Rich CuO Nanowires Fabrication via Laser Post-Treatment of Laser-Textured Copper Substrate
Abstract
1. Introduction
2. Results
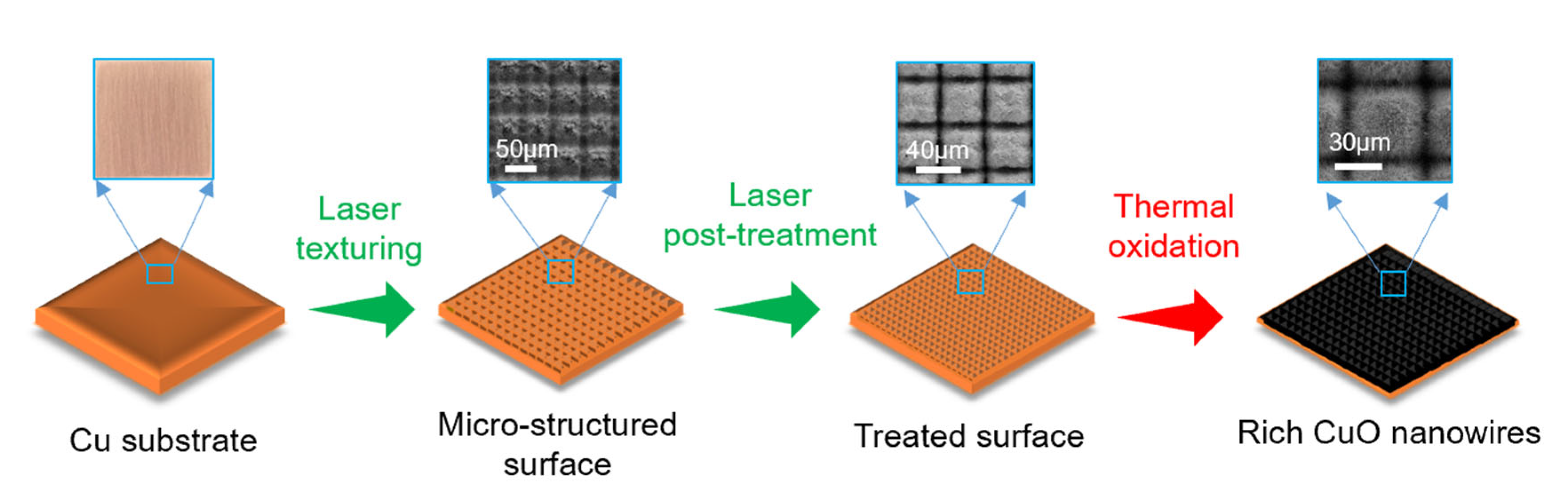
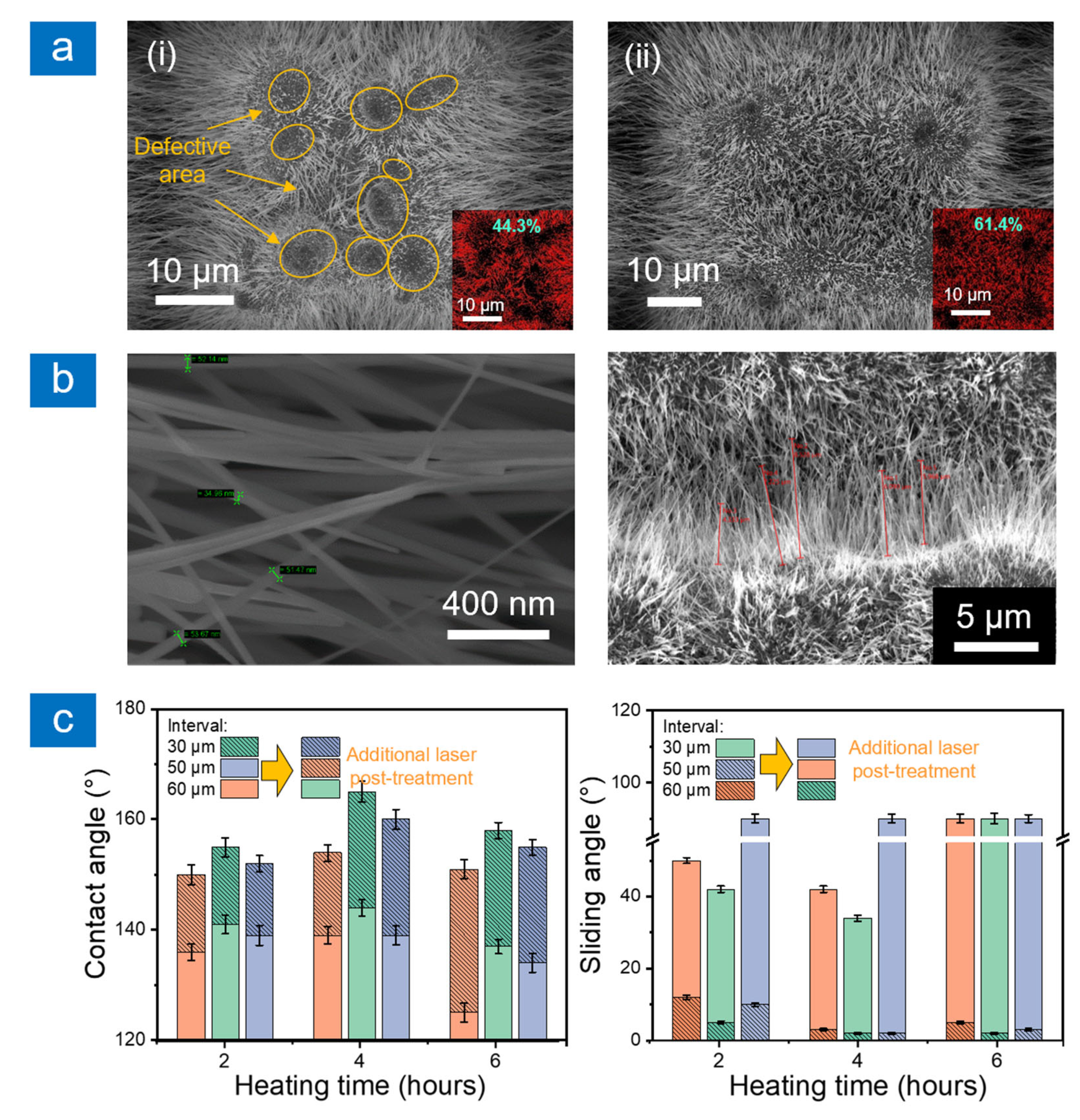
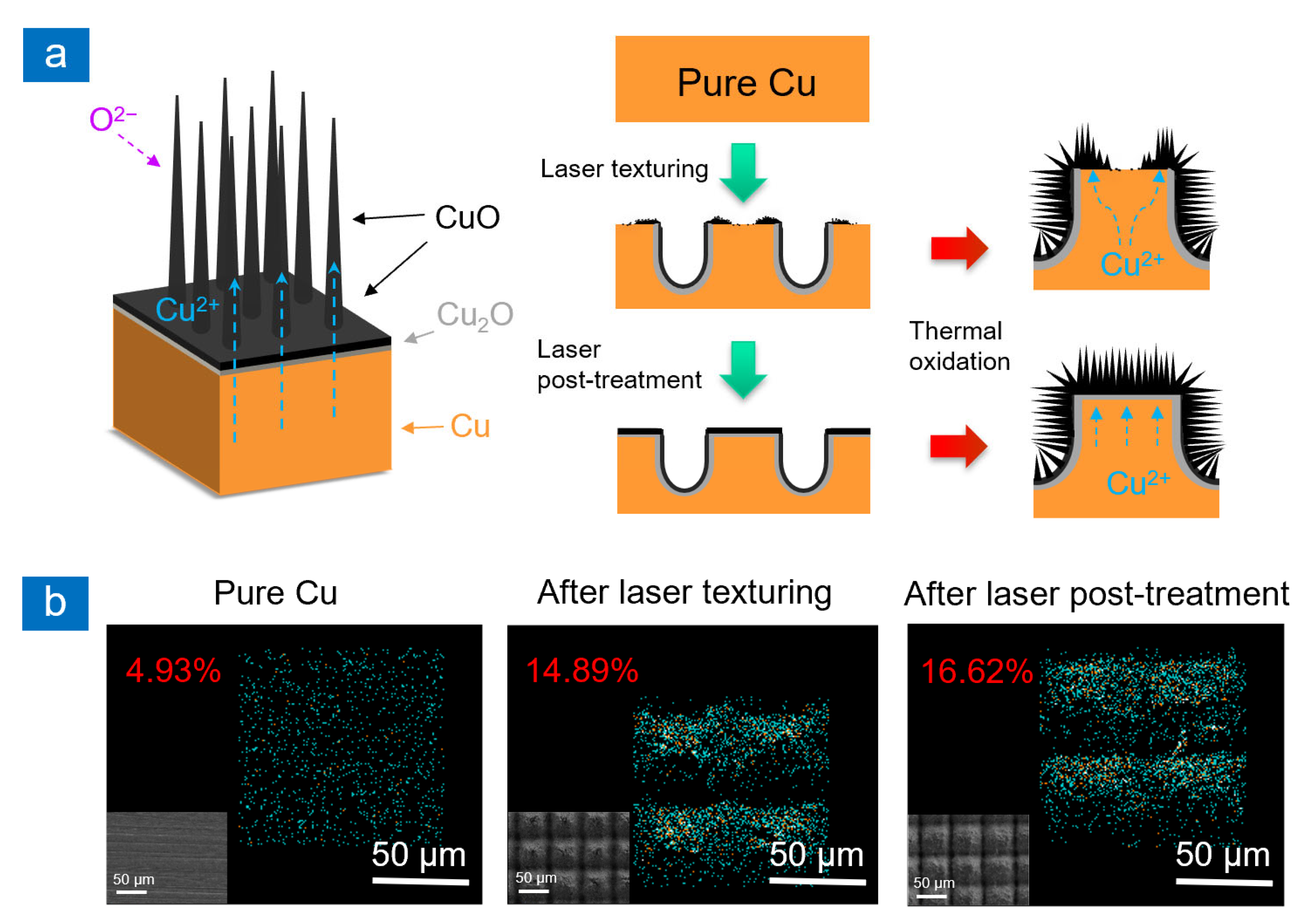
2.1. Surface Morphology and Wettability
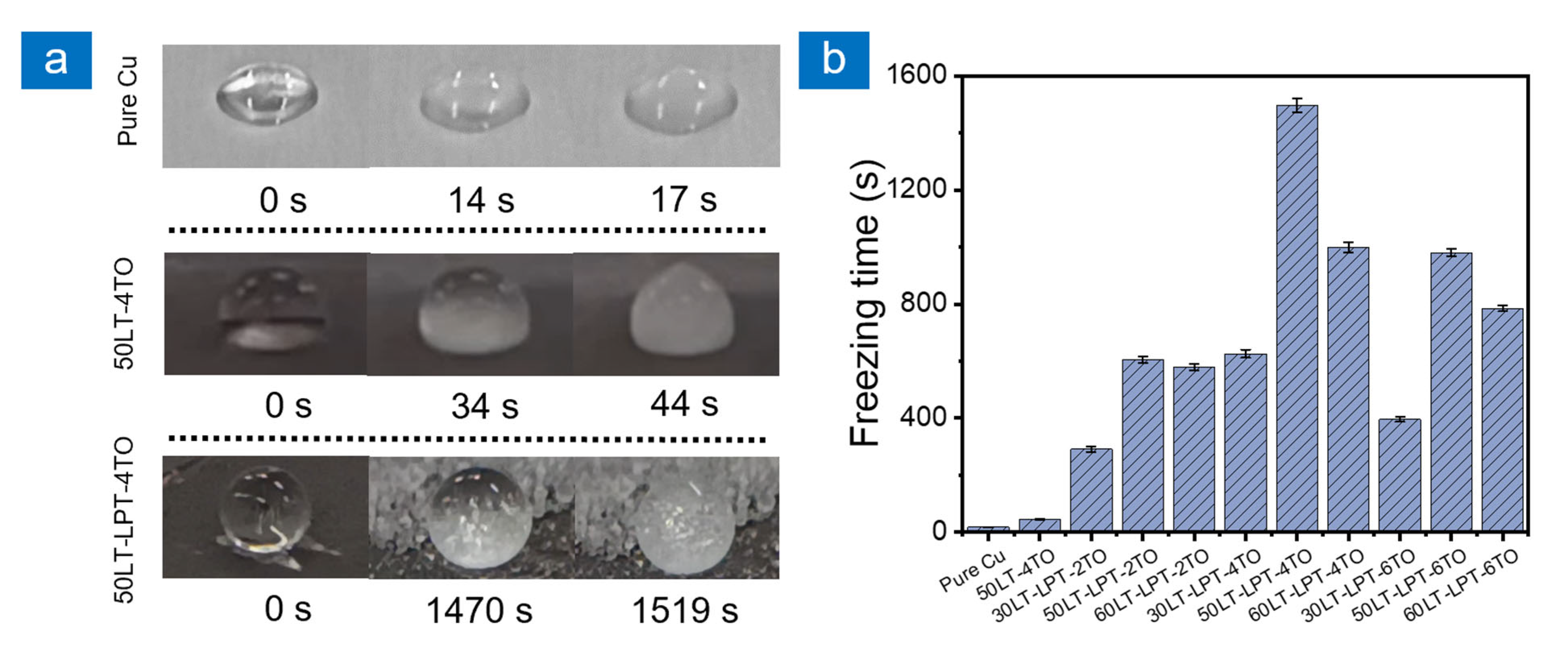
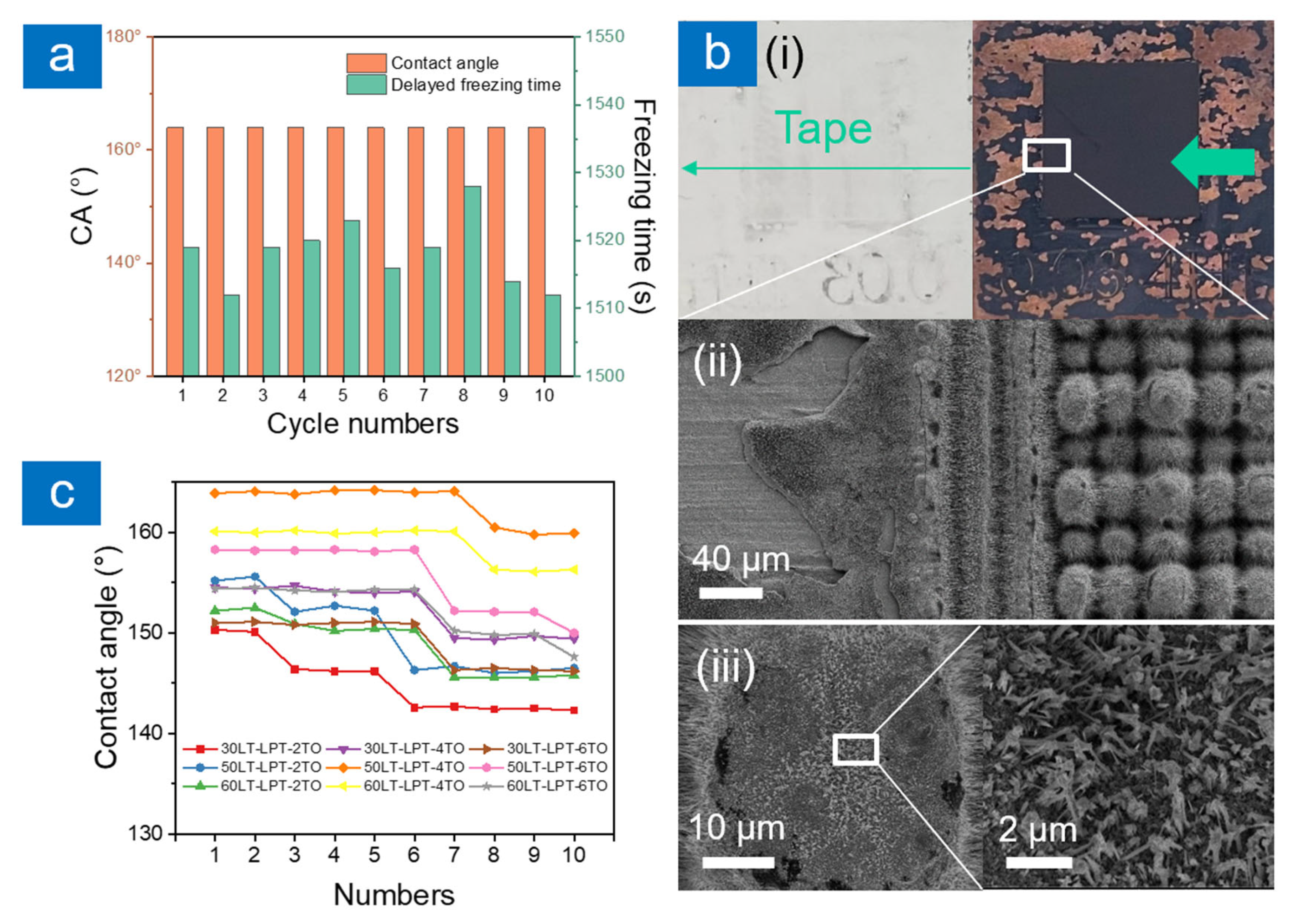
2.2. Anti-Icing Performance
2.3. Durability
3. Materials and Methods
3.1. Sample Preparation
3.2. Surface Characterization
3.3. Anti-Icing Performance Evaluation
3.4. Durability Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdulshaheed, A.A.; Wang, P.; Huang, G.; Li, C. High performance copper-water heat pipes with nanoengineered evaporator sections. Int. J. Heat Mass Transf. 2019, 133, 474–486. [Google Scholar] [CrossRef]
- Yoon, B.Y.; Jung, G.; Lim, C.S. A Study on the Corrosion of Cu-Ni Alloy in Chlorinated Seawater for Marine Applications. Corros. Sci. Technol. 2018, 17, 176–182. [Google Scholar]
- Long, J.; Wu, Y.; Gong, D.; Fan, P.; Jiang, D.; Zhang, H.; Zhong, M. Femtosecond Laser Fabricated Superhydrophobic Copper Surfaces and Their Anti-Icing Properties. Chin. J. Lasers 2015, 42, 706002. [Google Scholar] [CrossRef]
- Jiang, W.; He, J.; Xiao, F.; Yuan, S.; Lu, H.; Liang, B. Preparation and Antiscaling Application of Superhydrophobic Anodized CuO Nanowire Surfaces. Ind. Eng. Chem. Res. 2015, 54, 6874–6883. [Google Scholar] [CrossRef]
- Shu, Y.; Zhang, Y.; Zhang, J. First-principles analysis of properties of Cu surfaces. Acta Phys. Sin. 2012, 61, 016108. [Google Scholar] [CrossRef]
- Liang, C.; Lin, K.; Cheng, P. Effect of Au/Pd bilayer metallization on solder/Cu interfacial reaction and growth kinetics of Cu Sn intermetallic compounds during solid-state aging. Surf. Coat. Technol. 2017, 319, 55–60. [Google Scholar] [CrossRef]
- Vogel, G. Creeping corrosion of copper on printed circuit board assemblies. Microelectron. Reliab. 2016, 64, 650–655. [Google Scholar] [CrossRef]
- Oh, G.H.; Joo, S.J.; Jeong, J.W.; Kim, H.S. Effect of plasma treatment on adhesion strength and moisture absorption characteristics between epoxy molding compound/silicon chip (EMC/chip) interface. Microelectron. Reliab. 2019, 92, 63–72. [Google Scholar] [CrossRef]
- Nekouei, R.K.; Pahlevani, F.; Rajarao, R.; Golmohammadzadeh, R.; Sahajwalla, V. Two-step pre-processing enrichment of waste printed circuit boards: Mechanical milling and physical separation. J. Clean. Prod. 2018, 184, 1113–1124. [Google Scholar] [CrossRef]
- Zou, S.; Li, X.; Dong, C.; Ding, K.; Xiao, K. Electrochemical migration, whisker formation, and corrosion behavior of printed circuit board under wet H2S environment. Electrochim. Acta 2013, 114, 363–371. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, Y.; He, W. A combination of self-assembled monolayer and hydrophobic conformal coating for anti-corrosion of Cu/NiP/Au 3D circuitry in artificial sweat solution. Surf. Coat. Technol. 2017, 320, 126–131. [Google Scholar] [CrossRef]
- Ghosh, S. Three-dimensional microplate formation with evaporating nanoparticle suspensions on superhydrophobic surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 901–906. [Google Scholar] [CrossRef]
- Han, L.; Zhao, X.; Shen, Y. Study of the effect of thermal conductivity on the anti-icing performance of coated fabrics. Text. Res. J. 2020, 90, 2035–2045. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Q.; Jin, R.; Lin, Z.; Qiu, H.; Xu, Y. Mechanism analysis and durability evaluation of anti-icing property of superhydrophobic surface. Int. J. Heat Mass Transf. 2020, 156, 119768. [Google Scholar] [CrossRef]
- Zhu, K.; Li, X.; Su, J.; Li, H.; Zhao, Y.; Yuan, X. Improvement of anti-icing properties of low surface energy coatings by introducing phase-change microcapsules. Polym. Eng. Sci. 2018, 58, 973–979. [Google Scholar] [CrossRef]
- Jiang, X.; Fan, C.; Xie, Y. New method of preventing ice disaster in power grid using expanded conductors in heavy icing area. IET Gener. Transm. Distrib. 2018, 13, 536–543. [Google Scholar] [CrossRef]
- Zheng, M.; Wu, S.; Wang, C.; Li, Y.; Ma, Z.; Peng, L. A Study on Evaluation and Application of Snowmelt Performance of Anti-Icing Asphalt Pavement. Appl. Sci. 2017, 7, 583. [Google Scholar] [CrossRef]
- Sarajcev, I.; Majstrovic, M.; Medic, I. Calculation of losses in electric power cables as the base for cable temperature analysis. Adv. Comput. Methods Heat Transf. VI 2000, 3, 529–537. [Google Scholar]
- Mishchenko, L.; Hatton, B.; Bahadur, V.; Taylor, J.A.; Krupenkin, T.; Aizenberg, J. Design of Ice-free Nanostructured Surfaces Based on Repulsion of Impacting Water Droplets. ACS Nano 2010, 4, 7699–7707. [Google Scholar] [CrossRef]
- Guo, P.; Zheng, Y.; Wen, M.; Song, C.; Lin, Y.; Jiang, L. Icephobic/anti-icing properties of micro/nanostructured surfaces. Adv. Mater. 2012, 24, 2642–2648. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Wang, W.; Xu, C.; Ren, L. Superhydrophobic Copper Surface Textured by Laser for Delayed Icing Phenomenon. Langmuir 2020, 36, 1075–1082. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, R.; Yan, H.; Lin, Y.; Huang, W.; Yuan, G.; Cui, J. Bioinspired robust top-perforated micro-conical array of TC4 surface fabricated by pulsed laser ablation for enhanced anti-icing property. J. Mater. Sci. 2022, 57, 8890–8903. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Z.; He, J. CuO/Cu based superhydrophobic and self-cleaning surfaces. Scr. Mater. 2016, 118, 60–64. [Google Scholar] [CrossRef]
- Zhan, Y.; Ruan, M.; Li, W.; Li, H.; Hu, L.; Ma, F.; Yu, Z.; Feng, W. Fabrication of anisotropic PTFE superhydrophobic surfaces using laser microprocessing and their self-cleaning and anti-icing behavior. Colloids Surf. A Physicochem. Eng. Asp. 2017, 535, 8–15. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Y.; Liao, R.; Wang, L.; Gao, X. Fabrication of a Porous Slippery Icephobic Surface and Effect of Lubricant Viscosity on Anti-Icing Properties and Durability. Coatings 2020, 10, 896. [Google Scholar] [CrossRef]
- Dou, W.; Wu, J.; Gu, T.; Wang, P.; Zhang, D. Preparation of super-hydrophobic micro-needle CuO surface as a barrier against marine atmospheric corrosion. Corros. Sci. 2018, 131, 156–163. [Google Scholar] [CrossRef]
- Mirzanamadi, R.; Hagentoft, C.E.; Johansson, P. Coupling a Hydronic Heating Pavement to a Horizontal Ground Heat Exchanger for harvesting solar energy and heating road surfaces. Renew. Energy 2020, 147, 447–463. [Google Scholar] [CrossRef]
- Kirner, S.V.; Hermens, U.; Mimidis, A.; Skoulas, E.; Florian, C.; Hischen, F.; Plamadeala, C.; Baumgartner, W.; Winands, K.; Mescheder, H.; et al. Mimicking bug-like surface structures and their fluid transport produced by ultrashort laser pulse irradiation of steel. Appl. Phys. A 2017, 123, 754. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, L.; Wang, D.; Lin, X.; Chen, Y. Fabrication of biomimetic superhydrophobic surface based on nanosecond laser-treated titanium alloy surface and organic polysilazane composite coating. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 515–524. [Google Scholar] [CrossRef]
- Xu, K.; Yan, H.; Tan, C.F.; Lu, Y.; Li, Y.; Ho, G.W.; Ji, R.; Hong, M. Hedgehog Inspired CuO Nanowires/Cu2O Composites for Broadband Visible-Light-Driven Recyclable Surface Enhanced Raman Scattering. Adv. Opt. Mater. 2018, 6, 1701167. [Google Scholar] [CrossRef]
- Khew, S.; Tan, C.; Yan, H.; Lin, S.; Thian, E.; Zhou, R.; Hong, M. Nanosecond laser ablation for enhanced adhesion of CuO nanowires on copper substrate and its application for oil-water separation. Appl. Surf. Sci. 2019, 465, 995–1002. [Google Scholar] [CrossRef]
- Yan, X.; Huang, Z.; Sett, S.; Oh, J.; Cha, H.; Li, L.; Feng, L.; Wu, Y.; Zhao, C.; Orejon, D.; et al. Atmosphere-Mediated Superhydrophobicity of Rationally Designed Micro/Nanostructured Surfaces. ACS Nano 2019, 13, 4160–4173. [Google Scholar] [CrossRef]
- Wenzel, R. Resistance of solid surfaces to wetting by water. Trans. Faraday Soc. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, Y.S.X.; Hong, M. Directional sliding of water: Biomimetic snake scale surfaces. Opto-Electron. Adv. 2021, 4, 210008. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, D.; Chen, Y.; Jin, Q.; Wang, G. Enhanced Field Emission from UV-Illuminated CuO Nanowires Fabricated by Thermal Oxidation of Cu Film. Nano 2016, 11, 1650056. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Xu, D.; Wang, Z.; Li, X.; Zhang, K. Facile large-scale synthesis of vertically aligned CuO nanowires on nickel foam: Growth mechanism and remarkable electrochemical performance. J. Mater. Chem. A 2014, 2, 3865–3874. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, D.; Hung, T.; Zhang, K. Facile synthesis, growth mechanism and reversible superhydrophobic and superhydrophilic properties of non-flaking CuO nanowires grown from porous copper substrates. Nanotechnology 2013, 24, 065602. [Google Scholar] [CrossRef]
- Cao, F.; Jia, S.; Zheng, H.; Zhao, L.; Liu, H.; Li, L.; Zhao, L.; Hu, Y.; Gu, H.; Wang, J. Thermal-induced formation of domain structures in CuO nanomaterials. Phys. Rev. Mater. 2017, 1, 053401. [Google Scholar] [CrossRef]
- Jiang, X.; Herricks, T.; Xia, Y. CuO Nanowires Can Be Synthesized by Heating Copper Substrates in Air. Nano Lett. 2002, 2, 1333–1338. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, W.; Feng, X.; Feng, L.; Yue, G.; Wang, Y. Bio-inspired fabrication of copper oxide nanowire films with switchable wettability via a facile thermal oxidation method. RSC Adv. 2015, 5, 26107–26113. [Google Scholar] [CrossRef]
- Zhang, K.; Rossi, C.; Tenailleau, C.; Alphonse, P.; Chane-Ching, J.Y. Synthesis oflarge-area and aligned copper oxide nanowires from copper thin film on silicon substrate. Nanotechnology 2007, 18, 275607. [Google Scholar] [CrossRef]
- Fraggelakis, F.; Tsibidis, G.D.; Stratakis, E. Ultrashort pulsed laser induced complex surface structures generated by tailoring the melt hydrodynamics. Opto-Electron. Adv. 2022, 5, 210052. [Google Scholar] [CrossRef]
- Tang, M.; Shim, V.; Pan, Z.Y.; Choo, Y.S.; Hong, M.H. Laser ablation of metal substrates for super-hydrophobic effect. J. Laser Micro Nanoeng. 2011, 6, 6. [Google Scholar] [CrossRef]
- Yan, H.; Rashid, M.R.; Khew, S.Y.; Li, F.; Hong, M. Wettability transition of laser textured brass surfaces inside different mediums. Appl. Surf. Sci. 2018, 427, 369–375. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Yuan, G.; Zhou, R.; Huang, W.; Hong, M. Rich CuO Nanowires Fabrication via Laser Post-Treatment of Laser-Textured Copper Substrate. Inorganics 2022, 10, 236. https://doi.org/10.3390/inorganics10120236
Chen Z, Yuan G, Zhou R, Huang W, Hong M. Rich CuO Nanowires Fabrication via Laser Post-Treatment of Laser-Textured Copper Substrate. Inorganics. 2022; 10(12):236. https://doi.org/10.3390/inorganics10120236
Chicago/Turabian StyleChen, Zhekun, Gongfa Yuan, Rui Zhou, Weipeng Huang, and Minghui Hong. 2022. "Rich CuO Nanowires Fabrication via Laser Post-Treatment of Laser-Textured Copper Substrate" Inorganics 10, no. 12: 236. https://doi.org/10.3390/inorganics10120236
APA StyleChen, Z., Yuan, G., Zhou, R., Huang, W., & Hong, M. (2022). Rich CuO Nanowires Fabrication via Laser Post-Treatment of Laser-Textured Copper Substrate. Inorganics, 10(12), 236. https://doi.org/10.3390/inorganics10120236






