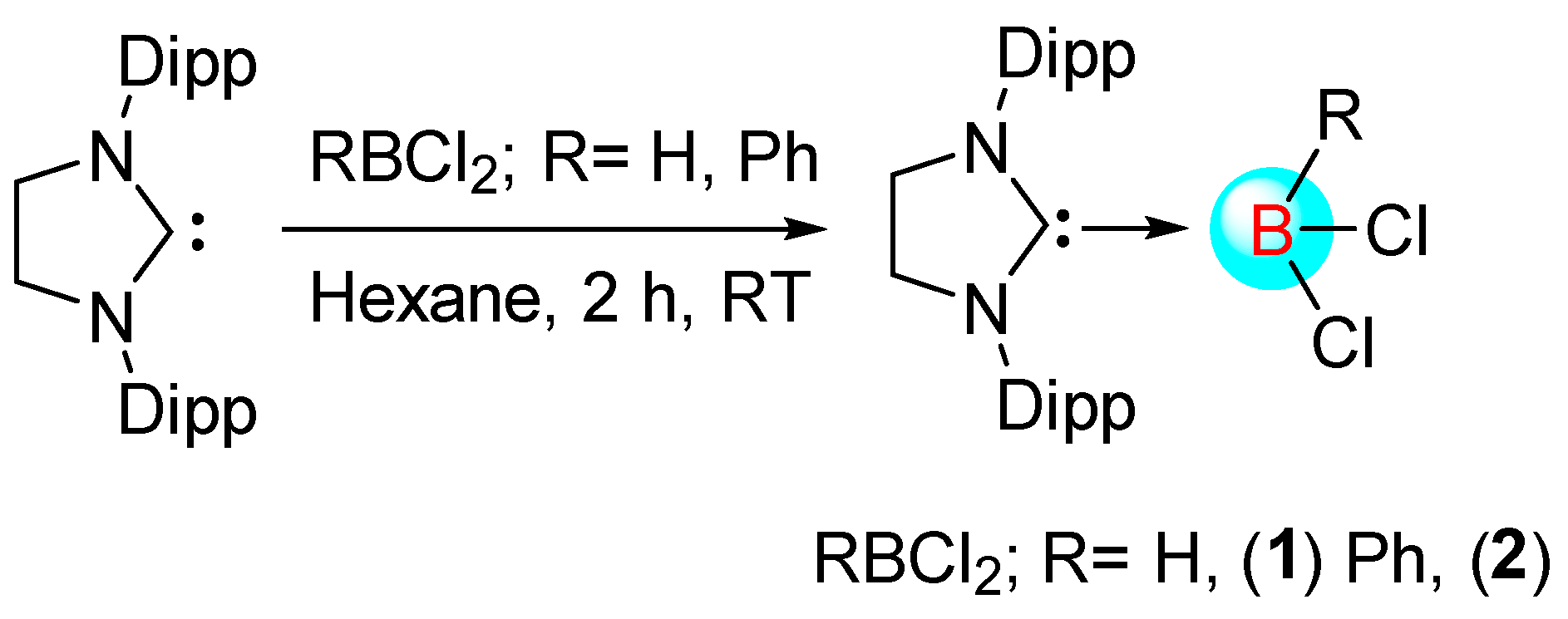
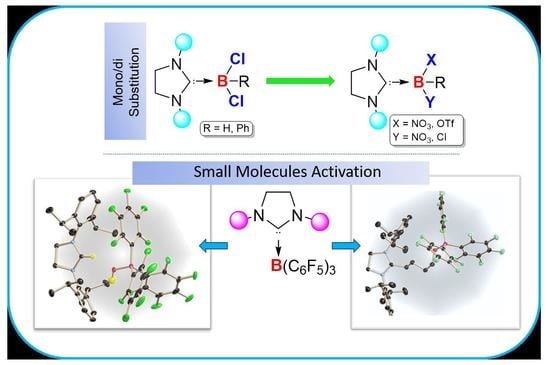
1. Introduction
While less than two decades ago, the N-heterocyclic carbene-borane adducts were conceived rare and exotic, they are now readily accessible owing to rapid synthetic development and constitute an important class of compounds because they display different chemistry from the most existing classes of boron compounds such as boranes or borates [
1]. Since the unveiling of the concept that NHC·boranes undergo nucleophilic substitution at boron by the groups of Fensterbank, Lacôte, Malacria, and Curran, the chemistry of these systems has flourished [
2,
3,
4,
5,
6]. However, these systems largely rely on imidazole-2-ylidene type carbenes. Braunschweig and coworkers recently broadened the range of Lewis base by demonstrating nucleophilic addition and substitution of cyclic alkyl amino carbene (
cAAC) BH
3 adduct [
7]. In our previous works, we have shown the nucleophilic substitution at 5-SIDipp·MeBCl
2 (5-SIDipp = 1,3,-bis-(2,6-diisopropylphenyl)imidazolin-2-ylidine) [
8]. Further, we have explored the substitution at 6-SIDipp∙BH
3 (6-SIDpp = 1,3-di(2,6-diisopropylphenyl) tetrahydropyrimidine-2-ylidene) center and the introduction of rare functional groups such as -OTf, –NO
3 [
9]. Although the saturated NHCs are more nucleophilic than its unsaturated analogue, the earlier work on the synthesis of more than two dozen of NHC∙borane and haloborane complexes relied on the unsaturated five-membered NHC [
1,
2,
3,
4,
5,
6,
10,
11,
12,
13,
14,
15,
16]. Clearly, the extension of unexplored carbenes in NHC·borane chemistry is desirable, as it may lead to the discovery of a range of interesting new applications. Due to our current interest in 5-SIDipp [
17,
18,
19], we have prepared here haloboranes 5SIDipp·BHCl
2,
1 and 5SIDipp·BPhCl
2,
2 and studied their substitution reactions with AgOTf, AgNO
3, and water. Furthermore, we have shown that the combination 5-SIDipp and B(C
6F
5)
3 led to the activation of THF and diethyl ether via frustrated Lewis pair (FLP) way. Our results are reported herein.
2. Results and Discussion
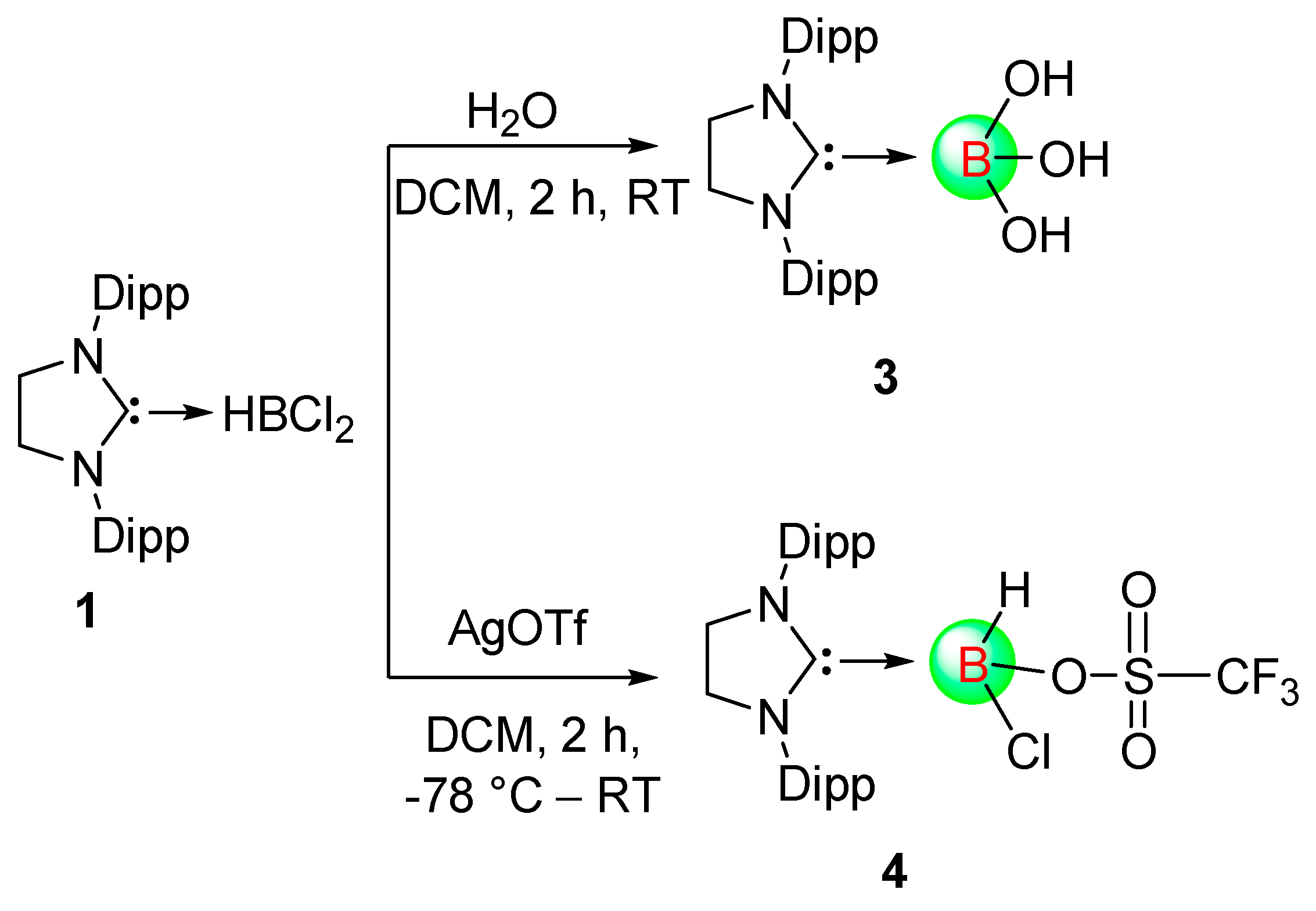
In our previous work, we have reported the first carbene MeBCl
2 adduct via salt metathesis procedure [
8]. In this work, we have prepared the 5SIDipp·haloborane adducts and shown their reactivities towards nucleophilic substitution. The addition of BHCl
2∙dioxane in the solution of 5SIDipp in
n-hexane gives the white precipitation of 5-SIDipp·BHCl
2,
1 at room temperature (
Scheme 1). The precipitate was further dissolved in toluene and dichloromethane to afford colorless crystals of
1 at −36 °C. The
11B NMR spectrum of
1 displays a resonance at 6.9 ppm as a sharp singlet. The backbone four protons appeared at 4.08 ppm in the
1H NMR spectrum.
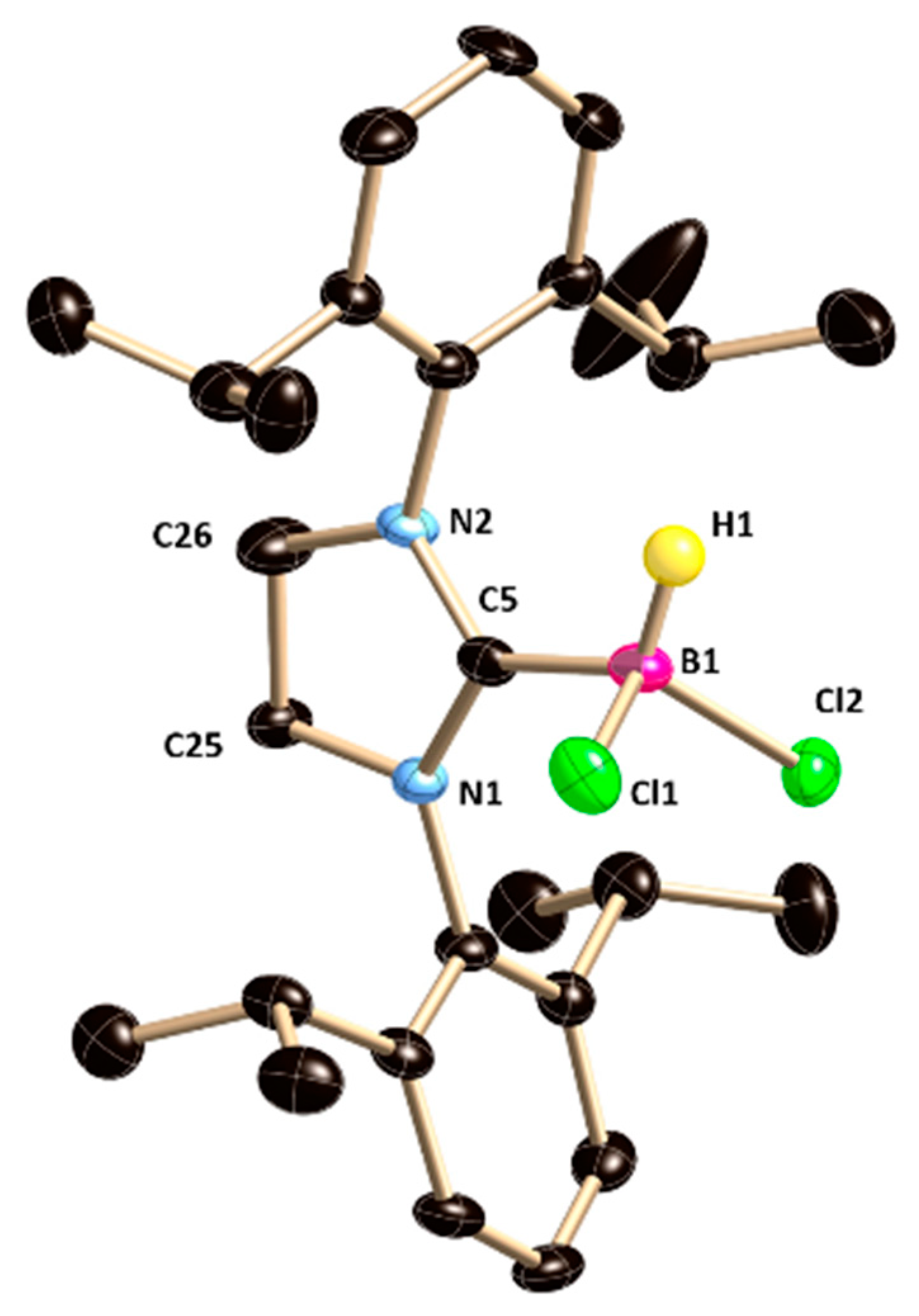
1 is characterized by single-crystal X-ray diffraction studies (
Figure 1).
1 is crystalized in the monoclinic
P2
1/
n space group. The B–C bond length in
1 [1.628(7) Å] is in good accordance with the previously reported 5-SIDipp·MeBCl
2 [1.6261(19) Å], but considerably longer compared to that in the 5-SIDipp·BH
3 [1.593(4) Å] [
8]. The increase in the bond length can be ascribed to the enhancement of steric hindrance at the central boron atom. The average B–Cl distance is 1.86 Å, which matches with the previously reported carbene-haloborane adducts (NHC·BCl
3, NHC·BRCl
2, and NHC·BR
2Cl).
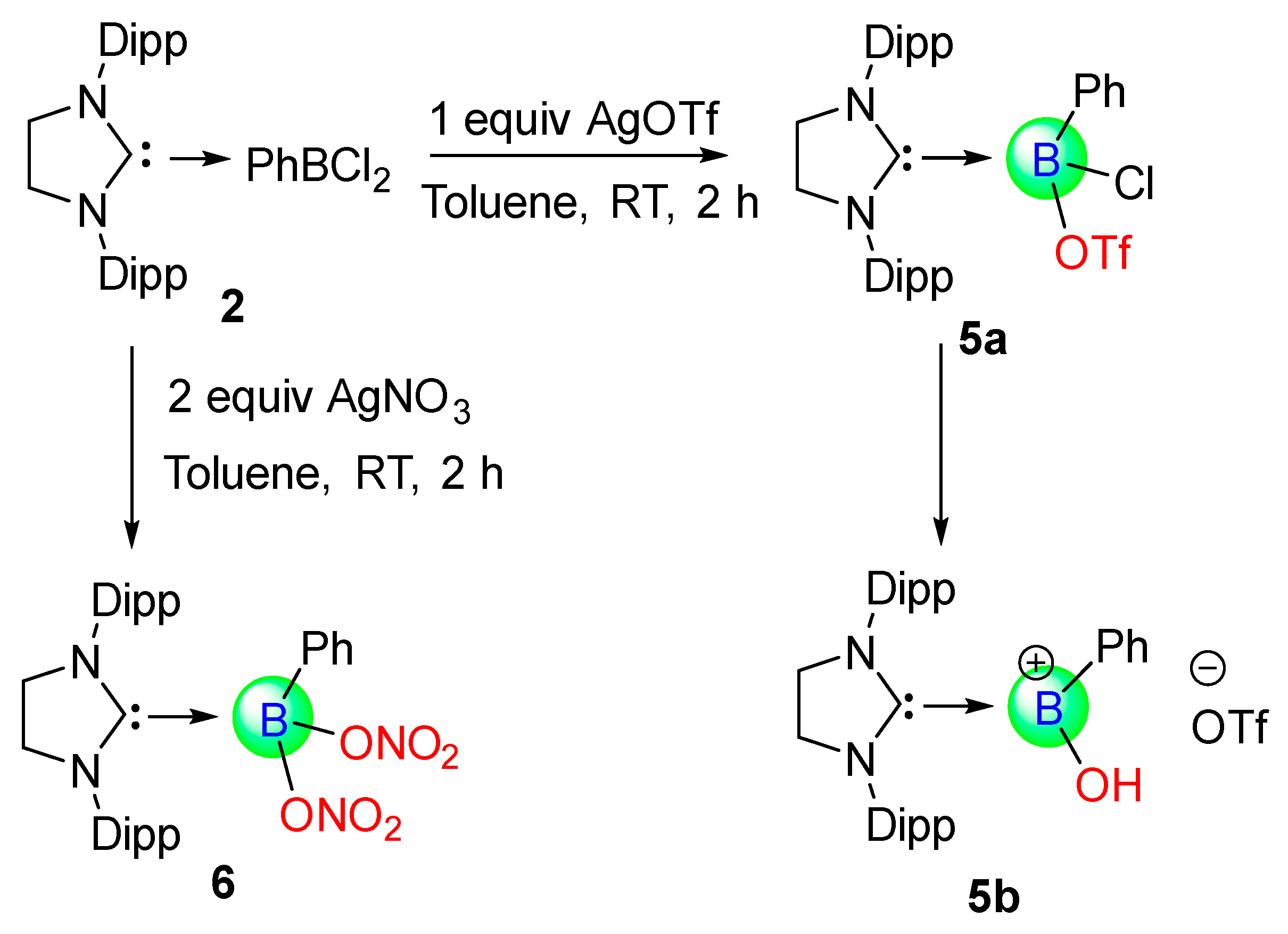
The reaction of 1.1 equivalent of PhBCl
2 with 5-SIDipp in
n-hexane gives an immediate white precipitate formation of 5-SIDipp∙PhBCl
2 (
2) (
Scheme 1). The
11B NMR spectrum of
2 shows one resonance at 1.8 ppm.
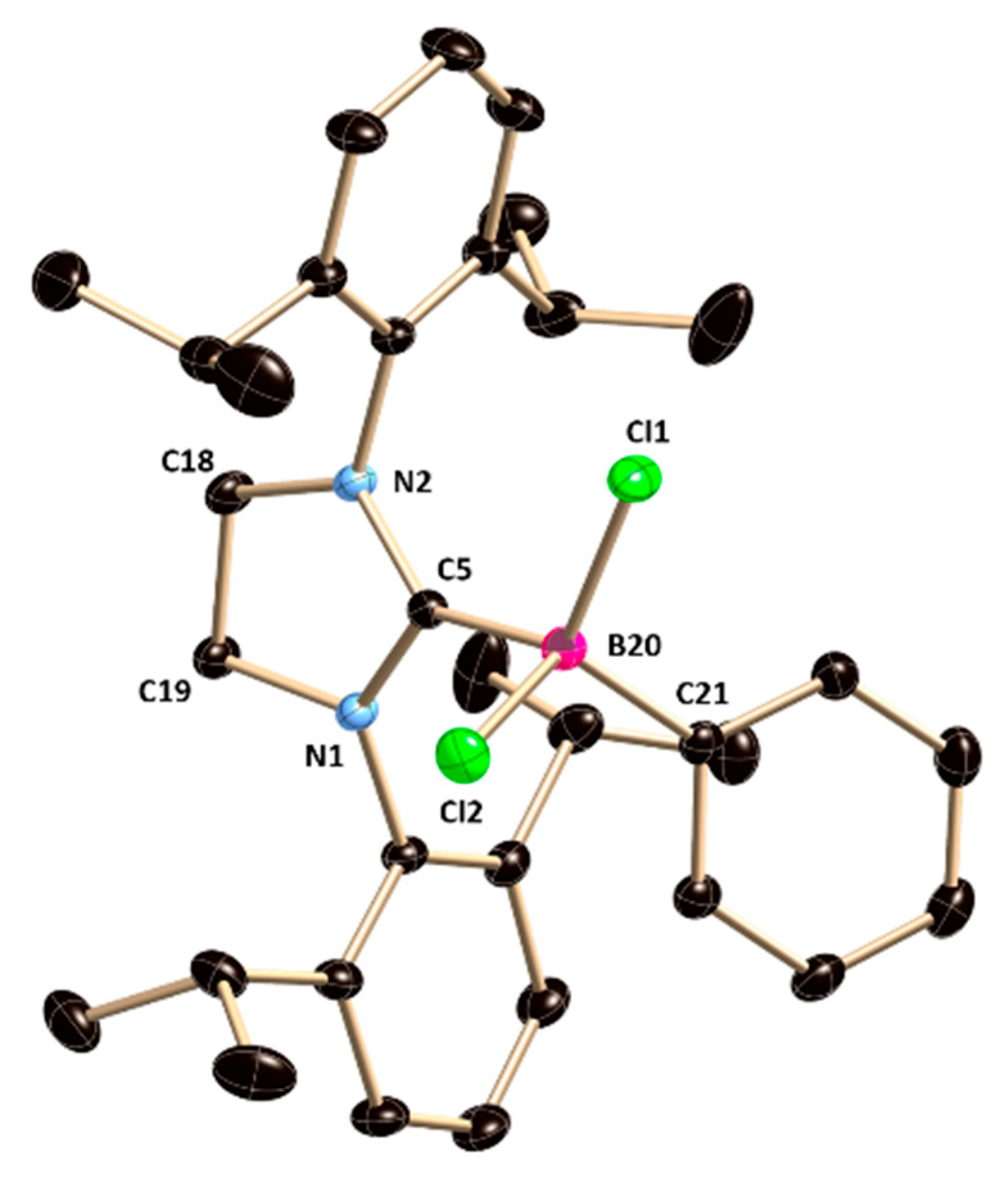
2 crystallizes in the monoclinic
P2
1/n space group (
Figure 2). The carbene carbon atom C5 is tri-coordinated and features a trigonal-planar geometry, and the boron atom connected with the C5 atom adopts a tetrahedral geometry. The B−C
NHC bond distance is 1.6661(10) Å, which is slightly longer in comparison to that in
1 due to the steric congestion of the phenyl group at the boron center. The B–Cl bonds are 1.8992(8) Å and 1.8698(8) Å, which match with the previously reported B–Cl bond distance [
8].
Treatment of 1.05 equivalents of water in a dichloromethane solution of
1 hydrolyzes all the B–H and B–Cl bonds and forms NHC-stabilized boric acid, 5-SIDipp∙B(OH)
3,
3 (
Scheme 2) exclusively. In our earlier work, we have reported the isolation of 6-SIDipp·B(OH)
3 as a minor product from the reaction of Br
2/H
2O with 6-SIDipp·BH
3 [
9]. Replacement of the chloride and the hydride groups by hydroxide moieties in
3 is accompanied by an upfield shift in the
11B NMR spectrum (−1.6 ppm) from that of
1.
3 crystallizes in the monoclinic
P2
1/
c space group (
Figure 3). The central boron-carbon distance is 1.650(5) Å, which is marginally shorter compared to that in 6-SIDipp·B(OH)
3. The average B– (OH) bond distances are 1.38 Å.
Further, we added silver triflate to a dichloromethane solution of
1 at −78 °C, which replaced one of the labile chlorine atoms by the triflate group (
Scheme 2). In the
11B NMR spectrum of
4, the resonance for the central boron atom appears at −3.4 ppm. The resonance at −76.7 ppm in the
19F NMR is characteristic of the triflate group attached to the central boron atom. Colorless crystals of
4 suitable for X-ray diffraction studies were grown from a saturated toluene solution at 4 °C. The constitution of
4 was authenticated by a single-crystal X-ray study (
Figure 4).
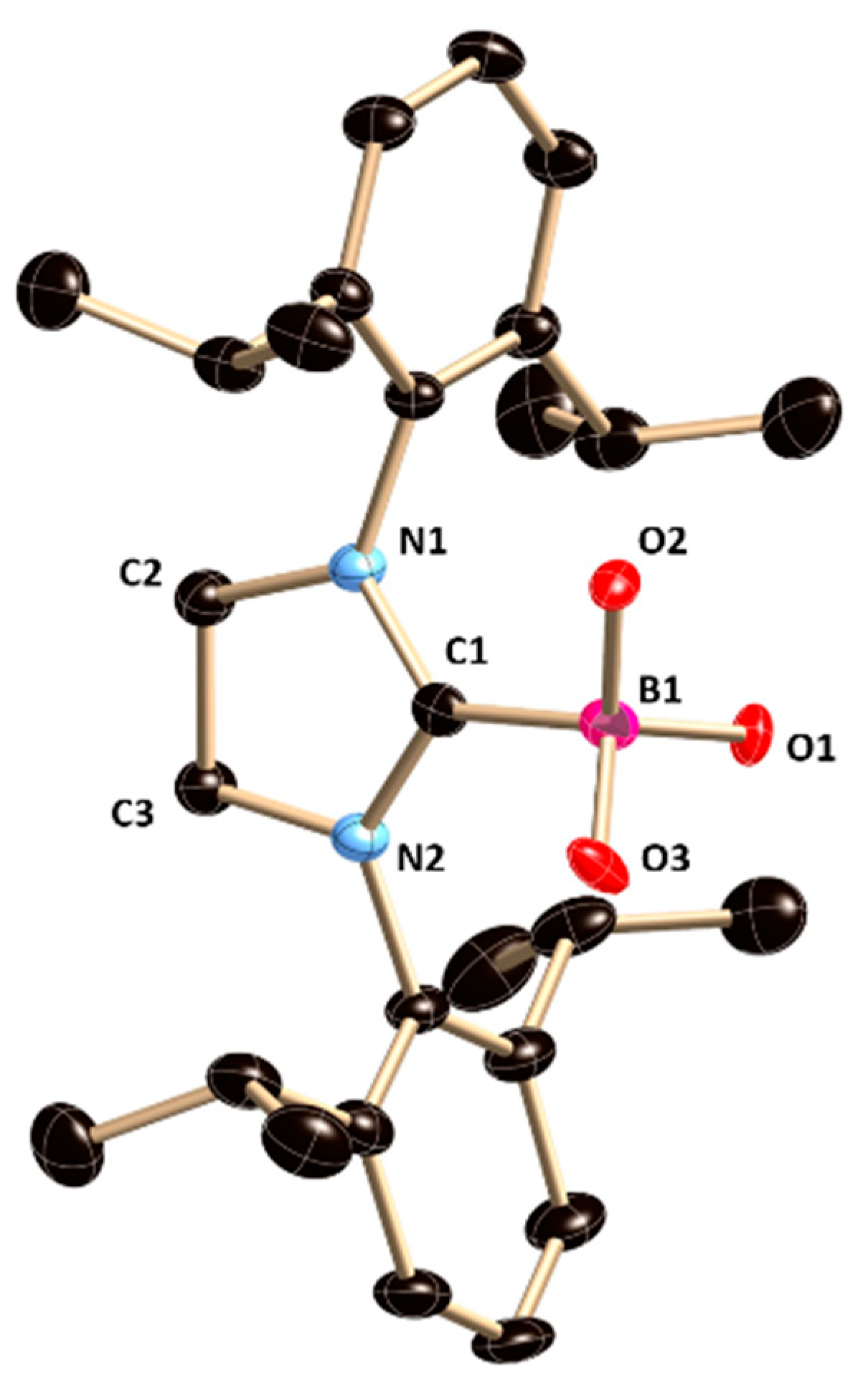
4 crystallizes in the monoclinic space group
P2
1/
n. The relevant bond length and angles are given in the legend of
Figure 4. All the four substituents on the central boron atom are different in
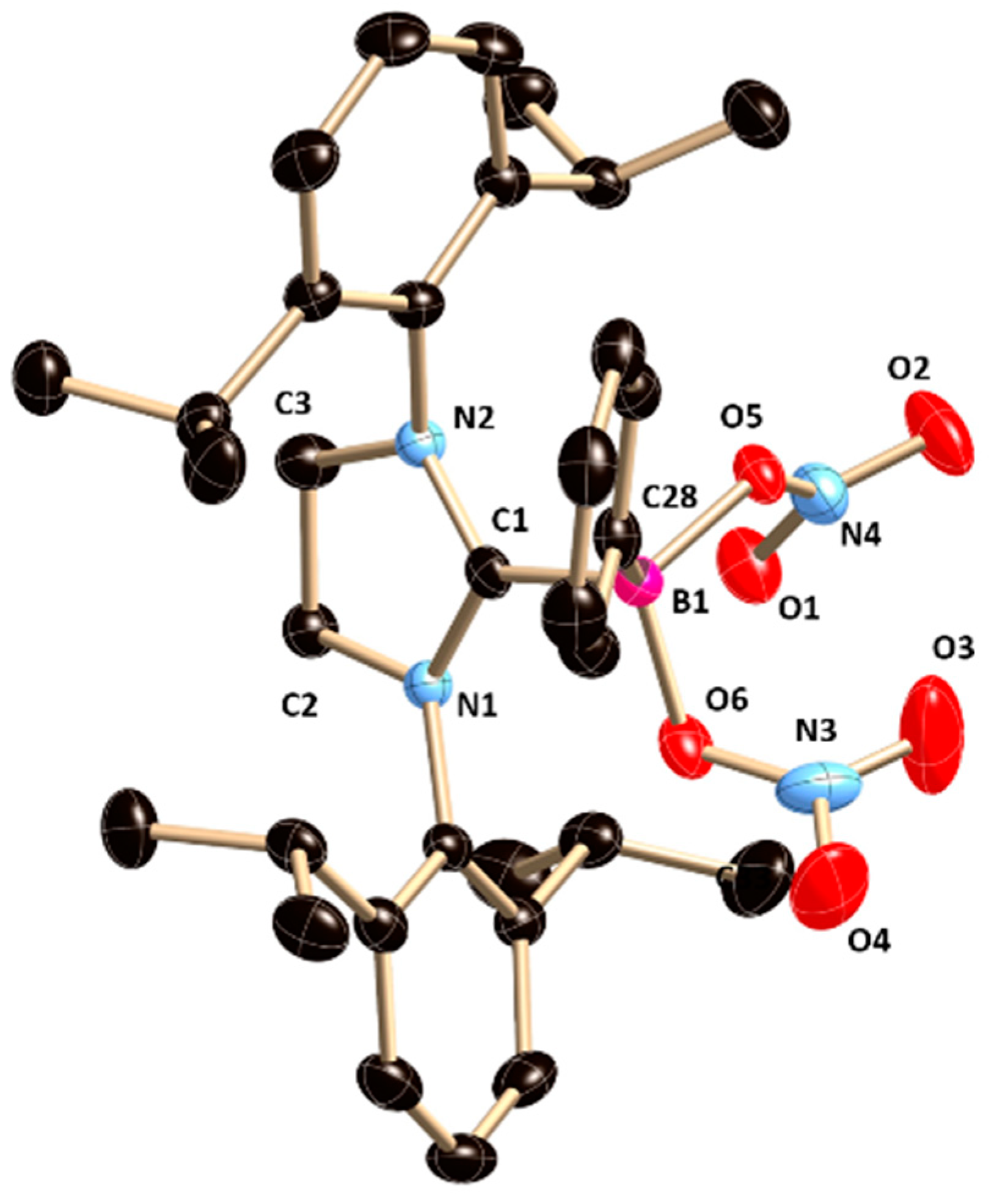
4. The central boron atom (B1) lies slightly below the plane of an imidazolinium ring (torsion angles (deg): C(2)−N(1)−C(1)−B(1) = 174.4(2) and C(3)−N(2)−C(1)−B(1) −166.5(2)) and the B−O bond is not orthogonal to the plane of the imidazolinium ring with the torsion angles N1−C1−B1−O1 = −41.6 (2)° and N2−C1−B1−O1 = 148.4 (2)°. The B−OTf bond length is 1.515(2) Å, which is in good agreement with the B−O bond length in our previously reported 5-SIDipp∙BMeOTfCl (B1−O3 1.503(3) Å) [
8].
Treatment of one and two equivalents of AgOTf and AgNO
3 with
2 in toluene afforded
mono-triflate and
di-nitro substituted 5-SIDipp·boranes, respectively. Substitution of one and two chlorine atoms from the tetra coordinated boron atom of
2 resulted in the formation of 5-SIDipp·BPhCl(OTf),
5a, and 5-SIDipp-BPh(ONO
2)
2,
6 (
Scheme 3). The functional groups such as nitrate and triflate are rarely found to bind with the boron atom [
5,
9,
20,
21,
22,
23,
24,
25,
26].
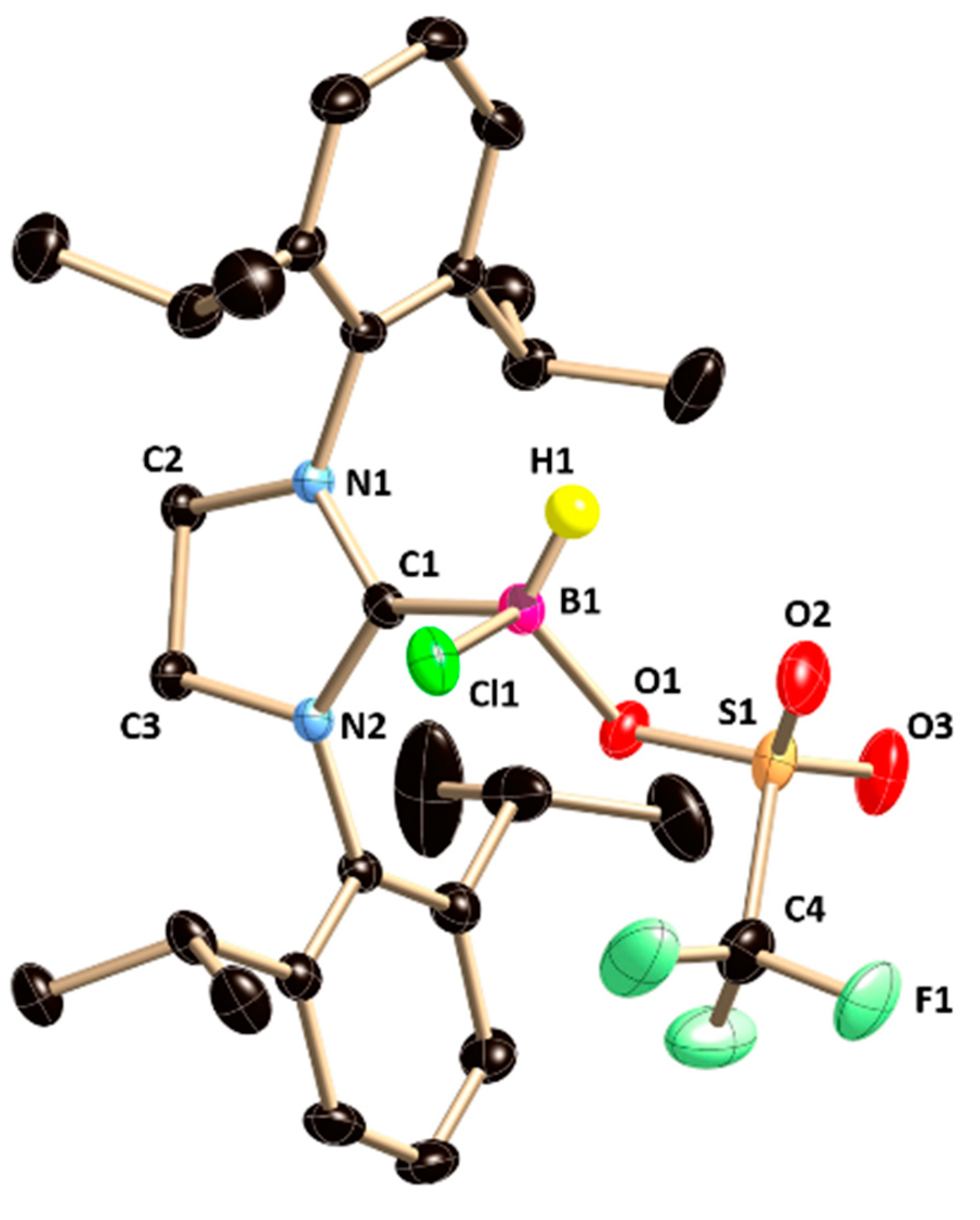
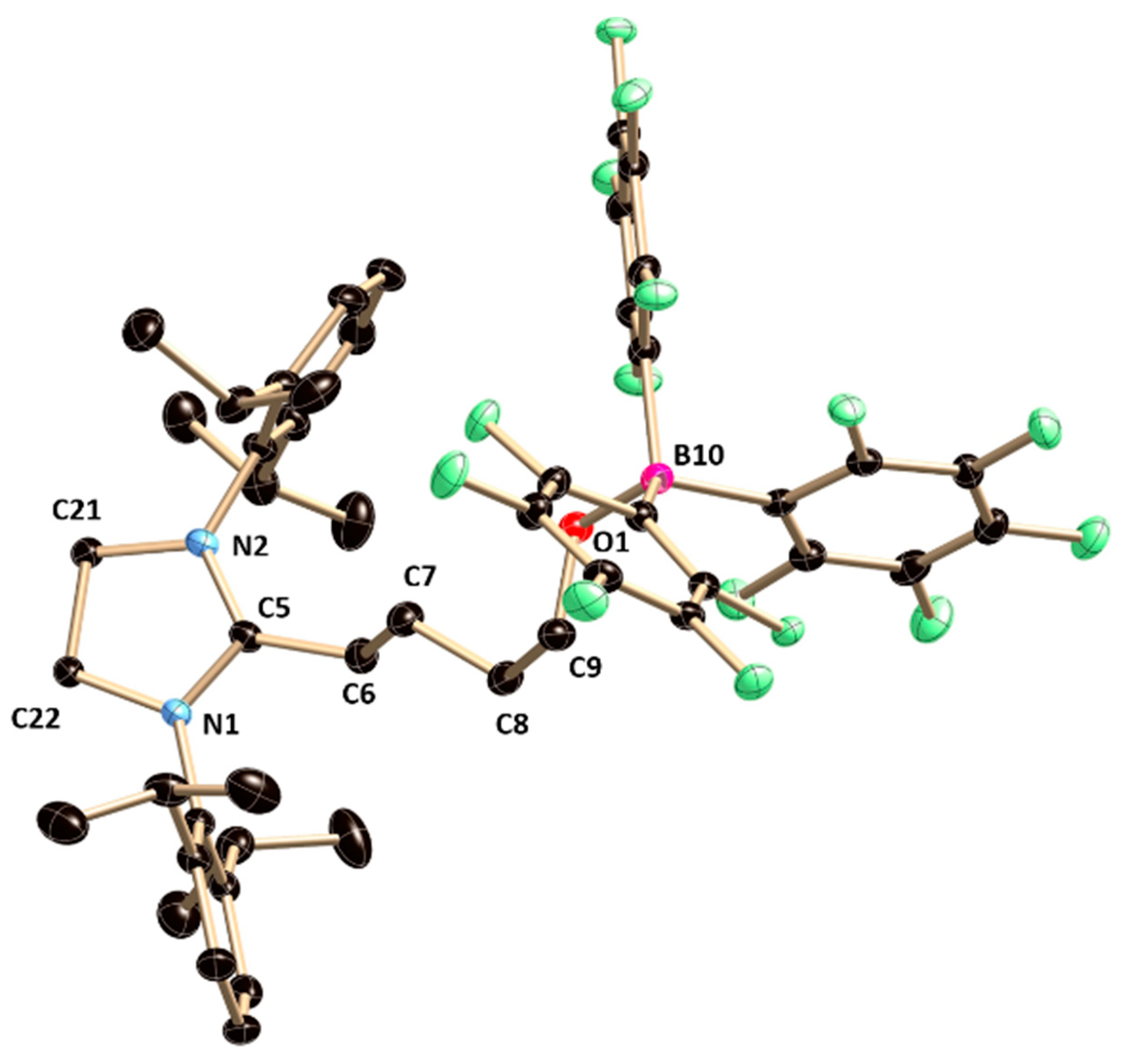
5a crystallizes in the monoclinic
P2
1/
n space group (
Figure 5). The boron atom lies on a tetrahedral geometry, which can be confirmed from the bond angles around the boron atoms in
5a (C5–B2–Cl1 100.54(11), C5–B2–O1 106.51(13), and C5–B2–C7 118.55(14)). The B–C
NHC bond length in
5a (1.648(2) Å) is in well agreement with that in
2. The B–O and B–Cl bonds in
5a is almost orthogonal to the plane (torsion angle: N1–C5–B2–Cl1 76.96(17)°, N2-C5–B2–Cl1 −89.6(2)° and torsion angle: N1–C5–B2–O1 −170.96(14)°, N2–C5–B2–O1 22.5(2)°, respectively). However, the spectroscopic characterization of
5a becomes complicated because of solvent-mediated slow hydrolysis. The only signal in the
11B NMR spectra of the product,
5b appears at 30.9 ppm as a singlet, which is indicative of a three-coordinated boron center, instead of a four-coordinated boron, as expected in
5a. We regrow the crystals from the NMR tube and realized that there is hydrolysis taking place at the B–Cl bond with adventitious water leading to 5-SIDipp stabilized borenium cation, with a triflate as a counter anion (
5b). The constitution of
5b rationalizes the resonance at 30.9 ppm in the
11B NMR spectrum. Although the formation of the
5b clearly can be seen from the molecular structure (
Figure S2), but due to low-quality data we refrain from discussing its structural parameters. However, even after repeated attempts, we were unable to stop this hydrolysis and hence, could not characterize
5a spectroscopically. The CF
3 group of the triflate moiety in
5b resonates at −78.6 ppm in the
19F NMR, which is slightly different from the resonances of triflates bound to the boron atom (−76.7 ppm in
4) and is characteristic of the free triflate anion.
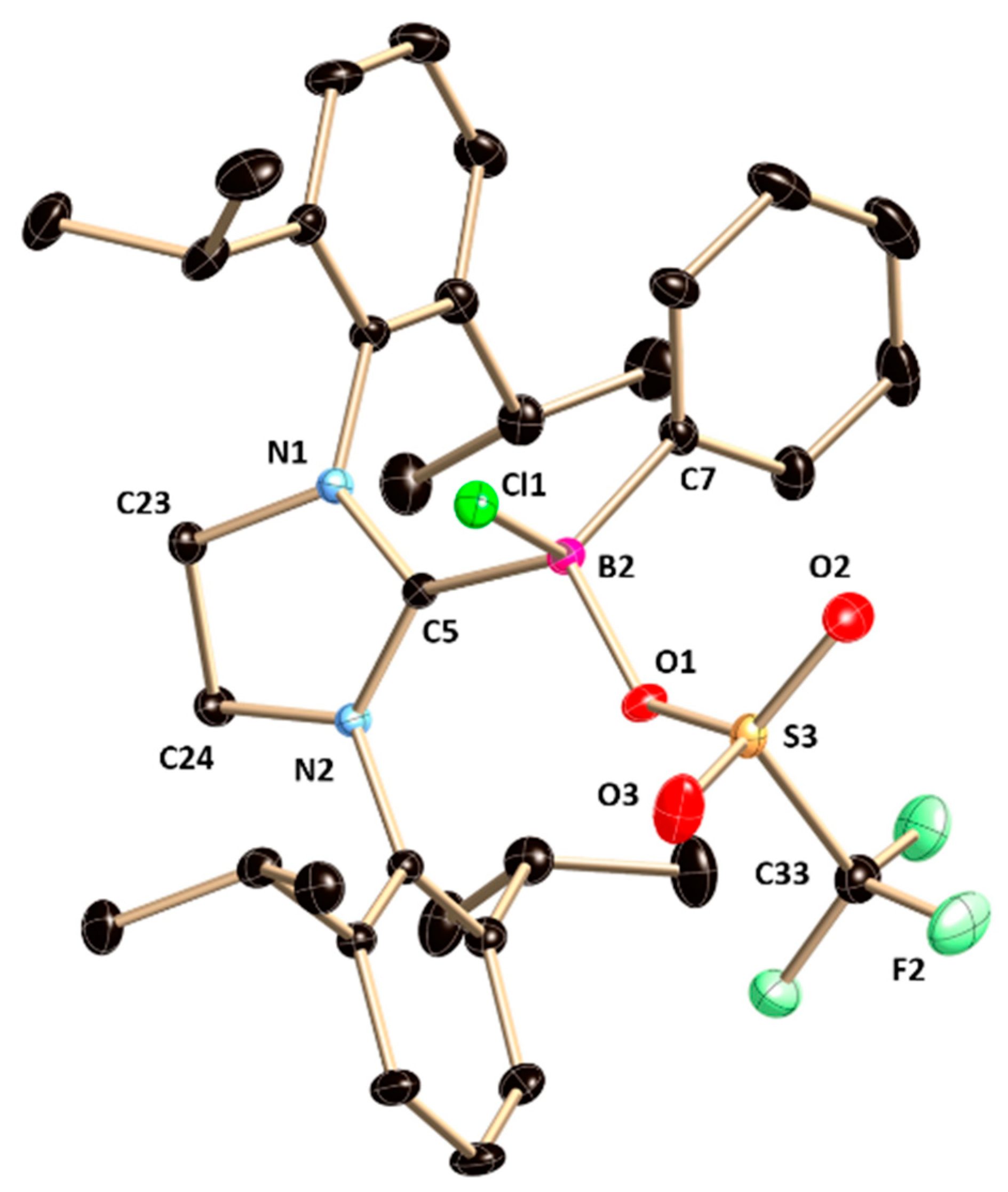
In the
11B|NMR,
6 shows resonance at 4.2 ppm, shifted slight low-field with respect to that in
2 (1.9 ppm), presumably due to electron-withdrawing nature of the ONO
2 moieties. The solid-state structure of
6 also was confirmed by X-ray crystal analysis.
6 crystallizes in the monoclinic
P2
1/
c space group (
Figure 6) and important structural parameters are given in the legend of
Figure 6.
The combination of N-heterocyclic carbene and B(C
6F
5)
3 has been exploited in FLP chemistry [
27]. In our previous work, we have demonstrated the adduct formation between 5-SIDipp and B(C
6F
5)
3 [
8]. We have prepared the adduct in toluene/
n-hexane. When we performed the same reaction in THF or diethyl ether, it led to the activation of those etheral solvents. The THF solution of the 5-SIDipp·B(C
6F
5)
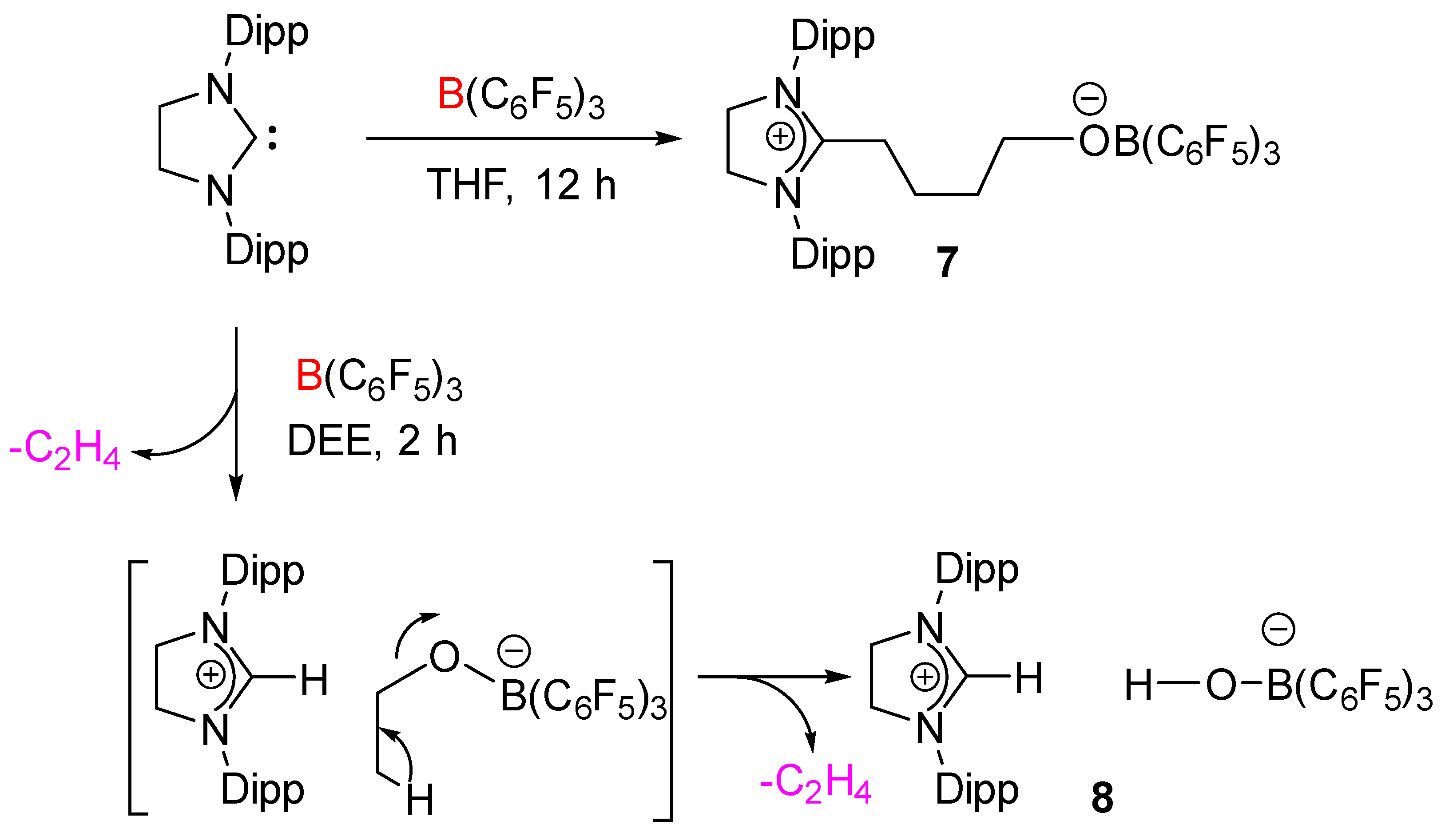
3 was kept for 12 h at room temperature, which afforded the zwitterionic species
7 in quantitative yield as a white solid (
Scheme 4). The molecular structure of
7 was additionally established by X-ray diffraction analysis (
Figure 7).
7 crystallizes in the triclinic
P space group. One of the C–O bonds in the THF molecule is cleaved, and as a result, the THF ring becomes acyclic and inserts between the Lewis pairs. Similar to the case for 5-SIDipp·B(C
6F
5)
3,
7 is not stable in solution at room temperature, so we were unable to satisfactorily characterize it by NMR spectroscopy. The
11B NMR resonance at −2.8 ppm is similar to those established for the tetra-coordinated boron compounds. The C5 atom adopts a trigonal planar geometry, which is confirmed by the sum of the bond angles [N1–C5–N2 112.29(15)°, N1–C5–C6 125.21(15)°, N2–C5-C6 122.50(14)°]. The C5–C6 bond distance is marginally shorter compared to the adjacent C–C bond (C5–C6 1.501(2) Å and C6–C7 1.539(2) Å). The boron atom adopts a tetrahedral geometry. The C–O (1.404(2) Å) and the B–O (1.453(2) Å) bond distances are similar to the other previously reported structures [
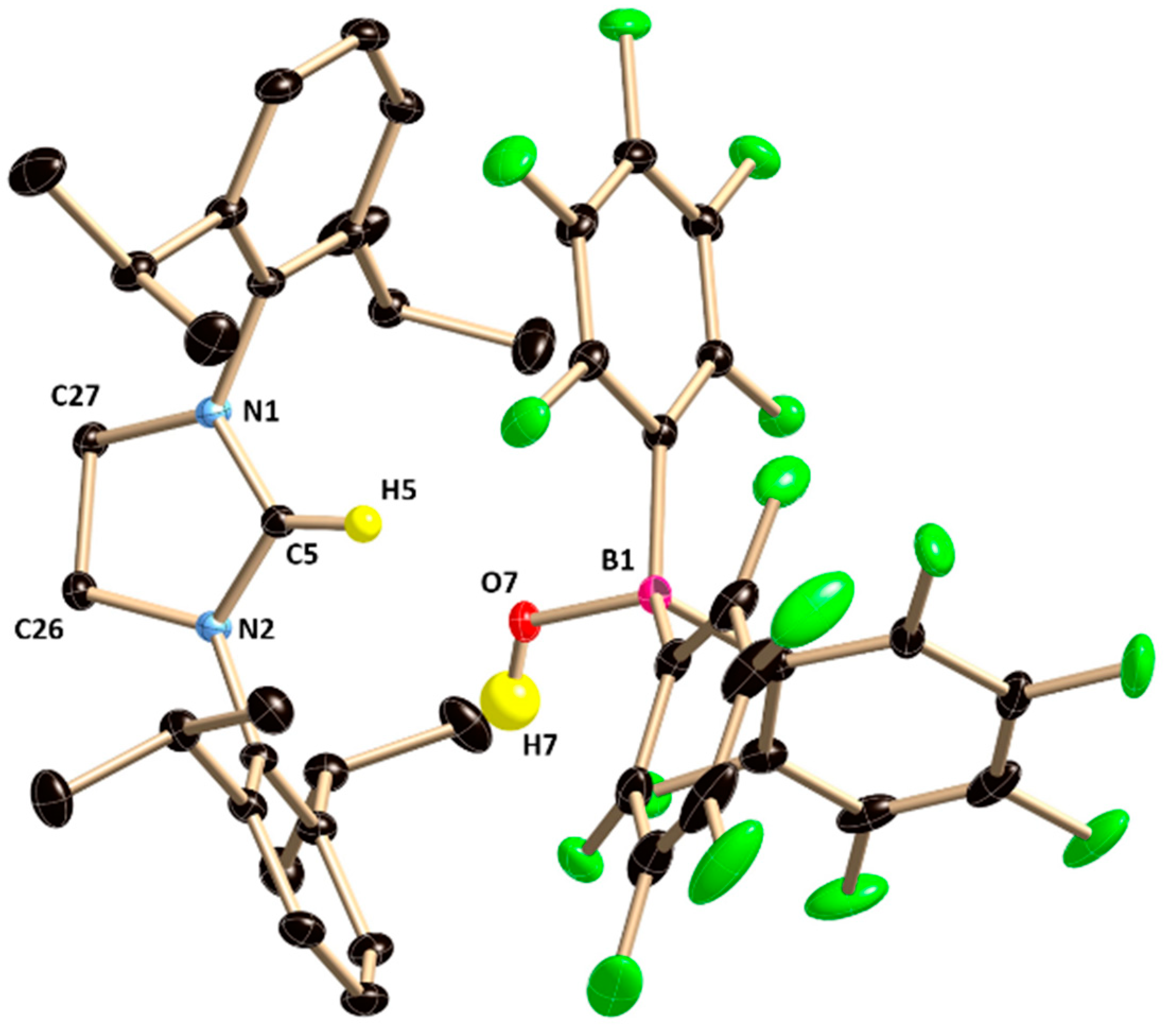
28]. This reactivity was extended to diethyl ether (DEE), which resulted in the isolation of imidazolinium salt with a borate counter-anion (
8). This compound presumably results from activation of the C–O bond of the diethyl ether with concomitant elimination of two ethylene molecules (
Scheme 4). A signal at 8.9 ppm in the
1H NMR spectrum confirms the presence of an imidazolinium cation. The
11B NMR displays the characteristic resonance at −4.2 ppm, which can be assigned to a tetrahedral [(HO)B(C
6F5)
3] anion. X-ray crystallographic analysis later confirmed the structure of
8 (
Figure 8).
4. General Procedures and Instrumentation
All manipulations were carried out in an inert atmosphere of argon using standard Schlenk techniques and in argon filled glove box. The solvents, especially toluene, tetrahydrofuran, dichloromethane, and
n-hexane were purified by MBRAUN solvent purification system MB SPS-800. Other chemicals were purchased from Sigma Aldrich and TCI Chemicals and were used without further purification. The starting material, 5-SIDipp, was synthesized by using the literature procedure [
29].
1H,
13C,
11B NMR, and
19F spectra were recorded in CDCl
3, using Bruker Avance DPX 400, or a Bruker Avance DPX 500 spectrometer. CDCl
3 was dried by distillation over CaH
2. Chemical shifts (δ) are given in ppm. NMR spectra were referenced to external SiMe
4 (
1H and
13C), BF
3·OEt
2 (
11B), CFCl
3 (
19F) respectively.
1: A slightly excess of BHCl2·dioxane (0.20 mL, 0.58 mmol) was added to a 10 mL hexane solution of 5-SIDipp (0.20 g, 0.48 mmol) at room temperature in a Schlenk flask. Stirring the resulted mixture for 2 h at room temperature resulted in the generation of a white precipitate. Colorless crystals of 1 were isolated after keeping the white powder in the mixture of 1 mL dichloromethane and 2 mL toluene solution at −36 °C. Yield = 0.20 g (90%).
1H NMR (400 MHz, 298 K, CDCl3):
δ = 1.29 (d,
J = 6.88 Hz, 12 H, CH(C
H3)
2), 1.40 (d,
J = 6.63 Hz, 12 H, CH(C
H3)
2), 3.17 (sept,
J = 6.75 Hz, 4 H, C
H(CH
3)
2), 4.08 (s, 4 H, NC
H2C
H2N), 7.23 (d,
J = 7.75 Hz, 4 H, Ar–
H), 7.40 (t,
J = 7.75 Hz, 2 H, Ar–
H) ppm (
Figure S1).
13C{1H} NMR (101 MHz, 298 K, CDCl3):
δ = 23.4, 26.0, 28.9, 53.1, 124.4, 129.8, 133.2, 146.1 ppm (
Figure S2).
11B{1H} NMR (128 MHz, 298 K, CDCl3): δ = 6.9 (s, 1B,
BHCl
2) ppm (
Figure S3).
2. A slightly excess of BPhCl2·dioxane (0.090 g, 0.58 mmol) was added to a 10 mL hexane solution of 5-SIDipp (0.20 g, 0.48 mmol) at room temperature in a flask. Stirring the resulted mixture for further 2 h at room temperature accessed a white precipitate. Colorless crystals of 2 were isolated after keeping the white powder in the mixture of 1 mL dichloromethane and 2 mL toluene solution. Yield = 0.24 g (90%).
1H NMR (400 MHz, 298 K, CDCl3):
δ = 1.42 (d,
J = 6.72 Hz, 12 H, CH(C
H3)
2), 1.51 (d,
J = 6.65 Hz, 12 H, CH(C
H3)
2), 3.39 (sept,
J = 6.75 Hz, 4 H, C
H(CH
3)
2), 4.22 (s, 4 H, NC
H2C
H2N), 6.95 (d,
J = 6.88 Hz, 2 H,
ortho–
H of B-
Ph), 7.25 (d,
J = 7.75 Hz, 4 H, Ar–
H), 7.26 (bs, 1 H,
para-
H of B–
Ph), 7.44 (t,
J = 7.73 Hz, 2 H, Ar–
H), 7.49 (t,
J = 7.88 Hz,
meta–
H of B–
Ph) ppm (
Figure S4).
13C{1H} NMR (101 MHz, 298 K, CDCl3):
δ = 23.2, 26.2, 28.9, 53.9, 124.2, 129.6, 133.5, 135.2, 145.7 ppm (
Figure S5).
11B{1H} NMR (128 MHz, 298 K, CDCl3): δ = 1.8 (s, 1 B,
BPhCl
2) ppm (
Figure S6).
3: 1.05 equivalent of water (0.01 g, 0.54 mmol) was added drop by drop to a 15 mL dichloromethane solution of 1 (0.20 g, 0.53 mmol) at room temperature in a flask. Stirring the resulted mixture for further 2 h at room temperature accessed a clear solution. The reaction mixture was concentrated to 5 mL and kept at 4 °C to obtain the colorless crystals of 3. Yield = 0.36 g (80%).
1H NMR (400 MHz, 298 K, CDCl3):δ = 1.31 (d,
J = 6.97 Hz, 12 H, CH(C
H3)
2), 1.35 (d,
J = 6.85 Hz, 12 H, CH(C
H3)
2), 3.07 (sept,
J = 6.85 Hz, 4 H, C
H(CH
3)
2), 4.06 (s, 4 H, NC
H2C
H2N), 7.23 (d,
J = 7.70 Hz, 4 H, Ar–
H), 7.39 (t,
J = 7.75 Hz, 2 H, Ar–
H) ppm (
Figure S7).
13C{1H} NMR (101 MHz, 298 K, CDCl3):
δ = 23.4, 25.4, 28.9, 53.3, 67.9, 124.2, 129.6, 133.1, 146.0 ppm (
Figure S8).
11B{1H} NMR (128 MHz, 298 K, CDCl3): δ = −1.6 (s, 1 B,
B(OH)
3) ppm (
Figure S9).
4: A DCM solution (20 mL) of 1 (0.47 g, 1 mmol) was added dropwise to a DCM solution (20 mL) of previously weighed AgOTf (0.25 g, 1 mmol) at −78 °C in the absence of light. A white precipitate of AgCl was formed immediately, and it was filtered through frit filtration after the reaction mixture was warmed to room temperature. The colorless toluene solution was concentrated (5 mL) and kept for crystallization at 4 °C, which afforded colorless crystals of 4 after 1−2 day(s). Yield = 0.48 g (82%).
1H NMR (400 MHz, 298 K, CDCl3):
δ = 1.31 (d,
J = 6.88 Hz, 12 H, CH(C
H3)
2), 1.40 (d,
J = 6.63 Hz, 12 H, CH(C
H3)
2), 3.13 (sept,
J = 7.63 Hz, 4 H, C
H(CH
3)
2), 4.11 (s, 4 H, NC
H2C
H2N), 7.20 (d,
J = 7.38 Hz, 4 H, Ar–
H), 7.42 (t,
J = 7.63 Hz, 2 H, Ar–
H) ppm (
Figure S10).
13C{1H} NMR (101 MHz, 298 K, CDCl3):
δ = 21.4, 23.2, 25.9, 26.3, 28.9, 53.4, 124.7, 128.2, 130.2, 132.4, 137.8, 145.7, 145.9 ppm (
Figure S11).
11B{1H} NMR (128 MHz, 298 K, CDCl3): δ = 3.4 (s, 1 B,
BH(OTf)Cl) ppm (
Figure S12).
19F{1H} NMR (377 MHz, 298 K, CDCl3): δ = −76.7 (s, 3 F, OSO
2C
F3) ppm (
Figure S13).
5b: A toluene solution (20 mL) of 2 (0.55 g, 1 mmol) was added dropwise to a toluene solution (20 mL) of previously weighed AgOTf (0.25 g, 1 mmol) at −30 °C in the absence of light. A white precipitate of AgCl formed immediately, and it was filtered through frit filtration after the reaction mixture was warmed to room temperature. The colorless toluene solution was concentrated (5 mL) and was kept for crystallization at 4 °C, which afforded colorless crystals of 5b after 1 day. Yield = 0.25 g (45%).
1H NMR (400 MHz, 298 K, CDCl3):
δ = 1.24 (d,
J = 6.88 Hz, 12 H, CH(C
H3)
2), 1.38 (d,
J = 6.63 Hz, 12 H, CH(C
H3)
2), 2.99 (sept,
J = 6.75 Hz, 4 H, C
H(CH
3)
2), 4.59 (s, 4 H, NC
H2C
H2N), 7.29 (d,
J = 7.75 Hz, 4 H, Ar–
H), 7.48 (t,
J = 7.75 Hz, 2 H, Ar–
H), 7.52 (t,
J = 7.50 Hz, 2 H,
meta-H of B–
Ph), 7.61 (t,
J = 7.38 Hz,
para–H of B–
Ph), 8.25 (d,
J = 6.75 Hz, 2 H,
ortho–H of B–
Ph) ppm (
Figure S14).
13C{1H} NMR (101 MHz, 298 K, CDCl3):
δ = 21.4, 23.8, 25.1, 29.2, 54.7, 125.0, 127.9, 129.0, 135.6, 137.8, 146.2 ppm (
Figure S15).
11B{1H} NMR (128 MHz, 298 K, CDCl3): δ = 30.9 (s, 1 B,
BPh(OH)) ppm (
Figure S16).
19F{1H} NMR (377 MHz, 298 K, CDCl3): δ = −78.6 (s, 3 F, OSO
2C
F3) ppm (
Figure S17).
6: A toluene solution (20 mL) of 2 (0.55 g, 1 mmol) was added dropwise to a toluene solution (20 mL) of previously weighed AgNO3 (0.34 g, 2 mmol) at −30 °C in the absence of light. A white precipitate of AgCl was formed slowly, and it was filtered via frit filtration after 6 h. The colorless toluene solution was concentrated (5 mL) and was kept for crystallization at 4 °C, which afforded colorless crystals of 6 after 2 days. Yield = 0.37 g (62%).
1H NMR (400 MHz, 298 K, CDCl3):
δ = 1.12 (d,
J = 6.75 Hz, 12 H, CH(C
H3)
2), 1.20 (d,
J = 6.75 Hz, 12 H, CH(C
H3)
2), 3.13 (sept,
J = 6.75 Hz, 4 H, C
H(CH
3)
2), 4.15 (s, 4 H, NC
H2C
H2N), 6.50 (d,
J = 6.88 Hz, 2 H,
ortho–H of B–
Ph), 6.89 (t,
J = 7.38 Hz, 2 H,
meta–H of B–
Ph), 7.01 (t,
J = 7.25 Hz,
para−H of B–
Ph), 7.24 (d,
J = 7.88 Hz, 4 H, Ar–
H), 7.46 (t,
J = 7.75 Hz, 2 H, Ar–
H), ppm (
Figures S18 and S19).
13C{1H} NMR (101 MHz, 298 K, CDCl3):
δ = 22.4, 26.8, 28.8, 53.8, 124.6, 127.1, 131.6, 134.5, 146.2 ppm (
Figure S20).
11B{1H} NMR (128 MHz, 298 K, CDCl3): δ = 4.2 (s, 1 B,
BPh(NO
3)
2) ppm (
Figure S21).
7: A THF solution (5 mL) of 5-SIDipp (0.382 g, 1 mmol) was added dropwise to a THF solution (20 mL) of previously weighed B(C6F5)3 (0.512 g, 1 mmol) at room temperature. The reaction mixture turned to a clear colorless solution immediately and run for 12 h. The solution was dried completely and 3 mL of toluene solution was added to dissolve the white solid product. Colorless crystals of 7 were afforded after keeping the toluene solution for crystallization at 4 °C after 2 days. Yield = 0.45 g (46%). The formation of 7 was accompanied by some other side products, which could not be identified. Hence, we did not have a spectroscopically pure product to record the 1H and 13C NMR.
11B{1H} NMR (128 MHz, 298 K, CDCl3):
δ = −2.8 (s, 1 B,
B(C
6F
5)
3) ppm (
Figure S23).
8: A diethyl ether solution (5 mL) of 5-SIDipp (0.382 g, 1 mmol) was added dropwise to a diethyl ether solution (5 mL) of previously weighed B(C6F5)3 (0.512 g, 1 mmol) at room temperature. The reaction mixture turned to a clear colorless solution immediately and run for 12 h. The solution was dried completely and 3 mL of toluene solution was added to dissolve the white solid product. Colorless crystals of 8 were afforded after keeping the toluene solution for crystallization at 4 °C after 1 day. Yield = 0.78 g (85%).
1H NMR (400 MHz, 298 K, CDCl3):
δ = 1.20 (d,
J = 6.88 Hz, 12 H, CH(C
H3)
2), 1.35 (d,
J = 6.88 Hz, 12 H, CH(C
H3)
2), 2.87 (sept,
J = 6.75 Hz, 4 H, C
H(CH
3)
2), 4.39 (s, 4 H, NC
H2C
H2N), 7.29 (d,
J = 7.75 Hz, 4 H, Ar–
H), 7.54 (t,
J = 7.88 Hz, 2 H, Ar–
H), 8.90 (s, 1 H, N–C
H–N) ppm (
Figure S24).
13C{1H} NMR (101 MHz, 298 K, CDCl3):
δ = 23.9 24.4, 29.4, 53.6, 60.8 125.2, 128.6, 131.9, 145.6, 160.3 ppm (
Figure S25).
11B{1H} NMR (128 MHz, 298 K, CDCl3):
δ = −4.2 (s, 1 B, HO–
B(C
6F
5)
3) ppm (
Figure S26).
19F{1H} NMR (377 MHz, 298 K, CDCl3):
δ = −135.6, −162.4, −166.1 (15 F, B(C
6F5)
3) ppm (
Figure S27).