1. Introduction
Silicon carbide has been used for a variety of applications and proposed for many others. Examples include high temperature heat exchangers, structural components of nuclear fission or fusion reactors, abrasives, automobile components such as break disks, body armor, gas turbine components, high temperature catalysis support, and structural components for highly corrosive and/or extremely high temperature environments [
1,
2,
3,
4]. Silicon carbide is an attractive material due to its superior properties when compared to other ceramics. Properties such as its high hardness, superior strength and toughness, high melting point of ~2700 °C, low density (3.2 g/cm
3), high thermal conductivity (300–400 W/mK at room temperature, which is comparable to some metals), thermal shock resistance, diffusion coefficient that is negligible below 1800 °C, and corrosion resistance, all make it a very attractive material option for structural applications [
1,
5,
6]. However, for some of the same characteristics, silicon carbide is one of the most difficult ceramics to fabricate and sinter into desirably shaped components. To sinter silicon carbide conventionally, temperatures above 1800 °C combined with pressures above 50 MPa as well as some sintering additives (B, C, Al
2O
3) up to 10 wt% are necessary [
6,
7,
8]. The difficulty in processing has limited the use of silicon carbide for decades. To overcome this, many techniques such as reaction bonding [
9], liquid phase sintering [
10], chemical vapor deposition [
11], and spark plasma sintering [
12] have been developed for a variety of applications. However, most still remain very costly and are only considered when economically viable.
Formation of high temperature and advanced silicon based non-oxide ceramics can be also be achieved through the use of organometallic preceramic polymers [
13]. An alternative approach for simple low temperature fabrication of SiC is to use a preceramic polymer such as polycarbosilane to form a matrix for SiC powders and has been shown to successfully consolidate SiC powders into rectangular pellets at relatively low temperatures between 1000 °C and 1400 °C without pressure [
14]. In this process which entails heating in an inert atmosphere, polycarbosilane crosslinks, undergoes pyrolysis, and converts from the organic polymer to inorganic amorphous SiC, which may then crystallize depending on the final sintering temperature [
15,
16,
17,
18,
19]. This process is very similar to how some SiC/SiC composites are manufactured with the difference that here the matrix volume fraction is less than 5%. This method has recently gained more attention for its ability to be used for additive manufacturing [
20,
21,
22]. One of the most widely used of SiC preceramics is allylhydridopolycarbosilane (AHPCS) that possess higher densification efficiency, superior rheological characteristics, greater mass yield (70 to 75%), and produce near-stoichiometric SiC [
23,
24,
25]. Extensive work in literature has utilized AHPCS for a variety of applications such as making SiC components or bonding them [
26,
27,
28]. Others have shown the application of similar preceramics to form SiC coatings [
29]. Finally, and most recently, work on additive manufacturing of SiC-filled preceramics has gained significant attention. Despite the relatively extensive prior work in the literature, few studies have investigated the basic materials processing property relationships and detailed compositional analysis of such systems. In addition, a unifying name for the process does not exist. Here, we propose the term pyrolysis bonded silicon carbide (PBSC) as a parallel to the well-known and investigated reaction bonded silicon carbide (RBSC). Pyrolysis bonding of silicon carbide is a near net shape process, which is advantageous for manufacturing purposes. Moreover, the ability to produce complex shapes and the low processing temperature (~850 °C) allows the possibility for a variety of processing techniques for simple, low-cost production of structural components.
We have previously shown that the mechanical properties of PBSC such as hardness and fracture strength decrease by approximately 30% and 20%, respectively, when the material is exposed to temperatures above 1500 °C, which given the high temperature stability of SiC, should not occur [
30]. The decrease in mechanical properties has been linked to a mass loss of about 5 to 6%, which has been observed around the same temperature through thermogravimetric analysis. Furthermore, thermogravimetric analysis of the stating powders have shown a mass loss of a few percent. The mass loss observed through thermogravimetric analysis and the degradation of Vickers hardness and fracture strength from the previous study is summarized in
Figure 1. One of the main goals of this work is to analyze compositional and mechanical changes of the PBSC material as it is exposed to higher temperatures, and shed light on the origins of this phenomenon. Furthermore, possible methods to eliminate this degradation and to improve the mechanical properties of the material by pre-treatments of the starting powders are investigated.
2. Experimental Procedure
As described in the previous publication [
30], a bimodal mixture of starting powders was used in order to enhance the final density of the PBSC material. However, the sample preparation here is further simplified and has shown no negative effects on the final pellets. In brief, 99.8% β-SiC powder of nominal sizes 1 µm (Alfa Aesar, Ward Hill, MA, USA) and 16.9 µm (Superior Graphite, Chicago, IL, USA) were mixed with 10 wt% of the SiC preceramic polymer, AHPCS (SMP-10, Starfire Systems Inc., Schenectady, NY, USA). The particle size distribution of the starting powders can be found in our previous study [
30]. Approximately 1 gram of n-Hexane 99+% (Acros Organics, NJ, USA) was added per gram of mixed powders as a solvent. Hexane is a compatible solvent for AHPCS recommended by the manufacturer and its primary purpose here is for dilution and to facilitate homogeneous mixing of the polymer with the starting powders. The slurry was then manually shaken for 10 minutes in a comminution vial after which it was dried and the mixture was uniaxially pressed into pellets at 200 MPa using a 7 mm die. The pellets after pressing look very similar to the fired pellet shown in figure in
Section 3.1. The pellets were then placed on an alumina tray with sacrificial SiC powder and fired in a tube furnace (CM Furnaces Inc., Bloomfield, NJ, USA) with flowing argon (10 standard cubic foot per hour, SCFH). The following heating schedule, recommended by the manufacturer for one step direct pyrolysis, was used: room temperature to 250 °C (2 °C/min), 250 °C to 650 °C (1 °C/min), 650 °C to maximum temperature of 850 °C (3 °C/min), held for 1 hour, and cooled to room temperature (5 °C/min).
The compositional changes of the material were investigated as it was exposed to higher temperatures, with one pellet cut into 4 quadrants to minimize sample to sample variation effects. Three pieces were annealed at different temperatures (1000, 1100, and 1200 °C) inside a tube furnace under a vacuum of ~200 mTorr for 1 hour and one piece was left untreated as the control. The control sample is referred to as the 850 °C sample in this work. Since temperatures above 1200 °C were not possible under vacuum due to furnace limitations, another pellet was heat treated in a tube furnace at 1500 °C for 1 hour under flowing argon (10 SCFH) to mimic similar non-oxidative environment. Relative densities were calculated by dividing the density of the pellets by the theoretical density of 3.2 g/cm3 for SiC.
To investigate strategies to improve the mechanical properties of PBSC and eliminate mechanical degradation when exposed to higher temperatures, powders were treated prior to the pellet synthesis. Heat treatment (above 1500 °C) under an inert atmosphere, acid treatment (using hydrofluoric acid) to remove the native oxide layer, and a combination of both were investigated. Ten pellets were synthesized as control to increase statistical certainty in standard PBSC samples and 6 pellets were synthesized for each treatment group using powders that were heat treated, acid treated, or heat treated and then acid treated. For the powder heat treatment, the fine and the coarse powders were put in two separate alumina boats and heat treated simultaneously with flowing argon atmosphere (10 SCFH) for 1 h at 1550 °C with 200 °C/h ramp rates. The acid etching of powders was done using a 10% HF solution. While many different methods can be used for removing the native oxide, HF is the most commonly used, simplest, and easily controlled for treating powders. A 50:50 w/w mixture of fine and coarse SiC powder was added to 75 mL of the HF solution. The mixture was manually stirred for 30 seconds and left to settle for 5 minutes. The HF solution was then poured out and the solution was diluted, resettled, and finally centrifuged to recover as much of the powder as possible. The resulting powder was then dried under a nitrogen atmosphere and used to make pellets, as described above. All drying processes were done under nitrogen flow to prevent re-oxidation of the powders.
Transmission x-ray diffraction patterns were collected at the x-ray powder diffraction (XPD) beamline, 28-ID-2, at the National Synchrotron Light Source-II (NSLS II). Patterns were collected in transmission made with X-ray wavelength of 0.1852 Å (66.9 keV). The samples had a thickness of ~1 mm and were rotated during collection to simultaneously probe and average the data from different areas of the samples as well as many grains to obtain powder patterns. The 2D XRD patterns were integrated and reduced to a 1D pattern using IGOR Irena [
31] and Nika [
32] software packages. A LaB
6 powder was used as a calibration standard for the XRD data analysis. The MDI JADE X-ray diffraction database was used to identify the crystal structures and phases present in the samples. Samples were later cut and the cut surfaces were polished down to ~100 nm roughness using diamond lapping films to analyze the microstructure of the material inside the bulk of the pellets, rather than their as-synthesized surfaces. The morphological changes of the samples were analyzed using a JOEL 7600F SEM operated at 5 kV. A Renishaw inVia Raman Microscope with a 532 nm diode laser was also used to collect spectra from the polished surfaces.
In order to understand the degradation of the mechanical properties at higher temperatures, nano-indentation was used to probe the local mechanical properties and to resolve the mechanical properties of the individual components of the composite. Temperature treated samples were cut and polished down to ~100 nm roughness and the nano-indentations were done by Nanoscience Instruments (Phoenix, AZ, USA) using an iNano nanoindenter. Arrays of one hundred indentations were done on the 850 °C and the 1500 °C samples, after which, scanning electron microscopy was used to identify the location of the indents.
The Vickers hardness was measured with a conventional Vickers indentation method (Buehler MicroMet 3, Lake Bluff, IL, USA) using a load of 1 kg and applying Equation (1):
where
F is the load (kg) and
d is the average length (millimeters) of the indentation diagonals. Each pellet was indented five times. All samples were then heat treated to 1500 °C under flowing argon and the measurements were repeated.
3. Results and Discussion
The diffraction patterns from the PBSC samples treated at 850, 1000, 1100, 1200, and 1500 °C are shown in
Figure 2. In all samples, only three crystalline phases were identified. The material is composed of β-SiC with a small fraction of α-SiC and some graphite. It is noteworthy that previous XRD studies using benchtop XRD instruments were only able to detect the β-SiC phase [
30,
33,
34]. Using the software Match!—Phase Identification from Powder Diffraction (Crystal Impact GbR, Bonn, Germany), phase weight fractions for the 850 °C samples were calculated to be β-SiC (90.5%), α-SiC (8.8%), and graphite (0.7%). The same calculations for the 1500 °C samples revealed β-SiC (92.1%), α-SiC (7.1%), and graphite (0.9%) as phases present. Overall, the XRD results show a relatively high level of purity and less than 1 wt% carbon impurities which is a major advantage of PBSC. However, the small changes in the phase fractions are within error and not significant enough to be responsible for the 5 to 6% mass loss and the degradation of mechanical properties summarized in
Figure 1. The crystalline components of the material remain unchanged by high temperature exposure, with the exception of some conversion of the α-SiC to β-SiC due to the known phase transition below 2000 °C [
35,
36,
37]. However, a clear reduction in the background of the data with increased temperature can be observed in
Figure 2. Since the patterns are not normalized, the decreased background is indicative of the loss of amorphous contents in the material.
The SEM analysis of all samples showed similar microstructure with large grains from the ~16 µm starting powder surrounded by small grains from the ~1 µm starting powder. The microstructure of the material is such that the large grains are surrounded by a matrix of small grains and the polymer derived SiC that bonds the particles together. Thus, in this discussion the areas with the small grains and polymer derived SiC will be referred to as the
matrix and the large SiC particles as
large grains. As expected, the most noticeable difference was observed in the 1500 °C sample.
Figure 3 shows the SEM images of the “as synthesized” control sample (850 °C) and the sample that was heat treated at 1500 °C. The 850 °C sample appears denser and there are few scattered pores such as the bright area in the upper right corner of the image. Furthermore, the particles in the matrix area of the surface are polished and at the same level of the large grain surfaces. In contrast, the 1500 °C sample appears rough in the matrix areas. The large grains are seen to be polished while the matrix is not. This finding supports two conclusions; (1) the 5% mass loss appears to occur in the matrix regions and (2) the degradation of mechanical properties in the matrix (due to mass loss, crystallization, etc.) has led to particle pull out from the matrix regions during the polishing process.
In a previous study, it was hypothesized that the mass loss was due to carbothermal reduction of amorphous species [
29]. Since the mass loss is measured to be at least 5% and the XRD data in
Figure 2 does not show any significant changes other than a reduction in the background, it can be concluded that the amorphous assumption is correct. Furthermore, in the 1200 °C samples, SEM images revealed the growth of whiskers as shown in
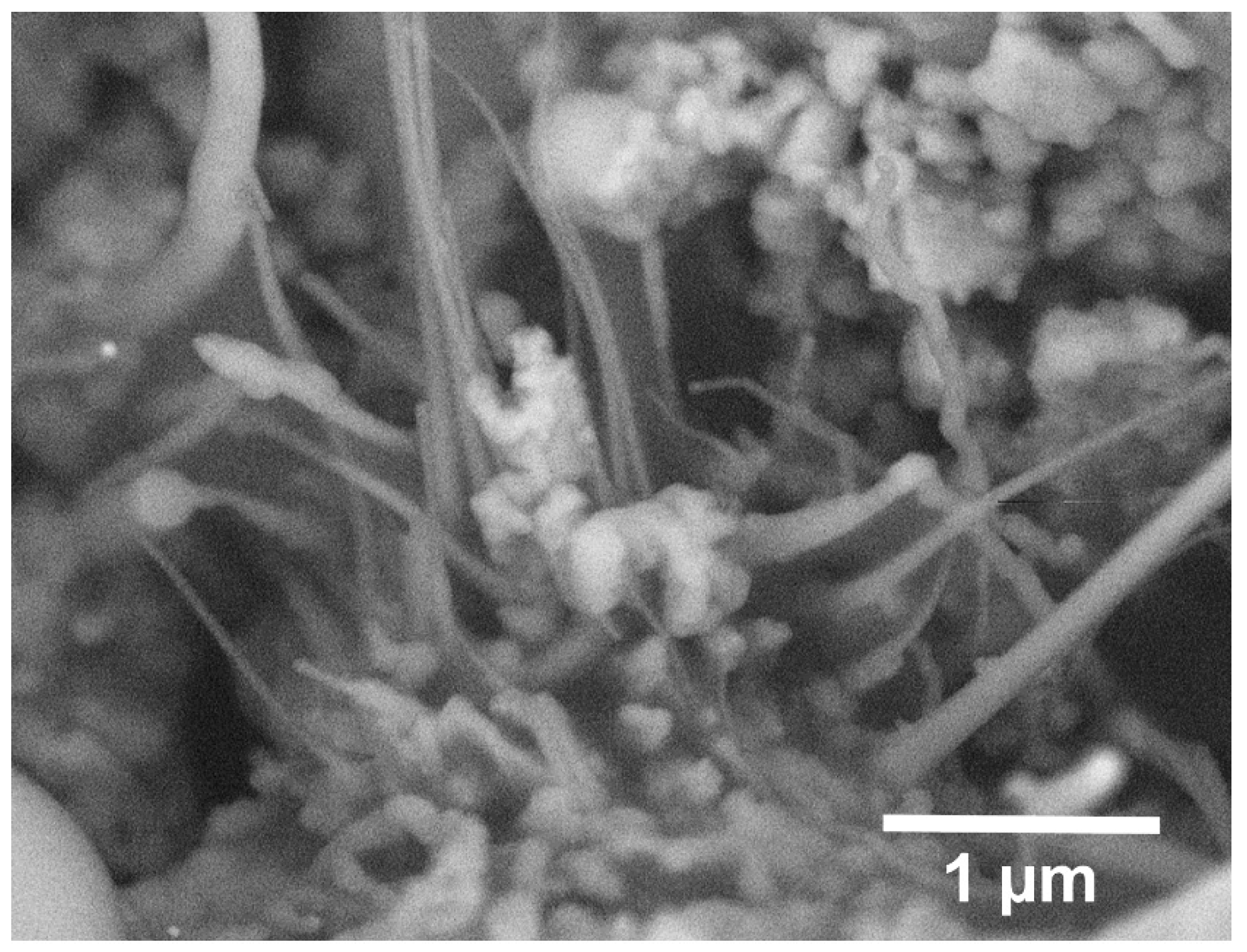
Figure 4 inside the pores throughout the bulk. These whiskers are most likely composed of SiC and the result of a carbothermal reduction process that is well documented in literature at temperatures ranging from 1200 to 1450 °C [
38,
39,
40,
41]. While the phase identity of these whiskers are not directly confirmed here and is beyond the scope of this paper, given the chemical constituents of the system, temperatures and PO2 levels experienced, as well as the fact that no other phase was detected in the detailed synchrotron XRD analysis of these samples, we can make this conclusion with a fairly good level of confidence. The necessary conditions are pores, silica (the native oxide of the powders), and carbon (remnant from the preceramic polymer), all of which are present in this system. Yao et al. describe the mechanism with the following chemical reactions [
39]:
With the net reaction as follows:
Raman spectra of the samples are shown in
Figure 5. A straight line background subtraction was performed on the spectra. SiC has a peak reported at ~795 cm
−1 and is attributed to the Si-C transverse optical (TO) mode [
42]. There are also peaks at ~1315 cm
−1 for disordered carbon (D) and at ~1600 cm
−1 for ordered graphite (G) [
42,
43]. The spectra collected from the large grain areas remain unchanged with different treatment temperatures. However, the D and the G peaks of the matrix areas show a gradual decrease with increasing temperature and disappear in the 1500 °C sample. This supports the hypothesis that the mass loss is due to the loss of mostly free remnant carbon from the preceramic polymer in the matrix areas. Rahman et al. and Zunjarrao et al. investigated fully AHPCS derived SiC bodies, observed the mass loss of pyrolyzed AHPCS in the above 1200 °C regime, and attributed it to the loss of silicon oxycarbides (SiO
xC
y) or just oxygen, respectively [
44,
45]. It is important to note that analysis of the mass loss in this temperature regime was not extensive in either of the studies. However, given the results here, and that Moraes found the carbon to silicon ratio after pyrolysis to be 1.13, attribution of the mass loss to remnant amorphous carbon is more plausible [
46].
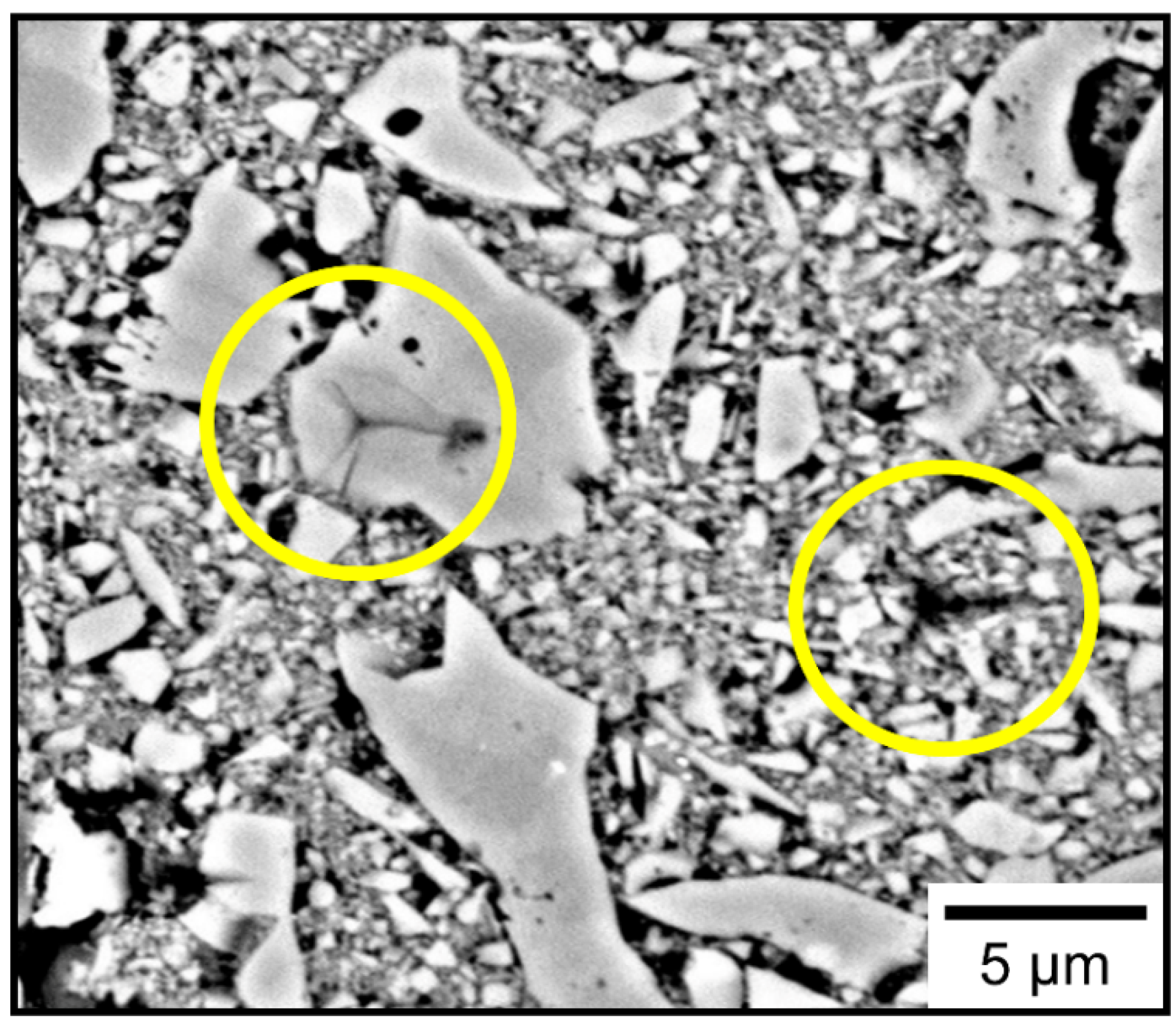
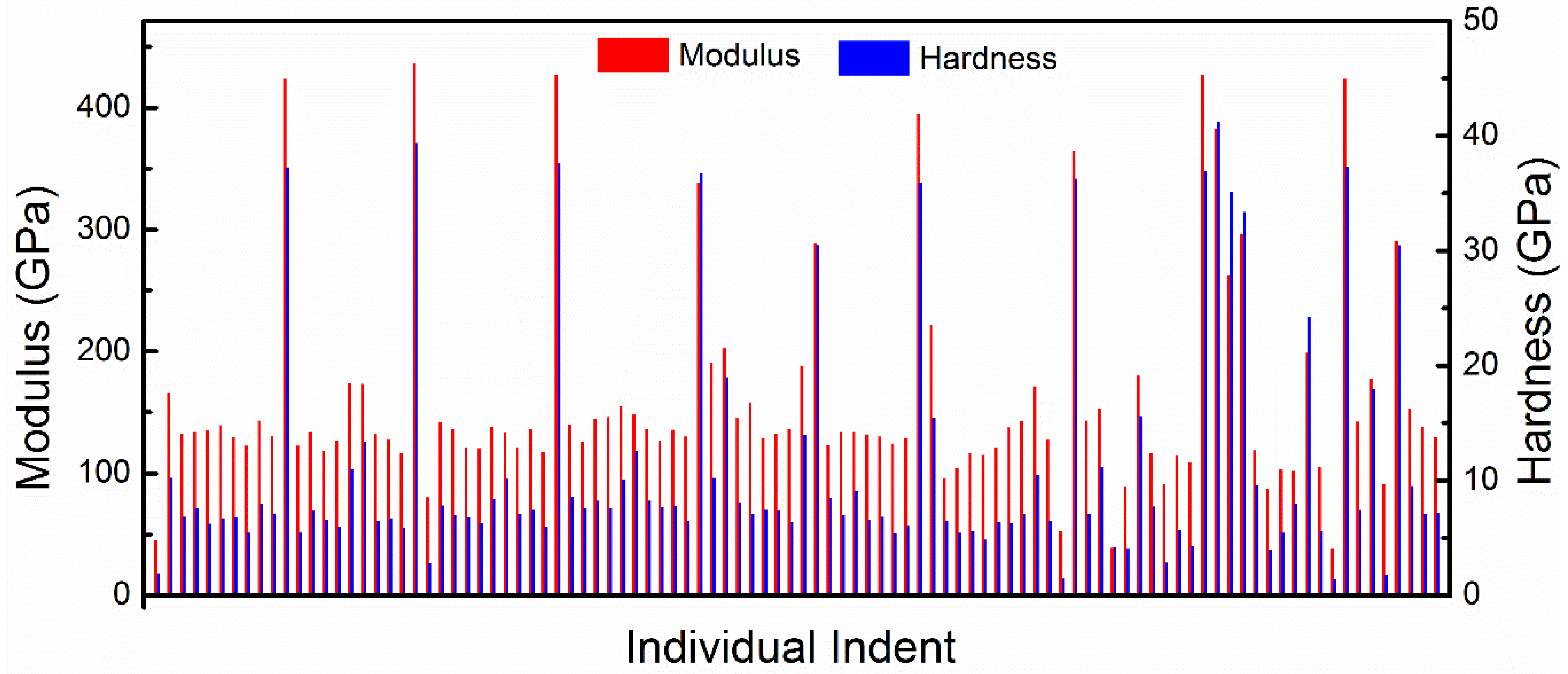
Nanoindentation measurements were able to probe the local mechanical properties of the large grains and the matrix. A sample SEM image of the array of the indentations is shown in
Figure 6. Each indentation was imaged and was linked to their corresponding data. As expected, the indents on the large single grains showed much higher hardness and modulus while the indents in the matrix showed values that were about an order of magnitude lower. The data for the 850 °C sample is shown in
Figure 7. This clear bimodal distribution in the data allowed for separation and analysis of measurements for the large grains and the matrix for hundreds of indentations, without the need to identify each indent through SEM. The data for the 850 °C and the 1500 °C samples were analyzed by separating the two distributions. Some individual indentation data appeared to be statistical outliers in either of the two distributions, which correspond to indents that were either at the edge of the large grains or encountered a large grain below the surface half way through the indent. The software Minitab was used to separate the data, remove the outliers, and the results are summarized in
Table 1. Standard deviation is reported as error.
The elastic modulus for dense silicon carbide ranges from 390 to 450 GPa and its nano-hardness measures from 32 to 40 GPa in the literature [
1,
6]. The data for the large grains is in good agreement with dense SiC as expected. The matrix hardness values are 7.5 and 5.4 for the 850 °C and the 1500 °C samples, respectively. These values are slightly higher than the previously measured Vickers hardness of PBSC which is about 5.3 GPa when synthesized at 930 °C and 3.3 GPa when exposed to 1500 °C [
30]. The slight increase is expected since nanoindentation generally shows higher values than micro hardness through Vickers indentation [
6]. Nanoindentaion of fully AHPCS derived SiC has been done in literature by Rahman et al. and more extensively by Zunjarrao et al. [
44,
45]. The hardness decreased while the modulus remained fairly unaffected at temperatures above 1200 °C which is in agreement with the results here [
45]. Literature comparison with Vickers hardness will be discussed later. The degradation of hardness (28% decrease), when exposed to 1500 °C, is in agreement with what was previously observed [
30]. The hardness values of the large grains also show a decrease of 22%. However, this value may not be correct since the large grains are measured while embedded in a matrix that is now ~30% weaker and the decrease may be far exaggerated. The deconvolution and full resolution of the mechanical properties of this mixture is beyond the scope of this paper. However, the hypothesis that the degradation of the mechanical properties is due to a weaker matrix holds. It must be noted that the degree of crystallinity (increased with higher temperatures) affects the mechanical properties. However, Rahman et al. concluded the mechanical properties are enhanced with increasing crystallinity [
44]. Therefore, the degradation investigated here which is well linked to the mass loss is not likely to have resulted from crystallinity but from the loss of free carbon remnant from the preceramic polymer is further supported by the data.
3.1. Effect of Powder Pre-Treatment
The final part of this work investigated the effect of prior treatment of the starting powders (such as heat treatment, etching and removal of the oxide layer, or both) on the mechanical properties of PBSC. The mixture prepared with the etched powders (after the drying of hexane) was less viscous than conventional green body mixtures (dough-like consistency as opposed to conventional ceramic powders with binder additives). It is believed that the altered rheology is the result of drastically different polymer-SiO
2 versus polymer-SiC surface interactions. The lower viscosity prevented pressing pellets using the etched powders as the mixtures would ooze out or discharge from the die at 200 MPa. Consequently, for those pellets, very small pressures on the order of 10 MPa were used to give the desired shape of the pellets. The other two conditions, (heat treated/heat treated and later HF treated) showed no noticeable differences in the processing other than some degree of agglomeration in the powders. The control, heat treated, and heat and HF treated pellets all showed similar final average relative densities ranging from 76 to 79% with standard deviations < 2% (theoretical density of 3.21 g/cm
3 as 100%). However, the HF-only treated samples had an average density of 85.6% with 3% standard deviation. This ~6% increase in relative density is a major improvement for the low temperature pressure-less pyrolysis bonding process. The increased density may be attributed to two factors. The enhanced flowability of the mixture may improve the packing of the particles in the green state [
47]. Conversely, the removal of SiO
2, which has a theoretical density of 2.1 g/cm
3, lower than the 3.2 g/cm
3 of SiC, may play a role. For example, a 1 µm spherical particle with 30 nm oxide layer, which is not out of range for SiC [
48], would result in about 3% lower relative density. This inherent difference rapidly increases with thicker oxide layer or non-spherical particles with increased surface/volume ratio which is the case for SiC powders.
Figure 8 shows the Vickers hardness measurements of the control pellets as well as of those synthesized using pretreated powders. It must be noted that the bimodal effect seen in the nanoindentation results is not observed here because the Vickers indents are sufficiently large that they incorporate both the large grains and the matrix yielding an overall bulk property. The control pellets show hardness values of 4.59 GPa in agreement with the previous study [
30]. In comparison, cold isostatic pressed samples that were conventionally sintered at 2150 °C with similar porosity of 20% are shown to have a Vickers hardness of 7.4 GPa [
49]. Furthermore, the control samples exposed to 1500 °C also showed a lower value of 3.74 GPa again in agreement with previous measurements [
30]. The samples synthesized using the HF treated powder showed a very large increase to 9.37 GPa in hardness values. This value is in good agreement with 10 GPa, which has been reported for the conventionally sintered samples with 15% porosity and sintered at 2150 °C [
49]. The exponential decay of hardness as a function of porosity is described by Snead et al. with the following equation with a 7% uncertainty [
6].
here,
Hv is the Vickers hardness in GPa and
Vp is the volume fraction of porosity.
As mentioned earlier, the HF treated samples had a higher relative density compared to control samples. This corresponds to lower volume fraction of porosity (~14%), as opposed to 21 to 24% for the control, assuming that the lower than theoretical density is only due to porosity. Considering these hardness values, the hardness of the HF treated samples deviates less than 10% form the model, which is close to the anticipated 7% uncertainty. However, the control samples show more than 30% deviation than what the model (Equation (8)) predicts. This indicates that the hardness of the control samples is not only affected by the porosity, but also by a weaker grain to grain bonding, most likely as a result of the native oxide layers of the particles.
The pellets synthesized using heat treated and those using heat and HF treated powders showed no appreciable difference with the controls. The results from the heat and HF treated powders are not as expected but should, in theory, be similar to those of only HF treated powders. One possible hypothesis is that the agglomeration and coarsening observed in all of the heat treated powders, as earlier mentioned, is playing a role. It is possible that the agglomeration is inhibiting the HF solution from efficiently removing oxide layers since agglomerates were also observed after the etching process for the heat treated samples. Regardless, this observation requires further investigation to be fully understood. In all samples, the temperature exposure of 1500 °C showed a significant degradation in hardness values as well as ~5 to 6% mass loss, which was known and was the motivation behind this work. However, it is noteworthy that the pellets with HF treated powders were so much harder that even after the exposure to 1500 °C they still had hardness values of around 6.86 GPa. This value is significantly higher than the unexposed controls, which is a major improvement in the material. The enhanced performance is most likely due to the improved bonding of the polymer derived SiC with the clean oxide free surfaces of the SiC powders and slightly better density contributed to the green mixture rheology. It is important to note that HRTEM, EELS, XPS and other surface sensitive characterization would be necessarily to thoroughly quantify the extent and characteristics of the native oxide and verify its removal. However, both the presence of the native oxide (ranging from a few to tens of nanometers) and its complete removal by HF etching are well documented and accepted in the literature [
48,
50,
51]. The enhanced bonding is expected to improve other properties such as strength, toughness, and thermal conductivity and will need further investigation along with direct TEM evidence of bonding quality which is beyond the scope of this work. Since the removal of the oxide layer did not eliminate the mass loss or the degradation of mechanical properties after exposure to 1500 °C, it can be concluded that although the carbothermal reduction process of the native oxides is present, it is not the main mechanism responsible for the mass loss. The direct oxidation of the amorphous free carbon remnant from the preceramic polymer is the main cause of the mass loss. The free carbon scavenges oxygen from the native oxide layers or atmosphere, whichever is more favorable. In order to prevent the direct oxidation of free carbon the PO
2 levels in the processing furnaces must be below ~10
−16 atm as predicted by the Ellingham diagram at temperatures around 1500 °C, which is hard to achieve in alumina tubes. Therefore, it is concluded that to improve the high temperature stability of PBSC the issue of remnant free carbon must be addressed by improvements in the preceramic polymer.