Chlorophyll Fluorescence Imaging (CFI) and Laser-Induced Breakdown Spectroscopy (LIBS) Applied to Investigate Tomato Plants Infected by the Root Knot Nematode (RKN) Meloidogyne incognita and Tobacco Plants Infected by Cymbidium Ringspot Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tomato Plant and Phytoparasitic Nematode
2.2. Tobacco Plant and Cymbidium Ringspot Virus
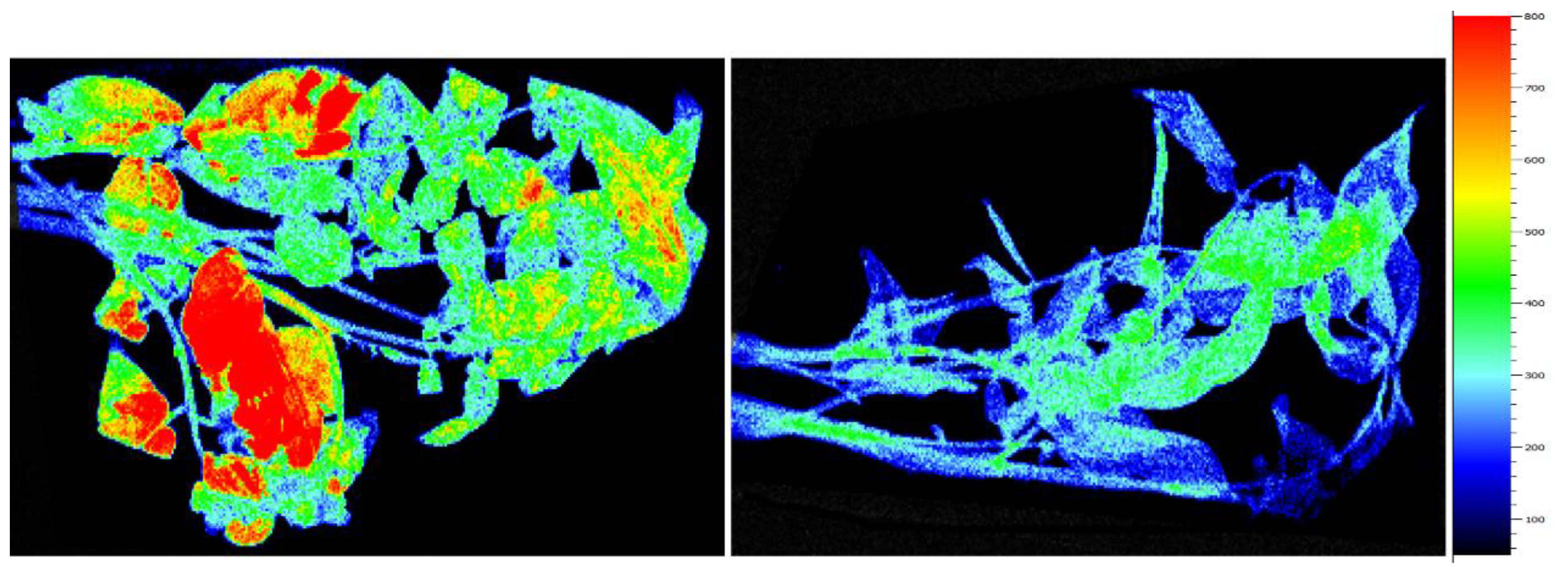
2.3. Chlorophyll Fluorescence Imaging (CFI)
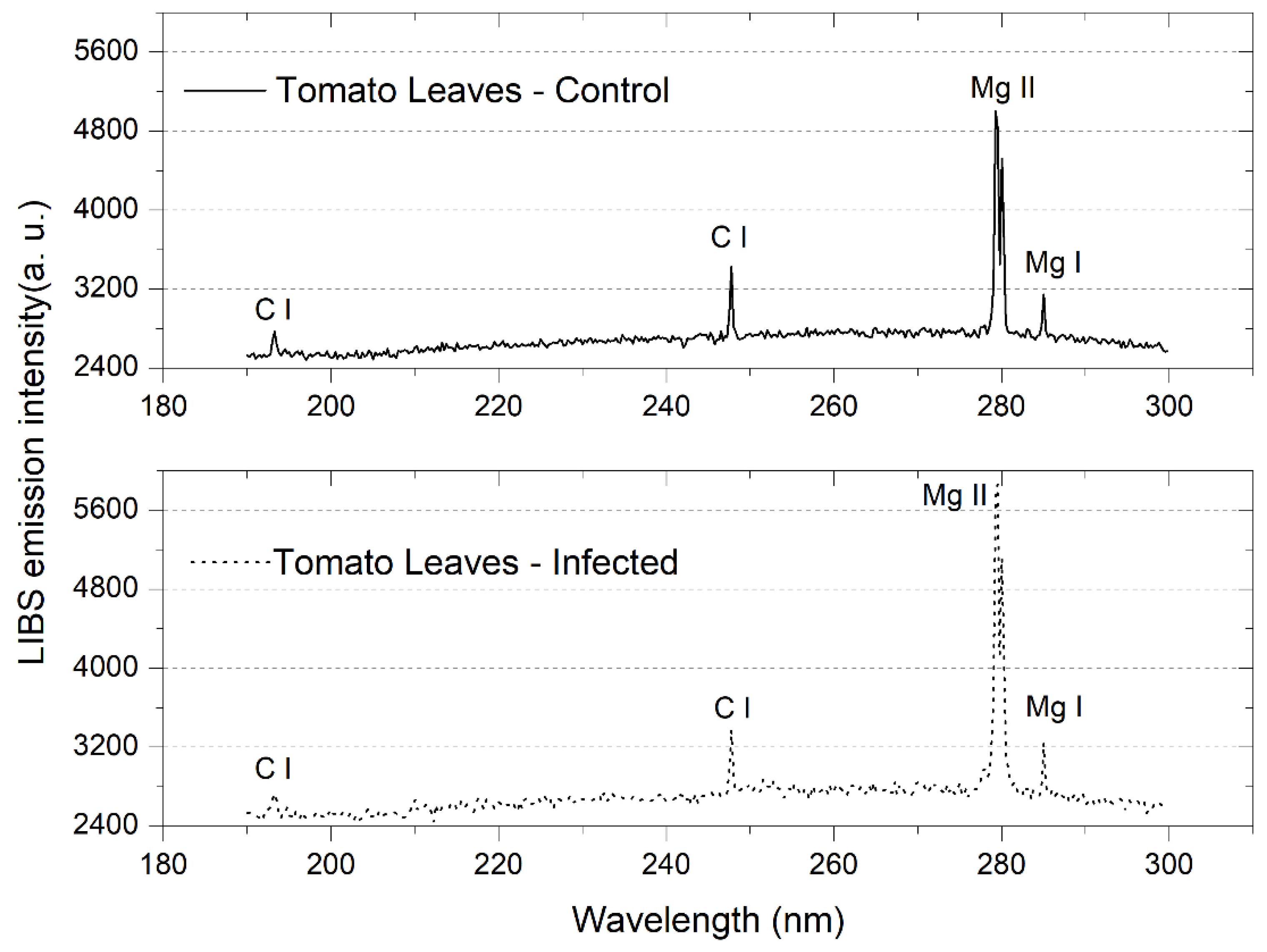
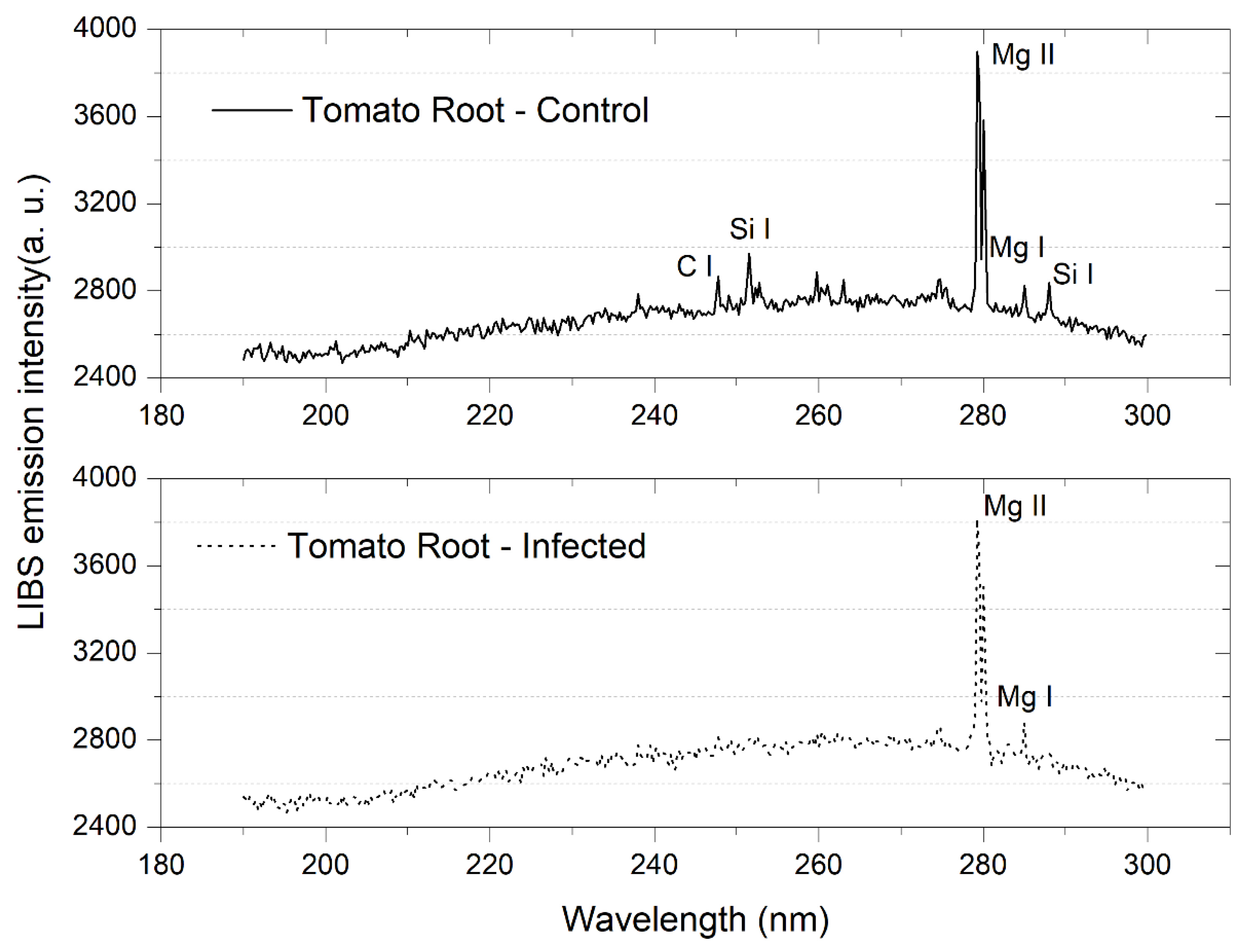
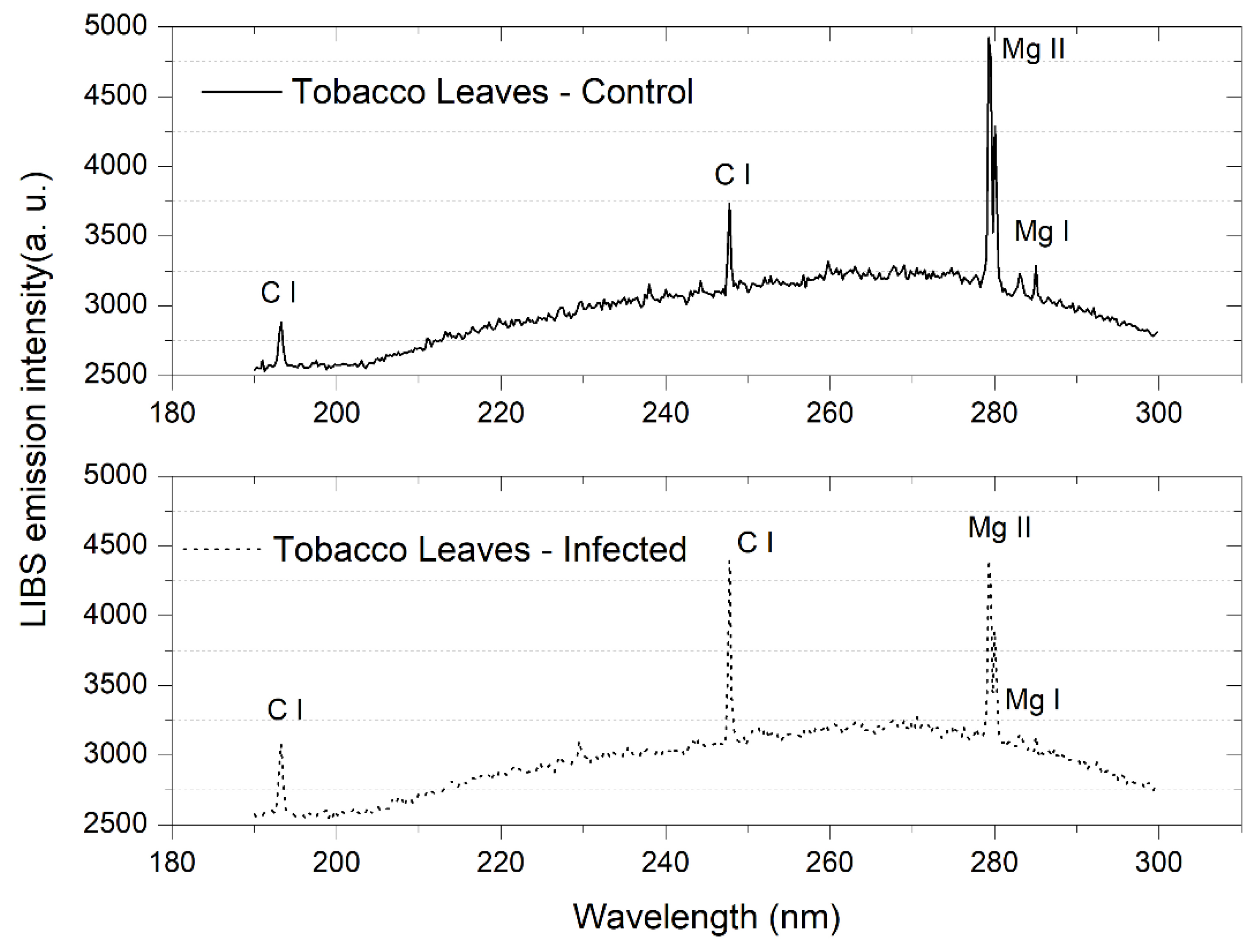
2.4. Laser-Induced Breakdown Spectroscopy (LIBS)
2.5. Multivariate Analysis
2.6. Chlorophyll Measurement
3. Results and discussion
3.1. Tomato Leaves, Stems and Roots
3.2. Tobacco Leaves
3.3. Multivariate Analysis
- Tomato stems—KNN with 3PCs: 100% accuracy, 100% precision and 100% sensitivity;
- Tomato roots—SVM with 2PCs: 52.4% accuracy, 50% precision and 50% sensitivity;
- Tomato leaves—SVM with 4PCS: 84.2% accuracy, 88.9% precision and 80% sensitivity;
- Tobacco leaves—SVM with 3PCs: 94.4% accuracy, 91.7% precision and 100% sensitivity.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vandvik, V.; Skarpaas, O.; Klanderud, K.; Telford, R.J.; Halbritter, H.; Goldberg, D.E. Biotic rescaling reveals importance of species interactions for variation in biodiversity responses to climate change. Proc. Natl. Acad. Sci. USA 2020, 117, 22858–22865. [Google Scholar] [CrossRef] [PubMed]
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, P.; Accotto, G.P.; Hanafy, M.S.; Pantaleo, V. Viruses and phytoparasitic nematodes of Cicer A. L.: Biotechnological approaches in interaction studies for sustainable control. Front. Plant Sci. 2018, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Lovey, S.; Bird, F. The influence of nematodes on photosynthesis in tomato plants. Physiol. Plant Pathol. 1973, 3, 525–529. [Google Scholar] [CrossRef]
- Fujimoto, T.; Abe, H.; Mizukubo, T.; Seo, S. Phytol, a Constituent of Chlorophyll, Induces Root-Knot Nematode Resistance in Arabidopsis via the Ethylene Signaling Pathway. Mol. Plant Microbe Interact. 2021, 34, 279–285. [Google Scholar] [CrossRef]
- Alon, D.M.; Hak, H.; Bornstein, M.; Pines, G.; Spiegelman, Z. Differential Detection of the Tobamoviruses Tomato Mosaic Virus (ToMV) and Tomato Brown Rugose Fruit Virus (ToBRFV) Using CRISPR-Cas12a. Plants 2021, 10, 1256. [Google Scholar] [CrossRef]
- Singh, V.; Sharma, N.; Singh, S. A review of imaging techniques for plant disease detection. Artif. Intell. Agric. 2020, 4, 229–242. [Google Scholar] [CrossRef]
- Leonetti, P.; Ghasemzadeh, A.; Consiglio, A.; Gursinsky, T.; Behrens, S.-E.; Pantaleo, V. Endogenous activated small interfering RNAs in virus-infected Brassicaceae crops show a common host gene-silencing pattern affecting photosynthesis and stress response. New Phytol. 2021, 229, 1650–1664. [Google Scholar] [CrossRef]
- Grishina, A.; Sherstneva, O.; Grinberg, M.; Zdobnova, T.; Ageyeva, M.; Khlopkov, A.; Sukhov, V.; Brilkina, A.; Vodeneev, V. Pre-Symptomatic Detection of Viral Infection in Tobacco Leaves Using PAM Fluorometry. Plants 2021, 10, 2782. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advance techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Tauseef, A.; Khalilullah, A.; Uddin, I. Role of MgO nanoparticles in the suppression of Meloidogyne incognita, infecting cowpea and improvement in plant growth and physiology. Exp. Parasitol. 2021, 220, 108045. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, S.M.; Lindqvist-Reis, P.; Persson, D.P.; Marttila, S.; Grenville-Briggs, L.J.; Andreasson, E. Visualising the ionome in resistant and susceptible plant-pathogen interactions. Plant J. 2021, 108, 870–885. [Google Scholar] [CrossRef] [PubMed]
- Molinari, S.; Akbarimotlagh, M.; Leonetti, P. Tomato root colonization by exogenously inoculated arbuscular mycorrhizal fungi induces resistance against Root-Knot Nematodes in a dose-dependent manner. Int. J. Mol. Sci. 2022, 23, 8920. [Google Scholar] [CrossRef] [PubMed]
- Senesi, G.S.; Harmon, R.S.; Hark, R. Field-portable and handheld laser-induced breakdown spectroscopy: Historical review, current status and future prospects. Spectrochim. Acta Part B 2021, 175, 106013. [Google Scholar] [CrossRef]
- Kaiser, J.; Novotny, K.; Martin, M.Z.; Hrdlicka, A.; Malina, R.; Hartl, M.; Adam, V.; Kizek, R. Trace elemental analysis by laser-induced breakdown spectroscopy biological applications. Surf. Sci. Rep. 2012, 67, 233–243. [Google Scholar] [CrossRef]
- Peng, J.; Liu, F.; Zhou, F.; Song, K.; Zhang, C.; Ye, L.; He, Y. Challenging applications for multi-element analysis by laser-induced breakdown spectroscopy in agriculture: A review. TrAC Trends Anal. Chem. 2016, 85, 260–272. [Google Scholar] [CrossRef]
- Markiewicz-Keszycka, M.; Cama-Moncunill, X.; Casado-Gavalda, M.P.; Dixit, Y.; Cama-Moncunill, R.; Cullen, P.J.; Sullivan, C. Laser-induced breakdown spectroscopy (LIBS) for food analysis: A review. Trends Food Sci. Technol. 2017, 65, 80–93. [Google Scholar] [CrossRef]
- de Carvalho, G.G.A.; Braga Bueno Guerra, M.; Adame, A.; Seimi Nomura, C.; Oliveira, P.V.; de Carvalho, H.W.P.; Santos, D., Jr.; Nunes, L.C.; Krug, F.J. Recent advances in LIBS and XRF for the analysis of plants. J. Anal. At. Spectrom. 2018, 33, 919–944. [Google Scholar] [CrossRef]
- Senesi, G.S.; Cabral, J.; Menegatti, C.R.; Marangoni, B.; Nicolodelli, G. Recent advances and future trends in LIBS applications to agricultural materials and their food derivatives: An overview of developments in the last decade (2010–2019). Part II. Crop plants and their food derivatives. TrAC Trends Anal. Chem. 2019, 118, 453–469. [Google Scholar] [CrossRef]
- Pereira, F.M.V.; Milori, D.M.B.P.; Venancio, A.L.; de Russo, M.S.T.; Martins, P.K.; Freitas-Astúa, J. Evaluation of the effects of Candidatus Liberibacter asiaticus on inoculated citrus plants using laser-induced breakdown spectroscopy (LIBS) and chemometrics tools. Talanta 2010, 83, 351–356. [Google Scholar] [CrossRef]
- Sankaran, S.; Ehsani, R.; Morgan, K.T. Detection of anomalies in citrus leaves using laser-induced breakdown spectroscopy (LIBS). Appl. Spectrosc. 2015, 69, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Song, K.; Zhu, H.; Kong, W.; Liu, F.; Shen, T.; He, Y. Fast detection of tobacco mosaic virus infected tobacco using laser-induced breakdown spectroscopy. Sci. Rep. 2017, 7, 44551. [Google Scholar] [CrossRef] [PubMed]
- Ranulfi, A.C.; Senesi, G.S.; Caetano, J.B.; Meyer, M.C.; Magalhaes, A.B.; Villas-Boas, P.R.; Milori, D.M.B.P. Nutritional characterization of healthy and Aphelenchoides besseyi infected soybean leaves by laser-induced breakdownspectroscopy (LIBS). Microchem. J. 2018, 141, 118–126. [Google Scholar] [CrossRef]
- Molinari, S.; Leonetti, P. Bio-control agents activate plant immune response and prime susceptible tomato against root-knot nematodes. PLoS ONE 2019, 14, e0213230. [Google Scholar]
- Molinari, S.; Fanelli, E.; Leonetti, P. Expression of tomato salicylic acid (SA)-responsive pathogenesis-related genes in Mi-1-mediated and SA-induced resistance to root-knot nematodes. Mol. Plant Pathol. 2014, 15, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Byrd, D.W.; Kirkpatrick, T., Jr.; Barker, K.R. An improved technique for clearing and staining plant tissue for detection of nematodes. J. Nematol. 1983, 15, 142–143. [Google Scholar]
- Menegatti, C.R.; Nicolodelli, G.; Senesi, G.S.; Silva, O.A.; Filho, H.J.I.; Villas-Boas, P.R.; Marangoni, B.S.; Milori, D.M.B.P. Semiquantitative analysis of mercury in landfill leachates using double-pulse laser-induced breakdown spectroscopy. Appl. Opt. 2017, 56, 3730–3735. [Google Scholar] [CrossRef]
- Marangoni, B.S.; Silva, K.S.G.; Nicolodelli, G.; Senesi, G.S.; Cabral, J.S.; Villas-Boas, P.R.; Silva, C.S.; Teixeira, P.C.; Nogueira, A.R.A.; Benites, V.M.; et al. Phosphorous quantification in fertilizers using laser induced breakdown spectroscopy (LIBS): A methodology of analysis to correct physical matrix effects. Anal. Methods 2016, 8, 78–82. [Google Scholar] [CrossRef]
- Larios, G.; Ribeiro, M.; Arruda, C.; Oliveira, S.L.; Canassa, T.; Baker, M.J.; Marangoni, B.S.; Ramos, C.; Cena, C. A new strategy for canine visceral leishmaniasis diagnosis based on FTIR spectroscopy and machine learning. J. Biophotonics 2021, 14, e202100141. [Google Scholar] [CrossRef]
- Allegretta, I.; Marangoni, B.S.; Manzari, P.; Porfido, C.; Terzano, R.; De Pascale, O.; Senesi, G.S. Macro-classification of meteorites by portable energy dispersive X-ray fluorescence spectroscopy (pED-XRF), principal component analysis (PCA) and machine learning algorithms. Talanta 2020, 212, 120785. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Melakeberhan, H.; Ferris, H. Impact of Meloidogyne incognita on Physiological Efficiency of Vitis vinifera. J. Nematol. 1989, 21, 74–80. [Google Scholar] [PubMed]
- Lu, P.; Davis, R.F.; Kemerait, R.C.; van Iersel, M.W.; Scherm, H. Physiological Effects of Meloidogyne incognita Infection on Cotton Genotypes with Differing Levels of Resistance in the Greenhouse. J. Nematol. 2014, 46, 352–359. [Google Scholar] [PubMed]
- Udalova, Z.V.; Zinovieva, S.V. Effect of Salicylic Acid on the Oxidative and Photosynthetic Processes in Tomato Plants at Invasion with Root-Knot Nematode Meloidogyne incognita (Kofoid Et White, 1919) Chitwood, 1949. Dokl. Biochem. Biophys. 2019, 488, 350–353. [Google Scholar] [CrossRef]
- Udalova, Z.V.; Folmanis, G.E.; Fedotov, M.A.; Pelgunova, L.A.; Krysanov, E.Y.; Khasanov, F.K.; Zinovieva, S.V. Effects of Silicon Nanoparticles on Photosynthetic Pigments and Biogenic Elements in Tomato Plants Infected with Root-Knot Nematode Meloidogyne incognita. Dokl. Biochem. Biophys. 2020, 495, 329–333. [Google Scholar] [CrossRef]
- Niyogi, K.K. Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 333–359. [Google Scholar] [CrossRef]
- Labudda, M.; Różańska, E.; Czarnocka, W.; Sobczak, M.; Dzik, J.M. Systemic changes in photosynthesis and reactive oxygen species homeostasis in shoots of Arabidopsis thaliana infected with the beet cyst nematode Heterodera schachtii. Mol. Plant Pathol. 2018, 19, 1690–1704. [Google Scholar] [CrossRef] [PubMed]
- NIST Database. 2022. Available online: https://physics.nist.gov/PhysRefData/ASD/LIBS (accessed on 16 May 2022).
- Luo, T.; Fan, T.; Liu, Y.; Rothbart, M.; Yu, J.; Zhou, S.; Grimm, B.; Luo, M. Thioredoxin redox regulates ATPase activity of magnesium chelatase CHLI subunit and modulates redox-mediated signaling in tetrapyrrole biosynthesis and homeostasis of reactive oxygen species in pea plants. Plant Physiol. 2012, 159, 118–130. [Google Scholar] [CrossRef]
- Shimura, H.; Pantaleo, V.; Ishihara, T.; Myojo, N.; Inaba, J.; Sueda, K.; Burgyán, J.; Masuta, C. A viral satellite RNA induces yellow symptoms on tobacco by targeting a gene involved in chlorophyll biosynthesis using the RNA silencing machinery. PLoS Pathog. 2011, 7, e1002021. [Google Scholar] [CrossRef]
- Wang, B.; Hajano, J.-U.-D.; Ren, Y.; Lu, C.; Wang, X. iTRAQ-based quantitative proteomics analysis of rice leaves infected by Rice stripe virus reveals several proteins involved in symptom formation. Virol. J. 2015, 12, 99. [Google Scholar] [CrossRef]
- Poll, J.; Marhan, S.; Haase, S.; Hallmann, J.; Kandeler, E.; Ruess, L. Low amounts of herbivory by root-knot nematodes affect microbial community dynamics and carbon allocation in the rhizosphere. FEMS Microbiol. Ecol. 2007, 62, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Ancín, M.; Larraya, L.; Florez-Sarasa, I.; Bénard, C.; Fernández-San Millán, A.; Veramendi, J.; Gibon, Y.; Fernie, A.R.; Aranjuelo, I.; Farran, I. Overexpression of thioredoxin m in chloroplasts alters carbon and nitrogen partitioning in tobacco. J. Exp. Bot. 2021, 72, 4949–4964. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.M.; Jones, J.B. The role of magnesium in plant disease. Plant Soil 2013, 368, 73–85. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senesi, G.S.; De Pascale, O.; Marangoni, B.S.; Caires, A.R.L.; Nicolodelli, G.; Pantaleo, V.; Leonetti, P. Chlorophyll Fluorescence Imaging (CFI) and Laser-Induced Breakdown Spectroscopy (LIBS) Applied to Investigate Tomato Plants Infected by the Root Knot Nematode (RKN) Meloidogyne incognita and Tobacco Plants Infected by Cymbidium Ringspot Virus. Photonics 2022, 9, 627. https://doi.org/10.3390/photonics9090627
Senesi GS, De Pascale O, Marangoni BS, Caires ARL, Nicolodelli G, Pantaleo V, Leonetti P. Chlorophyll Fluorescence Imaging (CFI) and Laser-Induced Breakdown Spectroscopy (LIBS) Applied to Investigate Tomato Plants Infected by the Root Knot Nematode (RKN) Meloidogyne incognita and Tobacco Plants Infected by Cymbidium Ringspot Virus. Photonics. 2022; 9(9):627. https://doi.org/10.3390/photonics9090627
Chicago/Turabian StyleSenesi, Giorgio Saverio, Olga De Pascale, Bruno Spolon Marangoni, Anderson Rodrigues Lima Caires, Gustavo Nicolodelli, Vitantonio Pantaleo, and Paola Leonetti. 2022. "Chlorophyll Fluorescence Imaging (CFI) and Laser-Induced Breakdown Spectroscopy (LIBS) Applied to Investigate Tomato Plants Infected by the Root Knot Nematode (RKN) Meloidogyne incognita and Tobacco Plants Infected by Cymbidium Ringspot Virus" Photonics 9, no. 9: 627. https://doi.org/10.3390/photonics9090627





