Polynuclear Cu(I) and Ag(I) Complexes of 1,3-Diisobutyl Thiourea, Synthesis, Crystal Structure and Antioxidant Potentials
Abstract
1. Introduction
2. Experimental
2.1. General
2.2. X-ray Diffraction Crystallography
2.3. Syntheses of Complexes
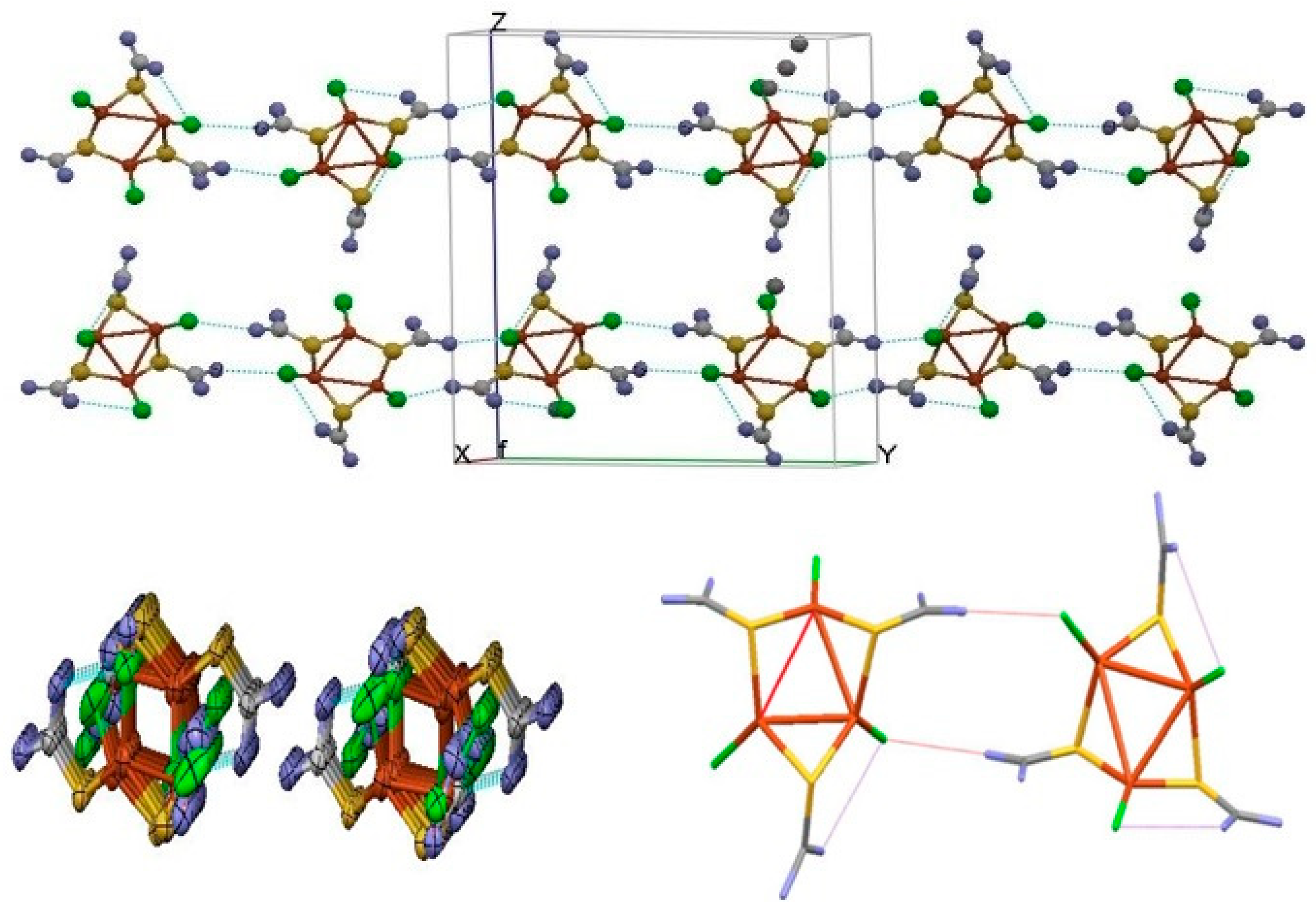
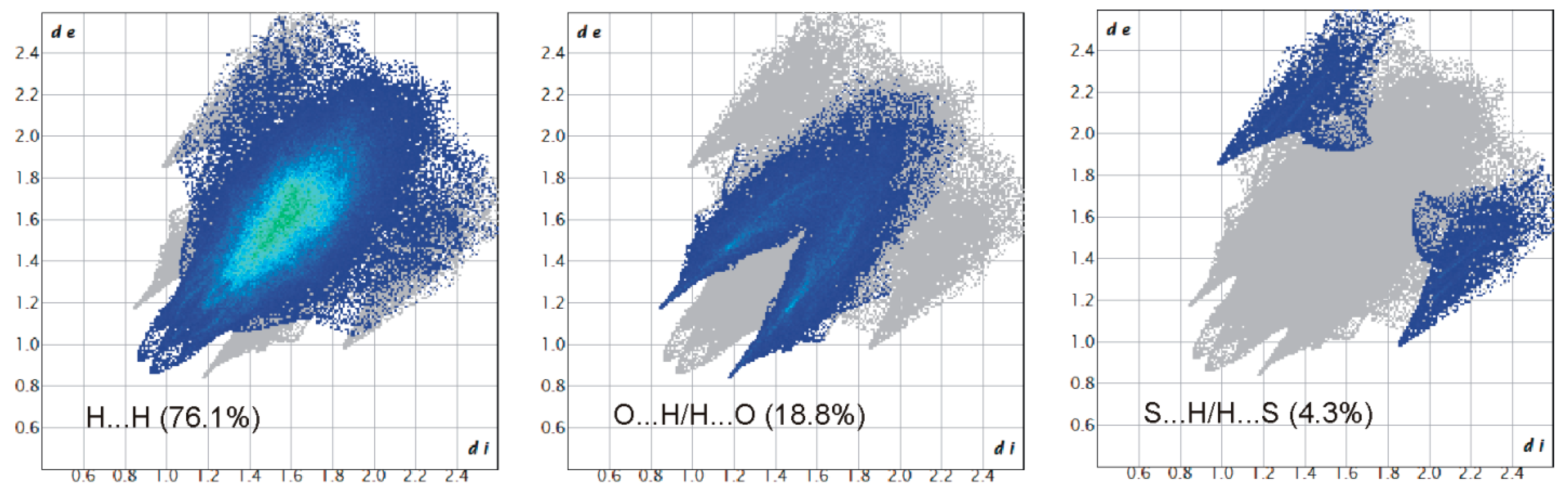
2.4. Hirshfeld Surface Analysis
2.5. Antioxidant Assay
3. Results and Discussion
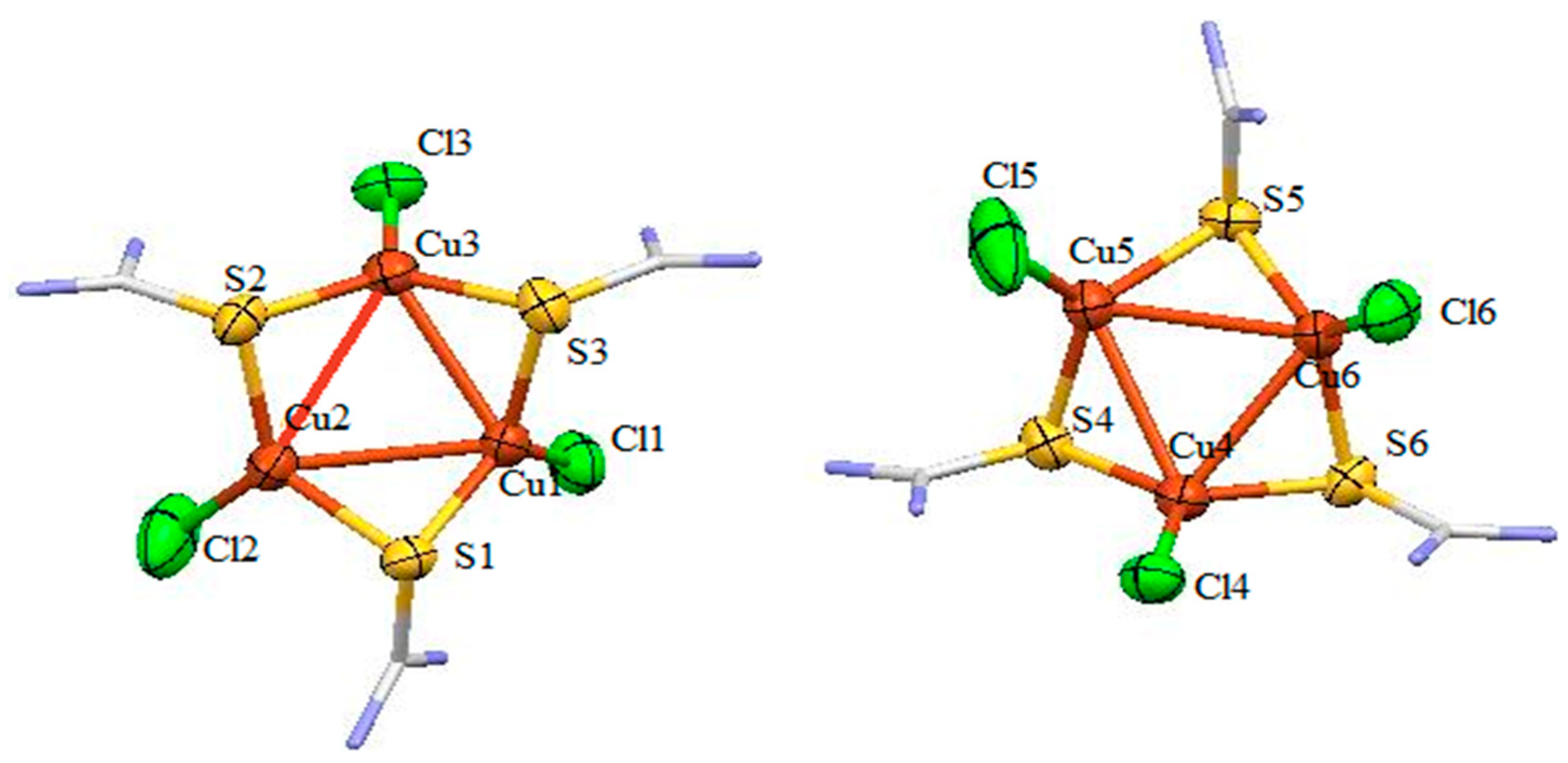
3.1. Description of Crystal Structure of [Cu3L3Cl3] (1)
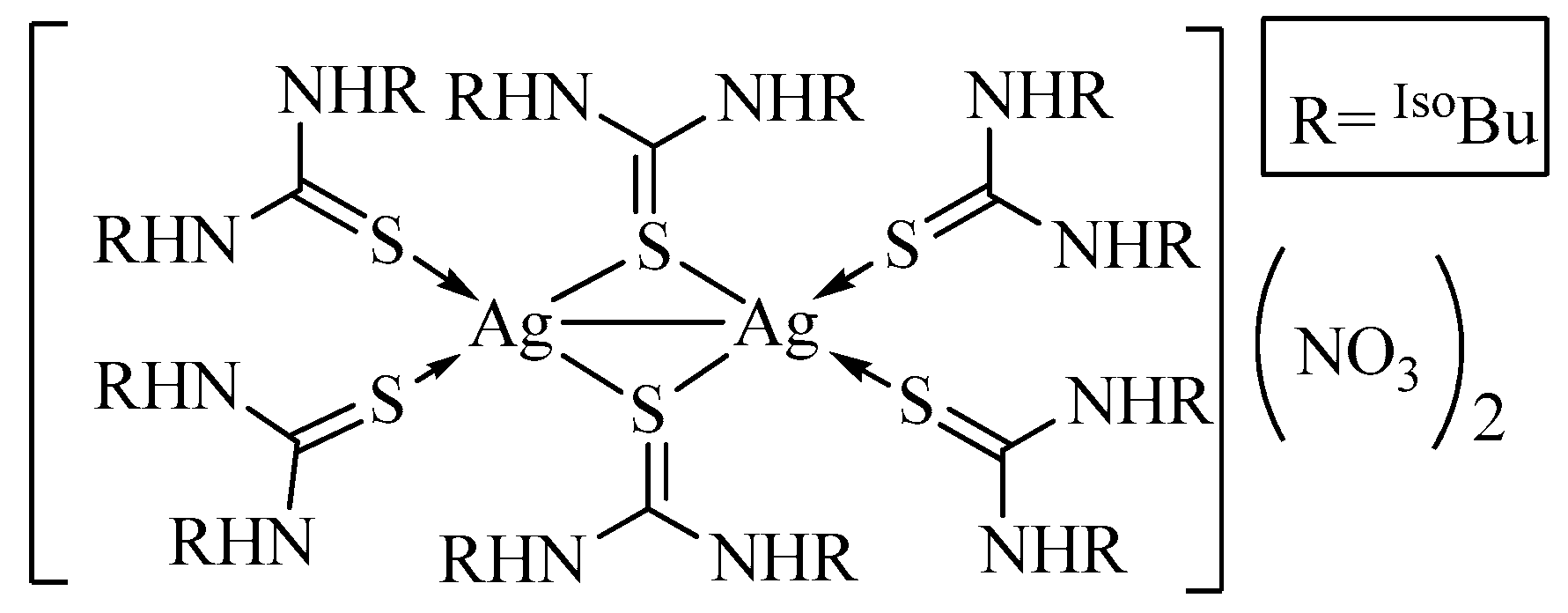
3.2. Description of Crystal Structure of [Ag2L6](NO3)2 (2)
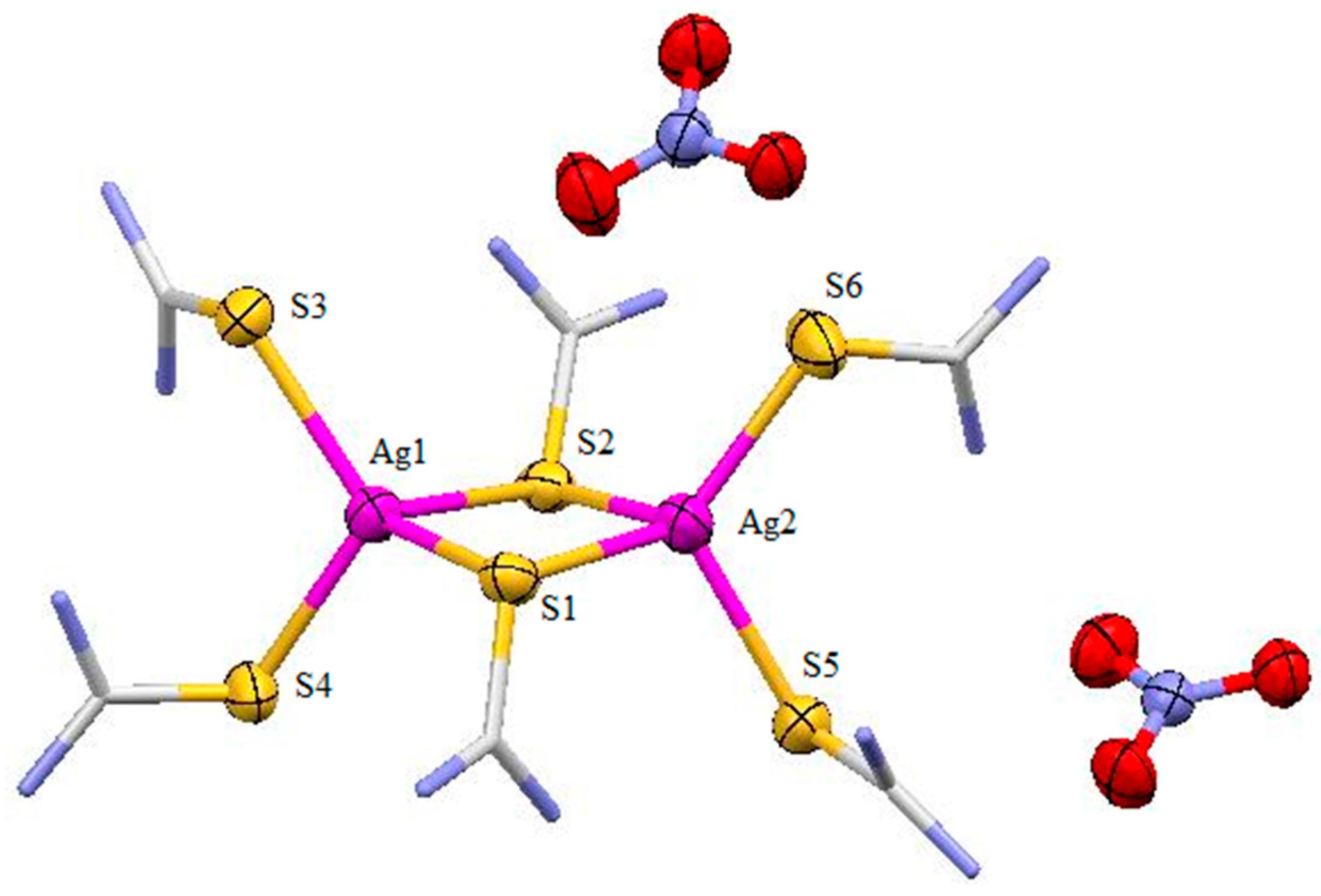
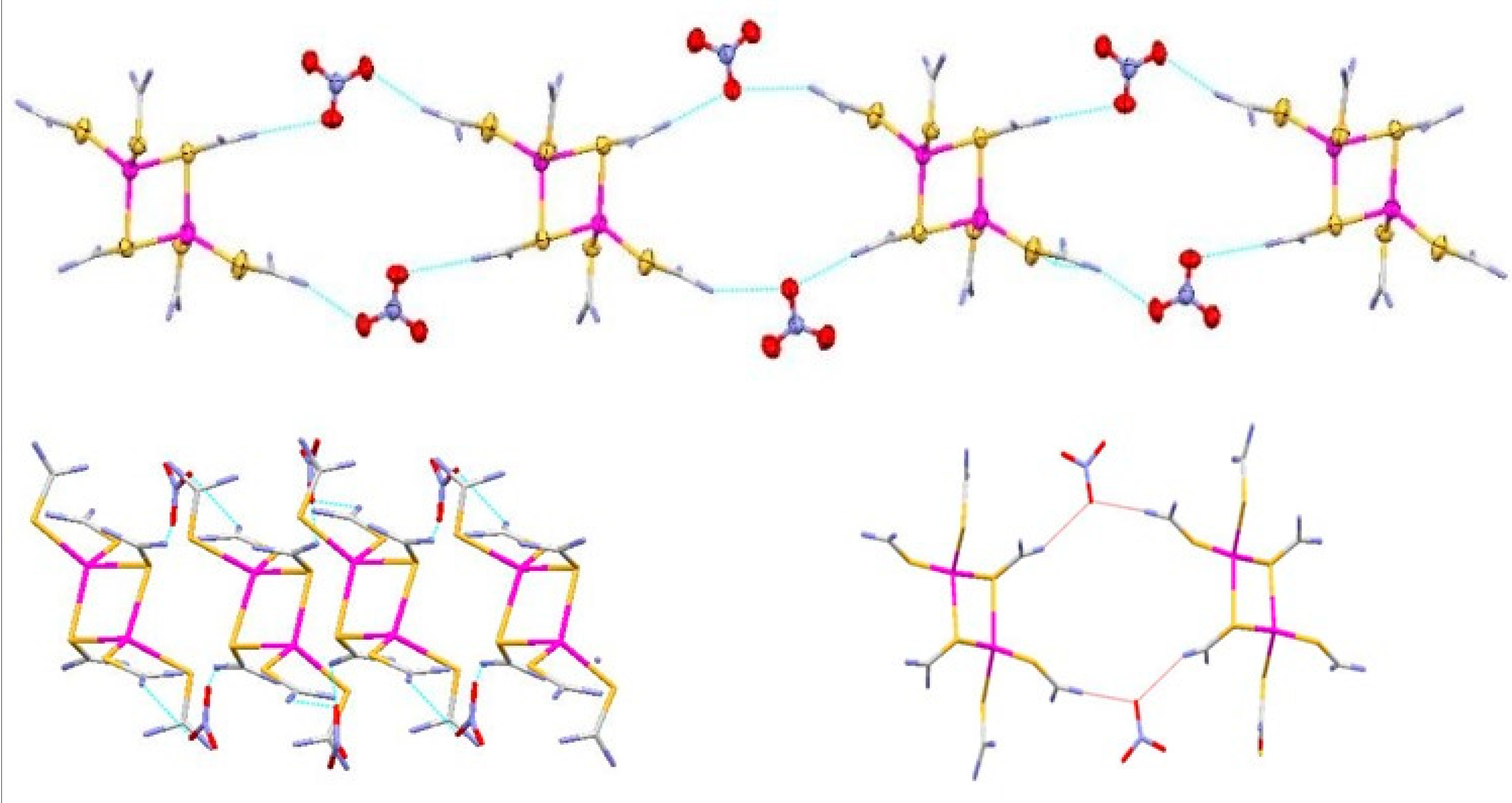

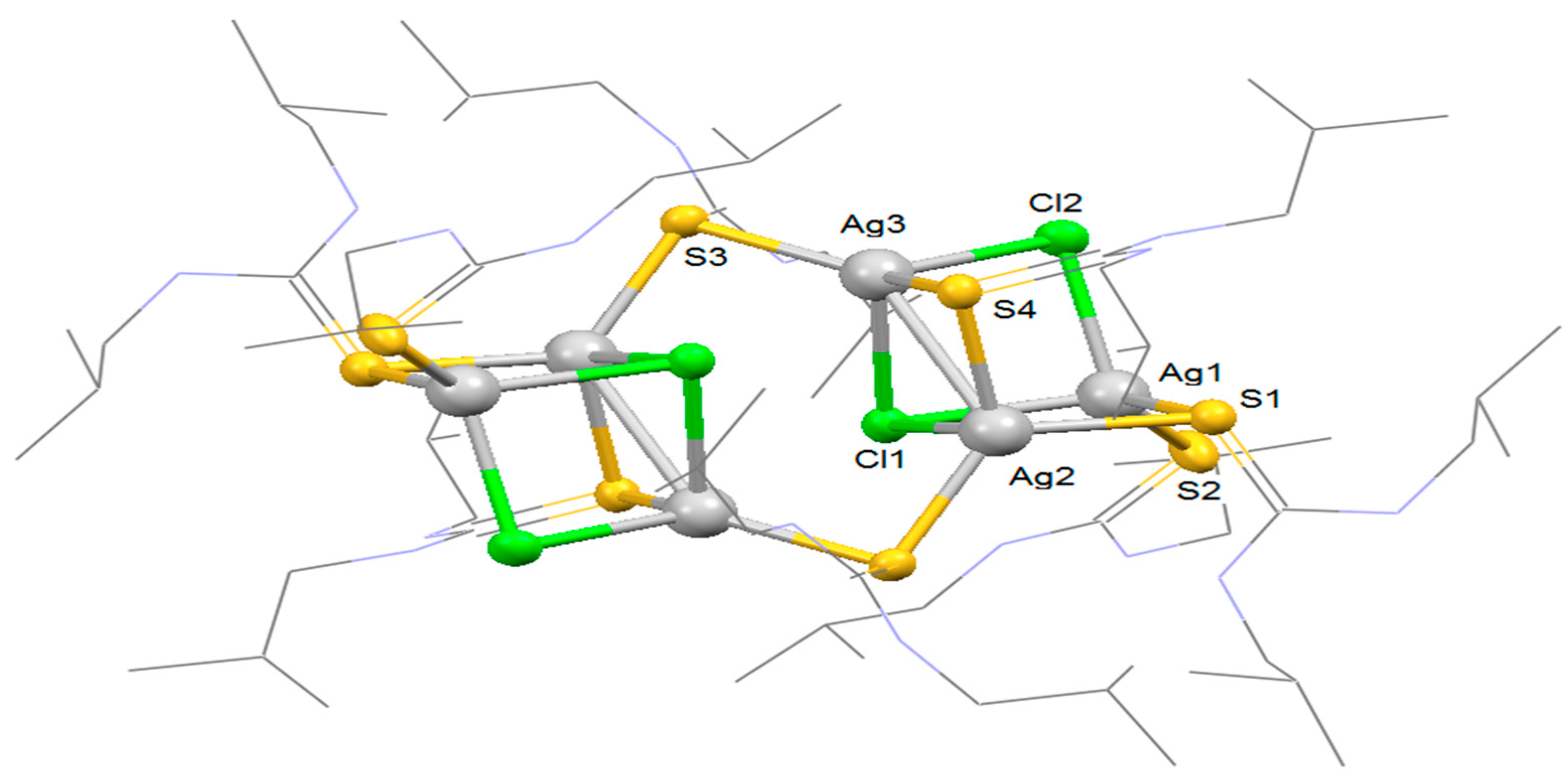
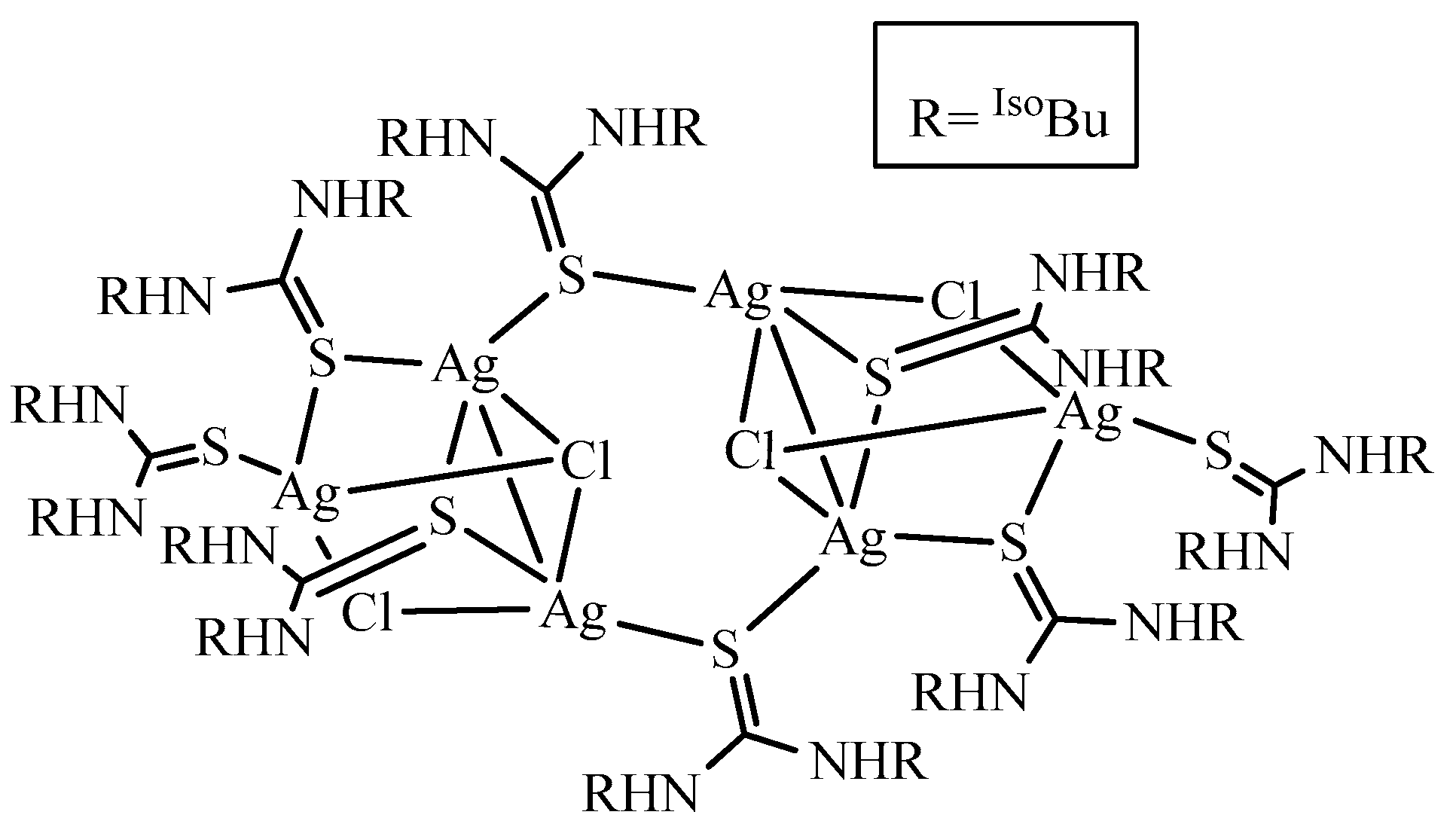
3.3. Description of Crystal Structure of [Ag6L8Cl4] (3)


| Compound | Atoms | Bond Length | Atoms | Bond Angle |
|---|---|---|---|---|
| 1 | Cu1-S1 | 2.221(17) | S1-Cu1-Cl1 | 123.17(7) |
| Cu1-Cl1 | 2.233(19) | S1-Cu1-S3 | 117.41(6) | |
| Cu1-S3 | 2.2697(18) | S3-Cu1-Cl1 | 119.21(7) | |
| Cu1-Cu2 | 2.889(10) | Cu1-Cu2-Cu3 | 54.87(3) | |
| 2 | Ag1-S4 | 2.5329(15) | S4-Ag1-S3 | 113.30(5) |
| Ag1-S3 | 2.5568(14) | S4-Ag1-S1 | 113.51(5) | |
| Ag1-S1 | 2.5941(14) | S3-Ag1-S1 | 115.49(5) | |
| Ag1-S2 | 2.6451(10) | S4-Ag1-S2 | 100.16(5) | |
| Ag1-Ag2 | 3.3539(6) | S3-Ag1-S2 | 112.00(5) | |
| 3 | Ag1-S1 | 2.602(15) | S2-Ag1-Cl1 | 113.13(5) |
| Ag1-S2 | 2.446(14) | Cl1-Ag1-S1 | 107.23(6) | |
| Ag1-Cl1 | 2.462(13) | S2-Ag1-Cl2 | 119.42(5) | |
| Ag1-Ag2 | 3.120(6) | S1-Ag1-Cl2 | 100.30(7) | |
| Ag2-S1 | 2.543(11) | S1-Ag1-S2 | 107.23(5) | |
| Ag2-S4 | 2.642(10) | Ag2-S3-Ag3 | 93.88(5) | |
| Ag2-S3 | 2.507(14) | Ag3-Cl2-Ag1 | 89.43(6) | |
| Ag3-S4 | 2.505(15) | Ag1-Cl1-Ag2 | 72.01 | |
| Ag3-S3 | 2.529(12) | Ag1-S1-Ag2 | 89.17 |
| 1 | 2 | 3 | |
|---|---|---|---|
| Empirical formula | C54H120N12Cu6Cl6S6 | C54H120N14Ag2O6S6 | C72H160N16Ag6Cl6S8 |
| Formula weight | 1603.97 | 1479 | 2366.57 |
| Temperature (K) | 296 | 296 | 296 |
| Wavelength (Å) | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Triclinic | Triclinic | Monoclinic |
| Space group | P-1 | P-1 | P 21/c |
| Aa | 11.016(4) | 11.611(6) | 11.7020(11) |
| Bb | 18.227(7) | 15.499(8) | 16.5310(14) |
| Ac | 21.634(8) | 24.149(13) | 28.0730(3) |
| Aα | 89.832(2) | 99.159(3) | 90 |
| Ββ | 86.125(2) | 102.357(2) | 99.096(5) |
| Γγ | 81.288(2) | 108.098(3) | 90 |
| Volume Å3 | 4284.0(3) | 3988.8(4) | 5362.3(9) |
| μ (mm−1) | 1.83 | 0.70 | 1.422 |
| Z | 2 | 2 | 2 |
| Density (Mg m−3) | 1.243 | 1.277 | 1.466 |
| F (0, 0, 0) | 1562 | 1624 | 2432 |
| (h, k, l) min | (−13, −22, −27) | −14, −18, −30 | (−13, −19, −33) |
| (h, k, l) max | (13, 22, 27) | 14, 19, 30 | (13, 19, 33) |
| Theta (max) | 26.5 | 27.0 | 24.999 |
| R (reflection) | 0.069 (17650) | 0.057 (17072) | 0.0853 (5485) |
| wR2 | 0.236 | 0.180 | 0.1706 |
3.4. Determination of Antioxidant Activities by DPPH Radical Scavenging Test
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, E.; Khan, S.; Gul, Z.; Muhammad, M. Medicinal Importance, Coordination Chemistry with Selected Metals (Cu, Ag, Au) and Chemosensing of Thiourea Derivatives. A Review. Crit. Rev. Anal. Chem. 2021, 51, 812–834. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Khan, E.; Said, M.; Khan, G.S.; Syed, M.G.; Noor, A.; Zahoor, M.; Ullah, R.; Bari, A. Complexes of 1, 3-diisobutyl thiourea with copper (I), zinc (II) and mercury (II): Their antioxidant and antibacterial evaluation. Crystals 2021, 11, 989. [Google Scholar] [CrossRef]
- Rahman, F.U.; Bibi, M.; Altaf, A.A.; Tahir, M.N.; Ullah, F.; Khan, E. Zn, Cd and Hg complexes with unsymmetric thiourea derivatives; syntheses, free radical scavenging and enzyme inhibition essay. J. Mol. Struct. 2020, 1211, 128096. [Google Scholar] [CrossRef]
- Rahman, F.U.; Bibi, M.; Khan, E.; Shah, A.B.; Muhammad, M.; Tahir, M.N.; Shahzad, A.; Ullah, F.; Zahoor, M.; Alamery, S. Thiourea derivatives, simple in structure but efficient enzyme inhibitors and mercury sensors. Molecules 2021, 26, 4506. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.A.; Badshah, A.; Tahir, M.N.; Khan, E. Gold (I), silver (I) and copper (I) complexes of 2, 4, 6-trimethylphenyl-3-benzoylthiourea; synthesis and biological applications. Polyhedron 2020, 181, 114485. [Google Scholar] [CrossRef]
- Noor, A.; Qayyum, S.; Jabeen, F.; Rehman, A.U. Mononuclear Tricoordinate Copper (I) and Silver (I) Halide Complexes of a Sterically Bulky Thiourea Ligand and a Computational Insight of Their Interaction with Human Insulin. Molecules 2022, 27, 4231. [Google Scholar] [CrossRef]
- Mykhalichko, B.M.; Temkin, O.N.; Mys’kiv, M. Polynuclear complexes of copper(I) halides: Coordination chemistry and catalytic transformations of alkynes. Russ. Chem. Rev. 2000, 69, 957–984. [Google Scholar] [CrossRef]
- Levitsky, M.M.; Bilyachenko, A.N.; Shul’pin, G.B. Oxidation of C-H compounds with peroxides catalyzed by polynuclear transition metal complexes in Si- or Ge-sesquioxane frameworks: A review. J. Organomet. Chem. 2017, 849–850, 201–218. [Google Scholar] [CrossRef]
- Roy, P.; Dhara, K.; Manassero, M.; Banerjee, P. Di-, Tetra-, and Polynuclear Copper (II) Complexes: Active Catalysts for Oxidation of Toluene and Benzene. Eur. J. Inorg. Chem. 2008, 2008, 4404–4412. [Google Scholar] [CrossRef]
- Musina, E.I.; Shamsieva, A.V.; Strelnik, I.D.; Gerasimova, T.P.; Krivolapov, D.B.; Kolesnikov, I.E.; Grachova, E.V.; Tunik, S.P.; Bannwarth, C.; Grimme, S.; et al. Synthesis of novel pyridyl containing phospholanes and their polynuclear luminescent copper(i) complexes. Dalton Trans. 2016, 45, 2250–2260. [Google Scholar] [CrossRef]
- Kobielska, P.A.; Telford, R.; Rowlandson, J.; Tian, M.; Shahin, Z.; Demessence, A.; Ting, V.P.; Nayak, S. Polynuclear Complexes as Precursor Templates for Hierarchical Microporous Graphitic Carbon: An Unusual Approach. ACS Appl. Mater. Interfaces 2018, 10, 25967–25971. [Google Scholar] [CrossRef] [PubMed]
- Puyo, M.; Lebon, E.; Vendier, L.; Kahn, M.L.; Fau, P.; Fajerwerg, K.; Lepetit, C. Topological Analysis of Ag–Ag and Ag–N Interactions in Silver Amidinate Precursor Complexes of Silver Nanoparticles. Inorg. Chem. 2020, 59, 4328–4339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, L. Macrocycle-Encircled Polynuclear Metal Clusters: Controllable Synthesis, Reactivity Studies, and Applications. Acc. Chem. Res. 2018, 51, 2535–2545. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, F.R.G.; Nunes, J.H.B.; de Carvalho, M.A.; Ribeiro, M.A.; de Paiva, P.P.; Banzato, T.P.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Lustri, W.R.; Martins, D.O.; et al. Polynuclear copper (II) complexes with nalidixic acid hydrazones: Antiproliferative activity and selectivity assessment over a panel of tumor cells. Inorg. Chim. Acta 2019, 484, 491–502. [Google Scholar] [CrossRef]
- Sun, W.H.; Li, K.H.; Liu, H.; Gu, Y.T.; Zhang, Y.; You, Z.L.; Li, W. Synthesis, Characterization, Crystal Structures, and Antibacterial Activity of Polynuclear Nickel (II) and Copper (II) Complexes with Similar Tridentate Schiff Bases. Russ. J. Coord. Chem. 2017, 43, 693–699. [Google Scholar] [CrossRef]
- Semerci, F.; Yeşilel, O.Z.; Soylu, M.S.; Keskin, S.; Büyükgüngör, O. A two-dimensional photoluminescent cadmium (II) coordination polymer containing a new coordination mode of pyridine-2,3-dicarboxylate: Synthesis, structure and molecular simulations for gas storage and separation applications. Polyhedron 2013, 50, 314–320. [Google Scholar] [CrossRef]
- Mei, G.; Li, Z.; Zhang, H.; Li, G.; Meng, X. Synthesis, Structures, and Properties of Two Novel Zn (II) Polynuclear Complexes. Synth. React. Inorg. Met. Org. Nano Met. Chem. 2010, 40, 163–169. [Google Scholar] [CrossRef]
- Roubeau, O.; Clérac, R. Rational Assembly of High-Spin Polynuclear Magnetic Complexes into Coordination Networks: The Case of a [Mn4] Single-Molecule Magnet Building Block. Eur. J. Inorg. Chem. 2008, 2008, 4325–4342. [Google Scholar] [CrossRef]
- Papaefstathiou, G.S.; Perlepes, S.P. Families of Polynuclear Manganese, Cobalt, Nickel and Copper Complexes Stabilized by Various Forms of Di-2-pyridyl Ketone. Comments Inorg. Chem. 2002, 23, 249–274. [Google Scholar] [CrossRef]
- Yao, L.; Niu, G.; Li, J.; Gao, L.; Luo, X.; Xia, B.; Liu, Y.; Du, P.; Li, D.; Chen, C.; et al. Circularly Polarized Luminescence from Chiral Tetranuclear Copper(I) Iodide Clusters. J. Phys. Chem. Lett. 2020, 11, 1255–1260. [Google Scholar] [CrossRef]
- Haasnoot, J.G. Mononuclear, oligonuclear and polynuclear metal coordination compounds with 1,2,4-triazole derivatives as ligands. Coord. Chem. Rev. 2000, 200–202, 131–185. [Google Scholar] [CrossRef]
- Bhowmik, P.; Chattopadhyay, S.; Drew, M.G.B.; Ghosh, A. A polynuclear helical and two dinuclear copper (II) complexes of a monocondensed N, N, O donor Schiff base with pseudohalides as co-ligand. Inorg. Chim. Acta 2013, 395, 24–32. [Google Scholar] [CrossRef]
- Escuer, A.; Esteban, J.; Perlepes, S.P.; Stamatatos, T.C. The bridging azido ligand as a central “player” in high-nuclearity 3d-metal cluster chemistry. Coord. Chem. Rev. 2014, 275, 87–129. [Google Scholar] [CrossRef]
- Marvaud, V.; Decroix, C.; Scuiller, A.; Tuyèras, F.; Guyard-Duhayon, C.; Vaissermann, J.; Marrot, J.; Gonnet, F.; Verdaguer, M. Hexacyanometalate Molecular Chemistry: Di-, Tri-, Tetra-, Hexa- and Heptanuclear Heterobimetallic Complexes; Control of Nuclearity and Structural Anisotropy. Chem. A Eur. J. 2003, 9, 1692–1705. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-L.; Qin, C.; Wang, E.-B.; Su, Z.-M. Metal Nuclearity Modulated Four-, Six-, and Eight-Connected Entangled Frameworks Based on Mono-, Bi-, and Trimetallic Cores as Nodes. Chem. A Eur. J. 2006, 12, 2680–2691. [Google Scholar] [CrossRef]
- Papatriantafyllopoulou, C.; Manessi-Zoupa, E.; Escuer, A.; Perlepes, S.P. The sulfate ligand as a promising “player” in 3d-metal cluster chemistry. Inorg. Chim. Acta 2009, 362, 634–650. [Google Scholar] [CrossRef]
- Ahmad, S.; Isab, A.A.; Perzanowski, H.P. Silver (I) complexes of thiourea. Transit. Met. Chem. 2002, 27, 782–785. [Google Scholar] [CrossRef]
- Safin, D.A.; Babashkina, M.G.; Bolte, M.; Pape, T.; Hahn, F.E.; Verizhnikov, M.L.; Bashirov, A.R.; Klein, A. Polynuclear and mixed-ligand mononuclear CuI complexes with N-thiophosphorylated thioureas and 1,10-phenanthroline or PPh3. Dalton Trans. 2010, 39, 11577–11586. [Google Scholar] [CrossRef]
- Safin, D.A.; Babashkina, M.G.; Bolte, M.; Garcia, Y. Homoleptic polynuclear CuI and AgI complexes of N-thiophosphorylated thioureas o-RO(O)CC6H4NHC(S)NHP(S)(OiPr)2 (R = Me, Et). CrystEngComm 2012, 14, 774–778. [Google Scholar] [CrossRef]
- Metlushka, K.E.; Sadkova, D.N.; Nikitina, K.A.; Pashagin, A.V.; Khrizanforov, M.N.; Budnikova, Y.H.; Morozov, V.I.; Islamov, D.R.; Latypov, S.K.; Kataeva, O.N.; et al. Synthesis of the first chiral polynuclear copper(i) complex based on (R)-1-(1-phenyl) ethyl-3-(O,O-diethylthiophosphoryl)thiourea and its characterization in the solid state and solution. New J. Chem. 2020, 44, 3224–3231. [Google Scholar] [CrossRef]
- Li, G.; Che, D.-J.; Li, Z.-F.; Zhu, Y.; Zou, D.-P. Versatile coordination patterns in the reaction system of N-benzoyl-N′-(2-pyridyl) thiourea with CuCl2. Their reaction conditions, systematic isolation and crystal structures. New J. Chem. 2002, 26, 1629–1633. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Thang, P.C.; Rodenstein, A.; Kirmse, R.; Abram, U. Bipodal Acylthiourea Ligands as Building Blocks for Bi-, Tetra-, and Polynuclear Oxorhenium (V) Complexes. Inorg. Chem. 2011, 50, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, S.; Shukla, P.; Tripathi, S.; Rivière, E.; Mallah, T.; Rajaraman, G.; Shanmugam, M. Substituted versus Naked Thiourea Ligand Containing Pseudotetrahedral Cobalt (II) Complexes: A Comparative Study on Its Magnetization Relaxation Dynamics Phenomenon. Inorg. Chem. 2018, 57, 3371–3386. [Google Scholar] [CrossRef] [PubMed]
- Luckay, R.C.; Sheng, X.; Strasser, C.E.; Raubenheimer, H.G.; Safin, D.A.; Babashkina, M.G.; Klein, A. Competitive bulk liquid membrane transport and solvent extraction of some metal ions using RC(S)NHP(X)(OiPr)2 (X = O, S) as ionophores. Formation of the polynuclear complex of [Ag(N≡C–NP(S)(OiPr)2)]n. Dalton Trans. 2009, 39, 8227–8236. [Google Scholar] [CrossRef] [PubMed]
- Altaf, A.A.; Shahzad, A.; Gul, Z.; Khan, S.A.; Badshah, A.; Tahir, M.N.; Zafar, Z.I.; Khan, E. Synthesis, crystal structure, and DFT calculations of 1, 3-diisobutyl thiourea. J. Chem. 2015, 2015, 913435. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Herbst-Irmer, R.; Sheldrick, G.M. Refinement of Twinned Structures with SHELXL97. Acta Crystallogr. Sect. B 1998, 54, 443–449. [Google Scholar] [CrossRef]
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Kuhn, A.; Köhler, J.; Lotsch, B.V. Single-crystal X-ray structure analysis of the superionic conductor Li10GeP2S12. Phys. Chem. Chem. Phys. 2013, 15, 11620–11622. [Google Scholar] [CrossRef]
- Spek, A. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Turner, M.; McKinnon, J.J.; Wolff, S.; Grimwood, D.; Spackman, P.; Jayatilaka, D.; Spackman, M. CrystalExplorer17; University of Western Australia: Perth, Australia, 2017; Volume 108, p. 76730. [Google Scholar]
- Yuan, C.; Du, L.; Jin, Z.; Xu, X. Storage stability and antioxidant activity of complex of astaxanthin with hydroxypropyl-β-cyclodextrin. Carbohydr. Polym. 2013, 91, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Bombicz, P.; Mutikainen, I.; Krunks, M.; Leskelä, T.; Madarász, J.; Niinistö, L. Synthesis, vibrational spectra and X-ray structures of copper(I) thiourea complexes. Inorg. Chim. Acta 2004, 357, 513–525. [Google Scholar] [CrossRef]
- Battaini, G.; Granata, A.; Monzani, E.; Gullotti, M.; Casella, L. Biomimetic Oxidations by Dinuclear and Trinuclear Copper Complexes. In Advances in Inorganic Chemistry; van Eldik, R., Reedijk, J., Eds.; Academic Press: Cambridge, MA, USA, 2006; Volume 58, pp. 185–233. [Google Scholar]
- Ahmad, S.; Altaf, M.; Stoeckli-Evans, H.; Rüffer, T.; Lang, H.; Mufakkar, M.; Waheed, A. Crystal Structures of Trinuclear Chlorido(N,N′-diethylthiourea)copper(I) and a Second Polymorph of Iodidotris(N,N′-diethylthiourea)copper(I). J. Chem. Crystallogr. 2010, 40, 639–645. [Google Scholar] [CrossRef]
- Isab, A.A.; Nawaz, S.; Saleem, M.; Altaf, M.; Monim-ul-Mehboob, M.; Ahmad, S.; Evans, H.S. Synthesis, characterization and antimicrobial studies of mixed ligand silver(I) complexes of thioureas and triphenylphosphine; crystal structure of {[Ag(PPh3)(thiourea)(NO3)]2·[Ag(PPh3)(thiourea)]2(NO3)2}. Polyhedron 2010, 29, 1251–1256. [Google Scholar] [CrossRef]
- Mironov, I.; Tsvelodub, L. Stability of Mononuclear and Binuclear Silver (I) Complexes with Thiourea in Aqueous Solution. Russ. J. Inorg. Chem. 1996, 41, 228–232. [Google Scholar]
- Samadov, A.S.; Mironov, I.V.; Gorichev, I.G.; Kovalchukova, O.V.; Stepnova, A.F. Study of Silver(I) Complex Formation with Some Thiourea Derivatives in Aqueous Solution. Russ. J. Gen. Chem. 2020, 90, 2111–2114. [Google Scholar] [CrossRef]
- Pakawatchai, C.; Sivakumar, K.; Fun, H.-K. Bis(N,N′-dimethylthiourea-S)silver(I) Perchlorate and Tris(N,N′-dimethylthiourea-S)silver(I) Perchlorate. Acta Crystallogr. Sect. C 1996, 52, 1954–1957. [Google Scholar] [CrossRef]
- Choudhary, M.A.; Ahmad, S.; Aslam, M.; Saleem, M.; Tahir, M.N.; Fettouhi, M.; Isab, A.A. Synthesis, crystal structure and antimicrobial activities of a dinuclear silver (I) complex of bis (diphenylphosphano) methane and thiourea. Z. Nat. B 2019, 74, 745–750. [Google Scholar]
- Fernandes, J.A.; Segura, D.F.; Netto, A.V.G.; Frem, R.C.G.; Mauro, A.E.; Paz, F.A.A. A Tetranuclear Silver–Thiourea Cluster. J. Chem. Crystallogr. 2015, 45, 410–418. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Pakawatchai, C.; Saithong, S.; Skelton, B.W.; White, A.H. 1:1 complexes of silver(i) thiocyanate with (substituted) thiourea ligands. Dalton Trans. 2009, 14, 2588–2598. [Google Scholar] [CrossRef]
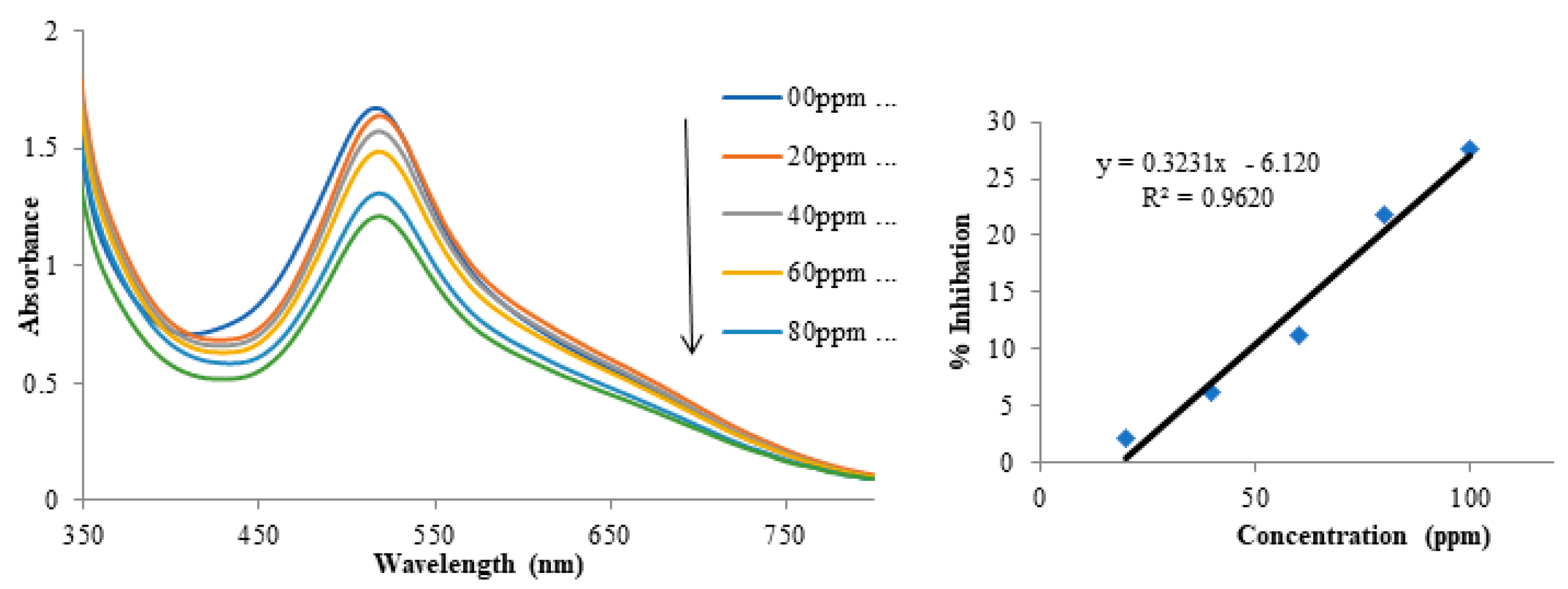
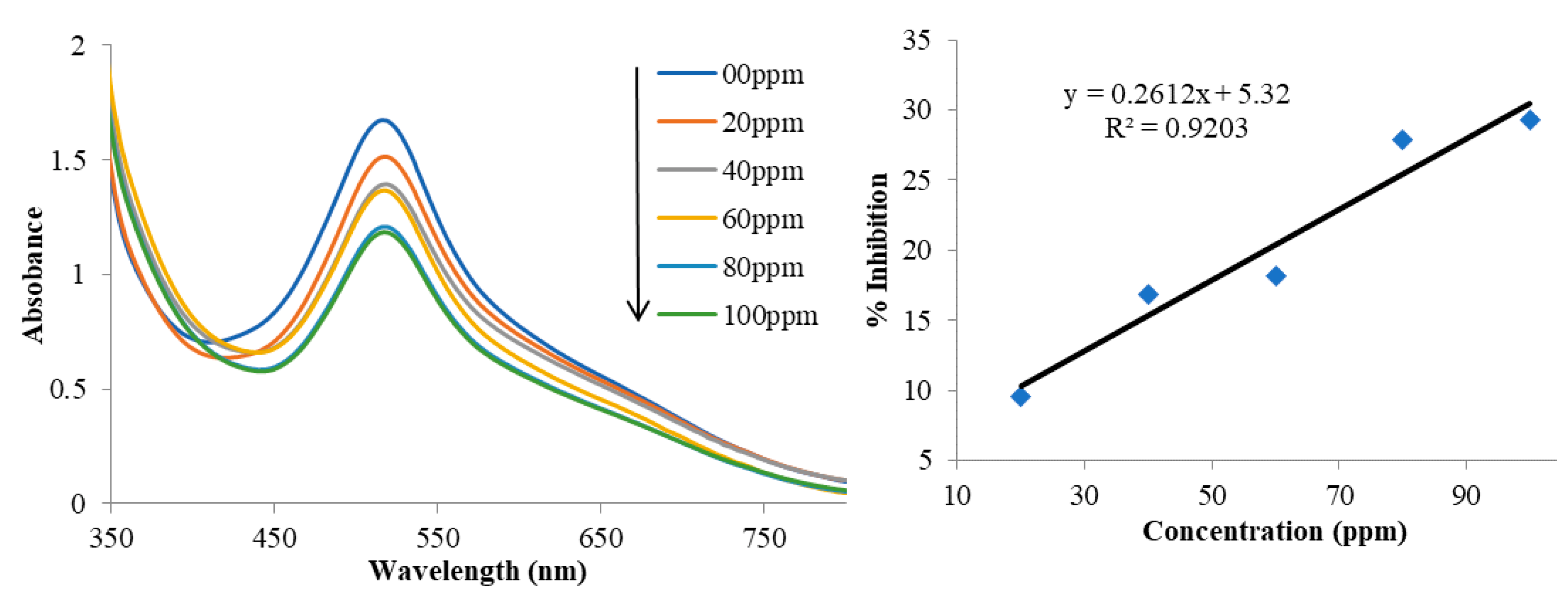
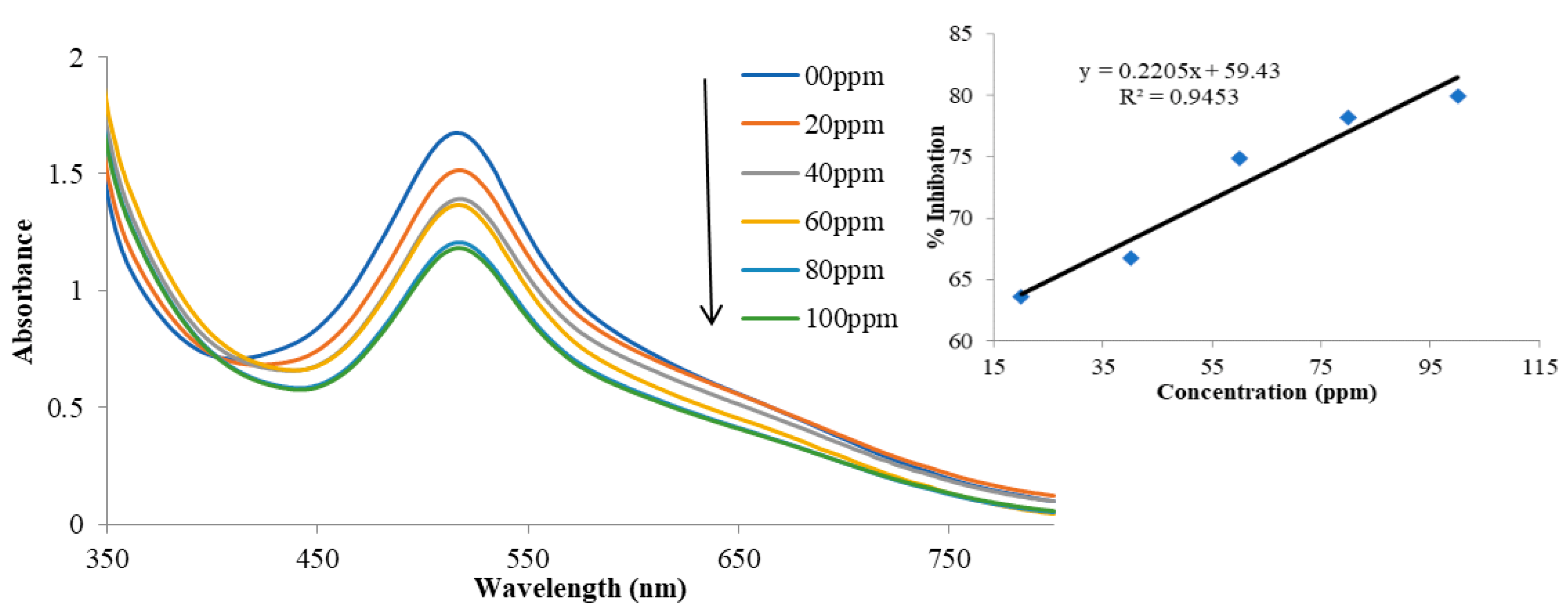

| Concentration (ppm) | 1 | 2 | 3 |
|---|---|---|---|
| 20 | 5.2 | 9.4 | 6.5 |
| 40 | 13.7 | 16.8 | 12.5 |
| 60 | 18.2 | 18.2 | 15.6 |
| 80 | 19.2 | 27.9 | 16.0 |
| 100 | 19.5 | 29.3 | 19.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noor, A.; Shahzad, A.; Khan, E.; Tahir, M.N.; Khan, G.S.; Rashid, A.u.; Said, M. Polynuclear Cu(I) and Ag(I) Complexes of 1,3-Diisobutyl Thiourea, Synthesis, Crystal Structure and Antioxidant Potentials. Inorganics 2022, 10, 185. https://doi.org/10.3390/inorganics10110185
Noor A, Shahzad A, Khan E, Tahir MN, Khan GS, Rashid Au, Said M. Polynuclear Cu(I) and Ag(I) Complexes of 1,3-Diisobutyl Thiourea, Synthesis, Crystal Structure and Antioxidant Potentials. Inorganics. 2022; 10(11):185. https://doi.org/10.3390/inorganics10110185
Chicago/Turabian StyleNoor, Awal, Adnan Shahzad, Ezzat Khan, Muhammad Nawaz Tahir, Gul Shahzada Khan, Amin ur Rashid, and Muhammad Said. 2022. "Polynuclear Cu(I) and Ag(I) Complexes of 1,3-Diisobutyl Thiourea, Synthesis, Crystal Structure and Antioxidant Potentials" Inorganics 10, no. 11: 185. https://doi.org/10.3390/inorganics10110185
APA StyleNoor, A., Shahzad, A., Khan, E., Tahir, M. N., Khan, G. S., Rashid, A. u., & Said, M. (2022). Polynuclear Cu(I) and Ag(I) Complexes of 1,3-Diisobutyl Thiourea, Synthesis, Crystal Structure and Antioxidant Potentials. Inorganics, 10(11), 185. https://doi.org/10.3390/inorganics10110185










