1. Introduction
Acid rain is a major problem for animals, plants, buildings, and also for the top glass of photovoltaic (PV) solar panels and greenhouses [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22]. Acid rain is considered one of the most dangerous factors of local pollution. This rain possesses higher levels of hydrogen ions (H
+) because of the contamination of sulfuric and nitric acids. Northeast America (Menz, Seip, 2004), Central Europe (Ilomuanya 2019, Menz, Seip, 2004), Malaysia (Omar, Rindam 2011), and China (Zhang 2017) have been identified as the largest acid rain affected regions in the world [
1,
2,
3,
4,
5,
6,
7,
8].
The pollutants from air such as NOx, NH
3, and H
2S can mix with water from atmosphere to form acid rain [
1]. The pH of water is neutral (pH 6–7). Generally, any rain with a pH below six is considered acid rain. For example, the pH value of lemon juice is two, and the pH of vinegar is three. Typical pH values of acid rain for anthropogenic emissions may be in the range of 3.5–5 (Kim et al., 2007) [
1,
2]. Water with a pH of 4 is about 20 times more acidic than normal rain.
Acid rain not only damages the natural ecosystem, but also man-made materials [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24]. Buildings, streets, outdoor sculptures, glass isolators for electric field distribution, etc. have been subject to attack by weathering: sun, wind, frost, and the effects of rain [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24]. Acid rain can accelerate the rate of this damage. Sometimes, acid rain is called “the unseen plague” [
1]. The effects of acid rain must be reduced for the welfare of the global ecosystem [
1].
The influence of different types of acid rain (sulfuric acid type acid rain, nitric acid type acid rain, and mixed type acid rain) on the stability of semi-flexible pavement, have been reported [
7].
Du (2020) studied the effects of mixed acid rain with different molar ratios of SO
42− and NO
3− (0:1, 1:0, 2:1, 1:1, and 1:2) on stream leaf breakdown through a microcosm experiment [
10]. A significant inhibition of leaf breakdown rate was observed when the ratio was 1:2, with reduced microcosm pH, fungal biomass, and enzyme activities, as well as the frequencies of hub generally found in the fungal community [
10].
Du (2022) showed the combined effects of acid rain and ZnO nanoparticles on plant litter decomposition through a microcosm experiment [
8]. The decomposition rate of plant litter was increased by 123–204% by co-exposures [
8].
Livingston [
11] reported that acid rain damages the cultural heritage, particularly outdoor marble and bronze sculptures. This study involved the development of pH vs. SO
42− phase diagrams for marble (calcium carbonate) and bronze (copper-tin) under ambient conditions [
11]. They used modeling of the acid neutralization for pH in the 3.5–6 range typical in wet deposition [
11]. In the case of marble, the theoretical results for Ca
2+ ions lost due to acid rain neutralization is 0.158 mmol/L compared to 10.5 mmol/L by dry deposition. For bronze, the Cu
2+ ion losses are theoretically 0.21 mmol/L and practically 47.3 mmol/L. Therefore, dry deposition effects have the potential to dominate over wet deposition effects [
11].
Most of the historic buildings in Malaysia commonly use building materials such as (1) timber, (2) stone, (3) brick, and (4) plaster in their construction [
5]. Acid rain is characterized as erosive and decolorate. It impacts construction materials like iron, zinc roofing, and limestone [
5]. Acid rain causes the discoloration of paint and rocks such as limestone, reducing the aesthetical value of buildings and bridges [
5]. An analysis of the status of acid deposition has shown some slight variation in pH values of the rainfall between regions in Malaysia [
5]. The pH level of rain was between 4.2 and 4.4 [
5].
Medieval stained-glass windows constitute a part of cultural heritage that has been exposed to environmental damage over centuries [
12]. This provides an exceptional opportunity to analyze the durability of glass and to identify the long-term environmental corrosion processes on glass [
12]. In the case of historic buildings, both rocks and glasses have been exposed to the same atmospheric conditions, which allows for a comparison of the corrosion mechanisms on both materials [
12]. Medieval stained glass (13th–15th centuries) from three restoration works (the Santa Maria del Mar and the Pedralbes Monastery church in Barcelona, and the Tarragona Cathedral in the northwestern Mediterranean Region) have been studied to characterize glass decay [
12]. The stained-glass windows were built around 1330, and since that time, the monastery and church have been under the auspices of the Barcelona municipality. There are very well-preserved sets of stained glass with exceptional preservation of original glass pieces in their medieval lead frameworks [
12]. Over the centuries, later damage and repairs have led to a mixture of old glass panels with new ones, the most important modifications probably dating from 1808 and 1939 [
12]. The windows show original black-fired designs [
12]. Electron microprobe analysis provided evidence of two types of glass: an Na-rich type (stable, Mediterranean, of Roman-like tradition), and a K-Ca-rich type, similar to coeval Central European medieval stained glasses [
12]. The defects defined as destructive (micro- and meso-pitting) and constructive (patina and micro-crust) were observed the on glass surfaces [
12]. This type of decay in the K-Ca-rich glasses is in terms of thickness reduction in flat glass was at an order of magnitude less than that that commonly found in Central European countries with a continental climate [
12]. Macroscopic and microstructural studies allowed for the identification of biodeterioration decay, with chemical effects similar to those for pure chemical hydration leaching and corrosion, associated with biomineralization by unspecific sulfate (gypsum, syngenite) and calcite mineralization, as well as bio-induced (weddellite, whewellite, etc.) mineralization [
12]. The authors established the following succession of bands from unaltered glass to the surface: a gel-like hydrated silica glass (depleted in most elements), beige-orange patina, and a sulfate weathering crust mostly consisting of gypsum and, locally, with interstitial K-Ca (syngenite) or K-Mg sulfates [
12]. Some extreme pathologies such as dramatic thinning of glass pieces, significant physical decay, and the development of generalized corrosions and associated thick crusts are absent. Several factors can be invoked to explain this. Widespread use of Na-rich glass, characterized by silica contents around 60% in weight, is a primary factor that explains the good preservation of these stained-glass pieces; the role of silica as a network-forming agent has also been clearly shown [
12]. Therefore, it seems useful to concentrate on the K-rich glasses present in the same windows to determine if the decay processes and pathologies are comparable to those described for Central European medieval windows. The lesser degree of decay in the Mediterranean Region can be interpreted in terms of different stages of the same processes [
12]. This implies that differences in decay are related to different kinetics of similar processes related to the same agents [
12]. The basic chemistry of the process of stained-glass decay is well known: first, attack by water allows for the formation of hydroxides, cation migration, and loss of a superficial film by leaching and hydration. Next, the subsequent conversion of these hydroxides to carbonates by carbon dioxide in the atmosphere takes place, and finally, the conversion of those carbonates to sulfates occurs [
12]. During this process, the surface of the glass can develop several microchemical environments from case to case, as a function of the different chemistry of stained glasses [
12]. It is not easy to quantify the decay in medieval stained glasses, since they do not display a homogeneous thickness as does industrial glass [
12]. The measured thickness of the K and K-Ca glasses is around 2–3 mm on average, while Na glass can be thicker (3 to 4 mm) [
12]. The authors concluded that the decay effects are on around 20 to 30 times greater for glasses in continental climates with an Atlantic influence where the mean rainfall is in the range of 750–900 mm/year [
12].
While the issue of maintenance has been highlighted, building and stained-glass window conservation should also be taken into consideration.
A sustainable and green energy supply is required for human life and the economic development of populations all over the world. Photovoltaic (PV) materials convert sunlight into electrical energy. A single PV device is known as a solar cell. The life of solar cell operation is about 10 years. Thin-film solar cells are of great interest because of their high-cost effectiveness as compared with monocrystalline cells [
9]. PV solar cells suffer from a loss in efficiency due to corrosion of the top glass [
9]. The top glasses used for solar cells are borosilicate or soda-lime glasses. They are characterized by high optical transmittance at low cost [
9]. It was discovered that atmospheric water vapor adsorbed on the glass surface can lead to corrosion of the glass surface [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24]. The chemical resistance of glass is conventionally designated as the opposition of the glass subjected to attack from aqueous solutions and atmospheric agents (water vapor, CO
2, NOx, SO
2) [
9,
23,
24]. Depending on the region of the world, the acid rain can contain sulfuric acid (H
2SO
4), nitric acid (HNO
3), and carbonic acid(H
2CO
3) in different concentrations. The alteration of glass is based on its reaction with adsorbed or condensate water. Such corrosion is not as effective, but becomes much stronger under the action of temperature (60–90 °C). The solar cells working temperature is 100 °C. The top glasses of solar cells are materials resistant to weak acids, and the greatest damage is done by nitric acid.
The formation of nitric acid from gases and rain is shown in the following reactions:
Using the isotopic water vapor (H
218O) for hydration experiments performed at 90 °C, Kudriavtsev (2019) demonstrated that the interaction between water vapor and borosilicate differed from the interaction between water vapor and soda-lime glass [
9]. In the first case, the formation of a hydrogenated layer was observed over time, due to the reaction of water with the glass network only at the surface and subsequent reactions between protons and glass modifiers (oxides of trivalent metals) with the formation of hydroxides [
9]. The water vapor adsorbed on the surface of soda-lime glass resulted in the ion-exchange reaction between H and alkali metals (Na, K) in the glass, with the formation of a hydrated layer that grew with time [
9].
When the glass surface is in contact with an aqueous acidic medium, the H+ ions of the medium can replace alkaline and transitional atoms (M) from the glass surface [
9,
23].
An experiment to simulate the real process of solar cell glass superstrate corrosion occurring in open air was conducted using the hydration of borosilicate glass in water vapor with 100% humidity at 88 °C over a period of two months [
9]. When water molecules penetrate into the soda-lime silicate glass, sodium and potassium leave the near surface layer and form a “sediment layer” of alkali salts on the surface [
9]. In the case of glass hydration in water, the alkalis dissolve in water, leaving the surface unpolluted [
9]. In the case of borosilicate glass, the transmittance decreased insignificantly during the hydration experiment [
9]. The difference in the observed effects of corrosion can be explained by the difference in the mechanisms of the water vapor–glass surface interactions in those glasses [
9]. It is important to notice that the water vapor–glass interaction differs significantly from the water–glass interaction [
9]. The authors observed a strong participation of alkali metals in the glass corrosion process for both types of glasses [
9]. For the alkali-free borosilicate glass Corning 1737 hydrated under the same conditions and during the same period, neither hydration nor hydrogenation can be observed [
9]. Thus, alkali-free glasses should be used for photovoltaic applications [
9].
In order to protect glass surface against acid rain, thin films with chosen compositions and anticorrosive properties can be deposited on glass using different methods.
It is well known that Ta2O5 is an oxide with anticorrosive properties, but it is expensive and cannot form glass by itself. ZnO is an oxide known as a glass former and exhibiting good optical properties.
The literature reports the use of crystalline ZnO films, amorphous and crystalline Ta
2O
5 films, Zn doped Ta
2O
5 films, ZnO/Ta
2O
5 successive crystalline films, and ZnO-Ta
2O
5 deposited on alloys with Co-Cr support [
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33].
Electron beam technology (e-gun) is a deposition technique for producing high-purity coatings in a short time [
29,
30]. The advantages are as follows: (1) the e-beam source is capable of heating oxides to high temperatures (up to 3000 °C); (2) films deposited by electron beam evaporation can maintain the purity of the oxide; (3) source and support types can be created in a variety of sizes. This method uses vacuum at a pressure of 10
−5 to 10
−6 torr. The most common technologies for physical deposition are PVD and sputtering [
21,
25,
26,
28,
29,
30,
31,
32,
33]. The angle of incidence flux influences the properties of the film. The quartz crystal microbalance controls the coating growth, reporting the thickness and evaporation rate.
Corbella studied thin films of Ta
2O
5 deposited by RF-magnetron reactive sputtering from a tantalum oxide target [
26]. Fused silica, alumina glasses, and WO
3/ITO on glass were used as substrates. The amorphous optical band gap energy values ranged between 4.10 and 4.25 eV [
26]. The variation of the process parameters leads to the possibility of using these films as solid electrolytes for electrochromic devices built on glass substrates [
26].
Ta
2O
5/ZnO heterojunction for metal-oxide-semiconductor (MOS) structured devices was fabricated by using radio-frequency magnetron sputtering and ion beam sputtering technologies on a α-sapphire substrate [
27]. The band alignments of ZnO with other oxides are the key issues for predicting its interface behaviors and determining the carrier [
27]. The reported band gap values are 3.97 eV for Ta
2O
5, 3.26 eV for ZnO, the conduction band offset is 0.33 ± 0.03 eV, and valence band offset is 0.38 ± 0.03 eV, indicating a type I band alignment [
27].
Physical vapor deposition (PVD) or chemical vapor deposition (CVD) lead to films that have an amorphous phase (a-Ta
2O
5), or two distinct crystal phases depending on the annealing, that is, an orthorhombic b-Ta
2O
5 and a hexagonal d-Ta
2O
5 [
28]. Sputtered films are smooth and of good structural quality and uniform thickness [
28]. A test film annealed in air at 800 °C for 1 h yielded well-defined XRD patterns. The samples become nanocrystalline, with a dominant orthorhombic phase (JCPDS: 25-0922) [
28]. Thermal stresses in the thicker samples after annealing were significant enough to cause cracking, and this effect contributes to volume scattering in the sample, thus affecting the near-IR transmittance [
28].
Ta
2O
5 and ZnO thin films deposited by e-gun technology on Co-Cr alloy disks manufactured by Direct Metal Laser Sintering were studied for dental crowns and implants [
29]. The Co-Cr alloy was thermally treated in a furnace for 30 min at 900 °C [
29,
30]. The films obtained were crystalline, with nano-particles between 11 and 25 nm [
29]. The best thin film (Ta
2O
5 (50%) and ZnO (50%)) for dental applications presented uniform grains, having the diameter of the nanometer order [
29].
The literature data reported a band gap of 4.18 eV for Ta
2O
5 films [
31] and 3.25eV for ZnO [
27,
32]. Ta
2O
5 is an interesting material with a very high refractive index (n ≈ 2.2, λ = 633 nm), a wide band gap (Eg = 4.2 eV), and a transparent nature in a wide wavelength range from 300 nm to 2 μm [
32].
ZnO-doped tantalum oxide films (doping concentration 0, 1, 3, and 5 wt.%) have been prepared using a pulsed laser deposition technique in a reactive oxygen atmosphere, and the films are annealed at temperatures of 973 and 1173 K [
33]. XRD analysis shows that the ZnO-doped films annealed at 973 K are crystalline in nature; whereas, the annealed counterpart of pure Ta
2O
5 is amorphous. On annealing at 1173 K, the undoped film shows good crystallinity; whereas, the ZnO doped film presents a decline in crystallinity compared to that of the films annealed at a temperature of 973 K [
33]. Ta
2O
5 nanoring of diameters around 700 nm have been observed in the AFM micrographs of 3 wt.% and 5 wt.% ZnO-doped Ta
2O
5 films [
33]. The band gap increases with increasing ZnO doping and increasing treatment temperature.
The purpose of this article is to obtain a thin film for the top glass of the solar cells in order to be protective against simulated acid rain on a vitreous structure (glass). The film must be transparent in the visible range and must have a band gap up to 3.2 eV. The e-gun technology is used to obtain a thin film, with anticorrosive and optical properties, on a borosilicate glass substrate.
3. Results
Figure 1 presents the XRD patterns of the glass substrate, glass coated with thin film, before and after acid corrosion. The possible applications as a protective film for the top glass of solar cells or as a protective film for windows require the vitreous state of the film. The thin film with a composition of 50%ZnO50%Ta
2O
5 was the one exhibiting a vitreous structure at a higher tantalum oxide content. All paterns show a wide peak characteristic of a vitreous state. After corrosion, the shape is not modified, which shows the good chemical resistance of the film to the corrosion of nitric acid 65%. In the case of low chemical resistance of the glasses after an acid attack on the surface, small crystallites or other compounds appear that can be observed in the XRD diagrams. The absence of salts or other compounds on the glass surface provide for a good optical transmittance.
Figure 2 shows the SEM image and EDS spectrum of the 50%ZnO50%Ta
2O
5 film deposited on a borosilicate glass support using e-gun technology.
The images of the film at a magnification of 10,000× show a glassy aspect of the surface, without particles. The images show darker areas—probably richer in tantalum oxide—and lighter areas—probably higher in zinc oxide.
The EDS data from confirm the composition of the film. The elemental analysis provides the following results: 47.74 wt.% OK, 9.54 wt.% ZnL, 42.72 wt.% TaM.
Figure 3 and
Figure 4 show the topographic profile created on the surface using optical profilometry measurements.
The film was investigated using wide area film surface topography, and profiles were mapped by the white light interferometry technique using a Filmetrics Profilm 3D optical profiler. By analyzing the 2D and 3D image obtained, along with the color scale, it is shown that the film roughness is 1.6 nm and the thickness is 131 nm. After corrosion, the surface of the thin film presents small holes, as can be seen in
Figure 4. The film roughness increased to 3.9 nm, and the thickness decreased to 107 nm. The image of the film after corrosion shows small defects on the surface, along with an increased roughness.
Figure 5 shows the UV-Vis spectra measured for the borosilicate glass substrate and 50%ZnO50%Ta
2O
5 film before and after corrosion. In the visible region between 380 nm and 800 nm, the optical transmittance of the films is above 75%, which means that these films are transparent. The optical transmittance varies from ~75 to 95% due to the thin film interference effects resulting from the overlaying of light reflected on both sides of the thin film [
31]. The maximum and minima on the spectra come from the constructive and destructive interference effects. Within a fixed wavelength range, the numbers of both maxima and minima on each spectrum depend on the optical thickness [
31]. In the case of the thin film, the transmittance decreases insignificantly during the corrosion experiment. The transmission decreases a bit in the lower wavelength region, possibly due to absorption caused by oxygen deficiency or a lack of ion assistance during thin film growth. In the UV region, below 380 nm, a sharp decrease in optical transmittance occurs. This behaviour can be explained due to substrate absorption in the UV region and to the heavy absorption of light that is generally associated with the optical transitions through the energy gap of tantalum oxide [
31]. The thin film bands move to a lower wavelength in the ultraviolet region, from 250 nm to 240 nm, and to a higher wavelength in the visible region, from 585 nm to 685 nm. The results from UV-VIS can be correlated with data obtained from the profilometer, and the changes in the optical spectrum can be explained by an increased roughness at the surface of the film after corrosion.
The optical direct band gap of the thin film is calculated according to the methods outlined in the literature [
31,
32]. When the thin film is non-crystalline, Tauc plots can be used to determine the optical direct band gap.
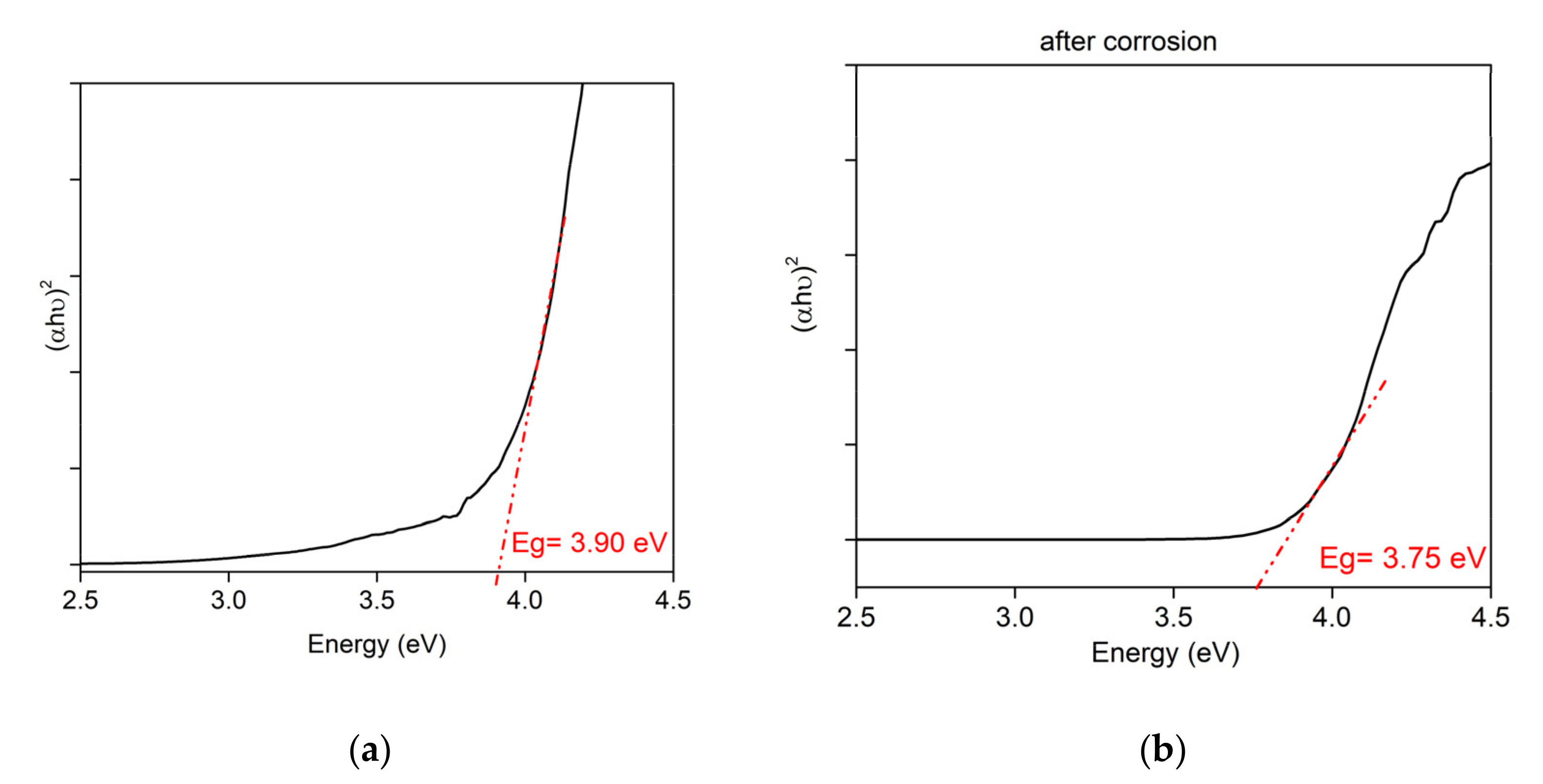
Figure 6 presents the Tauc plot (αhν)
2 vs. energy (hν) for the film before and after corrosion. The band gap value is obtained from the intersection of the tangent of the absorption edge (straight line in the graph) with the
x-axis.
The formulas used for axes of Tauc plot are:
where α is the linear absorption coefficient and t is the film thickness.
The optical direct band gap of the thin film was 3.9 eV for the 50%ZnO50%Ta2O5 film before corrosion and 3.7 eV after corrosion. The film exhibits a better band gap than ZnO and is obtained at a lower cost than the Ta2O5 film. The thin film can be used for the protection of solar cells due to the band gap above 3.2 eV. Due to its high transmittance, the thin film can also be used to protect historical glass windows.