Influence of Ag Nanowires with Different Morphologies on Light Trapping Abilities and Optoelectronic Properties of Ag Nanowires/ZnO:Al Nanorods Composite Films
Abstract
:1. Introduction
2. Experimental Method
3. Results and Discussion
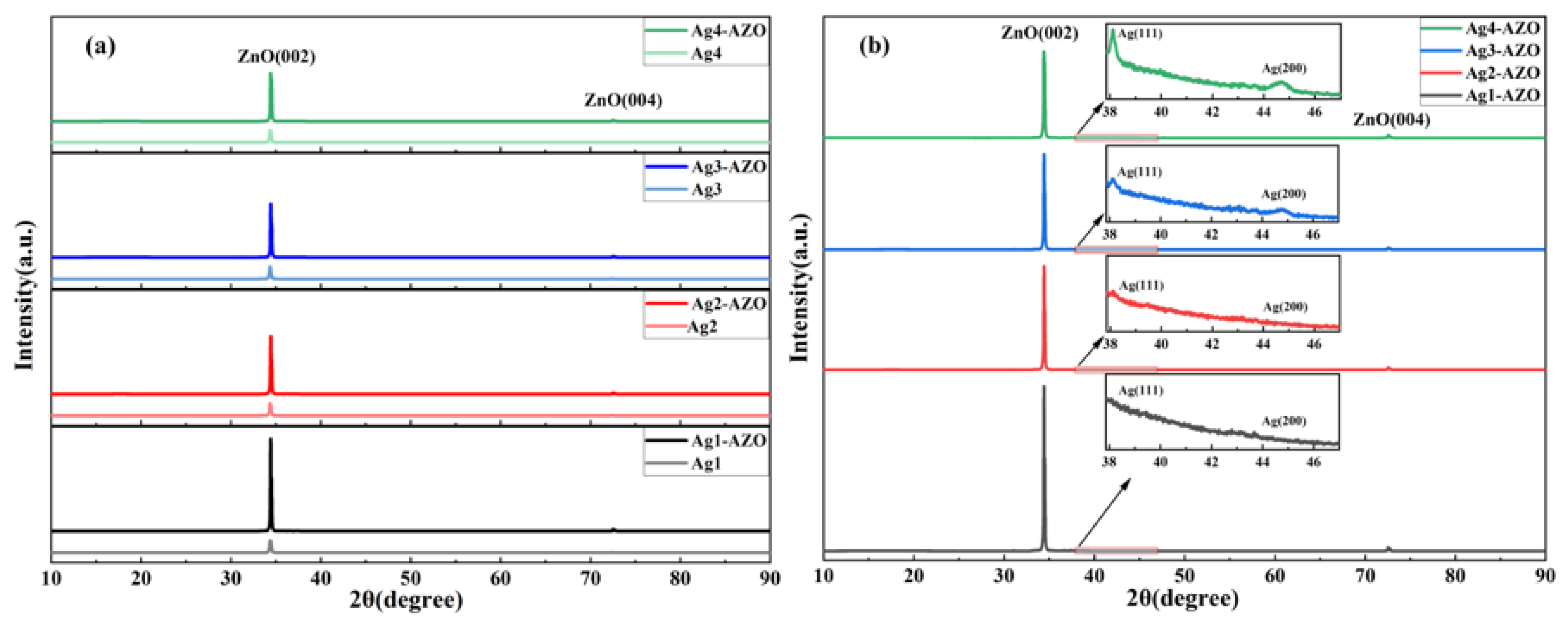
3.1. Structural Properties
3.2. Surface Morphology
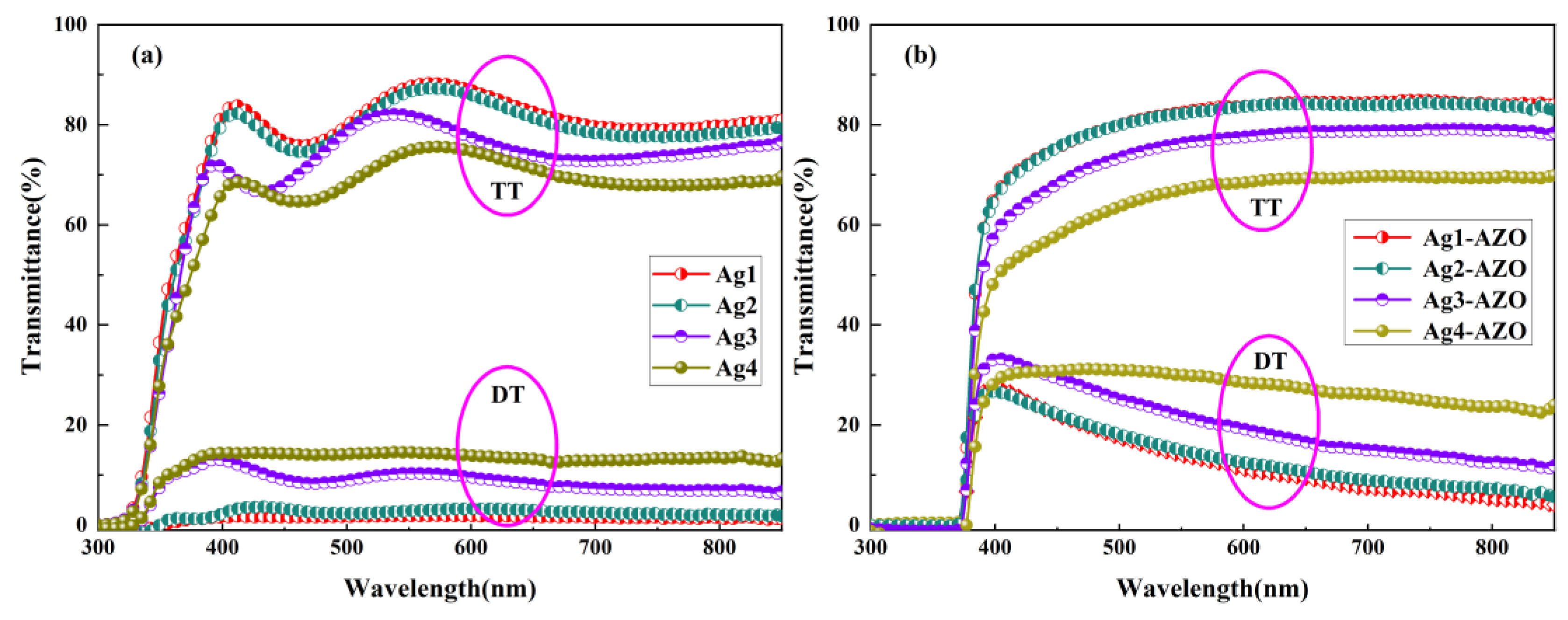
3.3. Optical Properties
3.4. Light Trapping Ability
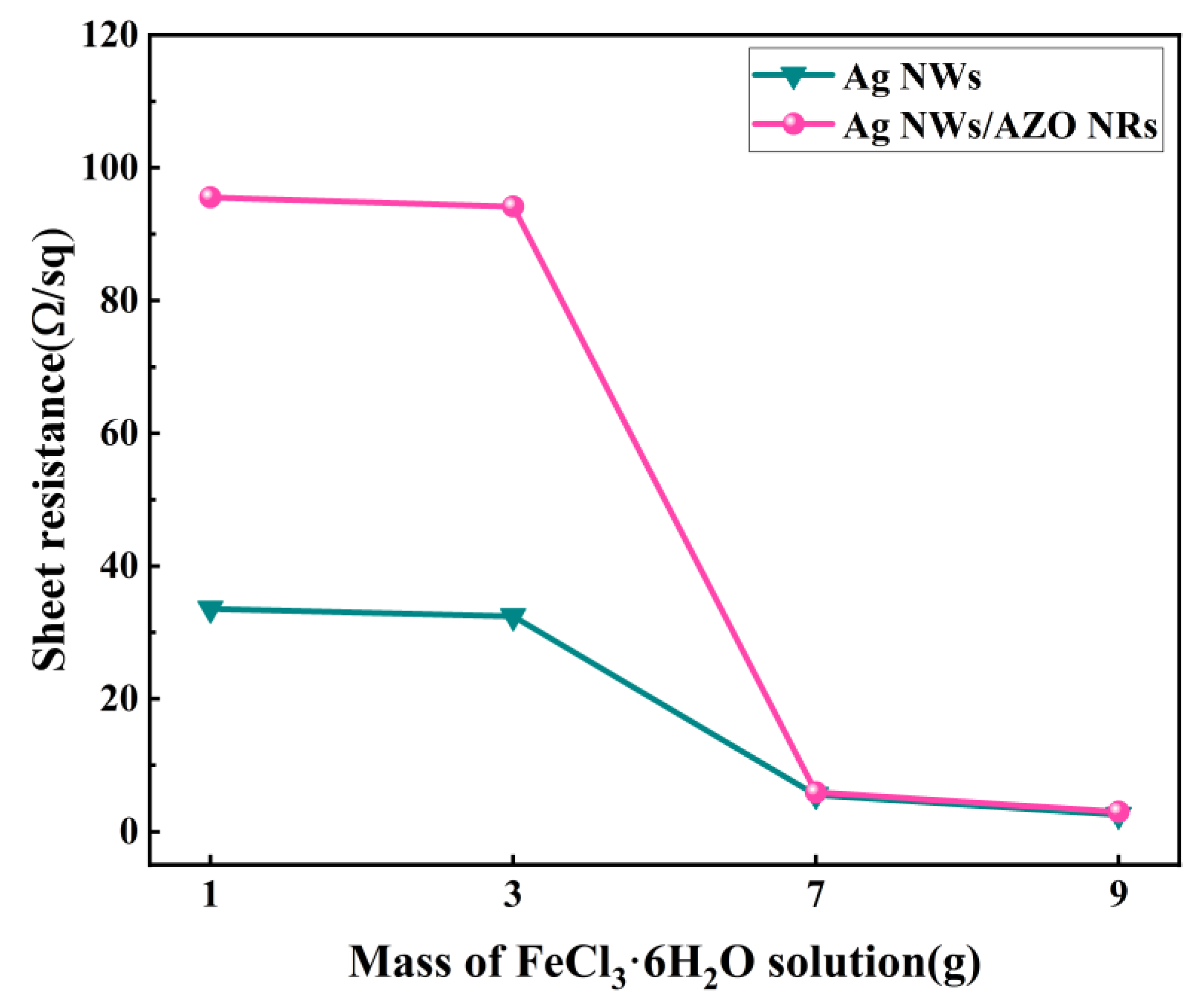
3.5. Electrical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, Q.H.; Chen, C.Y.; Liao, X.B.; Xiang, X.B.; Cao, X.M.; Ingler, W.; Adiga, N.; Deng, X.M. Spectroscopic aspects of front transparent conductive films for a-Si thin film solar cells. J. Appl. Phys. 2010, 107, 034505. [Google Scholar] [CrossRef]
- Kao, Y.C.; Ou, S.L.; Wu, F.L.; Horng, R.H. Performance enhancement of III–V multi-junction solar cells using indium-tin-oxide electrodes. Thin Solid Films 2016, 612, 36–40. [Google Scholar] [CrossRef]
- Dai, P.; Lu, J.Y.; Tan, M.; Wang, Q.S.; Wu, Y.Y.; Ji, L.; Bian, L.F.; Lu, S.L.; Yang, H. Transparent conducting indium-tin-oxide (ITO) film as full front electrode in III–V compound solar cell. Chin. Phys. B 2017, 26, 037305. [Google Scholar] [CrossRef]
- Asemi, M.; Ahmadi, M.; Ghanaatshoar, M. Preparation of highly conducting Al-doped ZnO target by vacuum heat-treatment for thin film solar cell applications. Ceram. Int. 2018, 44, 12862–12868. [Google Scholar] [CrossRef]
- Liu, Z.X.; Yang, C.; Ge, D.H.; Yang, P. Optoelectronic properties of AZO/ZnO bilayer. J. Alloys Compd. 2020, 816, 152531. [Google Scholar] [CrossRef]
- Kumar, A.; Ahmad, I. Role of defects and microstructure on the electrical properties of solution-processed Al-doped ZnO transparent conducting films. Appl. Phys. A Mater. Sci. Process. 2020, 126, 598. [Google Scholar] [CrossRef]
- Hüpkes, J.; Jost, G.C.E.; Merdzhanova, T.; Owen, J.I.; Zimmermann, T. Coupling and trapping of light in thin-film solar cells using modulated interface textures. Appl. Sci. 2019, 9, 4648. [Google Scholar] [CrossRef]
- Hertel, K.; Hüpkes, J.; Pflaum, C. An image processing approach to approximating interface textures of microcrystalline silicon layers grown on existing aluminum-doped zinc oxide textures. Opt. Express 2013, 21, A977–A990. [Google Scholar] [CrossRef]
- Das, G.; Bose, S.; Sharma, J.R.; Mukhopadhyay, S.; Barua, A.K. Texturization of ZnO:Al surface by reactive ion etching in SF6/Ar, CHF3/Ar plasma for application in thin film silicon solar cells. J. Mater. Sci. Mater. Electron. 2018, 29, 6206–6214. [Google Scholar] [CrossRef]
- Jiang, Q.J.; Lu, J.G.; Zhang, J.; Yuan, Y.L.; Cai, H.; Hu, L.; Feng, L.S.; Lu, B.; Pan, X.H.; Ye, Z.Z. Texture-etched broad surface features of double-layered ZnO:Al transparent conductive films for high haze values. J. Alloys Compd. 2014, 596, 107–112. [Google Scholar] [CrossRef]
- Hussain, S.Q.; Le, A.H.T.; Mallem, K.; Park, H.; Ju, M.; Kim, Y.; Cho, J.; Park, J.; Kim, Y.; Yi, J. Using the light scattering properties of multi-textured AZO films on inverted hemisphere textured glass surface morphologies to improve the efficiency of silicon thin film solar cells. Appl. Surf. Sci. 2018, 447, 866–875. [Google Scholar] [CrossRef]
- Nowak, R.E.; Vehse, M.; Sergeev, O.; von Maydell, K.; Agert, C. ZnO nanorod arrays as light trapping structures in amorphous silicon thin-film solar cells. Sol. Energy Mater. Sol. Cells 2014, 125, 305–309. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, D.Q.; Wang, Y.; Tao, C.L.; Jia, H.B.; Zhao, W.P. Efficient light trapping from nanorod-like single-textured Al-doped ZnO transparent conducting films. Coatings 2021, 11, 513. [Google Scholar] [CrossRef]
- Preston, C.; Xu, Y.L.; Han, X.G.; Munday, J.N.; Hu, L.B. Optical haze of transparent and conductive silver nanowire films. Nano Res. 2013, 6, 461–468. [Google Scholar] [CrossRef]
- Yu, X.M.; Yu, X.; Zhang, J.J.; Zhang, D.K.; Ni, J.; Cai, H.K.; Zhang, D.X.; Zhao, Y. Investigation of light transmission and scattering properties in silver nanowire mesh transparent electrodes. Mater. Lett. 2015, 145, 219–223. [Google Scholar] [CrossRef]
- Bari, B.; Lee, J.; Jang, T.; Won, P.; Ko, S.H.; Alamgir, K.; Arshad, M.; Guo, L.J. Simple hydrothermal synthesis of very-long and thin silver nanowires and their application in high quality transparent electrodes. J. Mater. Chem. A 2016, 4, 11365–11371. [Google Scholar] [CrossRef]
- Zhan, K.; Su, R.; Bai, S.H.; Yu, Z.H.; Cheng, N.; Wang, C.L.; Xu, S.; Liu, W.; Guo, S.S.; Zhao, X.Z. One-pot stirring-free synthesis of silver nanowires with tunable lengths and diameters via a Fe3+ & Cl− co-mediated polyol method and their application as transparent conductive films. Nanoscale 2016, 8, 18121–18133. [Google Scholar]
- Jiu, J.; Araki, T.; Wang, J.; Nogi, M.; Sugahara, T.; Nagao, S.; Koga, H.; Suganuma, K.; Nakazawa, E.; Hara, M.; et al. Facile synthesis of very-long silver nanowires for transparent electrodes. J. Mater. Chem. A 2014, 2, 6326–6330. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, Y.W.; Kim, J.E.; Yoon, S.W.; Kim, T.Y.; Noh, J.S.; Suh, K.S. Synthesis of dimension-controlled silver nanowires for highly conductive and transparent nanowire films. Acta Mater. 2015, 83, 84–90. [Google Scholar] [CrossRef]
- Lin, J.Y.; Hsueh, Y.L.; Huang, J.J.; Wu, J.R. Effect of silver nitrate concentration of silver nanowires synthesized using a polyol method and their application as transparent conductive films. Thin Solid Films 2015, 584, 243–247. [Google Scholar] [CrossRef]
- Wang, Y.F.; Song, J.M.; Bai, L.S.; Yang, F.; Han, B.; Guo, Y.J.; Dai, B.T.; Zhao, Y.; Zhang, X.D. Management of light trapping capability of AZO film for Si thin film solar cells-via tailoring surface texture. Sol. Energy Mater. Sol. Cells 2018, 179, 401–408. [Google Scholar] [CrossRef]
- Yu, X.M.; Yu, X.; Chen, L.Q.; Zhang, J.J.; Long, Y.Q.; Zhe, L.; Hu, J.F.; Zhang, H.Q.; Wang, Y.N. Investigation the scattering properties of silver nanowires with different densities. Opt. Mater. 2018, 84, 490–497. [Google Scholar] [CrossRef]
- Hussain, S.Q.; Yen, C.; Khan, S.; Kwon, G.D.; Kim, S.; Ahn, S.; Le, A.H.T.; Park, H.; Velumani, S.; Yi, J. Uniform 3D hydrothermally deposited zinc oxide nanorods with high haze ratio. Mater. Sci. Semicond. Process. 2015, 37, 99–104. [Google Scholar] [CrossRef]
- Shan, C.C.; Zhao, M.; Jiang, D.Y.; Li, Q.; Li, M.; Zhou, X.; Duan, Y.H.; Wang, N.; Deng, R. Improved responsivity performance of ZnO film ultraviolet photodetectors by vertical arrays ZnO nanowires with light trapping effect. Nanotechnology 2019, 30, 305703. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, C.; Hu, D.; Wang, Y.; Zhu, J.; Liu, J. Influence of Ag Nanowires with Different Morphologies on Light Trapping Abilities and Optoelectronic Properties of Ag Nanowires/ZnO:Al Nanorods Composite Films. Photonics 2022, 9, 628. https://doi.org/10.3390/photonics9090628
Tao C, Hu D, Wang Y, Zhu J, Liu J. Influence of Ag Nanowires with Different Morphologies on Light Trapping Abilities and Optoelectronic Properties of Ag Nanowires/ZnO:Al Nanorods Composite Films. Photonics. 2022; 9(9):628. https://doi.org/10.3390/photonics9090628
Chicago/Turabian StyleTao, Chunlei, Daqiang Hu, Ying Wang, Jiang Zhu, and Jian Liu. 2022. "Influence of Ag Nanowires with Different Morphologies on Light Trapping Abilities and Optoelectronic Properties of Ag Nanowires/ZnO:Al Nanorods Composite Films" Photonics 9, no. 9: 628. https://doi.org/10.3390/photonics9090628



