Integrative Metallomics Studies of Toxic Metal(loid) Substances at the Blood Plasma–Red Blood Cell–Organ/Tumor Nexus
Abstract
1. Introduction
2. Critical Importance of Studying Toxic Metal(loid) Substances in the Bloodstream (Footnote: From Now on We Use the Term ‘Substances’ When We Refer to Both Toxic Metal(loid) Species and Metallodrugs)
3. General Considerations Pertaining to Interactions of Toxic Metal(loid) Substances in Humans
4. Bioinorganic Chemistry of Toxic Metal(loid) Substances in the Bloodstream In Vitro/In Vivo
5. Toxic Metal(loid) Species at the Plasma–RBC–Organ Nexus
6. Metallodrugs/Novel Metal(loid) Complexes at the Plasma–RBC–Organ–Tumor Nexus
6.1. Stability of Metallodrugs/Novel Metal(loid) Complexes in Plasma
6.2. Metallodrugs-/Novel-Metal(loid)-Complexes-Induced Rupture of RBCs
6.3. Assessment of the Selectivity of Metallodrugs/Novel Metal(loid) Complexes to Cancer Cells
7. Integrative Metallomics Studies
| Toxic Metal(loid) Species in Biological Fluid | Metallomics Tool(s) | Investigated Species | Obtained Information | Reference |
|---|---|---|---|---|
| Blood plasma (in vitro) | SEC-ICP-AES | Cd2+ | Cd2+-driven displacement of Zn2+ from a Zn metalloprotein | [16] |
| SEC-ICP-AES | CH3Hg+ | formation of hCys-CH3Hg+ complexes | [84] | |
| Red blood cell cytosol (in vitro) | SEC-ICP-AES and XAS | Hg2+, CH3Hg+, CH3CH2Hg+, Cd2+ | formation of stable complexes of Hg2+, CH3Hg+ and CH3CH2Hg+ with hemoglobin | [75] |
| SEC-ICP-AES and XAS | CH3Hg+ and (GS)2AsSe- | formation of (GS)2AsSe-HgCH3 | [90] | |
| Bile (in vivo) | XAS | injection of hamsters with AsIII and SeIV | detection of (GS)2AsSe- | [81] |
| Metallodrug in Biological Fluid/Compartment | Metallomics Tool | Investigated Metallodrug | Obtained Information | Reference |
| Blood plasma (in vitro) | SEC-ICP-AES | cisplatin and carboplatin, Titanocref | stability/degree of hydrolysis/degradation | [76] [63] |
| SEC-ICP-AES | cisplatin | formation of complexes between a cisplatin-derived hydrolysis product and thiosulfate | [129] | |
| SEC-ICP-AES | cisplatin | formation of complex between a cisplatin-derived hydrolysis product and N-acetyl-L-cysteine | [128] | |
| SEC-ICP-AES | cisplatin | formation of complexes between a cisplatin-derived hydrolysis product and D-methionine | [127] | |
| SEC-ICP-AES | cisplatin | modulation of the metabolism of cisplatin in serum of cancer patients with human serum albumin (HSA) | [141] | |
| SEC-ICP-AES | (2,2′:6′2″-terpyridine) platinum (II) complexes | binding to rabbit serum albumin | [131] | |
| SEC-ICP-AES | cisplatin and NAMI-A | comparative metabolism | [142] | |
| Whole blood (in vitro) | SEC-ICP-AES SEC-GFAAS | no metallodrugs investigated | dose-dependent effect of metallodrug on RBC lysis | [99] [132] |
| Healthy and cancer cells (cell culture) | scICP-MS | cisplatin | selectivity of metallodrug | [133] |
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sousa, F.L.; Neunkirchen, S.; Allen, J.F.; Lane, N.; Martin, W.F. Lokiarcheon is hydrogen dependent. Nat. Microbiol. 2016, 16034, 1–3. [Google Scholar]
- Li, J.J.; Pawitwar, S.S.; Rosen, B.P. The organoarsenical biocycle and the primordial antibiotic methylarsenite. Metallomics 2016, 8, 1047–1055. [Google Scholar] [CrossRef]
- Vandal, G.M.; Fitzgerald, W.F.; Boutron, C.F.; Candelone, J.-P. Variations in mercury deposition to Antarctica over the past 34,000 years. Nature 1993, 362, 621–6623. [Google Scholar] [CrossRef]
- McConnell, J.R.; Edwards, R. Coal burning leaves toxic heavy metal legacy in the Arctic. Proc. Natl. Acad. Sci. USA 2008, 105, 12140–12144. [Google Scholar] [CrossRef] [PubMed]
- Izatt, R.M.; Izatt, S.R.; Bruening, R.L.; Izatt, N.E.; Moyer, B.A. Challenges to achievement of metal sustainability in our high-tech society. Chem. Soc. Rev. 2014, 43, 2451–2475. [Google Scholar] [CrossRef]
- Campbell, P.G.C.; Gailer, J. Effects of Non-essential Metal Releases on the Environment and Human Health. In Metal Sustainability: Global Challenges, Consequences and Prospects; Izatt, R.M., Ed.; John Wiley & Sions Ltd.: Chichester, UK, 2016; pp. 221–252. [Google Scholar]
- Llanos, E.J.; Leal, W.; Luu, D.H.; Jost, J.; Stadler, P.F.; Restrepo, G. Exploration of the chemical space and its three historical regimes. Proc. Natl. Acad. Sci. USA 2019, 116, 12660–12665. [Google Scholar] [CrossRef]
- Stern, F. Paul Ehrlich: The Founder of Chemotherapy. Angew. Chem. Int. Ed. 2004, 43, 4254–4261. [Google Scholar] [CrossRef]
- Marzo, T.; La Mendola, D. Strike a balance: Between metals and non-metals, metalloids as a source of anit-infective agents. Inorganics 2021, 9, 46. [Google Scholar] [CrossRef]
- Bolli, R. William Harvey and the discovery of the circulation of the blood. Circul. Res. 2019, 124, 1300–1302. [Google Scholar]
- Gailer, J. Reactive selenium metabolites as targets of toxic metals/metalloids in mammals: A molecular toxicological perspective. Appl. Organometal. Chem. 2002, 16, 701–707. [Google Scholar] [CrossRef]
- Sarpong-Kumankomah, S.; Gailer, J. Application of a novel metallomics tool to probe the fate of metal-based anticancer drugs in blood plasma: Potential, challenges and prospects. Curr. Top. Med. Chem. 2021, 21, 48–58. [Google Scholar] [CrossRef]
- Doroudian, M.; Gailer, J. Toxic metal(loid) species at the blood-organ interface. In Environmental and Biochemical Toxicology; Turner, R.J., Gailer, J., Eds.; DeGruyter: Munich, Germany, 2022; Volume 1, p. 335. [Google Scholar]
- Zheng, X.; Cheng, Y.; Wang, C. Global mapping of metalloproteomes. Biochemistry 2021, 60, 3507–3514. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Sun, H. Metalloproteomics for biomedical research: Methodology and applications. Annu. Rev. Biochem. 2022, 91, 449–473. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ariza, J.L.; Jahromi, E.Z.; Gonzalez-Fernandez, M.; Garcia-Barrera, T.; Gailer, J. Liquid chromatography-inductively coupled plasma-based metallomic approaches to probe health-relevant interactions between xenobiotics and mammalian organisms. Metallomics 2011, 3, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Theiner, S.; Schoeberl, A.; Schweikert, A.; Keppler, B.K.; Koellensperger, G. Mass spectrometry techniques for imaging and detection of metallodrugs. Curr. Opin. Chem. Biol. 2021, 61, 123–134. [Google Scholar] [CrossRef]
- Arruda, M.A.Z.; Jesus, J.R.D.; Blindauer, C.A.; Stewart, A.J. Speciomics as a concept involving chemical speciation and omics. J. Proteom. 2022, 263, 104615. [Google Scholar] [CrossRef] [PubMed]
- Pacyna, J.M. Monitoring and assessment of metal contaminants in the air. In Toxicology of Metals; Chang, L.W., Ed.; CRC Press: Boca Raton, Florida, FL, USA, 1996; pp. 9–28. [Google Scholar]
- Jonsson, S.; Andersson, A.; Nilsson, M.B.; Skyllberg, U.L.; Lundberg, E.; Schaefer, J.K.; Akerblom, S.; Bjoern, E. Terrestrial dischanrges mediate trophic shifts and enhance methylmercury accumulation in estuarine biota. Sci. Adv. 2017, 3, e1601239. [Google Scholar] [CrossRef] [PubMed]
- Steffen, W.; Crutzen, P.J.; McNeill, J.R. The anthropocene: Are humans now overwhelming the great forces of nature? Ambio 2007, 36, 614–621. [Google Scholar] [CrossRef]
- Kasozi, K.I.; Otim, E.O.; Ninsiima, H.I.; Zirintunda, G.; Tamale, A.; Ekou, J.; Musoke, G.H.; Muyinda, R.; Matama, K.; Mujinga, R.; et al. An analysis of heavy metals contamination and estimating the daily intakes of vegetables in Uganda. Toxicol. Res. Appl. 2021, 5, 1–15. [Google Scholar] [CrossRef]
- Borchers, A.; Teuber, S.S.; Keen, C.L.; Gershwin, M.E. Food safety. Clin. Rev. Allergy Immunol. 2010, 39, 95–141. [Google Scholar] [CrossRef]
- Schartup, A.T.; Thackray, C.P.; Qureshi, A.; Dassuncao, C.; Gillespie, K.; Hanke, A.; Sunderland, E.M. Climate change and overfishing increase neurotoxicant in marine predators. Nature 2019, 572, 648–650. [Google Scholar] [CrossRef] [PubMed]
- Dufault, R.; LeBlanc, B.; Schnoll, R.; Cornett, C.; Schweitzer, L.; Wallinga, D.; Hightower, J.; Patrick, L.; Lukiw, W.J. Mercury from chlor-alkali plants: Measured concentrations in food product sugar. Environ. Health 2009, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- McFarland, M.J.; Hauer, M.E.; Reuben, A. Half of US population exposed to adverse lead levels in early childhood. Proc. Natl. Acad. Sci. USA 2022, 119, e2118631119. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Zheng, X.Y.; Chung, K.F.; Zhong, N.S. Impact of air pollution on the burden of chronic respiratory diseases in China: Time for urgent action. Lancet 2016, 388, 1939–1951. [Google Scholar] [CrossRef]
- Mills, N.L.; Donaldson, K.; Hadoke, P.W.; Boon, N.A.; MacNee, W.; Cassee, F.R.; Sandstroem, T.; Blomberg, A.; Newby, D.E. Adverse cardionvascular effects of air pollution. Nat. Clin. Pract. Card. 2009, 6, 36–44. [Google Scholar] [CrossRef]
- Steckling, N.; Tobollik, M.; Plass, D.; Hornberg, C.; Ericson, B.; Fuller, R.; Bose-O’Reilly, S. Global burden of disease of mercury used in artisanal small-scale gold mining. Ann. Glob. Health 2017, 83, 234–247. [Google Scholar] [CrossRef]
- Guney, M.; Zagury, G.J. Contamination by ten harmful elements in toys and children’s jewelry bought on the North American market. Environ. Sci. Technol. 2013, 47, 5921–5930. [Google Scholar] [CrossRef]
- Travasso, C. Skin whitening cream may contain mercury, and lipstick may contain chromium and nickel, Indian study shows. Brit. Med. J. 2014, 348, g1330. [Google Scholar] [CrossRef]
- Hamann, C.R.; Boonchai, W.; Wen, L.; Sakanashi, E.; Chu, C.-Y.; Hamann, K.; Hamann, C.P.; Sinniah, K.; Hamann, D.; Linda, L. Spectrometric analysis of mercury content in 549 skin-lightening products: Is mercury toxicity a hidden global health hazard? J. Am. Acad. Dermayol. 2013, 70, 281–287. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.; Balde, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef]
- Trasande, L.; Liu, Y. Reducing the staggering costs of environmental disease in children, estimated at $76.6 billion in 2008. Health Affair. 2011, 30, 863–870. [Google Scholar] [CrossRef] [PubMed]
- North, A.E.; Sarpong-Kumankomah, S.; Bellavie, A.R.; White, W.M.; Gailer, J. Environmentally relevant concentrations of aminopolycarboxylate chelating agents mobilize Cd from humic acid. J. Environ. Sci. 2017, 57, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, T.J.; Macklin, M.G. Modeling long-term contamination in river systems from historical metal mining. Geology 2003, 31, 451–454. [Google Scholar] [CrossRef]
- Pruvot, C.; Douay, F.; Herve, F.; Waterlot, C. Heavy metals in soil, crops and grass as a source of human exposure in the former mining areas. J. Soils Sediments 2006, 6, 215–220. [Google Scholar] [CrossRef]
- Lamborg, C.H.; Hammerschmidt, C.R.; Bowman, K.L.; Swarr, G.J.; Munson, K.M.; Ohnemus, D.C.; Lam, P.J.; Heimbuerger, L.-E.; Rijkenberg, M.J.A.; Saito, M.A. A global ocean inventory of anthropogenic mercury based on water column measurements. Nature 2014, 512, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Streets, D.G.; Horowitz, H.M.; Jacob, D.J.; Lu, Z.; Levin, L.; ter Schure, A.F.H.; Sunderland, E.M. Total mercury released to the environment by human activities. Environ. Sci. Technol. 2017, 51, 5969–5977. [Google Scholar] [CrossRef]
- Ogunseitan, O.A. Mercury safety reform in the 21st centrury: Advancing the new framework for toxic substance control. Environ. Sci. Policy Sustain. Dev. 2017, 59, 4–13. [Google Scholar] [CrossRef]
- Hawkings, J.R.; Linhoff, B.S.; Wadham, J.L.; Stibal, M.; Lamborg, C.H.; Carling, G.T.; Lamarche-Gagnon, G.; Kohler, T.J.; Ward, R.; Hendry, K.R.; et al. Large subglacial source of mercury from the southwestern margin of the Greenland Ice Sheet. Nat. Geosci. 2021, 14, 496–502. [Google Scholar] [CrossRef]
- Schaefer, H.R.; Dennis, S.; Fitzpatrick, S. Cadmium. Minimization strategies to reduce dietary exposure. J. Food Sci. 2020, 85, 260–267. [Google Scholar] [CrossRef]
- Sebastian, A.; Prasad, M.N.V. Cadmium minimization in rice: A review. Agron. Sustain. Dev. 2014, 34, 155–173. [Google Scholar] [CrossRef]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Cristol, D.A.; Brasso, R.L.; Condon, A.M.; Fovarque, R.E.; Friedman, S.L.; Hallinger, K.K.; Monroe, A.P.; White, A.E. The movement of aquatic mercury through terrestrial food webs. Science 2008, 320, 335. [Google Scholar] [CrossRef] [PubMed]
- Obrist, D.; Agnan, Y.; Jiskra, M.; Olson, C.L.; Colegrove, D.P.; Hueber, J.; Morre, C.W.; Sonke, J.E.; Helmig, D. Tundra uptake of atmospheric elemental mercury drives Arctic mercury pollution. Nature 2017, 547, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Nyanza, E.C.; Bernier, F.P.; Martin, J.W.; Manyama, M.; Hatfield, J.; Dewey, D. Effetcs of prenatal expsoure and co-exposure to metallic or metalloid elements on early infant neurodevelopment outcomes in areas with small-scale gold mining activities in Northern Tanzania. Environ. Int. 2021, 149, 106104. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.; Frisbie, S.; Sarkar, B. Exposure to multiple metals from groundwater-a global crisis: Geology, climate change, health effects, testing, and mitigation. Metallomics 2011, 3, 874–908. [Google Scholar] [CrossRef]
- Sanchez-Rodas, D.; Sanchez de la Campa, A.; Martinez, M.M. The role of metalloids (As, Sb) in airborne particulate matter related to air pollution. In Environmental and Biochemical Toxicology; Turner, R.J., Gailer, J., Eds.; DeGruyetr: Munich, Germany, 2022; pp. 169–189. [Google Scholar]
- Turner, R.J. Toxicity of nanomaterials. In Environmental and Biochemical Toxicology; DeGruyter: Munich, Germany, 2022; pp. 221–247. [Google Scholar]
- Rappaport, S.M. Discovering environmental causes of disease. J. Epidemiol. Community Health 2012, 66, 99–102. [Google Scholar] [CrossRef]
- Weiss, B.; Clarkson, T.W.; Simon, W. Silent latency period in methylmercury poisoning and in neurodegenerative disease. Environ. Health Perspect. 2002, 110, 851–854. [Google Scholar] [CrossRef]
- Sturla, S.J.; Boobis, A.R.; Fitzgerald, R.E.; Hoeng, J.; Kavlock, R.J.; Schirmer, K.; Whelan, M.; Wilks, M.F.; Peitsch, M.C. Systems toxicology: From basic research to risk assessment. Chem. Res. Toxicol. 2014, 27, 314–329. [Google Scholar] [CrossRef]
- Marth, J.D. A unified vision of the building blocks of life. Nature Cell. Biol. 2008, 10, 1015–1016. [Google Scholar] [CrossRef]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effetcs of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef] [PubMed]
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Sooriyaarachchi, M.; Morris, T.T.; Gailer, J. Advanced LC-analysis of human plasma for metallodrug metabolites. Drug Discov. Today Technol. 2015, 16, e24–e30. [Google Scholar] [CrossRef] [PubMed]
- Hambley, T.W. Metal-based therapeutics. Science 2007, 318, 1392–1393. [Google Scholar] [CrossRef] [PubMed]
- Poirier, A.E.; Ruan, Y.; Walter, S.D.; Franco, E.L.; Villeneuve, P.J.; King, W.D.; Volesky, K.D.; O’Sullivan, D.E.; Friedenreich, C.M.; Brenner, D.R. The future burden of cancer in Canada: Long-term cancer incidence projections. Cancer Epidemiol. 2019, 59, 199–207. [Google Scholar] [CrossRef]
- Casini, A.V.A.; Meier-Menches, S.M. Metal-Based Anticancer Agents; Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Kutwin, M.; Sawosz, E.; Jaworski, S.; Kurantowicz, N.; Strojny, B.; Chwalibog, A. Structural damage of chicken red blood cells exposed to platinum nanoparticles and cisplatin. Nanoscale Res. Lett. 2014, 9, 257. [Google Scholar] [CrossRef]
- Sarpong-Kumankomah, S.; Contel, M.; Gailer, J. SEC hyphenated to a multielement-specific detector unravels the degradation pathway of a bimetallic anticancer complex in human plasma. J. Chromatogr. B 2020, 1145, 122093. [Google Scholar] [CrossRef]
- Gandin, V.; Ceresca, C.; Esposito, G.; Indraccolo, S.; Porchia, M.; Tisano, F.; Santini, C.; Pellei, M.; Marzano, C. Therapeutic potential of the phosphino Cu(I) complex (HydroCuP) in the treatment of solid tumors. Sci. Rep. 2017, 7, 13936. [Google Scholar] [CrossRef]
- Marcus, W.L.; Rispin, A.S. Threshold carcinogenicity using arsenic as an example. In Advances in Modern Environmental Toxicology; Cothern, C.R., Mehlmans, M.A., Marcus, W.L., Eds.; Princeton Scientific Publishing Company, Inc: Princeton, NJ, USA, 1988; Volume 15, pp. 133–158. [Google Scholar]
- Kitchin, K.T. The role of protein binding of trivalent arsenicals in arsenic carcinogenesis and toxicity. J. Inorg. Biochem. 2008, 102, 532–539. [Google Scholar] [CrossRef]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef]
- Anderson, N.L.; Anderson, N.G. The human plasma proteome: History, character, and diagnostic prospects. Mol. Cell. Proteom. 2002, 1, 845–867. [Google Scholar] [CrossRef] [PubMed]
- Bridle, T.G.; Kumarathasan, P.; Gailer, J. Toxic metal species and ‘endogenous’ metalloproteins at the blood-organ interface: Analytical and bioinorganic aspects. Molecules 2021, 26, 3408. [Google Scholar] [CrossRef] [PubMed]
- Righetti, P.G.; Boschetti, E. The Proteominer and the fortyniners: Searching for gold nuggest in the proteomic arena. Mass Spec. Rev. 2008, 27, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Gailer, J. Metal species in biology: Bottom-up and top-down LC approaches in applied toxicological research. ISRN Chromatography 2013, 2013, 21. [Google Scholar] [CrossRef][Green Version]
- Sarpong-Kumankomah, S.; Miller, K.; Gailer, J. Biological chemistry of toxic metals and metalloids, such as arsenic, cadmium and mercury. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 2006–2020. [Google Scholar]
- Gailer, J. Arsenic-selenium and mercury-selenium bonds in biology. Coord. Chem. Rev. 2007, 251, 234–254. [Google Scholar] [CrossRef]
- Kerek, E.; Hassanin, M.; Prenner, E.J. Inorganic mercury and cadmium induce rigidity in eukaryotic lipid extracts while mercury also ruptures red blood cells. Biochim. Biophys. Acta 2018, 1860, 710–717. [Google Scholar] [CrossRef]
- Gibson, M.A.; Sarpong-Kumankomah, S.; Nehzati, S.; George, G.N.; Gailer, J. Remarkable differences in the biochemical fate of Cd2+, Hg2+, CH3Hg+ and thimerosal in red blood cell lysate. Metallomics 2017, 9, 1060–1072. [Google Scholar] [CrossRef]
- Sooriyaarachchi, M.; Narendran, A.; Gailer, J. Comparative hydrolysis and plasma binding of cis-platin and carboplatin in human plasma in vitro. Metallomics 2011, 3, 49–55. [Google Scholar] [CrossRef]
- Manley, S.A.; Gailer, J. Analysis of the plasma metalloproteome by SEC-ICP-AES: Bridging proteomics and metabolomics. Expert. Rev. Proteom. 2009, 6, 251–265. [Google Scholar] [CrossRef]
- Gailer, J.; George, G.N.; Pickering, I.J.; Prince, R.C.; Ringwald, S.C.; Pemberton, J.E.; Glass, R.S.; Younis, H.S.; DeYoung, D.W.; Aposhian, H.V. A metabolic link between arsenite and selenite: The Seleno-bis(S-glutathionyl) Arsinium Ion. J. Am. Chem. Soc. 2000, 122, 4637–4639. [Google Scholar] [CrossRef]
- Gailer, J.; George, G.N.; Pickering, I.J.; Madden, S.; Prince, R.C.; Yu, E.Y.; Denton, M.B.; Younis, H.S.; Aposhian, H.V. Structural basis of the antagonism between inorganic mercury and selenium in mammals. Chem. Res. Toxicol. 2000, 13, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Manley, S.A.; George, G.N.; Pickering, I.J.; Glass, R.S.; Prenner, E.J.; Yamdagni, R.; Wu, Q.; Gailer, J. The seleno bis (S-glutathionyl) arsinium ion is assembled in erythrocyte lysate. Chem. Res. Toxicol. 2006, 19, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, O.; LaPorte, P.F.; Singh, S.P.; Langan, G.; Fleming, D.E.B.; Spallholz, J.; Alauddin, M.; Ahsan, H.; Ahmed, S.; Gailer, J.; et al. Selenium-mediated arsenic excretion in mammals: A synchrotron-based study of whole-body distribution and tissue-specific chemistry. Metallomics 2017, 9, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Gailer, J.; Ruprecht, L.; Reitmeir, P.; Benker, B.; Schramel, P. Mobilization of exogenous and endogenous selenium to bile after the intravenous administration of environmentally relevant doses of arsenite to rabbits. Appl. Organometal. Chem. 2004, 18, 670–675. [Google Scholar] [CrossRef]
- Maret, W.; Moulis, J.M. The bioinorganic chemistry of cadmium in the context of its toxicity. In Cadmium: From Toxicity to Essentiality, Metal Ions in Life Sciences; Sigel, A.S.H., Sigel, R.K.O., Eds.; Springer Science+Business Media Dordrecht: Basel, Switzerland, 2013; Volume 25, pp. 1–29. [Google Scholar]
- Bridle, T.G.; Doroudian, M.; White, W.; Gailer, J. Physiologically relevant hCys concentrations mobilize MeHg from rabbit serum albumin to form MeHg-hCys complexes. Metallomics 2022, 14, mfac010. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, J.; Leonard, S.S.; Rao, K.M.K. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Rad. Biol. Med. 2004, 36, 1434–1443. [Google Scholar] [CrossRef]
- Sarpong-Kumankomah, S.; Gibson, M.A.; Gailer, J. Organ damage by toxic metals is critically determined by the bloodstream. Coord. Chem. Rev. 2018, 374, 376–386. [Google Scholar] [CrossRef]
- Thijssen, S.; Maringwa, J.; Faes, C.; Lambrichts, I.; Van Kerkhove, E. Chronic exposure of mice to environmentally relevant, low doses of cadmium leads to early renal damage, not predicted by blood or urine cadmium levels. Toxicology 2007, 229, 145–156. [Google Scholar] [CrossRef]
- Bakir, F.; Damluji, S.F.; Amin-Zaki, L.; Murtafdha, M.; Khalidi, A.; Al-Rawi, N.Y.; Tikriti, S.; Dhahir, H.I.; Clarkson, T.W.; Smith, J.C.; et al. Methylmercury poisoning in Iraq. Science 1973, 181, 230–241. [Google Scholar] [CrossRef]
- Nordberg, G.F. Historical perspectives on cadmium toxicity. Toxicol. Appl. Pharmacol. 2009, 238, 192–200. [Google Scholar] [CrossRef]
- Korbas, M.; Percy, A.J.; Gailer, J.; George, G.N. A possible molecular link between the toxicological effects of arsenic, selenium and methylmercury: Methylmercury(II) seleno bis (S-glutathionyl) arsenic(III). J. Biol. Inorg. Chem. 2008, 13, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Rother, R.P.; Bell, L.; Hillmen, P.; Gladwin, M.T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin. J. Am. Med. Assoc. 2005, 293, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Kostic, M.M.; Ognjanovic, B.; Dimitrijevic, S.; Zikic, R.V.; Stajn, A.; Rosic, G.L.; Zivkovic, R.V. Cadmium-induced changes of antioxidant and metabolic status in red blood cells of rats: In vivo effects. Eur. J. Haematol. 1993, 51, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Oguma, E.; Kayama, F. Cadmium induces anaemia through interdependent progress of hemolysis, body iron accumulation, and insufficient erythropoietin production in rats. Toxicol. Sci. 2011, 122, 198–210. [Google Scholar] [CrossRef]
- Manley, S.A.; Byrns, S.; Lyon, A.W.; Brown, P.; Gailer, J. Simultaneous Cu-, Fe-, and Zn-specific detection of metalloproteins contained in rabbit plasma by size-exclusion chromatography-inductively coupled plasma atomic emission spectroscopy. J. Biol. Inorg. Chem. 2009, 14, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Sarpong-Kumankomah, S.; Knox, K.B.; Kelly, M.E.; Hunter, G.; Popescu, B.; Nichol, H.; Kopciuk, K.; Ntanda, H.; Gailer, J. Quantification of human plasma metalloproteins in multiple sclerosis, ischemic stroke and health controls reveals an association of haptoglobin-hemoglobin complexes with age. PLoS ONE 2022, 17, e0262160. [Google Scholar] [CrossRef]
- Suzuki, K.T.; Sunaga, H.; Kobayashi, E.; Shimojo, N. Mercaptoalbumin as a selective cadmium-binding protein in rat serum. Toxicol. Appl. Pharmacol. 1986, 86, 466–473. [Google Scholar] [CrossRef]
- Massai, L.; Pratesi, A.; Gailer, J.; Marzo, T.; Messori, L. The cisplatin/serum albumin systen: A reappraisal. Inorg. Chim. Acta 2019, 495, 118983. [Google Scholar] [CrossRef]
- de Magalhanes Silva, M.; de Araujo Dantas, M.D.; de Sila Filho, R.C.; dos Santos Sales, M.V.; de Almeida Xavier, J.; Leite, A.C.R.; Goulart, M.O.F.; Grillo, L.A.M.; de Barros, W.A.; de Fatima, A.; et al. Toxicity of thimerosal in biological systems: Conformational changes in human hemoglobin, decrease of oxygen binding, increase of protein glycation and amyloid formation. Intern. J. Biol. Macromol. 2020, 154, 661–671. [Google Scholar] [CrossRef]
- Sarpong-Kumankomah, S.; Gailer, J. Identification of a haptoglobin-hemoglobin complex in human blood plasma. J. Inorg. Biochem. 2019, 201, 110802. [Google Scholar] [CrossRef]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A red carpet for iron metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Gebicka, L.; Krych-Madej, J. The role of catalases in the prevention/promotion of oxidative stress. J. Inorg. Biochem. 2019, 197, 110699. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef] [PubMed]
- Ordonez, Y.N.; Montes-Bayon, M.; Blanco-Gonzalez, E.; Sanz-Medel, A. Quantitative analysis and simultaneous activity measurements of Cu, Zn-superoxide dismutase in red blood cells by HPLC-ICPMS. Anal. Chem. 2010, 82, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-Y.; Fauman, E.B.; Petersen, A.-K.; Krumsiek, J.; Santos, R.; Huang, J.; Arnold, M.; Erte, I.; Forgetta, V.; Yang, T.-P.; et al. An atlas of genetic influences on human blood metabolites. Nat. Genet. 2014, 46, 543–550. [Google Scholar] [CrossRef]
- Thomas, D.J.; Smith, J.C. Effects of coadministered low-molecular weight thiol compounds on short-term distribution of methylmercury in the rat. Toxicol. Appl. Pharmacol. 1982, 62, 104–110. [Google Scholar] [CrossRef]
- Simmons-Wills, T.A.; Koh, A.S.; Clarkson, T.W. Transport of a neurotoxicant by molecualr mimicry: The methylmercury-L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT 2. Biochem. J. 2002, 367, 239–246. [Google Scholar] [CrossRef]
- Jakubowski, H. Homocysteine modification in protein structure/function and human disease. Physiol. Rev. 2019, 99, 555–604. [Google Scholar] [CrossRef]
- Hill, A.; Gailer, J. Linking molecular targets of Cd in the bloodstream to organ-based adverse health effects. J. Inorg, Biochem. 2021, 216, 111279. [Google Scholar] [CrossRef]
- Costa, L.G.; Aschner, M.; Vitalone, A.; Syversen, T.; Soldin, O.P. Developmental neuropathology of environmental agents. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 87–110. [Google Scholar] [CrossRef]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Bruno, P.M.; Liu, Y.; Park, G.Y.; Murai, J.; Koch, C.E.; Eisen, T.J.; Pritchard, J.R.; Pommier, Y.; Lippard, S.J.; Hemann, M.T. A subset of platinum-containing chemotherapeutic agents kill cells by inducing ribosome biogenesis stress rather that by engaging a DNA damage response. Nat. Med. 2017, 23, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Prosser, K.E.; Harrypersad, S.; MacNeil, G.A.; Panchmatia, R.; Thompson, J.R.; Sinha, S.; Warren, J.J.; Walsby, C.J. Activation by oxidation: Ferrocene-functionalized Ru(II)-arene complexes with anticancer, antibacterial, and antioxidant properties. Inorg. Chem. 2018, 57, 15247–15261. [Google Scholar] [CrossRef]
- Morrison, C.N.; Prosser, K.E.; Stokes, R.W.; Cordes, A.; Metzler-Nolte, N.; Cohen, S.M. Expanding medicinal chemistry into 3D space: Metallofragments as 3D scaffolds for fragment-based drug discovery. Chem. Sci. 2020, 11, 1216–1225. [Google Scholar] [CrossRef]
- Prosser, K.E.; Stokes, R.W.; Cohen, S.M. Evaluation of 3-dimensionality in approved and experimental drug space. ACS Med. Chem. Lett. 2020, 11, 1292–1298. [Google Scholar] [CrossRef]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef]
- Kenny, R.G.; Marmion, C.J. Toward multi-targeted platinum and ruthenium drugs-a new paradigm in cancer drug treatment regimens? Chem. Rev. 2019, 119, 1058–1137. [Google Scholar] [CrossRef]
- Fronik, P.; Poetsch, I.; Kastner, A.; Mendrina, T.; Hager, S.; Hohenwallner, K.; Schueffl, H.; Herndler-Brandstetter, D.; Koellensperger, G.; Rampler, E.; et al. Structure-activity relationships of triple-action platinum(IV) prodrugs with albumin-binding properties and immunomodulating ligands. J. Med. Chem. 2021, 64, 12132–12151. [Google Scholar] [CrossRef]
- Englinger, B.; Pirker, C.; Heffeter, P.; Terenzi, A.; Kowol, C.R.; Keppler, B.K.; Berger, W. Metal drugs and the anticancer immune response. Chem. Rev. 2019, 119, 1519–1624. [Google Scholar] [CrossRef]
- Hambley, T.W. Transporter and protease mediated delivery of platinum complexes for precision oncology. J. Biol. Inorg. Chem. 2019, 24, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Bruijnincx, P.C.A.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Moretto, J.; Chauffert, B.; Ghiringhelli, F.; Aldrich-Wright, J.R.; Bouyer, F. Discrepancy between in vitro and in vivo antitumor effect of a new platinum(II) metallointercalator. Invest. New Drugs 2011, 29, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Kalke, R.; Li, X.-F. Interaction of oxaliplatin, cisplatin, and carboplatin with hemoglobin and the resulting release of a heme group. Chem. Res. Toxicol. 2004, 17, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Timerbaev, A.R.; Hartinger, C.G.; Aleksenko, S.S.; Keppler, B.K. Interactions of antitumor metallodrugs with serum proteins: Advances in characterization using modern analytical methodology. Chem. Rev. 2006, 106, 2224–2248. [Google Scholar] [CrossRef]
- Levine, K.E.; Young, D.J.; Afton, S.E.; Harrington, J.M.; Essader, A.S.; Weber, F.X.; Fernando, R.A.; Thayer, K.; Hatch, E.E.; Robinson, V.C.; et al. Development, validation, and application of an ultra-performance liquid chromatography-sector field inductively coupled plasma mass spectrometry method for simultaneous determination of six organotin compounds in human serum. Talanta 2015, 140, 115–121. [Google Scholar] [CrossRef]
- Entwisle, J.; Malinovsky, D.; Dunn, P.J.H.; Goenaga-Infante, H. Hg isotope ratio measurements of methylmercury in fish tissues using HPLC with off line cold vapour generation MC-ICPMS. J. Anal. At. Spetcrom. 2018, 33, 1645–1654. [Google Scholar] [CrossRef]
- Sooriyaarachchi, M.; Narendran, A.; White, W.H.; Gailer, J. Chemoprotection by D-methionine against cis-platin-induced side-effects: Insight from in vitro studies using human plasma. Metallomics 2014, 6, 532–541. [Google Scholar] [CrossRef]
- Sooriyaarachchi, M.; Narendran, A.; Gailer, J. N-acetyl-L-cysteine modulates the metabolism of cis-platin in human plasma in vitro. Metallomics 2013, 5, 197–207. [Google Scholar] [CrossRef]
- Sooriyaarachchi, M.; Narendran, A.; Gailer, J. The effect of sodium thiosulfate on the metabolism of cis-platin in human plasma in vitro. Metallomics 2012, 4, 960–967. [Google Scholar] [CrossRef]
- Sooriyaarachchi, M.; George, G.N.; Pickering, I.J.; Narendran, A.; Gailer, J. Tuning the metabolism of the anticancer drug cisplatin with chemoprotective agents to improve its safety and efficacy. Metallomics 2016, 8, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Harper, B.W.J.; Morris, T.T.; Gailer, J.; Aldrich-Wright, J.R. Probing the interaction of bisintercalating (2,2’:6’2ʺ)platinum(II) complexes with glutathione and rabbit plasma. J. Inorg. Biochem. 2016, 163, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Sarpong-Kumankomah, S.; Egorov, A.; Gailer, J. Sample preparation of blood plasma enables baseline separation of iron metalloproteins by SEC-GFAAS. J. Chromatogr. B 2020, 1147, 122147. [Google Scholar] [CrossRef] [PubMed]
- Corte Rodriguez, M.; Alvarez-Fernandez Garcia, R.; Blanco, E.; Bettmer, J.; Montes-Bayon, M. Quantitative evaluation of cisplatin uptake in sensitive and resistant individual cells by single-cell ICP-MS (sc-ICP-MS). Anal. Chem. 2017, 89, 11491–11497. [Google Scholar] [CrossRef]
- Temaj, G.; Chichiarelli, S.; Eufemi, M.; Altieri, F.; Hadziselimovic, R.; Farooki, A.A.; Yaylim, I.; Saso, L. Ribosome-directed therapies in cancer. Biomedicines 2022, 10, 2088. [Google Scholar] [CrossRef]
- He, L.; Chen, T.; You, Y.; Hu, H.; Zheng, W.; Kwong, W.-L.; Zou, T.; Che, C.-M. A cabcer-targeted nanosystem for delivery of Gold(III) complexes: Enhanced selectivity and apoptosis-inducing efficacy of a gold (III) porphyrin complex. Angew. Chem. Int. Ed. 2014, 53, 12532–12536. [Google Scholar]
- Soignet, S.L.; Maslak, P.; Wang, Z.-G.; Jhanwar, S.; Calleja, E.; Dardashti, L.J.; Corso, D.; DeBlasio, A.; Gabrilove, J.; Scheinberg, D.A.; et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N. Engl. J. Med. 1998, 339, 1341–1348. [Google Scholar] [CrossRef]
- Gierasch, L.M.; Gershenson, A. Post-reductionist protein science, or putting Humpty Dumpty back together again. Nat. Chem. Biol. 2009, 5, 774–777. [Google Scholar] [CrossRef]
- Mani, U.; Prasad, A.K.; Kumar, V.S.; Lal, K.; Kanojia, R.; Chaudhari, B.P.; Murthy, R.C. Effect of fly ash on biochemical and histomorphological changes in rat liver. Ecotoxicol. Environ. Saf. 2007, 68, 126–133. [Google Scholar] [CrossRef]
- Puris, E.; Gynther, M.; Auriola, S.; Huttunen, K.M. L-type amino acid transporter I as a target for drug delivery. Pharm. Res. 2020, 37, 88. [Google Scholar] [CrossRef]
- Xiong, X.; Liu, L.-Y.; Mao, Z.-W.; Zou, T. Approaches towards understanding the mechanism-of-action of metallodrugs. Coord. Chem. Rev. 2022, 453, 214311. [Google Scholar] [CrossRef]
- Morris, T.T.; Ruan, Y.; Lewis, V.A.; Narendran, A.; Gailer, J. Fortification of blood plasma from cancer patients with human serum albumin decreases the concentration of cis-platin derived toxic hydrolysis products in vitro. Metallomics 2014, 6, 2034–2041. [Google Scholar] [CrossRef] [PubMed]
- Sooriyaarachchi, M.; Wedding, J.A.; Harris, H.H.; Gailer, J. Simultaneous observation of the metabolism of cisplatin and NAMI-A in human plasma in vitro by SEC-ICP-AES. Metallomics 2014, 19, 1049–1053. [Google Scholar] [CrossRef] [PubMed]
- Au, F.; Bielecki, A.; Blais, E.; Fisher, M.; Cakmak, S.; Basak, A.; Gomes, J.; Arbuckle, T.E.; Fraser, W.D.; Vincent, R.; et al. Blood metal levels and third trimester maternal plasma matrix metalloproteinases (MMPs). Chemosphere 2016, 159, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef]
- Orr, S.E.; Bridges, C.C. Chronic kideny disease and exposure to nephrotoxic metals. Int. J. Mol. Sci. 2017, 18, 1039. [Google Scholar] [CrossRef] [PubMed]
- Nogara, P.A.; Oliveira, C.S.; Schmitz, G.L.; Piquini, P.C.; Farina, M.; Aschner, M.; Rocha, J.B.T. Methylmercury’s chemistry: From the environment to the mammalian brain. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129284. [Google Scholar] [CrossRef]
- Saha, A.L.; Kumar, V.; Tiwari, J.; Rawat, S.; Singh, J.; Bauddh, K. Electronic waste and their leachates impact on human health and environment: Global ecological threat and management. Environ. Technol. Innov. 2021, 24, 102049. [Google Scholar]
- Bjorklund, G.; Tinkov, A.A.; Dadar, M.; Rahman, M.M.; Chirumbolo, S.; Skalny, A.V.; Skalnaya, M.G.; Haley, B.E.; Ajsuvakova, O.P.; Aaseth, J. Insights into the potential role of mercury in Alzheimer’s disease. J. Mol. Neurosci. 2019, 67, 511–533. [Google Scholar] [CrossRef]
- Chapman, L.; Chan, H.M. The influence of nutrition on methyl mercury intoxication. Environ. Health Perspect. 2000, 108, 29–56. [Google Scholar]
- Rossignol, D.A.; Genius, S.J.; Frye, R.E. Environmental toxicants and autism spetrum disorders: A systematic review. Transl. Psychiatry 2014, 4, e360. [Google Scholar] [CrossRef] [PubMed]

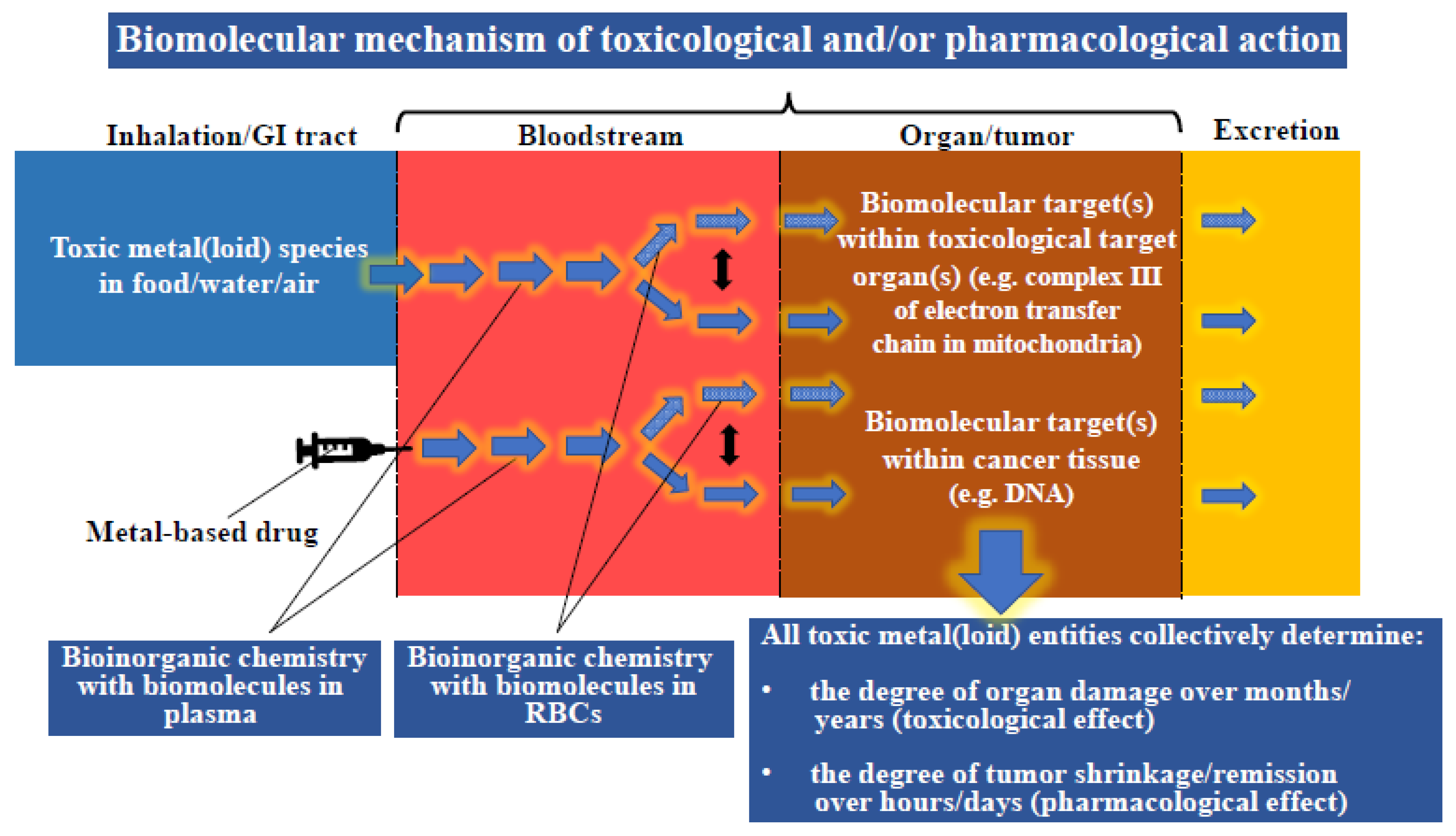
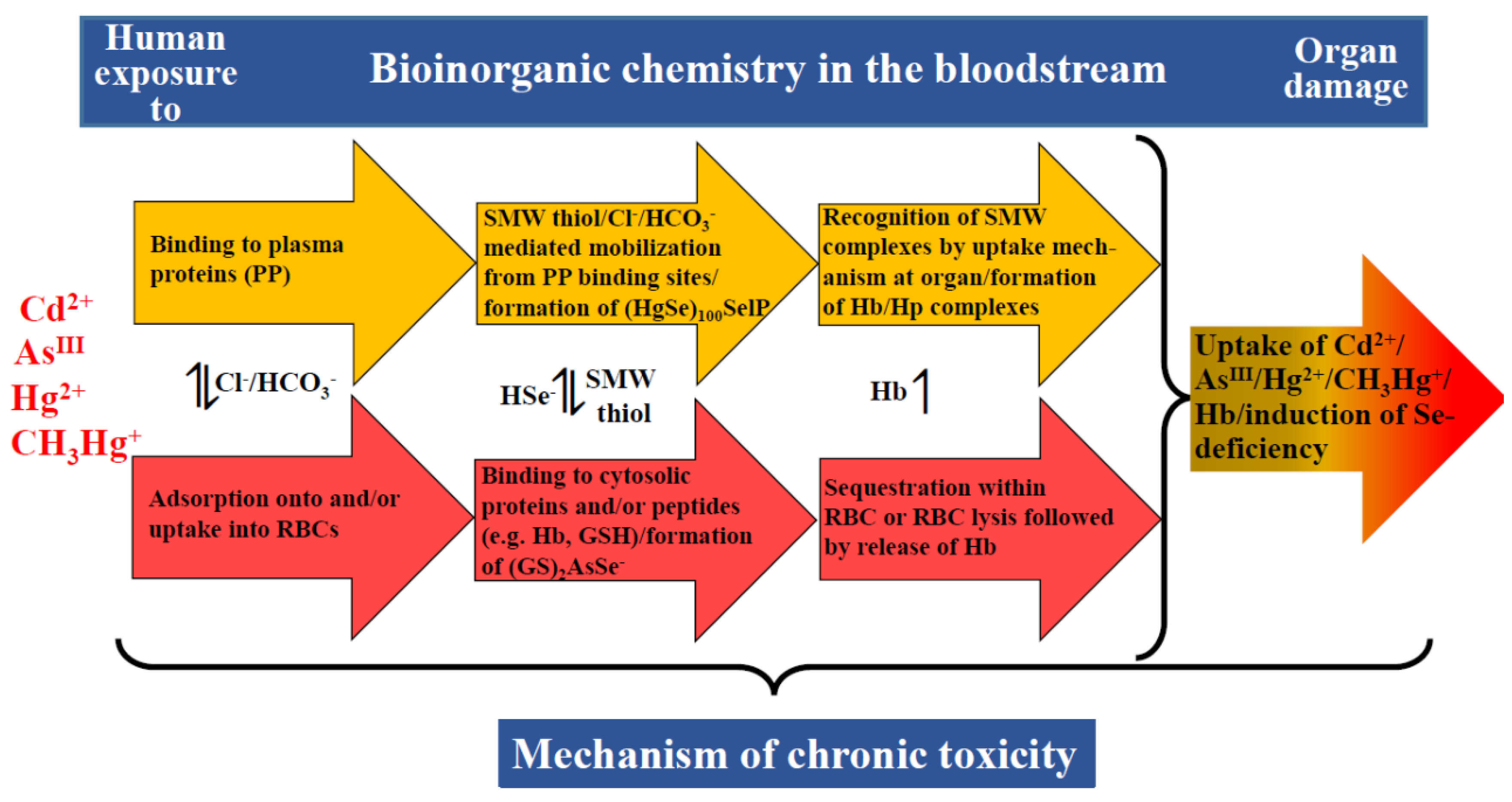
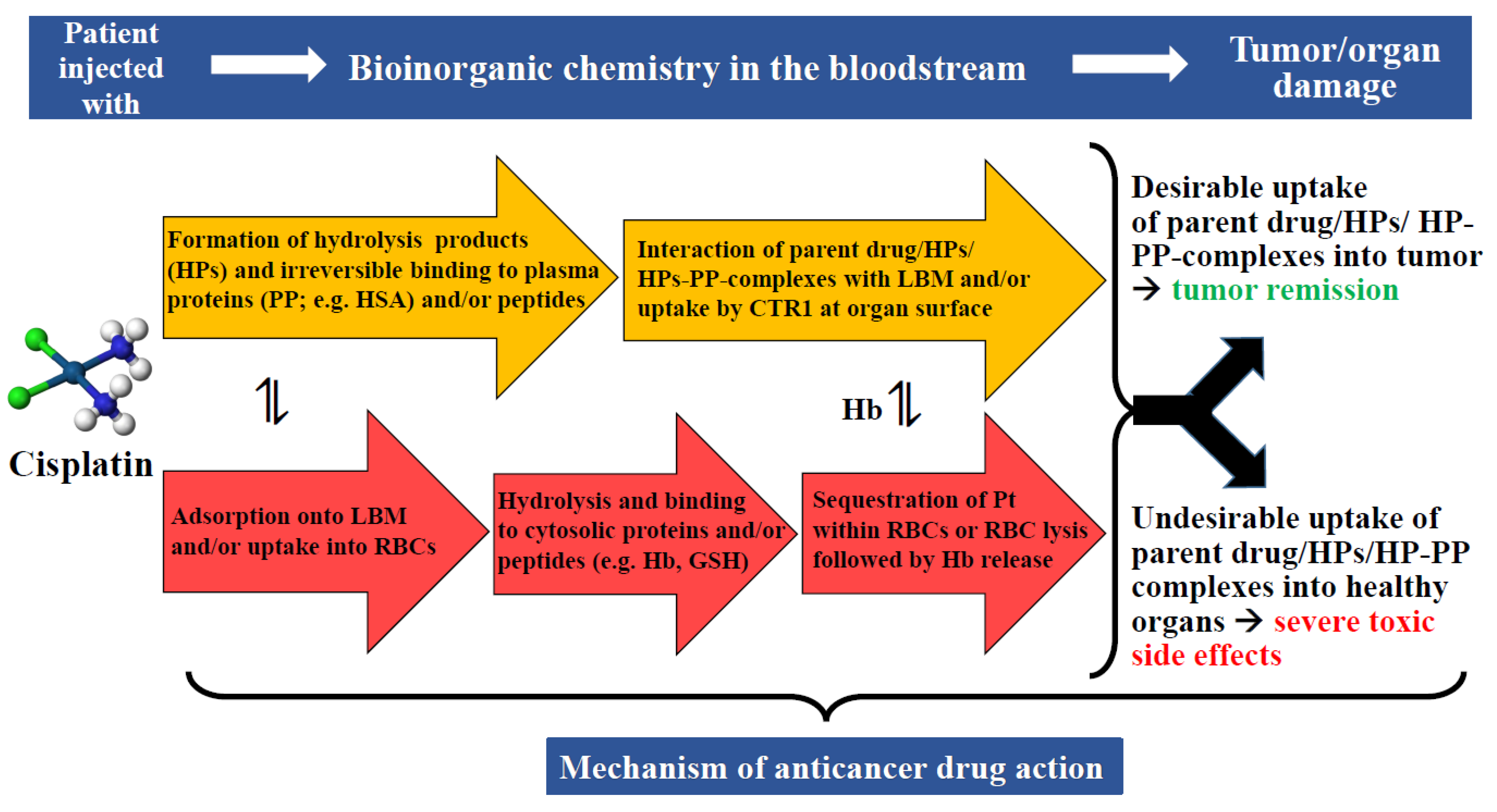
| Metal | Metalloprotein or Biomolecules which Contain Bound Metal(s) | Molecular Mass (kDa) | Number of Metal Atoms Bound per Protein | Reference |
|---|---|---|---|---|
| Fe | Ferritin | 450 | <4500 | [94] |
| Transferrin | 79.9 | 1 | [94] | |
| Haptoglobin–Hemoglobin complex | 86–900 | 2 | [99] | |
| Cu | Blood coagulation factor V | 330 | 1 | [94] |
| Transcuprein | 270 | 0.5 | [94] | |
| Ceruloplasmin | 132 | 6 | [94] | |
| Albumin | 66 | 1 | [94] | |
| Extracellular Superoxide Dismutase | 165 | 4 | [94] | |
| Peptides and amino acids | <5 | - | [94] | |
| Zn | α2 macroglobulin | 725 | 5 | [94] |
| Albumin | 66 | 1 | [94] | |
| Extracellular Superoxide Dismutase | 165 | 4 | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doroudian, M.; Gailer, J. Integrative Metallomics Studies of Toxic Metal(loid) Substances at the Blood Plasma–Red Blood Cell–Organ/Tumor Nexus. Inorganics 2022, 10, 200. https://doi.org/10.3390/inorganics10110200
Doroudian M, Gailer J. Integrative Metallomics Studies of Toxic Metal(loid) Substances at the Blood Plasma–Red Blood Cell–Organ/Tumor Nexus. Inorganics. 2022; 10(11):200. https://doi.org/10.3390/inorganics10110200
Chicago/Turabian StyleDoroudian, Maryam, and Jürgen Gailer. 2022. "Integrative Metallomics Studies of Toxic Metal(loid) Substances at the Blood Plasma–Red Blood Cell–Organ/Tumor Nexus" Inorganics 10, no. 11: 200. https://doi.org/10.3390/inorganics10110200
APA StyleDoroudian, M., & Gailer, J. (2022). Integrative Metallomics Studies of Toxic Metal(loid) Substances at the Blood Plasma–Red Blood Cell–Organ/Tumor Nexus. Inorganics, 10(11), 200. https://doi.org/10.3390/inorganics10110200







