Review of Virus Inactivation by Visible Light
Abstract
1. Introduction
2. Materials and Methods
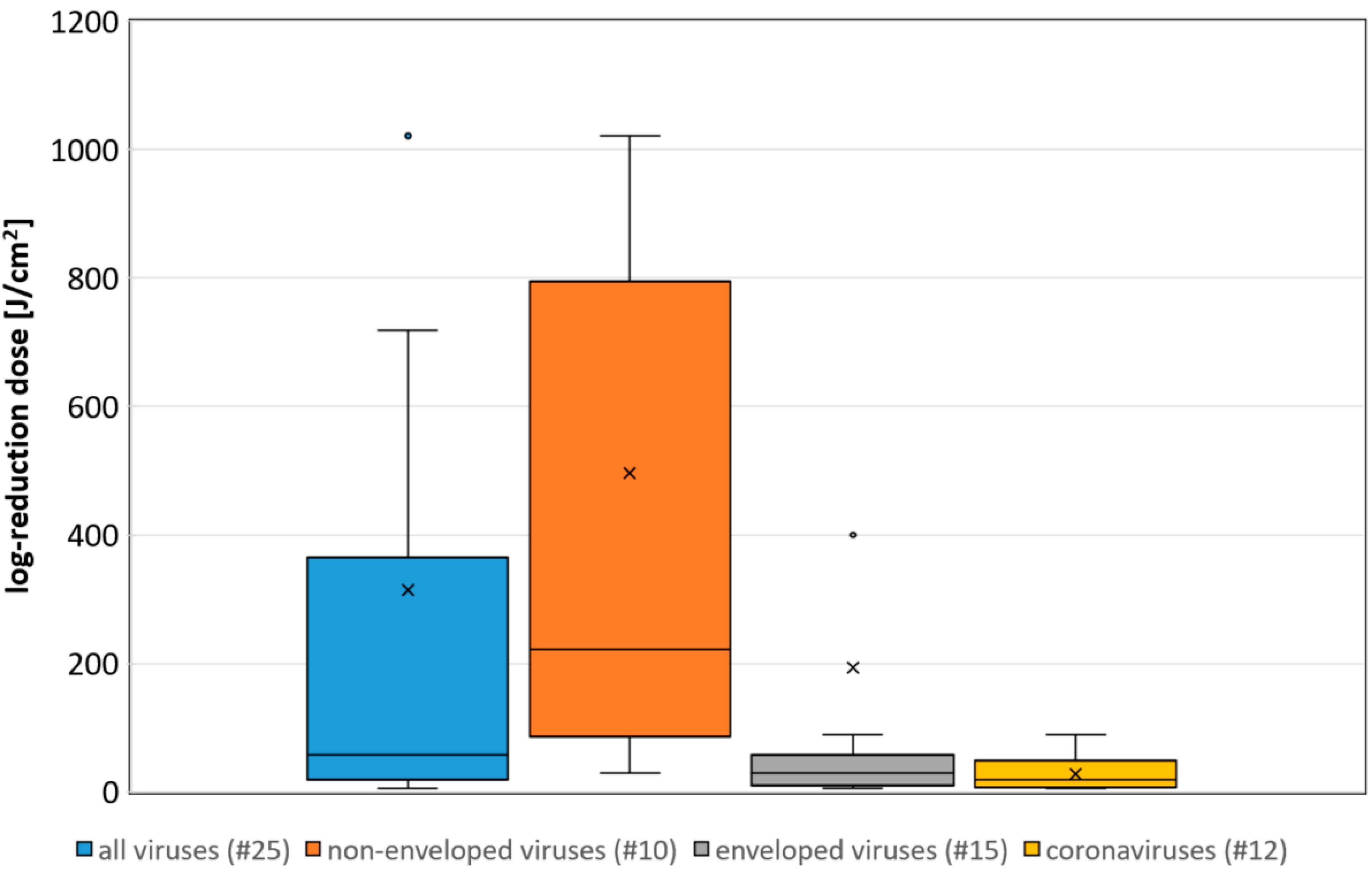
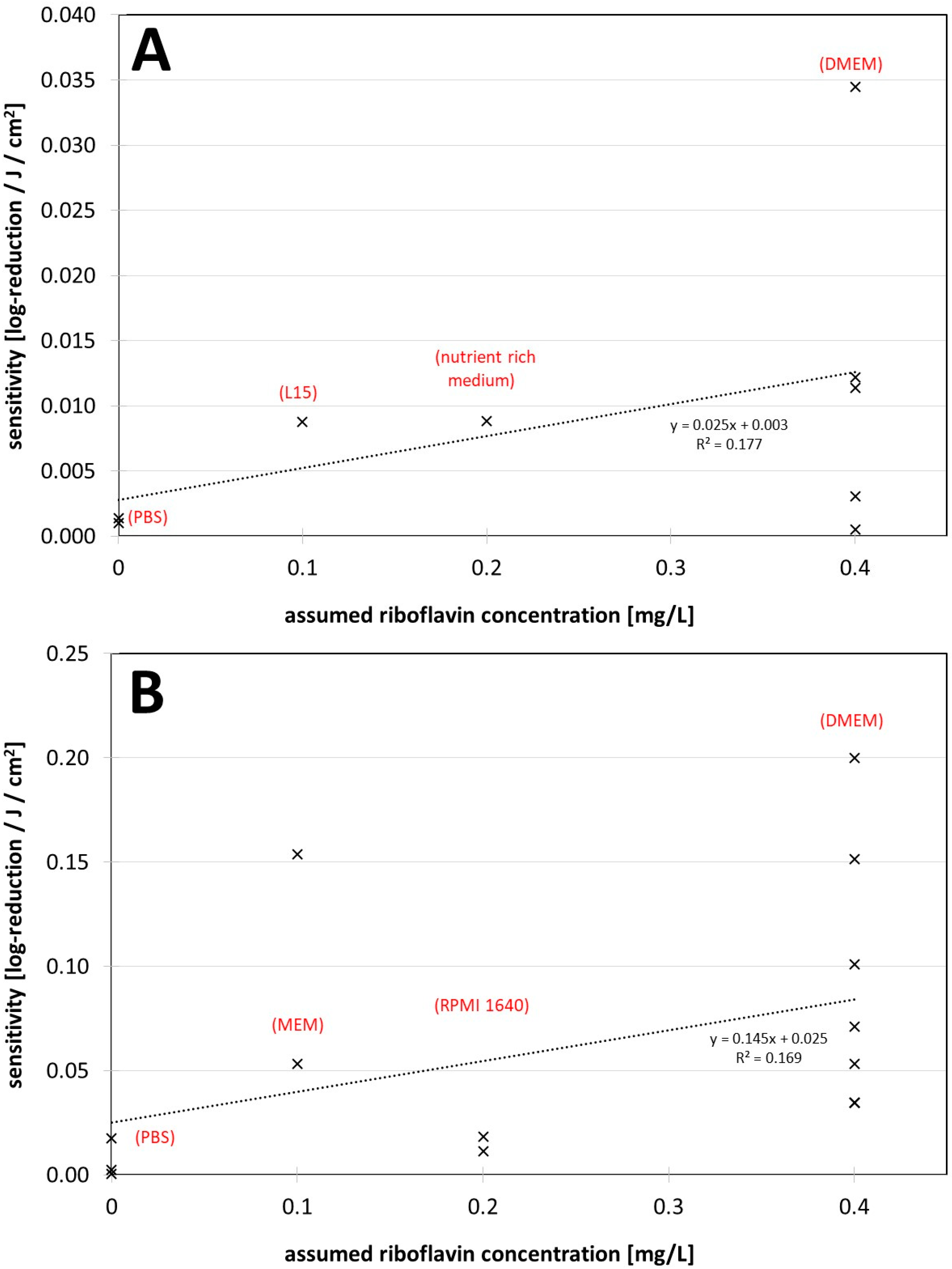
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronavirus Resource Center. COVID-19 Dashboard: (Global Map). Available online: https://coronavirus.jhu.edu/map.html (accessed on 3 January 2022).
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Kampf, G.; Voss, A.; Scheithauer, S. Inactivation of coronaviruses by heat. J. Hosp. Infect. 2020, 105, 348–349. [Google Scholar] [CrossRef] [PubMed]
- Kratzel, A.; Todt, D.; V’kovski, P.; Steiner, S.; Gultom, M.; Thao, T.T.N.; Ebert, N.; Holwerda, M.; Steinmann, J.; Niemeyer, D.; et al. Inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 by WHO-Recommended Hand Rub Formulations and Alcohols. Emerg. Infect. Dis. 2020, 26, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Hessling, M.; Hönes, K.; Vatter, P.; Lingenfelder, C. Ultraviolet irradiation doses for coronavirus inactivation—Review and analysis of coronavirus photoinactivation studies. GMS Hyg. Infect. Control 2020, 15, 8. [Google Scholar] [CrossRef]
- Hessling, M.; Hoenes, K.; Lingenfelder, C. Selection of parameters for thermal coronavirus inactivation—A data-based recommendation. GMS Hyg. Infect. Control 2020, 15, 16. [Google Scholar] [CrossRef]
- Ashkenazi, H.; Malik, Z.; Harth, Y.; Nitzan, Y. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Immunol. Med. Microbiol. 2003, 35, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Guffey, J.S.; Wilborn, J. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomed. Laser Surg. 2006, 24, 684–688. [Google Scholar] [CrossRef]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G. High-intensity narrow-spectrum light inactivation and wavelength sensitivity of Staphylococcus aureus. FEMS Microbiol. Lett. 2008, 285, 227–232. [Google Scholar] [CrossRef]
- Feuerstein, O.; Ginsburg, I.; Dayan, E.; Veler, D.; Weiss, E.I. Mechanism of visible light phototoxicity on Porphyromonas gingivalis and Fusobacterium nucleatum. Photochem. Photobiol. 2005, 81, 1186–1189. [Google Scholar] [CrossRef]
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial blue light inactivation of Pseudomonas aeruginosa by photo-excitation of endogenous porphyrins: In vitro and in vivo studies. Lasers Surg. Med. 2016, 48, 562–568. [Google Scholar] [CrossRef]
- Plavskii, V.Y.; Mikulich, A.V.; Tretyakova, A.I.; Leusenka, I.A.; Plavskaya, L.G.; Kazyuchits, O.A.; Dobysh, I.I.; Krasnenkova, T.P. Porphyrins and flavins as endogenous acceptors of optical radiation of blue spectral region determining photoinactivation of microbial cells. J. Photochem. Photobiol. B 2018, 183, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Spath, A.; Leibl, C.; Gollmer, A.; Regensburger, J.; Tabenski, L.; Hiller, K.-A.; Maisch, T.; Schmalz, G. Blue light kills Aggregatibacter actinomycetemcomitans due to its endogenous photosensitizers. Clin. Oral Investig. 2014, 18, 1763–1769. [Google Scholar] [CrossRef] [PubMed]
- Hessling, M.; Spellerberg, B.; Hoenes, K. Photoinactivation of bacteria by endogenous photosensitizers and exposure to visible light of different wavelengths—A review on existing data. FEMS Microbiol. Lett. 2016, 364, fnw270. [Google Scholar] [CrossRef]
- Tomb, R.M.; White, T.A.; Coia, J.E.; Anderson, J.G.; MacGregor, S.J.; Maclean, M. Review of the Comparative Susceptibility of Microbial Species to Photoinactivation Using 380-480 nm Violet-Blue Light. Photochem. Photobiol. 2018, 94, 445–458. [Google Scholar] [CrossRef]
- Kleinpenning, M.M.; Smits, T.; Frunt, M.H.A.; van Erp, P.E.J.; van de Kerkhof, P.C.M.; Gerritsen, R.M.J.P. Clinical and histological effects of blue light on normal skin. Photodermatol. Photoimmunol. Photomed. 2010, 26, 16–21. [Google Scholar] [CrossRef]
- McDonald, R.S.; Gupta, S.; Maclean, M.; Ramakrishnan, P.; Anderson, J.G.; Macgregor, S.J.; Meek, R.M.D.; Grant, M.H. 405 nm Light exposure of osteoblasts and inactivation of bacterial isolates from arthroplasty patients: Potential for new disinfection applications? Eur. Cell. Mater. 2013, 25, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Dong, J.; Yin, H.; Zhang, G. Blue light therapy to treat candida vaginitis with comparisons of three wavelengths: An in vitro study. Lasers Med. Sci. 2020, 35, 1329–1339. [Google Scholar] [CrossRef]
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-light irradiation regulates proliferation and differentiation in human skin cells. J. Investig. Dermatol. 2010, 130, 259–269. [Google Scholar] [CrossRef]
- Bumah, V.V.; Masson-Meyers, D.S.; Awosika, O.; Zacharias, S.; Enwemeka, C.S. The viability of human cells irradiated with 470-nm light at various radiant energies in vitro. Lasers Med. Sci. 2021, 36, 1661–1670. [Google Scholar] [CrossRef]
- Makdoumi, K.; Hedin, M.; Bäckman, A. Different photodynamic effects of blue light with and without riboflavin on methicillin-resistant Staphylococcus aureus (MRSA) and human keratinocytes in vitro. Lasers Med. Sci. 2019, 34, 1799–1805. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Grant, M.H. Differential sensitivity of osteoblasts and bacterial pathogens to 405-nm light highlighting potential for decontamination applications in orthopedic surgery. J. Biomed. Opt. 2014, 19, 105001. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Huang, Y.-Y.; Yin, R.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Tegos, G.P.; Hamblin, M.R. Blue light rescues mice from potentially fatal Pseudomonas aeruginosa burn infection: Efficacy, safety, and mechanism of action. Antimicrob. Agents Chemother. 2013, 57, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, Y.; Gupta, A.; Huang, Y.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Baer, D.G.; Hamblin, M.R.; Dai, T. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: Implications for prophylaxis and treatment of combat-related wound infections. J. Infect. Dis. 2014, 209, 1963–1971. [Google Scholar] [CrossRef] [PubMed]
- Grzelak, A.; Rychlik, B.; Bartosz, G. Light-dependent generation of reactive oxygen species in cell culture media. Free Radic. Biol. Med. 2001, 30, 1418–1425. [Google Scholar] [CrossRef]
- Ruane, P.H.; Edrich, R.; Gampp, D.; Keil, S.D.; Leonard, R.L.; Goodrich, R.P. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion 2004, 44, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Marschner, S.; Goodrich, R. Pathogen Reduction Technology Treatment of Platelets, Plasma and Whole Blood Using Riboflavin and UV Light. Transfus. Med. Hemother. 2011, 38, 8–18. [Google Scholar] [CrossRef]
- Faddy, H.M.; Prow, N.A.; Fryk, J.J.; Hall, R.A.; Keil, S.D.; Goodrich, R.P.; Marks, D.C. The effect of riboflavin and ultraviolet light on the infectivity of arboviruses. Transfusion 2015, 55, 824–831. [Google Scholar] [CrossRef]
- Faddy, H.M.; Fryk, J.J.; Watterson, D.; Young, P.R.; Modhiran, N.; Muller, D.A.; Keil, S.D.; Goodrich, R.P.; Marks, D.C. Riboflavin and ultraviolet light: Impact on dengue virus infectivity. Vox Sang. 2016, 111, 235–241. [Google Scholar] [CrossRef]
- Elikaei, A.; Hosseini, S.M.; Sharifi, Z. Inactivation of model viruses and bacteria in human fresh frozen plasma using riboflavin and long wave ultraviolet rays. Iran. J. Microbiol. 2017, 9, 50–54. [Google Scholar]
- Callahan, S.M.; Wonganan, P.; Obenauer-Kutner, L.J.; Sutjipto, S.; Dekker, J.D.; Croyle, M.A. Controlled inactivation of recombinant viruses with vitamin B2. J. Virol. Methods 2008, 148, 132–145. [Google Scholar] [CrossRef][Green Version]
- Keil, S.D.; Ragan, I.; Yonemura, S.; Hartson, L.; Dart, N.K.; Bowen, R. Inactivation of severe acute respiratory syndrome coronavirus 2 in plasma and platelet products using a riboflavin and ultraviolet light-based photochemical treatment. Vox Sang. 2020, 115, 495–501. [Google Scholar] [CrossRef]
- Zhou, Z.-Y.; Bi, X.-X. Experimental studies on the inactivation of HBV in blood via riboflavin photochemical treatment. Exp. Ther. Med. 2017, 13, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Dulbecco, R.; Freeman, G. Plaque production by the polyoma virus. Virology 1959, 8, 396–397. [Google Scholar] [CrossRef]
- Moore, G.E. Culture of Normal Human Leukocytes. JAMA 1967, 199, 519. [Google Scholar] [CrossRef] [PubMed]
- Eagle, H. Propagation in a fluid medium of a human epidermoid carcinoma, strain KB. Proc. Soc. Exp. Biol. Med. 1955, 89, 362–364. [Google Scholar] [CrossRef]
- Martin, C.B.; Wilfong, E.; Ruane, P.; Goodrich, R.; Platz, M. An action spectrum of the riboflavin-photosensitized inactivation of Lambda phage. Photochem. Photobiol. 2005, 81, 474–480. [Google Scholar] [CrossRef]
- Terrosi, C.; Anichini, G.; Docquier, J.D.; Gori Savellini, G.; Gandolfo, C.; Pavone, F.S.; Cusi, M.G. Efficient Inactivation of SARS-CoV-2 and Other RNA or DNA Viruses with Blue LED Light. Pathogens 2021, 10, 1590. [Google Scholar] [CrossRef]
- Tomb, R.M.; Maclean, M.; Coia, J.E.; Graham, E.; McDonald, M.; Atreya, C.D.; MacGregor, S.J.; Anderson, J.G. New Proof-of-Concept in Viral Inactivation: Virucidal Efficacy of 405 nm Light Against Feline Calicivirus as a Model for Norovirus Decontamination. Food Environ. Virol. 2016, 9, 159–167. [Google Scholar] [CrossRef]
- Tomb, R.M.; Maclean, M.; Herron, P.R.; Hoskisson, P.A.; MacGregor, S.J.; Anderson, J.G. Inactivation of Streptomyces phage C31 by 405 nm light: Requirement for exogenous photosensitizers? Bacteriophage 2014, 4, e32129. [Google Scholar] [CrossRef]
- Ho, D.T.; Kim, A.; Kim, N.; Roh, H.J.; Chun, W.-K.; Lee, Y.; Kim, D.-H. Effect of blue light emitting diode on viral hemorrhagic septicemia in olive flounder (Paralichthys olivaceus). Aquaculture 2020, 521, 735019. [Google Scholar] [CrossRef]
- Rathnasinghe, R.; Jangra, S.; Miorin, L.; Schotsaert, M.; Yahnke, C.; Garcίa-Sastre, A. The virucidal effects of 405 nm visible light on SARS-CoV-2 and influenza A virus. Sci. Rep. 2021, 11, 19470. [Google Scholar] [CrossRef] [PubMed]
- Kingsley, D.; Kuis, R.; Perez, R.; Basaldua, I.; Burkins, P.; Marcano, A.; Johnson, A. Oxygen-dependent laser inactivation of murine norovirus using visible light lasers. Virol. J. 2018, 15, 117. [Google Scholar] [CrossRef]
- Tsugita, A.; Okada, Y.; Uehara, K. Photosensitized inactivation of ribonucleic acids in the presence of riboflavin. Biochim. Biophys. Acta—Nucleic Acids Protein Synth. 1965, 103, 360–363. [Google Scholar] [CrossRef]
- Kingsley, D.H.; Perez-Perez, R.E.; Boyd, G.; Sites, J.; Niemira, B.A. Evaluation of 405-nm monochromatic light for inactivation of Tulane virus on blueberry surfaces. J. Appl. Microbiol. 2018, 124, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Skinner, H.H.; Bradish, C.J. Exposure to light as a source of error in the estimation of the infectivity of virus suspensions. J. Gen. Microbiol. 1954, 10, 377–397. [Google Scholar] [CrossRef]
- Appleyard, G. The photosensitivity of Semliki Forest and other viruses. J. Gen. Virol. 1967, 1, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Stasko, N.; Kocher, J.F.; Annas, A.; Henson, I.; Seitz, T.S.; Miller, J.M.; Arwood, L.; Roberts, R.C.; Womble, T.M.; Keller, E.G.; et al. Visible blue light inhibits infection and replication of SARS-CoV-2 at doses that are well-tolerated by human respiratory tissue. Sci. Rep. 2021, 11, 20595. [Google Scholar] [CrossRef]
- Cutchins, E.C.; Dayhuff, T.R. Photoinactivation of measles virus. Virology 1962, 17, 420–425. [Google Scholar] [CrossRef]
- de Santis, R.; Luca, V.; Näslund, J.; Ehmann, R.K.; de Angelis, M.; Lundmark, E.; Nencioni, L.; Faggioni, G.; Fillo, S.; Amatore, D.; et al. Rapid inactivation of SARS-CoV-2 with LED irradiation of visible spectrum wavelengths. J. Photochem. Photobiol. 2021, 8, 100082. [Google Scholar] [CrossRef]
- Biasin, M.; Strizzi, S.; Bianco, A.; Macchi, A.; Utyro, O.; Pareschi, G.; Loffreda, A.; Cavalleri, A.; Lualdi, M.; Trabattoni, D.; et al. UV-A and UV-B Can Neutralize SARS-CoV-2 Infectivity. medRxiv 2021. [Google Scholar] [CrossRef]
- Gardner, A.; Ghosh, S.; Dunowska, M.; Brightwell, G. Virucidal Efficacy of Blue LED and Far-UVC Light Disinfection against Feline Infectious Peritonitis Virus as a Model for SARS-CoV-2. Viruses 2021, 13, 1436. [Google Scholar] [CrossRef] [PubMed]
- Zavestovskaya, I.N.; Guschin, V.A.; Nikiforova, M.A.; Siniavin, A.E.; Russu, L.I.; Cheshev, E.A.; Koromyslov, A.L.; Tupitsyn, I.M.; Fronya, A.A.; Grigoryeva, M.S. Experimental investigation of the effect ov UVA radiation on the coronavirus infective properties. Bull. Lebedev Phys. Inst. 2021, 48, 195–199. [Google Scholar] [CrossRef]
- Enwemeka, C.S.; Bumah, V.V.; Mokili, J.L. Pulsed blue light inactivates two strains of human coronavirus. J. Photochem. Photobiol. B 2021, 222, 112282. [Google Scholar] [CrossRef]
- Lau, B.; Becher, D.; Hessling, M. High Intensity Violet Light (405 nm) Inactivates Coronaviruses in Phosphate Buffered Saline (PBS) and on Surfaces. Photonics 2021, 8, 414. [Google Scholar] [CrossRef]
- Zupin, L.; Caracciolo, I.; Tricarico, P.M.; Ottaviani, G.; D’Agaro, P.; Crovella, S. Photobiomodulation therapy reduces viral load and cell death in ZIKV-infected glioblastoma cell line. Lasers Med. Sci. 2018, 33, 2011–2013. [Google Scholar] [CrossRef] [PubMed]
- Zupin, L.; Caracciolo, I.; Tricarico, P.M.; Ottaviani, G.; D’Agaro, P.; Crovella, S. Antiviral properties of blue laser in an in vitro model of HSV-1 infection. Microbiol. Immunol. 2018, 62, 477–479. [Google Scholar] [CrossRef]
- Vatter, P.; Hoenes, K.; Hessling, M. Photoinactivation of the Coronavirus Surrogate phi6 by Visible Light. Photochem. Photobiol. 2021, 97, 122–125. [Google Scholar] [CrossRef]
- Vatter, P.; Hoenes, K.; Hessling, M. Blue light inactivation of the enveloped RNA virus Phi6. BMC Res. Notes 2021, 14, 187. [Google Scholar] [CrossRef]
- Cartwright, S.F. A cytopathic virus causing a transmissible gastroenteritis in swine. II. Biological and serological studies. J. Comp. Pathol. 1966, 76, 95–106. [Google Scholar] [CrossRef]
- Wallis, C.; Melnick, J.L. Irreversible photosensitization of viruses. Virology 1964, 23, 520–527. [Google Scholar] [CrossRef]
- Wallis, C.; Trulock, S.; Melnick, J.L. Inherent photosensitivity of herpes virus and other enveloped viruses. J. Gen. Virol. 1969, 5, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Nemo, G.J.; Cutchins, E.C. Effect of visible light on canine distemper virus. J. Bacteriol. 1966, 91, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.B.; Porter, C.D. Inactivation of murine leukaemia virus by exposure to visible light. Virology 2005, 341, 321–329. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Booth, J.C.; Stern, H. Photodynamic inactivation of rubella virus. J. Med. Microbiol. 1972, 5, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Zupin, L.; Gratton, R.; Fontana, F.; Clemente, L.; Pascolo, L.; Ruscio, M.; Crovella, S. Blue photobiomodulation LED therapy impacts SARS-CoV-2 by limiting its replication in Vero cells. J. Biophotonics 2021, 14, e202000496. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Almeida, A. Photodynamic inactivation of mammalian viruses and bacteriophages. Viruses 2012, 4, 1034–1074. [Google Scholar] [CrossRef] [PubMed]
- Wiehe, A.; O’Brien, J.M.; Senge, M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019, 18, 2565–2612. [Google Scholar] [CrossRef]
| (A) Non-Enveloped Viruses | Irradiation Wavelength | Medium during Irradiation | Assumed Riboflavin Concentration [mg/L] | 90% Reduction Dose [J/cm2] |
| lambda phage (dsDNA) | 410 nm | PBS + riboflavin | 18.8 | 4 * [37] |
| PBS | 0 | no reduction after 5 J/cm2 [37] | ||
| adenovirus (dsDNA) | 420 nm | DMEM + FCS | 0.4 | 29 [38] |
| feline calcivirus (ssRNA) | 405 nm | PBS + riboflavin + tyrosine, tryptophan, pyridoxine and folic acid | 0.4 | 82 [39] |
| nutrient rich medium (DMEM + FCS +...) | 0.4 | 88 [39] | ||
| PBS + riboflavin | 0.4 | 329 [39] | ||
| PBS | 0.0 | 719 [39] | ||
| phi C31 (dsDNA) | 405 nm | nutrient rich medium | 0.2 | 113 [40] |
| PBS | 0.0 | 1021 [40] | ||
| viral haemorrhagic septicaemia virus (ssRNA) | 405 nm | L15 + FCS | 0.1 | 114 [41] |
| encephalomyocarditis virus (ssRNA) | 405 nm + blue/white | DMEM + FCS + PBS (ratio unknown) | ? | 178 [42] |
| murine norovirus (ssRNA) | 408 (cw laser) | DMEM + riboflavin | 1.4 | 491 * [43] |
| DMEM | 0.4 | 1976 [43] | ||
| tobacco mosaic virus (ssRNA) | white light | acetate buffer + riboflavin | 0.05 | reduction observed [44] |
| acetate buffer | 0 | no reduction [44] | ||
| Tulane virus (ssRNA) | 405 nm | blueberry surface + riboflavin | reduction [45] | |
| blueberry surface | no reduction after 7.6 J/cm2 [45] | |||
| foot and mouth disease virus (ssRNA) | daylight and artificial light | phosphate saline | 0 | medium reduction [46] |
| poliovirus type 1 (ssRNA) | fluorescent lamp | gelatin saline | 0 | weak reduction [47] |
| human rhinovirus 1b (ssRNA) | 425 nm | DMEM + FCS | 0.4 | no reduction after 45 J/cm2 [48] |
| poliovirus type 3 (ssRNA) | white light of incandescent bulb | medium 199 | 0.01 | no reduction [49] |
| (B) Enveloped Viruses | Irradiation Wavelength | Medium during Irradiation | Assumed Riboflavin Concentration [mg/L] | 90% Reduction Dose [J/cm2] |
| SARS-CoV-2 (ssRNA, coronavirus) | 425 nm | DMEM + FCS | 0.4 | 5 [48] |
| MEM + FCS | 0.1 | 18.8 [48] | ||
| inside vero cells | ? | 6.4 * [48] | ||
| inside epithelial cells | ? | 6.7 * [48] | ||
| SARS-CoV-2 (ssRNA, coronavirus) | 410 nm + blue/white | MEM + FCS (DMEM @ FOI laboratory?) | 0.1 | 6.5 blue (<490 nm) 12.6 total irradiation [50] |
| SARS-CoV-2 (ssRNA, coronavirus) | 405 nm + blue/white | DMEM | 0.4 | 6.6 [51] |
| SARS-CoV-2 (ssRNA, coronavirus) | 405 nm + blue/white | DMEM + FCS + PBS (ratio unknown) | ? | 7.5 (<420 nm) [42] |
| SARS-CoV-1 (ssRNA, coronavirus) | 425 nm | DMEM + FCS | 0.4 | 9.9 [48] |
| feline infectious peritonitis virus (ssRNA, coronavirus) | 405 nm | DMEM + FCS | 0.4 | 14.1 [52] |
| metal wet | 12 * [52] | |||
| metal dry | 20.3 * [52] | |||
| paper wet | 10.8 * [52] | |||
| paper dry | 13 * [52] | |||
| plastic wet | 14.4 * [52] | |||
| plastic dry | 31.8 * [52] | |||
| MERS-CoV (ssRNA, coronavirus) | 425 nm | DMEM + FCS | 0.4 | 18.8 [48] |
| influenza A virus (ssRNA) | 405 nm + blue/white | DMEM + FCS + PBS (ratio unknown) | ? | 23.5 [42] |
| respiratory syncytial virus (ssRNA) | 420 nm | DMEM + FCS | 0.4 | 29 [38] |
| SARS-CoV-2 (ssRNA, coronavirus) | 420 nm | DMEM + FCS | 0.4 | 29 [38] |
| BCoV (ssRNA, coronavirus) | 401 nm | DMEM + FCS | 0.4 | 29 [53] |
| HCoV-229E (ssRNA, coronavirus) | 405 nm (pulsed) | RPMI 1640 | 0.2 | 55 [54] |
| BCoV (ssRNA, coronavirus) | 405 nm | (consumed) RPMI 1640 diluted 1:10 in PBS | 0 | 57.5 [55] |
| steel surface | 96 * [55] | |||
| zika virus (ssRNA) | 445 nm (cw laser) | unknown medium | ? | 64 [56] |
| HCoV-229E (ssRNA, coronavirus) | 405 nm | RPMI 1640 | 0.2 | 89 [54] |
| herpes simplex virus Type 1 (dsDNA) | 445 nm (pulsed) | unknown medium | 112 [57] | |
| phi 6 (dsRNA) | 405 | PBS/SMG | 0 | 400 [58] |
| phi 6 (dsRNA) | 455 | PBS | 0 | 2130 [59] |
| semliki forest virus (ssRNA) | daylight and fluorescent lamp | gelatin saline + riboflavin | 2 | very strong reduction [47] |
| gelatin saline | 0 | strong reduction [47] | ||
| sindbis virus (ssRNA) | daylight and fluorescent lamp | gelatin saline | 0 | strong reduction [47] |
| Murray Valley encephalitis virus (ssRNA) | fluorescent lamp | gelatin saline | 0 | strong reduction [47] |
| transmissible gastroenteritis virus (ssRNA, coronavirus) | daylight | unknown medium | ? | strong reduction [60] |
| influenza B virus (ssRNA) | daylight and artificial light | phosphate saline | 0 | strong reduction [46] |
| vesicular stomatitis virus (ssRNA) | daylight and artificial light | phosphate saline | 0 | strong reduction [46] |
| measles morbillivirus (ssRNA) | white light of fluorescent lamp | salt solutions | 0 | strong reduction [61] |
| herpes simplex virus (dsDNA, JES strain) | white light of fluorescent lamp | MEM | 0.1 | strong reduction [62] |
| riboflavin solution | 0.1 | strong reduction [62] | ||
| salt solutions | 0 | reduction [62] | ||
| distilled water | 0 | no reduction [62] | ||
| canine distemper virus (ssRNA) | artificial visible light | MEM | 0.1 | strong reduction [63] |
| riboflavin solution | 0.1 | strong reduction [63] | ||
| salt solutions | 0 | reduction [63] | ||
| measles morbillivirus (ssRNA) | white light of incandescent bulb | Eagle’s basal medium | 0.1 | strong reduction [49] |
| distilled water | 0 | reduction [49] | ||
| murine leukaemia virus (ssRNA) | 420–430 nm | OptiMEM | 0.1 | reduction [64] |
| rubella virus (ssRNA) | white light of incandescent bulb | PBS | 0 | reduction [65] |
| influenza A virus (ssRNA) | fluorescent lamp | gelatin saline | 0 | reduction [47] |
| parainfluenza virus type 3 (ssRNA) | white light of fluorescent lamp | salt solutions | 0 | reduction [61] |
| SARS-CoV-2 (ssRNA, coronavirus) | 450, 454, 470 nm | inside vero cells | ? | reduction [66] |
| DMEM + FCS | 0.4 | no reduction after 20 J/cm2 [66] | ||
| Newcastle disease virus (ssRNA) | daylight and artificial light | phosphate saline | 0 | weak reduction [46] |
| vaccinia virus (dsDNA) | white light of incandescent bulb | Eagle’s basal medium | 0.1 | weak reduction [49] |
| vaccinia virus (dsDNA) | daylight and artificial light | phosphate saline | 0 | weak reduction [46] |
| influenza A virus (ssRNA) | daylight and artificial light | phosphate saline | 0 | weak reduction [46] |
| fowl plague virus (ssRNA) | daylight and artificial light | phosphate saline | 0 | no reduction [46] |
| rabbit pox virus (dsDNA) | fluorescent lamp | gelatin saline | 0 | no reduction [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hessling, M.; Lau, B.; Vatter, P. Review of Virus Inactivation by Visible Light. Photonics 2022, 9, 113. https://doi.org/10.3390/photonics9020113
Hessling M, Lau B, Vatter P. Review of Virus Inactivation by Visible Light. Photonics. 2022; 9(2):113. https://doi.org/10.3390/photonics9020113
Chicago/Turabian StyleHessling, Martin, Bernhard Lau, and Petra Vatter. 2022. "Review of Virus Inactivation by Visible Light" Photonics 9, no. 2: 113. https://doi.org/10.3390/photonics9020113
APA StyleHessling, M., Lau, B., & Vatter, P. (2022). Review of Virus Inactivation by Visible Light. Photonics, 9(2), 113. https://doi.org/10.3390/photonics9020113





