Abstract
Retinal arterial macroaneurysm (RAM) can be defined as an acquired round or fusiform dilation of a retinal artery. RAMs frequently remain stable but, in some cases, can complicate with macular exudation or hemorrhage, resulting in symptomatic disease. While a watch-and-wait approach is the standard option in asymptomatic RAMs, there is no universal agreement regarding treatment of symptomatic cases and randomized clinical trials are warranted. Anti-VEGF intravitreal injections can reduce exudation, albeit multiple treatments may be necessary. Hence, laser treatment may be a better choice to provide a durable control of symptoms while anti-VEGF therapy should be preferred for lesions adjacent to the fovea. Indirect laser is recommended because there is a decreased danger of RAM rupture and hemorrhage. Furthermore, subthreshold laser seems to be comparable to conventional laser in terms of efficacy outcomes.
1. Introduction
Retinal arterial macroaneurysm (RAM) can be defined as an acquired round or fusiform dilation within the first three branches of the central retinal artery [1]. RAM tends to develop at the site of an arteriolar bifurcation or arteriovenous crossing. The most reported location is the superior-temporal quadrant albeit RAM can develop at the nasal arteries and, more rarely, at the cilioretinal arteries or optic nerve head [1,2,3,4,5,6].
The epidemiology of RAM has been thoroughly analyzed by several studies, revealing that they are strongly associated with female sex, systemic hypertension and atherosclerosis, and branch retinal vein occlusion [2,3,4,5,6,7,8,9]. Although the exact pathophysiological mechanisms leading to RAM development are not completely delineated, the most solid interpretation is based on the chronic vessel damage due to atherosclerosis which could in turn lead to progressive fibrotic degeneration of the vessel wall [3,4,6,7]. The reduced wall elasticity combined with the elevated luminal pressure, due to systemic hypertension, would result in the development of an arterial aneurysmal dilation. An alternative theory suggests that emboli or even intra-arterial thrombosis could determine mechanical damages to the endothelium or the adventitial leading to aneurysm formation and subsequent rupture [6,10]. It is worth mentioning that RAM can also occur in young patients, such as in Familial Retinal Arterial Macroaneurysm (FRAM), a rare inherited disorder caused by recessive variants in the Insulin-like Growth Factor Binding Protein 7 (IGFBP7) gene [11,12,13,14].
2. Clinical Presentation
RAMs frequently remain stable over time, with a natural history of thrombosis followed by spontaneous involution [3]. However, they can exudate, thus causing macular edema, and/or enlarge and subsequently rupture, resulting in extensive hemorrhages [15,16,17]. Therefore, the diagnosis can be made on routine examination when asymptomatic but also in consequence of the occurrence of a sudden and painless loss of vision. The clinical picture may vary according to the specific complication [6].
RAM can be classified into quiescent and symptomatic, either exudative or hemorrhagic [18]. The latter group accounts for approximately two-thirds of all symptomatic cases [19]. Hemorrhages related to RAM rupture can be further classified based on their location: vitreous (10–23%), preretinal (31–57%), subinternal limiting membrane (22–53%), intraretinal (26–52%) or subretinal (47–76%). Half of the cases present as multilevel hemorrhage, defined as occurring simultaneously in at least two locations [19,20]. Eyes with vitreous, preretinal, or intraretinal hemorrhage tend to show a better visual prognosis than those with exudation alone or subretinal hemorrhage [19].
3. Multimodal Imaging
Even though the simple biomicroscopic fundus examination allows the direct identification of RAM in most cases, the gold standard for the diagnosis of RAM is fluorescein angiography (FA), in which a focal saccular or fusiform dilation shows rapid filling in the arterial phase (Figure 1A,B). Dye leakage in the late phases of the study signals active exudation (Figure 2). When a thick preretinal hemorrhage is present, an area of blocked fluorescence around the aneurysm is observed. In those cases, indocyanine green angiography (ICGA) is useful, due to the greater penetration of near-infrared wavelength [21,22,23]. Dye leakage can be found also on ICGA, suggesting damage to the arterial wall [6]. Optical Coherence Tomography (OCT) scans taken at the level of a quiescent macroaneurysm show a round hyperreflective wall with a hyporeflective lumen located in the inner retinal layers, albeit the reflectivity of its innermost part can variable depending on whether the aneurysmal sac is perfused or thrombosed (Figure 3). Moreover, OCT can easily unveil the development of macular complications, such as exudation and hemorrhage, and occlusion after laser treatment, usually detectable as homogeneous high reflectance [24,25]. It is noteworthy that macular subretinal or intraretinal fluid can be due to the presence of RAM located far away from the fovea [6]. Linearity without undulation of the retinal pigment epithelium on OCT allows a differential diagnosis with aneurysmal type 1 neovascularization, which can show a hemorrhagic appearance similar to that of a ruptured RAM [17]. En-face OCT may prove helpful in planning laser treatment, as it allows precise measurement of RAM size in a coronal plane at a selected depth within the retina, assessment of its relationship with extravasated fluid or blood, and demarcation of the external and internal border of the wall on the basis of reflectivity differences [26]. OCT-Angiography has been shown able to detect perfused RAMs, even if obscured by hemorrhage, without resorting to dye injection (Figure 1C) [27,28,29,30,31]. However, its sensitivity is still limited to a predefined range of flow speeds, so that lesions with a turbulent flow may go undetected [32]. More recently, adaptive optics scanning light ophthalmoscope (AOSLO) helped to elucidate the mechanisms of RAM formation and thrombosis in vivo [10,33].
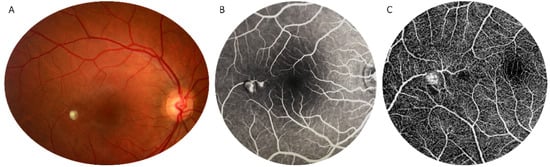
Figure 1.
Multimodal imaging in retinal arterial macroaneurysm. Round dilation of a temporal branch central retinal artery near the fovea on color fundus photograph (A). Early-phase fluorescein angiography detects dye filling of the aneurysmal sac but not leakage (B). Optical Coherence Tomography Angiography detects blood flow within the retinal arterial macroaneurysm (C).
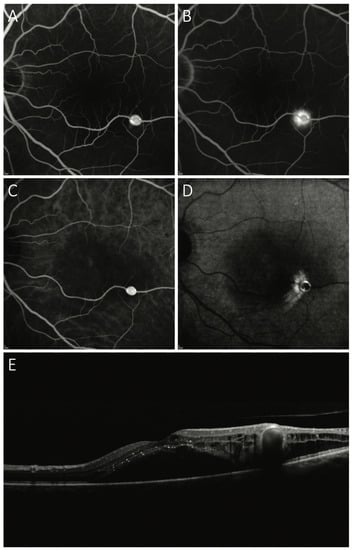
Figure 2.
Exudative retinal arterial macroaneurysm. Rapid arterial filling of the aneurysmal sac on early phase fluorescein angiography (A) and indocyanine green angiography (C). Perilesional dye leakage is highlighted by late phase fluorescein angiography (B) and indocyanine green angiography (D). Optical Coherence Tomography scan passing through the fovea and macroaneurysm detects subfoveal and intraretinal exudation (E).
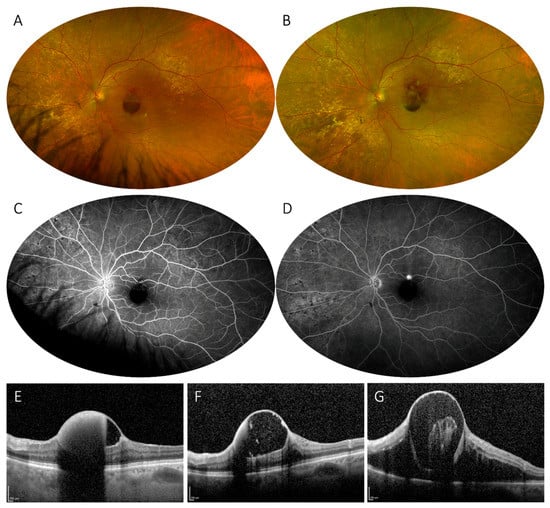
Figure 3.
Hemorrhagic retinal arterial macroaneurysm. Ultrawide-field imaging of a subinternal limiting membrane hemorrhage secondary to a ruptured macroaneurysm on a superior-temporal branch of the central retinal artery (A), showing a partially obliterated lesion on early phase fluorescein angiography (C) and subsequent filling in late phases (D). At 2-month of follow-up, development of subretinal hemorrhage and reduction of the subinternal limiting membrane hemorrhage can be detected (B). Optical Coherence Tomography scans taken at baseline (E), 1-month (F), and 2-month (G) follow-up confirm the reduction of the pre-existing hemorrhage and subsequent re-rupture and hemorrhage.
4. Treatment
4.1. General Management
At present, there is no consensus regarding treatment indications in RAM and several options are available: both laser and intravitreal (IV) pharmacologic therapy have been proposed as effective therapeutic options for macular exudative phenomena caused by RAM and also surgery may be needed in cases of extensive hemorrhage [5]. However, about two-thirds of RAMs spontaneously close, and half of symptomatic patients show vision improvement during observation [19]. Therefore, caution should be taken when considering to actively treat a patient with RAM while a watch and wait approach is the standard option if the RAM is asymptomatic. Treatment should be reserved for exudative or hemorrhagic RAMs, which can cause significant visual impairment if the central macula is involved and progressive photoreceptor damage due to persistent subretinal/intraretinal blood or fluid [16,34]. Anyhow, the visual prognosis of RAM is good regardless of the initial presentation and even after macular complications, if an individualized approach is correctly undertaken [35].
4.2. Laser Treatment
The most commonly employed approach is conventional direct or indirect laser photocoagulation with a visible endpoint (threshold). Laser photocoagulation leads to RAM closure in almost all cases (87–100%) and at least 1 line of visual acuity gain in almost 75% (65–80%) of patients, suggesting a preeminent role for laser in the treatment of RAM, especially in hemorrhagic ones [19].
No consensus exists about laser modality and setting for the treatment of RAM. Conventional direct photocoagulation using argon/KTP 532 nm green laser or 577 nm yellow diode is generally effective in sealing the aneurysmal sac and resolving the exudative manifestations. Large spots (500 μm) with long exposure time (500 ms), at the lowest power necessary to create a light burn directly on the lesion are recommended [36].
However, since the aneurysm’s walls are already thin and weakened, direct laser treatment may be burdened by some complications, including hemorrhage and branch retinal artery occlusion [37]; therefore, indirect treatment is generally employed (using 100 μm spot diameter, 200 ms exposure, and 100–300 mW power) [36]. This approach is focused on the incompetent retinal capillaries surrounding the RAM which display significant dye leakage on FA and are responsible for exudative phenomena, while also circumscribing the lesion with laser burns in order to confine the extent of hemorrhages in case of rupture; it may also reduce blood flow to the aneurysmal sac through a photothermal effect on vascular factors [36].
Another conventional laser therapy approach is navigated laser photocoagulation. The currently commercially available navigated laser system (Navilas; OD-OS GmbH, Teltow, Germany) incorporates a digital fundus camera (providing color fundus imaging, infrared imaging, and fluorescein angiography) and a diode-pumped solid-state (532 or 577 nm) laser. The system allows laser preplanning using multimodal imaging and retinal tracking during treatment to stabilize the position of laser spots [38]. A small comparative study on conventional direct photocoagulation for RAM proved that the navigated approach has similar efficacy and safety compared to the standard one while requiring less energy and number of burns [39]. Nevertheless, all kinds of threshold laser treatment may lead to potential sequelae, including increased retinal exudation, enlargement of laser scar, subretinal neovascularization and fibrosis, and vitreoretinal interface diseases [6].
A different approach can be pursued with the use of subthreshold laser treatment, which has been developed to minimize the adverse aspects of conventional laser, by reducing the duration of exposure and using a subvisible clinical endpoint (laser burn), while obtaining a comparable outcome (Figure 4). The mechanisms by which it works are not clearly understood but are probably related to the beneficial effects of sub-lethal retinal hyperthermia and mediated by heat shock proteins. In a pilot study, Battaglia Parodi et al. used an infrared diode laser (810 nm) with 125 μm spot diameter, 300 ms exposure time, 15% duty cycle, and 1400 mW power to deliver laser spots on both the entire RAM lesion and the surrounding area, with no free space in-between [40]. Subthreshold laser treatment was capable to achieve anatomical and functional improvement [40], which was subsequently demonstrated to be equivalent to conventional indirect treatment in a randomized clinical trial [41]. Thus, subthreshold laser is safe and effective to treat RAM with symptomatic macular involvement and it can also be used to safely treat RAMs with associated vitreoretinal pathology, in which conventional laser treatment cannot be considered an appropriate choice because of its potentially adverse thermal effects [42].
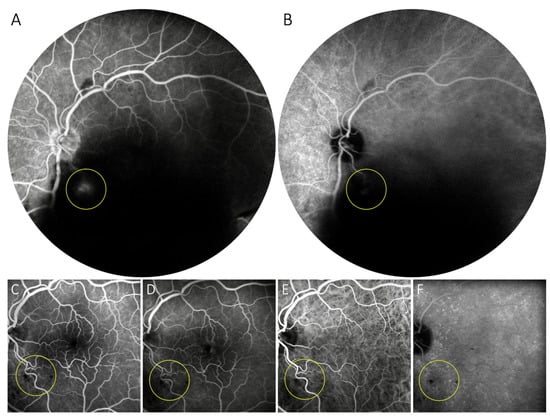
Figure 4.
Symptomatic retinal arterial macroaneurysm treated with subthreshold laser. Despite a massive vitreous hemorrhage, dye leakage from a saccular arterial dilation on the inferior-temporal major arterial division near the optic disk can be detected on lase-phase fluorescein angiography (A), but only minimally on intermediate-phase indocyanine green angiography (B). Four months after a single session of subthreshold micropulse laser treatment of the lesion, no aneurysmal dilation, dye leakage or laser can be seen at the same location on fluorescein (C,D) or indocyanine green angiography (E,F).
Laser hyaloidotomy and internal limiting membranotomy using Nd-YAG may be used to quickly resolve hemorrhages settling under the hyaloid membrane and/or internal limiting membrane which can be slow to resorb and cause macular scarring and epiretinal membrane formation [43,44]. In contrast to conventional retinal photocoagulation, which is performed using a beam with constant power (continuous mode), the laser is operated at Q-switch mode so that every emission is composed of many short bursts with high peak power. The energy level is set between 1.9–11.5 mJ and the beam is directed at the lower margin of the hemorrhage, away from the fovea. Few openings are made until a stream of blood to the vitreous cavity is seen. However, this procedure is controversial because of the risk of macular damage and dense vitreous hemorrhage; moreover, hemorrhage recurrence is not rare [36].
4.3. Intravitreal Pharmacologic Therapy
The rationale for treating exudative RAMs using IV anti-vascular endothelial growth factor (VEGF) antibodies relies on their ability to obtain tighter junctions between endothelial cells of the aneurysmal sac, alongside remodeling the affected vessel wall and luminal thrombosis [33]. Intravitreal bevacizumab for exudative complications of RAM was first reported in 2009 [45]. Since then, there have been several studies on the use of anti-VEGF agents (bevacizumab, ranibizumab, aflibercept, conbercept) for both hemorrhagic and exudative complications of RAM [46,47,48]. Symptomatic RAM responds well to a limited number of injections (1 to 3) [49,50,51,52]. In their landmark prospective study, Pichi et al. found that three monthly bevacizumab injections led to aneurysm closure, resolution of macular edema and hard exudates, and significant visual improvement in nearly all 38 treated eyes [50]. However, as a single IV is often sufficient to achieve a complete RAM closure, it is still questioned if all patients should receive a triple anti-VEGF injection in a standardized manner or rather follow a pro re nata regimen [47].
Despite retrospective comparative studies highlighted no significant differences in the final visual outcome between IV bevacizumab and mere observation, albeit the former likely hastens the resolution of edema and hemorrhage [49,53], a recent meta-analysis reported that anti-VEGF agents lead to RAM closure (93–100%) and visual acuity improvement (74–100%) in a higher proportion of patients when compared to observation and even laser. Interestingly, the visual prognosis of RAM treated with anti-VEGF was better in exudative than in hemorrhagic ones [19].
It still holds that in complicated RAM cases due to recurrent macular edema or hemorrhage after multiple anti-VEGF injections, focal laser photocoagulation is generally needed for sealing vessel leak and vision stabilization [54]. For that reason, combined protocols with IV anti-VEGF and focal laser photocoagulation have been proposed and successfully investigated [44,55,56]. One retrospective study suggested that combined therapy may lead to a better functional and anatomical outcome than monotherapy [57], although further studies are needed to determine which modality is the most safe and effective.
Regarding ruptured RAMs with hemorrhagic manifestations, anti-VEGF agents can be considered a treatment option, although their natural course generally proceeds with progressive resorption of the hemorrhage and fibrosis of the aneurysm to a restitutio ad integrum [58], particularly in cases with foveal intraretinal hemorrhage that have a poor visual prognosis and may be ineligible for laser treatment [53]. However, some authors argued that anti-VEGF in these patients has little rationale since hemorrhage from RAM, unlike exudation, is generally a one-time event that leads to fibrosis and occlusion of the lesion and visual loss due to subfoveal blood toxicity towards photoreceptors and retinal pigment epithelium. Thus, early surgical evacuation of subfoveal blood may the best option for these patients [51].
To our knowledge, IV corticosteroid use has been reported only in one patient affected by FRAM [59] and in three affected by adult-onset Coats’ disease [60]. In these cases, persistent macular edema poorly responding to intravitreal ranibizumab and aflibercept showed marked improvement after intravitreal dexamethasone implantation (Ozurdex®).
4.4. Surgical Treatment
The majority of vitreous hemorrhages secondary to RAM generally do not require surgical intervention, with a natural history of resorption in 6 to 10 weeks. However, when the etiology of the vitreous hemorrhage is unknown or it does not resolve, vitrectomy should be considered [36,61]. This may not only accelerate vision improvement but also enhance the detection of concomitant macular complications.
On the other hand, subretinal macular hemorrhage due to RAM rupture can damage photoreceptors and lead to vision-threatening macular holes, if left untreated [62,63,64,65]. Indeed, good visual outcomes have been accomplished with gas tamponade in conjunction with the use of tissue plasminogen activator (tPA), with or without surgical drainage and/or vitrectomy [35,66,67,68,69,70,71,72,73,74]. Perfluorocarbon gas is injected into the eye to physically displace the macular hemorrhage. Intravitreal or subretinal tPA may be used to aid spontaneous blood clearing as well as surgical drainage. Unfortunately, vitreous hemorrhage and deterioration of subretinal hemorrhage may occur as an immediate complication of gas injection and intravitreal tPA for the treatment of subretinal hemorrhage secondary to a ruptured RAM [75,76]. As a result, removal of extensive subretinal bleeding remains contentious.
5. Conclusions
While a watch-and-wait approach is the standard option in asymptomatic RAMs, there is no universal agreement regarding treatment of symptomatic cases and randomized clinical trials are warranted. Treatment should be reserved for exudative or hemorrhagic RAMs. Anti-VEGF intravitreal injections can reduce exudation, albeit multiple treatments may be necessary. Hence, laser treatment may be a better choice to provide a durable control of symptoms while anti-VEGF therapy should be preferred for lesions adjacent to the fovea. Indirect laser is recommended because there is a decreased danger of RAM rupture and hemorrhage. Furthermore, subthreshold laser seems to be comparable to conventional laser in terms of efficacy outcomes.
Author Contributions
Conceptualization, M.G., F.B., M.B.P.; methodology, L.B., M.B.P., M.G., F.B.; writing—original draft preparation, L.B., M.G., A.A. (Alessio Antropoli), A.A. (Alessandro Arrigo); writing—review and editing, F.B., M.B.P.; supervision, F.B., M.B.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
F. Bandello is consultant for Abbvie, Alimera, Bayer, Boehringer-Ingelheim, Fidia Sooft, Hofmann La Roche, Novartis, Ntc Pharma, Oxurion Nv, SIFI. M. Battaglia Parodi is consultant for Novartis. All other authors have no disclosures to declare.
References
- Robertson, D.M. Macroaneurysms of the Retinal Arteries. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1973, 77, OP55–OP67. [Google Scholar] [PubMed]
- Lewis, R.A.; Norton, E.W.; Gass, J.D. Acquired arterial macroaneurysms of the retina. Br. J. Ophthalmol. 1976, 60, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Rabb, M.F.; Gagliano, D.A.; Teske, M.P. Retinal arterial macroaneurysms. Surv. Ophthalmol. 1988, 33, 73–96. [Google Scholar] [CrossRef]
- Abdel-Khalek, M.N.; Richardson, J. Retinal macroaneurysm: Natural history and guidelines for treatment. Br. J. Ophthalmol. 1986, 70, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Goldhagen, B.E.; Goldhardt, R. Retinal Arterial Macroaneurysms: Updating Your Memory on RAM Management. Curr. Ophthalmol. Rep. 2019, 7, 73–79. [Google Scholar] [CrossRef]
- Battaglia Parodi, M. Aneurysm Retinal Arterial. In Encyclopedia of Ophthalmology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 93–95. [Google Scholar]
- Brown, D.M.; Sobol, W.M.; Folk, J.C.; Weingeist, T.A. Retinal arteriolar macroaneurysms: Long-term visual outcome. Br. J. Ophthalmol. 1994, 78, 534–538. [Google Scholar] [CrossRef]
- Panton, R.W.; Goldberg, M.F.; Farber, M.D. Retinal arterial macroaneurysms: Risk factors and natural history. Br. J. Ophthalmol. 1990, 74, 595–600. [Google Scholar] [CrossRef]
- Parodi, M.B.; Bondel, E.; Saviano, S.; Ravalico, G. Branch retinal vein occlusion after spontaneous obliteration of retinal arterial macroaneurysm. Retina 1998, 18, 378–379. [Google Scholar] [CrossRef]
- Ishikura, M.; Muraoka, Y.; Kadomoto, S.; Nishigori, N.; Murakami, T.; Ooto, S.; Tsujikawa, A. Retinal arterial macroaneurysm rupture caused by dissection-like change in the vessel wall. Am. J. Ophthalmol. Case Rep. 2022, 25, 101346. [Google Scholar] [CrossRef]
- Dhindsa, H.S.; Abboud, E.B. Familial retinal arterial macroaneurysms. Retina 2002, 22, 607–615. [Google Scholar] [CrossRef]
- Abu-Safieh, L.; Abboud, E.B.; Alkuraya, H.; Shamseldin, H.; Al-Enzi, S.; Al-Abdi, L.; Hashem, M.; Colak, D.; Jarallah, A.; Ahmad, H.; et al. Mutation of IGFBP7 Causes Upregulation of BRAF/MEK/ERK Pathway and Familial Retinal Arterial Macroaneurysms. Am. J. Hum. Genet. 2011, 89, 313–319. [Google Scholar] [CrossRef]
- Alkuraya, H.; Patel, N.; Ibrahim, N.; Al-Ghamdi, B.; Alsulaiman, S.M.; Nowilaty, S.R.; Abboud, E.; Alturki, R.; Alkharashi, A.; Eyaid, W.; et al. Phenotypic delineation of the retinal arterial macroaneurysms with supravalvular pulmonic stenosis syndrome. Clin. Genet. 2019, 97, 447–456. [Google Scholar] [CrossRef]
- Khan, A.O.; Pichi, F.; Neri, P.; Abboud, E.B. Retinal arteriolar macroaneurysms with supravalvular pulmonic stenosis in the United Arab Emirates. Ophthalmic Genet. 2021, 43, 58–63. [Google Scholar] [CrossRef]
- Palestine, A.G.; Robertson, D.M.; Goldstein, B.G. Macroaneurysms of the Retinal Arteries. Am. J. Ophthalmol. 1982, 93, 164–171. [Google Scholar] [CrossRef]
- Tsujikawa, A.; Sakamoto, A.; Ota, M.; Oh, H.; Miyamoto, K.; Kita, M.; Yoshimura, N. Retinal structural changes associated with retinal arterial macroaneurysm examined with optical coherence tomography. Retina 2009, 29, 782–792. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Muraoka, Y.; Nishigori, N.; Ishikura, M.; Kadomoto, S.; Miyata, M.; Murakami, T.; Ooto, S.; Tsujikawa, A. Detection and characteristics of unruptured retinal arterial macroaneurysms. Retina 2022, 42, 1909–1914. [Google Scholar] [CrossRef]
- Moosavi, R.A.; Fong, K.C.S.; Chopdar, A. Retinal artery macroaneurysms: Clinical and fluorescein angiographic features in 34 patients. Eye 2005, 20, 1011–1020. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, X.-Y.; Meng, L.-H.; Zhang, W.-F.; Chen, Y.-X. Clinical characteristics of retinal arterial macroaneurysms and prognosis of different interventions. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 260, 439–450. [Google Scholar] [CrossRef]
- Kester, E.; Walker, E. Retinal arterial macroaneurysm causing multilevel retinal hemorrhage. Optom. J. Am. Optom. Assoc. 2009, 80, 425–430. [Google Scholar] [CrossRef]
- Schneider, U.; Wagner, A.L.; Kreissig, I. Indocyanine Green Videoangiography of Hemorrhagic Retinal Arterial Macroaneurysms. Ophthalmologica 1997, 211, 115–118. [Google Scholar] [CrossRef]
- Gomez-Ulla, F.; Gonzalez, F.; Torreiro, M.G.; Perez, R.; Des, J. Indocyanine green angiography in isolated primary retinal arterial macroaneurysms. Acta Ophthalmol. Scand. 1998, 76, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Townsend-Pico, W.A.; Meyers, S.M.; Lewis, H. Indocyanine green angiography in the diagnosis of retinal arterial macroaneurysms associated with submacular and preretinal hemorrhages: A case series. Am. J. Ophthalmol. 2000, 129, 33–37. [Google Scholar] [CrossRef]
- Lee, E.K.; Woo, S.J.; Ahn, J.; Park, K.H. Morphologic characteristics of retinal arterial macroaneurysm and its regression pattern on spectral-domain optical coherence tomography. Retina 2011, 31, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.; Soiberman, U.; Loewenstein, A.; Goldstein, M. Heidelberg spectral-domain optical coherence tomographic findings in retinal artery macroaneurysm. Retina 2012, 32, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Hanhart, J.; Strassman, I.; Rozenman, Y. En face imaging of retinal artery macroaneurysms using swept-source optical coherence tomography. Retin. Cases Brief Rep. 2017, 11, 211–216. [Google Scholar] [CrossRef]
- Alnawaiseh, M.; Schubert, F.; Nelis, P.; Wirths, G.; Rosentreter, A.; Eter, N. Optical coherence tomography (OCT) angiography findings in retinal arterial macroaneurysms. BMC Ophthalmol. 2016, 16, 120. [Google Scholar] [CrossRef]
- Chang, V.S.; Schwartz, S.G.; Flynn, H.W. Optical Coherence Tomography Angiography of Retinal Arterial Macroaneurysm before and after Treatment. Case Rep. Ophthalmol. Med. 2018, 2018, 1–4. [Google Scholar] [CrossRef]
- Breazzano, M.P.; Fernández-Avellaneda, P.; Freund, K.B. Swept-Source Optical Coherence Tomography Angiography of Retinal Arterial Macroaneurysm with Overlying Hemorrhage. JAMA Ophthalmol. 2019, 137, e190247. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Hung, J.-H. OCT Angiography of Retinal Arterial Macroaneurysm. Ophthalmol. Retin. 2020, 4, 1180. [Google Scholar] [CrossRef]
- Astroz, P.; Miere, A.; Cohen, S.Y.; Querques, G.; Souied, E.H. Optical coherence tomography angiography in the diagnosis and follow-up of retinal arterial macroaneurysms. Retin. Cases Brief Rep. 2021, 15, 1–4. [Google Scholar] [CrossRef]
- Arrigo, A.; Aragona, E.; Parodi, M.B.; Bandello, F. Quantitative approaches in multimodal fundus imaging: State of the art and future perspectives. Prog. Retin. Eye Res. 2022, 101111. [Google Scholar] [CrossRef]
- Kadomoto, S.; Muraoka, Y.; Uji, A.; Ooto, S.; Murakami, T.; Tsujikawa, A. Hemodynamic and structural changes in retinal arterial macroaneurysm after intravitreal anti-vascular endothelial growth factor injection. Am. J. Ophthalmol. Case Rep. 2021, 23, 101182. [Google Scholar] [CrossRef]
- Hughes, E.L.; Dooley, I.J.; Kennelly, K.P.; Doyle, F.; Siah, W.F.; Connell, P. Angiographic features and disease outcomes of symptomatic retinal arterial macroaneurysms. Graefe's Arch. Clin. Exp. Ophthalmol. 2016, 254, 2203–2207. [Google Scholar] [CrossRef]
- Koinzer, S.; Heckmann, J.; Tode, J.; Roider, J. Long-term, therapy-related visual outcome of 49 cases with retinal arterial macroaneurysm: A case series and literature review. Br. J. Ophthalmol. 2015, 99, 1345–1353. [Google Scholar] [CrossRef]
- Amaro, M.; Ferreira, A. Retinal Macroaneurysm. In LASER Manual in Ophthalmology-Fundamentals and Laser Clinical Practice; Henriques, J., Duarte, A., Quintão, T., Eds.; SPILM Portuguese Medical Laser Society Publishing: Lisbon, Portugal, 2017; pp. 277–279. [Google Scholar]
- Russell, S.R.; Folk, J.C. Branch Retinal Artery Occlusion After Dye Yellow Photocoagulation of an Arterial Macroaneurysm. Am. J. Ophthalmol. 1987, 104, 186–187. [Google Scholar] [CrossRef]
- Kozak, I.; Oster, S.F.; Cortes, M.A.; Dowell, D.; Hartmann, K.; Kim, J.S.; Freeman, W.R. Clinical Evaluation and Treatment Accuracy in Diabetic Macular Edema Using Navigated Laser Photocoagulator NAVILAS. Ophthalmology 2011, 118, 1119–1124. [Google Scholar] [CrossRef]
- Maltsev, D.S.; Kulikov, A.N.; Uplanchiwar, B.; Lima, L.H.; Chhablani, J. Direct navigated laser photocoagulation as primary treatment for retinal arterial macroaneurysms. Int. J. Retin. Vitr. 2018, 4, 28. [Google Scholar] [CrossRef]
- Parodi, M.B.; Iacono, P.; Ravalico, G.; Bandello, F. Subthreshold laser treatment for retinal arterial macroaneurysm. Br. J. Ophthalmol. 2010, 95, 534–538. [Google Scholar] [CrossRef]
- Parodi, M.B.; Iacono, P.; Pierro, L.; Papayannis, A.; Kontadakis, S.; Bandello, F.M. Subthreshold Laser Treatment Versus Threshold Laser Treatment for Symptomatic Retinal Arterial Macroaneurysm. Investig. Opthalmology Vis. Sci. 2012, 53, 1783–1786. [Google Scholar] [CrossRef]
- Rajabian, F.; Arrigo, A.; Grazioli, A.; Falcomatà, B.; Bandello, F.; Parodi, M.B. Retinal arterial macroaneurysm associated with macular pucker. Eur. J. Ophthalmol. 2019, 30, NP74–NP78. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Kou, H.-K. Krypton laser membranotomy in the treatment of dense premacular hemorrhage. Can. J. Ophthalmol. 2004, 39, 761–766. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Lin, L.-Y.; Chang, P.-Y.; Chen, F.-T.; Mai, E.L.C.; Wang, J.-K. Laser and Anti–Vascular Endothelial Growth Factor Agent Treatments for Retinal Arterial Macroaneurysm. Asia Pac. J. Ophthalmol. 2017, 6, 444–449. [Google Scholar] [CrossRef]
- Chanana, B.; Azad, R.V. Intravitreal bevacizumab for macular edema secondary to retinal macroaneurysm. Eye 2008, 23, 493–494. [Google Scholar] [CrossRef] [PubMed]
- Oztas, Z.; Nalcaci, S.; Akkin, C. Intravitreal aflibercept for ruptured retinal arterial macroaneurysm. Int. J. Ophthalmol. 2017, 10, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Chatziralli, I.; Maniatea, A.; Koubouni, K.; Parikakis, E.; Mitropoulos, P. Intravitreal Ranibizumab for Retinal Arterial Macroaneurysm: Long-Term Results of a Prospective Study. Eur. J. Ophthalmol. 2016, 27, 215–219. [Google Scholar] [CrossRef]
- Lin, Z.; Hu, Q.; Wu, Y.; Xu, J.; Zhang, Q. Intravitreal ranibizumab or conbercept for retinal arterial macroaneurysm: A case series. BMC Ophthalmol. 2019, 19, 18. [Google Scholar] [CrossRef]
- Cho, H.J.; Rhee, T.K.; Kim, H.S.; Han, J.I.; Lee, D.W.; Cho, S.W.; Kim, J.W. Intravitreal Bevacizumab for Symptomatic Retinal Arterial Macroaneurysm. Am. J. Ophthalmol. 2013, 155, 898–904.e1. [Google Scholar] [CrossRef]
- Pichi, F.; Morara, M.; Torrazza, C.; Manzi, G.; Alkabes, M.; Balducci, N.; Vitale, L.; Lembo, A.; Ciardella, A.P.; Nucci, P. Intravitreal Bevacizumab for Macular Complications from Retinal Arterial Macroaneurysms. Am. J. Ophthalmol. 2013, 155, 287–294.e1. [Google Scholar] [CrossRef]
- Kishore, K. Intravitreal Bevacizumab for Symptomatic Retinal Arterial Macroaneurysm. Am. J. Ophthalmol. 2014, 157, 260. [Google Scholar] [CrossRef]
- Mansour, A.M.; Foster, R.E.; Gallego-Pinazo, R.; Moschos, M.M.; Sisk, R.A.; Chhablani, J.; Rojanaporn, D.; Sujirakul, T.; Arevalo, J.F.; Lima, L.H.; et al. Intravitreal anti-vascular endothelial growth factor injections for exudative retinal arterial macroaneurysms. Retina 2019, 39, 1133–1141. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.W.; Kim, C.G.; Lew, Y.J.; Cho, H.J. Influence of bevacizumab therapy and intraretinal hemorrhage in long-term outcomes of hemorrhagic retinal arterial macroaneurysm. Sci. Rep. 2021, 11, 14246. [Google Scholar] [CrossRef]
- Leung, E.; Reddy, A.; Vedula, A.; Flynn, H. Serial bevacizumab injections and laser photocoagulation for macular edema associated with a retinal artery macroaneurysm. Clin. Ophthalmol. 2015, 9, 601–609. [Google Scholar] [CrossRef][Green Version]
- Cahuzac, A.; Scemama, C.; Mauget-Faysse, M.; Sahel, J.-A.; Wolff, B. Retinal Arterial Macroaneurysms: Clinical, Angiographic, and Tomographic Description and Therapeutic Management of a Series of 14 Cases. Eur. J. Ophthalmol. 2015, 26, 36–43. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, C.; Cao, G.; Xu, X.; Wang, J. Outcomes of combined treatments in patients with retinal arterial macroaneurysm. Indian J. Ophthalmol. 2021, 69, 3564. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, C.Z.; Lee, S.U.; Lee, S.J. Combined Intravitreal Bevacizumab and Laser Photocoagulation to Treat Retinal Arterial Macroaneurysms. J. Korean Ophthalmol. Soc. 2021, 62, 1053–1060. [Google Scholar] [CrossRef]
- Chen, K.-J. Natural Course of Ruptured Retinal Arterial Macroaneurysm. Ophthalmol. Retin. 2020, 4, 629. [Google Scholar] [CrossRef]
- AlZaid, A.; Magliyah, M.; Schatz, P.; Al-Dhibi, H. Long-term resolution of chronic macular edema after a single dose of intravitreal dexamethasone in familial retinal arterial macroaneurysm. Ophthalmic Genet. 2020, 41, 394–396. [Google Scholar] [CrossRef]
- Dave, A.D.; Thavikulwat, A.T.; De Silva, T.; Wiley, H.E.; Keenan, T.D.; Wong, W.T.; Cukras, C.A. Longitudinal characterization and treatment response of retinal arterial macroaneurysms in adult-onset coats disease. Am. J. Ophthalmol. Case Rep. 2022, 27, 101647. [Google Scholar] [CrossRef]
- Zhao, P.; Hayashi, H.; Oshima, K.; Nakagawa, N.; Ohsato, M. Vitrectomy for macular hemorrhage associated with retinal arterial macroaneurysm. Ophthalmology 2000, 107, 613–617. [Google Scholar] [CrossRef]
- Tashimo, A.; Mitamura, Y.; Ohtsuka, K.; Okushiba, U.; Imaizumi, H.; Takeda, M. Macular hole formation following ruptured retinal arterial macroaneurysm. Am. J. Ophthalmol. 2003, 135, 487–492. [Google Scholar] [CrossRef]
- Tonotsuka, T.; Imai, M.; Saito, K.; Iijima, H. Visual prognosis for symptomatic retinal arterial macroaneurysm. Jpn. J. Ophthalmol. 2003, 47, 498–502. [Google Scholar] [CrossRef]
- Sato, R.; Yasukawa, T.; Hirano, Y.; Ogura, Y. Early-onset macular holes following ruptured retinal arterial macroaneurysms. Graefe's Arch. Clin. Exp. Ophthalmol. 2008, 246, 1779–1782. [Google Scholar] [CrossRef] [PubMed]
- Sagara, N.; Kawaji, T.; Koshiyama, Y.; Inomata, Y.; Fukushima, M.; Tanihara, H. Macular hole formation after macular haemorrhage associated with rupture of retinal arterial macroaneurysm. Br. J. Ophthalmol. 2009, 93, 1337–1340. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, K.; Ozturk, F.; Ozcan, P.Y. Treatment of multilevel macular hemorrhage secondary to retinal arterial macroaneurysm with submacular tissue plasminogen activator. Eur. J. Ophthalmol. 2012, 22, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Shiraga, F.; Shirakata, Y.; Morizane, Y.; Kimura, S.; Hirakata, A. Subretinal injection of recombinant tissue plasminogen activator for submacular hemorrhage associated with ruptured retinal arterial macroaneurysm. Graefe's Arch. Clin. Exp. Ophthalmol. 2014, 253, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Pierre, M.; Mainguy, A.; Chatziralli, I.; Pakzad-Vaezi, K.; Ruiz-Medrano, J.; Bodaghi, B.; Loewenstein, A.; Ambati, J.; de Smet, M.D.; Tadayoni, R.; et al. Macular Hemorrhage Due to Age-Related Macular Degeneration or Retinal Arterial Macroaneurysm: Predictive Factors of Surgical Outcome. J. Clin. Med. 2021, 10, 5787. [Google Scholar] [CrossRef]
- Humayun, M.; Lewis, H.; Flynn, H.W.; Sternberg, P.; Blumenkranz, M.S. Management of submacular hemorrhage associated with retinal arterial macroaneurysms. Am. J. Ophthalmol. 1998, 126, 358–361. [Google Scholar] [CrossRef]
- van Zeeburg, E.J.T.; Cereda, M.G.; van Meurs, J.C. Recombinant tissue plasminogen activator, vitrectomy, and gas for recent submacular hemorrhage displacement due to retinal macroaneurysm. Graefe's Arch. Clin. Exp. Ophthalmol. 2012, 251, 733–740. [Google Scholar] [CrossRef]
- Cakir, M.; Cekiç, O.; Yilmaz, F. Pneumatic displacement of acute submacular hemorrhage with and without the use of tissue plasminogen activator. Eur. J. Ophthalmol. 2010, 20, 565–571. [Google Scholar] [CrossRef]
- Hillenkamp, J.; Surguch, V.; Framme, C.; Gabel, V.-P.; Sachs, H.G. Management of submacular hemorrhage with intravitreal versus subretinal injection of recombinant tissue plasminogen activator. Graefe's Arch. Clin. Exp. Ophthalmol. 2009, 248, 5–11. [Google Scholar] [CrossRef]
- Ohji, M.; Saito, Y.; Hayashi, A.; Lewis, J.M.; Tano, Y. Pneumatic Displacement of Subretinal Hemorrhage Without Tissue Plasminogen Activator. Arch. Ophthalmol. 1998, 116, 1326–1332. [Google Scholar] [CrossRef]
- Ibanez, H.; Williams, D.F.; Thomas, M.A.; Ruby, A.J.; Meredith, T.A.; Boniuk, I.; Grand, M.G. Surgical Management of Submacular Hemorrhage. A Series of 47 Consecutive Cases. Arch. Ophthalmol. 1995, 113, 62–69. [Google Scholar] [CrossRef]
- Kokame, G.T. Vitreous hemorrhage after intravitreal tissue plasminogen activator (t-PA) and pneumatic displacement of submacular hemorrhage. Am. J. Ophthalmol. 2000, 129, 546–547. [Google Scholar] [CrossRef]
- Tsuiki, E.; Kusano, M.; Kitaoka, T. Complication associated with intravitreal injection of tissue plasminogen activator for treatment of submacular hemorrhage due to rupture of retinal arterial macroaneurysm. Am. J. Ophthalmol. Case Rep. 2019, 16, 100556. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
