A High-Performing Nanostructured Ir Doped-TiO2 for Efficient Photocatalytic Degradation of Gaseous Toluene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
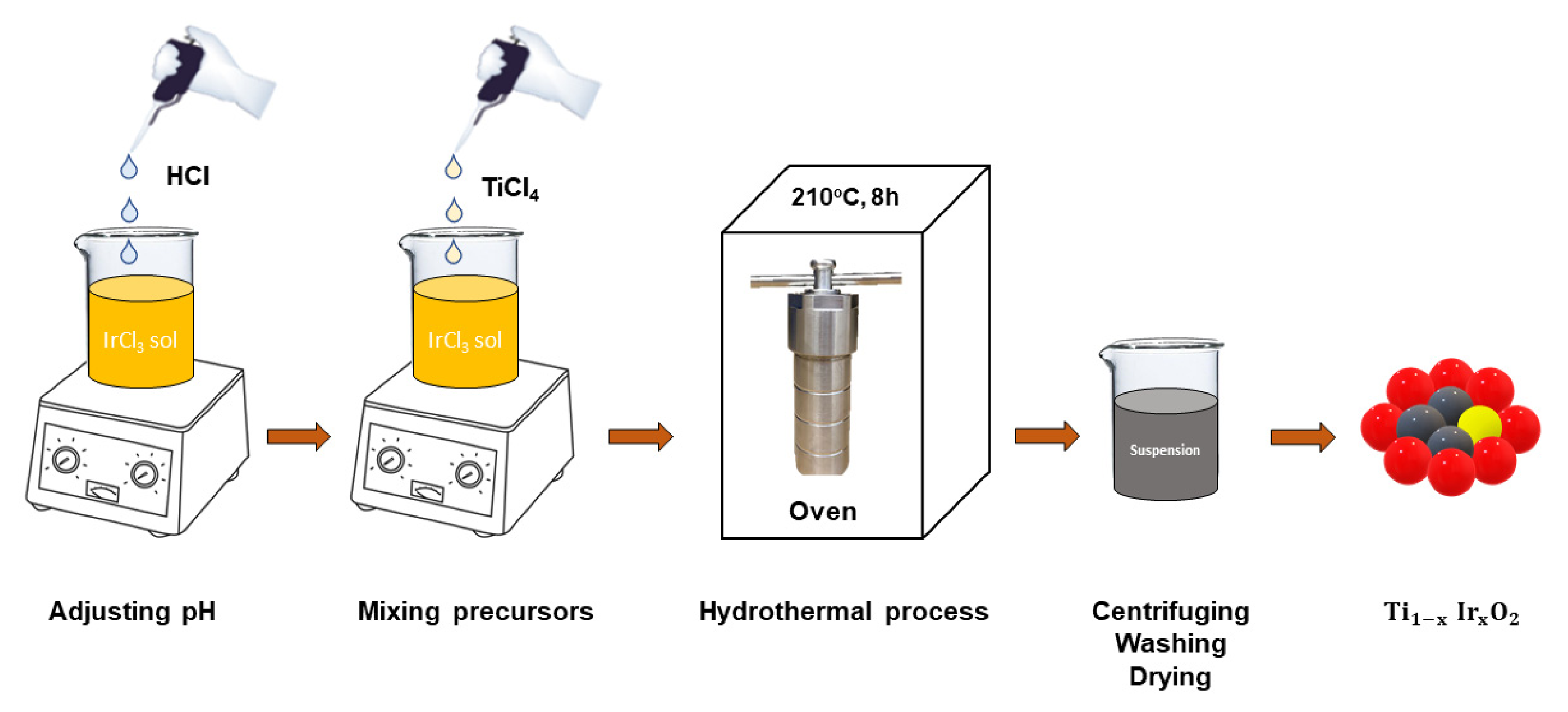
2.2. Synthesis of Ir-Doped Samples
2.3. Sample Characterization
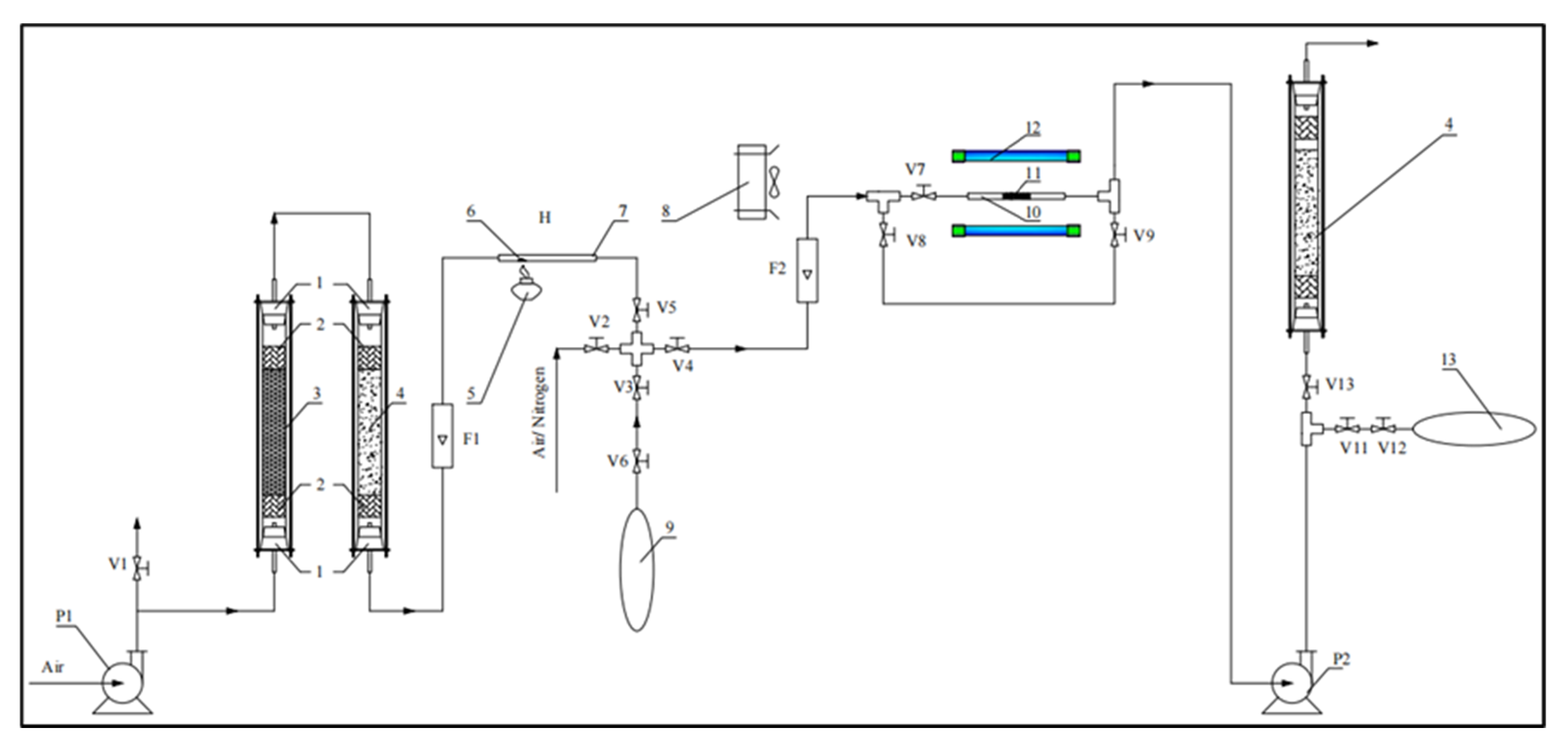
2.4. Toluene Degradation Laboratory System
- The air bag was connected to V3 valve. Then, V6 valve of the air bag was opened. F1 flow meter was adjusted to the rate of 1 L/min while other valves were closed. The glass wool was padded into the tubes.
- A sample drop (containing 0.02 mL of toluene and 0.11 mL distilled water) was put in the reaction tube. Then, the alcohol burner was used to evaporate the drop in 1 min.
- V3 and V5 valves were opened to collect the air in 03 min. When the bag was full, V6 valve was closed, the P1 compressor was turned off and then other valves were closed.
- A measure of 0.1 g of synthesized photocatalyst was placed evenly in the reaction tube. Then, the cooling fan and UV lamps were turned on 10 min before reacting to stabilize the light source.
- Another air bag was connected to V11 valve and V12 valve of this airbag was also opened.
- P2 compressor and F2 flow meter were adjusted to desired flow rates. Then, V3, V4, V7, and V11 valves were opened, while V5, V6, V8, V9, and V13 valves were closed.
- The air was collected in T (minutes) needed for investigation. When the bag containing outlet gas was full, V11, V12 valves were closed. Then, the bag was removed, then P2 compressor and UV lamps were turned off.
- After reactions, the tube containing sample drop and reaction tube were cleaned. The system was also cleaned by blowing air through it.
- The air bags were labeled and sent to the analyzing center for gas chromatography analysis.
3. Results and Discussion
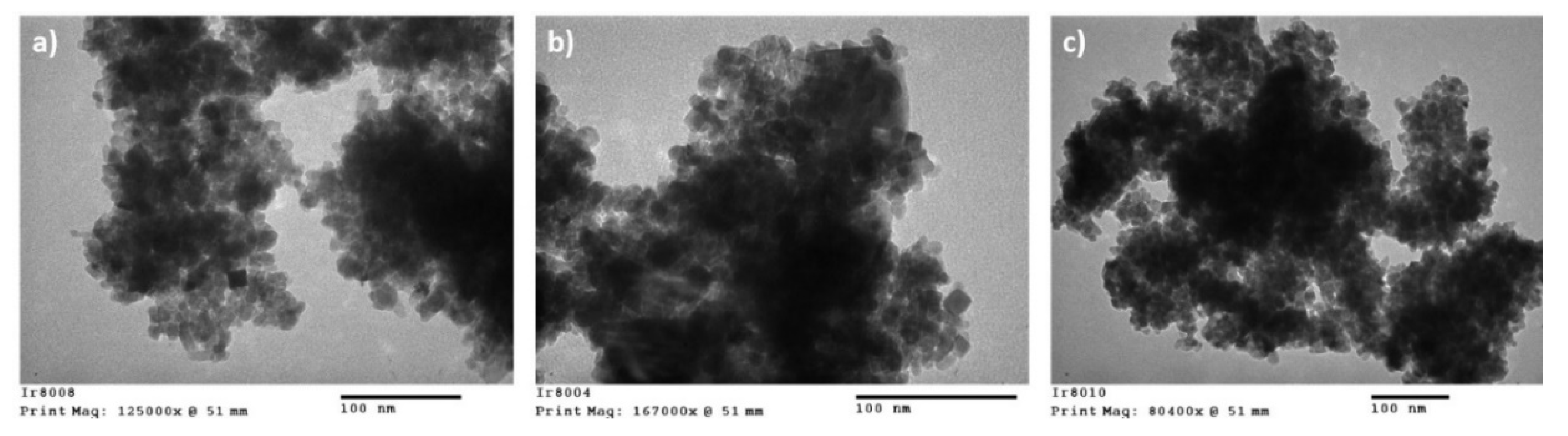
Material Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, K.; Wang, C.; Xue, S.; Li, W.; Liu, J.; Li, L. The identification, health risks and olfactory effects assessment of VOCs released from the wastewater storage tank in a pesticide plant. Ecotoxicol. Environ. Saf. 2019, 184, 109665. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Donelli, D.; Barbieri, G.; Valussi, M.; Maggini, V.; Firenzuoli, F. Forest Volatile Organic Compounds and Their Effects on Human Health: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2020, 17, 6506. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Improving indoor air quality, health and performance within environments where people live, travel, learn and work. Atmos. Environ. 2018, 200, 90–109. [Google Scholar] [CrossRef] [Green Version]
- Tomatis, M.; Moreira, M.T.; Xu, H.; Deng, W.; He, J.; Parvez, A.M. Removal of VOCs from waste gases using various thermal oxidizers: A comparative study based on life cycle assessment and cost analysis in China. J. Clean. Prod. 2019, 233, 808–818. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Yang, Z.; He, Z.; Wang, P.; Yan, Y.; Ran, J. Hydrophobic modified activated carbon using PDMS for the adsorption of VOCs in humid condition. Sep. Purif. Technol. 2020, 239, 116517. [Google Scholar] [CrossRef]
- Yadav, A.A.; Kang, S.-W.; Hunge, Y.M. Photocatalytic degradation of Rhodamine B using graphitic carbon nitride photocatalyst. J. Mater. Sci. Mater. Electron. 2021, 32, 15577–15585. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.-W.; Kim, H. Photocatalytic degradation of tetracycline antibiotics using hydrothermally synthesized two-dimensional molybdenum disulfide/titanium dioxide composites. J. Colloid Interface Sci. 2022, 606, 454–463. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, X.; Pu, H.; Nishimoto, S.; Murakami, T.; Fujishima, A. Photochromism-based detection of volatile organic compounds by W-doped TiO2 nanofibers. J. Colloid Interface Sci. 2011, 362, 188–193. [Google Scholar] [CrossRef]
- Hinojosa-Reyes, M.; Arriaga, S.; Diaz-Torres, L.A.; Rodríguez-González, V. Gas-phase photocatalytic decomposition of ethylbenzene over perlite granules coated with indium doped TiO2. Chem. Eng. J. 2013, 224, 106–113. [Google Scholar] [CrossRef]
- Inturi, S.N.R.; Boningari, T.; Suidan, M.; Smirniotis, P.G. Visible-light-induced photodegradation of gas phase acetonitrile using aerosol-made transition metal (V, Cr, Fe, Co, Mn, Mo, Ni, Cu, Y, Ce, and Zr) doped TiO2. Appl. Catal. B Environ. 2014, 144, 333–342. [Google Scholar] [CrossRef]
- Huang, H.; Huang, H.; Zhang, L.; Hu, P.; Ye, X.; Leung, D.Y.C. Enhanced degradation of gaseous benzene under vacuum ultraviolet (VUV) irradiation over TiO2 modified by transition metals. Chem. Eng. J. 2015, 259, 534–541. [Google Scholar] [CrossRef]
- Oseghe, E.O.; Ndungu, P.G.; Jonnalagadda, S.B. Photocatalytic degradation of 4-chloro-2-methylphenoxyacetic acid using W-doped TiO2. J. Photochem. Photobiol. A Chem. 2015, 312, 96–106. [Google Scholar] [CrossRef]
- Binas, V.; Stefanopoulos, V.; Kiriakidis, G.; Papagiannakopoulos, P. Photocatalytic oxidation of gaseous benzene, toluene and xylene under UV and visible irradiation over Mn-doped TiO2 nanoparticles. J. Mater. 2018, 5, 56–65. [Google Scholar] [CrossRef]
- Li, X.; Guo, Z.; He, T. The doping mechanism of Cr into TiO2 and its influence on the photocatalytic performance. Phys. Chem. Chem. Phys. 2013, 15, 20037–20045. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.T.; Nguyen, Q.T.; Bui, T.H.L.; Tran, M.C.; Dang, T.P.; Tran, T.K.H. Synthesis and characterization of TiO2 photocatalyst doped by transition metal ions (Fe3+, Cr3+ and V5+). Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 15009. [Google Scholar] [CrossRef] [Green Version]
- Bartkowiak, A.; Korolevych, O.; Chiarello, G.; Makowska-Janusik, M.; Zalas, M. How Can the Introduction of Zr4+ Ions into TiO2 Nanomaterial Impact the DSSC Photoconversion Efficiency? A Comprehensive Theoretical and Experimental Consideration. Materials 2021, 14, 2955. [Google Scholar] [CrossRef] [PubMed]
- Kasach, A.A.; Kharytonau, D.S.; Paspelau, A.V.; Ryl, J.; Sergievich, D.S.; Zharskii, I.M.; Kurilo, I.I. Effect of TiO2 Concentration on Microstructure and Properties of Composite Cu–Sn–TiO2 Coatings Obtained by Electrodeposition. Materials 2021, 14, 6179. [Google Scholar] [CrossRef]
- Fernandes, S.; da Silva, J.C.G.E.; da Silva, L.P. Life cycle assessment of the sustainability of enhancing the photodegradation activity of TiO2 with metal-doping. Materials 2020, 13, 1487. [Google Scholar] [CrossRef] [Green Version]
- Elmehasseb, I.; Kandil, S.; Elgendy, K. Advanced visible-light applications utilizing modified Zn-doped TiO2 nanoparticles via non-metal in situ dual doping for wastewater detoxification. Optik 2020, 213, 164654. [Google Scholar] [CrossRef]
- Zou, F.; Hu, J.; Miao, W.; Shen, Y.; Ding, J.; Jing, X. Synthesis and Characterization of Enhanced Photocatalytic Activity with Li+-Doping Nanosized TiO2 Catalyst. ACS Omega 2020, 5, 28510–28516. [Google Scholar] [CrossRef] [PubMed]
- Babajani, N.; Jamshidi, S. Investigation of photocatalytic malachite green degradation by iridium doped zinc oxide nanoparticles: Application of response surface methodology. J. Alloy. Compd. 2018, 782, 533–544. [Google Scholar] [CrossRef]
- Dhanalakshmi, M.; Saravanakumar, K.; Prabavathi, S.L.; Abinaya, M.; Muthuraj, V. Fabrication of novel surface plasmon resonance induced visible light driven iridium decorated SnO2 nanorods for degradation of organic contaminants. J. Alloys Compd. 2018, 763, 512–524. [Google Scholar] [CrossRef]
- Rojas, J.V.; Castano, C.H. Radiolytic synthesis of iridium nanoparticles onto carbon nanotubes. J. Nanoparticle Res. 2014, 16, 1–5. [Google Scholar] [CrossRef]
- Pawluk, T.; Hirata, Y.; Wang, L. Studies of Iridium Nanoparticles Using Density Functional Theory Calculations. J. Phys. Chem. B 2005, 109, 20817–20823. [Google Scholar] [CrossRef]
- Atanasoska, L.; Gupta, P.; Deng, C.; Warner, R.; Larson, S.; Thompson, J. XPS, AES, and Electrochemical Study of Iridium Oxide Coating Materials for Cardiovascular Stent Application. ECS Trans. 2009, 16, 37–48. [Google Scholar] [CrossRef]
- Huynh, T.T.; Van Nguyen, A.; Pham, H.Q.; Bach, L.G.; Ho, V.T.T. Synthesis the New Nanostructure Ti0.7Ir0.3O2 via Low Temperature Hydrothermal Process. Appl. Mech. Mater. 2018, 876, 64–70. [Google Scholar] [CrossRef]
- Huynh, T.T.; Van Nguyen, A.; Pham, H.Q.; Vinh, N.H.; Bach, L.G.; Ho, V.T.T. One-Step Hydrothermal Synthesis of a New Nanostructure Ti0.7Ir0.3O2 for Enhanced Electrical Conductivity: The Effect of pH on the Formation of Nanostructure. J. Nanosci. Nanotechnol. 2018, 18, 6928–6933. [Google Scholar] [CrossRef]
- Van Nguyen, A.; Huynh, T.T.; Pham, H.Q.; Phan, V.T.T.; Nguyen, V.A.; Ho, V.T.T. Novel nanorod Ti0.7Ir0.3O2 prepared by facile hydrothermal process: A promising non-carbon support for Pt in PEMFCs. Int. J. Hydrog. Energy 2018, 44, 2361–2371. [Google Scholar] [CrossRef]
- Wang, S.; Liu, B.; Zhu, Y.; Ma, Z.; Liu, B.; Miao, X.; Ma, R.; Wang, C. Enhanced performance of TiO2-based perovskite solar cells with Ru-doped TiO2 electron transport layer. Sol. Energy 2018, 169, 335–342. [Google Scholar] [CrossRef]
- Savini, A.; Bucci, A.; Nocchetti, M.; Vivani, R.; Idriss, H.; Macchioni, A. Activity and Recyclability of an Iridium–EDTA Water Oxidation Catalyst Immobilized onto Rutile TiO2. ACS Catal. 2015, 5, 264–271. [Google Scholar] [CrossRef]
- Kumaresan, L.; Mahalakshmi, M.; Palanichamy, M.; Murugesan, V. Synthesis, characterization, and photocatalytic activity of Sr2+ doped TiO2 nanoplates. Ind. Eng. Chem. Res. 2010, 49, 1480–1485. [Google Scholar] [CrossRef]
- Holm, A.; Hamandi, M.; Simonet, F.; Jouguet, B.; Dappozze, F.; Guillard, C. Impact of rutile and anatase phase on the photocatalytic decomposition of lactic acid. Appl. Catal. B Environ. 2019, 253, 96–104. [Google Scholar] [CrossRef]
- Li, Z.; Shen, W.; He, W.; Zu, X. Effect of Fe-doped TiO2 nanoparticle derived from modified hydrothermal process on the photocatalytic degradation performance on methylene blue. J. Hazard. Mater. 2008, 155, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Caudillo-Flores, U.; Muñoz-Batista, M.J.; Cortés, J.A.; Fernández-García, M.; Kubacka, A. UV and visible light driven H2 photo-production using Nb-doped TiO2: Comparing Pt and Pd co-catalysts. Mol. Catal. 2017, 437, 1–10. [Google Scholar] [CrossRef]
- Munir, S.; Shah, S.M.; Hussain, H.; Khan, R.A. Effect of carrier concentration on the optical band gap of TiO2 nanoparticles. Mater. Des. 2016, 92, 64–72. [Google Scholar] [CrossRef]
- El Mragui, A.; Logvina, Y.; Da Silva, L.P.; Zegaoui, O.; Da Silva, J.C.E. Synthesis of Fe- and Co-Doped TiO2 with Improved Photocatalytic Activity Under Visible Irradiation Toward Carbamazepine Degradation. Materials 2019, 12, 3874. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Ohtani, B. Substitutionally rhodium(IV)-doped titania showing photocatalytic activity toward organics oxidation under visible-light irradiation. Catal. Today 2021, 380, 25–31. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, M.-H.; Yu, L.E. Hydrothermal synthesis and photocatalytic performance of metal-ions doped TiO2. Appl. Catal. A Gen. 2012, 413–414, 238–244. [Google Scholar] [CrossRef]
- Liu, S.; Guo, E.; Yin, L. Tailored visible-light driven anatase TiO2 photocatalysts based on controllable metal ion doping and ordered mesoporous structure. J. Mater. Chem. 2012, 22, 5031–5041. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y. Electrochemically Self-Doped TiO2 Nanotube Arrays for Supercapacitors. J. Phys. Chem. C 2014, 118, 5626–5636. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, P.; Zhang, S.; Huo, G.; Suo, Z.; Yue, Z.; Zhang, S.; Huang, W.; Zhu, B. Platinum and Iridium Oxide Co-modified TiO2 Nanotubes Array Based Photoelectrochemical Sensors for Glutathione. Nanomaterials 2020, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Termin, A.; Hoffmann, M.R. The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q.; Lamson, J.J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Yang, X.; Cao, C.; Erickson, L.; Hohn, K.; Maghirang, R.; Klabunde, K. Synthesis of visible-light-active TiO2-based photocatalysts by carbon and nitrogen doping. J. Catal. 2008, 260, 128–133. [Google Scholar] [CrossRef]
- Rodríguez, M.J.H.; Melián, E.P.; Díaz, O.G.; Araña, J.; Macías, M.; Orive, A.G.; Rodríguez, J.M.D. Comparison of supported TiO2 catalysts in the photocatalytic degradation of NOx. J. Mol. Catal. A Chem. 2016, 413, 56–66. [Google Scholar] [CrossRef]
- Sharotri, N.; Sharma, D.; Sud, D. Experimental and theoretical investigations of Mn-N-co-doped TiO2 photocatalyst for visible light induced degradation of organic pollutants. J. Mater. Res. Technol. 2019, 8, 3995–4009. [Google Scholar] [CrossRef]
- Qiu, X.; Miyauchi, M.; Sunada, K.; Minoshima, M.; Liu, M.; Lu, Y.; Li, D.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y.; et al. Hybrid CuxO/TiO2 Nanocomposites as Risk-Reduction Materials in Indoor Environments. ACS Nano 2012, 6, 1609–1618. [Google Scholar] [CrossRef]
- Zhong, L.; Brancho, J.J.; Batterman, S.; Bartlett, B.M.; Godwin, C. Experimental and modeling study of visible light responsive photocatalytic oxidation (PCO) materials for toluene degradation. Appl. Catal. B Environ. 2017, 216, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Hussain, M.; Russo, N.; Saracco, G. Studies on the activity and deactivation of novel optimized TiO2 nanoparticles for the abatement of VOCs. Chem. Eng. J. 2011, 175, 330–340. [Google Scholar] [CrossRef]
- Khan, R.; Kim, T.-J. Preparation and application of visible-light-responsive Ni-doped and SnO2-coupled TiO2 nanocomposite photocatalysts. J. Hazard. Mater. 2009, 163, 1179–1184. [Google Scholar] [CrossRef] [PubMed]


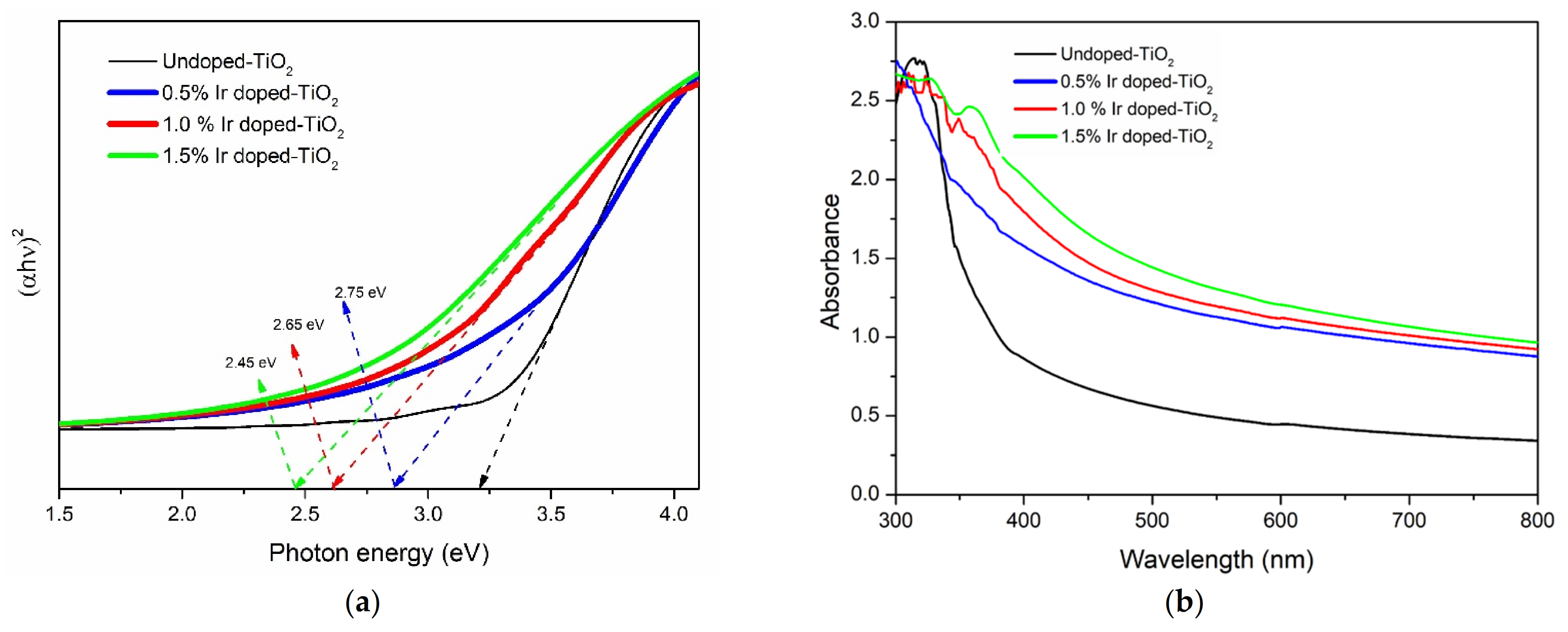
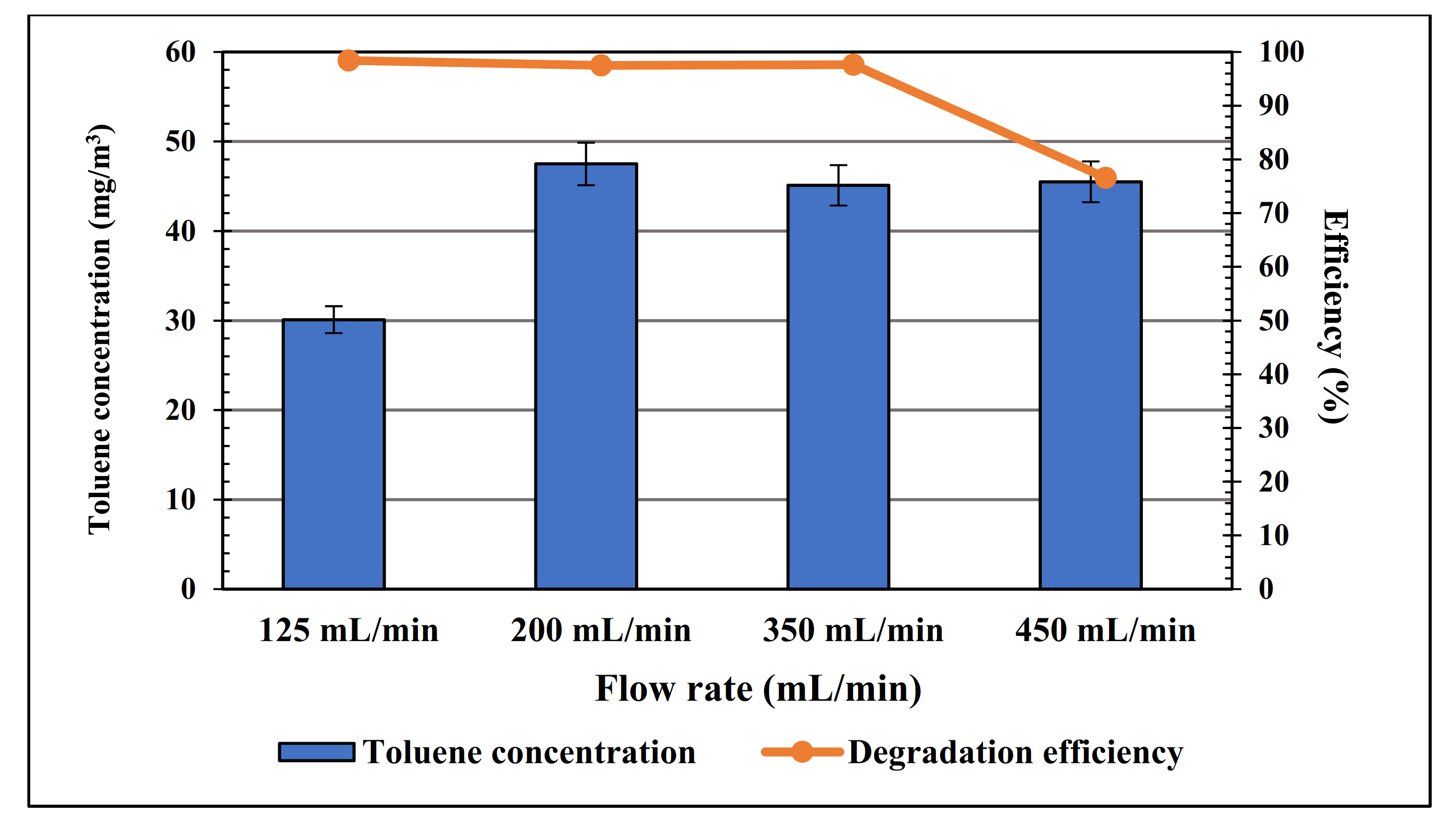
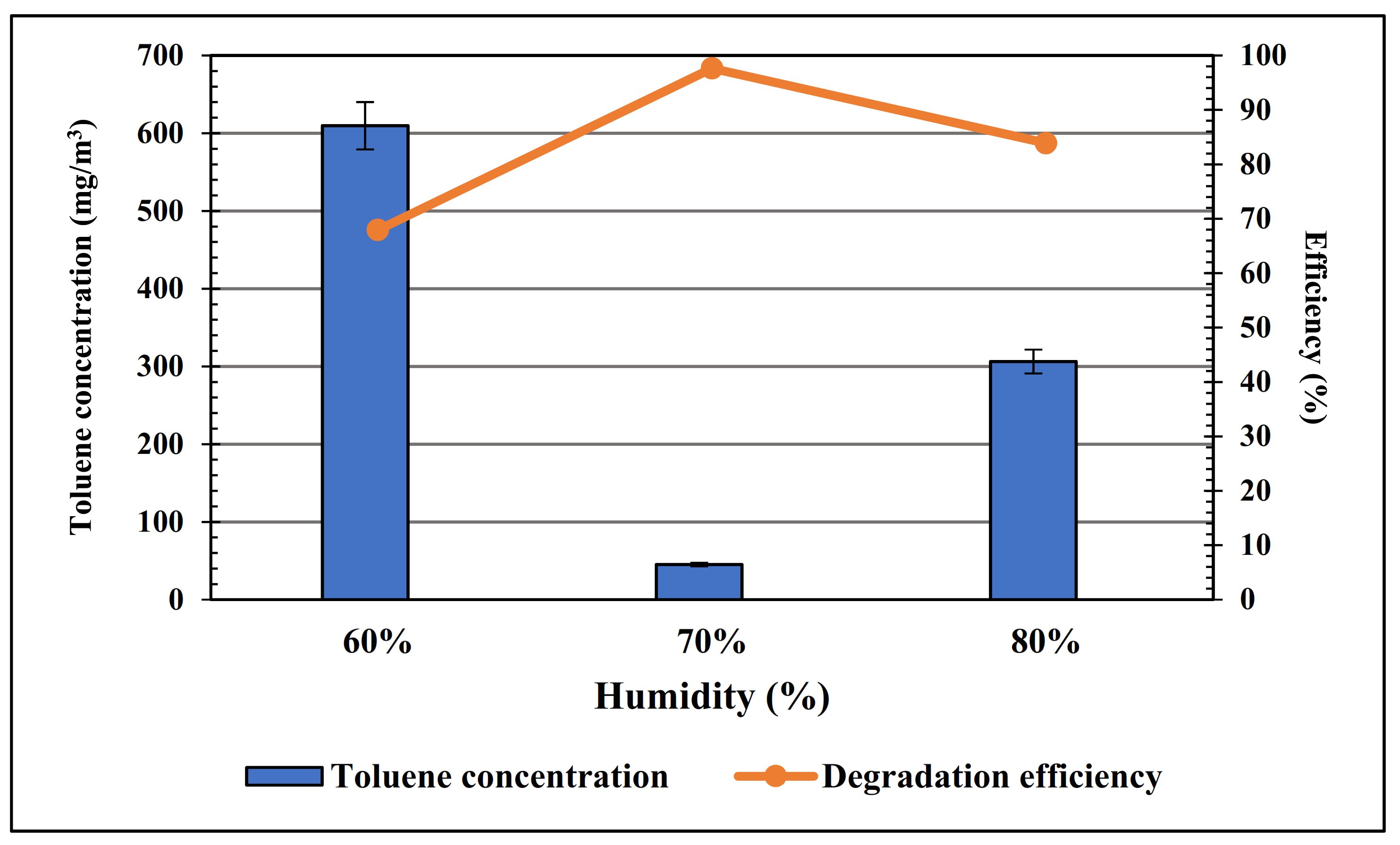
| No. | Sample | Mol% of Iridium |
|---|---|---|
| 1 | 0.5 mol% Ir-doped | 0.62% |
| 2 | 1.0 mol% Ir-doped | 1.15% |
| 3 | 1.5 mol% Ir-doped | 1.80% |
| Photocatalysts | Un-Doped TiO2 | P25 | P90 | PC105 | PC500 | Ir-Doped TiO2 | Fe-Doped TiO2 | |
|---|---|---|---|---|---|---|---|---|
| Properties | ||||||||
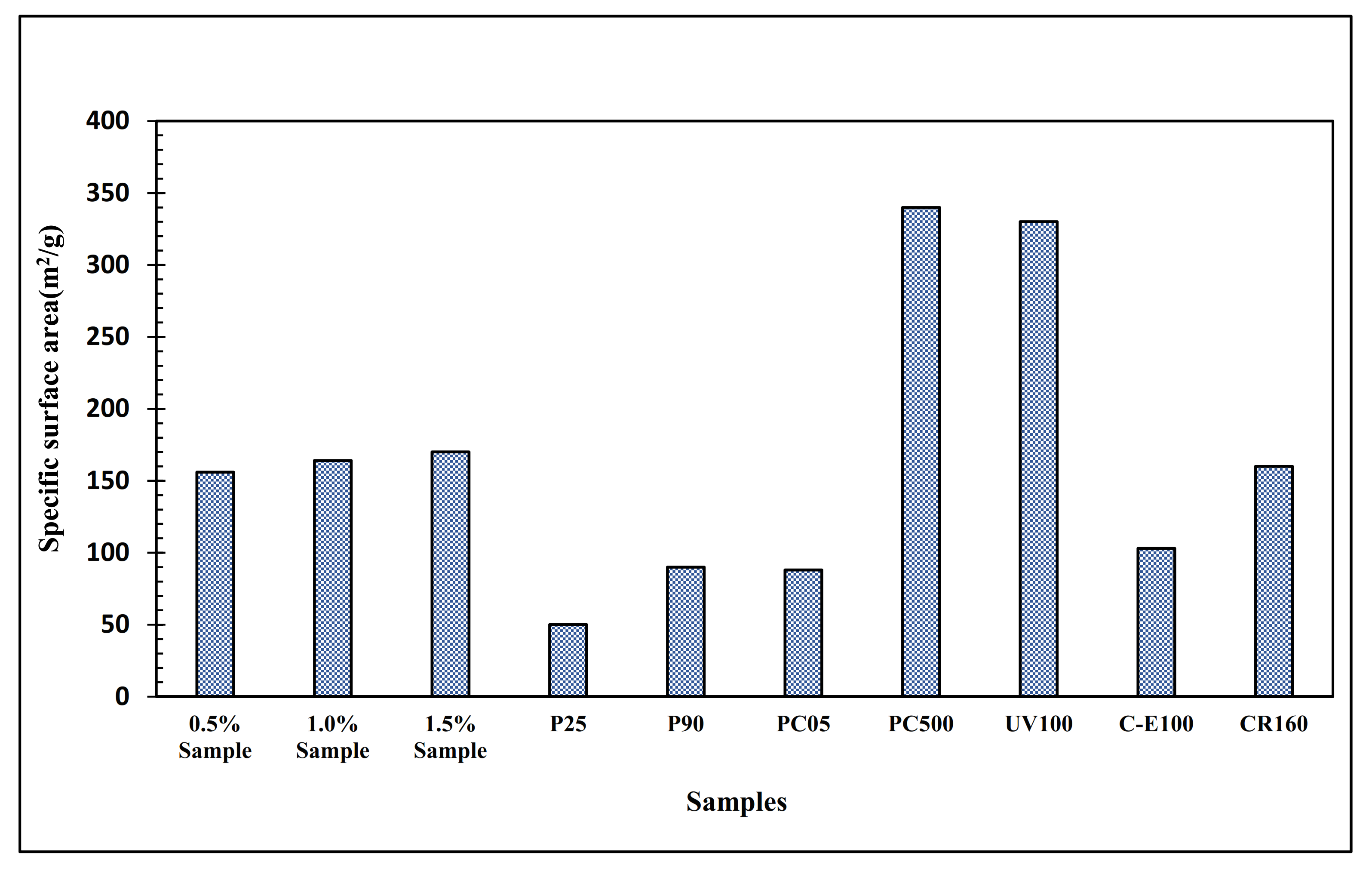
| Surface area (m2/g) | 50 | 50 | 90 | 88 | 340 | 156–170 | 103 | |
| Crystallite size (nm) | 20–30 | 21–30 | 14 | 15–25 | 5–10 | 10–15 | 13 | |
| References | [33] | [33] | [33] | [33] | [33] | This work | [34] | |
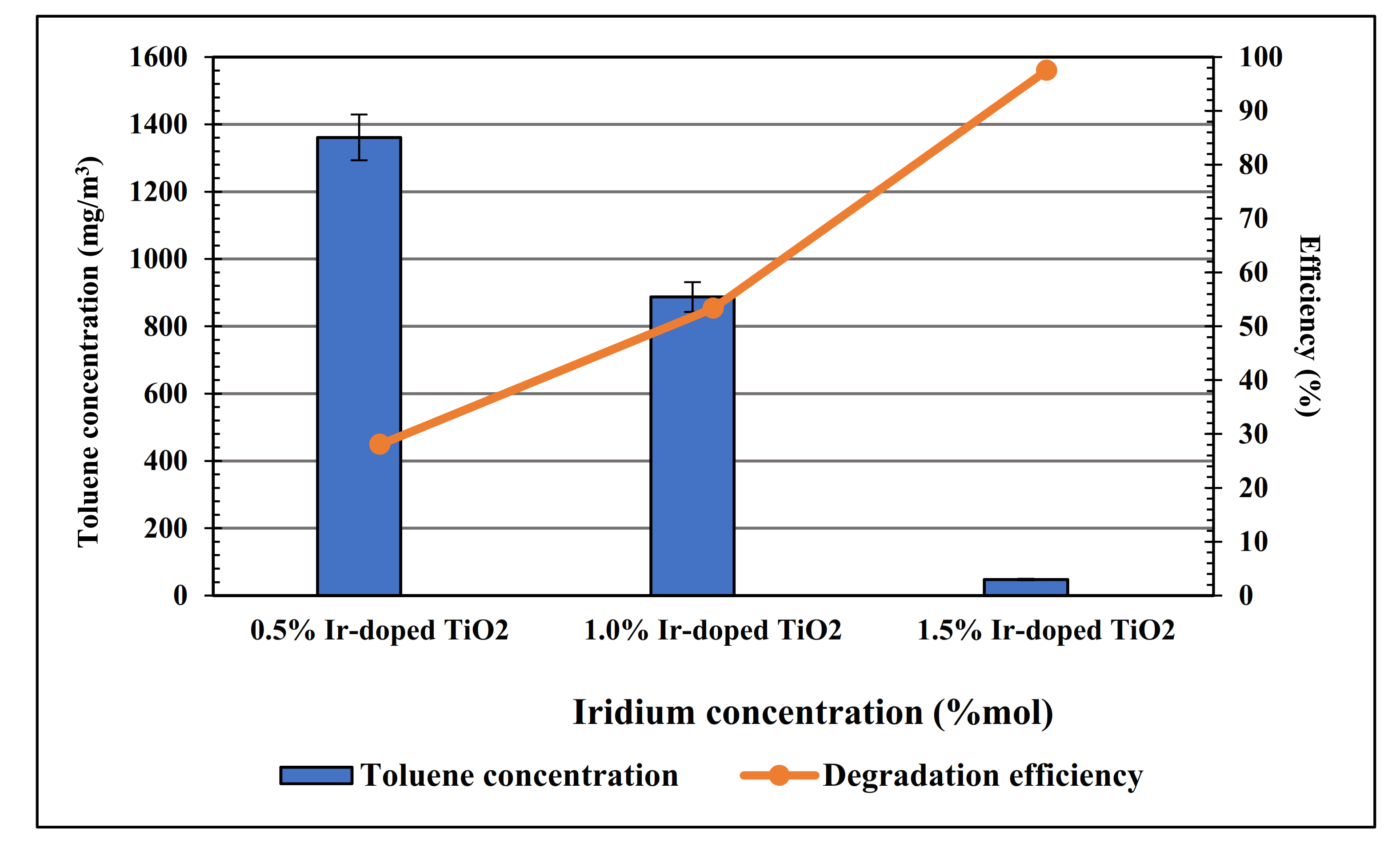
| No. | Sample | Toluene Degradation Efficiency (%) | |
|---|---|---|---|
| 1 | 0.5 mol% Ir-doped | 28.04 | 1.87% |
| 2 | 1.0 mol% Ir-doped | 53.34 | 34.67% |
| 3 | 1.5 mol% Ir-doped | 97.50 | 64.35% |
| No. | Materials | Synthesis Method | Parameters | Reaction Condition | Degradation Efficiency | References |
|---|---|---|---|---|---|---|
| 1 | Co-alloyed TiNbON photocatalyst. | Urea-glass synthesis method. | -Particle diameter: 1–2 μm. -Crystalline structure: irregularly shaped surfaces -Surface area: 40.76 m2/g. -Band gap: 2.3 eV. | -Toluene concentration: 1–5 ppm. -RH: 25–65%. -Irradiation: 42–95 (W/m2) (400–540 nm) | 58% | [46] |
| 2 | nanoparticles (TNPs) photocatalyst. | Sol-gel method. | -Particle diameter: 10–20 μm. -Crystalline structure: tetragonal. -Surface area: 151 m2/g. -Band gap: 3.17 eV. | -Toluene concentration: 200 ppm. -Irradiation: UVA (320–400 nm) and UVB (290–320 nm). | 40% | [47] |
| 3 | x (x = 0.1, 0.3, 0.5 wt%) photocatalyst. | Sol-gel method and wet-impregnation method. | -Crystallite size: 15–16 nm. -Crystalline structrure: crystalline structure. | -Toluene concentration: 177 ppm. -Irradiation: visible light source with minimum wavelength of 400 nm. | 51% | [48] |
| 4 | photocatalyst. | Commercial photocatalyst. | -Fine particles were coated on the activated carbon fibers.-Surface area: 999.6 m2/g. | -T = 25 ± 0.5 °C. -RH = 15, 30, 45, and 60%. -Irradiation: UV radiation with a primary wavelength at 254 nm | 14.2% | [41] |
| 5 | V-doped TiO2/PU (6 wt% V-TiO2) | Immobilization of amino titanosiloxane on activated PU combined with using NH4VO3 as precursor. | -Surface area: 192.5 m2/g -Bandgap: 2.83 eV for 6 wt% V-TiO2 | -T = 25 °C -RH = 50% -AFR = 200 mL/min -Irradiation: visible light source with minimum wavelength of 400 nm | 80% | [49] |
| 6 | MnOx-ZrO2 (MnOx-5% ZrO2) | Co-precipitation with NaOH of Mn3O4 and ZrO2 | -Crystallite size: 14.5 nm -Surface area: 85.4 m2/g. -Bandgap: 3.26 (eV) | -T = 25 °C -Irradiation: solar lamp (300 W; 10.7 mW/cm2) | 84% | [50] |
| 7 | TiO2-MnO2 | One-step anodic oxidation of Ti–Mn alloys in an ethylene glycol-based electrolyte | -Crystallite size: d = 76 ± 9 nm; l = 1.0 ± 1 μm -Crystalline structure: anatase -Surface area: 170 m2/g. | -T = 25 °C -Irradiation: 25 LEDS (wavelength at 465 nm) | 43% | [51] |
| 8 | Brookite TiO2-5% CeO2 | Thermohydrolysis of titanium tetrachloride | -Crystalline structure: brookite -Surface area: 66 m2/g. -Bandgap: 3.19 (eV) | -T = 25 °C -Irradiation: solar lamp (300 W; 10.7 mW/cm2) | 25% | [52] |
| 9 | Ir-doped photocatalyst. | Hydrothermal method. | -Particle diameter: 10–15 nm. -Crystalline structure: tetragonal. -Surface area: 170 m2/g. | -Toluene concentration: 1900 ppm. -RH: 60–80%. -Minimum wavelength: 255 nm | 97% | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, V.T.T.; Chau, D.H.; Bui, K.Q.; Nguyen, N.T.T.; Tran, T.K.N.; Bach, L.G.; Truong, S.N. A High-Performing Nanostructured Ir Doped-TiO2 for Efficient Photocatalytic Degradation of Gaseous Toluene. Inorganics 2022, 10, 29. https://doi.org/10.3390/inorganics10030029
Ho VTT, Chau DH, Bui KQ, Nguyen NTT, Tran TKN, Bach LG, Truong SN. A High-Performing Nanostructured Ir Doped-TiO2 for Efficient Photocatalytic Degradation of Gaseous Toluene. Inorganics. 2022; 10(3):29. https://doi.org/10.3390/inorganics10030029
Chicago/Turabian StyleHo, Van Thi Thanh, Dung Hung Chau, Khang Quang Bui, Ngan Thi Thanh Nguyen, Thi Kim Ngan Tran, Long Giang Bach, and Son Nguyen Truong. 2022. "A High-Performing Nanostructured Ir Doped-TiO2 for Efficient Photocatalytic Degradation of Gaseous Toluene" Inorganics 10, no. 3: 29. https://doi.org/10.3390/inorganics10030029
APA StyleHo, V. T. T., Chau, D. H., Bui, K. Q., Nguyen, N. T. T., Tran, T. K. N., Bach, L. G., & Truong, S. N. (2022). A High-Performing Nanostructured Ir Doped-TiO2 for Efficient Photocatalytic Degradation of Gaseous Toluene. Inorganics, 10(3), 29. https://doi.org/10.3390/inorganics10030029






