12-Vertex closo-3,1,2-Ruthenadicarbadodecaboranes with Chelate POP-Ligands: Synthesis, X-ray Study and Electrochemical Properties
Abstract
1. Introduction
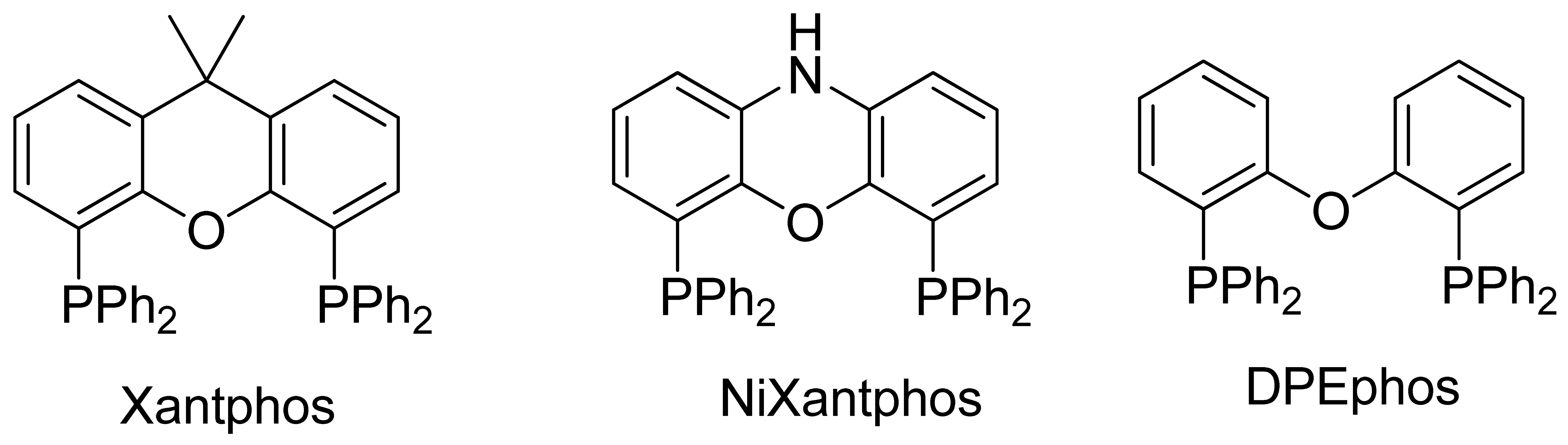
2. Results
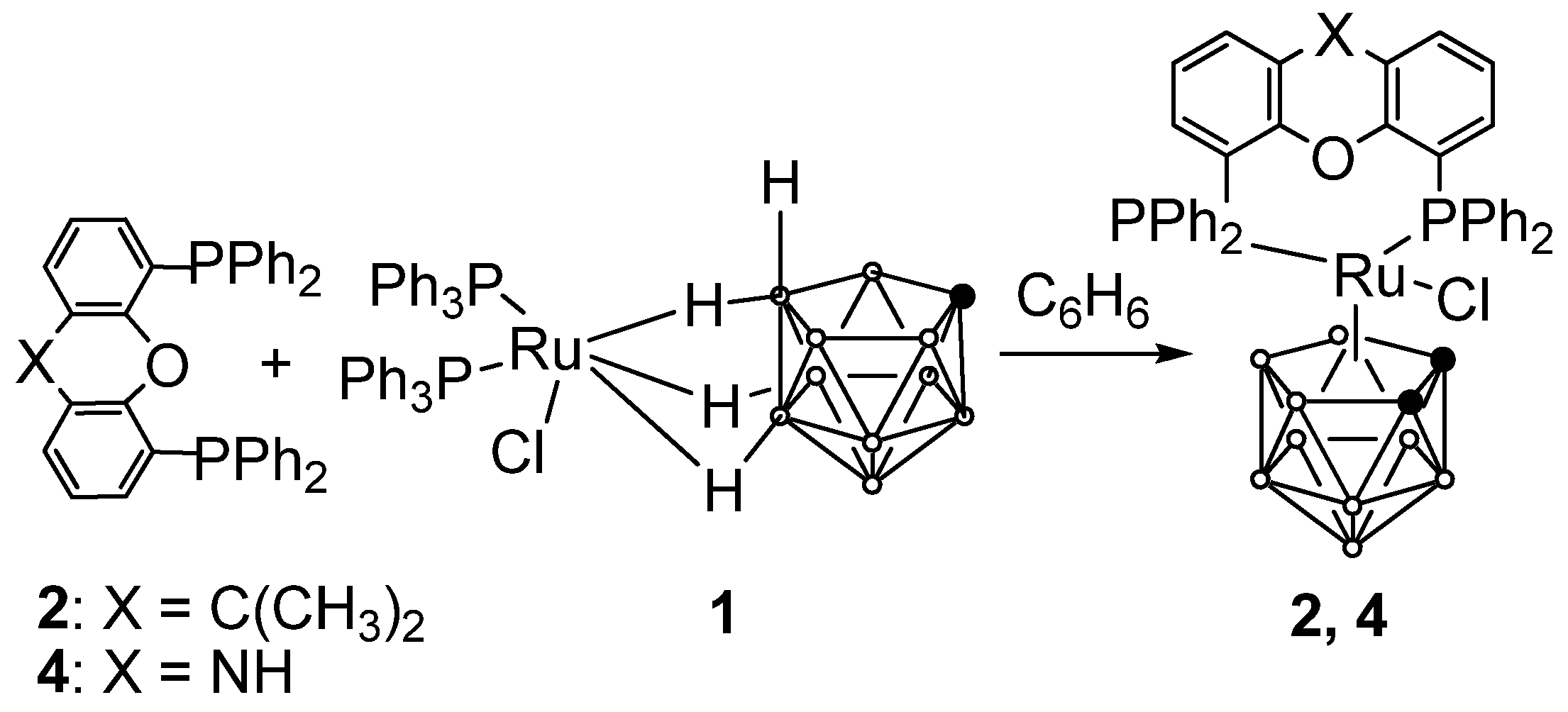
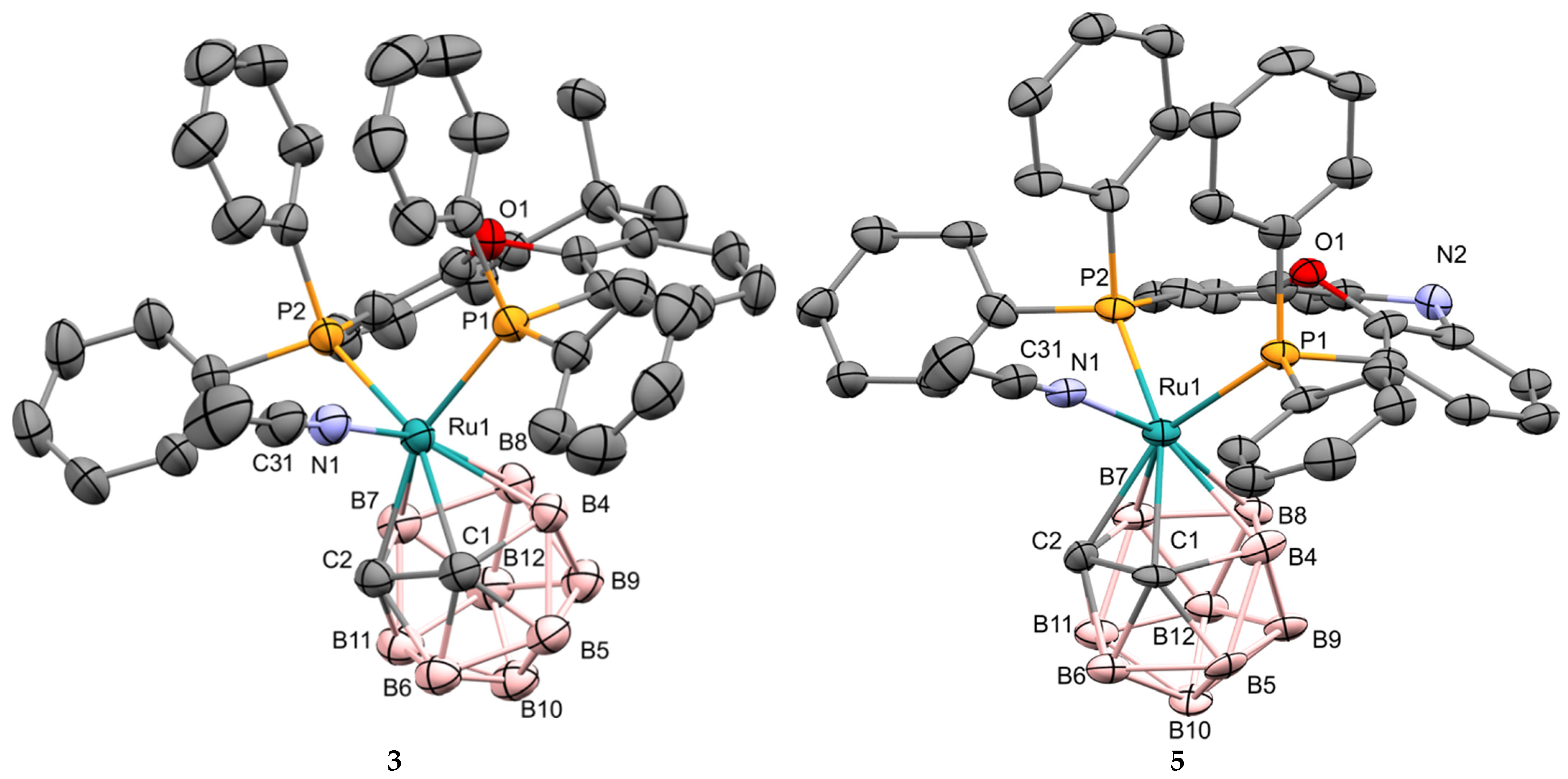
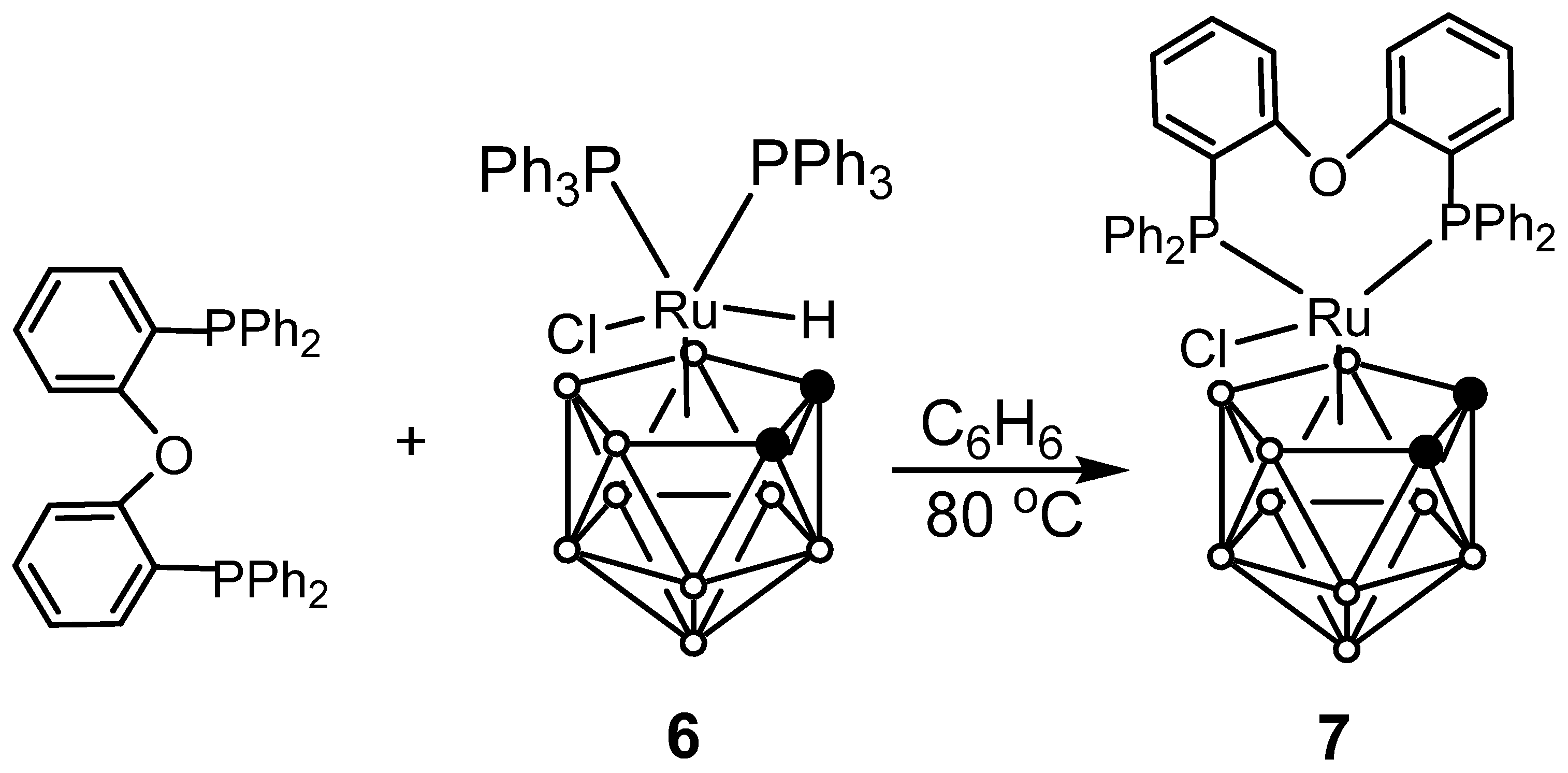
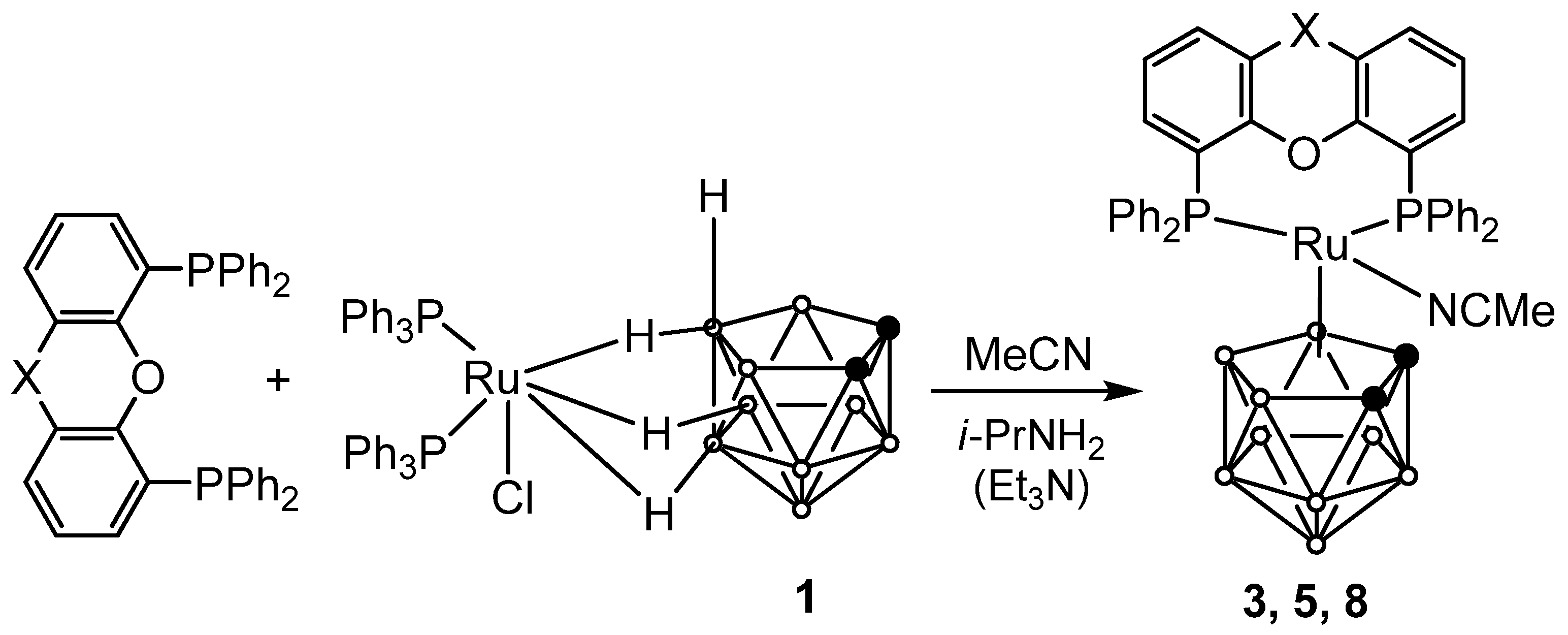
2.1. Synthesis of Novel Ruthenacarboranes
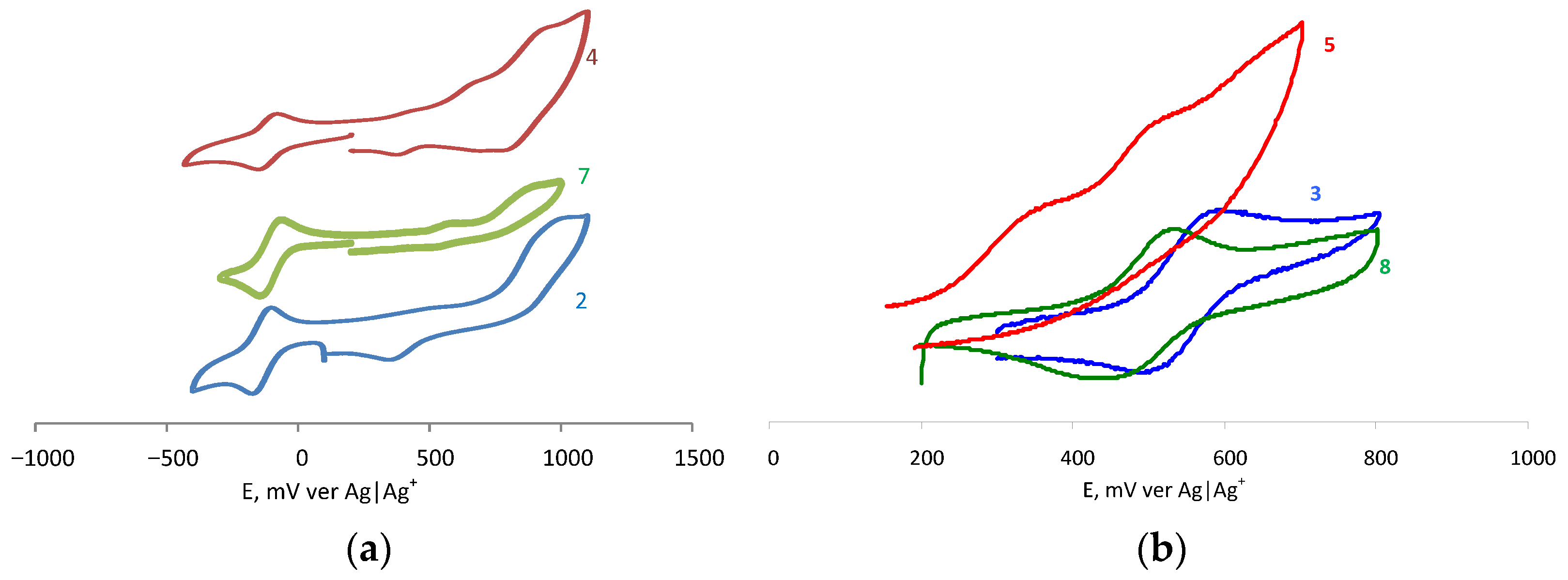
2.2. Electrochemical Studies
2.3. Catalysis of Radical Polymerization
2.4. DFT Studies of the Complexes
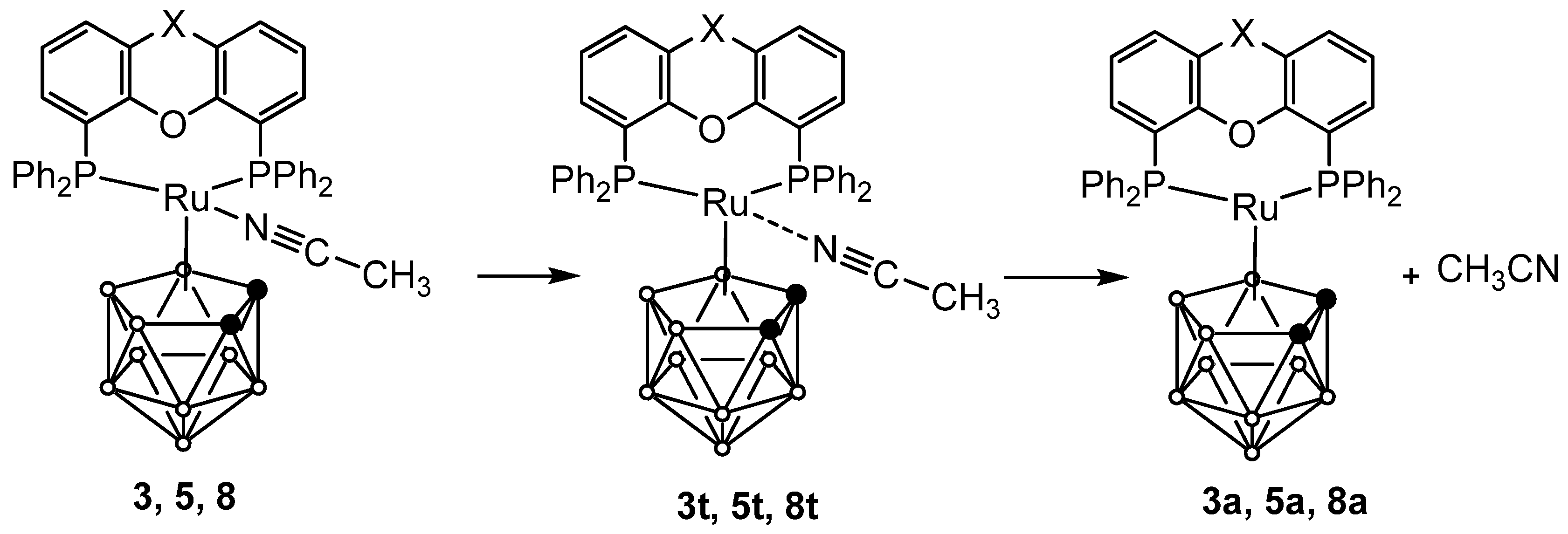
2.5. The Studies of Transformations of DPEphos-Based Complexes
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis of Novel Ruthenacarboranes
3.3. X-ray Diffraction Study
3.4. Quantum-Chemical Calculations
3.5. Polymerization Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorandi, F.; Matyjaszewski, K. Why do we need more active ATRP catalysts? Isr. J. Chem. 2019, 60, 108–123. [Google Scholar] [CrossRef]
- Bultz, E.; Ouchi, M.; Sawamoto, M.; Cunningham, M.F. “Smart” catalysis with thermoresponsive ruthenium catalysts for miniemulsion ru-mediated reversible deactivation radical polymerization cocatalyzed by smart iron cocatalysts. J. Polym. Sci. A Polym. Chem. 2019, 57, 305–312. [Google Scholar] [CrossRef]
- Grishin, I.D.; Zimina, A.M.; Anufriev, S.A.; Knyazeva, N.A.; Piskunov, A.V.; Dolgushin, F.M.; Sivaev, I.B. Synthesis and Catalytic Properties of Novel Ruthenacarboranes Based on nido-[5-Me-7,8-C2B9H10]2− and nido-[5,6-Me2-7,8-C2B9H9]2− Dicarbollide Ligands. Catalysts 2021, 11, 1409. [Google Scholar] [CrossRef]
- Zimina, A.M.; Knyazeva, N.A.; Balagurova, E.V.; Dolgushin, F.M.; Somov, N.V.; Vorozhtsov, D.L.; Malysheva, Y.B.; Grishin, I.D. Revising the chemistry of κ2-dppe-closo-RuC2B9H11 fragment: Synthesis of novel diamagnetic complexes and its transformations. J. Organomet. Chem. 2021, 946–947, 121908. [Google Scholar] [CrossRef]
- Penkal, A.M.; Somov, N.V.; Shchegravina, E.S.; Grishin, I.D. Ruthenium Diphosphine Closo-C2B9-Carborane Clusters with Nitrile Ligands: Synthesis and Structure Determination. J. Clust. Sci. 2019, 30, 1317–1325. [Google Scholar] [CrossRef]
- Penkal, A.M.; D’yachihin, D.I.; Somov, N.V.; Shchegravina, E.S.; Grishin, I.D. Synthesis of novel closo -carborane complexes of ruthenium (II) with triphenylphosphine or acetonitrile ligands via reduction of paramagnetic Ru(III) derivatives. J. Organomet. Chem. 2018, 872, 63–72. [Google Scholar] [CrossRef]
- D’yachikhin, D.I.; Grishin, I.D.; Dolgushin, F.M.; Godovikov, I.A.; Chizhevsky, I.T.; Grishin, D.F. Synthesis, isomerism, and catalytic properties of chelate ruthenium copper bimetallacarborane cluster exo-closo-(Ph3P)Cu(μ-H)Ru[Ph2P(CH2)4PPh2](η5-C2B9H11), in radical polymerization of methyl methacrylate. Russ. Chem. Bull. 2010, 59, 1145–1151. [Google Scholar] [CrossRef]
- Higashi, S.; Takenaka, H.; Ito, Y.; Oe, Y.; Ohta, T. Synthesis of RuCl2(xantphos)L (L = PPh3, P(OPh)3, DMSO) Complexes, and their Catalytic Activity for the Addition of Carboxylic Acids onto Olefins. J. Organomet. Chem. 2015, 791, 46–50. [Google Scholar] [CrossRef]
- Adams, G.M.; Weller, A.S. POP-type ligands: Variable coordination and hemilabile behaviour. Coord. Chem. Rev. 2018, 355, 150–172. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamashita, M.; Nozaki, K. Tandem Hydroformylation/Hydrogenation of Alkenes to Normal Alcohols Using Rh/Ru Dual Catalyst or Ru Single Component Catalyst. J. Am. Chem. Soc. 2012, 134, 18746–18757. [Google Scholar] [CrossRef]
- Venkateswaran, R.; Mague, J.T.; Balakrishna, M.S. Ruthenium(II) Complexes Containing Bis(2-(diphenylphosphino)phenyl) Ether and Their Catalytic Activity in Hydrogenation Reactions. Inorg. Chem. 2007, 46, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Deb, B.; Borah, B.J.; Sarmah, B.J.; Das, B.; Dutta, D.K. Dicarbonylruthenium(II) complexes of diphosphine ligands and their catalytic activity. Inorg. Chem. Commun 2009, 12, 868–871. [Google Scholar] [CrossRef]
- Grishin, I.D.; D’yachihin, D.I.; Piskunov, A.V.; Dolgushin, F.M.; Smolyakov, A.F.; Il’in, M.M.; Davankov, V.A.; Chizhevsky, I.T.; Grishin, D.F. Carborane complexes of ruthenium(III): Studies on thermal reaction chemistry and the catalyst design for atom transfer radical polymerization of methyl methacrylate. Inorg. Chem. 2011, 50, 7574–7585. [Google Scholar] [CrossRef] [PubMed]
- Grishin, I.D.; D’yachihin, D.I.; Turmina, E.S.; Dolgushin, F.M.; Smol’yakov, A.F.; Piskunov, A.V.; Chizhevsky, I.T.; Grishin, D.F. Mononuclear closo-ruthenacarborane complexes containing a rare eight-membered metal-diphosphine ring. J. Organomet. Chem. 2012, 721–722, 113–118. [Google Scholar] [CrossRef]
- Jones, J.J.; English, L.E.; Robertson, A.P.M.; Rosair, G.M.; Welch, A.J. Mixed-ligand (triphenylphosphine)ruthenium complexes of diphenylcarborane by ligand manipulation and an asymmetric, bimolecular “symbiotic” cluster. J. Organomet. Chem. 2018, 865, 65–71. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Markov, V.Y.; Sivaev, I.B. Synthesis and crystal structures of nickel(II) and palladium(II) complexes with o-carboranyl amidine ligands. Dalton Trans. 2021, 50, 4967–4975. [Google Scholar] [CrossRef]
- Tutusaus, O.; Vinas, C.; Nunez, R.; Teixidor, F.; Demonceau, A.; Delfosse, S.; Noels, A.F.; Mata, I.; Molins, E. The modulating possibilities of dicarbollide clusters: optimizing the Kharasch catalysts. J. Am. Chem. Soc. 2003, 125, 11830–11831. [Google Scholar] [CrossRef]
- Teixeira, R.G.; Marques, F.; Robalo, M.P.; Fontrodona, X.; Garcia, M.H.; Crich, G.S.; Vinas, C.; Valente, A. Ruthenium carboranyl complexes with 2,2′-bipyridine derivatives for potential bimodal therapy application. RSC Adv. 2020, 10, 16266–16276. [Google Scholar] [CrossRef]
- Van der Veen, L.A.; Keeven, P.H.; Schoemaker, G.C.; Reek, J.N.H.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Lutz, M.; Spek, A.L. Origin of the Bite Angle Effect on Rhodium Diphosphine Catalyzed Hydroformylation. Organometallics 2000, 19, 872–883. [Google Scholar] [CrossRef]
- Kostukovich, A.Y.; D’yachihin, D.I.; Dolgushin, F.M.; Smol’yakov, A.F.; Godovikov, I.A.; Chizhevsky, I.T. An Unusual Conversion of Paramagnetic [3-Cl-3,3,8-{Ph2P(CH2)nPPh-μ-(C6H4-ortho)}-1,2-(CH3)2-closo-3,1,2-RuIIIC2B9H8] (n = 3 and 4) to Form the First 18-Electron P-Phenylene ortho-Cycloboronated closo-Ruthenacarboranes with a Dioxygen Ligand. Molecules 2014, 19, 7094–7103. [Google Scholar] [CrossRef]
- Kirchner, K.; Mauthner, K.; Mereiter, K.; Schmid, R. Irreversible binding of dioxygen and other gases to a half-sandwich ruthenium(II) complex: X-ray structure of [Ru(η2-O2)(η5-C5Me5)(Ph2PCH2CH2PPh2)]PF6. J. Chem. Soc. Chem. Commun. 1993, 11, 892–894. [Google Scholar] [CrossRef]
- Chizhevsky, I.T.; Lobanova, I.A.; Bregadze, V.I.; Petrovskii, P.V.; Antonovich, V.A.; Polyakov, A.V.; Yanovsky, A.I.; Struchkov, Y.T. The first exo-nido-ruthenacarborane clusters. Synthesis and molecular structure of 5,6,10-[Cl(PPh3)2Ru]-5,6,10-μ-(H)3-10-H-7,8-C2B9H11. Mendeleev. Commun. 1991, 1, 47–49. [Google Scholar] [CrossRef]
- Cheredilin, D.N.; Balagurova, E.V.; Godovikov, I.A.; Solodovnikov, S.P.; Chizhevsky, I.T. Facile formation of exo-nido→closo-rearrangement products upon the replacement of PPh3 ligands with bis(diphenylphosphino)alkanes in “three-bridge” ruthenacarborane 5,6,10-[RuCl(PPh3)2]-5,6,10-(µ-H)3-10-H-exo-nido-7,8-C2B9H8. Russ. Chem. Bull. 2005, 54, 2535–2539. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystalstructure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for smallmolecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Pople, Gaussian 03, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Perdew, J.P.; Wang, Y. Accurate and simple analytic representation of the electron-gas correlation energy. Phys. Rev. B. 1992, 45, 13244–13249. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Kaltenberg, A.A.; Somov, N.V.; Malysheva, Y.B.; Knyazeva, N.A.; Piskunov, A.V.; Grishin, I.D. Novel carborane complexes of ruthenium with tridentate phosphine ligands: Synthesis and application in Atom Transfer Radical Polymerization. J. Organomet. Chem. 2020, 917, 121291. [Google Scholar] [CrossRef]
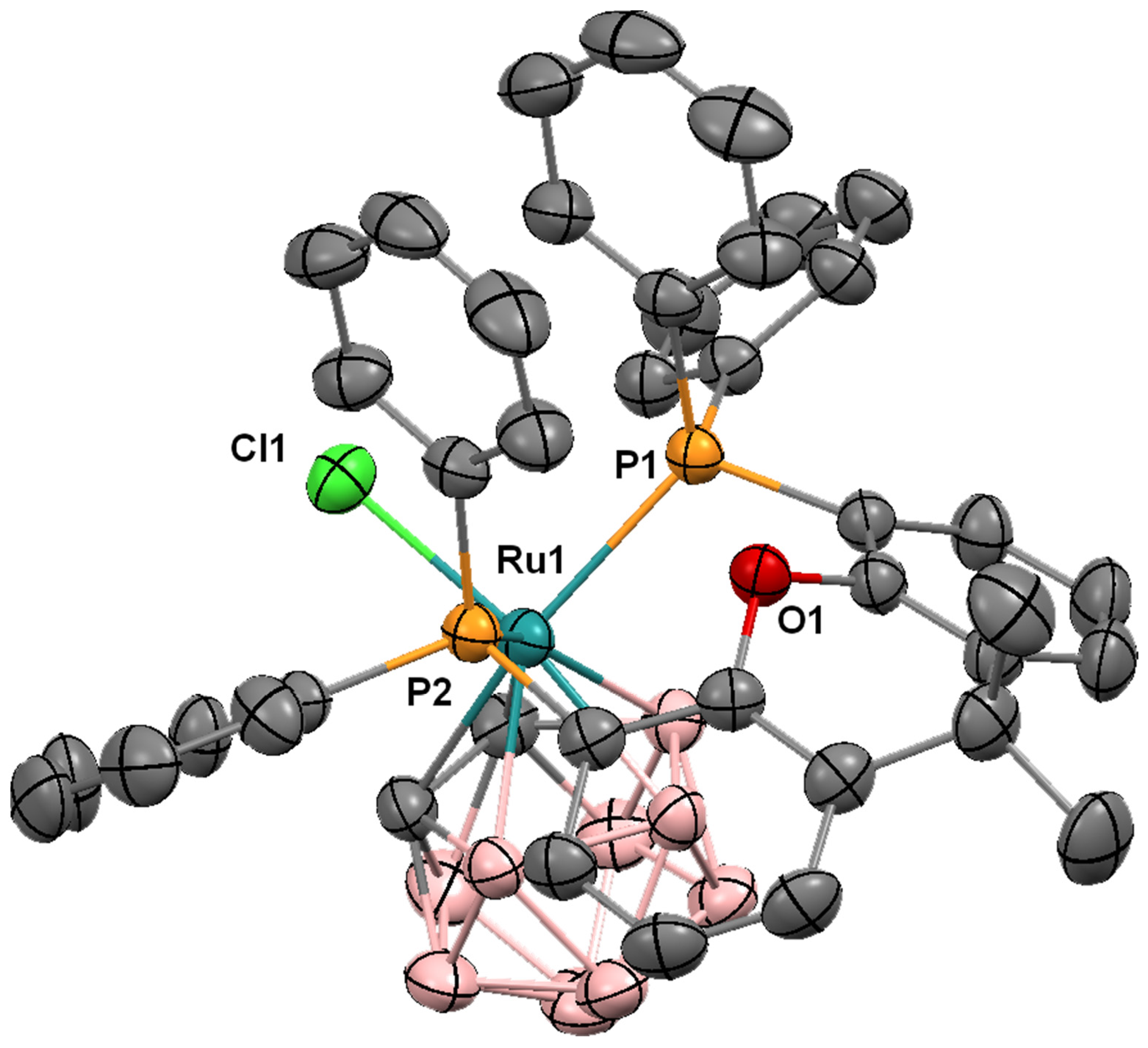
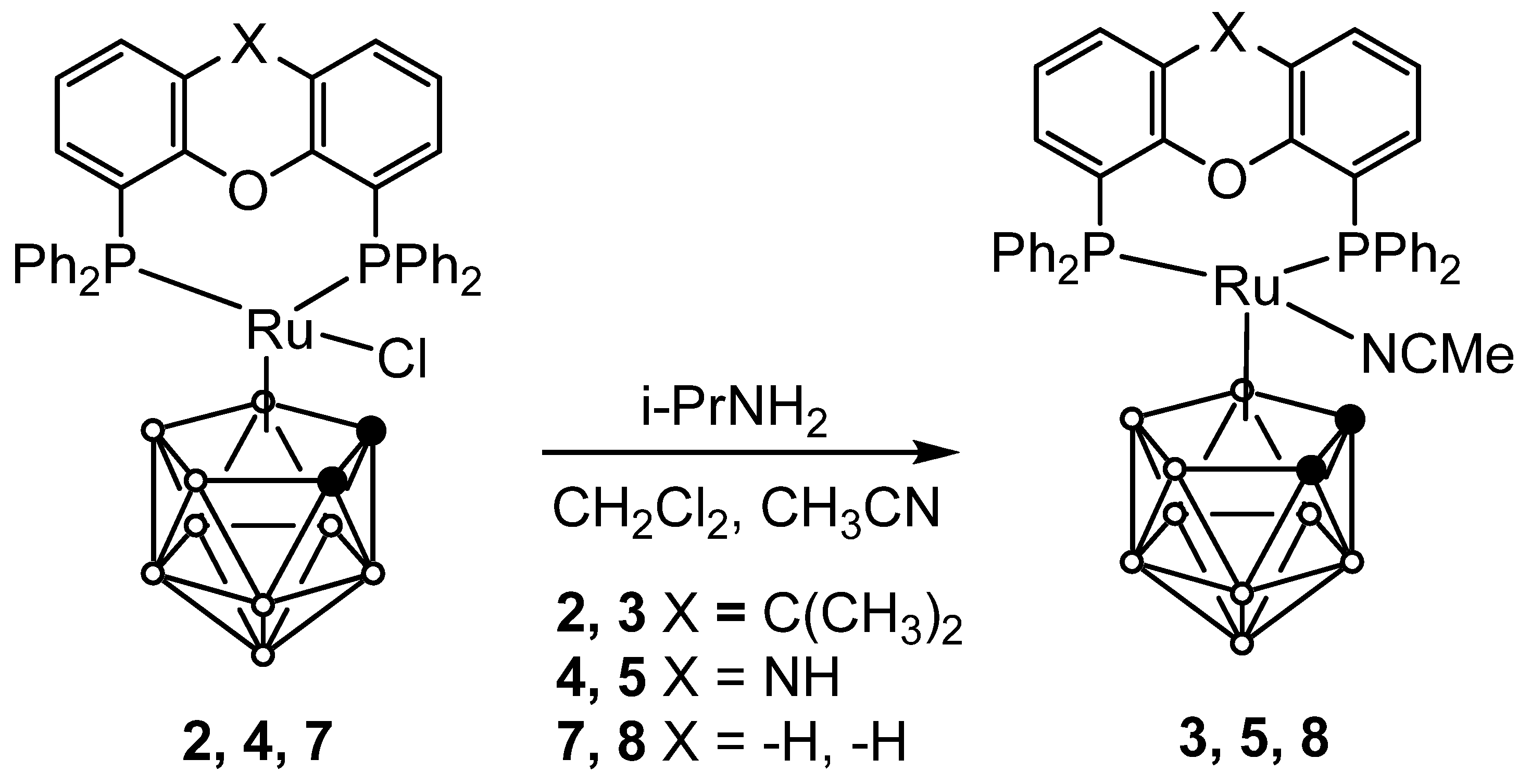




| Parameter | Compound | |||
|---|---|---|---|---|
| 2 | 3 | 5 | 9 | |
| Bond lengths, Ǻ | ||||
| Ru–P1 | 2.4016(6) | 2.3606(6) | 2.374(3) | 2.3629(7) |
| Ru–P2 | 2.4048(6) | 2.3666(6) | 2.358(3) | 2.3657(2) |
| Ru–Cl/N | 2.3831(7) | 2.064(2) | 2.064(10) | - |
| Ru–O1 | 3.5388(17) | 3.5028(16) | 3.456(8) | 3.6910(18) |
| Ru–O2 | - | - | - | 2.0399(18) |
| Ru–O3 | - | - | - | 2.0427(18) |
| Ru–C1 | 2.246(3) | 2.206(2) | 2.229(10) | 2.286(2) |
| Ru–C2 | 2.248(3) | 2.212(2) | 2.209(12) | 2.393(2 |
| Ru–B8 | 2.259(3) | 2.293(3) | 2.295(14) | 2.278(3) |
| Ru–B7 | 2.248(3) | 2.242(3) | 2.258(13) | 2.271(3) |
| Ru–B4 | 2.245(3) | 2.243(3) | 2.255(13) | 2.271(3) |
| C1–C2 | 1.604(4) | 1.631(3) | 1.643(15) | 1.595(4) |
| O2–O3 | - | - | - | 1.405(3) |
| Valence angles, deg. | ||||
| P1–Ru–P2 | 94.51(2) | 95.58(2) | 96.08(11) | 92.47(2) |
| P1–Ru–Cl/N | 91.65(2) | 89.99(6) | 91.9(3) | - |
| P2–Ru–Cl/N | 92.47(2) | 89.79(6) | 88.4(3) | - |
| Transition | Compound | ||||||
|---|---|---|---|---|---|---|---|
| 2 | 4 | 7 | 3 | 5 | 8 | ||
| M−/M | Epa, mV | −298 | −280 | −274 | - | - | - |
| Epc, mV | −369 | −360 | −350 | - | - | - | |
| E1/2, mV | −333 | −320 | −312 | - | - | - | |
| Epa−Epc, mV | 71 | 80 | 76 | - | - | - | |
| M/M+ | Epa, mV | 800 | 471 2 | 702 | 331 | 360 3 | 395 |
| Epc, mV | - | - | - | 242 | - | 300 | |
| E1/2, mV | - | - | - | 286 | - | 348 | |
| Epa−Epc, mV | - | - | - | 94 | - | 94 | |
| Complex | Type | Conversion | Mn × 10−3 | Mw/Mn | Mn,theor × 10−3 |
|---|---|---|---|---|---|
| 2 | 17-e | 61 | 21.4 | 1.5 | 24.4 |
| 3 | 18-e | 94 | 27.2 | 1.6 | 37.6 |
| 4 | 17-e | 70 | 23.5 | 1.7 | 28.0 |
| 5 | 18-e | 68 | 25.5 | 1.8 | 27.2 |
| 7 | 17-e | 68 | 52.6 | 2.2 | 27.2 |
| 8 | 18-e | 75 | 59.3 | 2.4 | 30.0 |
| Ligand | Energy Change in Reaction, kcal/mol | ||||||
|---|---|---|---|---|---|---|---|
| I | II | ||||||
| ΔrE | ΔrH | ΔrG | E(TS) | ΔrE | ΔrH | ΔrG | |
| Xantphos | 14.1 | 14.1 | 2.6 | 13.7 | 1.8 | 2.2 | 1.6 |
| NiXantphos | 14.6 | 14.6 | 3.1 | 14.0 | 1.0 | 1.4 | 1.0 |
| DPEphos | 8.9 | 8.7 | −1.9 | 15.1 | 15.1 | 15.8 | 13.1 |
| Compound | 2 | 3 | 5 | 9 |
|---|---|---|---|---|
| CCDC No | 2165075 | 2165074 | 2165064 | 2214909 |
| Empirical formula | C41H43B9ClOP2Ru 2 C6H6 | C43H46B9NOP2Ru 2 CH2Cl2 | C40H41B9N2P2Ru CH3CN | C38.75H40.50B9Cl1.50O3P2Ru |
| Molecular weight | 1003.72 | 1022.96 | 867.1 | 867.68 |
| Crystal size (mm) | 0.845 × 0.397 × 0.211 | 0.561 × 0.28 × 0.22 | 0.216 × 0.112 × 0.092 | 0.342 × 0.222 × 0.062 |
| Temperature (K) | 297(2) | 293(2) | 100(2) | 100(2) |
| Crystal system | Monoclinic | Triclinic | Triclinic | Monoclinic |
| Space group | P21/c | P21/c | ||
| a (Å) | 12.2652(2) | 11.3175(4) | 11.6015(14) | 12.9051(4) |
| b (Å) | 22.0922(2) | 11.6877(4) | 12.9626(16) | 17.3631(2) |
| c (Å) | 22.7267(4) | 18.8933(6) | 15.7089(15) | 23.3119(7) |
| β (deg) | 125.048(3) | 83.161(3) | 93.373(9) | 129.448(5) |
| V (Å3) | 5041.5(2) | 2468.62(15) | 2119.4(5) | 4033.6(3) |
| Z | 4 | 2 | 2 | 4 |
| Dcalcd (g.cm−3) | 1.322 | 1.376 | 1.359 | 1.429 |
| linear absorption µ (cm−1) | 4.66 | 6.35 | 4.83 | 6.05 |
| Tmin/Tmax | 0.801/0.925 | 0.809/0.913 | 0.599/1 | 0.92212/1 |
| Reflections collected | 105,544 | 39,245 | 14,935 | 105,579 |
| Independent reflections (Rint) | 15,316 (0.0373) | 12,224 (0.031) | 8323 (0.052) | 8224 |
| Observed reflections (I > 2σ(I)) | 12,773 | 10,834 | 5852 | 7511 |
| Number of parameters | 765 | 571 | 525 | 515 |
| R1 (on F for I > 2σ(I)) | 0.052 | 0.0435 | 0.1179 | 0.0377 |
| wR2 (on F2 for all data) | 0.1343 | 0.1263 | 0.2995 | 0.0930 |
| GOOF | 1.147 | 1.04 | 1.259 | 1.057 |
| Largest diff. peak/hole (e Å−3) | 0.922/−0.637 | 0.821/−0.958 | 3.376/−1.702 | 1.573/−0.848 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimina, A.M.; Somov, N.V.; Malysheva, Y.B.; Knyazeva, N.A.; Piskunov, A.V.; Grishin, I.D. 12-Vertex closo-3,1,2-Ruthenadicarbadodecaboranes with Chelate POP-Ligands: Synthesis, X-ray Study and Electrochemical Properties. Inorganics 2022, 10, 206. https://doi.org/10.3390/inorganics10110206
Zimina AM, Somov NV, Malysheva YB, Knyazeva NA, Piskunov AV, Grishin ID. 12-Vertex closo-3,1,2-Ruthenadicarbadodecaboranes with Chelate POP-Ligands: Synthesis, X-ray Study and Electrochemical Properties. Inorganics. 2022; 10(11):206. https://doi.org/10.3390/inorganics10110206
Chicago/Turabian StyleZimina, Anastasiya M., Nikolay V. Somov, Yulia B. Malysheva, Nadezhda A. Knyazeva, Alexander V. Piskunov, and Ivan D. Grishin. 2022. "12-Vertex closo-3,1,2-Ruthenadicarbadodecaboranes with Chelate POP-Ligands: Synthesis, X-ray Study and Electrochemical Properties" Inorganics 10, no. 11: 206. https://doi.org/10.3390/inorganics10110206
APA StyleZimina, A. M., Somov, N. V., Malysheva, Y. B., Knyazeva, N. A., Piskunov, A. V., & Grishin, I. D. (2022). 12-Vertex closo-3,1,2-Ruthenadicarbadodecaboranes with Chelate POP-Ligands: Synthesis, X-ray Study and Electrochemical Properties. Inorganics, 10(11), 206. https://doi.org/10.3390/inorganics10110206







