Ultrathin Films of Silver by Magnetron Sputtering
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
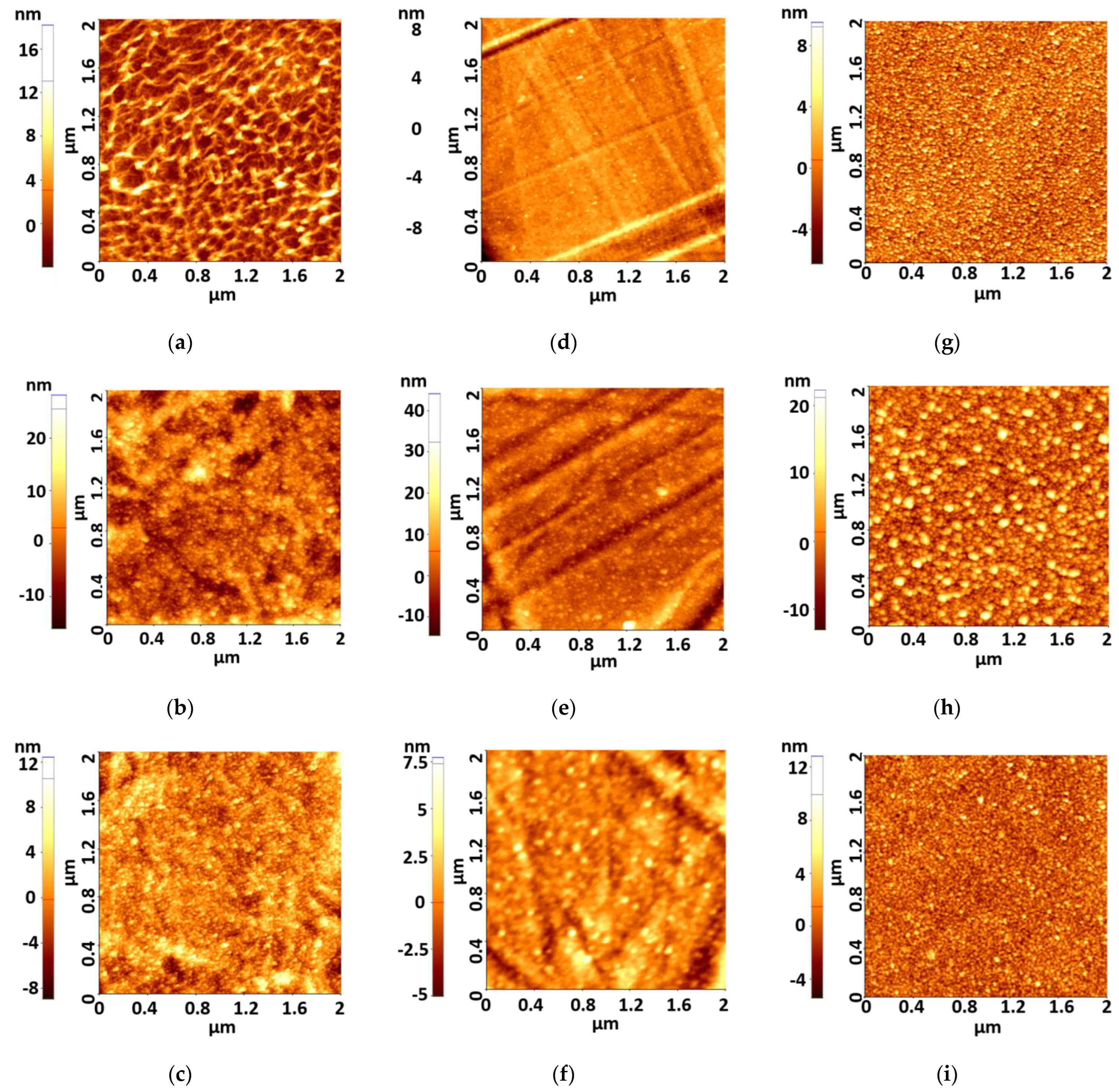
3.1. Atomic Force Microscopy (AFM)
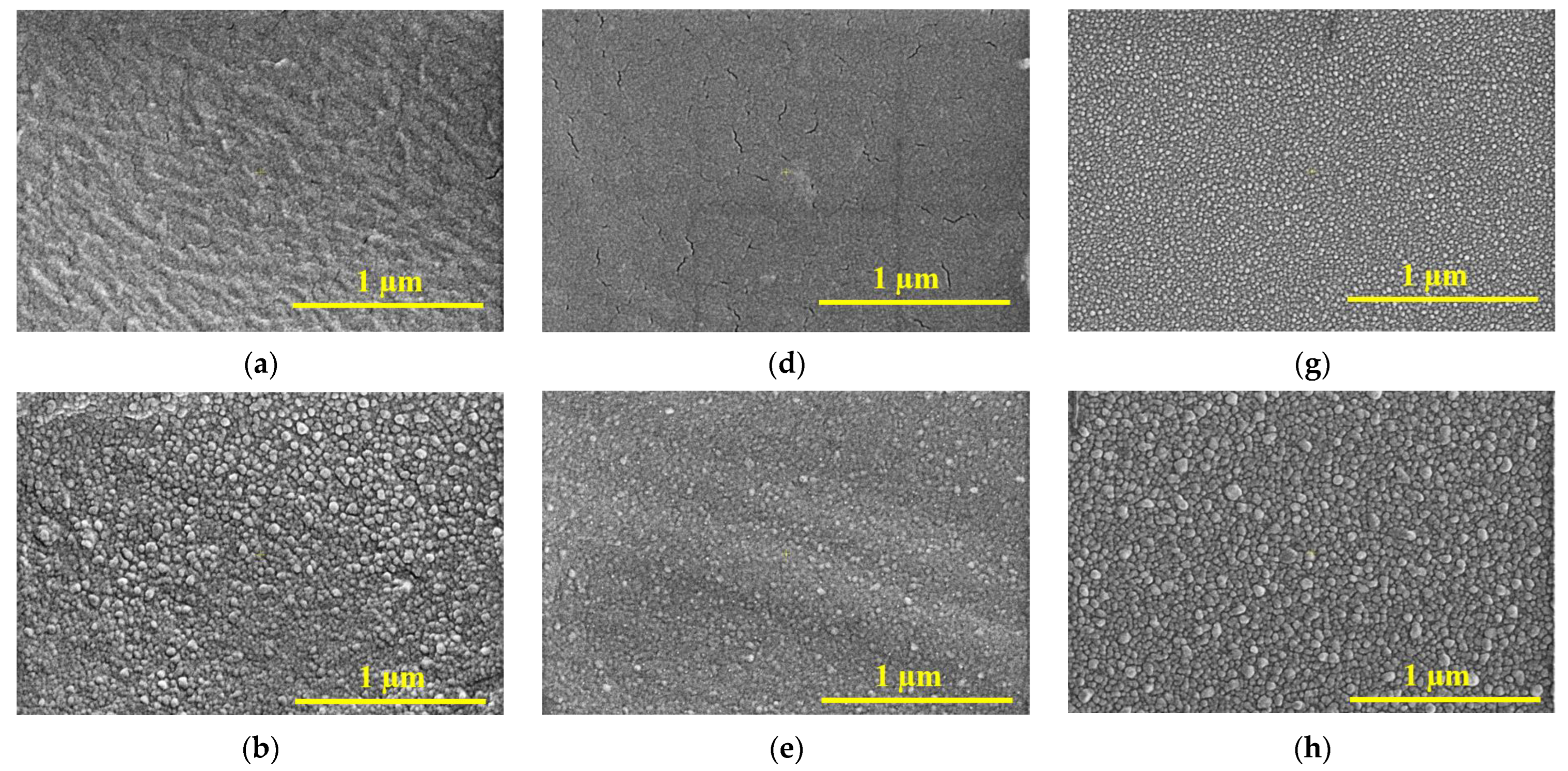
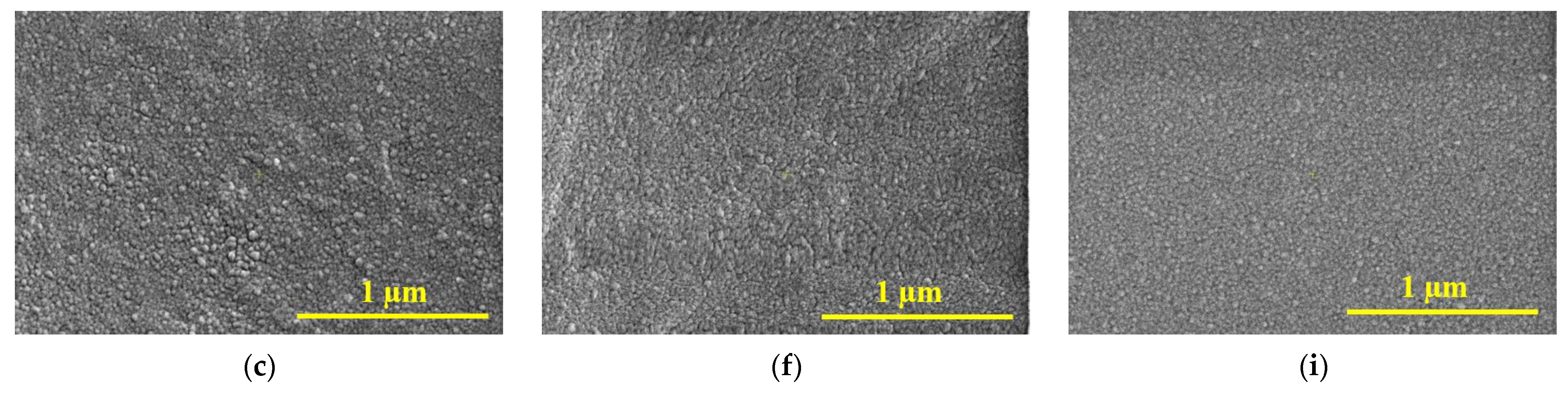
3.2. Scanning Electron Microscopy (SEM)
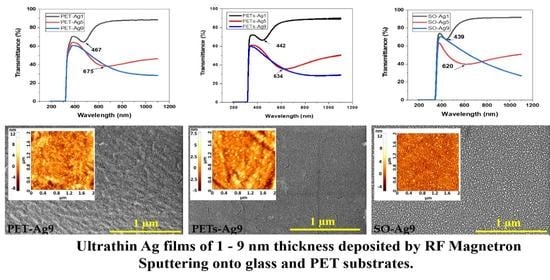
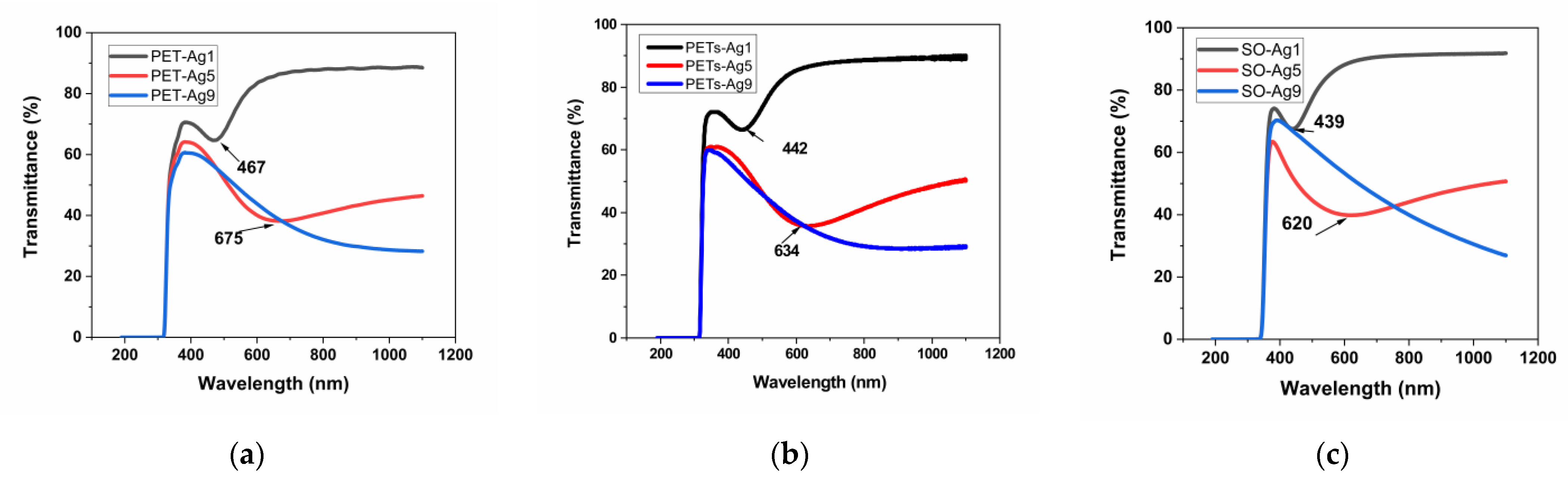
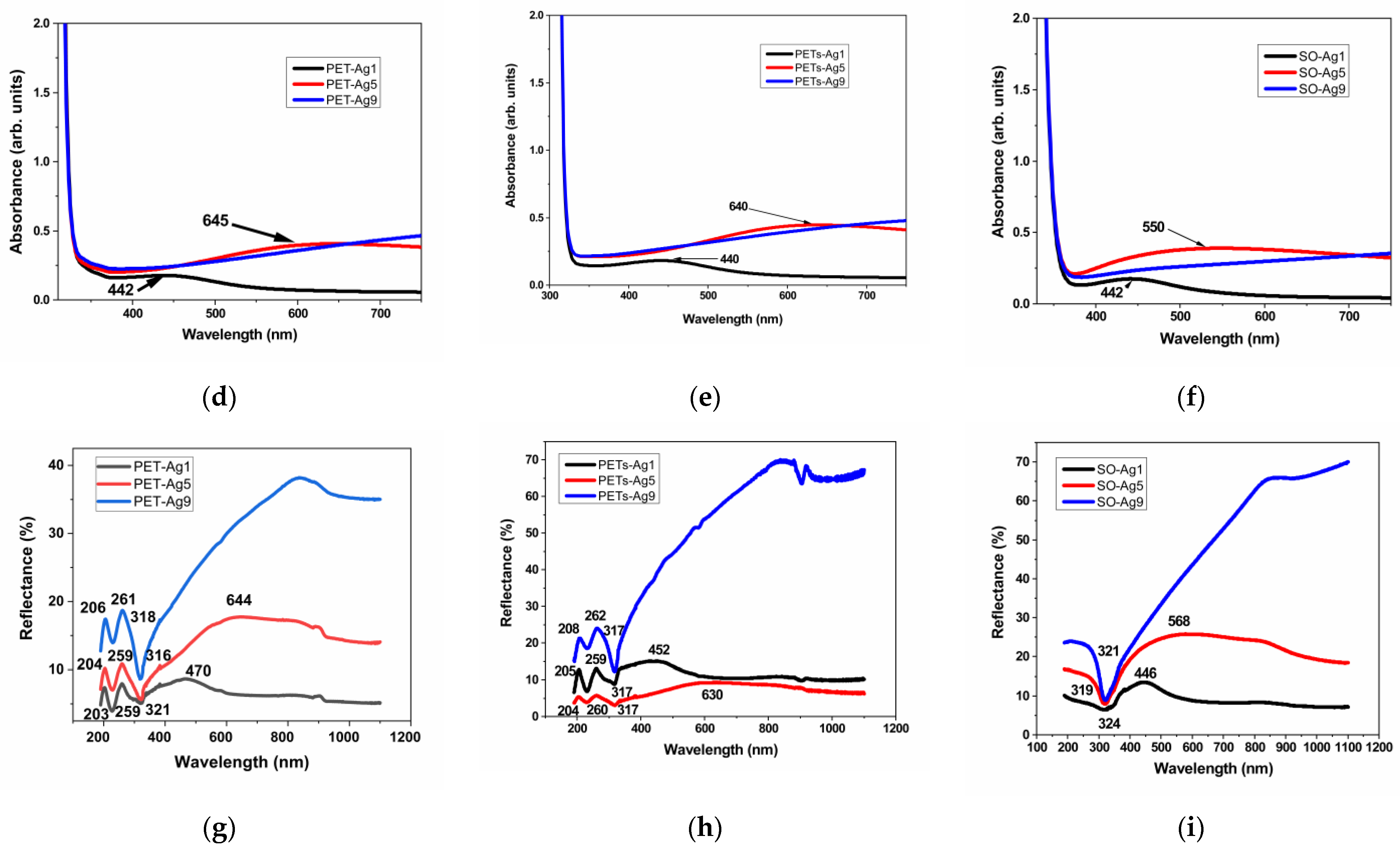
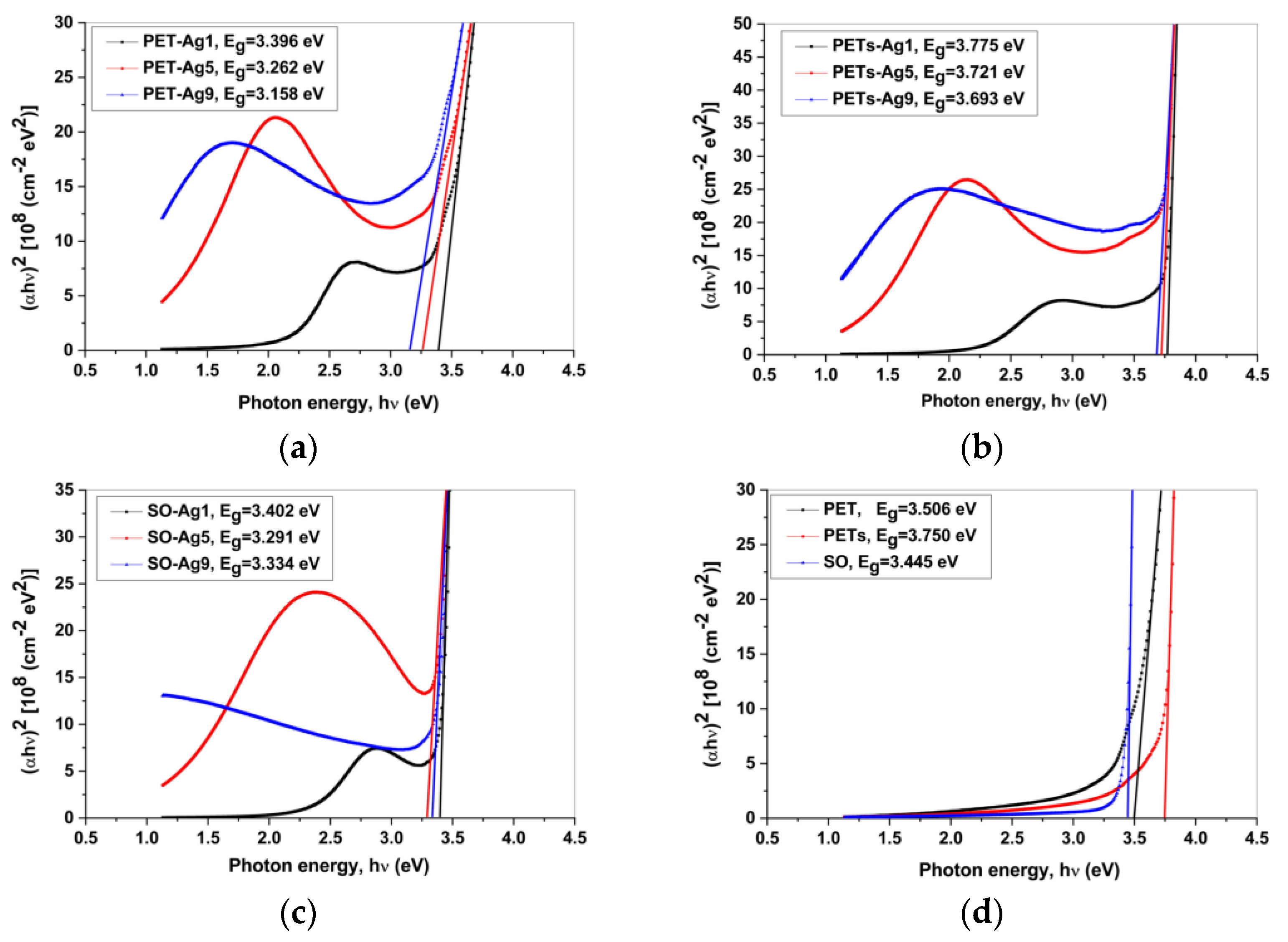
3.3. Optical Properties
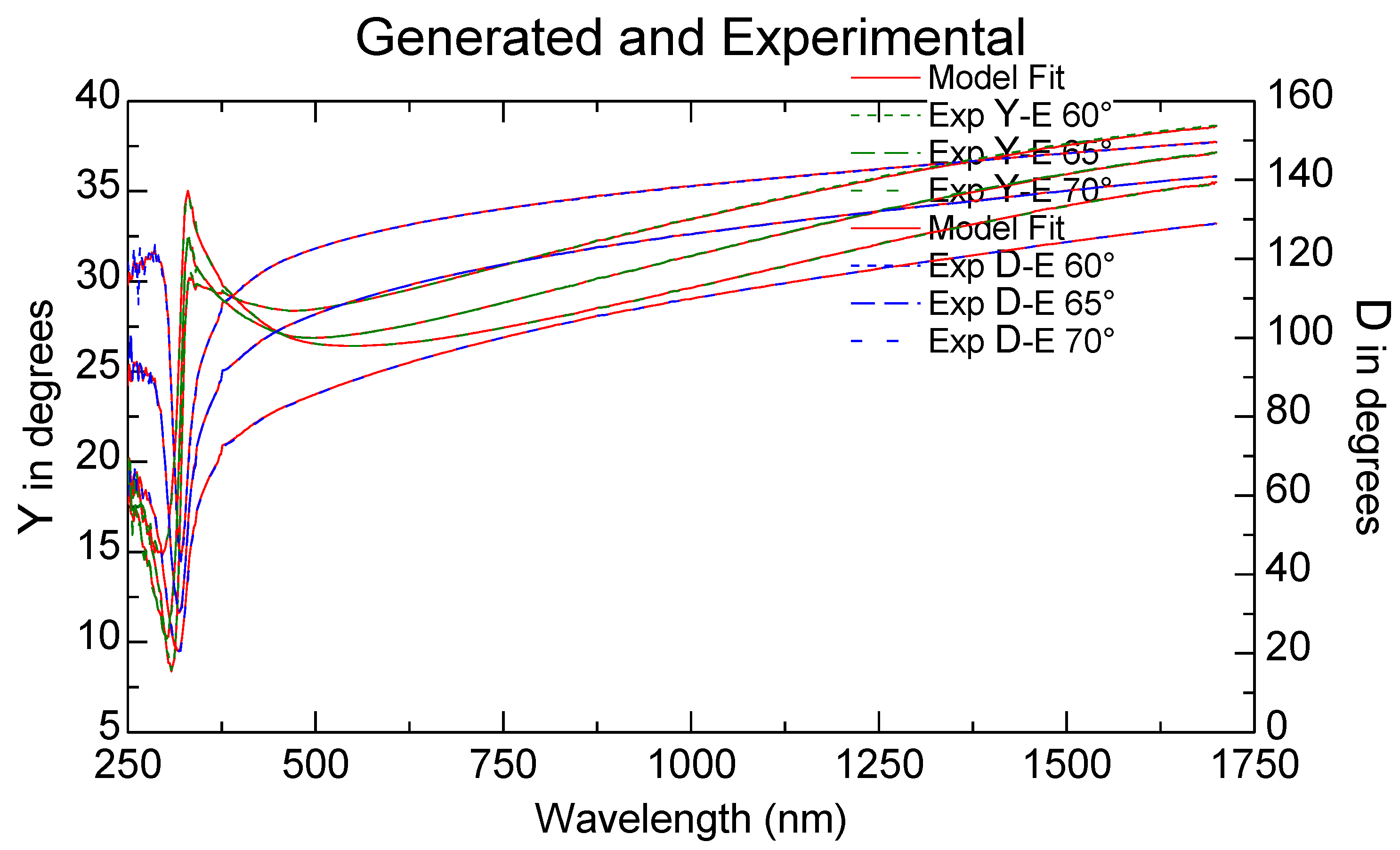
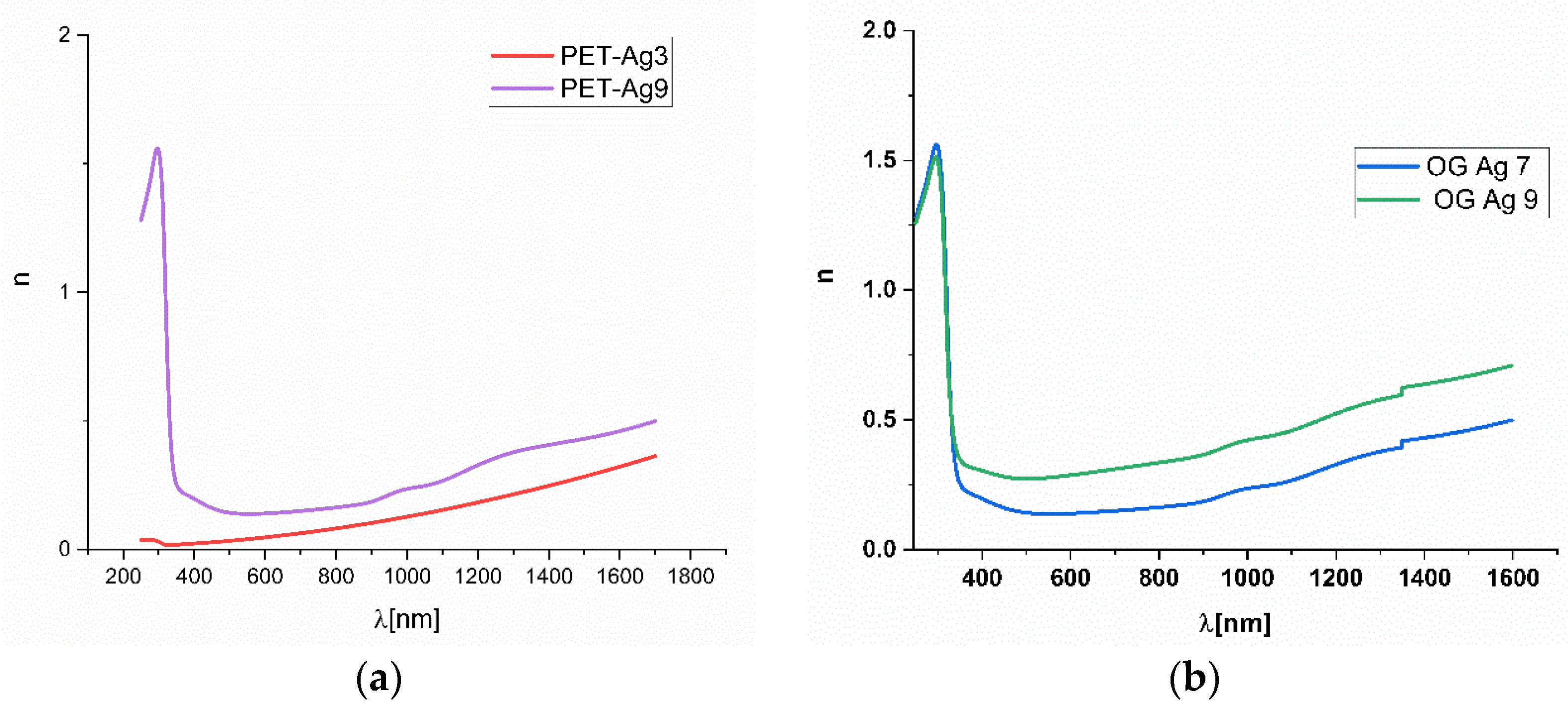
3.4. Ellipsometric Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jen, Y.-J.; Chen, C.-H.; Yu, C.-W. Deposited Metamaterial Thin Film with Negative Refractive Index and Permeability in the Visible Regime. Opt. Lett. 2011, 36, 1014. [Google Scholar] [CrossRef] [PubMed]
- Prokes, S.M.; Glembocki, O.J.; Cleveland, E.; Caldwell, J.D.; Foos, E.; Niinistö, J.; Ritala, M. Spoof-like Plasmonic Behavior of Plasma Enhanced Atomic Layer Deposition Grown Ag Thin Films. Appl. Phys. Lett. 2012, 100, 053106. [Google Scholar] [CrossRef]
- Gong, J.; Dai, R.; Wang, Z.; Zhang, Z. Thickness Dispersion of Surface Plasmon of Ag Nano-Thin Films: Determination by Ellipsometry Iterated with Transmittance Method. Sci. Rep. 2015, 5, 9279. [Google Scholar] [CrossRef] [PubMed]
- Jalili, S.; Hajakbari, F.; Hojabri, A. Effect of Silver Thickness on Structural, Optical and Morphological Properties of Nanocrystalline Ag/NiO Thin Films. J. Theor. Appl. Phys. 2018, 12, 15–22. [Google Scholar] [CrossRef]
- Axelevitch, A.; Gorenstein, B.; Golan, G. Investigation of Optical Transmission in Thin Metal Films. Phys. Procedia 2012, 32, 1–13. [Google Scholar] [CrossRef]
- Baburin, A.S.; Merzlikin, A.M.; Baryshev, A.V.; Ryzhikov, I.A.; Panfilov, Y.V.; Rodionov, I.A. Silver-Based Plasmonics: Golden Material Platform and Application Challenges [Invited]. Opt. Mater. Express 2019, 9, 611. [Google Scholar] [CrossRef]
- Hajakbari, F.; Ensandoust, M. Study of Thermal Annealing Effect on the Properties of Silver Thin Films Prepared by DC Magnetron Sputtering. Acta Phys. Pol. A 2016, 129, 680–682. [Google Scholar] [CrossRef]
- Marom, S.; Dorresteijn, M.; Modi, R.; Podestà, A.; Di Vece, M. Silver Nanoparticles from a Gas Aggregation Nanoparticle Source for Plasmonic Efficiency Enhancement in A-Si Solar Cells. Mater. Res. Express 2019, 6, 045012. [Google Scholar] [CrossRef]
- Nur-E-Alam, M.; Rahman, M.M.; Basher, M.K.; Vasiliev, M.; Alameh, K. Optical and Chromaticity Properties of Metal-Dielectric Composite-Based Multilayer Thin-Film Structures Prepared by RF Magnetron Sputtering. Coatings 2020, 10, 251. [Google Scholar] [CrossRef]
- Domaradzki, J.; Kaczmarek, D.; Mazur, M.; Wojcieszak, D.; Halarewicz, J.; Glodek, S.; Domanowski, P. Investigations of Optical and Surface Properties of Ag Single Thin Film Coating as Semitransparent Heat Reflective Mirror. Mater. Sci.-Pol. 2016, 34, 747–753. [Google Scholar] [CrossRef][Green Version]
- Zeng, S.; Baillargeat, D.; Ho, H.-P.; Yong, K.-T. Nanomaterials Enhanced Surface Plasmon Resonance for Biological and Chemical Sensing Applications. Chem. Soc. Rev. 2014, 43, 3426. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Schwartzkopf, M.; Roth, S.V.; Müller-Buschbaum, P. State of the Art of Ultra-Thin Gold Layers: Formation Fundamentals and Applications. Nanoscale Adv. 2022, 4, 2533–2560. [Google Scholar] [CrossRef] [PubMed]
- Colin, J.; Jamnig, A.; Furgeaud, C.; Michel, A.; Pliatsikas, N.; Sarakinos, K.; Abadias, G. In Situ and Real-Time Nanoscale Monitoring of Ultra-Thin Metal Film Growth Using Optical and Electrical Diagnostic Tools. Nanomaterials 2020, 10, 2225. [Google Scholar] [CrossRef] [PubMed]
- Grachev, S.; de Grazia, M.; Barthel, E.; Søndergård, E.; Lazzari, R. Real-Time Monitoring of Nanoparticle Film Growth at High Deposition Rate with Optical Spectroscopy of Plasmon Resonances. J. Phys. D Appl. Phys. 2013, 46, 375305. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, D.; Gu, D.; Kim, H.; Ling, T.; Wu, Y.-K.R.; Guo, L.J. An Ultrathin, Smooth, and Low-Loss Al-Doped Ag Film and Its Application as a Transparent Electrode in Organic Photovoltaics. Adv. Mater. 2014, 26, 5696–5701. [Google Scholar] [CrossRef]
- Bumai, Y.A.; Volobuev, V.S.; Valeev, V.F.; Dolgikh, N.I.; Lukashevich, M.G.; Khaibullin, R.I.; Nuzhdin, V.I.; Odzhaev, V.B. Optical Characteristics of Composites Obtained by Ion Implantation of Silver Ions in Polyethylene Terephthalate. J. Appl. Spectrosc. 2012, 79, 773–779. [Google Scholar] [CrossRef]
- Link, S.; Burda, C.; Wang, Z.L.; El-Sayed, M.A. Electron Dynamics in Gold and Gold–Silver Alloy Nanoparticles: The Influence of a Nonequilibrium Electron Distribution and the Size Dependence of the Electron–Phonon Relaxation. J. Chem. Phys. 1999, 111, 1255–1264. [Google Scholar] [CrossRef]
- Xu, G.; Tazawa, M.; Jin, P.; Nakao, S. Surface Plasmon Resonance of Sputtered Ag Films: Substrate and Mass Thickness Dependence. Appl. Phys. A 2005, 80, 1535–1540. [Google Scholar] [CrossRef]
- Charton, C.; Fahland, M. Optical Properties of Thin Ag Films Deposited by Magnetron Sputtering. Surface Coat. Technol. 2003, 174–175, 181–186. [Google Scholar] [CrossRef]
- Nakamura, H.; Shirakawa, Y.; Kitamura, H.; Yamada, T.; Shidara, Z.; Yokozuka, T.; Nguyen, P.; Takahashi, T.; Takahashi, S. Blended Polyethylene Terephthalate and Polyethylene Naphthalate Polymers for Scintillation Base Substrates. Radiat. Meas. 2013, 59, 172–175. [Google Scholar] [CrossRef]
- Elisa, M.; Stefan, R.C.; Vasiliu, I.C.; Iordache, S.M.; Iordache, A.-M.; Sava, B.A.; Boroica, L.; Dinca, M.C.; Filip, A.V.; Galca, A.C.; et al. A New Zinc Phosphate-Tellurite Glass for Magneto-Optical Applications. Nanomaterials 2020, 10, 1875. [Google Scholar] [CrossRef] [PubMed]
- Filip, A.; Musat, V.; Tigau, N.; Polosan, S.; Pimentel, A.; Ferreira, S.; Gomes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. ZnO Nanostructures Grown on ITO Coated Glass Substrate by Hybrid Microwave-Assisted Hydrothermal Method. Optik 2020, 208, 164372. [Google Scholar] [CrossRef]
- Shimakawa, K.; Singh, J.; O’Leary, S.K. Optical Properties of Disordered Condensed Matter. In Optical Properties of Materials and Their Applications; Singh, J., Ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 67–81. ISBN 978-1-119-50631-7. [Google Scholar]
- Oates, T.W.H.; Wormeester, H.; Arwin, H. Characterization of Plasmonic Effects in Thin Films and Metamaterials Using Spectroscopic Ellipsometry. Prog. Surface Sci. 2011, 86, 328–376. [Google Scholar] [CrossRef]
- Jiang, Y.; Pillai, S.; Green, M.A. Re-Evaluation of Literature Values of Silver Optical Constants. Opt. Express 2015, 23, 2133. [Google Scholar] [CrossRef] [PubMed]
- Tiwald, T.E.; Thompson, D.W.; Woollam, J.A.; Paulson, W.; Hance, R. Application of IR Variable Angle Spectroscopic Ellipsometry to the Determination of Free Carrier Concentration Depth Profiles. Thin Solid Films 1998, 313–314, 661–666. [Google Scholar] [CrossRef]
- Todorov, R.; Lozanova, V.; Knotek, P.; Černošková, E.; Vlček, M. Microstructure and Ellipsometric Modelling of the Optical Properties of Very Thin Silver Films for Application in Plasmonics. Thin Solid Films 2017, 628, 22–30. [Google Scholar] [CrossRef]
- Pascu Rovena; Dinescu Maria Spectroscopic Ellipsometry. Rom. Rep. Phys. 2012, 64, 135–142.
- Pascu, V.R. Bilayer Electrolyte Thin Films Gadolinia Doped Ceria /Yttria Stabilized Zirconia Prepared by Pulsed Laser Deposition for Electrochemichal Devices. Rev. Chim. 2020, 70, 4181–4186. [Google Scholar] [CrossRef]
- Palik, E.D. Handbook of Optical Constants of Solids II; Academic Press Inc.: San Diego, CA, USA, 1991; ISBN 978-0-12-544422-4. [Google Scholar]
| No. | Working Condition | Value |
|---|---|---|
| 1 | Quartz constant | 8.25 |
| 2 | Target density | 10,490 kg/m3 |
| 3 | Vacuum | 5.6 × 10−2 pascal |
| 4 | Ar pressure | 33.86 pascal |
| 5 | Active power | 1 W |
| 6 | Reactive power | 0 W |
| 7 | Intensity | 0.1 A |
| 8 | Substrate | Thick PET/thin PET/optical BK7 glass |
| 9 | Deposition speed | 0.1 × 10−10 m/s |
| 10 | Deposition time | 2–15 min |
| 11 | Thickness of deposited films | 1, 3, 5, 7, and 9 nm |
| 12 | Deposition temperature | Room temperature (RT) |
| Sample Code | Substrate | Substrate Thickness [mm] | Ag Film Deposition Thickness [nm] |
|---|---|---|---|
| PET-Ag1 | Thick PET | 0.5 | 1 |
| PET-Ag3 | Thick PET | 0.5 | 3 |
| PET-Ag5 | Thick PET | 0.5 | 5 |
| PET-Ag7 | Thick PET | 0.5 | 7 |
| PET-Ag9 | Thick PET | 0.5 | 9 |
| PETs-Ag1 | Thin PET | 0.15 | 1 |
| PETs-Ag3 | Thin PET | 0.15 | 3 |
| PETs-Ag5 | Thin PET | 0.15 | 5 |
| PETs-Ag7 | Thin PET | 0.15 | 7 |
| PETs-Ag9 | Thin PET | 0.15 | 9 |
| SO-Ag1 | Optical glass | 1.8 | 1 |
| SO-Ag3 | Optical glass | 1.8 | 3 |
| SO-Ag5 | Optical glass | 1.8 | 5 |
| SO-Ag7 | Optical glass | 1.8 | 7 |
| SO-Ag9 | Optical glass | 1.8 | 9 |
| //Sample Code | Roughness Rq [nm] | Sample Code | Roughness Rq [nm] | Sample Code | Roughness Rq [nm] |
|---|---|---|---|---|---|
| PET-Ag1 | 2.6 | PETs-Ag1 | 1.2 | SO-Ag1 | 1.9 |
| PET-Ag5 | 4.8 | PETs-Ag5 | 4.1 | SO-Ag5 | 4.6 |
| PET-Ag9 | 2.2 | PETs-Ag9 | 1.4 | SO-Ag9 | 1.9 |
| Sample Code | Transmittance SPR Minimum [nm] | Absorbance SPR Maximum [nm] | Reflectance SPR Maximum [nm] |
|---|---|---|---|
| PET-Ag1 | 467 ± 0.5 | 442 ± 0.5 | 470 ± 0.5 |
| PET-Ag5 | 675 ± 0.5 | 645 ± 0.5 | 644 ± 0.5 |
| PET-Ag9 | - | - | - |
| PETs-Ag1 | 442 ± 0.5 | 440 ± 0.5 | 452 ± 0.5 |
| PETs-Ag5 | 634 ± 0.5 | 640 ± 0.5 | 630 ± 0.5 |
| PETs-Ag9 | - | - | - |
| SO-Ag1 | 439 ± 0.5 | 442 ± 0.5 | 446 ± 0.5 |
| SO-Ag5 | 620 ± 0.5 | 550 ± 0.5 | 568 ± 0.5 |
| SO-Ag9 | - | - | - |
| Sample Code | Eg [eV] | ΔEg [eV] |
|---|---|---|
| PET-Ag1 | 3.396 ± 0.034 | 0.110 ± 0.001 |
| PET-Ag5 | 3.262 ± 0.033 | 0.242 ± 0.002 |
| PET-Ag9 | 3.158 ± 0.032 | 0.348 ± 0.003 |
| PET | 3.506 ± 0.035 | - |
| PETs-Ag1 | 3.775 ± 0.038 | −0.025 ± 0.001 |
| PETs-Ag5 | 3.721 ± 0.037 | 0.029 ± 0.001 |
| PETs-Ag9 | 3.693 ± 0.037 | 0.057 ± 0.001 |
| PETs | 3.750 ± 0.037 | - |
| SO-Ag1 | 3.402 ± 0.034 | 0.043 ± 0.001 |
| SO-Ag5 | 3.291 ± 0.033 | 0.154 ± 0.002 |
| SO-Ag9 | 3.334 ± 0.033 | 0.111 ± 0.001 |
| SO | 3.445 ± 0.034 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filip, A.V.; Sava, B.A.; Medianu, R.V.; Boroica, L.; Dinca, M.C.; Pascu, R.; Tigau, N.; Andrei, A.; Moldovan, A.; Dumitru, M.; et al. Ultrathin Films of Silver by Magnetron Sputtering. Inorganics 2022, 10, 235. https://doi.org/10.3390/inorganics10120235
Filip AV, Sava BA, Medianu RV, Boroica L, Dinca MC, Pascu R, Tigau N, Andrei A, Moldovan A, Dumitru M, et al. Ultrathin Films of Silver by Magnetron Sputtering. Inorganics. 2022; 10(12):235. https://doi.org/10.3390/inorganics10120235
Chicago/Turabian StyleFilip, Ana Violeta, Bogdan Alexandru Sava, Rares Victor Medianu, Lucica Boroica, Marius Catalin Dinca, Rovena Pascu, Nicolae Tigau, Andreea Andrei, Antoniu Moldovan, Marius Dumitru, and et al. 2022. "Ultrathin Films of Silver by Magnetron Sputtering" Inorganics 10, no. 12: 235. https://doi.org/10.3390/inorganics10120235
APA StyleFilip, A. V., Sava, B. A., Medianu, R. V., Boroica, L., Dinca, M. C., Pascu, R., Tigau, N., Andrei, A., Moldovan, A., Dumitru, M., Oane, M., & Eftimie, M. (2022). Ultrathin Films of Silver by Magnetron Sputtering. Inorganics, 10(12), 235. https://doi.org/10.3390/inorganics10120235