Dicarbocyanine Dye-Based Organic Photodiodes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
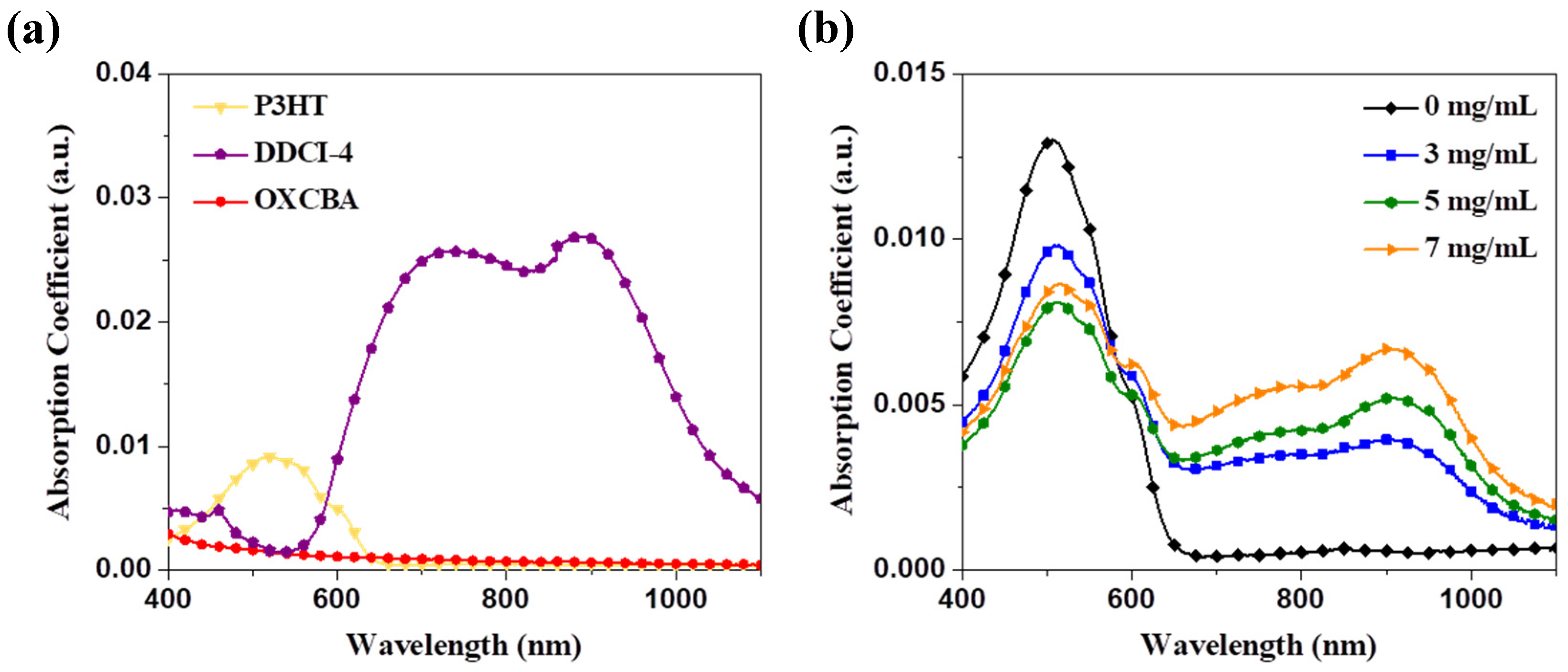
3.1. Strong Absorption in the NIR Region by Dicarbocyanine
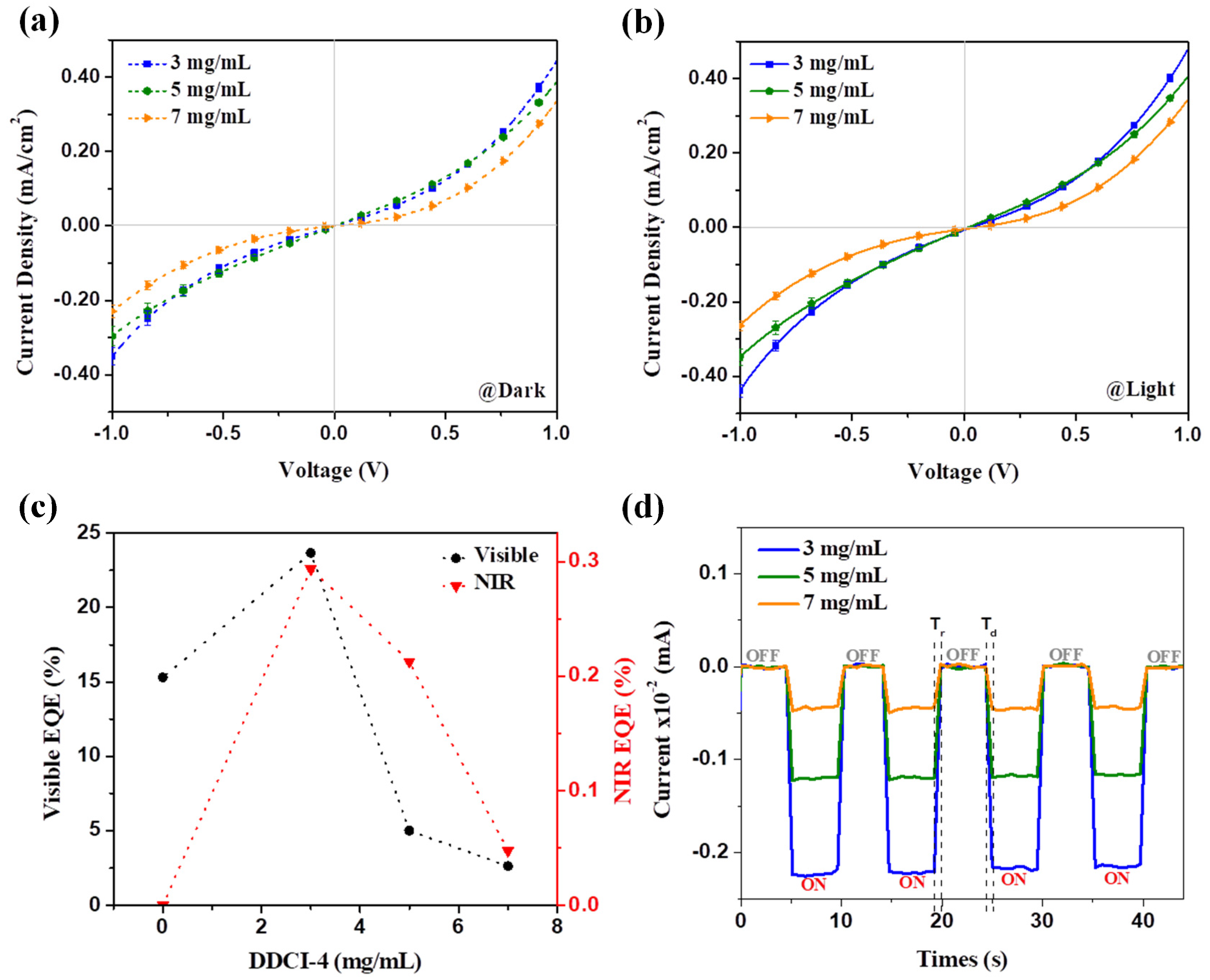
3.2. Photocurrent Performance Due to Dicarbocyanine
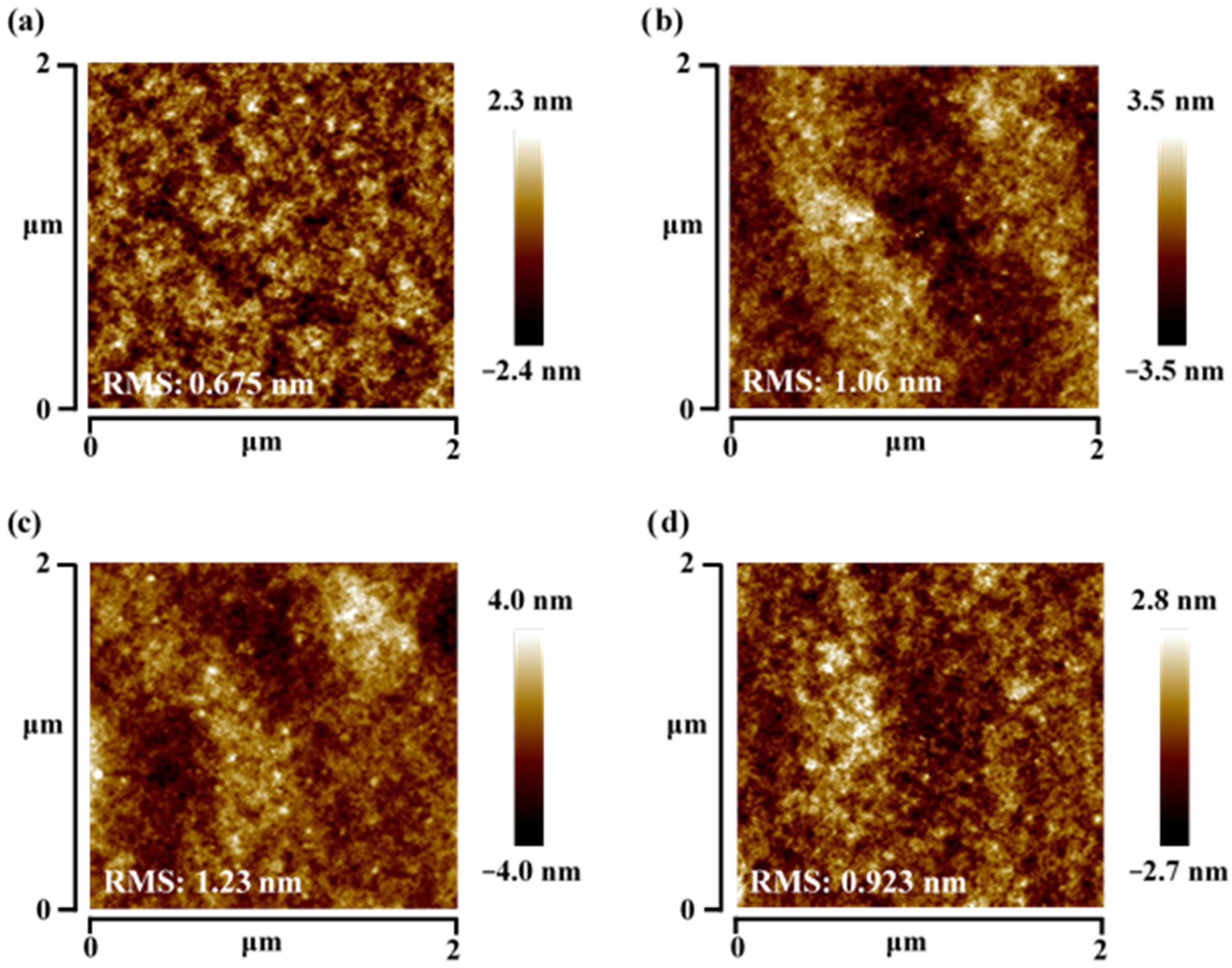
3.3. Rougher Surface with Dicarbocyanine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liess, A.; Arjona-Esteban, A.; Kudzus, A.; Albert, J.; Krause, A.M.; Lv, A.; Stolte, M.; Meerholz, K.; Würthner, F. Ultranarrow Bandwidth Organic Photodiodes by Exchange Narrowing in Merocyanine H- and J-Aggregate Excitonic Systems. Adv. Funct. Mater. 2019, 29, 1–9. [Google Scholar] [CrossRef]
- Osedach, T.P.; Iacchetti, A.; Lunt, R.R.; Andrew, T.L.; Brown, P.R.; Akselrod, G.M.; Bulović, V. Near-Infrared Photodetector Consisting of J-Aggregating Cyanine Dye and Metal Oxide Thin Films. Appl. Phys. Lett. 2012, 101. [Google Scholar] [CrossRef]
- Shapiro, B.I.; Nekrasov, A.D.; Manulik, E.V.; Krivobok, V.S.; Lebedev, V.S. Optical and Photoelectric Properties of Multichromic Cyanine Dye J-Aggregates. Quantum Electron. 2018, 48, 856–866. [Google Scholar] [CrossRef]
- Walker, B.J.; Dorn, A.; Bulović, V.; Bawendi, M.G. Color-Selective Photocurrent Enhancement in Coupled J-Aggregate/Nanowires Formed in Solution. Nano Lett. 2011, 11, 2655–2659. [Google Scholar] [CrossRef] [PubMed]
- Konstantatos, G.; Sargent, E.H. Nanostructured Materials for Photon Detection. Nat. Nanotechnol. 2010, 5, 391–400. [Google Scholar] [CrossRef]
- Qi, T.; Yu, Y.; Liu, J.; Jia, Y.; Ding, D. Enhanced Performance of Single-Walled Carbon Nanotube-Germanium Near-Infrared Photodetector by Doping with Au Nanoparticles. Photonics 2022, 9, 615. [Google Scholar] [CrossRef]
- Mishra, A.; Behera, R.K.; Behera, P.K.; Mishra, B.K.; Behera, G.B. Cyanines during the 1990s: A Review. Chem. Rev. 2000, 100, 1973–2011. [Google Scholar] [CrossRef]
- Caram, J.R.; Fidler, A.F.; Engel, G.S. Excited and Ground State Vibrational Dynamics Revealed by Two-Dimensional Electronic Spectroscopy. J. Chem. Phys. 2012, 137, 024507. [Google Scholar] [CrossRef]
- Yang, T.-S.; Chang, M.-S.; Chang, R.; Hayashi, M.; Lin, S.H.; Vöhringer, P.; Dietz, W.; Scherer, N.F. Femtosecond Pump-Probe Study of Molecular Vibronic Structures and Dynamics of a Cyanine Dye in Solution. J. Chem. Phys. 1999, 110, 12070–12081. [Google Scholar] [CrossRef][Green Version]
- Bricks, J.L.; Slominskii, Y.L.; Panas, I.D.; Demchenko, A.P. Fluorescent J-Aggregates of Cyanine Dyes: Basic Research and Applications Review. Methods Appl. Fluoresc. 2018, 6, 012001. [Google Scholar] [CrossRef]
- Zhang, H.; Jenatsch, S.; De Jonghe, J.; Nuësch, F.; Steim, R.; Véron, A.C.; Hany, R. Transparent Organic Photodetector Using a Near-Infrared Absorbing Cyanine Dye. Sci. Rep. 2015, 5, 9439. [Google Scholar] [CrossRef] [PubMed]
- Laryushkin, A.S.; Grishina, A.D.; Krivenko, T.V.; Savel’ev, V.V.; Rychwalski, R.W.; Vannikov, A.V. The Effect of Cyanine Dyes on Photorefractive Properties of Composites Based on Carbon Nanotubes. Prot. Met. Phys. Chem. Surfaces 2012, 48, 191–198. [Google Scholar] [CrossRef]
- Würthner, F.; Kaiser, T.E.; Saha-Möller, C.R. J-Aggregates: From Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials. Angew. Chem.-Int. Ed. 2011, 50, 3376–3410. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, R.; Isella, G.; Sanchez-Amores, A.; Neukom, S.; Neels, A.; Neumann, L.; Brenzikofer, A.; Dommann, A.; Urban, C.; Von Känel, H. Near Infrared Image Sensor with Integrated Germanium Photodiodes. J. Appl. Phys. 2011, 110, 023107. [Google Scholar] [CrossRef]
- Hisamuddin, S.N.; Abdullah, S.M.; Alwi, S.A.K.; Majid, S.R.; Anuar, A.; Sulaiman, K.; Tunmee, S.; Chanlek, N.; Bawazeer, T.M.; Alsoufi, M.S.; et al. Optimizing the Performance of P3HT-Based Photodetector by Tuning the Composition of OXCBA. Synth. Met. 2020, 268, 116506. [Google Scholar] [CrossRef]
- Alwi, S.A.K.; Hisamuddin, S.N.; Abdullah, S.M.; Anuar, A.; Rahim, A.H.A.; Majid, S.R.; Bawazeer, T.M.; Alsoufi, M.S.; Alsenany, N.; Supangat, A. Naphthalocyanine-Based Nir Organic Photodiode: Understanding the Role of Different Types of Fullerenes. Micromachines 2021, 12, 1383. [Google Scholar] [CrossRef]
- Coulter, J.B.; Birnie, D.P. Assessing Tauc Plot Slope Quantification: ZnO Thin Films as a Model System. Phys. Status Solidi Basic Res. 2018, 255, 1–7. [Google Scholar] [CrossRef]
- Wang, C.; Ouyang, L.; Xu, X.; Braun, S.; Liu, X.; Fahlman, M. Relationship of Ionization Potential and Oxidation Potential of Organic Semiconductor Films Used in Photovoltaics. Sol. RRL 2018, 2, 1–6. [Google Scholar] [CrossRef]
- Sworakowski, J. How Accurate Are Energies of HOMO and LUMO Levels in Small-Molecule Organic Semiconductors Determined from Cyclic Voltammetry or Optical Spectrosco. Synth. Met. 2018, 235, 125–130. [Google Scholar] [CrossRef]
- Guan, Z.L.; Bok Kim, J.; Loo, Y.L.; Kahn, A. Electronic Structure of the Poly(3-Hexylthiophene):Indene-C60 Bisadduct Bulk Heterojunction. J. Appl. Phys. 2011, 110, 043719. [Google Scholar] [CrossRef]
- Shekhar, H.; Solomeshch, O.; Liraz, D.; Tessler, N. Low Dark Leakage Current in Organic Planar Heterojunction Photodiodes. Appl. Phys. Lett. 2017, 111, 223301. [Google Scholar] [CrossRef]
- Hirade, M.; Adachi, C. Small Molecular Organic Photovoltaic Cells with Exciton Blocking Layer at Anode Interface for Improved Device Performance. Appl. Phys. Lett. 2011, 99, 2012–2015. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, X.; Li, H.; Qin, J.; Du, S.; Lu, X.; Tong, J.; Yang, C.; Li, J. Utilizing Non-Conjugated Small-Molecular Tetrasodium Iminodisuccinateas Electron Transport Layer Enabled Improving Efficiency of Organic Solar Cells. Opt. Mater. (Amst). 2022, 129, 112520. [Google Scholar] [CrossRef]
- Roslan, N.A.; Abu Bakar, A.; Bawazeer, T.M.; Alsoufi, M.S.; Alsenany, N.; Abdul Majid, W.H.; Supangat, A. Enhancing the Performance of Vanadyl Phthalocyanine-Based Humidity Sensor by Varying the Thickness. Sens. Actuators B Chem. 2019, 279, 148–156. [Google Scholar] [CrossRef]
- Ashraf Md Sabri, A.; Nafisah Hisamuddin, S.; Anis Khairani Alwi, S.; Adilah Roslan, N.; Minh Tam, N.; Bawazeer, T.M.; Alsoufi, M.S.; Alsenany, N.; Ahmad Ludin, N.; Supangat, A. Effect of Silver Nanowires on the Performance of VTP:PC71BM Organic Photodiodes. Mater. Lett. 2022, 324, 132685. [Google Scholar] [CrossRef]
- Spano, F.C. The Spectral Signatures of Frenkel Polarons In. Acc. Chem. Res. 2010, 43, 429–439. [Google Scholar] [CrossRef]
- Fidder, H.; Knoester, J.; Wiersma, D.A. Superradiant Emission and Optical Dephasing in J-Aggregates. Chem. Phys. Lett. 1990, 171, 529–536. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Wagner, W.; Stepanenko, V.; Ren, X.; Ogi, S.; Würthner, F. Near-IR Absorbing J-Aggregate of an Amphiphilic BF2-Azadipyrromethene Dye by Kinetic Cooperative Self-Assembly. Angew. Chem.-Int. Ed. 2017, 56, 5729–5733. [Google Scholar] [CrossRef]
- Abramavicius, D.; Palmieri, B.; Voronine, D.V.; Šanda, F.; Mukamel, S. Coherent Multidimensional Optical Spectroscopy of Excitons in Molecular Aggregates; Quasiparticle versus Supermolecule Perspectives. Chem. Rev. 2009, 109, 2350–2408. [Google Scholar] [CrossRef]
- Van Burgel, M.; Wiersma, D.A.; Duppen, K. The Dynamics of One-Dimensional Excitons in Liquids. J. Chem. Phys. 1995, 102, 20–33. [Google Scholar] [CrossRef]
- Möbius, D. Scheibe Aggregates. Adv. Mater. 1995, 7, 437–444. [Google Scholar] [CrossRef]
- Oishi, Y.; Arima, H.; Suehiro, K.; Era, M. Morphological and Optical Studies for a Multi Component Monolayer of Cyanine Dye and Fatty Acid. Thin Solid Films 2003, 438–439, 44–47. [Google Scholar] [CrossRef]
- Zhang, J.P.; Zhou, S.Y.; Chen, P.; Tsuneki, O.; Masaaki, H. The Effect of Solvent on the Optical Properties of Cyanine Dye Films. Dye. Pigment. 2001, 51, 93–101. [Google Scholar] [CrossRef]
- Murugesan, V.S.; Ono, S.; Tsuda, N.; Yamada, J.; Shin, P.K.; Ochiai, S. Characterization of Organic Thin Film Solar Cells of PCDTBT: PC71BM Prepared by Different Mixing Ratio and Effect of Hole Transport Layer. Int. J. Photoenergy 2015, 2015, 687678. [Google Scholar] [CrossRef]
- Liang, Z.; Tong, J.; Li, H.; Wang, Y.; Wang, N.; Li, J.; Yang, C.; Xia, Y. The Comprehensive Utilization of the Synergistic Effect of Fullerene and Non-Fullerene Acceptors to Achieve Highly Efficient Polymer Solar Cells. J. Mater. Chem. A 2019, 7, 15841–15850. [Google Scholar] [CrossRef]
- Grajower, M.; Levy, U.; Khurgin, J.B. The Role of Surface Roughness in Plasmonic-Assisted Internal Photoemission Schottky Photodetectors. ACS Photonics 2018, 5, 4030–4036. [Google Scholar] [CrossRef]
- Ma, Z.; Tang, Z.; Wang, E.; Andersson, M.R.; Inganäs, O.; Zhang, F. Influences of Surface Roughness of ZnO Electron Transport Layer on the Photovoltaic Performance of Organic Inverted Solar Cells. J. Phys. Chem. C 2012, 116, 24462–24468. [Google Scholar] [CrossRef]
- Xue, L.; Liu, X.; Wang, Q.; Yang, M.; Du, S.; Yang, C.; Tong, J.; Xia, Y.; Li, J. Improved Performance of Organic Solar Cells by Utilizing Green Non-Halogen Additive to Modulate Active-Layer Morphology. Energy Technol. 2022, 10, 2200504. [Google Scholar] [CrossRef]
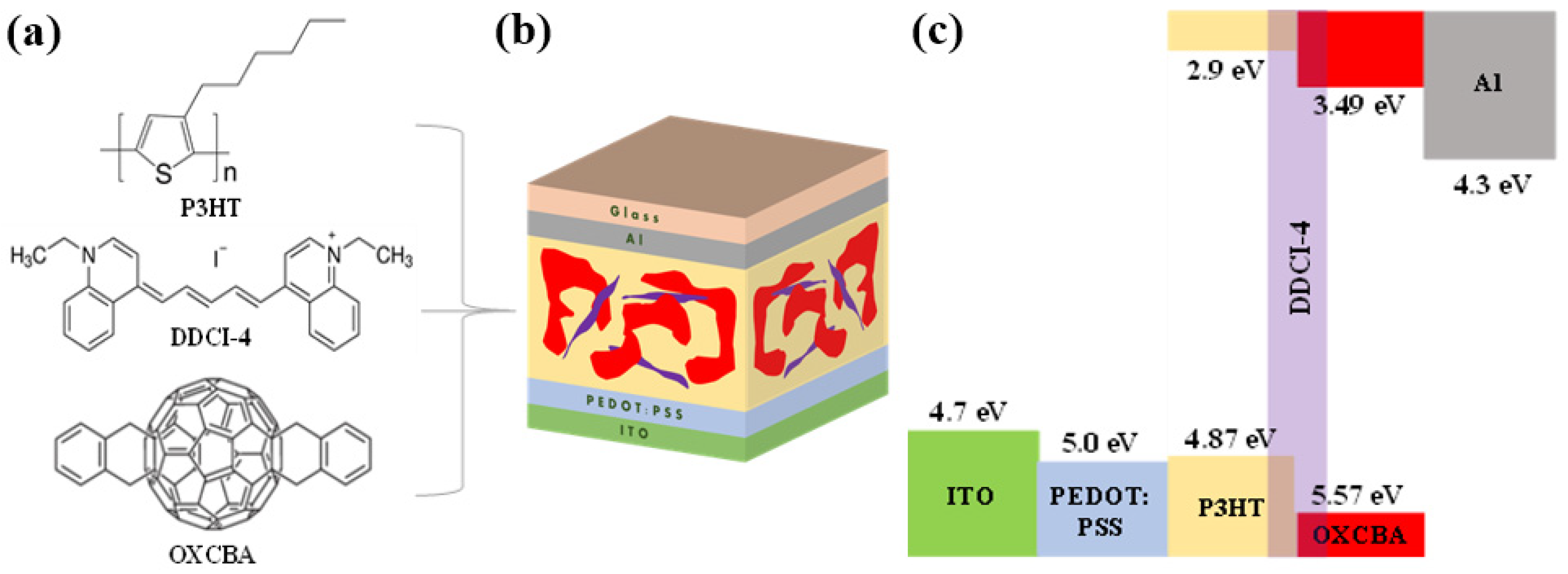

| Materials | HOMOonset (eV) | Cut-Off Energy (eV) | Work Function (eV) | HOMO Level (eV) | LUMO Level (eV) | Eg (eV) |
|---|---|---|---|---|---|---|
| P3HT | 0.93 | 35.56 | 3.94 | 4.87 | 2.90 | 1.97 |
| DDCI-4 | 0.72 | 35.55 | 3.95 | 4.67 | 3.46 | 1.21 |
| OXCBA | 1.33 | 35.26 | 4.24 | 5.57 | 3.49 | 2.08 |
| Materials | HOMO (eV) | LUMO (eV) | Eg (eV) | Ref |
|---|---|---|---|---|
| P3HT | 4.65 | 2.13 | 2.52 | [20] |
| 4.94 | 3.08 | 1.86 | [15] | |
| 4.87 | 2.90 | 1.97 | This work | |
| DDCI-4 | 4.67 | 3.46 | 1.21 | This work |
| OXCBA | 5.66 | 3.47 | 2.19 | [15] |
| 5.57 | 3.49 | 2.08 | This work |
| Devices | Jd (mA/cm2) | Jph (mA/cm2) | Jph/Jd | Rise Time, Tr (ms) | Decay Time, Td (ms) | R (mA/W) | D (×1010 Jones) |
|---|---|---|---|---|---|---|---|
| 3 mg/mL | 0.35 | 0.44 | 1.257 | 641 | 641 | 22 | 6.57 |
| 5 mg/mL | 0.29 | 0.35 | 1.207 | 641 | 641 | 17.5 | 5.74 |
| 7 mg/mL | 0.23 | 0.26 | 1.130 | 641 | 641 | 13 | 4.79 |
| Devices | Visible EQE (%) | NIR EQE (%) |
|---|---|---|
| 0 mg/mL | 15.3 | 0 |
| 3 mg/mL | 23.7 | 0.29 |
| 5 mg/mL | 5.0 | 0.21 |
| 7 mg/mL | 2.6 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Md Sabri, A.A.; Natashah, F.A.; Hisamuddin, S.N.; Roslan, N.A.; Bawazeer, T.M.; Alsoufi, M.S.; Supangat, A. Dicarbocyanine Dye-Based Organic Photodiodes. Photonics 2022, 9, 947. https://doi.org/10.3390/photonics9120947
Md Sabri AA, Natashah FA, Hisamuddin SN, Roslan NA, Bawazeer TM, Alsoufi MS, Supangat A. Dicarbocyanine Dye-Based Organic Photodiodes. Photonics. 2022; 9(12):947. https://doi.org/10.3390/photonics9120947
Chicago/Turabian StyleMd Sabri, Amirul Ashraf, Fadlan Arif Natashah, Syaza Nafisah Hisamuddin, Nur Adilah Roslan, Tahani M. Bawazeer, Mohammad S. Alsoufi, and Azzuliani Supangat. 2022. "Dicarbocyanine Dye-Based Organic Photodiodes" Photonics 9, no. 12: 947. https://doi.org/10.3390/photonics9120947
APA StyleMd Sabri, A. A., Natashah, F. A., Hisamuddin, S. N., Roslan, N. A., Bawazeer, T. M., Alsoufi, M. S., & Supangat, A. (2022). Dicarbocyanine Dye-Based Organic Photodiodes. Photonics, 9(12), 947. https://doi.org/10.3390/photonics9120947







