Synthesis of MOFs for RhB Adsorption from Wastewater
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Raw Materials
2.2. Preparation of Fe-MOFs
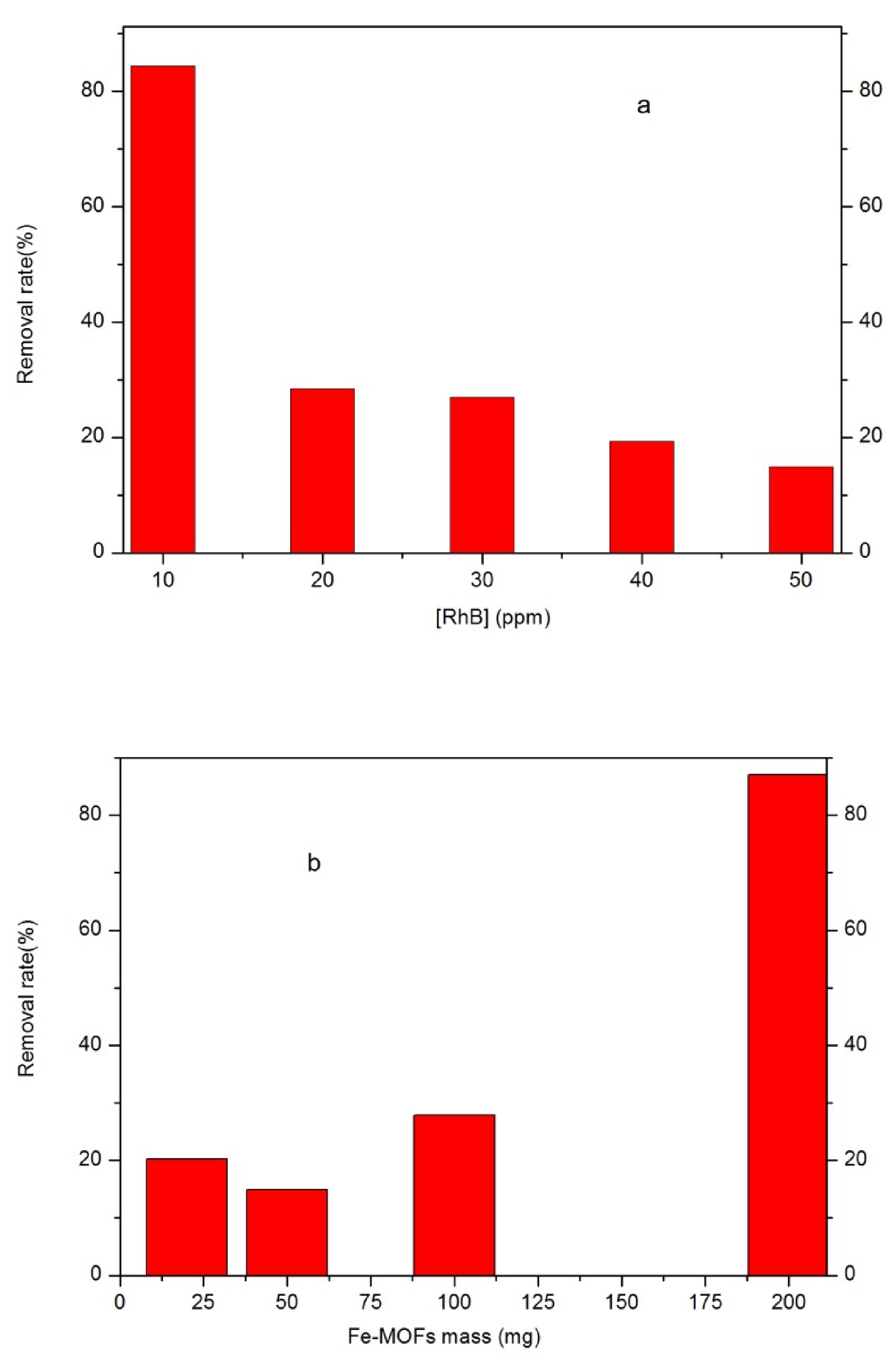
2.3. Removal of RhB
3. Results and Discussion
3.1. Preparation of Fe-MOFs
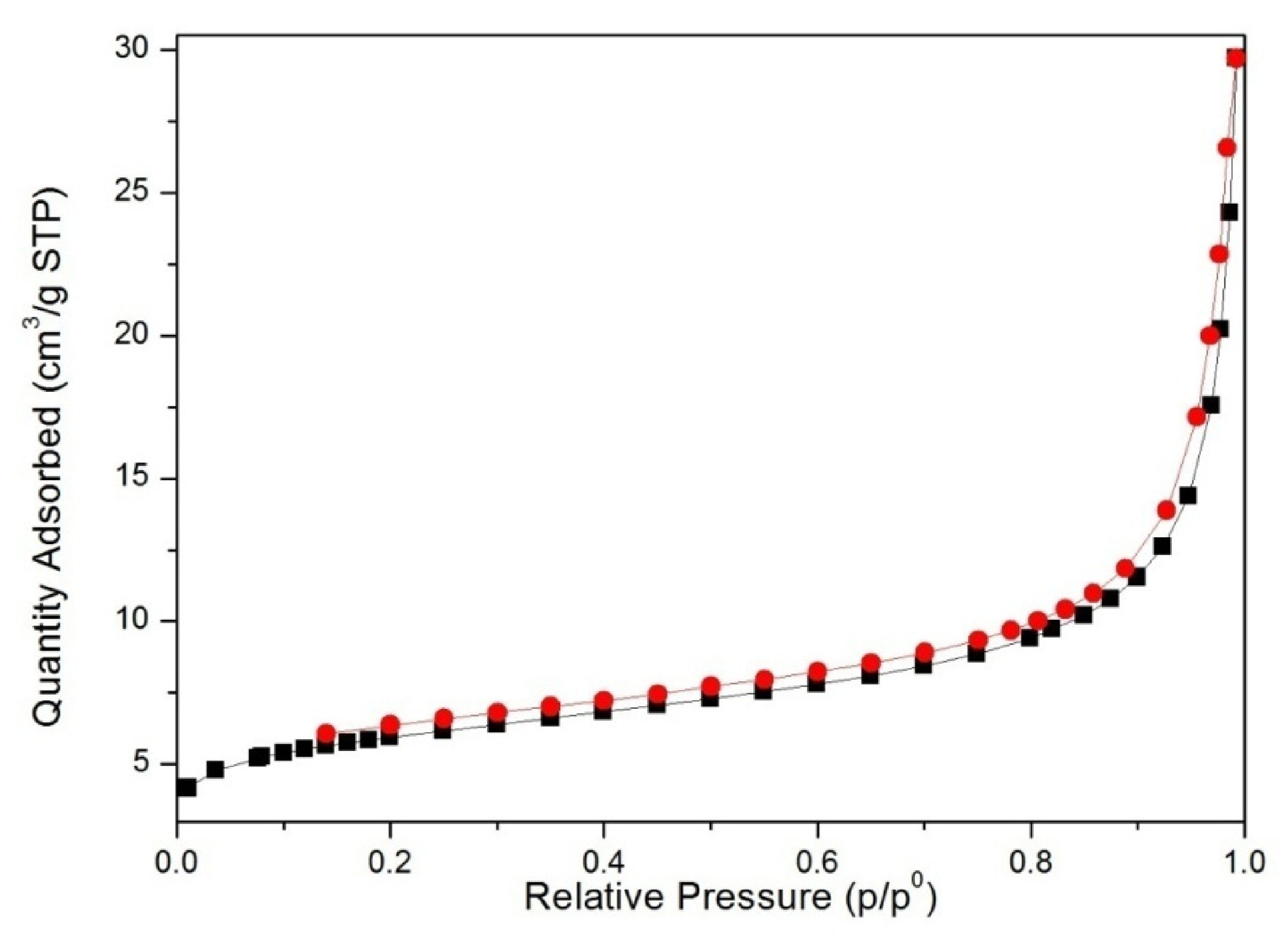
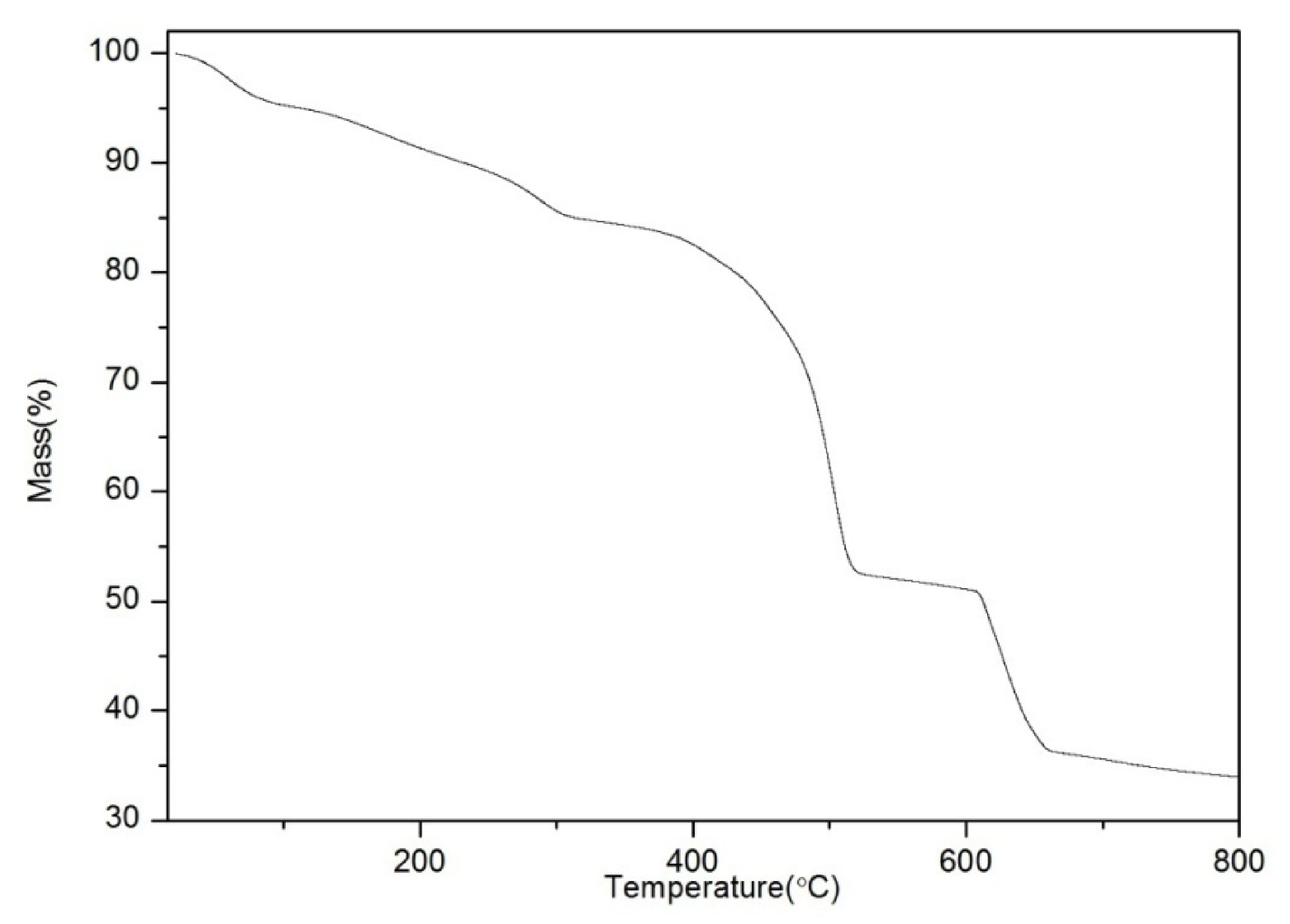
3.2. Structural Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azizullah, A.; Khattak, M.N.K.; Richter, P.; Häder, D.P. Water pollution in Pakistan and its impact on public health—A review. Environ. Int. 2011, 37, 479–497. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environmental Protection. Environmental Statistics Annual Report; Ministry of Environmental Protection: Beijing, China, 2015; pp. 15–16.
- Ruan, X.C.; Liu, M.Y.; Zeng, Q.F.; Ding, Y.H. Degradation and decolorization of reactive red X-3B aqueous solution by ozone integrated with internal micro electrolysis. Sep. Purif. Technol. 2010, 74, 195–201. [Google Scholar] [CrossRef]
- Parrott, J.L.; Bartlett, A.J.; Balakrishnan, V.K. Chronic toxicity of azo and anthracenedione dyes to embryo-larval fathead innow. Environ. Pollut. 2016, 210, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.H.; Wei, F.H.; Chen, H.L.; Chen, D.; Ding, B. Preparation of Zn-MOFs by microwave-assisted ball milling for removal of tetracycline hydrochloride and Congo red from wastewater. Green Process. Synth. 2021, 10, 125–133. [Google Scholar] [CrossRef]
- Tian, D.F.; Wang, Y.R.; Song, W. Degradation of Rhodamine B in aqueous solution by UV/PMS system, kinetics and reaction mechanism. Acta Sci. Circumstantiae 2018, 38, 1868–1876. [Google Scholar]
- Chen, M.; Shang, T.; Fang, W.; Diao, G. Study on adsorption and desorption properties of the starch grafted p-tert-butyl-calix[n] arene for butyl Rhodamine B solution. J. Hazard. Mater. 2011, 185, 914–921. [Google Scholar] [CrossRef]
- Sha, Z.; Sun, J.; Chan, H.S.O.; Jaenicke, S.; Wu, J. Enhanced photocatalytic activity of the Ag I/Ui O-66(Zr)composite for rhodamine B degradation under visible-light irradiation. ChemPlusChem 2015, 80, 1321–1328. [Google Scholar] [CrossRef]
- Han, Y.; Bai, C.; Zhang, L.; Wu, J.; Meng, H.; Xu, J.; Xu, Y.; Liang, Z.; Zhang, X. A facile strategy for fabricating Ag IMIL-53(Fe)composites, Superior interfacial contact and enhanced visible light photocatalytic performance. New J. Chem. 2018, 42, 3799–3807. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wu, Y.; Zeng, G.; Dong, H.; Chen, X.; Leng, L.; Wu, Z.; Peng, L. In situ synthesis of In2S3@MIL-125(Ti) core-shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis. Appl. Catal. B Environ. 2016, 186, 19–29. [Google Scholar] [CrossRef]
- Chen, D.; Feng, P.F.; Wei, F.H. Preparation of Fe(III)-MOFs by microwave-assisted ball for efficiently removing organic dyes in aqueous solutions under natural light. Chem. Eng. Process.-Process Intensif. 2019, 135, 63–67. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Shukla, S.; Khan, I.; Kang, S.M.; Haldorai, Y.; Tripathi, K.M.; Jung, S.; Chen, L.; Kim, T.; Huh, Y.S.; et al. A Sustainable Graphene Aerogel Capable of the Adsorptive Elimination of Biogenic Amines and Bacteria from Soy Sauce and Highly Efficient Cell Proliferation. ACS Appl. Mater. Interfaces 2019, 11, 43949–43963. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Li, J.R.; Lv, X.L.; Zhang, Y.Q.; Guo, G. Photocatalytic organic pollutants degradation in metal-organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, J.; Liu, D.; Lin, W. Nanoscale Metal-Organic Frameworks for Biomedical Imaging and Drug Delivery. Acc. Chem. Res. 2011, 44, 957–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.Y.; Qin, C.; Wang, X.L.; Su, Z.M. Metal-organic frameworks as potential drug delivery systems. Expert Opin. Drug Deliv. 2013, 10, 89–101. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon Dioxide Capture in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-Organic Framework Materials as Chemical Sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Sheel, R.; Kumari, P.; Panda, P.K.; Ansari, M.D.J.; Patel, P.; Singh, S.; Kumari, B.; Sarkar, B.; Mallick, M.A.; Verma, S.K. Molecular intrinsic proximal interaction infer oxidative stress and apoptosis modulated in vivo biocompatibility of Pniruri contrived antibacterial iron oxide nanoparticles with zebra fish. Environ. Pollut. 2020, 267, 115482. [Google Scholar] [CrossRef]
- Verma, S.K.; Jha, E.; Panda, P.K.; Thirumurugan, A.; Patro, S.; Parashar, S.K.S.; Suar, M. Molecular insights to alkaline based bio-fabrication of silver nanoparticles for inverse cytotoxicity and enhanced antibacterial activity. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 92, 807–818. [Google Scholar] [CrossRef]
- Kumari, S.; Kumari, P.; Panda, P.K.; Patel, P.; Jha, E.; Mallick, M.A.; Verma, S.K. Biocompatible biogenic silver nanoparticles interact with caspases on an atomic level to elicit apoptosis. Nanomedicine 2020, 15, 2119–2132. [Google Scholar]
- Mohan, A.; Dipallini, S.; Lata, S.; Mohanty, S.; Pradhan, P.K.; Patel, P.; Makkar, H.; Verma, S.K. Oxidative stress induced antimicrobial efficacy of chitosan and silver nanoparticles coated Gutta-percha for endodontic applications. Mater. Today Chem. 2020, 17, 100299. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barea, E.; Montoro, C.; Navarro, J.A. Toxic Gas Removal—Metal-Organic Frameworks for the Capture and Degradation of Toxic Gases and Vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Yang, Q.; Li, X.; Zeng, G.; Wang, D.; Niu, C.; Zhao, J.; An, H.; Xie, T.; Deng, Y. Hierarchical assembly of graphene-bridged Ag3PO4/Ag/BiVO4 (040) Z-scheme photocatalyst, An efficient, sustainable and heterogeneous catalyst with enhanced visible-light photoactivity towards tetracycline degradation under visible light irradiation. Appl. Catal. B 2017, 200, 330–342. [Google Scholar] [CrossRef]
- Wei, F.H.; Ren, Q.H.; Zhang, H.; Yang, L.L.; Chen, H.L.; Liang, Z.; Chen, D. Removal of tetracycline hydrochloride from wastewater by Zr/Fe-MOFs/GO composites. RSC Adv. 2021, 11, 9977–9984. [Google Scholar] [CrossRef]
- Wei, F.H.; Chen, D.; Liang, Z.; Zhao, S.Q.; Luo, Y. Synthesis and characterization of metal-organic frameworks fabricated by microwave-assisted ball milling for adsorptive removal of from aqueous solutions. RSC Adv. 2017, 7, 46520–46528. [Google Scholar] [CrossRef]
- Farha, O.K.; Hupp, J.T. Rational design, synthesis, purification, and activation of metal−organic framework materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Zhang, H.; Ren, Q.; Chen, H.; Yang, L.; Ding, B.; Yu, M.; Liang, Z. Removal of organic contaminants from wastewater with GO/MOFs composites. PLoS ONE 2021, 7, e0253500. [Google Scholar] [CrossRef]
- Tian, N.; Jia, Q.; Su, H.; Zhi, Y.; Ma, A.; Wu, J.; Shan, S. The synthesis of mesostructured NH2-MIL-101(Cr) and kinetic and thermodynamic study in tetracycline aqueous solutions. J. Porous Mater. 2016, 23, 1269–1278. [Google Scholar] [CrossRef]
- Hu, T.; Lv, H.; Shan, S.; Jia, Q.; Su, H.; Tian, N.; He, S. Porous structured MIL-101 synthesized with different mineralizers for adsorptive removal of oxytetracycline from aqueous solution. RSC Adv. 2016, 6, 73741–73747. [Google Scholar] [CrossRef]
- Wu, C.S.; Xiong, Z.H.; Li, C.; Zhang, J.M. Zeolitic imidazolate metal organic framework ZIF-8 with ultra-high adsorption capacity bound tetracycline in aqueous solution. Rsc Adv. 2015, 5, 82127–82137. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Ribeiro, R.S.; Gomes, H.T.; Ovejero, G.; García, J. Removal of antibiotic compounds by adsorption using glycerol-based carbon materials. Chem. Eng. J. 2016, 296, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Wei, F.; Chen, D.; Liang, Z.; Zhao, S. Comparison study on the adsorption capacity of Rhodamine B and Orange II on Fe-MOFs. Nanomaterials 2018, 8, 248. [Google Scholar] [CrossRef] [Green Version]


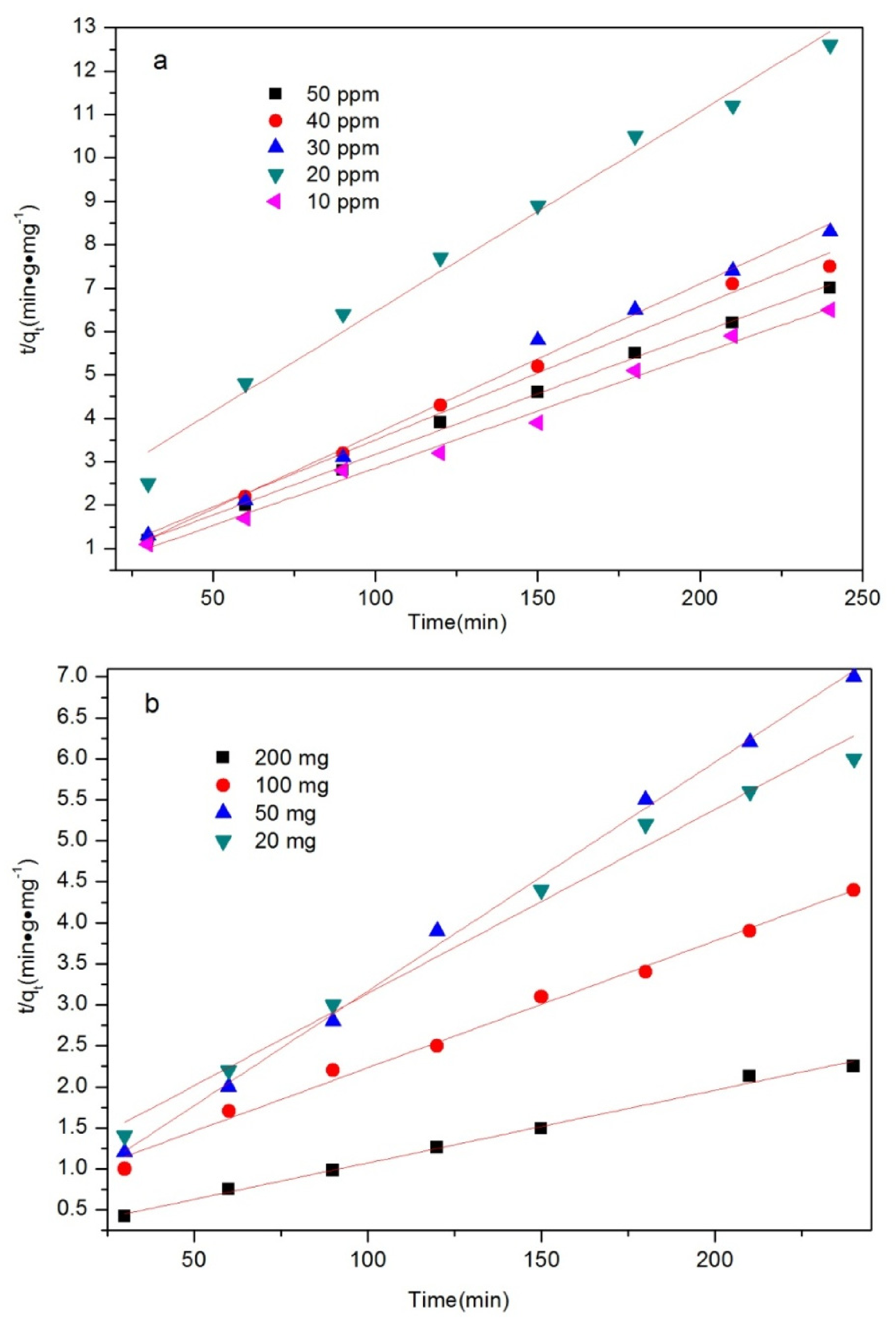
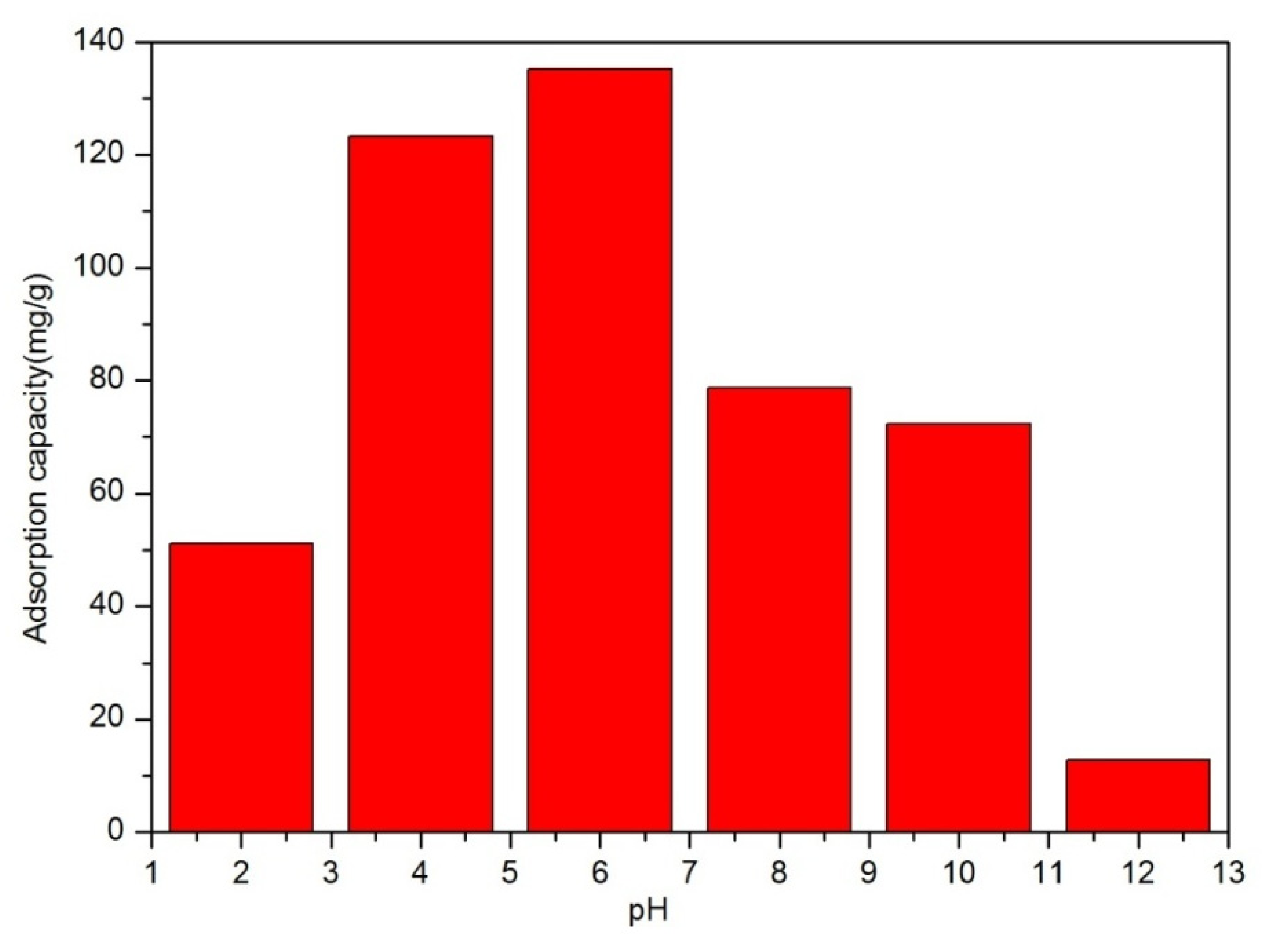
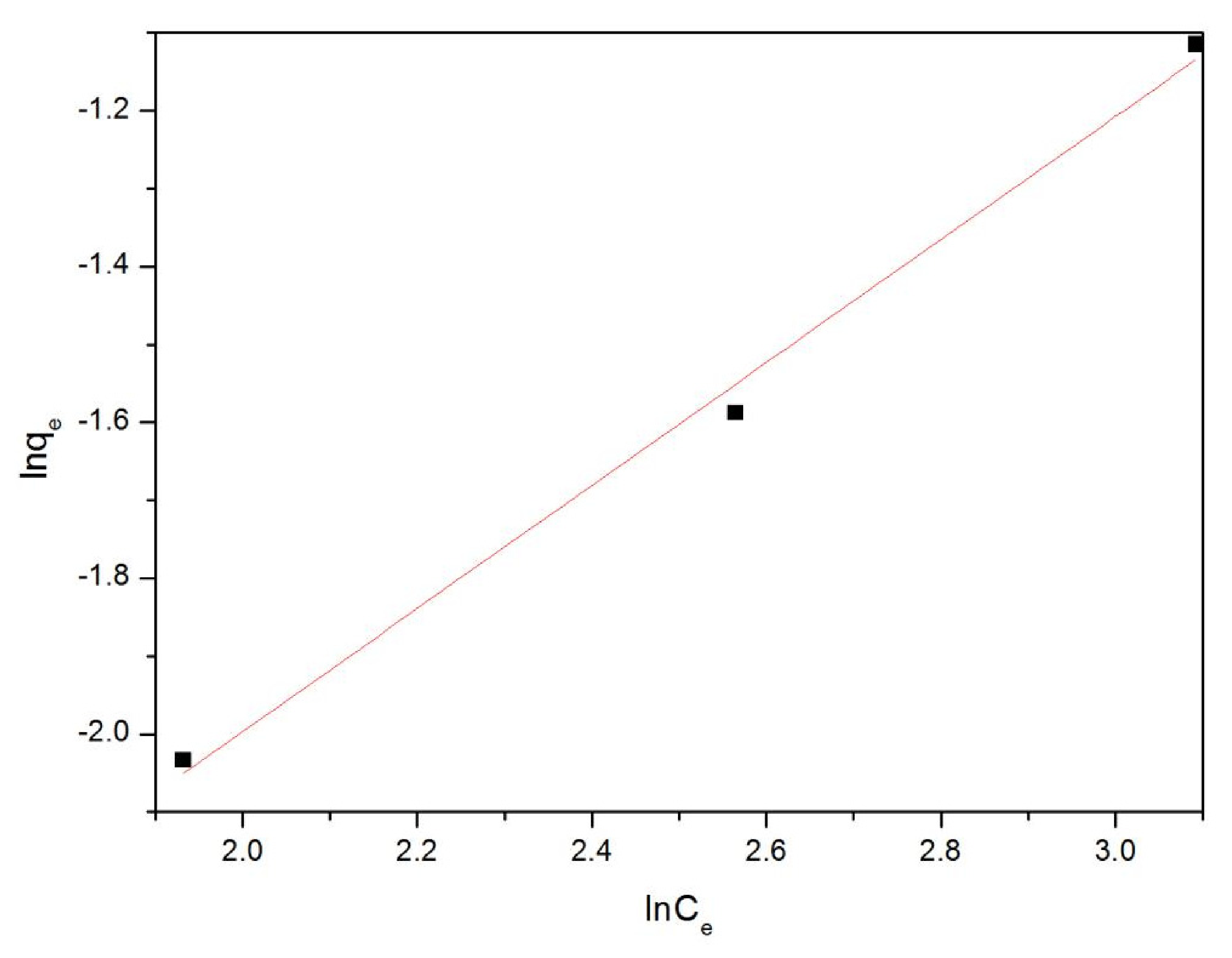
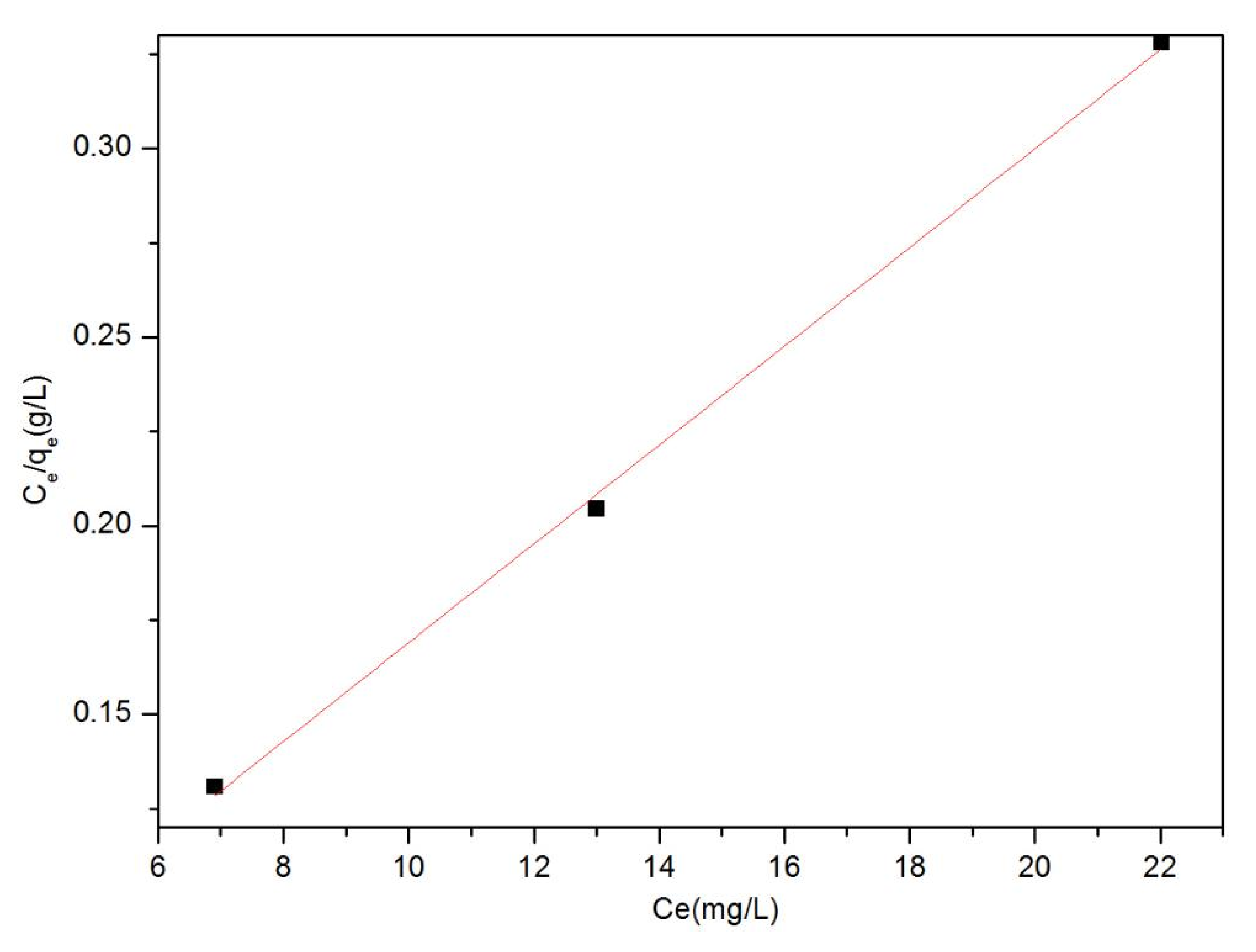
| Concentration (ppm) | K (g(mg min)−1) | R2 |
|---|---|---|
| 50 | 2.8 × 10−2 | 0.9976 |
| 40 | 3.1 × 10−2 | 0.992 |
| 30 | 3.5 × 10−2 | 0.992 |
| 20 | 4.6 × 10−2 | 0.983 |
| 10 | 2.6 × 10−2 | 0.9906 |
| Mass (mg) | R2 | K (g(mg min)−1) |
|---|---|---|
| 200 | 0.9941 | 8.8 × 10−3 |
| 100 | 0.9921 | 1.5 × 10−2 |
| 50 | 0.9976 | 2.8 × 10−2 |
| 20 | 0.987 | 2.2 × 10−2 |
| Adsorbent | Langmuir Isotherm | Freundlich Isotherm | ||
|---|---|---|---|---|
| K(L/mg) | R2 | K (mg/g)(L/mg)1/n | R2 | |
| Fe-MOFs | 0.0131 | 0.99783 | 0.78938 | 0.99031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Q.; Nie, M.; Yang, L.; Wei, F.; Ding, B.; Chen, H.; Liu, Z.; Liang, Z. Synthesis of MOFs for RhB Adsorption from Wastewater. Inorganics 2022, 10, 27. https://doi.org/10.3390/inorganics10030027
Ren Q, Nie M, Yang L, Wei F, Ding B, Chen H, Liu Z, Liang Z. Synthesis of MOFs for RhB Adsorption from Wastewater. Inorganics. 2022; 10(3):27. https://doi.org/10.3390/inorganics10030027
Chicago/Turabian StyleRen, Qinhui, Meng Nie, Lili Yang, Fuhua Wei, Bo Ding, Hongliang Chen, Zhengjun Liu, and Zhao Liang. 2022. "Synthesis of MOFs for RhB Adsorption from Wastewater" Inorganics 10, no. 3: 27. https://doi.org/10.3390/inorganics10030027
APA StyleRen, Q., Nie, M., Yang, L., Wei, F., Ding, B., Chen, H., Liu, Z., & Liang, Z. (2022). Synthesis of MOFs for RhB Adsorption from Wastewater. Inorganics, 10(3), 27. https://doi.org/10.3390/inorganics10030027






