Laser Ablation Synthesis and Characterization of Tb2O3 Nanoparticles for Magneto-Optical Ceramics
Abstract
1. Introduction
2. Results and Discussion
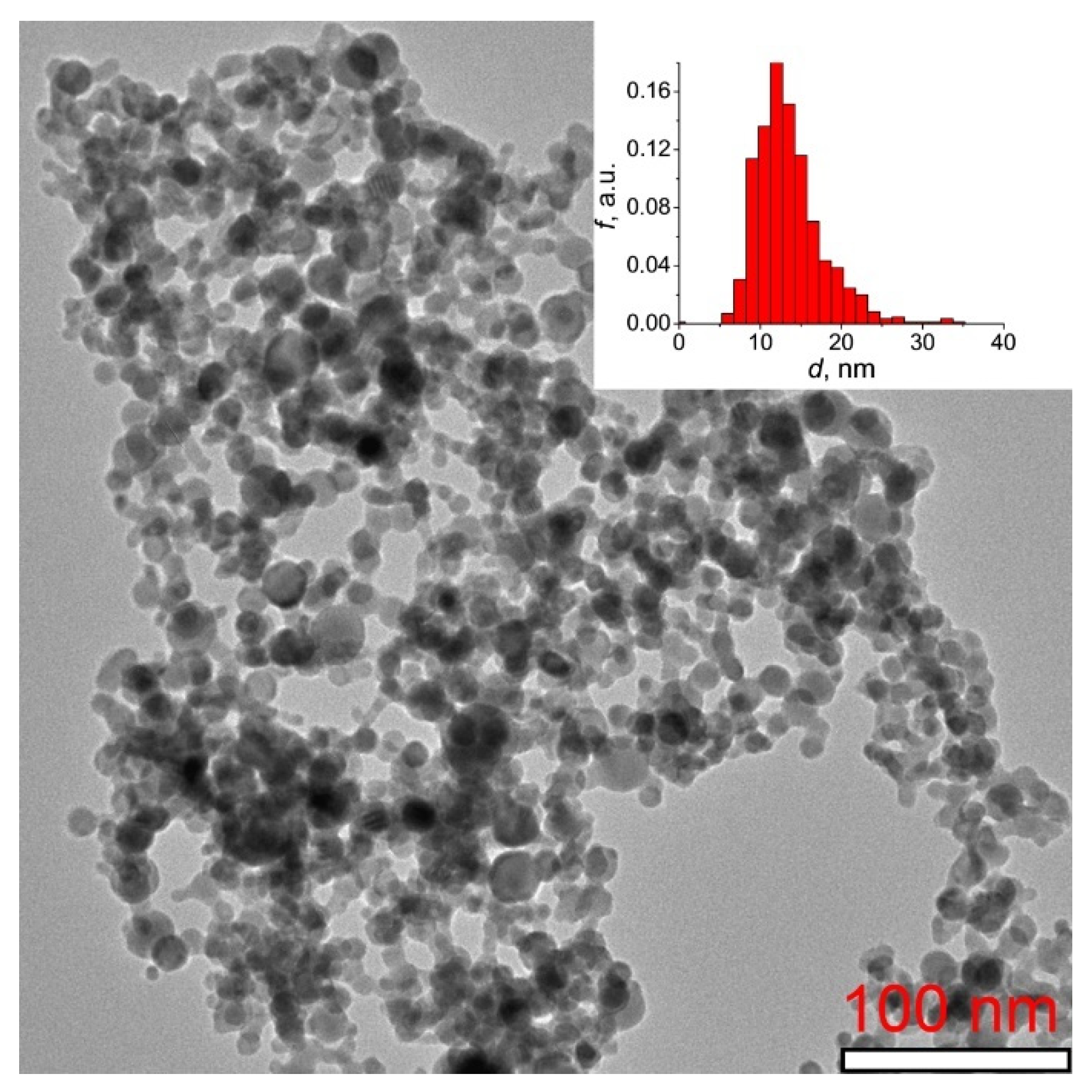
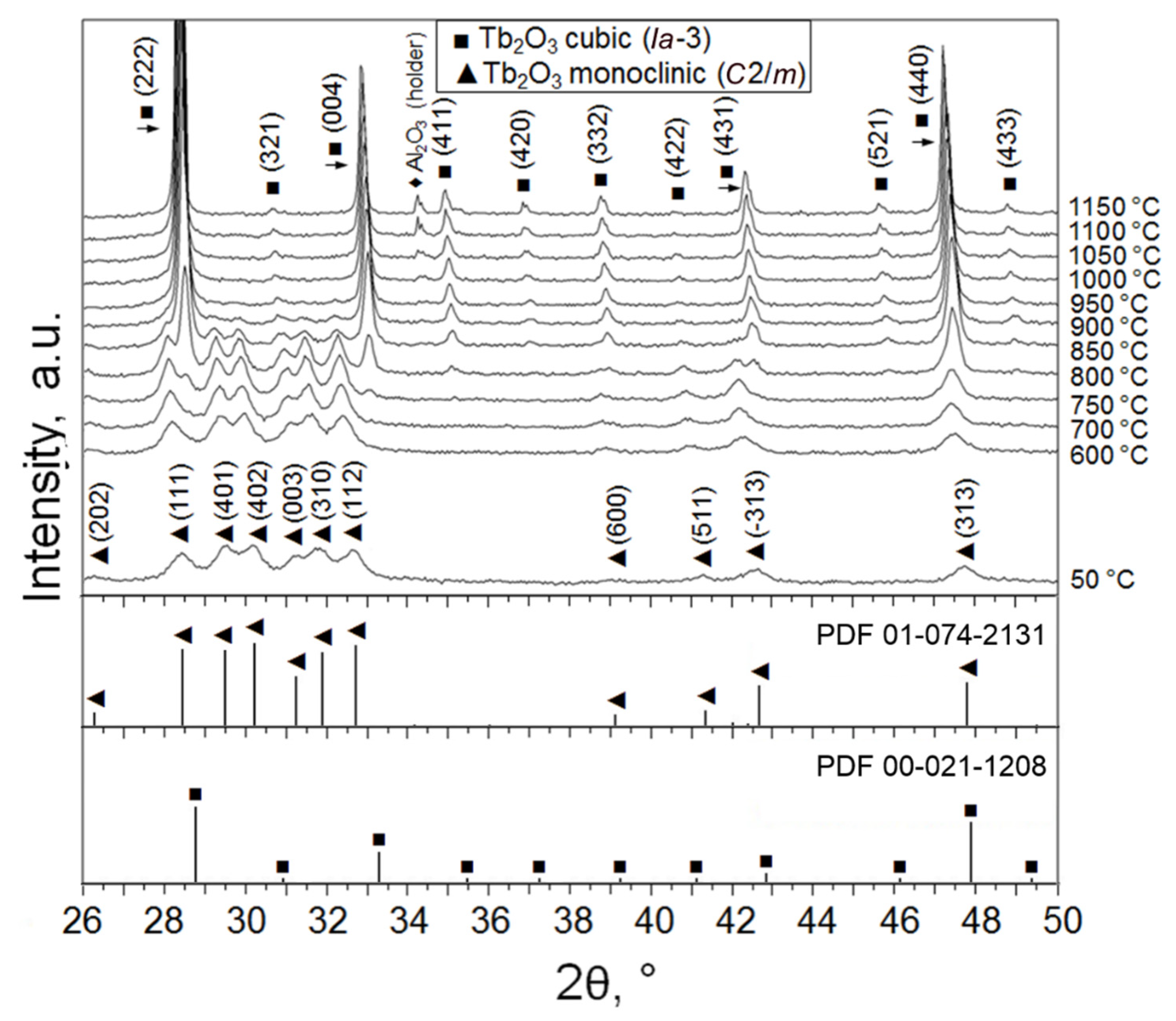
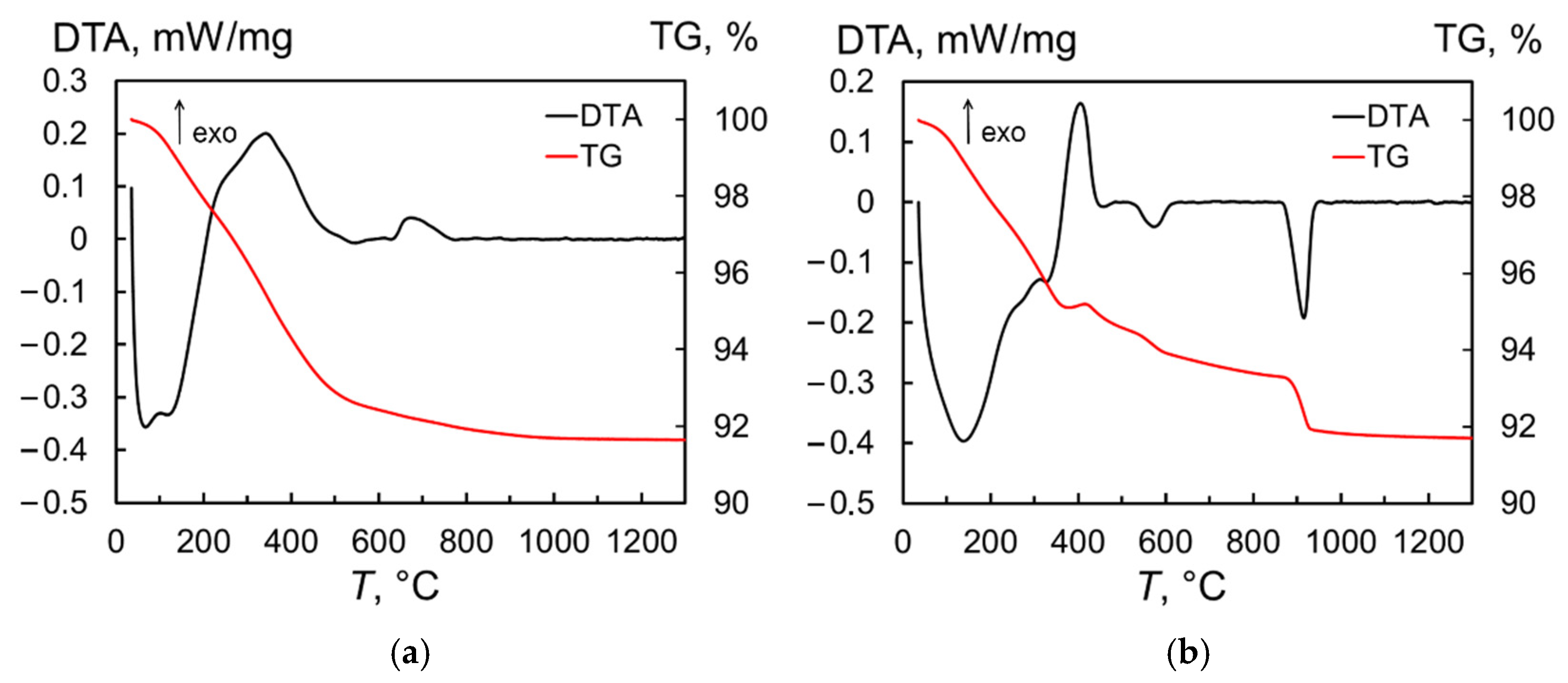
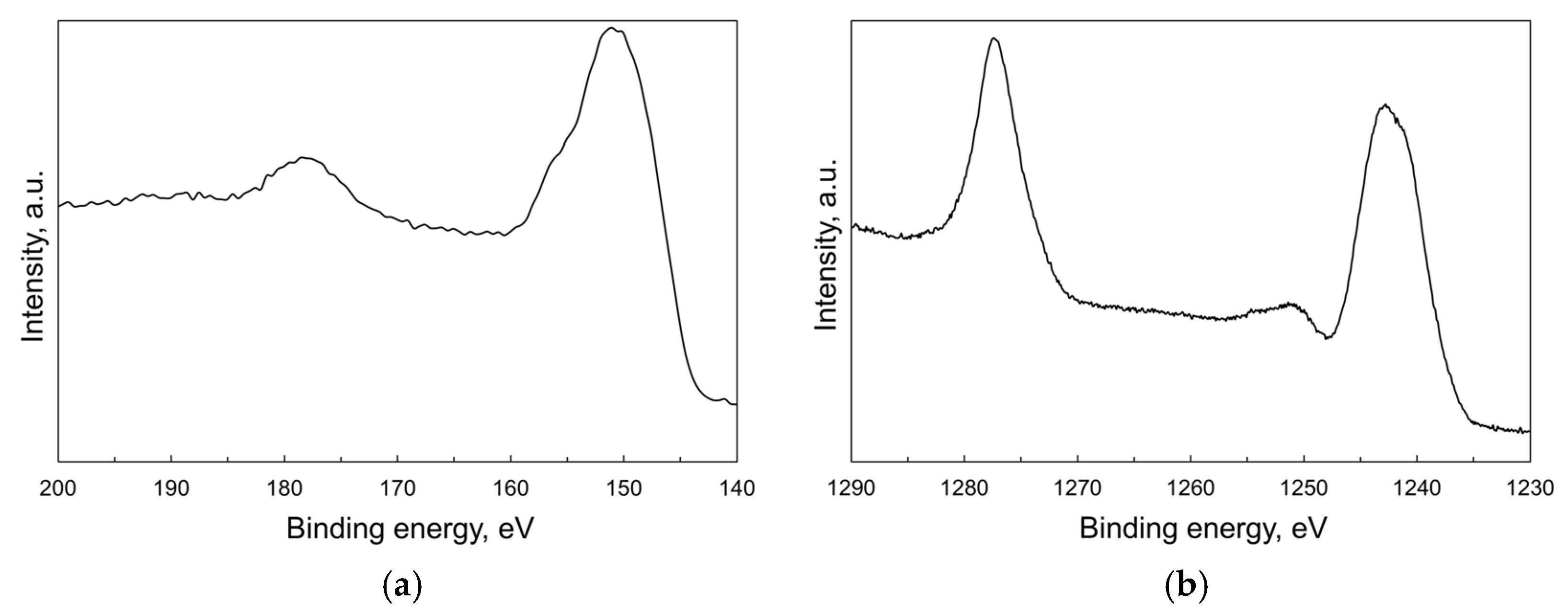
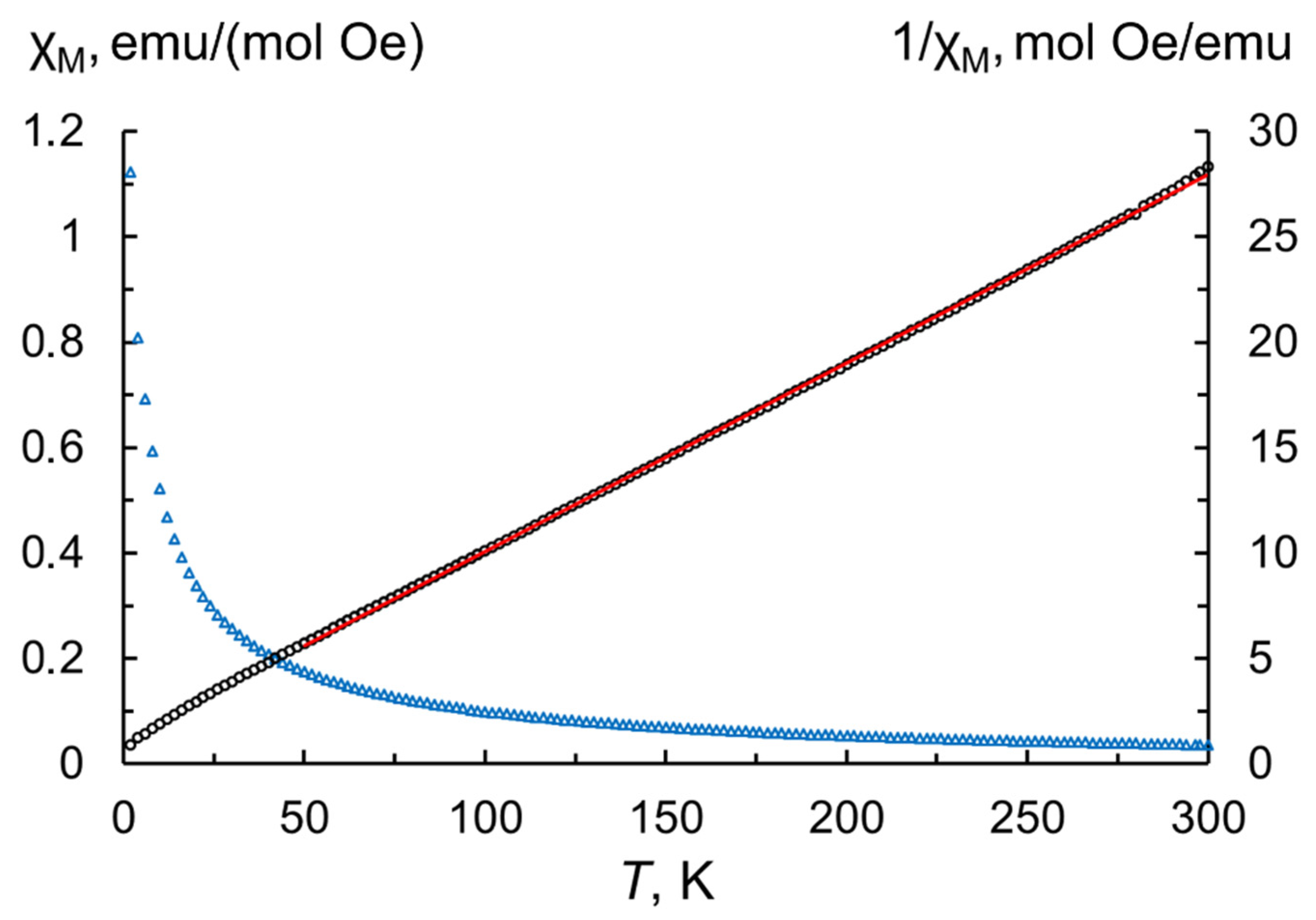
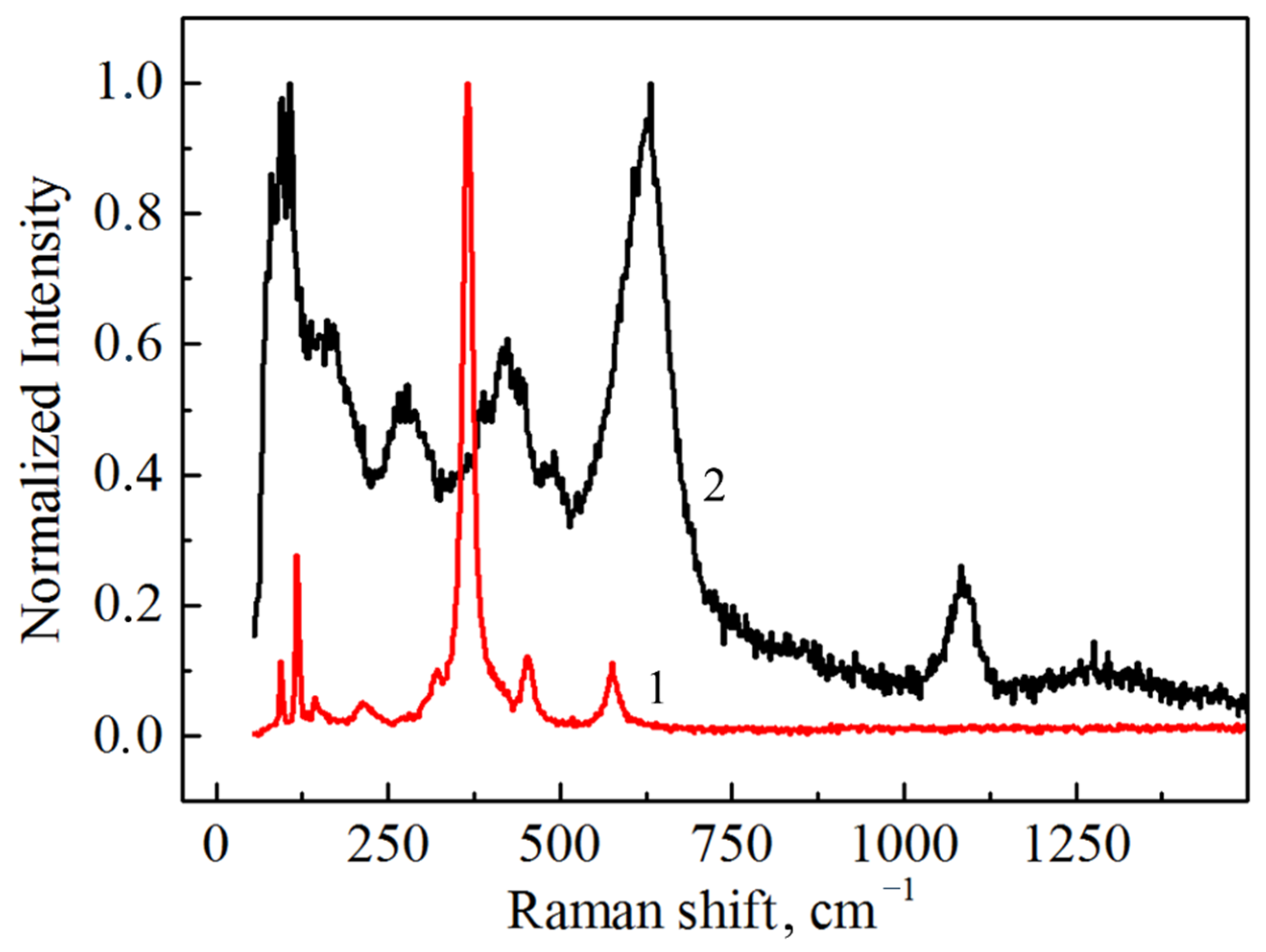
2.1. Properties of Tb2O3 Nanoparticles
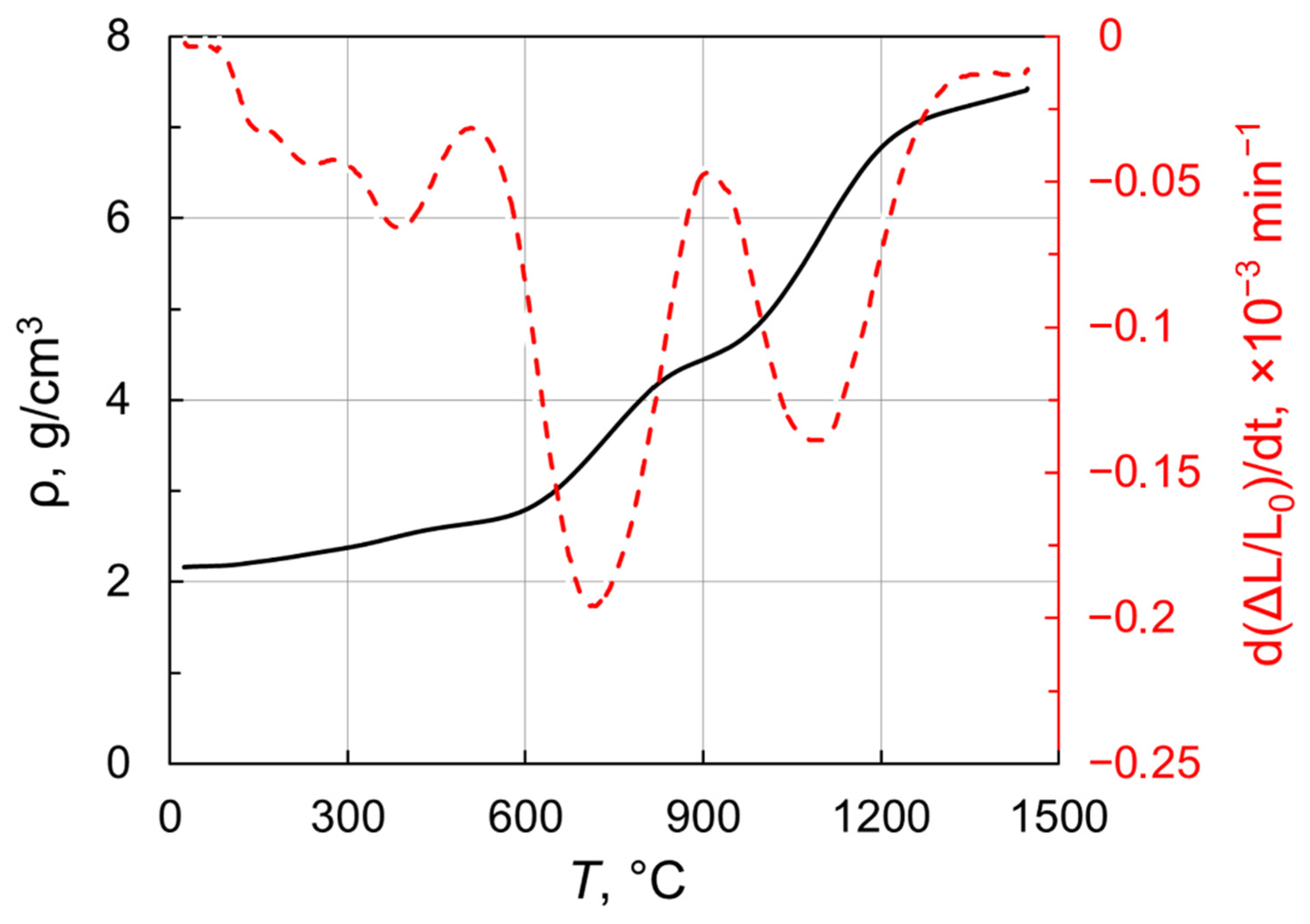
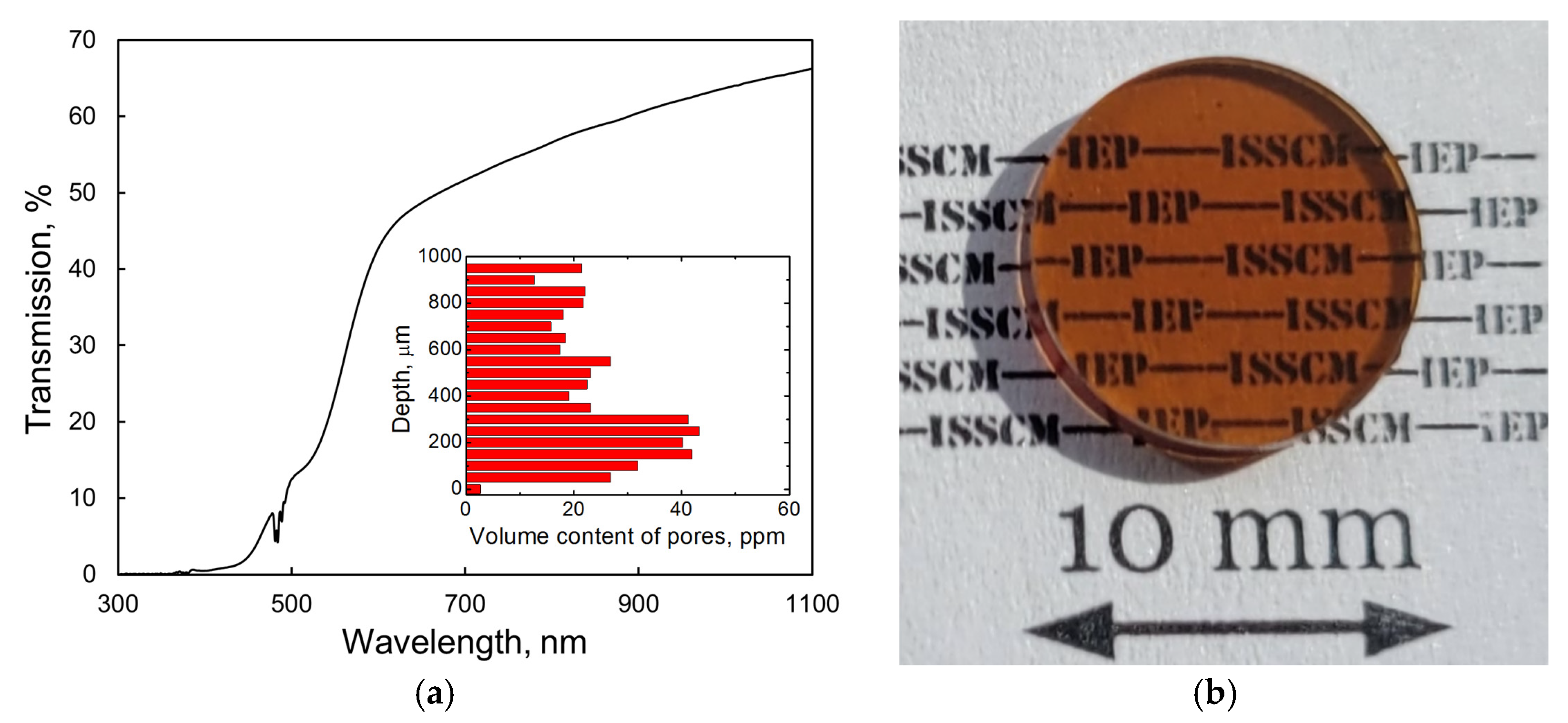
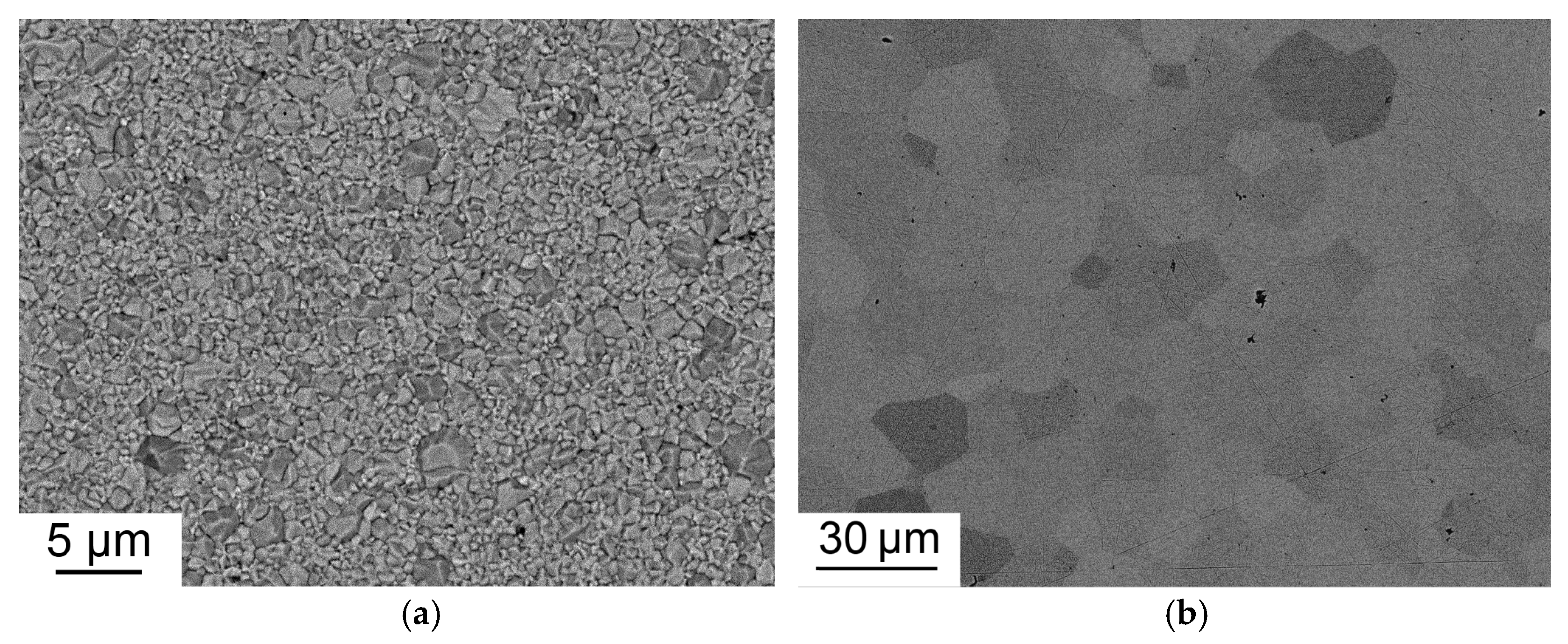
2.2. Characterization of the as-HIPed Tb2O3 Transparent Ceramics
3. Materials and Methods
3.1. Synthesis of Nanopowder
3.2. Fabrication of Tb2O3 Ceramic
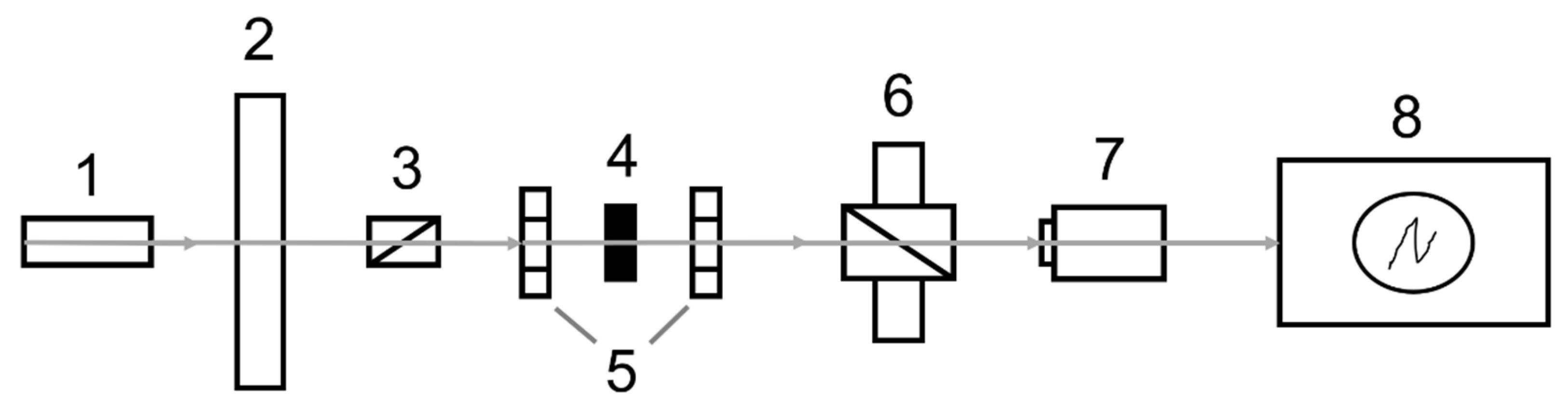
3.3. Characterization Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carothers, K.J.; Norwood, R.A.; Pyun, J. High Verdet constant materials for magneto-optical Faraday rotation: A Review. Chem. Mater. 2022, 34, 2531–2544. [Google Scholar] [CrossRef]
- Vojna, D.; Slezak, O.; Lucianetti, A.; Mocek, T. Verdet constant of magneto-active materials developed for high-power Faraday devices. Appl. Sci. 2019, 9, 3160. [Google Scholar] [CrossRef]
- Dai, J.; Li, J. Promising magneto-optical ceramics for high power Faraday isolators. Scr. Mater. 2018, 155, 78–84. [Google Scholar] [CrossRef]
- Veber, P.; Velazquez, M.; Gadret, G.; Rytz, D.; Peltz, M.; Decourt, R. Flux growth at 1230 °C of cubic Tb2O3 single crystals and characterization of their optical and magnetic properties. CrystEngComm 2015, 17, 492–497. [Google Scholar] [CrossRef]
- Ikesue, A.; Aung, Y.L.; Makikawa, S.; Yahagi, A. Polycrystalline (TbxY1−x)2O3 Faraday rotator. Opt. Lett. 2017, 42, 4399–4401. [Google Scholar] [CrossRef]
- Ikesue, A.; Aung, Y.L.; Makikawa, S.; Yahagi, A. Total performance of magneto-optical ceramics with a bixbyite structure. Materials 2019, 12, 421. [Google Scholar] [CrossRef]
- Balabanov, S.S.; Permin, D.A.; Rostokina, E.Y.; Egorov, S.V.; Sorokin, A.A.; Kuznetsov, D.D. Synthesis and structural characterization of ultrafine terbium oxide powders. Ceram. Int. 2017, 43, 16569–16574. [Google Scholar] [CrossRef]
- Zinkevich, M. Thermodynamics of rare earth sesquioxides. Prog. Mater. Sci. 2007, 52, 597–647. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Wang, J.; Wang, D.; Han, D.; Zhang, J.; Wang, S. Phase transformation process of Tb2O3 at elevated temperatures. Scr. Mater. 2019, 171, 108–111. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, D.; Xu, J.; Tian, T.; Jia, R.; Wang, Z. Fabrication and magneto-optical property of yttria stabilized Tb2O3 transparent ceramics. J. Eur. Ceram. Soc. 2019, 39, 5005–5009. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Wang, J.; Wang, D.; Han, D.; Zhang, J.; Wang, S. Preparation of (Tb1−xLux)2O3 transparent ceramics by solid solution for magneto-optical application. J. Eur. Ceram. Soc. 2021, 41, 2818–2825. [Google Scholar] [CrossRef]
- Balabanov, S.S.; Permin, D.A.; Rostokina, E.Y.; Palashov, O.V.; Snetkov, I.L. Characterizations of REE:Tb2O3 magneto-optical ceramics. Phys. Status Solidi B 2020, 257, 1900474. [Google Scholar] [CrossRef]
- Hu, D.; Li, X.; Zhang, L.; Snetkov, I.; Chen, P.; Dai, Z.; Balabanov, S.; Palashov, O.; Li, J. Terbium (III) oxide (Tb2O3) transparent ceramics by two-step sintering from precipitated powder. Magnetochemistry 2022, 8, 73. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Ma, J.; Ni, M.; Yang, F.; Liu, P.; Lee, K.Y.; Hsiang, H.-I.; Shen, D.; Tang, D. Fabrication of high-efficiency Yb:Y2O3 laser ceramics without photodarkening. J. Am. Ceram. Soc. 2022, 105, 3375–3381. [Google Scholar] [CrossRef]
- Yin, D.; Ma, J.; Liu, P.; Yao, B.; Wang, J.; Dong, Z.; Kong, L.B.; Tang, D. Submicron-grained Yb:Lu2O3 transparent ceramics with lasing quality. J. Am. Ceram. Soc. 2019, 102, 2587–2592. [Google Scholar] [CrossRef]
- Ikesue, A.; Aung, Y.L. Synthesis of Yb:YAG ceramics without sintering additives and their performance. J. Am. Ceram. Soc. 2017, 100, 26–30. [Google Scholar] [CrossRef]
- Wang, J.; Yin, D.; Ma, J.; Liu, P.; Wang, Y.; Dong, Z.; Kong, L.B.; Tang, D. Pump laser induced photodarkening in ZrO2-doped Yb:Y2O3 laser ceramics. J. Eur. Ceram. Soc. 2019, 39, 635–640. [Google Scholar] [CrossRef]
- Gaume, R.; He, Y.; Markosyan, A.; Byer, R.L. Effect of Si-induced defects on 1 µm absorption losses in laser-grade YAG ceramics. J. Appl. Phys. 2012, 111, 093104. [Google Scholar] [CrossRef]
- Fursikov, P.V.; Abdusalyamova, M.N.; Makhmudov, F.A.; Shairmardanov, E.N.; Kovalev, I.D.; Kovalev, D.Y.; Morgunov, R.B.; Koplak, O.V.; Volodin, A.A.; Khodos, I.I.; et al. Structural features and magnetic behavior of nanocrystalline powders of terbium oxide prepared by the thermal decomposition of terbium acetate in air. J. Alloys Compd. 2016, 657, 163–173. [Google Scholar] [CrossRef]
- Kai, F.; Bin, L.; Hongmei, C.; Shaofan, W.; Yan, W.; Yongxing, L. Synthesis of ultrafine TbO1.81 and Tb2O3 powders for magneto-optical application. J. Synth. Cryst. 2021, 50, 80–87. [Google Scholar]
- Abu-Zied, B.M.; Mohamed, A.-R.N.; Asiri, A.M. Effect of thermal treatment on the formation, textural and electrical conductivity properties of nanocrystalline Tb4O7. J. Nanosci. Nanotechnol. 2015, 15, 4487–4492. [Google Scholar] [CrossRef]
- Kurland, H.-D.; Grabow, J.; Stotzel, C.; Muller, F.A. Preparation of ceramic nanoparticles by CO2 laser vaporization. J. Ceram. Sci. Technol. 2014, 5, 275–280. [Google Scholar]
- Osipov, V.V.; Platonov, V.V.; Lisenkov, V.V.; Tikhonov, E.V.; Podkin, A.V. Study of nanoparticle production from yttrium oxide by pulse-periodic radiation of ytterbium fibre laser. Appl. Phys. A 2018, 124, 3. [Google Scholar] [CrossRef]
- Maksimov, R.N.; Shitov, V.A.; Platonov, V.V.; Yurovskikh, A.S.; Toci, G.; Patrizi, B.; Vannini, M.; Pirri, A. Hot isostatic pressing of transparent Yb3+-doped Lu2O3 ceramics for laser applications. Ceram. Int. 2021, 47, 5168–5176. [Google Scholar] [CrossRef]
- Baenziger, N.C.; Eick, H.A.; Schuldt, H.S.; Eyring, L. Terbium oxides. III. X-ray diffraction studies of several stable phases. J. Am. Chem. Soc. 1961, 83, 2219–2223. [Google Scholar] [CrossRef]
- MacChesney, J.B.; Williams, H.J.; Sherwood, R.C.; Potter, J.F. Preparation and low-temperature magnetic properties of the terbium oxides. J. Chem. Phys. 1966, 44, 596–601. [Google Scholar] [CrossRef]
- Velazquez, M.; Pechev, S.; Duttine, M.; Wattiaux, A.; Labrugere, C.; Veber, P.; Buffiere, S.; Denux, D. Point defect disorder in high-temperature solution grown Sr6Tb0.94Fe1.06(BO3)6 single crystals. J. Solid State Chem. 2018, 264, 91–97. [Google Scholar] [CrossRef]
- Sarma, D.D.; Rao, C.N.R. XPES studies of oxides of second- and third-row transition metals including rare earth. J. Electron Spectrosc. Relat. Phenom. 1980, 20, 25–45. [Google Scholar] [CrossRef]
- Blanco, G.; Pintado, J.M.; Bernal, S.; Cauqui, M.A.; Corchado, M.P.; Galtayries, A.; Ghijsen, J.; Sporken, R.; Eickhoff, T.; Drube, W. Influence of the nature of the noble metal (Rh,Pt) on the low-temperature reducibility of a Ce/Tb mixed oxide with application as TWC component. Surf. Interface Anal. 2002, 34, 120–124. [Google Scholar] [CrossRef]
- Glatzle, M.; Janka, O.; Svitlyk, V.; Chernyshov, D.; Bartsch, M.; Zacharias, H.; Pottgen, R.; Huppertz, H. The high-pressure oxide Tb3O5 and its non-centrosymmetric low-temperature polymorph—A comprehensive study. Chem. Eur. J. 2018, 24, 15236–15245. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, C. Observation of Eu2+ and Tb4+ in SrMgF4:Eu3+, Tb3+. Solid State Commun. 1995, 95, 319–322. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Neyts, K.A.; De Visschere, P.; Corlatan, D.; Pauwels, H.; Vercaemst, R.; Fiermans, L.; Poelman, D.; Van Meirhaeghe, R.L.; Lafrele, W.H.; et al. XPS Study of TbF, and TbOF Centres in ZnS. Phys. Status Solidi A 1994, 146, K67–K70. [Google Scholar] [CrossRef]
- Mugiraneza, S.; Hallas, A.M. Tutorial: A beginner’s guide to interpreting magnetic susceptibility data with the Curie-Weiss law. Commun. Phys. 2022, 5, 95. [Google Scholar] [CrossRef]
- Vickery, R.C.; Ruben, A. Magnetic susceptibilities of praseodymium and terbium oxides. J. Chem. Soc. 1959, 510–513. [Google Scholar] [CrossRef]
- Balabanov, S.S.; Permin, D.A.; Rostokina, E.Y.; Egorov, S.V.; Sorokin, A.A. Sinterability of nanopowders of terbia solid solutions with scandia, yttria, and lutetia. J. Adv. Ceram. 2018, 7, 362–369. [Google Scholar] [CrossRef]
- Ikesue, A.; Aung, Y.L.; Lupei, V. Ceramic Lasers; Cambridge University Press: Cambridge, UK, 2013; p. 152. [Google Scholar]
- Biswas, P.; Chakravarty, D.; Suresh, M.B.; Johnson, R.; Mohan, M.K. Fabrication of graphite contamination free polycrystalline transparent MgAl2O4 spinel by spark plasma sintering using platinum foil. Ceram. Int. 2016, 42, 18920–18923. [Google Scholar] [CrossRef]
- Wang, P.; Yang, M.; Zhang, S.; Tu, R.; Goto, T.; Zhang, L. Suppression of carbon contamination in SPSed CaF2 transparent ceramics by Mo foil. J. Eur. Ceram. Soc. 2017, 37, 4103–4107. [Google Scholar] [CrossRef]
- Gan, L.; Park, Y.-J.; Park, M.-J.; Kim, H.; Kim, J.-M.; Ko, J.-W.; Lee, J.-W. Facile fabrication of highly transparent yttria ceramics with fine microstructures by a hot-pressing method. J. Am. Ceram. Soc. 2015, 98, 2002–2004. [Google Scholar] [CrossRef]
- Chen, T.; Xu, Y.; Li, L.; Zhu, J.; Cong, M.; Song, J. Eliminate of colour center and growth of large-size Tb3Ga5O12 crystals. J. Cryst. Growth 2021, 562, 126090. [Google Scholar] [CrossRef]
- Ibanez, J.; Sans, J.A.; Cuenca-Gotor, V.; Oliva, R.; Gomis, O.; Rodríguez-Hernandez, P.; Munoz, A.; Rodriguez-Mendoza, U.; Velazquez, M.; Veber, P.; et al. Structural and lattice-dynamical properties of Tb2O3 under compression: A comparative study with rare earth and related sesquioxides. Inorg. Chem. 2020, 59, 9648–9666. [Google Scholar] [CrossRef]
- Candela, M.T.; Aguado, F.; Gonzalez-Lavín, J.; Gonzalez, J.A.; Valiente, R. Modification of the spectroscopic properties of Tb2O3 phosphor under the high-pressure phase transitions sequence. J. Alloys Compd. 2021, 859, 157899. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Slezak, O.; Yasuhara, R.; Lucianetti, A.; Mocek, T. Temperature-wavelength dependence of terbium gallium garnet ceramics Verdet constant. Opt. Mater. Express 2016, 6, 3683–3691. [Google Scholar] [CrossRef]
| Composition | Main Impurities | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Si | Ca | Ti | Fe | Zn | Ce | Nd | Gd | Dy | Yb | |
| Content, ppm | 2 | 10 | 3 | 5 | 10 | 2 | 50 | 7 | 4 | 5 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimov, R.N.; Osipov, V.V.; Karagedov, G.R.; Platonov, V.V.; Yurovskikh, A.S.; Orlov, A.N.; Spirina, A.V.; Shitov, V.A. Laser Ablation Synthesis and Characterization of Tb2O3 Nanoparticles for Magneto-Optical Ceramics. Inorganics 2022, 10, 173. https://doi.org/10.3390/inorganics10100173
Maksimov RN, Osipov VV, Karagedov GR, Platonov VV, Yurovskikh AS, Orlov AN, Spirina AV, Shitov VA. Laser Ablation Synthesis and Characterization of Tb2O3 Nanoparticles for Magneto-Optical Ceramics. Inorganics. 2022; 10(10):173. https://doi.org/10.3390/inorganics10100173
Chicago/Turabian StyleMaksimov, Roman N., Vladimir V. Osipov, Garegin R. Karagedov, Vyacheslav V. Platonov, Artem S. Yurovskikh, Albert N. Orlov, Alfiya V. Spirina, and Vladislav A. Shitov. 2022. "Laser Ablation Synthesis and Characterization of Tb2O3 Nanoparticles for Magneto-Optical Ceramics" Inorganics 10, no. 10: 173. https://doi.org/10.3390/inorganics10100173
APA StyleMaksimov, R. N., Osipov, V. V., Karagedov, G. R., Platonov, V. V., Yurovskikh, A. S., Orlov, A. N., Spirina, A. V., & Shitov, V. A. (2022). Laser Ablation Synthesis and Characterization of Tb2O3 Nanoparticles for Magneto-Optical Ceramics. Inorganics, 10(10), 173. https://doi.org/10.3390/inorganics10100173







