Probing Dynamic Variation of Layered Microstructure Using Backscattering Polarization Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
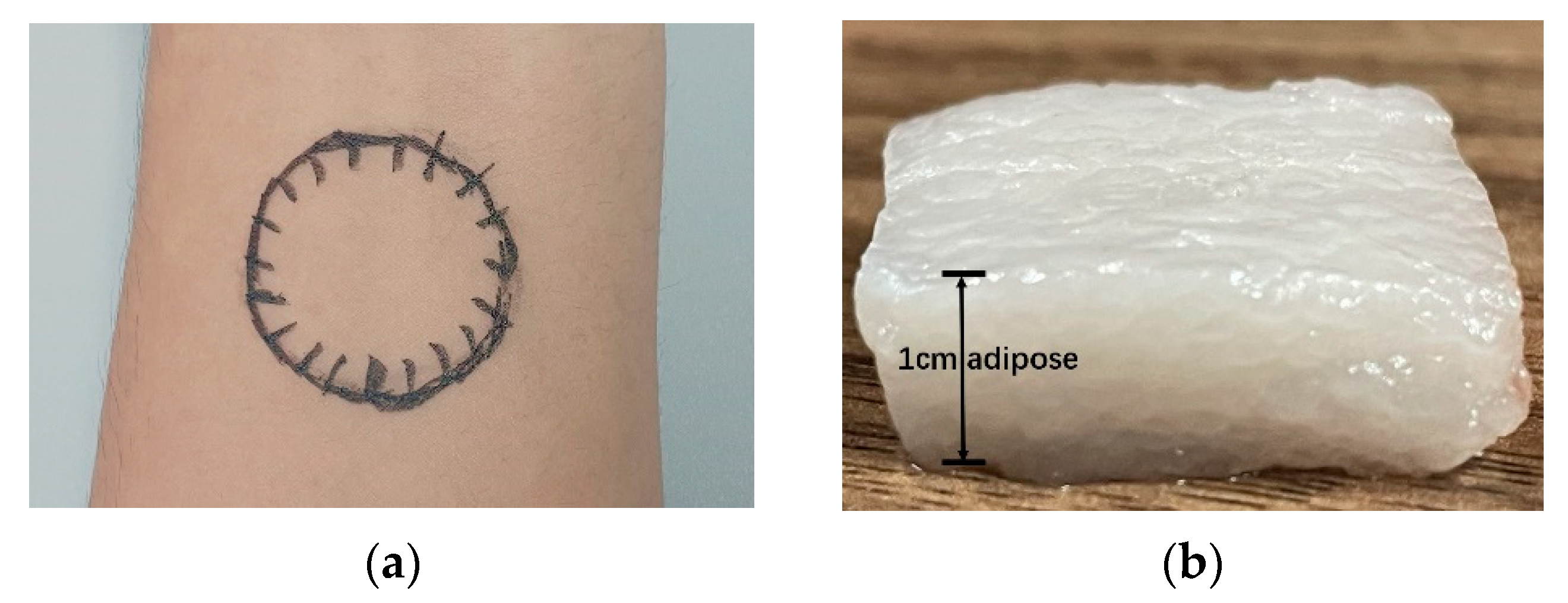
2.2. Tissue Samples Preparation
2.3. Polarization Parameters
2.4. Monte Carlo (MC) Simulation
3. Results and Discussions
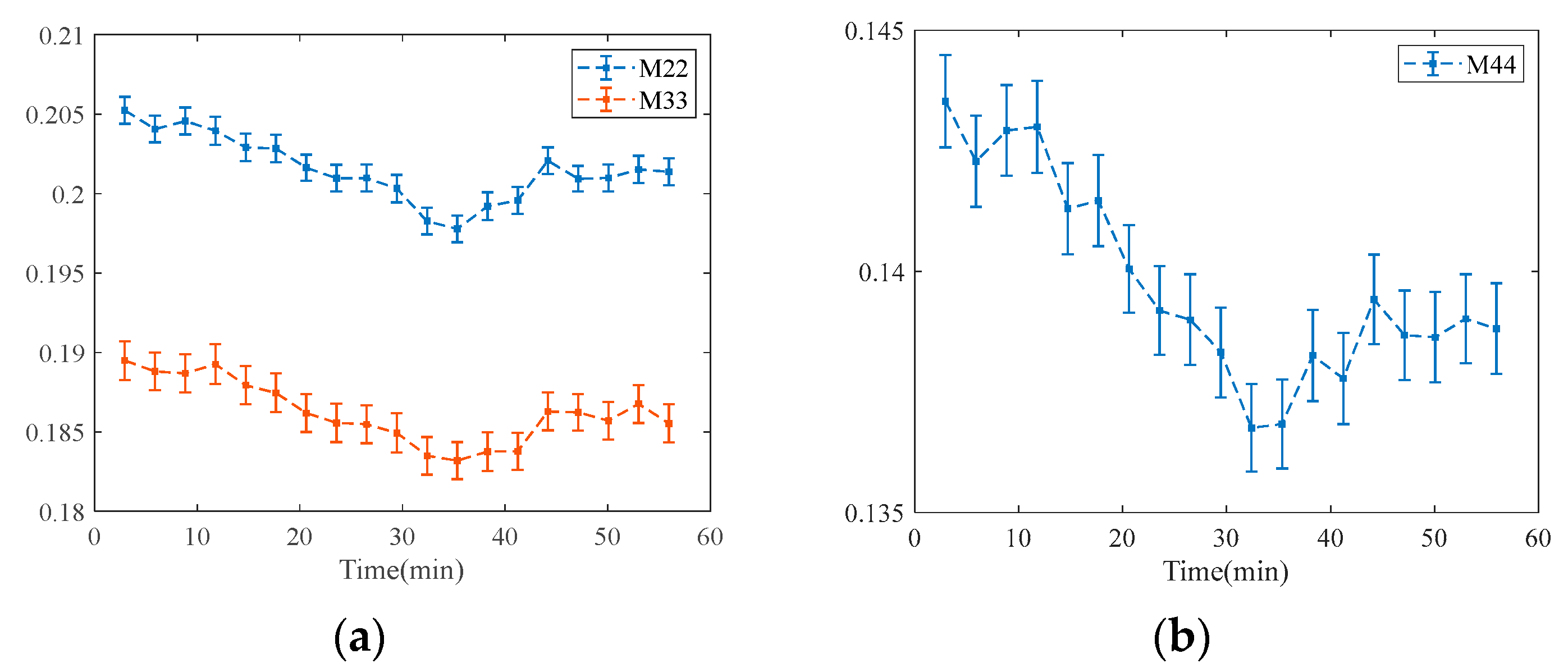
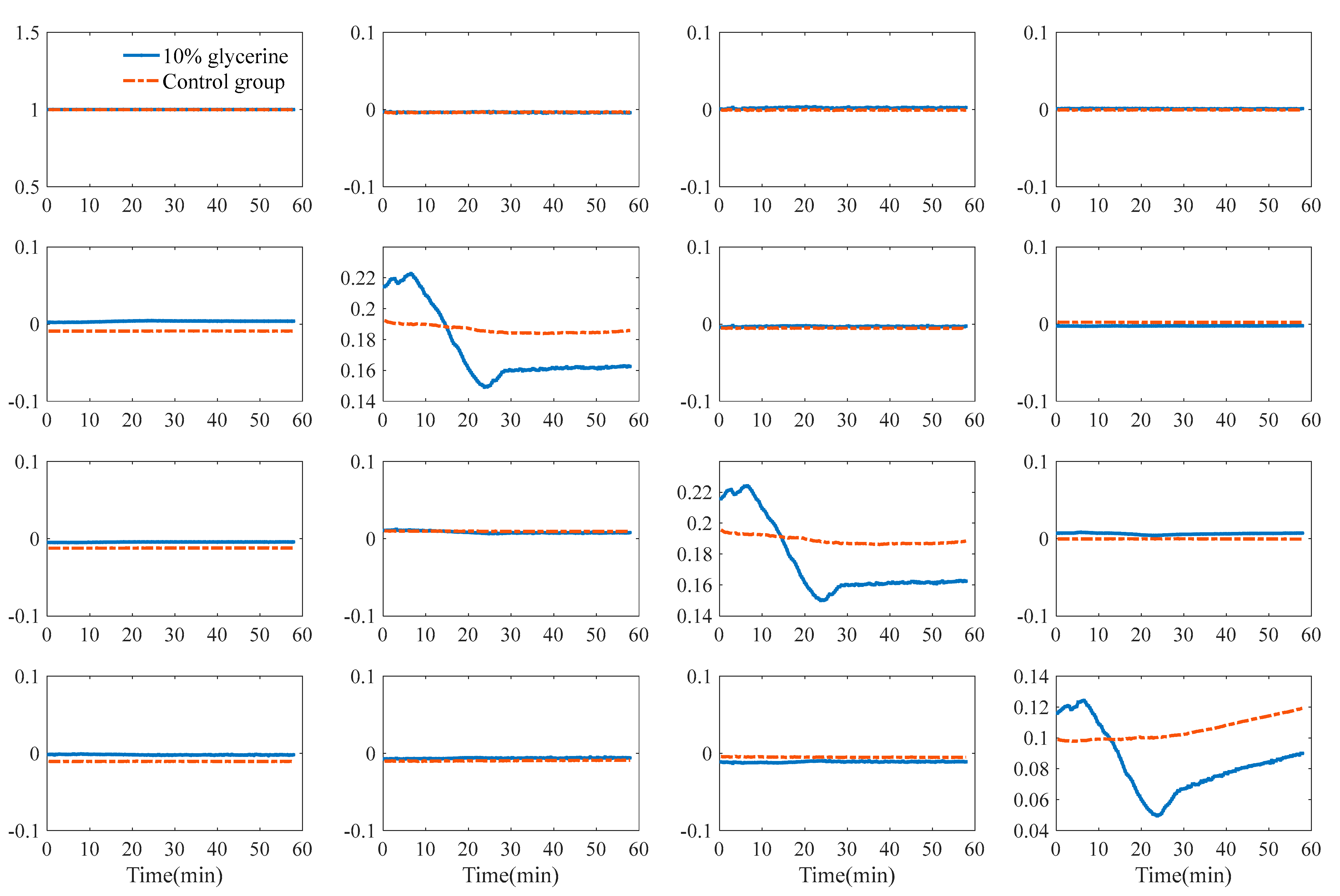
3.1. TOC Dynamics of Human Skin
3.2. Error Analysis at Different Time of Single MM Measurement
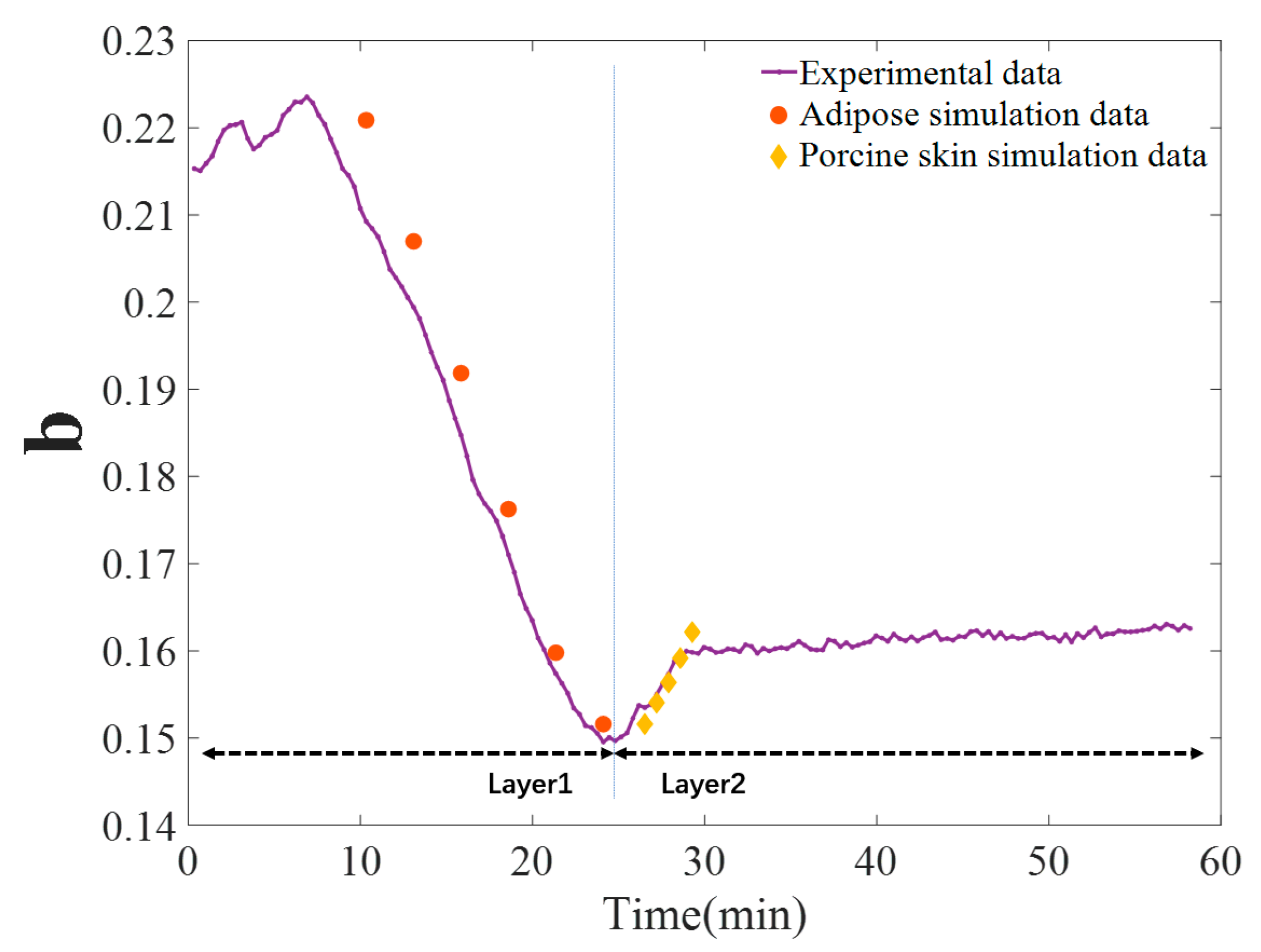
3.3. TOC Dynamics of Double-Layer Tissue
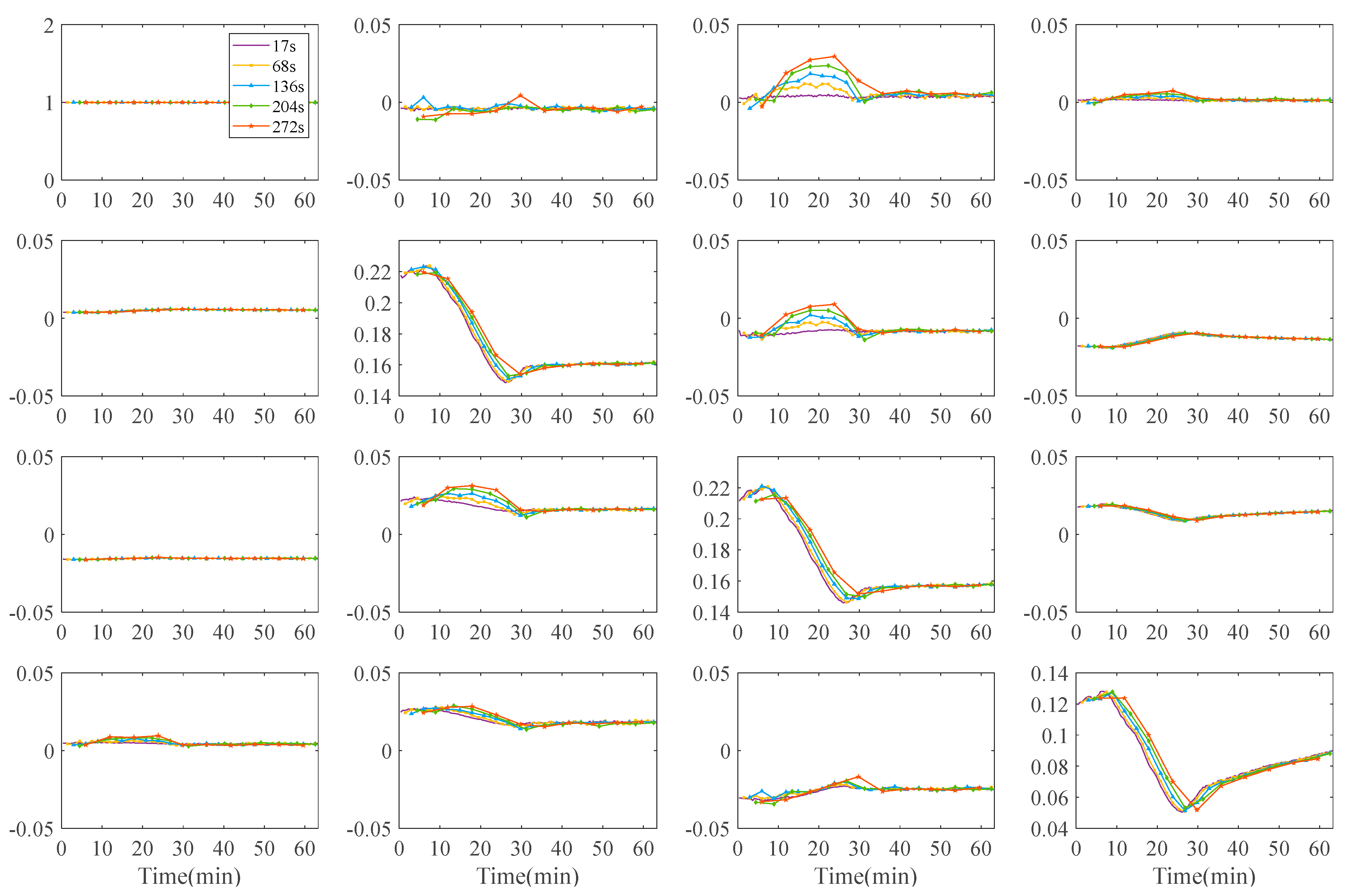
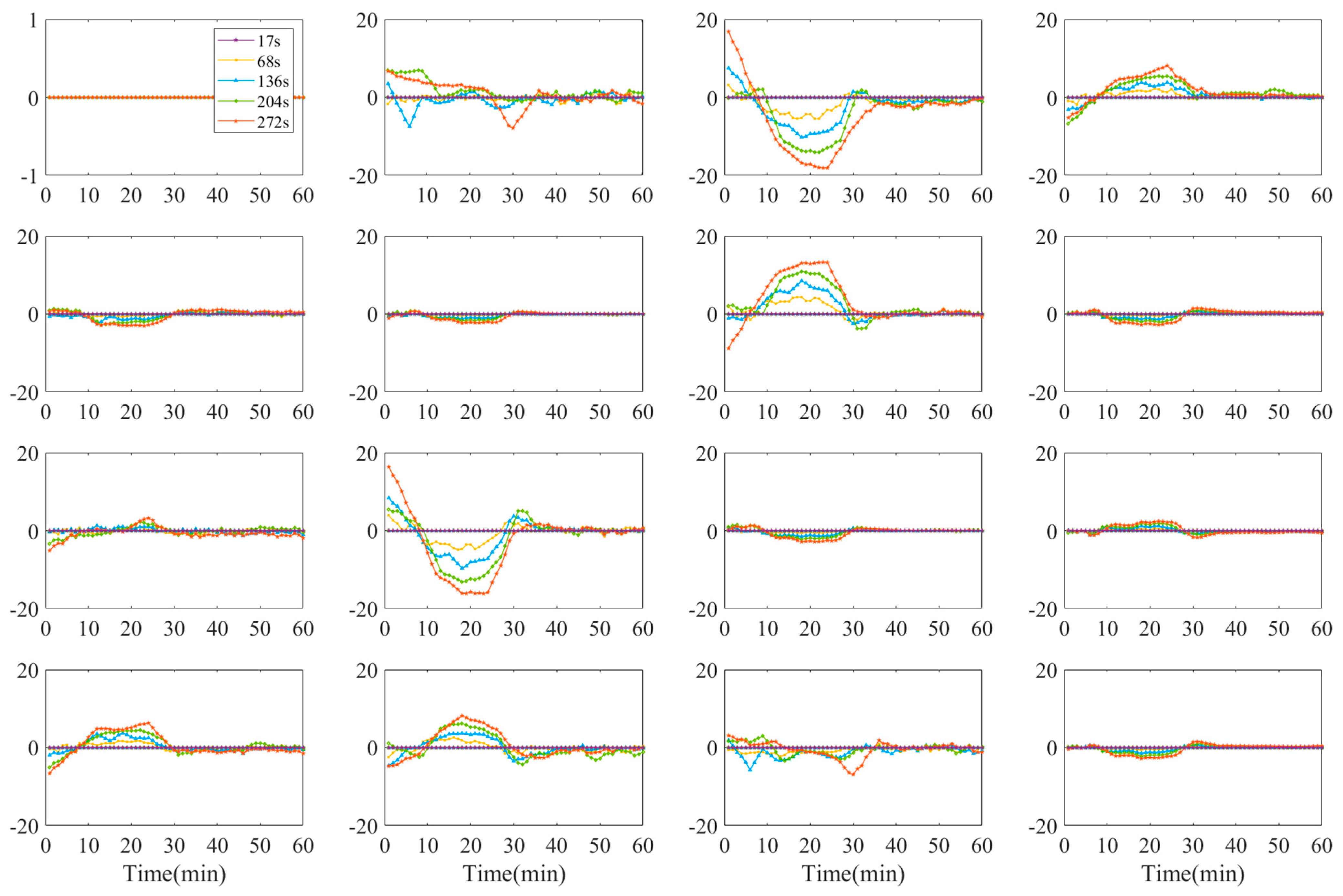
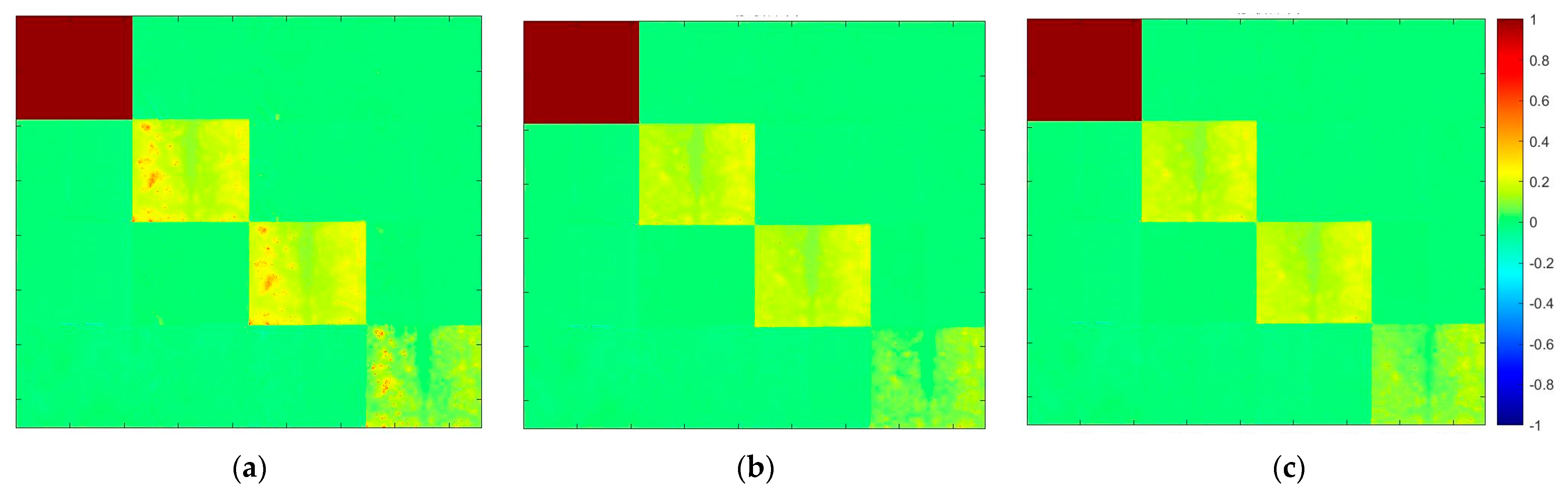
3.3.1. Mueller Matrix Elements of Double-Layer Tissue
3.3.2. Polarization Parameters of Single-Layer and Double-Layer Tissues
3.3.3. Monte Carlo (MC) Simulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gurjar, R.S.; Backman, V.; Perelman, L.T.; Georgakoudi, I.; Badizadegan, K.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Imaging human epithelial properties with polarized light-scattering spectroscopy. Nat. Med. 2001, 7, 1245–1248. [Google Scholar] [CrossRef]
- Kunnen, B.; Macdonald, C.; Doronin, A.; Jacques, S.; Eccles, M.; Meglinski, I. Application of circularly polarized light for non-invasive diagnosis of cancerous tissues and turbid tissue-like scattering media. J. Biophotonics 2015, 8, 317–323. [Google Scholar] [CrossRef]
- Ghosh, N.; Vitkin, A.I. Tissue polarimetry: Concepts, challenges, applications, and outlook. J. Biomed. Opt. 2011, 16, 110801. [Google Scholar] [CrossRef] [Green Version]
- Alali, S.; Vitkin, I.A. Polarized light imaging in biomedicine: Emerging Mueller matrix methodologies for bulk tissue assessment. J. Biomed. Opt. 2015, 20, 061104. [Google Scholar] [CrossRef]
- Ivanov, D.; Dremin, V.; Bykov, A.; Borisova, E.; Genova, T.; Popov, A.; Ossikovski, R.; Novikova, T.; Meglinski, I. Colon cancer detection by using Poincaré sphere and 2D polarimetric mapping of ex vivo colon samples. J. Biophotonics 2020, 13, e202000082. [Google Scholar] [CrossRef]
- Schucht, P.; Lee, H.R.; Mezouar, H.M.; Hewer, E.; Raabe, A.; Murek, M.; Zubak, I.; Goldberg, J.; Kövari, E.; Pierangelo, A. Visualization of white matter fiber tracts of brain tissue sections with wide-field imaging Mueller polarimetry. IEEE Trans. Med. Imaging 2020, 39, 4376–4382. [Google Scholar] [CrossRef]
- Ushenko, Y.A.; Peresunko, A.; Baku, B.A. A new method of Mueller-matrix diagnostics and differentiation of early oncological changes of the skin derma. Adv. Opt. Technol. 2010, 2010, 952423. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; He, H.; Sheng, W.; Wu, J.; Ma, H. A quantitative and non-contact technique to characterise microstructural variations of skin tissues during photo-damaging process based on Mueller matrix polarimetry. Sci. Rep. 2017, 7, 14702. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; Elson, D.S. A high definition Mueller polarimetric endoscope for tissue characterisation. Sci. Rep. 2016, 6, 25953. [Google Scholar] [CrossRef] [Green Version]
- Ushenko, Y.A.; Tomka, Y.Y.; Dubolazov, A.V.; Telen’ga, O.Y. Diagnostics of optical anisotropy changesin biological tissues using Müller matrix. Quantum Electron. 2011, 41, 273. [Google Scholar] [CrossRef]
- Huang, T.; Meng, R.; Qi, J.; Liu, Y.; Wang, X.; Chen, Y.; Liao, R.; Ma, H. Fast Mueller matrix microscope based on dual DoFP polarimeters. Opt. Lett. 2021, 46, 1676–1679. [Google Scholar] [CrossRef]
- He, H.; Liao, R.; Zeng, N.; Li, P.; Chen, Z.; Liu, X.; Ma, H. Mueller matrix polarimetry—an emerging new tool for characterizing the microstructural feature of complex biological specimen. J. Lightwave Technol. 2019, 37, 2534–2548. [Google Scholar] [CrossRef]
- Li, P.; Tariq, A.; He, H.; Ma, H. Characteristic Mueller matrices for direct assessment of the breaking of symmetries. Opt. Lett. 2020, 45, 706–709. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, N.; He, H.; Liu, C.; Du, E.; He, Y.; Ma, H. Retardance of bilayer anisotropic samples consisting of well-aligned cylindrical scatterers and birefringent media. J. Biomed. Opt. 2016, 21, 55002. [Google Scholar] [CrossRef] [Green Version]
- Azzam, R. Photopolarimetric measurement of the Mueller matrix by Fourier analysis of a single detected signal. Opt. Lett. 1978, 2, 148–150. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, Y.; Yao, Y.; Si, L.; Liu, Y.; He, H.; Ma, H. Probing layered structures by multi-color backscattering polarimetry and machine learning. Biomed. Opt. Express 2021, 12, 4324–4339. [Google Scholar] [CrossRef]
- Tuchin, V.V.; Maksimova, I.L.; Zimnyakov, D.A.; Kon, I.L.; Mavlyutov, A.H.; Mishin, A.A. Light propagation in tissues with controlled optical properties. J. Biomed. Opt. 1997, 2, 401–417. [Google Scholar] [CrossRef]
- Ke, M.-T.; Nakai, Y.; Fujimoto, S.; Takayama, R.; Yoshida, S.; Kitajima, T.S.; Sato, M.; Imai, T. Super-resolution mapping of neuronal circuitry with an index-optimized clearing agent. Cell Rep. 2016, 14, 2718–2732. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Shi, R.; Ma, N.; Tuchina, D.K.; Tuchin, V.V.; Zhu, D. Skin optical clearing potential of disaccharides. J. Biomed. Opt. 2016, 21, 081207. [Google Scholar] [CrossRef] [Green Version]
- Feng, W.; Zhang, C.; Yu, T.; Zhu, D. Quantitative evaluation of skin disorders in type 1 diabetic mice by in vivo optical imaging. Biomed. Opt. Express 2019, 10, 2996–3008. [Google Scholar] [CrossRef]
- Xu, J.; Ma, Y.; Yu, T.; Zhu, D. Quantitative assessment of optical clearing methods in various intact mouse organs. J. Biophotonics 2019, 12, e201800134. [Google Scholar] [CrossRef] [PubMed]
- Rakotomanga, P.; Soussen, C.; Khairallah, G.; Amouroux, M.; Zaytsev, S.; Genina, E.; Chen, H.; Delconte, A.; Daul, C.; Tuchin, V.; et al. Source separation approach for the analysis of spatially resolved multiply excited autofluorescence spectra during optical clearing of ex vivo skin. Biomed. Opt. Express 2019, 10, 3410–3424. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.S.; Lichtman, J.W. Clarifying tissue clearing. Cell 2015, 162, 246–257. [Google Scholar] [CrossRef] [Green Version]
- Zaytsev, S.M.; Amouroux, M.; Khairallah, G.; Bashkatov, A.N.; Tuchin, V.V.; Blondel, W.; Genina, E.A. Impact of optical clearing on ex vivo human skin optical properties characterized by spatially resolved multimodal spectroscopy. J. Biophotonics 2021, 15, e202100202. [Google Scholar] [CrossRef]
- Sdobnov, A.Y.; Darvin, M.; Genina, E.; Bashkatov, A.; Lademann, J.; Tuchin, V. Recent progress in tissue optical clearing for spectroscopic application. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 197, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, J.; Li, C.; Liu, Y.; Li, S.; Chen, H.; Sun, G.; Wang, Y. Application of Second Harmonic Generation and Two-photonFluorescence in the Distribution Tracing of Fluorescence Labelled Collagen. Acta Laser Biol. Sin. 2017, 26, 24–29. (In Chinese) [Google Scholar]
- Ma, S.; Sun, Y.; Yang, W.; Sun, G.; Li, S.; Jiang, Y.; Wang, Y.; Wang, Y. Repair Effect and Mechanism of Recombinant Human Collagen on Laser Induced Damage of Mice Skin. Acta Laser Biol. Sin. 2018, 27, 399–406. (In Chinese) [Google Scholar]
- Xie, Q.; Zeng, N.; Huang, Y.; Tuchin, V.V.; Ma, H. Study on the tissue clearing process using different agents by Mueller matrix microscope. Biomed. Opt. Express 2019, 10, 3269–3280. [Google Scholar] [CrossRef]
- Song, J.; Zeng, N.; Guo, W.; Guo, J.; Ma, H. Stokes polarization imaging applied for monitoring dynamic tissue optical clearing. Biomed. Opt. Express 2021, 12, 4821–4836. [Google Scholar] [CrossRef]
- Dong, Y.; Wan, J.; Si, L.; Meng, Y.; Dong, Y.; Liu, S.; He, H.; Ma, H. Deriving polarimetry feature parameters to characterize microstructural features in histological sections of breast tissues. IEEE Trans. Biomed. Eng. 2020, 68, 881–892. [Google Scholar] [CrossRef]
- Sun, M.; He, H.; Zeng, N.; Du, E.; Guo, Y.; Liu, S.; Wu, J.; He, Y.; Ma, H. Characterizing the microstructures of biological tissues using Mueller matrix and transformed polarization parameters. Biomed. Opt. Express 2014, 5, 4223–4234. [Google Scholar] [CrossRef] [Green Version]
- He, H.; He, C.; Chang, J.; Lv, D.; Wu, J.; Duan, C.; Zhou, Q.; Zeng, N.; He, Y.; Ma, H. Monitoring microstructural variations of fresh skeletal muscle tissues by Mueller matrix imaging. J. Biophotonics 2017, 10, 664–673. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dong, Y.; Wan, J.; He, H.; Aziz, T.; Ma, H. Polaromics: Deriving polarization parameters from a Mueller matrix for quantitative characterization of biomedical specimen. J. Phys. D Appl. Phys. 2021, 55, 34002. [Google Scholar] [CrossRef]
- Chen, D.; Zeng, N.; Xie, Q.; He, H.; Tuchin, V.V.; Ma, H. Mueller matrix polarimetry for characterizing microstructural variation of nude mouse skin during tissue optical clearing. Biomed. Opt. Express 2017, 8, 3559–3570. [Google Scholar] [CrossRef] [Green Version]
- Meng, R.; Shao, C.; Li, P.; Dong, Y.; Hou, A.; Li, C.; Lin, L.; He, H.; Ma, H. Transmission Mueller matrix imaging with spatial filtering. Opt. Lett. 2021, 46, 4009–4012. [Google Scholar] [CrossRef]
- Bashkatov, A.N.; Genina, E.A.; Tuchin, V.V. Optical properties of skin, subcutaneous, and muscle tissues: A review. J. Innov. Opt. Health Sci. 2011, 4, 9–38. [Google Scholar] [CrossRef]
- Tuchin, V.V. Optical immersion as a new tool for controlling the optical properties of tissues and blood. Laser Phys. 2005, 15, 1109–1136. [Google Scholar]
- Pierangelo, A.; Benali, A.; Antonelli, M.-R.; Novikova, T.; Validire, P.; Gayet, B.; De Martino, A. Ex-vivo characterization of human colon cancer by Mueller polarimetric imaging. Opt. Express 2011, 19, 1582–1593. [Google Scholar] [CrossRef]
- Novikova, T.; Pierangelo, A.; Manhas, S.; Benali, A.; Validire, P.; Gayet, B.; De Martino, A. The origins of polarimetric image contrast between healthy and cancerous human colon tissue. Appl. Phys. Lett. 2013, 102, 241103. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, T.; Shao, C.; Zhu, Y.; Huang, T.; Zhao, Q.; Sun, Y.; Wang, Y.; Ma, H. Probing Dynamic Variation of Layered Microstructure Using Backscattering Polarization Imaging. Photonics 2022, 9, 153. https://doi.org/10.3390/photonics9030153
Bu T, Shao C, Zhu Y, Huang T, Zhao Q, Sun Y, Wang Y, Ma H. Probing Dynamic Variation of Layered Microstructure Using Backscattering Polarization Imaging. Photonics. 2022; 9(3):153. https://doi.org/10.3390/photonics9030153
Chicago/Turabian StyleBu, Tongjun, Conghui Shao, Yuanhuan Zhu, Tongyu Huang, Qianhao Zhao, Yanan Sun, Yi Wang, and Hui Ma. 2022. "Probing Dynamic Variation of Layered Microstructure Using Backscattering Polarization Imaging" Photonics 9, no. 3: 153. https://doi.org/10.3390/photonics9030153
APA StyleBu, T., Shao, C., Zhu, Y., Huang, T., Zhao, Q., Sun, Y., Wang, Y., & Ma, H. (2022). Probing Dynamic Variation of Layered Microstructure Using Backscattering Polarization Imaging. Photonics, 9(3), 153. https://doi.org/10.3390/photonics9030153





