Esteban-Cubillo, A.; Pecharromán, C.; Aguilar, E.; Santarén, J.; Moya, J.S. Antibacterial activity of copper monodispersed nanoparticles into sepiolite. J. Mater. Sci. 2006, 41, 5208–5212. [Google Scholar] [CrossRef]
- Le Pape, H.; Solano-Serena, F.; Contini, P.; Devillers, C.; Maftah, A.; Leprat, P. Evaluation of the anti-microbial properties of an activated carbon fibre supporting silver using a dynamic method. Carbon N. Y. 2002, 40, 2947–2954. [Google Scholar] [CrossRef]
- Hu, C.H.; Xu, Z.R.; Xia, M.S. Antibacterial effect of Cu2+-exchanged montmorillonite on Aeromonas hydrophila and discussion on its mechanism. Vet. Microbiol. 2005, 109, 83–88. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Mahltig, B.; Fiedler, D.; Fischer, A.; Simon, P. Antimicrobial coatings on textiles–modification of sol–gel layers with organic and inorganic biocides. J. Sol-Gel Sci. Technol. 2010, 55, 269–277. [Google Scholar] [CrossRef]
- Poli, R.; Colleoni, C.; Calvimontes, A.; Polášková, H.; Dutschk, V.; Rosace, G. Innovative sol–gel route in neutral hydroalcoholic condition to obtain antibacterial cotton finishing by zinc precursor. J. Sol-Gel Sci. Technol. 2015, 74, 151–160. [Google Scholar] [CrossRef]
- Tarimala, S.; Kothari, N.; Abidi, N.; Hequet, E.; Fralick, J.; Dai, L.L. New approach to antibacterial treatment of cotton fabric with silver nanoparticle–doped silica using sol–gel process. J. Appl. Polym. Sci. 2006, 101, 2938–2943. [Google Scholar] [CrossRef]
- Mahltig, B.; Gutmann, E.; Meyer, D.C.; Reibold, M.; Dresler, B.; Günther, K.; Faßler, D.; Böttcher, H. Solvothermal preparation of metallized titania sols for photocatalytic and antimicrobial coatings. J. Mater. Chem. 2007, 17, 2367–2374. [Google Scholar] [CrossRef]
- Mahltig, B.; Fischer, A. Inorganic/organic polymer coatings for textiles to realize water repellent and antimicrobial properties-A study with respect to textile comfort. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1562–1568. [Google Scholar] [CrossRef]
- Rivero, P.J.; Goicoechea, J. Sol-Gel Technology for Antimicrobial Textiles; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; ISBN 9780081005859. [Google Scholar]
- Wang, S.; Hou, W.; Wei, L.; Jia, H.; Liu, X.; Xu, B. Antibacterial activity of nano-SiO2 antibacterial agent grafted on wool surface. Surf. Coatings Technol. 2007, 202, 460–465. [Google Scholar] [CrossRef]
- Mahltig, B.; Textor, T. Silver containing sol-gel coatings on polyamide fabrics as antimicrobial finish-description of a technical application process for wash permanent antimicrobial effect. Fibers Polym. 2010, 11, 1152–1158. [Google Scholar] [CrossRef]
- Li, Q.; Chen, S.-L.; Jiang, W.-C. Durability of nano ZnO antibacterial cotton fabric to sweat. J. Appl. Polym. Sci. 2007, 103, 412–416. [Google Scholar] [CrossRef]
- Rios, P.F.; Dodiuk, H.; Kenig, S.; McCarthy, S.; Dotan, A. Durable ultra-hydrophobic surfaces for self-cleaning applications. Polym. Adv. Technol. 2008, 19, 1684–1691. [Google Scholar] [CrossRef]
- Ikezawa, S.; Homyara, H.; Kubota, T.; Suzuki, R.; Koh, S.; Mutuga, F.; Yoshioka, T.; Nishiwaki, A.; Ninomiya, Y.; Takahashi, M.; et al. Applications of TiO2 film for environmental purification deposited by controlled electron beam-excited plasma. Thin Solid Films 2001, 386, 173–176. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Tabrizi, N.S. Kinetics of heterogeneous photocatalytic degradation of reactive dyes in an immobilized TiO2 photocatalytic reactor. J. Colloid Interface Sci. 2006, 295, 159–164. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N. Visible-Light-Driven N−F−Codoped TiO2 Photocatalysts. 1. Synthesis by Spray Pyrolysis and Surface Characterization. Chem. Mater. 2005, 17, 2588–2595. [Google Scholar] [CrossRef]
- Cermenati, L.; Pichat, P.; Guillard, C.; Albini, A. Probing the TiO2 Photocatalytic Mechanisms in Water Purification by Use of Quinoline, Photo-Fenton Generated OH• Radicals and Superoxide Dismutase. J. Phys. Chem. B 1997, 101, 2650–2658. [Google Scholar] [CrossRef]
- Nazari, A.; Montazer, M.; Rashidi, A.; Yazdanshenas, M.; Anary-Abbasinejad, M. Nano TiO2 photo-catalyst and sodium hypophosphite for cross-linking cotton with poly carboxylic acids under UV and high temperature. Appl. Catal. A Gen. 2009, 371, 10–16. [Google Scholar] [CrossRef]
- Keshmiri, M.; Mohseni, M.; Troczynski, T. Development of novel TiO2 sol–gel-derived composite and its photocatalytic activities for trichloroethylene oxidation. Appl. Catal. B Environ. 2004, 53, 209–219. [Google Scholar] [CrossRef]
- Fu, G.; Vary, P.S.; Lin, C.-T. Anatase TiO2 Nanocomposites for Antimicrobial Coatings. J. Phys. Chem. B 2005, 109, 8889–8898. [Google Scholar] [CrossRef]
- Chang, C.-C.; Lin, C.-K.; Chan, C.-C.; Hsu, C.-S.; Chen, C.-Y. Photocatalytic properties of nanocrystalline TiO2 thin film with Ag additions. Thin Solid Films 2006, 494, 274–278. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Lee, H.J.; Jeong, S.H. Preparation of nanocomposite fibers for permanent antibacterial effect. J. Mater. Sci. 2003, 38, 2143–2147. [Google Scholar] [CrossRef]
- Ayyad, O.; Muñoz-Rojas, D.; Oró-Solé, J.; Gómez-Romero, P. From silver nanoparticles to nanostructures through matrix chemistry. J. Nanopart. Res. 2010, 12, 337–345. [Google Scholar] [CrossRef]
- Yu, D.-G. Formation of colloidal silver nanoparticles stabilized by Na+–poly(γ-glutamic acid)–silver nitrate complex via chemical reduction process. Colloids Surf. B Biointerfaces 2007, 59, 171–178. [Google Scholar] [CrossRef]
- Nersisyan, H.H.; Lee, J.H.; Son, H.T.; Won, C.W.; Maeng, D.Y. A new and effective chemical reduction method for preparation of nanosized silver powder and colloid dispersion. Mater. Res. Bull. 2003, 38, 949–956. [Google Scholar] [CrossRef]
- Courrol, L.C.; de Oliveira Silva, F.R.; Gomes, L. A simple method to synthesize silver nanoparticles by photo-reduction. Colloids Surf. A Physicochem. Eng. Asp. 2007, 305, 54–57. [Google Scholar] [CrossRef]
- Xie, Y.; Ye, R.; Liu, H. Synthesis of silver nanoparticles in reverse micelles stabilized by natural biosurfactant. Colloids Surf. A Physicochem. Eng. Asp. 2006, 279, 175–178. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Won, S.W.; Cho, C.-W.; Kim, S.; Yun, Y.-S. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf. B Biointerfaces 2009, 73, 332–338. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; De Souza, G.I.H.; Alves, O.L.; Esposito, E. Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J. Biomed. Nanotechnol. 2007, 3, 203–208. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Mojtahedi, M.R.M.; Shoshtari, A.M.; Khosroshahi, A. Investigating the production and properties of Ag/TiO 2/PP antibacterial nanocomposite filament yarns. J. Text. Inst. 2010, 101, 204–213. [Google Scholar] [CrossRef]
- Dastjerdi, R.; Mojtahedi, M.R.M.; Shoshtari, A.M. Comparing the effect of three processing methods for modification of filament yarns with inorganic nanocomposite filler and their bioactivity against Staphylococcus aureus. Macromol. Res. 2009, 17, 378–387. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Proteomic Analysis of the Mode of Antibacterial Action of Silver Nanoparticles. J. Proteome Res. 2006, 5, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Hwang, Y.H.; Yi, S.C. Antibacterial properties of padded PP/PE nonwovens incorporating nano-sized silver colloids. J. Mater. Sci. 2005, 40, 5413–5418. [Google Scholar] [CrossRef]
- Jeong, S.H.; Yeo, S.Y.; Yi, S.C. The effect of filler particle size on the antibacterial properties of compounded polymer/silver fibers. J. Mater. Sci. 2005, 40, 5407–5411. [Google Scholar] [CrossRef]
- Stobie, N.; Duffy, B.; McCormack, D.E.; Colreavy, J.; Hidalgo, M.; McHale, P.; Hinder, S.J. Prevention of Staphylococcus epidermidis biofilm formation using a low-temperature processed silver-doped phenyltriethoxysilane sol–gel coating. Biomaterials 2008, 29, 963–969. [Google Scholar] [CrossRef]
- Wen, H.-C.; Lin, Y.-N.; Jian, S.-R.; Tseng, S.-C.; Weng, M.-X.; Liu, Y.-P.; Lee, P.-T.; Chen, P.-Y.; Hsu, R.-Q.; Wu, W.-F.; et al. Observation of Growth of Human Fibroblasts on Silver Nanoparticles. J. Phys. Conf. Ser. 2007, 61, 445–449. [Google Scholar] [CrossRef]
- Jia, X.; Ma, X.; Wei, D.; Dong, J.; Qian, W. Direct formation of silver nanoparticles in cuttlebone-derived organic matrix for catalytic applications. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 234–240. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Jeong, S.H. Preparation and characterization of polypropylene/silver nanocomposite fibers. Polym. Int. 2003, 52, 1053–1057. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Ilić, V.; Šaponjić, Z.; Vodnik, V.; Molina, R.; Dimitrijević, S.; Jovančić, P.; Nedeljković, J.; Radetić, M. Antifungal efficiency of corona pretreated polyester and polyamide fabrics loaded with Ag nanoparticles. J. Mater. Sci. 2009, 44, 3983–3990. [Google Scholar] [CrossRef]
- Potiyaraj, P.; Kumlangdudsana, P.; Dubas, S.T. Synthesis of silver chloride nanocrystal on silk fibers. Mater. Lett. 2007, 61, 2464–2466. [Google Scholar] [CrossRef]
- Simončič, B.; Klemenčič, D. Preparation and performance of silver as an antimicrobial agent for textiles: A review. Text. Res. J. 2016, 86, 210–223. [Google Scholar] [CrossRef]
- Mohamed, A.L.; El-Naggar, M.E.; Shaheen, T.I.; Hassabo, A.G. Laminating of chemically modified silan based nanosols for advanced functionalization of cotton textiles. Int. J. Biol. Macromol. 2017, 95, 429–437. [Google Scholar] [CrossRef]
- Tam, K.H.; Djurišić, A.B.; Chan, C.M.N.; Xi, Y.Y.; Tse, C.W.; Leung, Y.H.; Chan, W.K.; Leung, F.C.C.; Au, D.W.T. Antibacterial activity of ZnO nanorods prepared by a hydrothermal method. Thin Solid Films 2008, 516, 6167–6174. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, Y.; Povey, M.; York, D. ZnO nanofluids—A potential antibacterial agent. Prog. Nat. Sci. 2008, 18, 939–944. [Google Scholar] [CrossRef]
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646. [Google Scholar] [CrossRef]
- Karunakaran, C.; Rajeswari, V.; Gomathisankar, P. Enhanced photocatalytic and antibacterial activities of sol–gel synthesized ZnO and Ag-ZnO. Mater. Sci. Semicond. Process. 2011, 14, 133–138. [Google Scholar] [CrossRef]
- Ansari, M.A.; Khan, H.M.; Khan, A.A.; Sultan, A.; Azam, A. Characterization of clinical strains of MSSA, MRSA and MRSE isolated from skin and soft tissue infections and the antibacterial activity of ZnO nanoparticles. World J. Microbiol. Biotechnol. 2012, 28, 1605–1613. [Google Scholar] [CrossRef]
- Wang, H.; Xie, C.; Zhang, W.; Cai, S.; Yang, Z.; Gui, Y. Comparison of dye degradation efficiency using ZnO powders with various size scales. J. Hazard. Mater. 2007, 141, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Farouk, A.; Moussa, S.; Ulbricht, M.; Schollmeyer, E.; Textor, T. ZnO-modified hybrid polymers as an antibacterial finish for textiles. Text. Res. J. 2014, 84, 40–51. [Google Scholar] [CrossRef]
- Znaidi, L. Sol–gel-deposited ZnO thin films: A review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).