1. Introduction
Nanocrystalline titanium dioxide is currently being used in the production of high-tech products of a wide range. The modifications of TiO
2 that meet the requirements for unassuming and scalable production include anatase, rutile, brookite, and TiO
2(B) (bronze phase). Each of these TiO
2 polymorphs is characterized by its own character of the structural packing of TiO
6 octahedra. This causes a difference in physicochemical properties determining the scopes of application. In particular, among other polymorphs, anatase and rutile have gained much attention as photocatalysts, whereas TiO
2(B) is considered as high-performance anode for metal-ion batteries [
1,
2,
3]. However, the band gap of TiO
2 is 3.0–3.3 eV, which limits its effectiveness. Indeed, anatase/rutile nanocomposite as a photocatalyst is able to absorb only ultraviolet light (i.e., less than 5% of solar radiation). In the case of TiO
2(B), this circumstance causes insufficient electronic conductivity as an anode material for batteries.
Against this background, non-stoichiometric modifications of TiO
2 containing intrinsic and/or impurity structural defects (so-called color centers) attract significant attention due to their improved optoelectronic properties [
4,
5]. Doping with impurities of the cationic or anionic type is one of the promising ways to create defects in TiO
2. At the same time, such an approach is not devoid of disadvantages and limitations. For example, the presence of impurities can change the conditions of structural-phase transformations in TiO
2 during heating (lowering the temperature of such transitions), cause particle growth and a decrease in the specific surface area, etc. On the other hand, the formation of structural defects in TiO
2 can be achieved without changing the chemical composition (i.e., by a non-doping method) due to heat treatment under specific conditions–namely under a certain atmosphere. In most cases, a reducing atmosphere (a mixture of inert gases with hydrogen) is used for these purposes, which leads to the production of so-called self-doped TiO
2 containing Ti
3+ centers [
6,
7,
8]. However, working with hydrogen is unsafe and energy-consuming, and requires special skills and specialized equipment. Another approach to creating defects in TiO
2, which does not involve obvious danger, is heat treatment under inert gas (most often argon), nitrogen, or in vacuum [
9,
10,
11,
12,
13]. It is important to know that in this case, the type of atmosphere in which the heat treatment takes place determines not only the degree of TiO
2 deficiency, but also the methodology of the procedure (temperature, time, etc.), which in turn determines the energy consumption of the process as a whole.
In the discussed vein, it should also be noted that defective forms of TiO
2 have attracted the interest as magnetic semiconductors due to the presence of ferromagnetism at room temperature. At the same time, if the magnetic properties of nonstoichiometric TiO
2 derivatives in the anatase and rutile phases are described in the literature in detail [
14,
15,
16], then no attention is paid to the creation of magnetically diluted semiconductors based on non-doped defective TiO
2(B).
In the present work, the possibility of creating structural defects in nano-belt-like TiO2(B) by vacuum heat treatment is studied. The nature of defect centers and optoelectronic properties of synthesized nonstoichiometric titanium dioxide with a bronze structure were studied via the electron paramagnetic resonance method and ultraviolet, visible, and near-infrared spectrophotometry. The magnetic properties of defective TiO2(B) and their dependence on temperature are estimated.
2. Results and Discussion
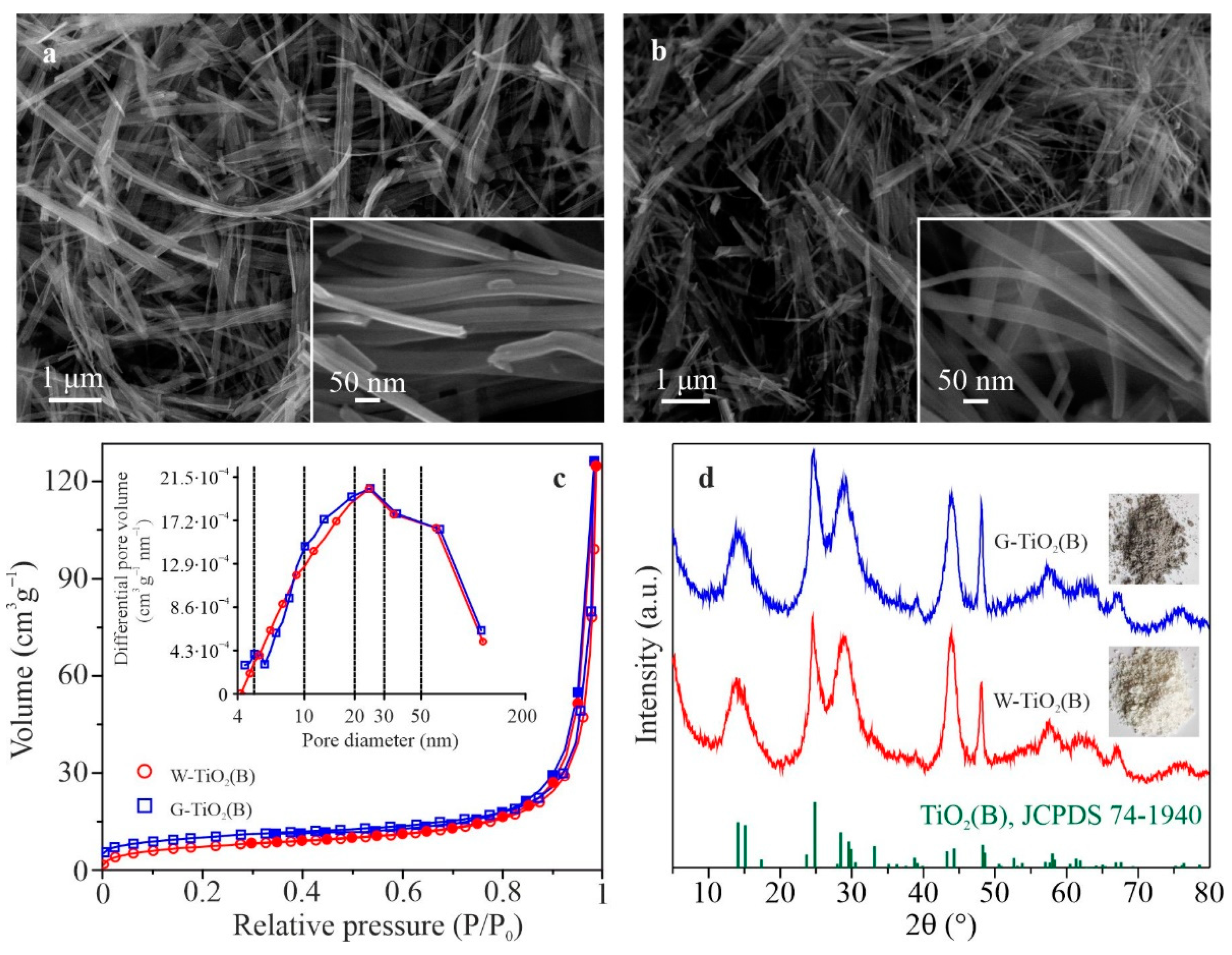
According to the scanning electron microscopy (SEM) studies (
Figure 1a,b), as-synthesized powdered materials have a similar morphology. Each sample consists of nanobelts with a width of 30–60 nm, thickness of 6–8 nm, and length of several micrometers. Low-temperature nitrogen adsorption/desorption isotherms of studied materials (
Figure 1b) with a hysteresis loop belong to the IV type (according to the IUPAC), indicating the capillary condensation in mesopores (size 2–50 nm). The hysteresis profile has the H1 type shape corresponding to cylindrical pores with open ends. The Brunauer-Emmett-Teller (BET) specific surface area of W-TiO
2(B) and G-TiO
2(B) samples is 26.4 and 36.1 m
2 g
–1, respectively. The Barrett-Joyner–Halenda (BJH) total pore volume of samples is about 0.19 cm
3 g
–1. The character of the pore size distribution curves (insert in
Figure 1c) indicates that mesopores predominate in both materials (at least 0.12 cm
3 g
–1 of the total pore volume).
Figure 1d shows X-ray powder diffraction (XRD) patterns and photo images of the appearance (corresponding inserts) for synthesized samples. The analysis of XRD data shows that phase composition of the products is presented by bronze titanium dioxide (JCPDS 74-1940) with a monoclinic unit cell (space group
C2/
m) having the parameters
a = 12.208 Å,
b = 3.749 Å,
c = 6.535 Å and
β = 107.36°. It should be noted that the XRD patterns of the W-TiO
2(B) and G-TiO
2(B) are close to each other, and hence their phase composition is determined by the conditions of hydrothermal synthesis and does not depend on the type of atmosphere in which the heat treatment is carried out.
Figure 2a represents the electron paramagnetic resonance (EPR) spectra of W-TiO
2(B) and G-TiO
2(B) recorded at –160 °C. The analyzed spectra contain an intense narrow symmetric component with
g = 2.0037(1), low-intensity asymmetric component with
g~4.32, and group of three nearby low-intensity resonances marked as “
a”, “
b” and “
c” (
Figure 2a, insert) with
g-factors of 2.063, 2.056, and 2.044, respectively. The
g-factor value of the main component in the EPR spectra is close to that of the electrons localized in oxygen vacancies of titanium dioxide (so-called
F-centers or color centers) [
17,
18]. On the other hand, absence or weak temperature dependence of integral intensity of the EPR signal are characteristic for spin resonance on conduction electrons [
19]. Taking into account these data, it can be assumed that in the studied samples, the wave function of conduction electrons makes the contribution on that of localized electrons (or vice versa). To deepen insight into this issue, it is necessary to perform EPR studies at lower (helium) temperatures. It should be noted that oxygen vacancies have a positive effect on the sodium storage performance of TiO
2, shortening the Na
+ diffusion path, facilitating the Na
+ diffusion, accelerating ion transport, and increasing electric conductivity [
20,
21]. At the same time, the concentration and size of defects should be taken into account for such materials. The
g-factors values of “
a”, “
b”, and “
c” resonances are close to that of Ȯ– and ȮH– radicals in titanium dioxide [
17]. The
g-factor of the asymmetric weak-field component in the spectra corresponds to ions having a 3d
5 configuration (i.e., Mn
2+ or Fe
3+), which are under the influence of crystal fields with a significant rhombic component [
22,
23,
24]. Taking into account the small width of this line, it should be considered as a resonance on trivalent iron ions (3d
5,
S = 5/2,
I = 0), which is present in precursors.
With an increase in temperature from –160 °C to room temperature, the
g-factor of the main component in the G-TiO
2 (B) EPR-spectrum increases to 2.0039(1). At the same time, its peak intensity, integral intensity, and width decrease by ~8%, ~20%, and 0.1 mT, respectively (
Figure 2b). For comparison, we note that the integral resonance intensity on Fe
3+ ions decreases by 2.5 times when the sample is heated from –160 °C to room temperature (
Figure 2b, insert).
With ultraviolet irradiation of G-TiO
2(B) performed at –160 °C, the peak intensity of the main component in the EPR spectrum increases with a saturating character at a constant width and the
g-factor (
Figure 2c). At the same time, a low-intensity resonance “
d” appears on the low-field wing of this component, the peak intensity of which increases slightly with increasing irradiation time. The contour of the difference between the spectra after and before UV irradiation has a two-component form, with the values of the
g-factors of the low- and high-field lines being 2.013 and 2.004, respectively (
Figure 2c, inset). The parameters of “
a”, “
b”, and “
c” components do not change during irradiation. The relaxation time of the EPR signal to its original form after irradiation (without reaching saturation of the main signal intensity) depends on both the time of UV exposure and temperature. For example, after 20 minutes of irradiation at –160 °C, the spectrum at the same temperature returns to its original form in ~10 hours, and at 20 °C, relaxation is completed after 0.5 hours.
According to literature [
25,
26,
27,
28,
29,
30,
31], the EPR signal relaxation taking a long time to return to its original shape after the cessation of UV irradiation (about a minute) is a sign of the existence of an excited metastable paramagnetic state, which is populated through other spin states with higher energy. Direct excitation or radiation from this state, in the first approximation of the perturbation theory, is prohibited, since it requires a change in the total spin of the system [
25,
26,
27]. At present, resonant absorption of the microwave field has been detected on the metastable states of many compounds [
25,
26,
27,
28,
29,
30,
31]. In view of the foregoing consideration, the two-peak spectrum revealed by subtracting the spectrum of the non-irradiated sample from that of the irradiated one (
Figure 2c, inset) can be interpreted as the spectrum of resonant transitions between the spin energy levels in the metastable state G-TiO
2(B).
Transitions from the metastable paramagnetic state to the ground diamagnetic state are possible if the approximations of the second and higher orders of the perturbation theory are taken into account. The spin –orbit interaction is one of the “channels” through which the thermal vibrations of ions affect the spin [
25,
27]. The amplitudes of thermal vibrations of lattice ions increase with temperature, which results in a reduction in the time the system is in a metastable state. This is the reason for the decrease with increasing temperature in the time it takes for the EPR spectrum to return to its original shape after the cessation of UV irradiation. The saturating nature of the increase in the intensity of the main EPR signal with the time of UV irradiation indicates that the filling of metastable spin states under the influence of irradiation prevails over their depletion due to transitions to the ground state. The correlation between the time of UV irradiation of the sample without reaching saturation of the intensity of the main signal of the EPR spectrum and the time of the subsequent return of the spectrum to its original shape after turning off the irradiation is a direct consequence of the dependence of the populations of metastable states on the irradiation time.
The mechanism of occurrence of oxygen vacancies in oxides during heat treatment due to treatment under gaseous medium, including a rarefied one, is described in [
32]. The thermodynamic equilibrium in such cases assumes that if the chemical potential of oxygen in the gas medium is changed, its chemical potential in the solid phase also varies. Thus, with a decrease in the partial pressure of oxygen over the oxide phase, the equalization of chemical potentials will be achieved due to a change in the oxygen concentration within it, including the lowering oxygen stoichiometry (oxygen release from the lattice into the gas medium). For titanium dioxide, this process can be represented by the following equation:
where
is oxygen atom in the TiO
2 lattice,
is anionic vacancy.
The slight deviation from stoichiometry for the W-TiO
2(B) sample can be explained by the inclusion of Fe
3+ ions in the crystallographic positions of Ti
4+ (apparently during the hydrothermal procedure), which is accompanied by the appearance of compensatory oxygen vacancies according to:
where
is trivalent iron ion in the position of tetravalent titanium. The W-TiO
2(B) should be attributed to the class of quasi-stoichiometric. Based on the preparation methodology, the same is true for G-TiO
2(B) sample (i.e., a small part of the oxygen vacancies contained in it is due to Fe
3+/Ti
4+ substitution).
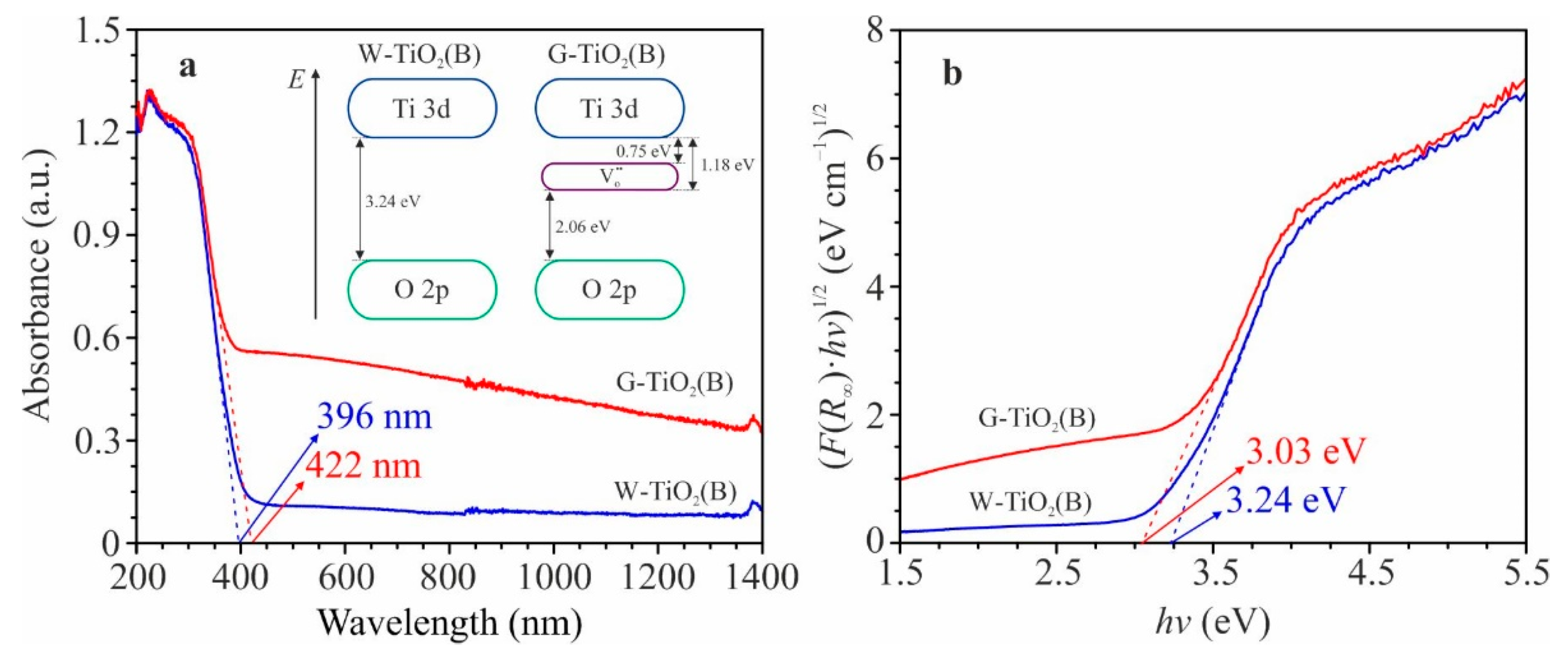
Figure 3a shows the UV-Vis-NIR absorption spectra for W-TiO
2(B) and G-TiO
2(B) samples. According to these data, the product obtained during heat treatment in air intensively absorbs UV (up to λ ~ 396 nm) and has a high reflection in the visible and near infrared spectral ranges. This is explained by the fact that TiO
2(B), which belongs to wide–band semiconductors, has a band gap of 3.1–3.3 eV [
33,
34,
35]. As a result of annealing in vacuum, the edge of the absorption band shifts towards the red part of the spectrum (bathochromic shift) up to 422 nm. Further, the intensity of electromagnetic radiation absorption increases (hyperchromic effect) significantly in the visible region and the near infrared range. The observed differences in optical activity are consistent with the color of the samples and are caused, based on the abovementioned data, by changes in the electronic structure of TiO
2(B)–specifically the occurrence of defects in its anionic sublattice.
To estimate the band gap of analyzed materials, the Tauc equation (α·
hν
= A (
hν –
Eg)
γ) was used for the case of indirect allowed transitions (γ = 2) from the valence band (O 2p) to the conduction band (Ti 3d), taking into account the Kubelka-Munk approximation (α ~
F(
R∞)).
Figure 3b shows the (
F(
R∞)·
hν)
1/2 against
hν graphs for W-TiO
2(B) and G-TiO
2(B) samples. The
Eg values were determined by extrapolating linear sections of these dependencies to the abscissa axis (approximation error ~ 0.02 eV). According to the obtained data, the band gap for defective TiO
2(B) (sample G-TiO
2(B)) is 3.03 eV, which is less than that (3.24 eV) for quasi–stoichiometric titanium dioxide (W-TiO
2(B)) obtained during annealing in an oxidizing atmosphere. The observed decrease in
Eg is related to the appearance of the energy levels induced by the oxygen-vacancy-related states, which are located on 0.75–1.18 eV away from the lower boundary of the conduction band [
36,
37] (insert to
Figure 3a; the schemes of band gaps are given without taking into account the iron ions and anionic defects induced by their entry into the lattice).
Interestingly, the UV-Vis-NIR data (
Figure S1) clearly show that annealing of TiO
2(B) under Ar and N
2 atmosphere as opposed to vacuum results in poorer optoelectronic properties. It seems that this is due to the lower concentration of anionic defects in the forming nonstoichiometric titanium dioxide lattice. In this way, it is important to note that as reported for TiO
2(B) prepared through annealing in argon [
8], oxygen deficiency is an important factor for designing high performance anodes based on TiO
2(B) for sodium-ion batteries because it not only rises electrical conductivity but also gives a lower energy barrier to sodiation, accelerating storage kinetics and preserving crystal structure upon cycling. Note that comparing with the Ar and N
2, heat treatment under vacuum does not require chemicals. Thus, such a method may be more advantageous in the development of advanced materials for the next generation of metal-ion batteries.
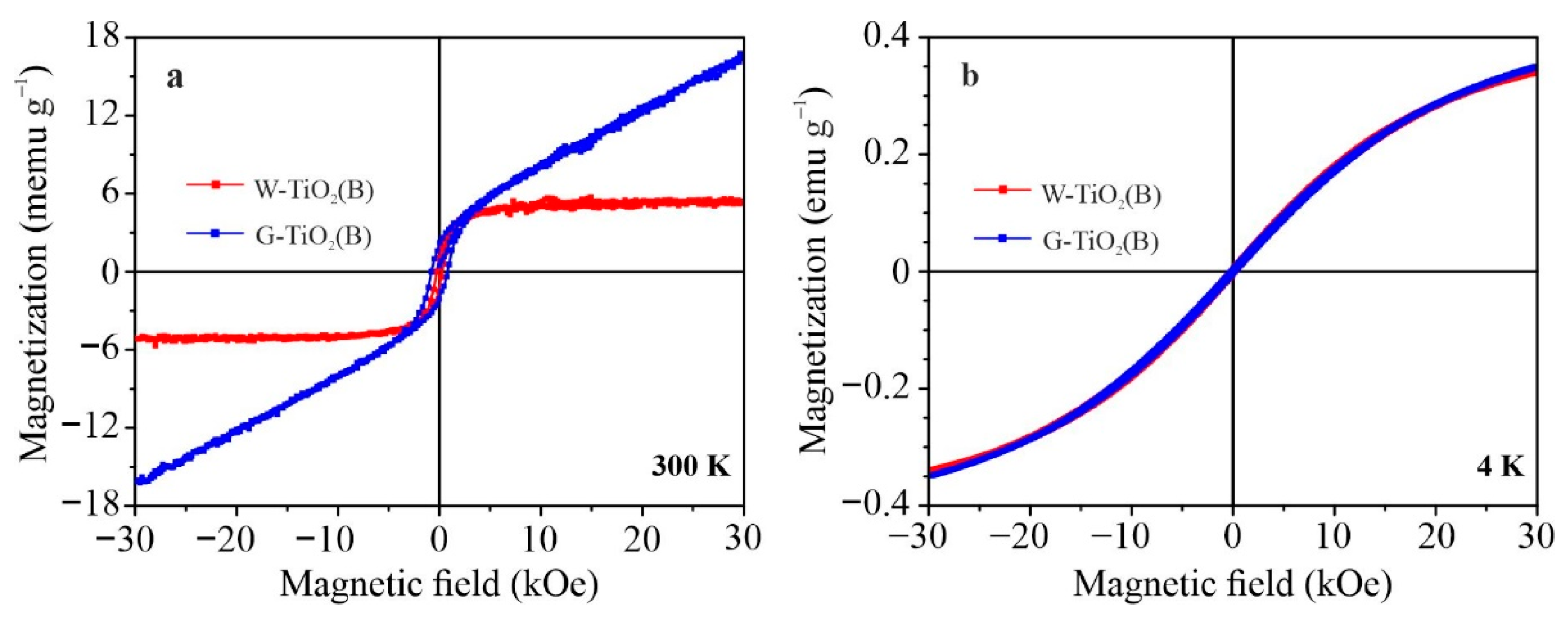
Figure 4a shows the field dependences at room temperature of samples obtained during heat treatment under oxidizing atmosphere and in vacuum. According to the data, both materials have a magnetic ordering under ambient conditions. At the same time, the hysteresis loop ceases to reach saturation and the coercive force increases from 270 Oe (W-TiO
2(B)) to 800 Oe (G-TiO
2(B)) when the parameters (medium) of final annealing are changed. Prior to the analysis of obtained results, it is necessary to take into account that the anatase precursor has a purity of 99.7% and the EPR data clearly confirm the presence of trivalent iron as impurity in the synthesized TiO
2(B) samples. This means that ferromagnetism in studied products can be associated, among other things, with magnetic moments localized on impurity atoms, which are ordered by indirect exchange interactions [
38,
39]. At the same time, obviously, the mentioned contribution is not decisive for G-TiO
2(B). Indeed, the increase in magnetization of TiO
2(B) as a result of heat treatment in vacuum indicates that the deficiency of crystal structure (presence of vacancies in the anionic sublattice) plays a predominant role for the occurrence of magnetic properties. According to [
40], the reason for ferromagnetic ordering in such materials (oxygen-deficient oxide semiconductors) is the spin splitting of a narrow vacancy zone located near the bottom of the conduction band by the Stoner mechanism. Within the scope of this model, ferromagnetism is spatially inhomogeneous in the sample and can exist only in the field of percolation of defects. Therefore, an increase in the total number of defects (i.e., oxygen vacancies in the case of G-TiO
2(B)) will result in the growth of spatial areas involved in ferromagnetic ordering, as a whole increasing the total magnetic moment of the substance. This is exactly the effect observed in the G-TiO
2(B) sample, which has the same composition as W-TiO
2(B), but contains a larger number of its own defects (oxygen vacancies).
When the temperature decreases to 4 K, the field dependences of W-TiO
2(B) and G-TiO
2(B) samples become similar (
Figure 4b). The observed phenomenon is explained by the fact that the electron population of the vacancy zone decreases and, hence, the contribution from the exchange interaction between electrons to the magnetic characteristics of the material reduces. In this way, the contribution from impurity defects becomes predominant. Since the amount of impurities in both samples is the same, the magnetic properties of them in low temperature region become similar.
3. Materials and Methods
The synthesis of materials was carried out using a combination hydrothermal technology, ion exchange process, and annealing. Initially, 0.26 g commercial anatase TiO2 with an average particle size of ~100 nm (99.7%, Alfa Aesar, Ward Hill, MA, USA) was dispersed in 40 mL NaOH aqueous solution (14 M) under continuous stirring for 1–2 h. Then, the dispersion was maintained for 72 h at a temperature of 170 °C in a 50 mL autoclave (stainless-steel reactor with a Teflon liner). After that, the reaction mixture was cooled to room temperature and the precipitate was separated by centrifugation. By rinsing the filtered product in a solution of HCl (0.05 M) taken in 5-fold excess, Na+ was exchanged by H+. The ion-exchange process was carried out for 72 h, and the HCl solution was replaced every 24 h. The resulting protonated form was separated from the mother liquor, washed with deionized water to a neutral pH, and dried at a temperature of 80 °C. The final stage of synthesis involved annealing in a tubular furnace at 350 °C for 3 h in an ambient atmosphere or in vacuum (1 Pa), resulting in samples of white (W-TiO2(B)) or gray (G-TiO2(B)) coloring, respectively.
The phase composition was studied by X-ray powder diffraction (XRD) on a Bruker D8-Advance (Billerica, MA, USA) with the CuKα-radiation in Bragg–Brentano geometry. Diffractograms were analyzed using the PDF-2 database (release 2015). The surface morphology was studied by scanning electron microscopy (SEM) using a Hitachi S5500 (Tokyo, Japan) microscope. The specific surface area, pore volume, and size were evaluated by the gas adsorption method on a Quantachrome Autosorb iQ (Boynton Beach, FL, USA) using nitrogen at 77 K with the Brunauer-Emmett-Teller (BET) and Barrett-Joyner–Halenda (BJH) models.
The nature of the paramagnetic centers was determined by electron paramagnetic resonance (EPR). EPR spectra were recorded on a JEOL JES-X 330 (Tokyo, Japan) in X-band frequencies with the following settings: microwave radiation power of 2 mW, modulation amplitude of 0.2 Hz, and modulation frequency of 100 kHz. Temperature-dependent measurements were carried out in a continuous stream of nitrogen using a JEOL ES-13060 DVT5 (Tokyo, Japan) unit. The samples were irradiated under ultraviolet (UV) light using a JEOL ES-USH500 (Tokyo, Japan) device. The integral intensities and values of g-factors for EPR lines were calibrated by these of the spin resonance signal on the conduction electrons of Li nanoparticles (g = 2.002293 ± 0.000003) in the reference sample LiF:Li, which do not change in the range from 2 to 400 K.
The optical properties and electronic band structure were studied by the ultraviolet, visible, and near-infrared spectrophotometry (UV-Vis-NIR). UV-Vis-NIR diffuse reflectance spectra were recorded in the A(λ) coordinate system (where A is the absorbance, λ is the wavelength) on a Shimadzu 2600 (Tokyo, Japan) spectrophotometer equipped by ISR-2600Plus integrating sphere unit at room temperature in the wavelength range 200–1400 nm (spectral slit width was 1 nm and a scanning step was 1 nm). Barium sulfate was applied as a non-absorbing standard.
The magnetic properties were studied at two temperatures of 300 and 4 K on a Quantum Design PPMS 9T (San Diego, CA, USA) with a VSM vibromagnetometer option. The range of variation of the external field was ± 30 kOe.