Improvement of Retinal Images Affected by Cataracts
Abstract
:1. Introduction
2. Background and Method
- -
- Grade I or non-cataract corresponding to clear lens or early cataract: The lens is still clear, but patients begin to feel visual discomfort; nevertheless, the retinal images are clear.
- -
- Grade II or mild cataract: Lens begins to be slightly opaque and eye fundus images are dimmed. Small details of fundus images begin to fade out.
- -
- Grade III or moderate cataract: The opacity of the lens has increased, and images are blurred.
- -
- Grade IV or severe cataract: The cataract is very dense and features of the eye fundus can be hardly detected.
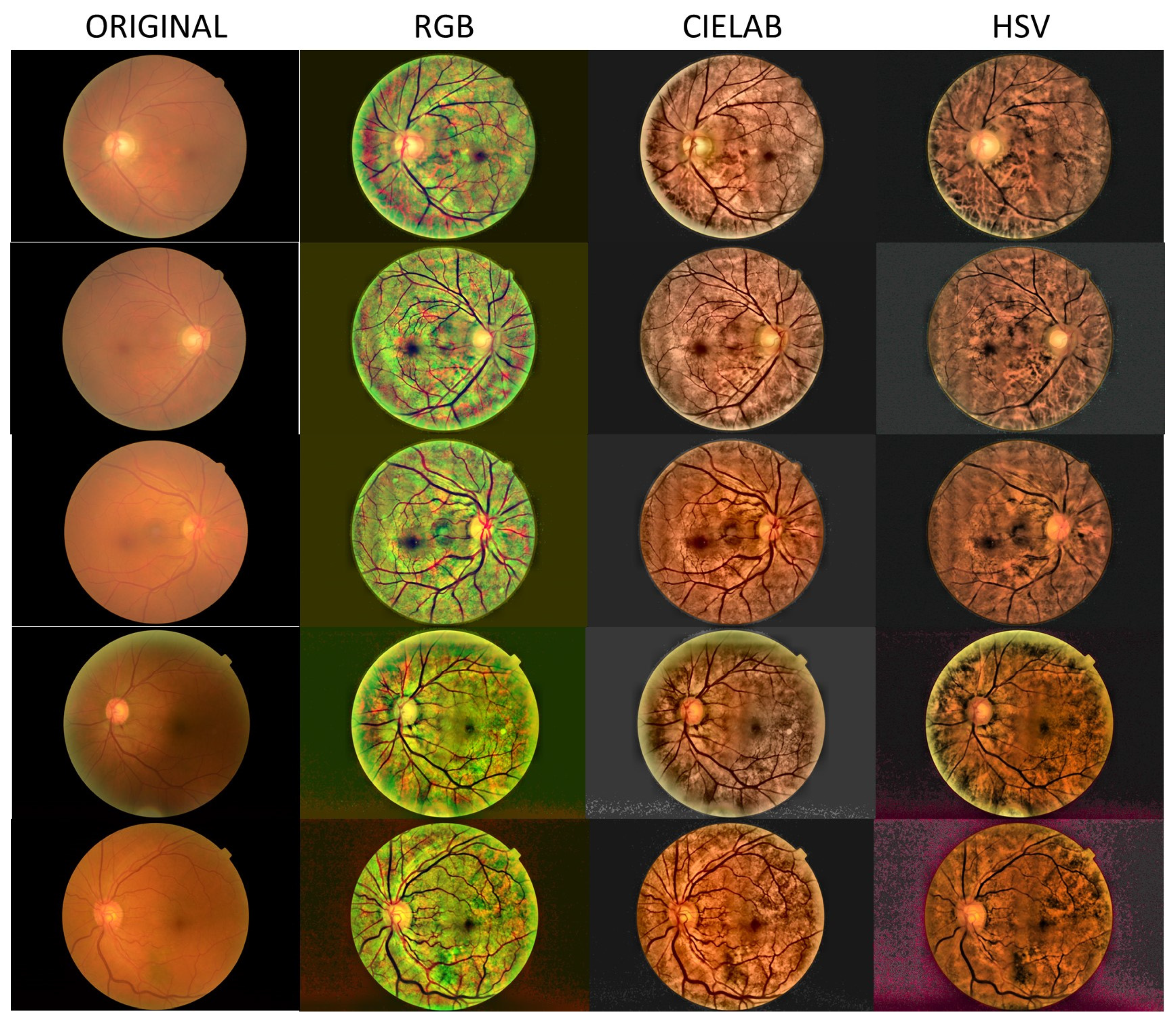
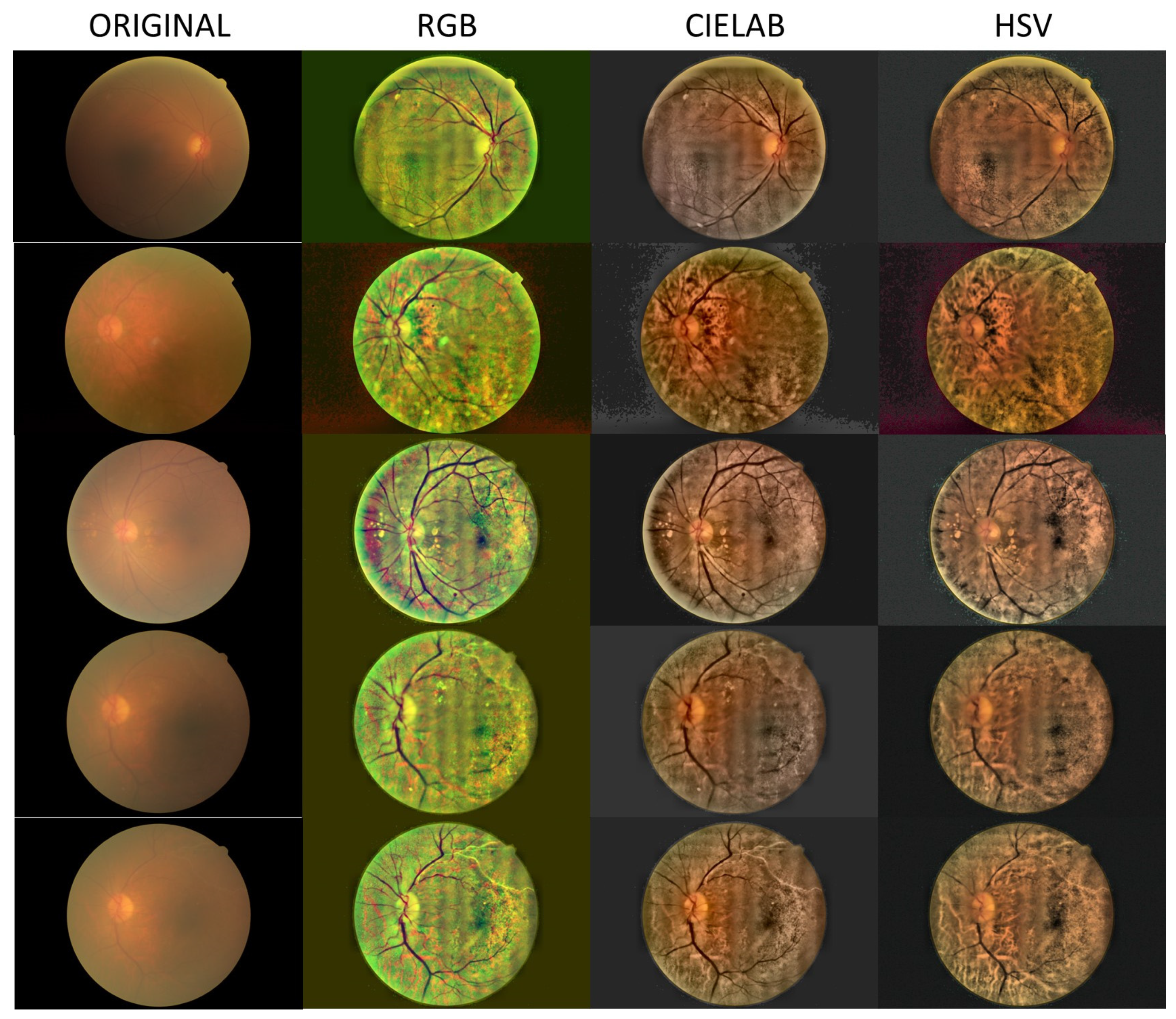
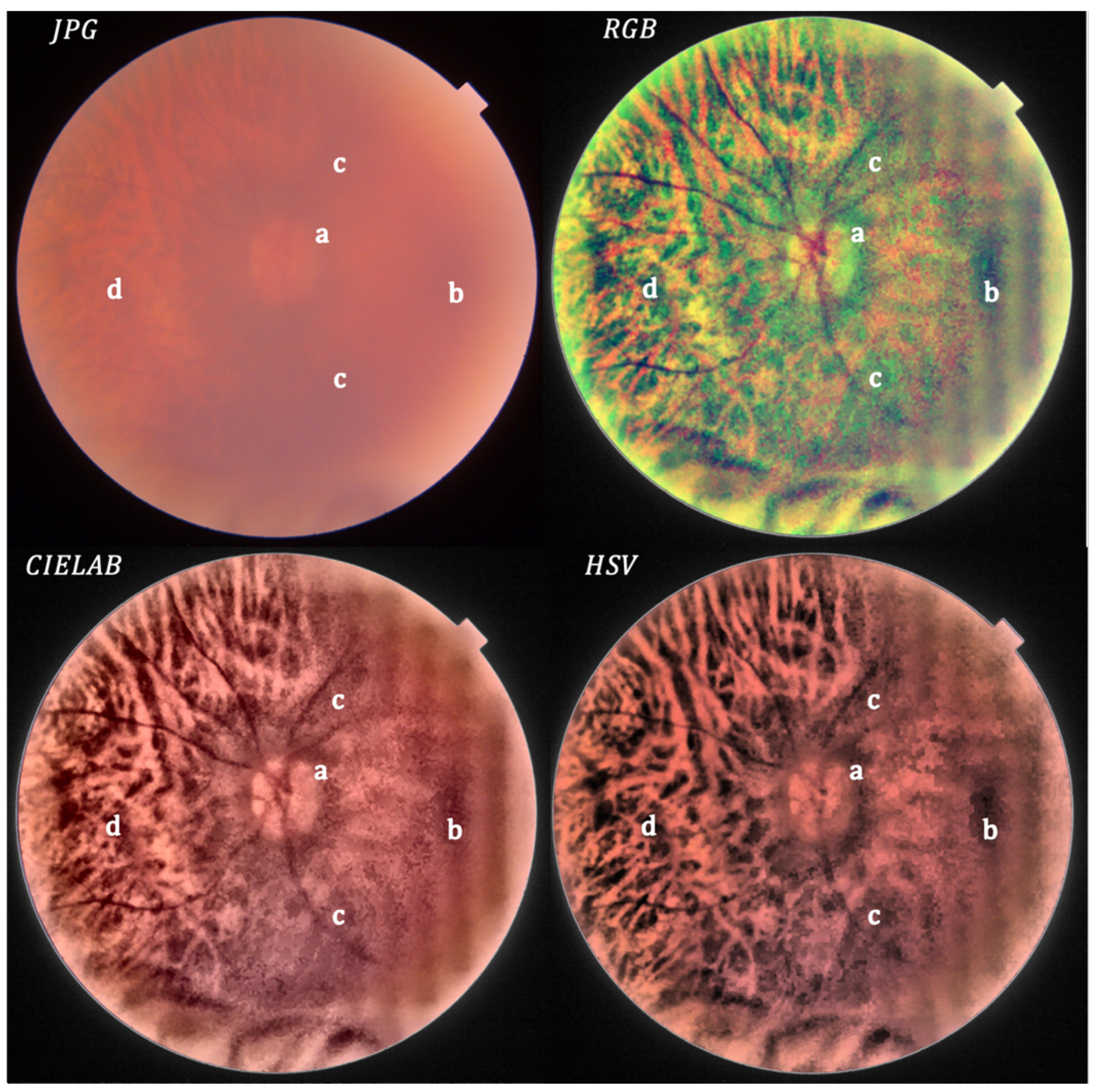
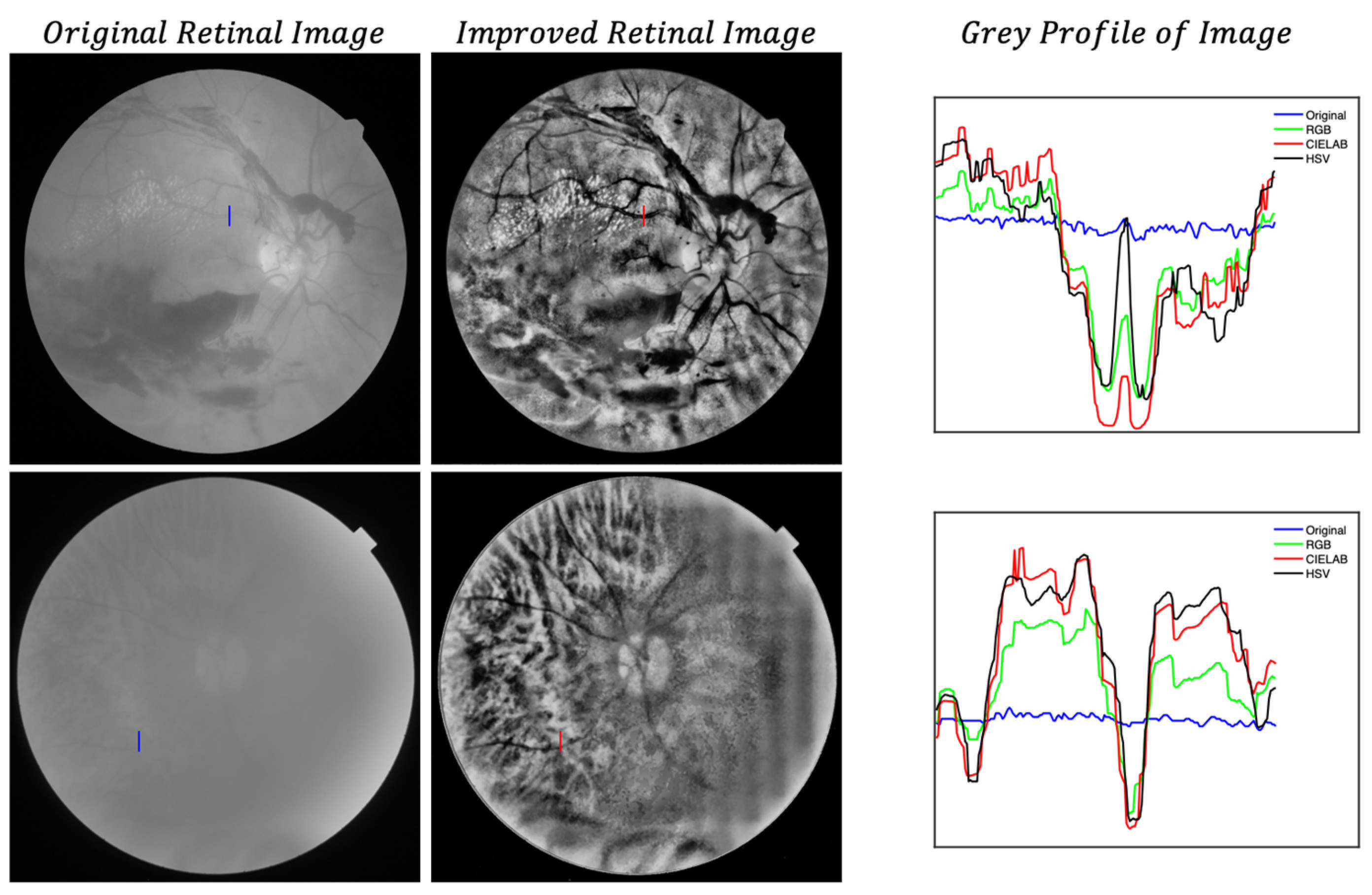
- Local histogram equalization is performed in the R and G channels of RGB, L channel for CIELAB, and V channel for HSV, respectively.
- We use the Matlab built-in function adapthisteq() that uses CLAHE to enhance the contrast in the channels. This function needs three input parameters: ‘NBins’ is number of bins of the histogram used to improve contrast, ‘NumTiles’ is the size of the rectangular region where the local contrast enhancement will be performed and ‘ClipLimit’ is a contrast factor that avoids the oversaturation in some regions of the image. Below, we explain how we chose the default values for retinal images affected by the same stage of cataract.
- Median filtering of processed channels for noise removal: We used the Matlab built-in function medfilt2() over a region of 11 × 11 pixels to remove high-frequency noise amplified during the local histogram equalization.
- RGB merging, and CIELAB or HSV to RGB conversion.
- -
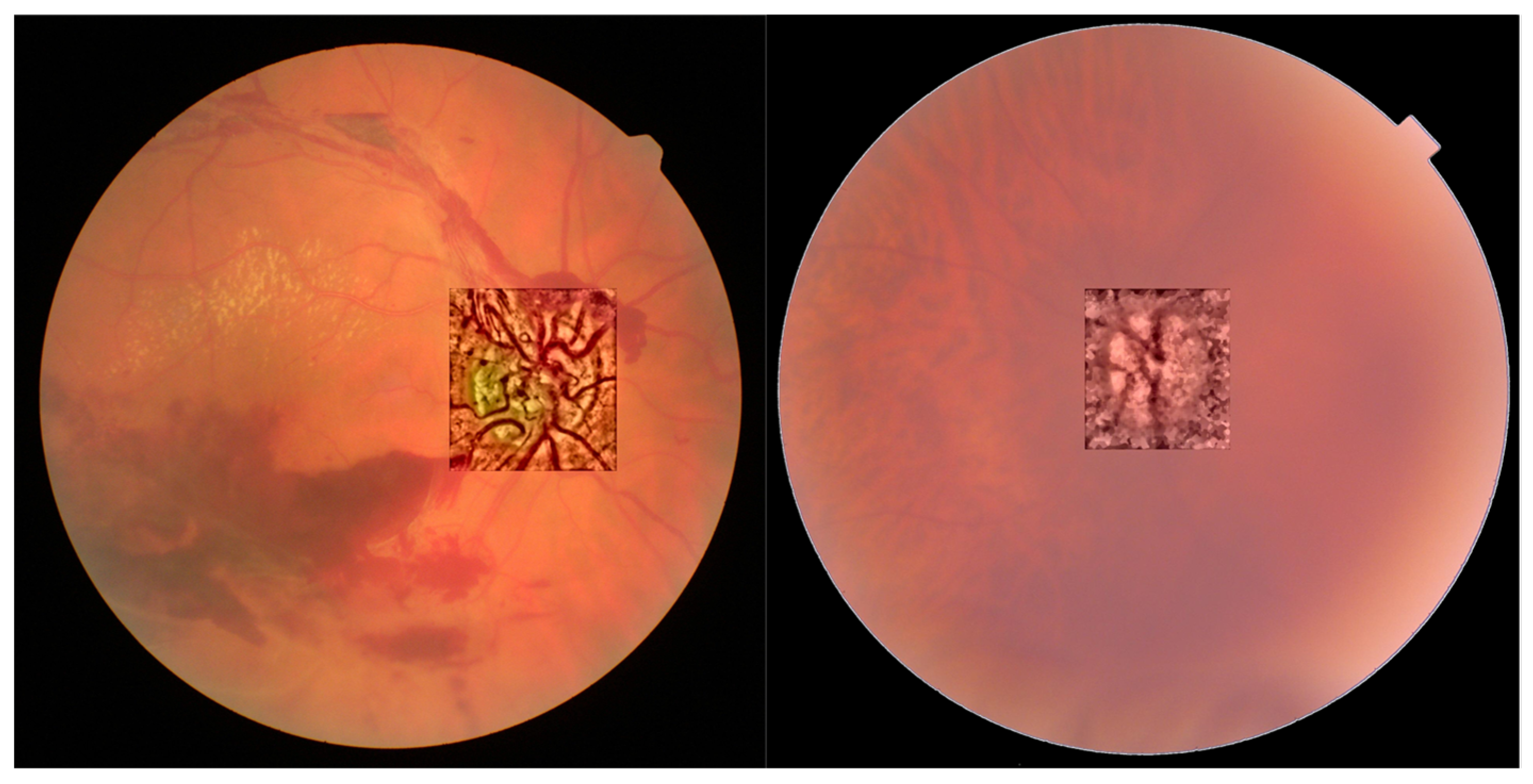
- NTiles and NBins: NTiles defines the block size or local region around a pixel for which the histogram is equalized. This size should be larger than the size of features to be preserved or enhanced. Moreover, the number of histogram bins used for histogram equalization within a block should be smaller than the number of pixels in the block. All images from both data sets are 8-bit images, and their size is 2592 × 1728 or 2464 × 1632 for the public dataset and 2976 × 2976 for our dataset. We used NTiles = 16 and NBins = 256 in all processed images presented in the manuscript. Larger values are not meaningful, and reducing the number of bins reduces the dynamic range of the output image and, therefore, the quality of enhanced details by limiting the change in intensity to improve contrast. For the images ROIs containing the optical disk presented in this work, we used NTiles = 8 and NBins = 256. Nevertheless, the developed interface allows the user to choose different values for NBins and NTiles depending on the bit depth and region of interest to be processed.
- -
- ClipLimit: This value limits the amplification of noise or artifacts avoiding peaks in the histogram. We have processed all images for both data sets and in those without cataracts or mild cataracts we found that ClipLimit values between 0.05 and 0.3 provided the best results being 0.1 the one that fitted for most of images. For moderate cataracts values range from 0.1 to 0.3 being 0.2 the one that fitted for most of them. For severe cataracts the range goes from 0.1 to 0.5 being 0.3 the one that provided best results for most of images. These values apply to all channels of the different color maps. We must point out here that the quality of processed images does not change dramatically with ClipLimit values in the above mentioned ranges, leaving the choice to the user.
- -
- In all cases we found that 0.2 can be considered as the default value for ROI’s of about 600 × 600 pixels containing the optical disk.
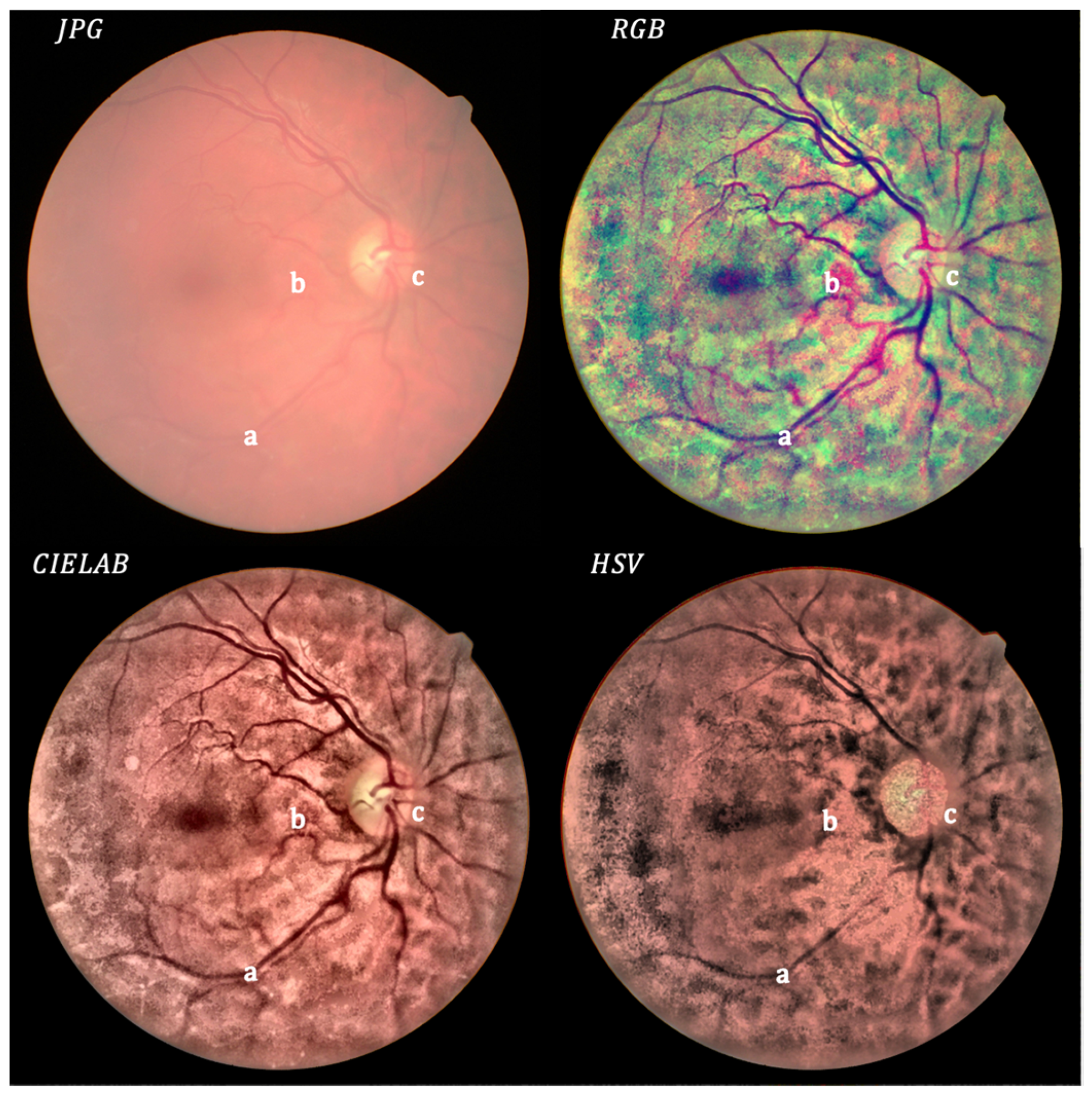
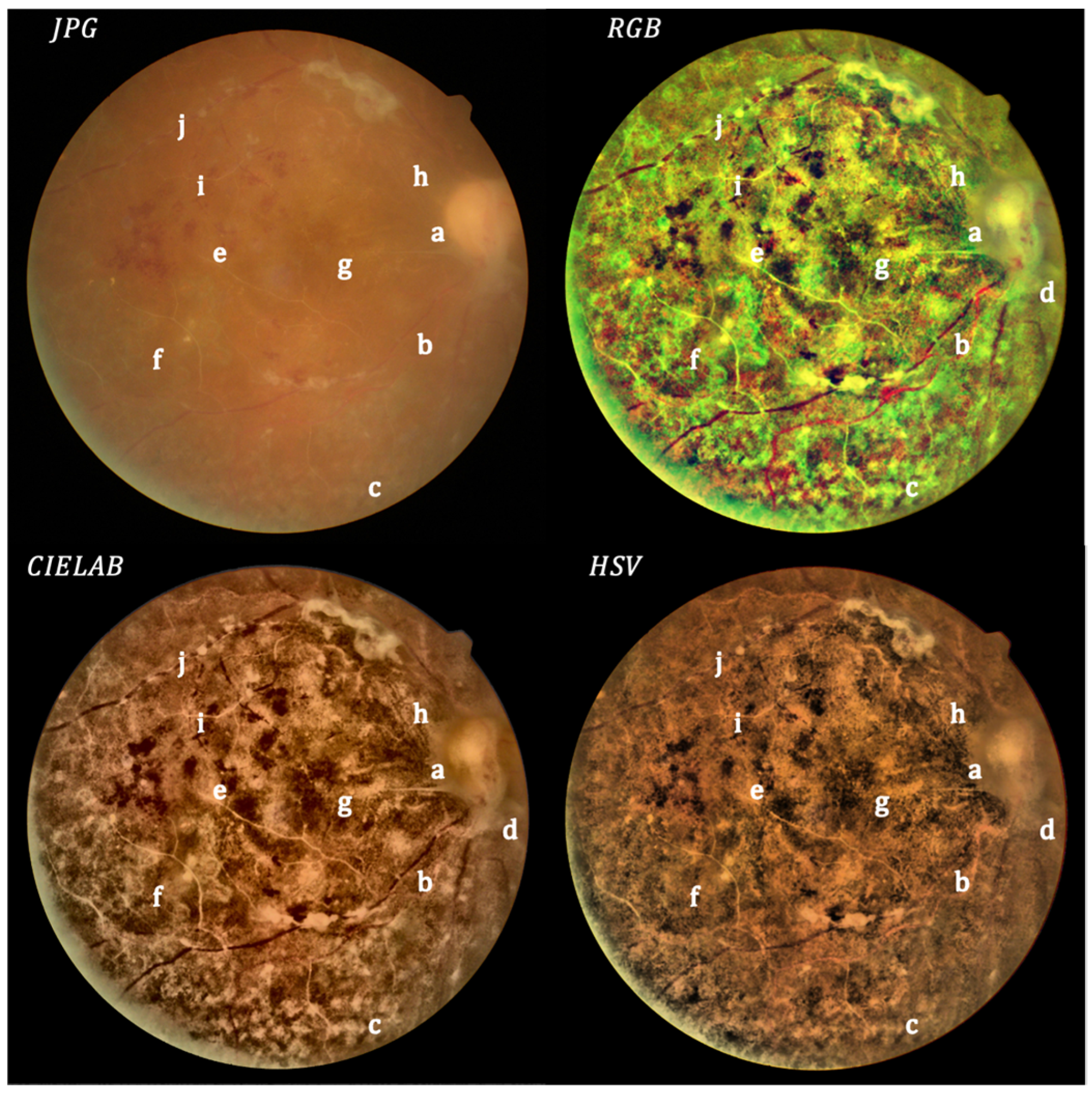
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lumbroso, B.; Rispoli, M.; Savastano, M.C. Clinical Applications of OCT SSADA Angiography in Everyday Clinical Practice. In Clinical OCT Angiography Atlas, 2nd ed.; Jaypee Brothers Medical Publishers: New Delhi, India, 2015. [Google Scholar]
- Tsang, S.H.; Sharma, T. Atlas of Inherited Retinal Diseases; Springer International Publishing: Cham, Switzerland, 2018; pp. 139–151. [Google Scholar]
- Wang, W.; Lo, A.C. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [Green Version]
- Maude, R.J.; Ahmed, B.W.; Rahman, A.H.M.W.; Rahman, R.; Majumder, M.I.; Menezes, D.B.; Abu Sayeed, A.; Hughes, L.; MacGillivray, T.J.; Borooah, S.; et al. Retinal changes in visceral leishmaniasis by retinal photography. BMC Infect. Dis. 2014, 14, 527. [Google Scholar] [CrossRef] [Green Version]
- Van den Berg, T.J. Intraocular light scatter, reflections, fluorescence and absorption: What we see in the slit lamp. Ophthalmic Physiol. Opt. 2018, 38, 6–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abràmoff, M.D.; Garvin, M.K.; Sonka, M. Retinal imaging and image analysis. IEEE Rev. Biomed. Engine 2010, 3, 169–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouhous, A.; Kemih, K. Novel encryption method based on optical time-delay chaotic system and a wavelet for data transmission. Opt. Laser Technol. 2018, 108, 162–169. [Google Scholar] [CrossRef]
- Salamat, N.; Missen, M.M.S.; Rashid, A. Diabetic retinopathy techniques in retinal images: A review. Artif. Intell. Med. 2019, 97, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Imran, S.A.; Arefeen, A.; Hasan, M.; Shahnaz, C. Source and camera independent ophthalmic disease recognition from fundus image using neural network. In Proceedings of the 2019 IEEE International Conference on Signal Processing, Information, Communication & Systems (SPICSCON), Dhaka, Bangladesh, 28–30 November 2019; pp. 59–63. [Google Scholar]
- Dash, J.; Bhoi, N. A thresholding-based technique to extract retinal blood vessels from fundus images. Future Comput. Inform. J. 2017, 2, 103–109. [Google Scholar] [CrossRef]
- Ramos-Soto, O.; Rodríguez-Esparza, E.; Balderas-Mata, S.E.; Oliva, D.; Hassanien, A.E.; Meleppat, R.K.; Zawadzki, R.J. An efficient retinal blood vessel segmentation in eye fundus images by using optimized top-hat and homomorphic filtering. Comput. Methods Programs Biomed. 2021, 201, 105949. [Google Scholar] [CrossRef] [PubMed]
- Meleppat, R.K.; Miller, E.B.; Manna, S.K.; Zhang, P.; Pugh, E.N., Jr.; Zawadzki, R.J. Multiscale Hessian filtering for enhancement of OCT angiography images. In Proceedings of the Ophthalmic Technologies XXIX, San Francisco, CA, USA, 2–7 February 2019; p. 108581K. [Google Scholar]
- Peli, E.; Peli, T. Restoration of retinal images obtained through cataracts. IEEE Trans. Med. Imaging 1989, 8, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Meleppat, R.K.; Ronning, K.E.; Karlen, S.J.; Burns, M.E.; Pugh, E.N., Jr.; Zawadzki, R.J. In vivo multimodal retinal imaging of disease-related pigmentary changes in retinal pigment epithelium. Sci. Rep. 2021, 11, 16252. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.; Oakley, J.P.; McLoughlin, N.; Nourrit, V. Enhancement of color retinal images in poor imaging conditions. In Proceedings of the 2012 IEEE International Conference on Imaging Systems and Techniques Proceedings, Manchester, UK, 16–17 July 2012; pp. 176–178. [Google Scholar]
- Shen, Z.; Fu, H.; Shen, J.; Shao, L. Modeling and Enhancing Low-Quality Retinal Fundus Images. IEEE Trans. Med. Imaging 2020, 40, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Pizer, S.M.; Johnston, R.E.; Ericksen, J.P.; Yankaskas, B.C.; Muller, K.E. Contrast-limited adaptive histogram equalization: Speed and effectiveness. In Proceedings of the First Conference on Visualization in Biomedical Computing, Atlanta, Georgia, 22–25 May 1990. [Google Scholar]
- Chylack, L.T.; Leske, M.C.; McCarthy, D.; Khu, P.; Kashiwagi, T.; Sperduto, R. Lens opacities classification system II (LOCS II). Arch. Ophthalmol. 1989, 107, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, H.; Zhang, Y.; Zhang, L.; Xu, L. Hierarchical method for cataract grading based on retinal images using improved Haar wavelet. Inf. Fusion 2020, 53, 196–208. [Google Scholar] [CrossRef]
- Cataract Dataset. Available online: https://www.kaggle.com/jr2ngb/cataractdataset/code (accessed on 2 February 2022).
- Zimmerman, J.B.; Pizer, S.M.; Staab, E.V.; Perry, J.R.; McCartney, W.; Brenton, B.C. An evaluation of the effectiveness of adaptive histogram equalization for contrast enhancement. IEEE Trans. Med. Imaging 1988, 7, 304–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummel, R. Image enhancement by histogram transformation. Comput. Graph. Image Process. 1977, 6, 184–195. [Google Scholar] [CrossRef]
- Zuiderveld, K. Contrast limited adaptive histogram equalization. In Graphics gems IV; Academic Press Professional, Inc.: San Diego, CA, USA, 1994; pp. 474–485. [Google Scholar]
- Stark, J.A. Adaptive image contrast enhancement using generalizations of histogram equalization. IEEE Trans. Image Process. 2000, 9, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robertson, A.R. The CIE 1976 color-difference formulae. Color Res. Appl. 1977, 2, 7–11. [Google Scholar] [CrossRef]
- Gonzalez, R.C.; Woods, R.E. Digital Image Processing, 2nd ed.; Prentice Hall: Hoboken, NJ, USA, 2002; pp. 282–330. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Amador, E.; Arines, J.; Charlón, P.; Garcia-Porta, N.; Abraldes, M.J.; Acosta, E. Improvement of Retinal Images Affected by Cataracts. Photonics 2022, 9, 251. https://doi.org/10.3390/photonics9040251
Gonzalez-Amador E, Arines J, Charlón P, Garcia-Porta N, Abraldes MJ, Acosta E. Improvement of Retinal Images Affected by Cataracts. Photonics. 2022; 9(4):251. https://doi.org/10.3390/photonics9040251
Chicago/Turabian StyleGonzalez-Amador, Enrique, Justo Arines, Pablo Charlón, Nery Garcia-Porta, Maximino J. Abraldes, and Eva Acosta. 2022. "Improvement of Retinal Images Affected by Cataracts" Photonics 9, no. 4: 251. https://doi.org/10.3390/photonics9040251
APA StyleGonzalez-Amador, E., Arines, J., Charlón, P., Garcia-Porta, N., Abraldes, M. J., & Acosta, E. (2022). Improvement of Retinal Images Affected by Cataracts. Photonics, 9(4), 251. https://doi.org/10.3390/photonics9040251




