Abstract
The preparation of novel nitrilium derivatives of closo-dodecaborate anion [B12H11NCR]−, R = n-C3H7, i-C3H7, 4-C6H4CH3, 1-C10H7 is described. Target compounds were obtained in good yields (up to 73%). The synthesis of target borylated nitrilium derivatives was characterised by the simplicity of the chemical apparatus and the absence of the necessity for the purification of desired compounds. The crystal structures of previously obtained [B12H11NCCH3]− and novel [B12H11NCC3H7]− were established with the help of X-ray structure analysis. DFT-analysis of several nitrilium derivatives [B12H11NCR]−, R = CH3, C3H7, 4-CH3C6H4 was carried out. The main peculiarities of the C≡N bond of the exo-polyhedral substituent were revealed in terms of bond lengths, bond orders and atomic charges. The LUMO orbitals of the systems considered were examined for understanding of the electrophilic nature of the nitrilium derivatives of the closo-dodecaborate anion.
1. Introduction
The closo-dodecaborate anion [B12H12]2− is one of the most important chemical species among boron cluster compounds [,]. This anion, as well as other closo-borates [BnHn]2−, has a number of remarkable properties, such as high thermal and chemical stability, and low toxicity [,,]. Furthermore, closo-borate anions and their derivatives have many potential applications in the design of catalytic systems [,,], coordination compounds [,,], solid electrolytes [,,,] etc. The main application of boron cluster compounds, however, is in medical research [,,,,,]. In addition, to the preparation of potential drugs for Boron Neutron Capture Therapy (BNCT), intensive work has been carried out to investigate the antiviral and antimicrobial properties of cluster-containing systems [].
Derivatives of closo-borate anions with exo-polyhedral B-N bonds are the most interesting, and most often studied, boron cluster compounds [,,]. Initially, most interest was focused on ammonium derivatives of closo-borate systems [BnHn−1NH3]− []. These compounds can be easily modified by substituting the hydrogen atom of the NH3-group. Various borylated alkylamines [,], amides [,], imines [] and amidines [] can be prepared by such methods.
Nitrilium derivatives are another important class of cluster systems with exo-polyhedral B-N bonds. Nitrilium derivatives of boron clusters contain a polarised N≡C bond and have similar chemical properties to transition metal complex compounds, with nitrile ligands [,,]. Nitrilium derivatives readily enter nucleophilic addition reactions. This peculiarity enables nitrilium derivatives to be applied as a starting point for obtaining different borylated systems. Various borylated imine amides, iminols, imidates, amidines, including those based on amino acids, and oligopeptides have been obtained on the basis of nitrilium derivatives [,].
Nitrilium derivatives have been obtained for closo-decaborate [,,,,], cobalt bis-dicarbolide [,,,] and nido-carborane systems [,,,]. Several approaches to the preparation of these systems have been established. All these approaches are based on electrophile-induced nucleophilic substitution [,,]. Several electrophilic inducers were used: CF3COOH, CF3SO3H, HgCl2 and AlCl3 []. Depending on reaction conditions and inducer nature, mono- or dinitrilium derivatives of cluster systems can be obtained.
In several works, nitrilium derivatives of closo-dodecaborate anions were prepared [B12H11NCR]−, R = CH3, CH2CH3 [,] and different reaction protocols were applied. In paper [], the most optimal method was established. This method was based on obtaining the target substances in a glass autoclave at an oil bath temperature above 150 °C. The CF3COOH was used as an electrophilic inducer. In the present work, this approach was extended to obtaining nitrile complexes [B12H11NCR]−, R = n-C3H7, i-C3H7, 4-C6H4CH3, 1-C10H7. The main goal of the current research was to establish the best conditions for the obtaining derivatives, with both aliphatic and aromatic substituents. In addition, theoretical investigation was devoted to the impact of boron clusters on the N≡C bond nature in target derivatives.
2. Results
2.1. Synthesis of closo-Dodecaborate Nitrilium Derivatives
As previously stated, the main goal of the present research was to establish the best conditions for the preparation of derivatives with both aliphatic and aromatic substituents. The approach used was one that had been employed by the authors in previous research []. This process involved obtaining the target substances in a glass autoclave at an oil bath temperature above 150 °C. The CF3COOH was used as an electrophilic inducer (Scheme 1). As in the case of the acetonitrile derivative, the complete conversion of the initial salt of closo-dodecaborate anion with tetrabutylammonium cations ((n-C4H9)4N)+ occurred in about 30 min. Increasing the reaction time had a negative effect on the yield of the target nitrile derivative due to formation of additional by-products such as carboxylic derivatives of closo-dodecaborate anion.
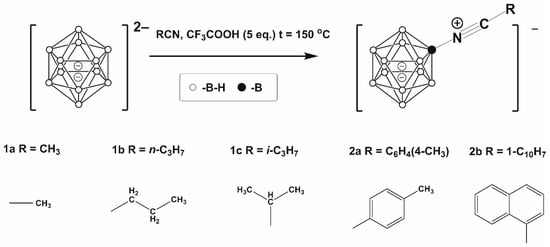
Scheme 1.
Scheme of preparation of [B12H11NCR]−.
The completeness of the reaction was monitored using 11B NMR. As in the case of [B12H11NCCH3]−, there were two signals observed in the spectra of the target compounds. A signal from the substituted boron atom lay in the range −12.0–−12.3 ppm; the signal from unsubstituted boron atoms B(2-12) lay in the range −14.5–−15.2.
The yields of nitriles [B12H11NCR]− obtained were in the range of 69–73%, in all cases except [B12H11NCR]−, R = C10H7 (yield = 36%). These values were lower than in the case of [B12H11NCCH3]−, relating to the increasing solubility of [B12H11NCR]− in the mixture of CH3COOH/CF3COOH, (this mixture was used for washing nitrilium products). In case of [B12H11NCR]−, R = C10H7 the low yields is additionally related to the reduced nucleophilic capacity of C7H10CN compared with other nitriles. The yields of the target products were at an acceptable level and losses of target products were compensated by the ease of their isolation.
Apart from 11B{H} NMR, the target closo-dodecaborate derivatives were characterised using 1H and 13C{H} NMR, IR and ESI MS methods. In 1H NMR spectra, signals from the tetrabutylammonium cation and a signal from the side R group of nitrilium derivatives were observed.
In 13C{H} NMR spectra, tetrabutylammonium cation signals were observed at 59.4, 24.4, 20.2 and 13.9 ppm for (N(n-C4H9)4)+. Signals from carbon atoms of the nitrilium group were in the interval 115.5–103.3 ppm in the case of nitrilium derivatives with aliphatic backbones R = n-C3H7, i-C3H7. These values were more positive than with [B12H11NCCH3]−. In the case of borylated nitriles with aromatic backbones R = 1-C10H7, 4-C6H4CH3, signals from carbon atoms of the nitrilium group were in the interval 103.3–104.2 ppm.
In the IR-spectra of [B12H11NCR]−, R = n-C3H7, i-C3H7, 4-C6H4CH3, 1-C10H7 there were the two most significant absorption bands: from BH valence vibrations in the range 2490–2500 cm−1 and from CN valence vibrations in the range 2304–2338 cm−1.
The target derivatives can be obtained without complex purification procedures. All derivatives obtained are stable in air and can be stored without specific conditions (such as an argon atmosphere or a vacuum). Over time, these compounds experience no more than slight hydrolysis processes of the nitrile group. These circumstances are additional advantages of utilising [B12H11NCR]− as potential molecular species for various applications.
2.2. X-ray Analysis
The crystals for X-ray diffraction experiments were obtained by isothermal evaporation of acetonitrile solutions of the corresponding derivative, in the presence of (C2H5)4NCl in case of [B12H11NCCH3]− and (C6H5)4PCl in case of [B12H11NCC3H7]−. The crystallographically independent part of the orthorhombic unit cell (space group Pbcn) of ((C2H5)4N)[B12H11NCCH3] salt contained the anion [B12H11NCCH3]−, which occupied a common position, and two independent halves of the (C2H5)4N+ cation, on the second order axis. The crystallographically independent part of the salt ((C6H5)4P)[B12H11NCn-C3H7] triclinic unit cell (P-1) contained one (C6H5)4P+ cation and a [B12H11NCn-C3H7]− anion each. The B-N bond length was 1.513(3) Å in compound 1 and 1.514(2) Å in salt 2; the C≡N bond lengths were 1.136(3) Å and 1.135(2) Å, respectively. The BNC angles in the compounds deviated slightly from unfolded and were 178.5(3)˚ and 174.4(2)˚, respectively. The average B-N and C≡N bond lengths in the [B12H11NCR]− (R = CH3, n-C3H7) anions were 1.514 Å and 1.128 Å (Figure 1).
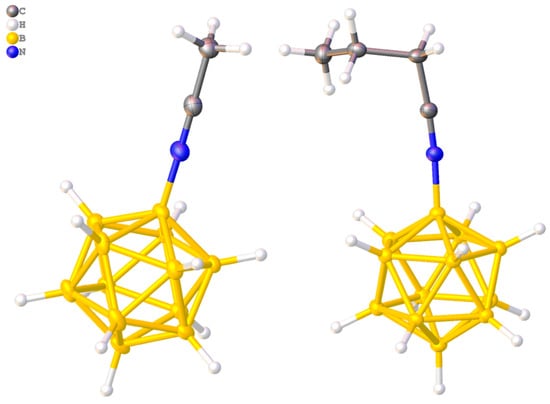
Figure 1.
Structures of [B12H11NCCH3]− and [B12H11NCC3H7]−.
In the crystal structure of compound 1a, cation-anion layers of [B12H11NCCH3]− anions and (C2H5)4N+ cations were formed, parallel to the plane ab (Figure 2a). The anions bound to each other in dimeric pairs by weak CH…HB(B) contacts were located sequentially above each other along the a-axis. The cations and anions were also connected by a network of weak CH…HB(B) interactions, which are shown as red spots on the Hirschfeld surface of the anion (Figure 2b). H…H contacts accounted for 92.5% of the anion surface, while H…C/C…H and H…N/N…H contacts accounted for 5.5% and 2.0% of the anion surface, respectively.
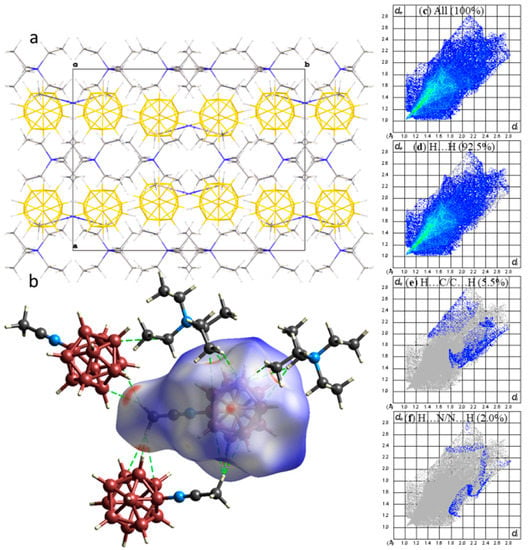
Figure 2.
(a) Packing diagram, (b) dnorm surface of ((C2H5)4N)[B12H11NCCH3]− anion, (c) full two-dimensional fingerprint plot for 1a, together with that delineated into (d) H…H, (e) H…C/C…H, and (f) H…N/N…H contacts.
In the packing of compound 1b, the picture is similar to that of compound 1a: the (C6H5)4P+ cations and [B12H11NCn-C3H7]− anions formed cation-anion layers parallel to the ab plane, which were bonded together, due to weak CH…HB(B) interactions (Figure 3a). The antiparallel [B12H11NCn-C3H7]− anions were similarly bonded by CH…HB(B) contacts between the α-methylene groups of the exo-polyhedral substituent and the boron backbone of the neighbouring anion (Figure 3b). The Hirshfeld surface fingerprint plot analysis shows that the H…H contacts accounted for 88.7% of the anion surface, whereas the H…C/C…H and H…N/N…H contacts accounted for 8.9% and 2.3% of the anion surface, respectively.
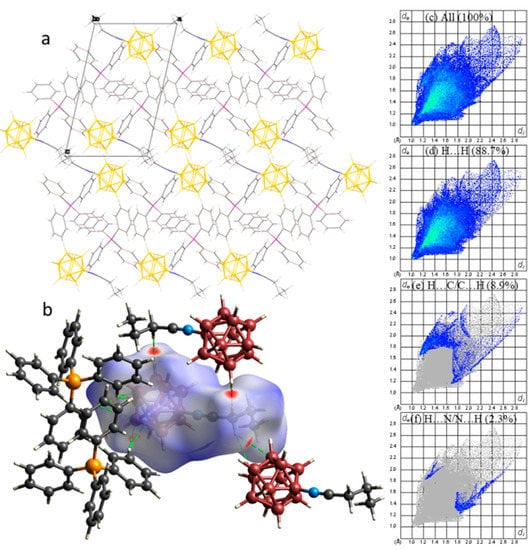
Figure 3.
(a) Packing diagram, (b) dnorm surface of ((C6H5)4P)[B12H11NCn-C3H7]− anion for compound 2, (c) full two-dimensional fingerprint plot for 2, together with that delineated into (d) H…H, (e) H…C/C…H, and (f) H…N/N…H contacts.
2.3. DFT Calculation
One of the main tasks of the present work was to reveal how the formation of the complex with closo-dodecaborate anion affected the properties of the nitrile group. To investigate this phenomenon, several nitriles RCN, R = CH3, n-C3H7, 4-CH3C6H4 and nitrilium derivatives [B12H11NCR]−, R = CH3, n-C3H7, 4-CH3C6H4 were chosen for theoretical study (Figure 4). All DFT calculations were carried out both in the gas phase and considering solvation effects.
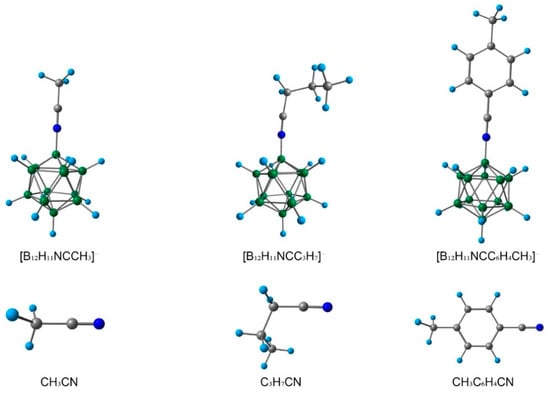
Figure 4.
Optimised structure of nitriles RCN, R = CH3, n-C3H7, CH3C6H4 and nitrilium derivatives [B12H11NCR]−, R = CH3, n-C3H7, CH3C6H4.
Firstly, the bond lengths of initial nitriles and borylated complexes were examined (Table 1). It is worth noting that the CN bond lengths were practically the same in the initial nitriles and their borylated analogues. For the estimation of bond orders, the Wiberg and Mayer approaches were used (Table 1). With the Mayer approach, the values of bond orders were higher than with the Wiberg approach. Both Wiberg and Mayer bond orders slightly decreased during the formation of the complex. These changes indicated that the nature of the interaction between nitrogen and carbon atoms had modified. The role of electrostatic interaction between carbon and nitrogen atoms was increased. The carbon atom of the nitrilium group of [B12H11NCR]− became much more positively charged than the initial nitrile. This increase in positive charge also indicated that the carbon atom of the nitrilium group should be much more easily attacked by nucleophile molecules. In the case of CH3CN and CH2Cl2 solutions, mean values of carbon atomic charges were higher than in the gas phase model. The nature of the R-substituent of [B12H11NCR]−, R = CH3, n-C3H7, 4-CH3C6H4 had a slight effect on the values of the bond lengths and atomic charges.

Table 1.
Main considered descriptors of nitriles RCN, R = CH3, C3H7, CH3C6H4 and nitrilium derivatives [B12H11NCR]−, R = CH3, C3H7, CH3C6H4.
The increase in electrophilicity can also be correlated with the energy of LUMO (lowest unoccupied orbital). The carbon atom of the nitrilium group makes the largest contribution to LUMO orbitals. As can be seen from the data obtained for the gas phase, the LUMO of [B12H11NCR]− had more positive values of energies than the initial nitriles. When solvation effects were taken into account, the opposite was true. The ratio of LUMO energies explains the much greater reactivity of activated nitriles. This pattern was common to all nitriles considered and their derivatives [B12H11NCR]−. The value of LUMO for [B12H11NC(4-C6H4CH3)]− was less positive than for [B12H11NCR]−, R = CH3, n-C3H7. In addition, the shape of LUMO for [B12H11NC(4-C6H4CH3)]− was quite different to [B12H11NCR]−, R = CH3, n-C3H7 (Figure 5). In addition to the carbon atom of the nitrilium group, atoms from the benzene ring also made a significant contribution to LUMO.
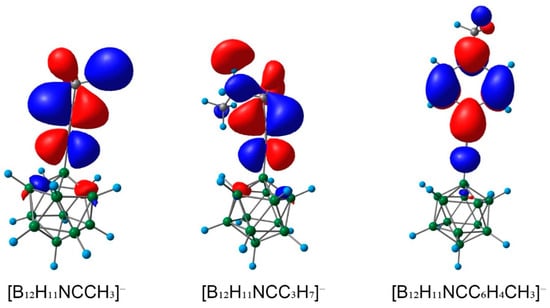
Figure 5.
LUMO of nitrilium derivatives [B12H11NCR]−, R = CH3, n-C3H7, 4-CH3C6H4.
The increased reactivity of borylated nitriles compared with initial nitriles is a well-known and established fact, which can be explained in terms of changes in the nature of the CN chemical bond upon formation of the target complex. In the case of nitrilium derivatives, the CN bond has a more ionic nature than that of initial nitriles. In addition, a change in the energy of the LUMO orbital during the formation of the nitrilium complex also plays a significant role. The decrease in LUMO energy during complex formation provides greater reactivity with nucleophile molecules. These effects are, however, characteristic only when solvent influence is taken into account. In the gas phase, uncoordinated nitriles have lower positive LUMO values than cluster derivatives.
3. Materials and Methods
3.1. IR Spectra
IR spectra of the compounds were recorded on an Infralum FT-08 IR Fourier spectrophotometer (NPF Lumex AP) in the region 400–4000 cm−1, with a resolution of 1 cm−1. Samples were prepared as thin film in CH2Cl2.
3.2. NMR Spectra
1H, 13C{H} and 11B{H} NMR spectra of solutions of the studied substances in CD3CN or CD2Cl2 were recorded on a Bruker MSL-300 pulsed Fourier spectrometer (Germany), at frequencies of 300.3, 75.49 and 96.32 MHz, respectively, with internal deuterium stabilisation. Tetramethylsilane or boron trifluoride ether was used as the external standard.
3.3. Electrospray Ionisation Mass Spectrometry (ESI-MS)
The LC system consisted of two LC-20AD pumps (Shimadzu, Japan) and an autosampler was coupled online with an LCMS-IT-TOF mass spectrometer equipped with an electrospray ionisation source (Shimadzu, Japan), The HRMS spectra were acquired in direct injection mode without column. The samples were prepared as CH3CN solutions. Detection parameters: Detector Voltage 1.55 kV; Nebulising Gas 1.50 L/min; CDL Temperature 200.0 °C.
3.4. X-ray Diffraction
The single-crystal X-ray diffraction data for 1 and 2 were collected using a three-circle Bruker D8 Venture (Centre of Joint Equipment of Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences), in φ and ω scan mode. The data were indexed and integrated using the SAINT program [No Title. Bruker, SAINT, Bruker AXS Inc., Madison, WI, 2018.]. Absorption correction based on measurements of equivalent reflections (SADABS) was applied []. The structures were determined by direct methods and refined using the full-matrix least squares technique on F2 with anisotropic displacement parameters for non-hydrogen atoms. The hydrogen atoms in all compounds were placed in calculated positions and refined with the riding model with fixed isotropic displacement parameters [Uiso(H) = 1.5Ueq(C) for the CH3-groups and 1.2Ueq(C) for the other groups].
All calculations were carried out using the SHELXTL program [] and OLEX2 program package []. For details, see Table S1 (Electronic Supporting Information).
The crystallographic data were deposited with the Cambridge Crystallographic Data Center, CCDC 2206019–2206020. Copies of this information may be obtained, free of charge, from the Director, CCDC, 12 Union Road, Cambridge CHB2 1EZ, UK (Fax: +44 1223 336033; e-mail: deposit@ccdc.cam.ac.uk or www.ccdc.cam.ac.uk).
As it stated previously the crystals for X-ray diffraction experiments were obtained by isothermal evaporation of acetonitrile solutions of the corresponding derivative, in the presence of (C2H5)4NCl or (C6H5)4PCl.
3.5. Hirshfeld Surface Analysis
The Crystal Explorer 17.5 program was [] used to analyse the interactions within the crystal. The donor–acceptor groups were visualised using a standard (high) surface resolution and dnorm surfaces were mapped over a fixed colour scale of −0.640 (red) to 0.986 (blue) a.u.
3.6. Computational Details
DFT calculations were made using the ORCA 4.2.1 program package []. Geometries of all model structures were fully optimised at the ωB97X-D3/def2-TZVPP level of theory [,]. All calculations were performed using the RIJCOSX approximation with the def2/J auxiliary basis set []. Tight criteria of SCF convergence (Tight SCF) were employed for the calculations. the keywords Grid5 FinalGrid6 GridX5 were used as parameters for the spatial integration grid All of the nitrilium derivatives considered had closed electron shells and the spin restricted approximation was applied. During the geometry optimisation procedure, symmetry operations were not applied for the structures considered. The Hessian matrices were calculated numerically for all model structures, to prove the location of correct minima on potential energy surfaces; (in all cases, no imaginary frequencies were found). Solvent effects were taken into account using the Solvation Model based on Density (SMD) []. The natural bond orbital (NBO) method (for calculation of Wiberg bond indices in Natural Atomic Orbitals) was employed using the NBO7 program package []. The Cartesian atomic coordinates for all optimised model structures of nitrlium derivatives of closo-dodecaborate anion are presented in the Supporting Information. The visualisation of optimised structures and their LUMO was performed with the help of the ChemCraft program (version 1.7) [].
Solvents of reagent and special purity grades Sigma-Aldrich and Panreac (99.7%), were used without any additional purification.
3.7. Synthesis of Nitrilium Derivatives of the closo-Dodecaborate Anion [B12H11NCR]− R=CH3, n-C3H7, i-C3H7, 4-C6H4CH3, 1-C10H7
((n-C4H9)4N)[B12H11(NCCH3)] (1a) was prepared by the known procedure []. 0.313 g (0.5 mmol) of ((n-C4H9)4N)2[B12H12] were dissolved in 10 mL of acetonitrile CH3CN. The solution was placed in a glass pressure vessel, it was purged with argon and then 0.3 mL trifluoroacetic acid CF3COOH was added. The reaction solution was heated to 150 °C. After 30 min of heating, the solution was cooled to room temperature and concentrated in a rotary evaporator. To this solution, 15 mL of glacial acetic acid CH3COOH was added. The precipitate was filtered through a glass filter and washed with 30 mL glacial acetic acid and 30 mL diethyl ether. The product was dried in vacuo. Yield: 76%.
((n-C4H9)4N)[B12H11(NCn-C3H7)] 1b
The method used was similar to 1a. Yield: 73%. 11B{H} NMR (ppm, CD2Cl2): −12.3 (s. 1B. B-N), −15.0 (s. 11B, B-H). 1H NMR (CD2Cl2. ppm): 2.5–0.0 (m. 11H, B-H), 3.15 (8H, (n-C4H9)4N), 1.61 (8H, (n-C4H9)4N), 1.45 (8H, (n-C4H9)4N), 1.01 (12H, (n-C4H9)4N), 2.87 (t. 2H, CH2CH2CH3. J = 7.0 Hz), 1.78 (m. 2H, CH2CH2CH3), 1.03(t. 3H, CH2CH2CH3 J = 7.4 Hz). 13C{H} NMR (CD2Cl2. ppm): 59.4 ((n-C4H9)4N), 24.4 ((n-C4H9)4N), 20.2 ((n-C4H9)4N), 13.9 (NBu4), 113.2 (NC-CH3), 20.6 (CH2CH2CH3), 18.9 (CH2CH2CH3), 13.3 (CH2CH2CH3). IR(CH2Cl2.cm−1): 2493 ν(B-H), 2338 ν(C≡N). MS(ESI) m/z: 210.2651 (A refers to the molecular weight of [B12H11(NCn-C3H7)]. Calculated for {[A]-} 210.2629).
((n-C4H9)4N)[B12H11(NCi-C3H7)] 1c
The method used was similar to 1a. Yield: 69%.
11B{H} NMR (CD2Cl2. ppm): −12.0 (s. 1B. B-N), −15.2 (s. 11B. B-H). 1H NMR (ppm, CD2Cl2): 2.5–0.0 (m. 11H. B-H), 3.15 (8H. (n-C4H9)4N), 1.61 (8H. (n-C4H9)4N), 1.45 (8H. (n-C4H9)4N), 1.01 (12H. (n-C4H9)4N), 3.28 (m. 1H. CH(CH3)2 J = 7.0 Hz), 1.38 (d. 6H. CH(CH3)2. J = 7.0 Hz). 13C{H} NMR (CD2Cl2. ppm): 59.4 ((n-C4H9)4N), 24.4 ((n-C4H9)4N), 20.2 ((n-C4H9)4N), 13.9 ((n-C4H9)4N), 115.5 (NC-CH3), 21.7 (CH(CH3)2), 18.3 (H(CH3)2), IR(CH2Cl2.cm−1): 2499 ν(B-H), 2338 ν(C≡N). MS(ESI) m/z: 210.2623 (A refers to the molecular weight of [B12H11(NCi-C3H7)]. Calculated for {[A]-} 210.2629).
((n-C4H9)4N)[B12H11(NCC6H4CH3)] 2a
A mixture of 0.313 g (0.5 mmol) of ((n-C4H9)4N)2[B12H12], and 5 g of NCC6H4CH3, was placed in an autoclave and then heated until melting in an argon stream. To the melt, 0.2 mL of CF3COOH was added. The autoclave was heated to 150 °C and then kept for 30 min. The solution was cooled until crystallisation. The resulting solid mixture was transferred into a round-bottom flask and the excess nitrile was distilled off in a vacuum. The product obtained was dissolved in 5 mL of CH3COOH/CF3COOH mixture and left for two hours. The resulting product was filtered off, washed with two portions of an acid mixture, 20 mL of cold diethyl ether, and dried in vacuo. The yield was 69%.
11B{H} NMR (ppm, CD2Cl2): −12.2 (s. 1B. B-N), −14.5 (s. 11B. B-H), 1H NMR (CD2Cl2. ppm): 2.5–0.0 (m. 11H. B-H), 3.16 (8H. (n-C4H9)4N), 1.64 (8H. (n-C4H9)4N), 1.43 (8H. (n-C4H9)4N), 1.01 (12H, (n-C4H9)4N), 7.76 (d. 2H. C6H4 J = 7.9 Hz), 7.43 (d. 2H, C6H4 J = 8.1 Hz), 2.49 (s. 3H, CH3). 13C{H} NMR (CD2Cl2. ppm): 59.5 ((n-C4H9)4N), 24.5 ((n-C4H9)4N), 20.2 ((n-C4H9)4N), 13.9 ((n-C4H9)4N), 149.4, 134.3, 131.2 108.8 (C6H4), 103.3 (N≡C), 22.7 (CH3). IR(CH2Cl2.cm−1): 2500 ν(B-H), 2305 ν(C≡N), 1600 ν(C=C). MS(ESI) m/z: 258.2666 (A refers to the molecular weight of [B12H11(NCC6H4CH3)]. Calculated for {[A]-} 258.2629).
((n-C4H9)4N)[B12H11(NC(1-C10H7))] 2b
The method used was similar to 2a. Yield: 36 %.
11B{H} NMR (CD3CN. ppm): −12.2 (s. 1B, B-N), -14.8 (s. 11B, B-H), 1H NMR (CD3CN. ppm): 2.5–0.0 (m. 11H. B-H), 3.05 (8H. (n-C4H9)4N), 1.61 (8H. (n-C4H9)4N), 1.34 (8H. (n-C4H9)4N), 0.94 (12H. (n-C4H9)4N), 8.5–7.5 (m. 7H, C10H7). 13C{H} NMR (CD3CN. ppm): 54.1 ((n-C4H9)4N), 26.4 ((n-C4H9)4N), 20.6 ((n-C4H9)4N), 14.0 ((n-C4H9)4N), 138.6, 137.6, 134.6, 134.1 133.8, 131.5, 130.7, 129.5, 126.5, 124.6 (C10H7), 104.2 (N≡C). IR(CH2Cl2.cm−1): 2502 ν(B-H), 2304 ν(C≡N), 1623, 1506 ν(C=C). MS(ESI) m/z: 294.2643 (A refers to the molecular weight of [B12H11(NCC10H7)]]. Calculated for {[A]-} 294.2629).
4. Conclusions
The present work featured a complex investigation of nitrilium derivatives of closo-dodecaborate anion [B12H11NCR]−, R = n-C3H7, i-C3H7, 4-C6H4CH3, 1-C10H7. The range of nitrilium derivatives of closo-dodecaborate anions was expanded, including derivatives with exo-polyhedral substituents with aromatic backbones. The target derivatives could be obtained without complex purification procedures. All derivatives obtained were stable in air and could be stored without specific conditions (such as an argon atmosphere or vacuum conditions). Simplicity of purification and storage makes nitrilium derivatives of closo-dodecaborate anion [B12H11NCR]− promising as a starting platform for the formation of inorganic systems with various useful applications, including use in biomedicine. The increased reactivity of borylated nitriles in comparison with initial nitriles was explained with the help of DFT calculation. The carbon atom of the nitrilium group of [B12H11NCR]− became much more positively charged than the initial nitrile. This increase in positive charge also indicates that the carbon atom of the nitrilium group should be much more easily attacked by nucleophile molecules. In addition, the energies of the LUMO orbitals drastically change during the formation of the nitrilium complex. The decrease in LUMO energy during complex formation provides greater reactivity with nucleophile molecules.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics10110196/s1, Figure S1: 11B{1H} NMR spectrum (Bu4N)[B12H11(NCnC3H7)] 1a; Figure S2: 1H NMR spectrum (Bu4N)[B12H11(NCnC3H7)] 1a; Figure S3: 13C NMR spectrum (Bu4N)[B12H11(NCnC3H7)] 1a; Figure S4: ESI-MS spectrum (Bu4N)[B12H11(NCnC3H7)] (negative area) 1a; Figure S5: 11B{1H} NMR spectrum (Bu4N)[B12H11(NCiC3H7)] 1b; Figure S6: 1H NMR spectrum (Bu4N)[B12H11(NCiC3H7)] 1b; Figure S7: 13C NMR spectrum (Bu4N)[B12H11(NCiC3H7)] 1b; Figure S8: ESI-MS spectrum (Bu4N)[B12H11(NCiC3H7)] (negative area) 1b; Figure S9: 11B{1H} NMR spectrum (Bu4N)[B12H11(NCC6H4CH3)] 2a; Figure S10: 1H NMR spectrum (Bu4N)[B12H11(NCC6H4CH3)] 2a; Figure S11: 13C NMR spectrum (Bu4N)[B12H11(NCC6H4CH3)] 2a; Figure S12: ESI-MS spectrum (Bu4N)[B12H11(NCC6H4CH3)] (negative area) 2a; Figure S13: 11B{1H} NMR spectrum (Bu4N)[B12H11(NCC10H7)] 2b; Figure S14: 1H NMR spectrum (Bu4N)[B12H11(NCC10H7)] 2b; Figure S15: 13C NMR spectrum (Bu4N)[B12H11(NCC10H7)] 2b; Figure S16: ESI-MS spectrum (Bu4N)[B12H11(NCC10H7)] (negative area) 2b; Table S1 Cartesian atomic coordinates of the calculated optimized equilibrium model structures. All coordinates are given in Angstrom units. Optimized equilibrium model structures in gas phase; Table S2 Cartesian atomic coordinates of the calculated optimized equilibrium model structures. All coordinates are given in Angstrom units. Optimized equilibrium model structures in dichloromethane; Table S3 Cartesian atomic coordinates of the calculated optimized equilibrium model structures. All coordinates are given in Angstrom units. Optimized equilibrium model structures in acetonitrile; Table S4 Crystal data and structure refinement for 1a and 1b.
Author Contributions
Manuscript conception, A.P.Z., I.N.K. and K.Y.Z.; writing and original draft preparation, A.V.N., I.N.K., A.S.N. and A.P.Z.; synthesis of derivatives A.V.N., A.P.Z., NMR analysis, N.A.S. and A.Y.B.; X-ray analysis, Hirshfeld analysis A.S.K.; quantum chemical calculations, I.N.K. and A.S.N.; editing, data analysis, and interpretation, A.P.Z., K.Y.Z. and N.T.K.; supervision, K.Y.Z. and N.T.K. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Ministry of Science and Higher Education of the Russian Federation. Agreement No 075-15-2020-792, unique contract identifier RF—190220X0031.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
A.S.N. is grateful to the RUDN University Strategic Academic Leadership Program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, X.; Yang, Z.; Chen, H.; Wang, Z.; Zhou, X.; Zhang, H. Progress in Three-Dimensional Aromatic-like Closo-Dodecaborate. Coord. Chem. Rev. 2021, 444, 214042. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I.; Sjöberg, S. Chemistry of Closo-Dodecaborate Anion [B12H12]2−: A Review. Collect. Czechoslov. Chem. Commun. 2002, 67, 679–727. [Google Scholar] [CrossRef]
- Ali, F.; Hosmane, N.S.; Zhu, Y. Boron Chemistry for Medical Applications. Molecules 2020, 25, 828. [Google Scholar] [CrossRef]
- Golub, I.E.; Filippov, O.A.; Kulikova, V.A.; Belkova, N.V.; Epstein, L.M.; Shubina, E.S. Thermodynamic Hydricity of Small Borane Clusters and Polyhedral Closo-Boranes. Molecules 2020, 25, 2920. [Google Scholar] [CrossRef]
- Tran, B.L.; Allen, T.N.; Bowden, M.E.; Autrey, T.; Jensen, C.M. Effects of Glymes on the Distribution of Mg(B10H10) and Mg(B12H12) from the Thermolysis of Mg(Bh4)2. Inorganics 2021, 9, 41. [Google Scholar] [CrossRef]
- Lavallo, V.; Wright, J.H.; Tham, F.S.; Quinlivan, S. Perhalogenated Carba-Closo-Dodecaborate Anions as Ligand Substituents: Applications in Gold Catalysis. Angew. Chem. Int. Ed. 2013, 52, 3172–3176. [Google Scholar] [CrossRef]
- Messina, M.S.; Axtell, J.C.; Wang, Y.; Chong, P.; Wixtrom, A.I.; Kirlikovali, K.O.; Upton, B.M.; Hunter, B.M.; Shafaat, O.S.; Khan, S.I.; et al. Visible-Light-Induced Olefin Activation Using 3D Aromatic Boron-Rich Cluster Photooxidants. J. Am. Chem. Soc. 2016, 138, 6952–6955. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yao, C.; Chen, H.; Fu, Y.; Xiang, C.; He, S.; Zhou, X.; Zhang, H. In Situ Nano Au Triggered by a Metal Boron Organic Polymer: Efficient Electrochemical N 2 Fixation to NH 3 under Ambient Conditions. J. Mater. Chem. A 2019, 7, 20945–20951. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Malinina, E.A.; Kuznetsov, N.T. Boron Cluster Anions and Their Derivatives in Complexation Reactions. Coord. Chem. Rev. 2022, 469, 214636. [Google Scholar] [CrossRef]
- Bolli, C.; Derendorf, J.; Jenne, C.; Scherer, H.; Sindlinger, C.P.; Wegener, B. Synthesis and Properties of the Weakly Coordinating Anion [Me3NB12Cl11]−. Chem. A Eur. J. 2014, 20, 13783–13792. [Google Scholar] [CrossRef]
- Bolli, C.; Derendorf, J.; Jenne, C.; Keßler, M. Halogenated Closo-Dodecaborate Anions Stabilize Weakly Bound [(Me3NH)3X]2+ (X = Cl, Br) Dications in the Solid State. Eur. J. Inorg. Chem. 2017, 2017, 4552–4558. [Google Scholar] [CrossRef]
- Teprovich, J.A.; Colón-Mercado, H.; Washington II, A.L.; Ward, P.A.; Greenway, S.; Missimer, D.M.; Hartman, H.; Velten, J.; Christian, J.H.; Zidan, R. Bi-Functional Li2B12H12 for Energy Storage and Conversion Applications: Solid-State Electrolyte and Luminescent down-Conversion Dye. J. Mater. Chem. A 2015, 3, 22853–22859. [Google Scholar] [CrossRef]
- Duchêne, L.; Lunghammer, S.; Burankova, T.; Liao, W.-C.; Embs, J.P.; Copéret, C.; Wilkening, H.M.R.; Remhof, A.; Hagemann, H.; Battaglia, C. Ionic Conduction Mechanism in the Na2(B12H12)0.5(B10H10)0.5 Closo -Borate Solid-State Electrolyte: Interplay of Disorder and Ion–Ion Interactions. Chem. Mater. 2019, 31, 3449–3460. [Google Scholar] [CrossRef]
- Gigante, A.; Duchêne, L.; Moury, R.; Pupier, M.; Remhof, A.; Hagemann, H. Direct Solution-Based Synthesis of Na4 (B12H12)(B10H10) Solid Electrolyte. ChemSusChem 2019, 12, 4832–4837. [Google Scholar] [CrossRef]
- Hagemann, H. Boron Hydrogen Compounds: Hydrogen Storage and Battery Applications. Molecules 2021, 26, 7425. [Google Scholar] [CrossRef]
- Barth, R.F.; Zhang, Z.; Liu, T. A Realistic Appraisal of Boron Neutron Capture Therapy as a Cancer Treatment Modality. Cancer Commun. 2018, 38, 36. [Google Scholar] [CrossRef]
- Hattori, Y.; Kusaka, S.; Mukumoto, M.; Uehara, K.; Asano, T.; Suzuki, M.; Masunaga, S.; Ono, K.; Tanimori, S.; Kirihata, M. Biological Evaluation of Dodecaborate-Containing L-Amino Acids for Boron Neutron Capture Therapy. J. Med. Chem. 2012, 55, 6980–6984. [Google Scholar] [CrossRef]
- Vaňková, E.; Lokočová, K.; Maťátková, O.; Křížová, I.; Masák, J.; Grüner, B.; Kaule, P.; Čermák, J.; Šícha, V. Cobalt Bis-Dicarbollide and Its Ammonium Derivatives Are Effective Antimicrobial and Antibiofilm Agents. J. Organomet. Chem. 2019, 899, 120891. [Google Scholar] [CrossRef]
- Iguchi, Y.; Michiue, H.; Kitamatsu, M.; Hayashi, Y.; Takenaka, F.; Nishiki, T.; Matsui, H. Tumor-Specific Delivery of BSH-3R for Boron Neutron Capture Therapy and Positron Emission Tomography Imaging in a Mouse Brain Tumor Model. Biomaterials 2015, 56, 10–17. [Google Scholar] [CrossRef]
- Avdeeva, V.V.; Garaev, T.M.; Breslav, N.V.; Burtseva, E.I.; Grebennikova, T.V.; Zhdanov, A.P.; Zhizhin, K.Y.; Malinina, E.A.; Kuznetsov, N.T. New Type of RNA Virus Replication Inhibitor Based on Decahydro-Closo-Decaborate Anion Containing Amino Acid Ester Pendant Group. JBIC J. Biol. Inorg. Chem. 2022, 27, 421–429. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Zhang, Y.; Liu, J.; van der Veen, S.; Duttwyler, S. The Closo-Dodecaborate Dianion Fused with Oxazoles Provides 3D Diboraheterocycles with Selective Antimicrobial Activity. Chem. A Eur. J. 2018, 24, 10364–10371. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, S.; Hattori, Y.; Uehara, K.; Asano, T.; Tanimori, S.; Kirihata, M. Synthesis of Optically Active Dodecaborate-Containing l-Amino Acids for BNCT. Appl. Radiat. Isot. 2011, 69, 1768–1770. [Google Scholar] [CrossRef] [PubMed]
- Knoth, W.H.; Sauer, J.C.; Miller, H.C.; Muetterties, E.L. Diazonium and Carbonyl Derivatives of Polyhedral Boranes. J. Am. Chem. Soc. 1964, 86, 115–116. [Google Scholar] [CrossRef]
- Dou, D.; Mavunkal, I.J.; Bauer, J.A.K.; Knobler, C.B.; Hawthorne, M.F.; Shore, S.G. Synthesis and Structure of Triethylammonium 2-(Acetonitrile)Nonahydro-Closo-Decaborate(1-). Inorg. Chem. 1994, 33, 6432–6434. [Google Scholar] [CrossRef]
- Mahfouz, N.; Ghaida, F.A.; El Hajj, Z.; Diab, M.; Floquet, S.; Mehdi, A.; Naoufal, D. Recent Achievements on Functionalization within Closo-Decahydrodecaborate [B10H10]2− Clusters. ChemistrySelect 2022, 7, e202200770. [Google Scholar] [CrossRef]
- John, K.C.; Kaczmarczyk, A.; Soloway, A.H. Alkylation and Acylation of B10H9NH3-. J. Med. Chem. 1969, 12, 54–57. [Google Scholar] [CrossRef]
- Grüner, B.; Bonnetot, B.; Mongeot, H. Synthesis of N- and B-Substituted Derivatives of Closo-Amino-Undecahydro-Dodecaborate(1-) Anion. Collect. Czechoslov. Chem. Commun. 1997, 62, 1185–1204. [Google Scholar] [CrossRef]
- Koo, M.-S.; Ozawa, T.; Santos, R.A.; Lamborn, K.R.; Bollen, A.W.; Deen, D.F.; Kahl, S.B. Synthesis and Comparative Toxicology of a Series of Polyhedral Borane Anion-Substituted Tetraphenyl Porphyrins. J. Med. Chem. 2007, 50, 820–827. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bruskin, A.B.; Nesterov, V.V.; Antipin, M.Y.; Bregadze, V.I.; Sjöberg, S. Synthesis of Schiff Bases Derived from the Ammoniaundecahydro-Closo-Dodecaborate(1−) Anion, [B12H11NHCHR]−, and Their Reduction into Monosubstituted Amines [B12H11NH2CH2R]-: A New Route to Water Soluble Agents for BNCT. Inorg. Chem. 1999, 38, 5887–5893. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Wang, T.; Liu, J.; Spingler, B.; Duttwyler, S. Synthesis and Structural Characterization of Amidine, Amide, Urea and Isocyanate Derivatives of the Amino-Closo-Dodecaborate Anion [B12H11NH3]−. Molecules 2018, 23, 3137. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Sivaev, I.B.; Bregadze, V.I. Nitrilium Derivatives of Polyhedral Boron Compounds (Boranes, Carboranes, Metallocarboranes): Synthesis and Reactivity. Phosphorus Sulfur Silicon Relat. Elem. 2019, 194, 983–988. [Google Scholar] [CrossRef]
- Luzyanin, K.V.; Haukka, M.; Bokach, N.A.; Kuznetsov, M.L.; Kukushkin, V.Y.; Pombeiro, A.J.L. Platinum(IV)-Mediated Hydrolysis of Nitriles Giving Metal-Bound Iminols. J. Chem. Soc. Dalt. Trans. 2002, 9, 1882–1887. [Google Scholar] [CrossRef]
- Kukushkin, V.Y.; Pombeiro, A.J.L. Additions to Metal-Activated Organonitriles. Chem. Rev. 2002, 102, 1771–1802. [Google Scholar] [CrossRef]
- Zhdanov, A.P.; Klyukin, I.N.; Bykov, A.Y.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Nucleophilic Addition of Alcohols to Anionic [2-B10H9NCR]−(R = Et, t-Bu): An Approach to Producing New Borylated Imidates. Polyhedron 2017, 123, 176–183. [Google Scholar] [CrossRef]
- Bolotin, D.S.; Burianova, V.K.; Novikov, A.S.; Demakova, M.Y.; Pretorius, C.; Mokolokolo, P.P.; Roodt, A.; Bokach, N.A.; Suslonov, V.V.; Zhdanov, A.P.; et al. Nucleophilicity of Oximes Based upon Addition to a Nitrilium Closo-Decaborate Cluster. Organometallics 2016, 35, 3612–3623. [Google Scholar] [CrossRef]
- Miller, H.C.; Hertler, W.R.; Muetterties, E.L.; Knoth, W.H.; Miller, N.E. Chemistry of Boranes. XXV. Synthesis Andc Chemistry of Base Derivatives of B10H102− and B12H122−. Inorg. Chem. 1965, 4, 1216–1221. [Google Scholar] [CrossRef]
- Zhizhin, K.Y.; Zhdanov, A.P.; Kuznetsov, N.T. Derivatives of Closo-Decaborate Anion [B10H10]2− with Exo-Polyhedral Substituents. Russ. J. Inorg. Chem. 2010, 55, 2089–2127. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Klyukin, I.N.; Zhdanov, A.P.; Grigor’ev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Synthesis of Nitrile Derivatives of the Closo-Decaborate and Closo-Dodecaborate Anions [BnHn–1NCR]– (n = 10, 12) by a Microwave Method. Russ. J. Inorg. Chem. 2021, 66, 139–145. [Google Scholar] [CrossRef]
- Voinova, V.V.; Selivanov, N.A.; Plyushchenko, I.V.; Vokuev, M.F.; Bykov, A.Y.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Grigoriev, M.S.; Rodin, I.A.; et al. Fused 1,2-Diboraoxazoles Based on Closo-Decaborate Anion–Novel Members of Diboroheterocycle Class. Molecules 2021, 26, 248. [Google Scholar] [CrossRef]
- Laskova, J.; Ananiev, I.; Kosenko, I.; Serdyukov, A.; Stogniy, M.; Sivaev, I.; Grin, M.; Semioshkin, A.; Bregadze, V.I. Nucleophilic Addition Reactions to Nitrilium Derivatives [B12H11NCCH3]− and [B12H11NCCH2CH3]−. Synthesis and Structures of Closo-Dodecaborate-Based Iminols, Amides and Amidines. Dalt. Trans. 2022, 51, 3051–3059. [Google Scholar] [CrossRef]
- Šícha, V.; Plešek, J.; Kvíčalová, M.; Císařová, I.; Grüner, B. Boron(8) Substituted Nitrilium and Ammonium Derivatives, Versatile Cobalt Bis(1,2-Dicarbollide) Building Blocks for Synthetic Purposes. Dalt. Trans. 2009, 5, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, E.V.; Stogniy, M.Y.; Suponitsky, K.Y.; Sivaev, I.B.; Bregadze, V.I. Synthesis of Boronated Amidines by Addition of Amines to Nitrilium Derivative of Cobalt Bis(Dicarbollide). Molecules 2021, 26, 6544. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, E.V.; Stogniy, M.Y.; Chekulaeva, L.A.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B.; Grin, M.A.; Mironov, A.F.; Bregadze, V.I. Synthesis and Reactivity of Propionitrilium Derivatives of Cobalt and Iron Bis(Dicarbollides). New J. Chem. 2020, 44, 15836–15848. [Google Scholar] [CrossRef]
- El Anwar, S.; Růžičková, Z.; Bavol, D.; Fojt, L.; Grüner, B. Tetrazole Ring Substitution at Carbon and Boron Sites of the Cobalt Bis(Dicarbollide) Ion Available via Dipolar Cycloadditions. Inorg. Chem. 2020, 59, 17430–17442. [Google Scholar] [CrossRef] [PubMed]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Anisimov, A.A.; Sivaev, I.B.; Bregadze, V.I. Nucleophilic Addition Reactions to the Ethylnitrilium Derivative of Nido -Carborane 10-EtCN-7,8-C2B9H11. New J. Chem. 2018, 42, 17958–17967. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Erokhina, S.A.; Suponitsky, K.Y.; Markov, V.Y.; Sivaev, I.B. Synthesis and Crystal Structures of Nickel(II) and Palladium(II) Complexes with o-Carboranyl Amidine Ligands. Dalt. Trans. 2021, 50, 4967–4975. [Google Scholar] [CrossRef] [PubMed]
- Stogniy, M.Y.; Erokhina, S.A.; Anisimov, A.A.; Suponitsky, K.Y.; Sivaev, I.B.; Bregadze, V.I. 10-NCCH2CH2OCH2CH2C N-7,8-C2B9H11: Synthesis and Reactions with Various Nucleophiles. Polyhedron 2019, 174, 114170. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Bogdanova, E.V.; Anufriev, S.A.; Sivaev, I.B. Synthesis of New Rhodacarborane [3,3-(1′,5′-COD)-8-PrNH=C(Et)NH-3,1,2-RhC2B9H10]. Russ. J. Inorg. Chem. 2022, 67, 1537–1544. [Google Scholar] [CrossRef]
- Calligaro, L.; Michelin, R.A.; Uguagliati, P. Mechanism of Nucleophilic Attack by Secondary Anilines on the Nitrile Group in Platinum(II) Ortho-Cyanobenzyl Complexes. Inorg. Chim. Acta 1983, 76, L83–L87. [Google Scholar] [CrossRef]
- Uguagliati, P.; Belluco, U.; Michelin, R.A.; Guerriero, P. Reactions of Coordinated Nitriles. I. Mechanism of Formation of Amidino Complexes of Attack of Primary Anilines of Pt(II) Ortho-Cyanobenzyl Complexes. Inorg. Chim. Acta 1984, 81, 61–67. [Google Scholar] [CrossRef]
- Ezhov, A.V.; Vyal’ba, F.Y.; Kluykin, I.N.; Zhdanova, K.A.; Bragina, N.A.; Zhdanov, A.P.; Zhizhin, K.Y.; Mironov, A.F.; Kuznetsov, N.T. Synthesis of New Bioinorganic Systems Based on Nitrilium Derivatives of Closo-Decaborate Anion and Meso-Arylporphyrins with Pendant Amino Groups. Macroheterocycles 2017, 10, 505–509. [Google Scholar] [CrossRef]
- Nelyubin, A.V.; Selivanov, N.A.; Bykov, A.Y.; Klyukin, I.N.; Novikov, A.S.; Zhdanov, A.P.; Karpechenko, N.Y.; Grigoriev, M.S.; Zhizhin, K.Y.; Kuznetsov, N.T. Primary Amine Nucleophilic Addition to Nitrilium Closo-Dodecaborate [B12H11NCCH3]−: A Simple and Effective Route to the New BNCT Drug Design. Int. J. Mol. Sci. 2021, 22, 13391. [Google Scholar] [CrossRef] [PubMed]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Electronic Supplementary Information (ESI). Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Glendening, E.D.; Badenhoop, J.K.; Reed, A.E.; Carpenter, J.E.; Bohmann, J.A.; Morales, C.M.; Karafiloglou, P.; Landis, C.R.; Weinhold, F. NBO 7.0: Natural Bond Orbital Analysis Program; University of Wisconsin: Madison, WI, USA, 2018. [Google Scholar]
- Chemcraft-Graphical Software for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com (accessed on 16 September 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).